Uncovering Key Factors in Graphene Aerogel-Based Electrocatalysts for Sustainable Hydrogen Production: An Unsupervised Machine Learning Approach
Abstract
1. Introduction
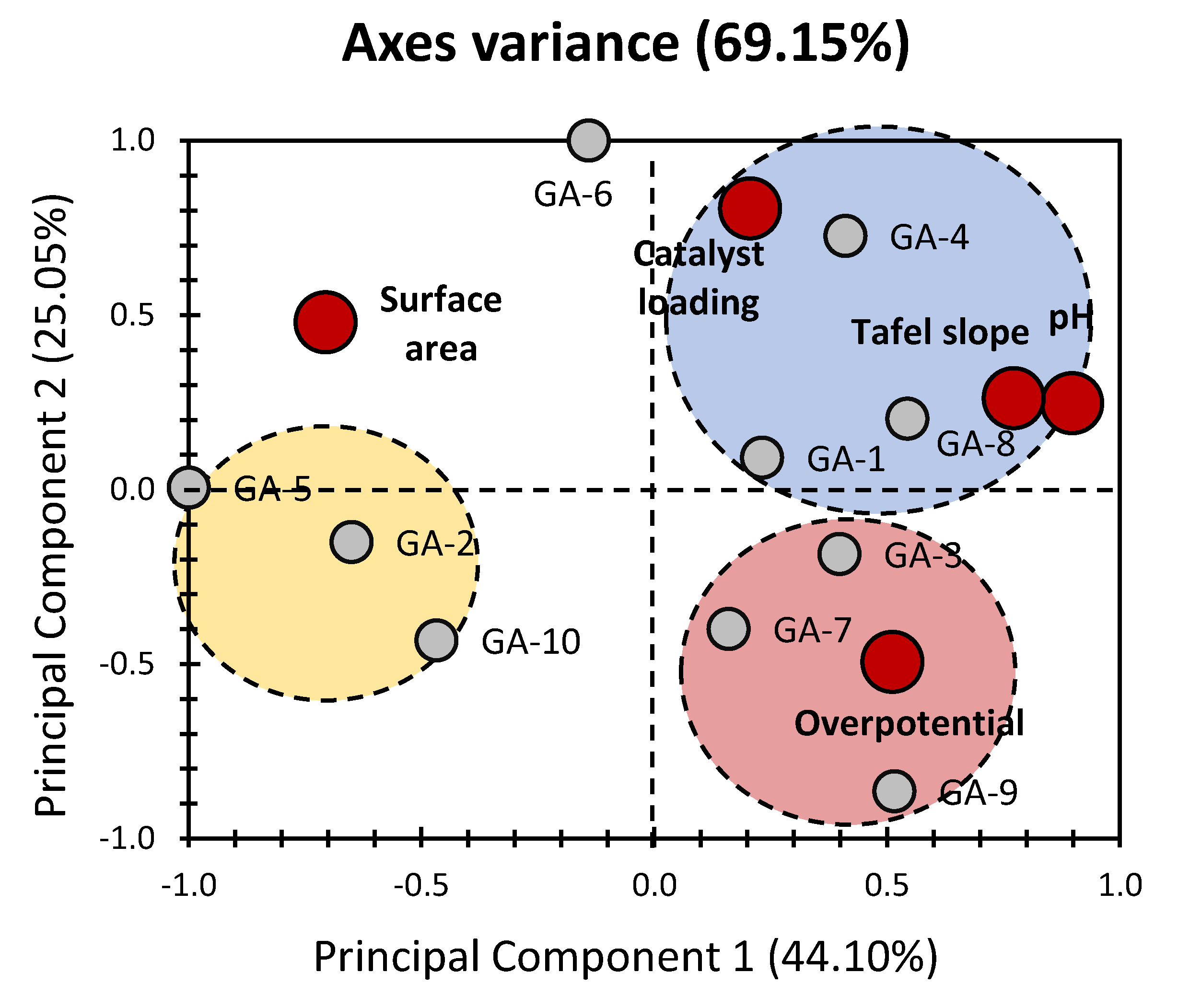
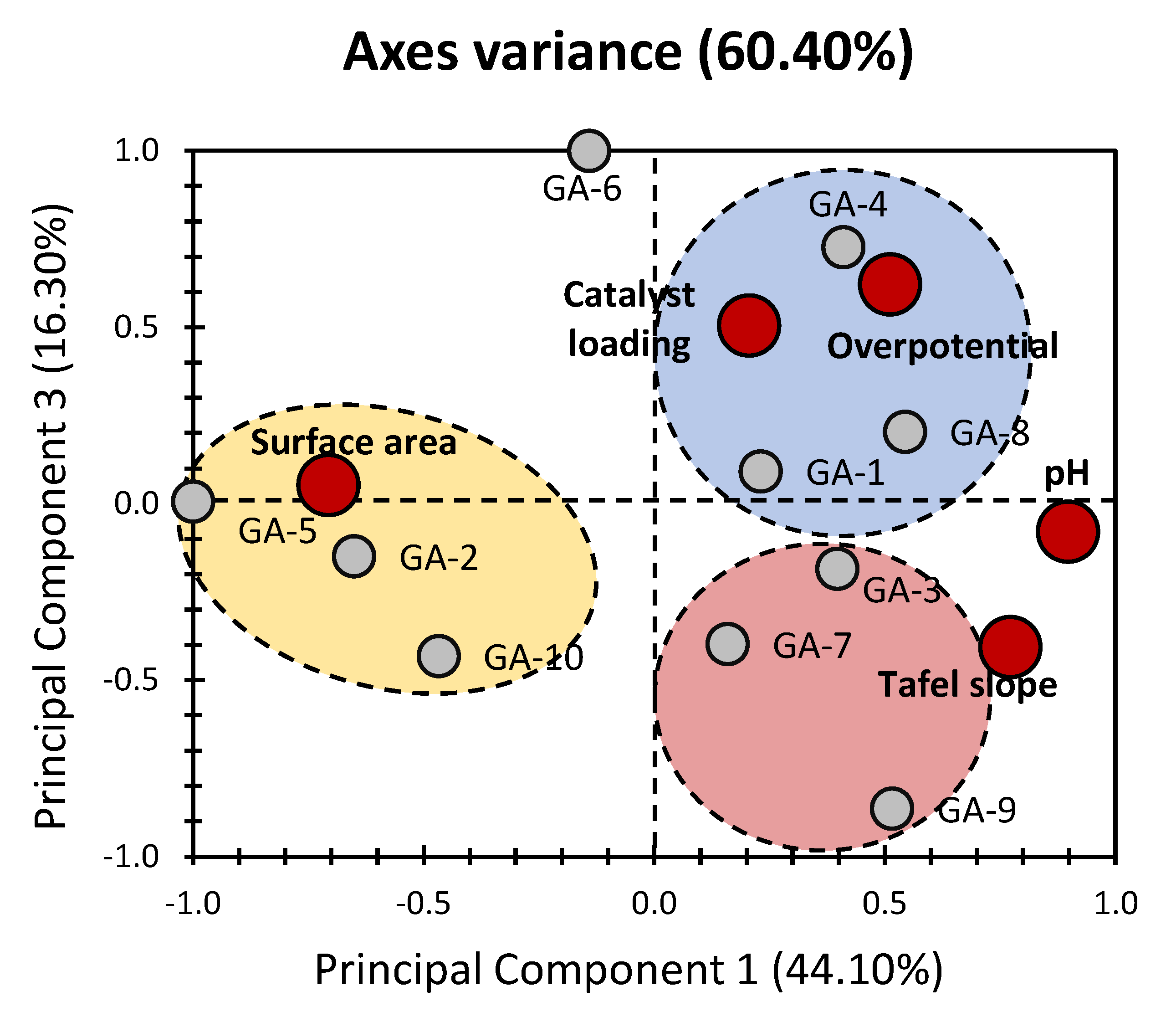
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Data Collection and Normalization
| Catalyst | Surface Area (m2 g−1) | pH | Catalyst Loading (µg cm−2) | Overpotential (mV) | Tafel Slope (mV dec−1) | References | |
|---|---|---|---|---|---|---|---|
| Ni3FeN/graphene aerogel | GA-1 | 171 | 14 | 500 | 94 | 90 | [38] |
| CoP/graphene aerogel | GA-2 | 532.2 | 0.1 | 280 | 121 | 61 | [39] |
| Ru/N-graphene aerogel | GA-3 | 244.8 | 13 | 100 | 145 | 109 | [40] |
| (Ni,Co)Se2/graphene aerogel | GA-4 | 123 | 14 | 2650 | 128 | 79 | [41] |
| Co-N-graphene aerogel | GA-5 | 466.6 | 0.1 | 275 | 50 | 33 | [42] |
| MoS2/graphene aerogel | GA-6 | 700 | 14 | 2000 | 120 | [43] | |
| CoP-C/graphene aerogel | GA-7 | 31.4 | 14 | 280 | 120 | 57 | [44] |
| WSe2/NiFe- LDH/N,S-graphene aerogel | GA-8 | 110 | 14 | 1000 | 122 | 112 | [45] |
| CoP-C/graphene aerogel | GA-9 | 31.4 | 14 | 280 | 225 | 66 | [44] |
| MoS2/graphene aerogel | GA-10 | 294 | 0.1 | 162 | 41 | [46] |
4.2. Principal Component Analysis (PCA)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaiswal, K.K.; Chowdhury, C.R.; Yadav, D.; Verma, R.; Dutta, S.; Jaiswal, K.S.; Sangmesh, B.; Karuppasamy, K.S.K. Renewable and Sustainable Clean Energy Development and Impact on Social, Economic, and Environmental Health. Energy Nexus 2022, 7, 100118. [Google Scholar] [CrossRef]
- Fund, S. Sustainable Development Goals. 2015. Available online: https://www.un.org/sustainabledevelopment/inequality (accessed on 29 December 2023).
- Al-Hamamre, Z.; Karimzadeh, Z.; Ji, S.; Choi, H.; Maleki, H. Aerogels-Inspired Based Photo and Electrocatalyst for Water Splitting to Produce Hydrogen. Appl. Mater. Today 2022, 29, 101670. [Google Scholar] [CrossRef]
- Ursua, A.; Gandia, L.M.; Sanchis, P. Hydrogen Production from Water Electrolysis: Current Status and Future Trends. Proc. IEEE 2011, 100, 410–426. [Google Scholar] [CrossRef]
- Caravaca, A.; Garcia-Lorefice, W.E.; Gil, S.; de Lucas-Consuegra, A.; Vernoux, P. Towards a Sustainable Technology for H2 Production: Direct Lignin Electrolysis in a Continuous-Flow Polymer Electrolyte Membrane Reactor. Electrochem. Commun. 2019, 100, 43–47. [Google Scholar] [CrossRef]
