Self-Assembled Aminated and TEMPO Cellulose Nanofibers (Am/TEMPO-CNF) Aerogel for Adsorptive Removal of Oxytetracycline and Chloramphenicol Antibiotics from Water
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Am/TEMPO-CNF
2.2. Adsorption Studies
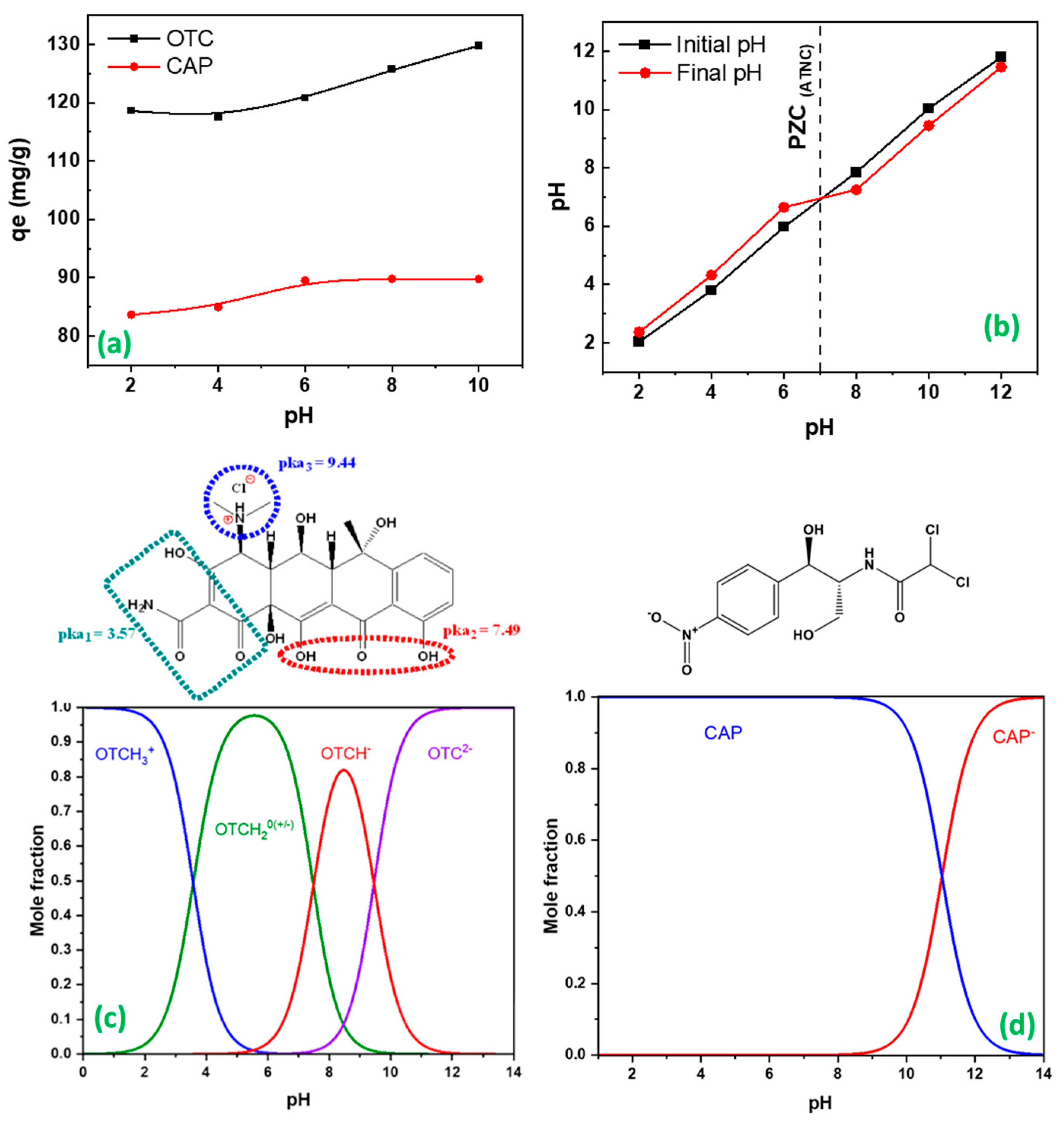
2.2.1. pH Influence
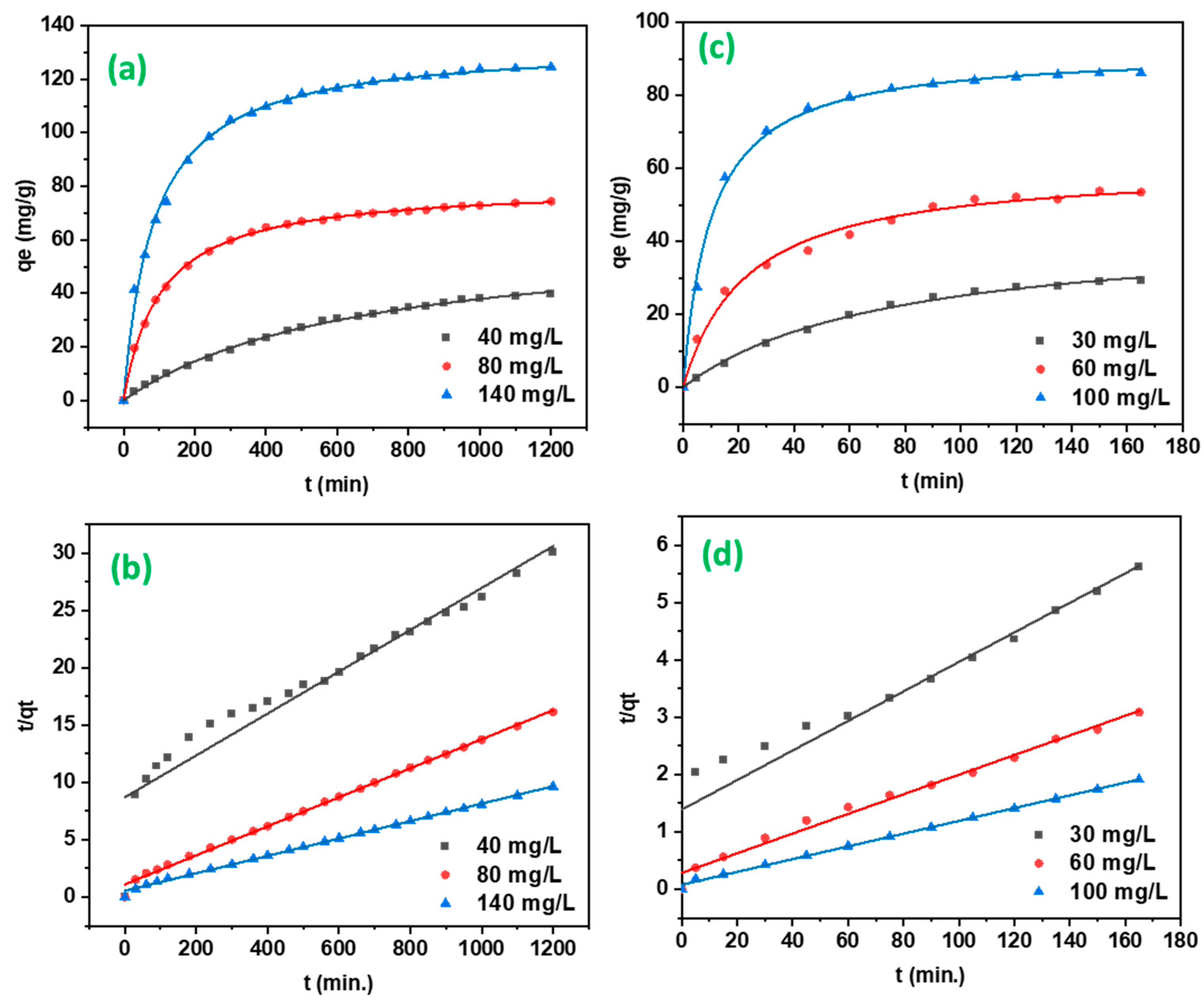
2.2.2. Adsorption Kinetics
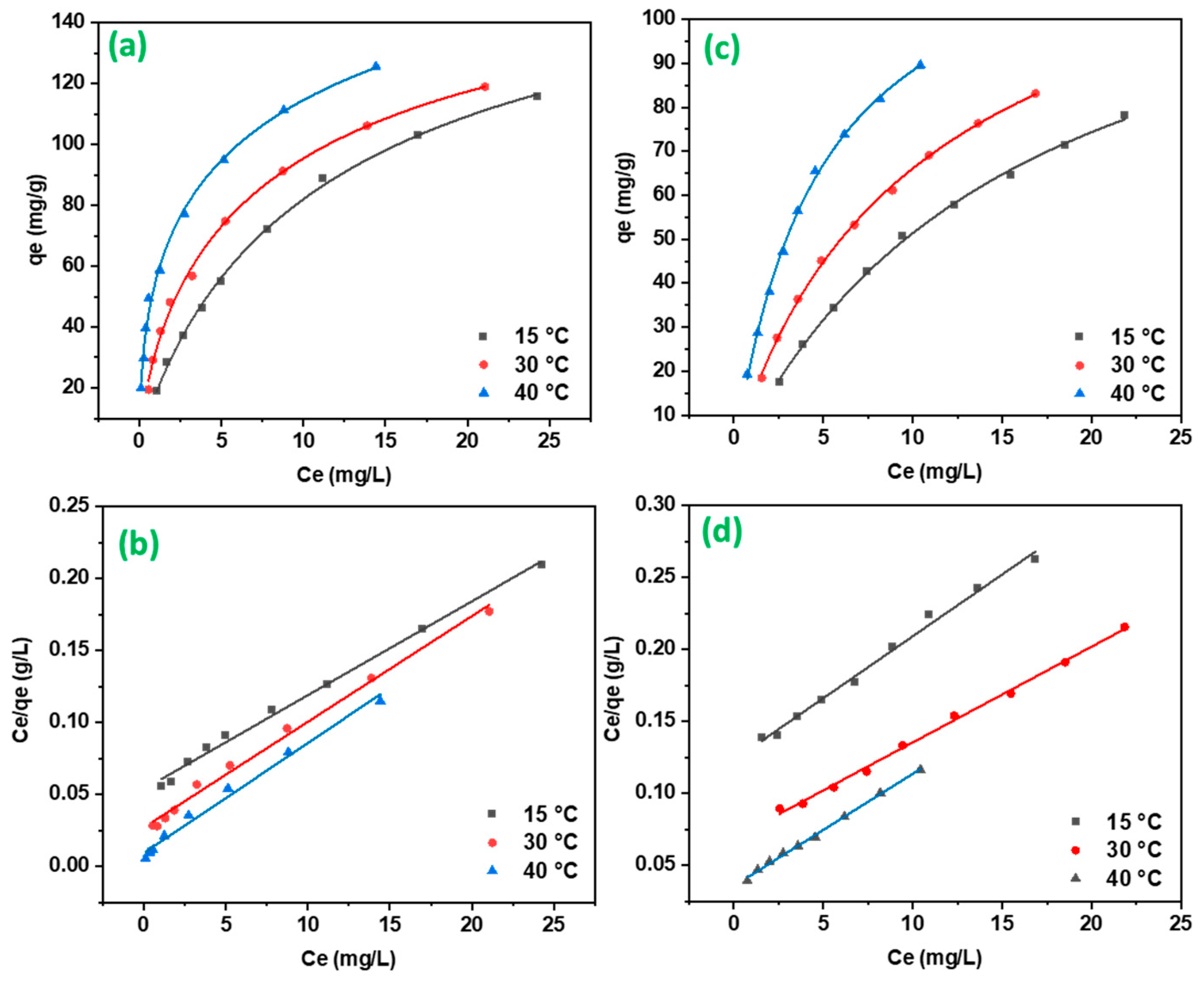
2.2.3. Adsorption Isotherms
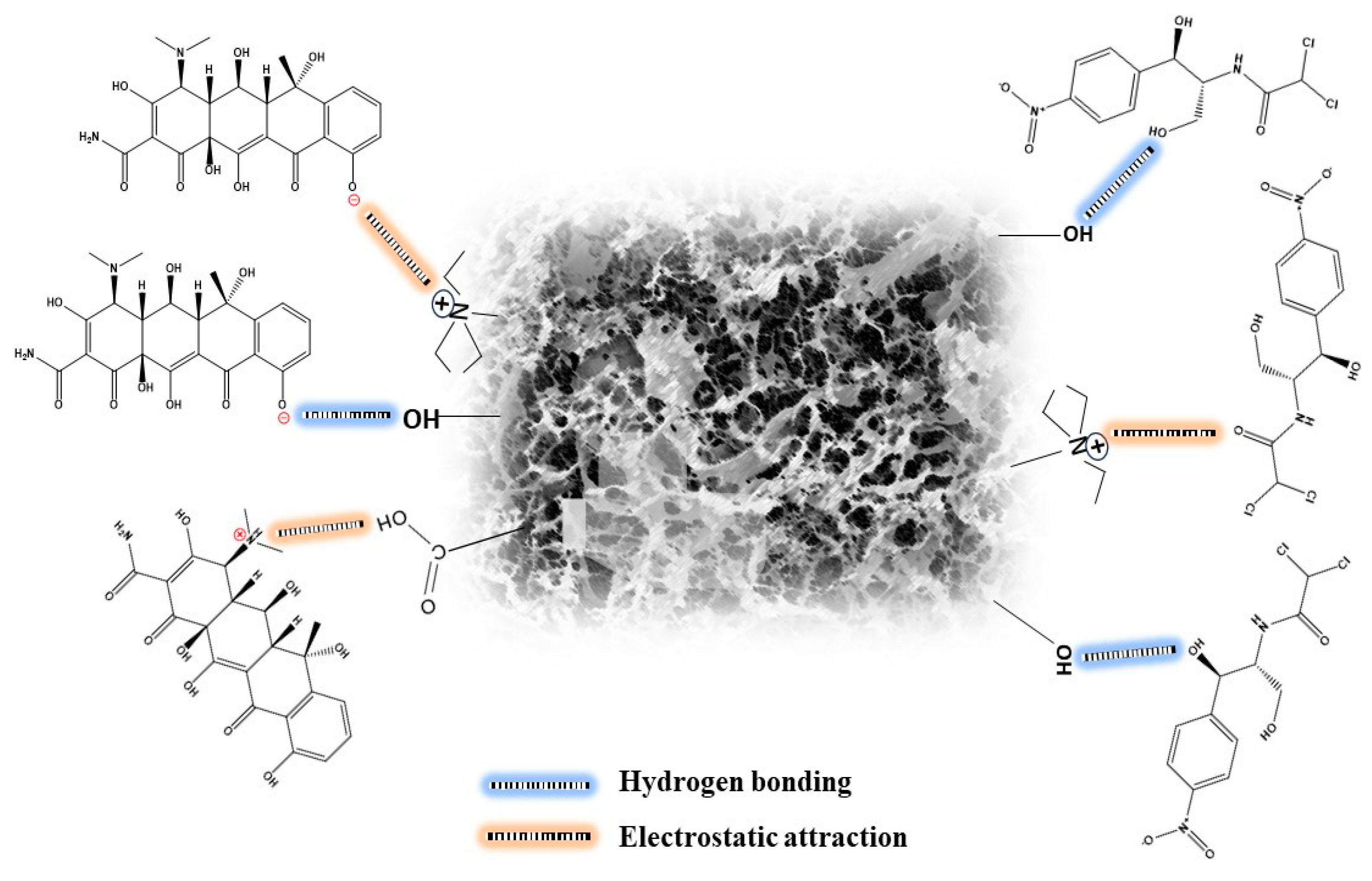
2.2.4. Removal Mechanism
2.2.5. Adsorption Thermodynamics
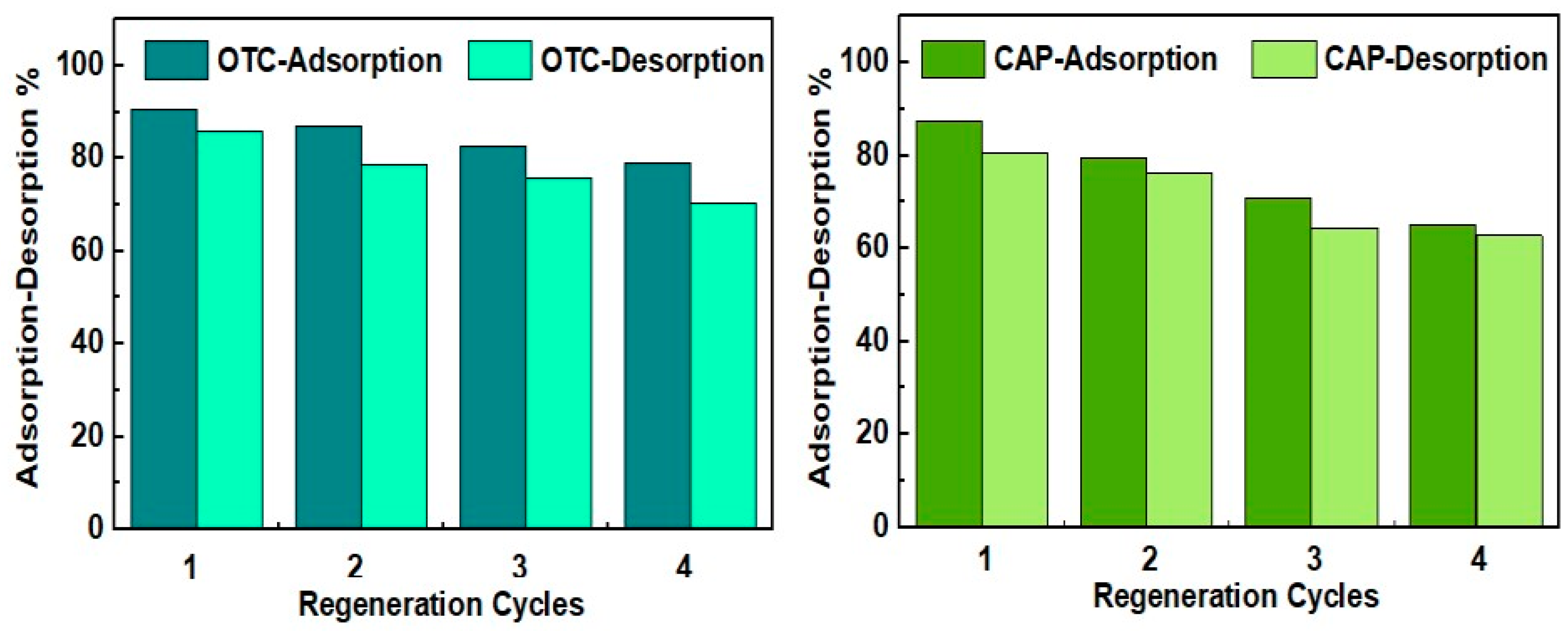
2.2.6. Regeneration Study
3. Conclusions
4. Materials and Methods
4.1. Materials
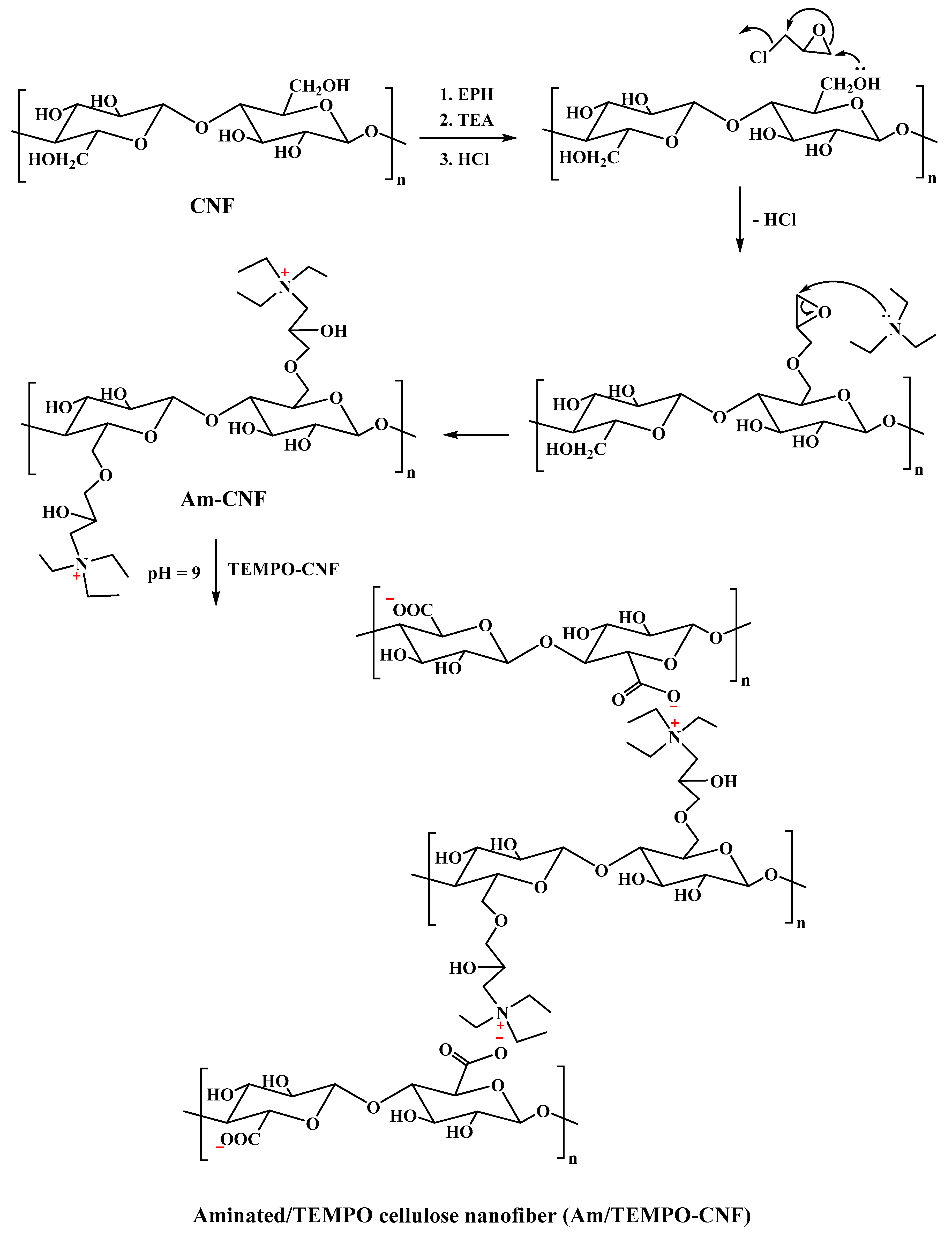
4.2. Aminated Cellulose Nanofiber (Am-CNF) Synthesis
4.3. Aminated/TEMPO Cellulose Nanofiber (Am/TEMPO-CNF) Aerogel Synthesis
4.4. Aerogel Characterization
4.5. Point of Zero Charge (PZC)
4.6. Adsorption Experiments
4.6.1. Effect of pH
4.6.2. Adsorption Kinetics
4.6.3. Adsorption Isotherms
4.6.4. Adsorption Thermodynamics
4.6.5. Reusability Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, Y.; Li, S.; Xie, P.; Chen, X.; Li, X.; Ho, S.-H. New insight into the concentration-dependent removal of multiple antibiotics by Chlorella sorokiniana. Bioresour. Technol. 2023, 385, 129409. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, R.; Pan, C.; Sun, Y.; Mai, B.; Li, Q.X. Antibiotics and food safety in aquaculture. J. Agric. Food Chem. 2020, 68, 11908–11919. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; He, S.; Shi, G.; Ma, Y.; Ruan, C.; Jin, X.; Chen, Q.; Liu, X.; Dai, H.; Chen, X. In-situ immobilization of ZIF-67 on wood aerogel for effective removal of tetracycline from water. Chem. Eng. J. 2021, 423, 130184. [Google Scholar] [CrossRef]
- Ruan, C.; Chen, G.; Ma, Y.; Du, C.; He, C.; Liu, X.; Jin, X.; Chen, Q.; He, S.; Huang, Y. PVA-assisted CNCs/SiO2 composite aerogel for efficient sorption of ciprofloxacin. J. Colloid Interface Sci. 2023, 630, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Cha, B.; Kim, N.; Yea, Y.; Han, J.; Yoon, Y.; Kim, S.; Park, C.M. Comprehensive evaluation of antibiotic tetracycline and oxytetracycline removal by Fe-metal organic framework/biopolymer-clay hydrogel. Ceram. Int. 2023, 49, 12201–12213. [Google Scholar] [CrossRef]
- Zhou, H.; Jiao, G.; Li, X.; Gao, C.; Zhang, Y.; Hashan, D.; Liu, J.; She, D. High capacity adsorption of oxytetracycline by lignin-based carbon with mesoporous structure: Adsorption behavior and mechanism. Int. J. Biol. Macromol. 2023, 234, 123689. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, A.P.; Kumar, S.; Giri, B.S.; Kim, K.-H. Antibiotic resistance in major rivers in the world: A systematic review on occurrence, emergence, and management strategies. J. Clean. Prod. 2019, 234, 1484–1505. [Google Scholar] [CrossRef]
- Ben, W.; Pan, X.; Qiang, Z. Occurrence and partition of antibiotics in the liquid and solid phases of swine wastewater from concentrated animal feeding operations in Shandong Province, China. Environ. Sci. Process. Impacts 2013, 15, 870–875. [Google Scholar] [CrossRef]
- Liu, X.; Pei, Y.; Cao, M.; Yang, H.; Li, Y. Magnetic CuFe2O4 nanoparticles anchored on N-doped carbon for activated peroxymonosulfate removal of oxytetracycline from water: Radical and non-radical pathways. Chemosphere 2023, 334, 139025. [Google Scholar] [CrossRef]