- Du, C.; Dinh, K.N.; Liang, Q.; Zheng, Y.; Luo, Y.; Zhang, J.; Yan, Q. Self-Assemble and In Situ Formation of Ni1−xFexPS3 Nanomosaic-Decorated MXene Hybrids for Overall Water Splitting. Adv. Energy Mater. 2018, 8, 1801127. [Google Scholar] [CrossRef]
- Bao, F.; Kemppainen, E.; Dorbandt, I.; Bors, R.; Xi, F.; Schlatmann, R.; van de Krol, R.; Calnan, S. Understanding the Hydrogen Evolution Reaction Kinetics of Electrodeposited Nickel-Molybdenum in Acidic, Near-Neutral, and Alkaline Conditions. ChemElectroChem 2021, 8, 195–208. [Google Scholar] [CrossRef]
- Xue, Y.; Li, J.; Xue, Z.; Li, Y.; Liu, H.; Li, D.; Yang, W.; Li, Y. Extraordinarily Durable Graphdiyne-Supported Electrocatalyst with High Activity for Hydrogen Production at All Values of pH. ACS Appl. Mater. Interfaces 2016, 8, 31083–31091. [Google Scholar] [CrossRef]
- Luo, F.; Zhang, Q.; Yu, X.; Xiao, S.; Ling, Y.; Hu, H.; Guo, L.; Yang, Z.; Huang, L.; Cai, W.; et al. Palladium Phosphide as a Stable and Efficient Electrocatalyst for Overall Water Splitting. Angew. Chem. Int. Ed. 2018, 57, 14862–14867. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, S.; Zhao, K.; Muqsit, A.; Tang, H.; Chang, L.; Zhao, H.; Gao, Y.; Tang, Z. Ultrathin Platinum Nanowires Grown on Single-Layered Nickel Hydroxide with High Hydrogen Evolution Activity. Nat. Commun. 2015, 6, 6430. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble Metal-Free Hydrogen Evolution Catalysts for Water Splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Chatenet, M.; Pollet, B.G.; Dekel, D.R.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R.D.; Bazant, M.Z.; Eikerling, M.; Staffell, I. Water Electrolysis: From Textbook Knowledge to the Latest Scientific Strategies and Industrial Developments. Chem. Soc. Rev. 2022, 51, 4583–4762. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-K.; Chung, D.Y.; Ko, D.; Sung, Y.-E.; Piao, Y. Three-Dimensional Carbon Foam/N-Doped graphene@MoS2 Hybrid Nanostructures as Effective Electrocatalysts for the Hydrogen Evolution Reaction. J. Mater. Chem. A 2016, 4, 12720–12725. [Google Scholar] [CrossRef]
- Du, R.; Zhao, Q.; Zhang, N.; Zhang, J. Macroscopic Carbon Nanotube-based 3D Monoliths. Small 2015, 11, 3263–3289. [Google Scholar] [CrossRef]