- Jin, W.; Feng, J.; Xing, W.; Tang, W. Significantly Enhanced Electrocatalytic Reduction of Chloramphenicol from Water Mediated by Fe–F–N Co-doped Carbon Materials. ACS EST Water 2023, 3, 1385–1394. [Google Scholar] [CrossRef]
- Lv, Y.; Han, L.; Shen, J.; Li, J.; Dong, H.; Hu, J.; He, Y.; Li, Y. Impact of environmental factors on the removal of chloramphenicol by zero-valent iron and pyrite mixture. Sep. Purif. Technol. 2024, 329, 125045. [Google Scholar] [CrossRef]
- Mokni, S.; Tlili, M.; Jedidi, N.; Hassen, A. Applicability of electrocoagulation process to the treatment of Ofloxacin and Chloramphenicol in aqueous media: Removal mechanism and antibacterial activity. J. Water Process Eng. 2022, 49, 103080. [Google Scholar] [CrossRef]
- Nguyen, L.M.; Nguyen, N.T.T.; Nguyen, T.T.T.; Nguyen, T.T.; Nguyen, D.T.C.; Tran, T.V. Occurrence, toxicity and adsorptive removal of the chloramphenicol antibiotic in water: A review. Environ. Chem. Lett. 2022, 20, 1929–1963. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yuan, X.; Wu, X.; Liu, L.; Guo, H.; Xu, K.; Zhang, L.; Du, G. Preparation of nanocellulose-based aerogel and its research progress in wastewater treatment. Molecules 2023, 28, 3541. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Fan, B.; Xiong, Y.; Jin, C.; Sun, Q.; Sheng, C. 3D assembly based on 2D structure of cellulose nanofibril/graphene oxide hybrid aerogel for adsorptive removal of antibiotics in water. Sci. Rep. 2017, 7, 45914. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Huang, H.; Liang, D.; Xie, Y.; Kong, F.; Yang, Q.; Fu, J.; Dou, Z.; Zhang, Q.; Meng, Z. Adsorption of tetracycline hydrochloride on layered double hydroxide loaded carbon nanotubes and site energy distribution analysis. Chem. Eng. J. 2022, 443, 136398. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, X.; Ding, R.; Huang, H.; Gao, X.; Liu, B. Post-synthesis introduction of dual functional groups in metal–organic framework for enhanced adsorption of moxifloxacin antibiotic. J. Colloid Interface Sci. 2023, 639, 59–67. [Google Scholar] [CrossRef]
- Pang, W.; Wang, Y.; Li, S.; Luo, Y.; Wang, G.; Hou, J.; Han, T.; Gao, Z.; Guo, Q.; Zhou, H. Novel magnetic graphoxide/biochar composite derived from tea for multiple SAs and QNs antibiotics removal in water. Environ. Sci. Pollut. Res. 2023, 30, 43215–43228. [Google Scholar] [CrossRef]
- Selvaraj, R.; Prabhu, D.; Kumar, P.S.; Rangasamy, G.; Murugesan, G.; Rajesh, M.; Goveas, L.C.; Varadavenkatesan, T.; Samanth, A.; Balakrishnaraja, R. Adsorptive removal of tetracycline from aqueous solutions using magnetic Fe2O3/activated carbon prepared from Cynometra ramiflora fruit waste. Chemosphere 2023, 310, 136892. [Google Scholar] [CrossRef]
- Ahmad, A.; Kamaruddin, M.A.; HPS, A.K.; Yahya, E.B.; Muhammad, S.; Rizal, S.; Ahmad, M.I.; Surya, I.; Abdullah, C. Recent Advances in Nanocellulose Aerogels for Efficient Heavy Metal and Dye Removal. Gels 2023, 9, 416. [Google Scholar] [CrossRef]
- Nan, Y.; Gomez-Maldonado, D.; Iglesias, M.C.; Whitehead, D.C.; Peresin, M.S. Valorized soybean hulls as TEMPO-oxidized cellulose nanofibril and polyethylenimine composite hydrogels and their potential removal of water pollutants. Cellulose 2023, 30, 3639–3651. [Google Scholar] [CrossRef]