- Katsounaros, I.; Cherevko, S.; Zeradjanin, A.R.; Mayrhofer, K.J.J. Oxygen Electrochemistry as a Cornerstone for Sustainable Energy Conversion. Angew. Chem. Int. Ed. 2014, 53, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Advancing the Electrochemistry of the Hydrogen-Evolution Reaction through Combining Experiment and Theory. Angew. Chem. Int. Ed. 2015, 54, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer Aerogels and Foams: Chemistry, Properties, and Applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef]
- Koo, S.H.; Li, D.J.; Yun, T.; Choi, D.S.; Lee, K.E.; Lee, G.Y.; Oh, Y.; Lim, J.; Padmajan Sasikala, S.; Lee, H.J.; et al. Cobalt Based Nanoparticles Embedded Reduced Graphene Oxide Aerogel for Hydrogen Evolution Electrocatalyst. Part. Part. Syst. Charact. 2019, 36, 1900090. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, J.; Pan, L.; Shi, Y.; Yu, G. Energy Gels: A Bio-Inspired Material Platform for Advanced Energy Applications. Nano Today 2016, 11, 738–762. [Google Scholar] [CrossRef]
- Maleki, H.; Hüsing, N. Current Status, Opportunities and Challenges in Catalytic and Photocatalytic Applications of Aerogels: Environmental Protection Aspects. Appl. Catal. B Environ. 2018, 221, 530–555. [Google Scholar] [CrossRef]
- Huo, Z.; Zhao, P.; Miu, P.; Ren, L.; Tan, B.; Feng, N.; Wan, H.; Guan, G. Enhanced Catalytic Oxidation of Soot over 3DOM LaMnO3 by Adding Ag and CeO2: Improving the Generation and Delivery of Active Oxygen Species. Appl. Surf. Sci. 2022, 600, 154204. [Google Scholar] [CrossRef]
- Zhai, G.; Wang, J.; Chen, Z.; Yang, S.; Men, Y. Highly Enhanced Soot Oxidation Activity over 3DOM Co3O4-CeO2 Catalysts by Synergistic Promoting Effect. J. Hazard. Mater. 2019, 363, 214–226. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Chen, G.; Zeng, G.; Chen, A.; Huang, Z.; Shi, J.; Huang, T.; Peng, M.; Hu, L. Three-Dimensional Graphene Supported Catalysts for Organic Dyes Degradation. Appl. Catal. B Environ. 2018, 228, 19–28. [Google Scholar] [CrossRef]
- Chen, J.; Wu, P.; Bu, F.; Gao, Y.; Liu, X.; Guan, C. 3D Printing Enhanced Catalysis for Energy Conversion and Environment Treatment. DeCarbon 2023, 2, 100019. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, P.; Chao, Y.; Yu, J.; Zhu, W.; Liu, Z.; Xu, C. Recent Advances in 3D Printing for Catalytic Applications. Chem. Eng. J. 2022, 433, 134341. [Google Scholar] [CrossRef]