- Do, N.H.; Truong, B.Y.; Nguyen, P.T.; Le, K.A.; Duong, H.M.; Le, P.K. Composite aerogels of TEMPO-oxidized pineapple leaf pulp and chitosan for dyes removal. Sep. Purif. Technol. 2022, 283, 120200. [Google Scholar] [CrossRef]
- Alothman, Z. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Van Hoomissen, D.J.; Vyas, S. Early events in the reductive dehalogenation of linear perfluoroalkyl substances. Environ. Sci. Technol. Lett. 2019, 6, 365–371. [Google Scholar] [CrossRef]
- Pei, A.; Butchosa, N.; Berglund, L.A.; Zhou, Q. Surface quaternized cellulose nanofibrils with high water absorbency and adsorption capacity for anionic dyes. Soft Matter 2013, 9, 2047–2055. [Google Scholar] [CrossRef]
- Li, D.; Lee, C.-S.; Zhang, Y.; Das, R.; Akter, F.; Venkatesan, A.K.; Hsiao, B.S. Efficient removal of short-chain and long-chain PFAS by cationic nanocellulose. J. Mater. Chem. A 2023, 11, 9868–9883. [Google Scholar] [CrossRef]
- Saini, S.; Yücel Falco, Ç.; Belgacem, M.N.; Bras, J. Surface cationized cellulose nanofibrils for the production of contact active antimicrobial surfaces. Carbohydr. Polym. 2016, 135, 239–247. [Google Scholar] [CrossRef]
- Soni, B.; Hassan, E.B.; Mahmoud, B. Chemical isolation and characterization of different cellulose nanofibers from cotton stalks. Carbohydr. Polym. 2015, 134, 581–589. [Google Scholar] [CrossRef]
- Mahendra, I.P.; Wirjosentono, B.; Tamrin, I.H.; Mendez, J.A. Thermal and Morphology Properties of Cellulose Nanofiber from TEMPO-oxidized Lower part of Empty Fruit Bunches (LEFB). Open Chem. 2019, 17, 526–536. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Wang, S.; Jiang, H.; Liu, S.; Yao, Y.; Zhang, T.; Li, Q. Synthesis and characterization of amine-modified spherical nanocellulose aerogels. J. Mater. Sci. 2018, 53, 13304–13315. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, M.; Jang, J.; Han, M.; Moon, Y.; Kim, J.; You, J.; Li, S.; Park, T.; Kim, J. Amino-functionalized nanocellulose aerogels for the superior adsorption of CO2 and separation of CO2/CH4 mixture gas. Carbohydr. Polym. 2024, 323, 121393. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Levanic, J.; Šenk, V.P.; Nadrah, P.; Poljanšek, I.; Oven, P.; Haapala, A. Analyzing TEMPO-oxidized cellulose fiber morphology: New insights into optimization of the oxidation process and nanocellulose dispersion quality. ACS Sustain. Chem. Eng. 2020, 8, 17752–17762. [Google Scholar] [CrossRef]
- Ferchichi, K.; Amdouni, N.; Chevalier, Y.; Hbaieb, S. Low-cost Posidonia oceanica bio-adsorbent for efficient removal of antibiotic oxytetracycline from water. Environ. Sci. Pollut. Res. 2022, 29, 83112–83125. [Google Scholar] [CrossRef] [PubMed]
- Punamiya, P.; Sarkar, D.; Rakshit, S.; Datta, R. Effectiveness of Aluminum-based Drinking Water Treatment Residuals as a Novel Sorbent to Remove Tetracyclines from Aqueous Medium. J. Environ. Qual. 2013, 42, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Liu, H. Removal of Chloramphenicol from Aqueous Solution Using Low-Cost Activated Carbon Prepared from Typha orientalis. Water 2018, 10, 351. [Google Scholar] [CrossRef]
- Mitchell, S.M.; Ullman, J.L.; Teel, A.L.; Watts, R.J. Hydrolysis of amphenicol and macrolide antibiotics: Chloramphenicol, florfenicol, spiramycin, and tylosin. Chemosphere 2015, 134, 504–511. [Google Scholar] [CrossRef]