- Ziegler, C.; Wolf, A.; Liu, W.; Herrmann, A.; Gaponik, N.; Eychmüller, A. Modern Inorganic Aerogels. Angew. Chem. Int. Ed. 2017, 56, 13200–13221. [Google Scholar] [CrossRef]
- Cai, B.; Eychmüller, A. Promoting Electrocatalysis upon Aerogels. Adv. Mater. 2019, 31, 1804881. [Google Scholar] [CrossRef]
- Bandar Abadi, M.; Weissing, R.; Wilhelm, M.; Demidov, Y.; Auer, J.; Ghazanfari, S.; Anasori, B.; Mathur, S.; Maleki, H. Nacre-Mimetic, Mechanically Flexible, and Electrically Conductive Silk Fibroin-MXene Composite Foams as Piezoresistive Pressure Sensors. ACS Appl. Mater. Interfaces 2021, 13, 34996–35007. [Google Scholar] [CrossRef]
- Mohanan, J.L.; Arachchige, I.U.; Brock, S.L. Porous Semiconductor Chalcogenide Aerogels. Science 2005, 307, 397–400. [Google Scholar] [CrossRef]
- Du, R.; Gao, X.; Feng, Q.; Zhao, Q.; Li, P.; Deng, S.; Shi, L.; Zhang, J. Microscopic Dimensions Engineering: Stepwise Manipulation of the Surface Wettability on 3D Substrates for Oil/Water Separation. Adv. Mater. 2016, 28, 936–942. [Google Scholar] [CrossRef]
- Du, R.; Hu, Y.; Hübner, R.; Joswig, J.-O.; Fan, X.; Schneider, K.; Eychmüller, A. Specific Ion Effects Directed Noble Metal Aerogels: Versatile Manipulation for Electrocatalysis and Beyond. Sci. Adv. 2019, 5, eaaw4590. [Google Scholar] [CrossRef]
- Park, H.; Kim, H.; Moon, G.; Choi, W. Photoinduced Charge Transfer Processes in Solar Photocatalysis Based on Modified TiO2. Energy Environ. Sci. 2016, 9, 411–433. [Google Scholar] [CrossRef]
- Zalitis, C.M.; Sharman, J.; Wright, E.; Kucernak, A.R. Properties of the Hydrogen Oxidation Reaction on Pt/C Catalysts at Optimised High Mass Transport Conditions and Its Relevance to the Anode Reaction in PEFCs and Cathode Reactions in Electrolysers. Electrochim. Acta 2015, 176, 763–776. [Google Scholar] [CrossRef]
- Obeid, E.; Lizarraga, L.; Tsampas, M.N.; Cordier, A.; Boréave, A.; Steil, M.C.; Blanchard, G.; Pajot, K.; Vernoux, P. Continuously Regenerating Diesel Particulate Filters Based on Ionically Conducting Ceramics. J. Catal. 2014, 309, 87–96. [Google Scholar] [CrossRef]
- Ďurovič, M.; Hnát, J.; Bouzek, K. Electrocatalysts for the Hydrogen Evolution Reaction in Alkaline and Neutral Media. A Comparative Review. J. Power Sources 2021, 493, 229708. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.-J. Hydrogen Production from Water Electrolysis: Role of Catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- Murshid, N.; Mouhtady, O.; Abu-Samha, M.; Obeid, E.; Kharboutly, Y.; Chaouk, H.; Halwani, J.; Younes, K. Metal Oxide Hydrogel Composites for Remediation of Dye-Contaminated Wastewater: Principal Component Analysis. Gels 2022, 8, 702. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, S.; Ren, J.; Jia, Y.A.; Chen, C.; Komarneni, S.; Yang, D.; Yao, X. Electronic Structure Tuning in Ni3FeN/r-GO Aerogel toward Bifunctional Electrocatalyst for Overall Water Splitting. ACS Nano 2018, 12, 245–253. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Huang, L.; Dong, S. 3D Graphene Aerogels Decorated with Cobalt Phosphide Nanoparticles as Electrocatalysts for the Hydrogen Evolution Reaction. ChemSusChem 2016, 9, 3049–3053. [Google Scholar] [CrossRef]
- Zhu, B.; Qu, C.; Gao, S.; Liang, Z.; Zhang, H.; Zou, R. Ultralow Loading Ruthenium Nanoparticles on Nitrogen-Doped Graphene Aerogel for Trifunctional Electrocatalysis. ChemCatChem 2018, 10, 1113–1121. [Google Scholar] [CrossRef]
- Xu, X.; Liang, H.; Ming, F.; Qi, Z.; Xie, Y.; Wang, Z. Prussian Blue Analogues Derived Penroseite (Ni,Co)Se2 Nanocages Anchored on 3D Graphene Aerogel for Efficient Water Splitting. ACS Catal. 2017, 7, 6394–6399. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, Y.; Guan, Y.; Xue, J.; Cui, L. Construction of a Cobalt-Embedded Nitrogen-Doped Carbon Material with the Desired Porosity Derived from the Confined Growth of MOFs within Graphene Aerogels as a Superior Catalyst towards HER and ORR. J. Mater. Chem. A 2016, 4, 15536–15545. [Google Scholar] [CrossRef]
- Worsley, M.A.; Shin, S.J.; Merrill, M.D.; Lenhardt, J.; Nelson, A.J.; Woo, L.Y.; Gash, A.E.; Baumann, T.F.; Orme, C.A. Ultralow Density, Monolithic WS2, MoS2, and MoS2/Graphene Aerogels. ACS Nano 2015, 9, 4698–4705. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Lu, X.; Selvaraj, M.; Wei, W.; Jiang, Z.; Ullah, N.; Liu, J.; Xie, J. MXP (M = Co/Ni)@ Carbon Core–Shell Nanoparticles Embedded in 3D Cross-Linked Graphene Aerogel Derived from Seaweed Biomass for Hydrogen Evolution Reaction. Nanoscale 2018, 10, 9698–9706. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chu, H.; Zhang, Z.; Dong, P.; Baines, R.; Ajayan, P.M.; Shen, J.; Ye, M. Integrated Energy Aerogel of N,S-rGO/WSe2/NiFe-LDH for Both Energy Conversion and Storage. ACS Appl. Mater. Interfaces 2017, 9, 32756–32766. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, X.; Zhang, J.; Liu, H.; Ahn, H.; Sun, K.; Wang, G. MoS2 Nanosheets Supported on 3D Graphene Aerogel as a Highly Efficient Catalyst for Hydrogen Evolution. Chem. A Eur. J 2015, 21, 15908–15913. [Google Scholar] [CrossRef]