- Peng, H.; Xiong, W.; Yang, Z.; Cao, J.; Jia, M.; Xiang, Y.; Hu, Q.; Xu, Z. Facile fabrication of three-dimensional hierarchical porous ZIF-L/gelatin aerogel: Highly efficient adsorbent with excellent recyclability towards antibiotics. Chem. Eng. J. 2021, 426, 130798. [Google Scholar] [CrossRef]
- Wu, Y.; Yue, Q.; Ren, Z.; Gao, B. Immobilization of nanoscale zero-valent iron particles (nZVI) with synthesized activated carbon for the adsorption and degradation of Chloramphenicol (CAP). J. Mol. Liq. 2018, 262, 19–28. [Google Scholar] [CrossRef]
- Liu, H.; Wei, Y.; Luo, J.; Li, T.; Wang, D.; Luo, S.; Crittenden, J.C. 3D hierarchical porous-structured biochar aerogel for rapid and efficient phenicol antibiotics removal from water. Chem. Eng. J. 2019, 368, 639–648. [Google Scholar] [CrossRef]
- Xing, W.; Liu, Q.; Wang, J.; Xia, S.; Ma, L.; Lu, R.; Zhang, Y.; Huang, Y.; Wu, G. High selectivity and reusability of biomass-based adsorbent for chloramphenicol removal. Nanomaterials 2021, 11, 2950. [Google Scholar] [CrossRef] [PubMed]
- Anthonysamy, S.I.; Yusop, M.F.M.; Ismail, H.; Ahmad, M.A. Chloramphenicol Removal from Aqueous Solution Using Sodium Bicarbonate-Impregnated Coconut Husk-Derived Activated Carbon: Optimization and Insight Mechanism Study. Arab. J. Sci. Eng. 2023, 48, 15999–16022. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Belhaj, D. Competitive sorption affinity of sulfonamides and chloramphenicol antibiotics toward functionalized biochar for water and wastewater treatment. Bioresour. Technol. 2017, 238, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Dai, J.; Dai, X.; Da, Z.; Yan, Y. Core–shell molecularly imprinted polymers based on magnetic chitosan microspheres for chloramphenicol selective adsorption. Monatshefte Für Chem. Chem. Mon. 2015, 146, 465–474. [Google Scholar] [CrossRef]