- Younes, K.; Kharboutly, Y.; Antar, M.; Chaouk, H.; Obeid, E.; Mouhtady, O.; Abu-Samha, M.; Halwani, J.; Murshid, N. Application of Unsupervised Learning for the Evaluation of Aerogels’ Efficiency towards Dye Removal—A Principal Component Analysis (PCA) Approach. Gels 2023, 9, 327. [Google Scholar] [CrossRef]
- Joliffe, I.; Morgan, B. Principal Component Analysis and Exploratory Factor Analysis. Stat. Methods Med. Res. 1992, 1, 69–95. [Google Scholar] [CrossRef]
- Younes, K.; Moghrabi, A.; Moghnie, S.; Mouhtady, O.; Murshid, N.; Grasset, L. Assessment of the Efficiency of Chemical and Thermochemical Depolymerization Methods for Lignin Valorization: Principal Component Analysis (PCA) Approach. Polymers 2022, 14, 194. [Google Scholar] [CrossRef]
- Gazo Hanna, E.; Younes, K.; Amine, S.; Roufayel, R. Exploring Gel-Point Identification in Epoxy Resin Using Rheology and Unsupervised Learning. Gels 2023, 9, 828. [Google Scholar] [CrossRef]
- Hanna, E.G.; Poitou, A.; Casari, P. Modeling the Interply Slip during Forming of Thermoplastic Laminates. Mater. Phys. Mech. 2018, 65, 22–36. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obeid, E.; Younes, K. Uncovering Key Factors in Graphene Aerogel-Based Electrocatalysts for Sustainable Hydrogen Production: An Unsupervised Machine Learning Approach. Gels 2024, 10, 57. https://doi.org/10.3390/gels10010057
Obeid E, Younes K. Uncovering Key Factors in Graphene Aerogel-Based Electrocatalysts for Sustainable Hydrogen Production: An Unsupervised Machine Learning Approach. Gels. 2024; 10(1):57. https://doi.org/10.3390/gels10010057
Chicago/Turabian StyleObeid, Emil, and Khaled Younes. 2024. "Uncovering Key Factors in Graphene Aerogel-Based Electrocatalysts for Sustainable Hydrogen Production: An Unsupervised Machine Learning Approach" Gels 10, no. 1: 57. https://doi.org/10.3390/gels10010057
APA StyleObeid, E., & Younes, K. (2024). Uncovering Key Factors in Graphene Aerogel-Based Electrocatalysts for Sustainable Hydrogen Production: An Unsupervised Machine Learning Approach. Gels, 10(1), 57. https://doi.org/10.3390/gels10010057