- Song, Y.; Sackey, E.A.; Wang, H.; Wang, H. Adsorption of oxytetracycline on kaolinite. PLoS ONE 2019, 14, e0225335. [Google Scholar] [CrossRef]
- Smata, A.; Yoshimura, C. One-step synthesis of magnetic–layered double hydroxide and its application for oxytetracycline removal from water. J. Environ. Chem. Eng. 2022, 10, 107819. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, S.; Li, J. Fast and highly efficient tetracyclines removal from environmental waters by graphene oxide functionalized magnetic particles. Chem. Eng. J. 2013, 225, 679–685. [Google Scholar] [CrossRef]
- Wei, M.; Zheng, H.; Zeng, T.; Yang, J.; Fang, X.; Zhang, C. Porous carbon aerogel derived from bacterial cellulose with prominent potential for efficient removal of antibiotics from the aquatic matrix. Water Sci. Technol. 2021, 84, 1896–1907. [Google Scholar] [CrossRef]
- Chen, M.; Yan, Z.; Luan, J.; Sun, X.; Liu, W.; Ke, X. π-π electron-donor-acceptor (EDA) interaction enhancing adsorption of tetracycline on 3D PPY/CMC aerogels. Chem. Eng. J. 2023, 454, 140300. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F.; Zhong, L.; Dong, Z.; Chen, C.; Xu, Z. Reusable and environmentally friendly cellulose nanofiber/titanium dioxide/chitosan aerogel photocatalyst for efficient degradation of tetracycline. Appl. Surf. Sci. 2023, 641, 158425. [Google Scholar] [CrossRef]
- Elsayed, I.; Schueneman, G.T.; El-Giar, E.M.; Hassan, E.B. Amino-Functionalized Cellulose Nanofiber/Lignosulfonate New Aerogel Adsorbent for the Removal of Dyes and Heavy Metals from Wastewater. Gels 2023, 9, 154. [Google Scholar] [CrossRef] [PubMed]

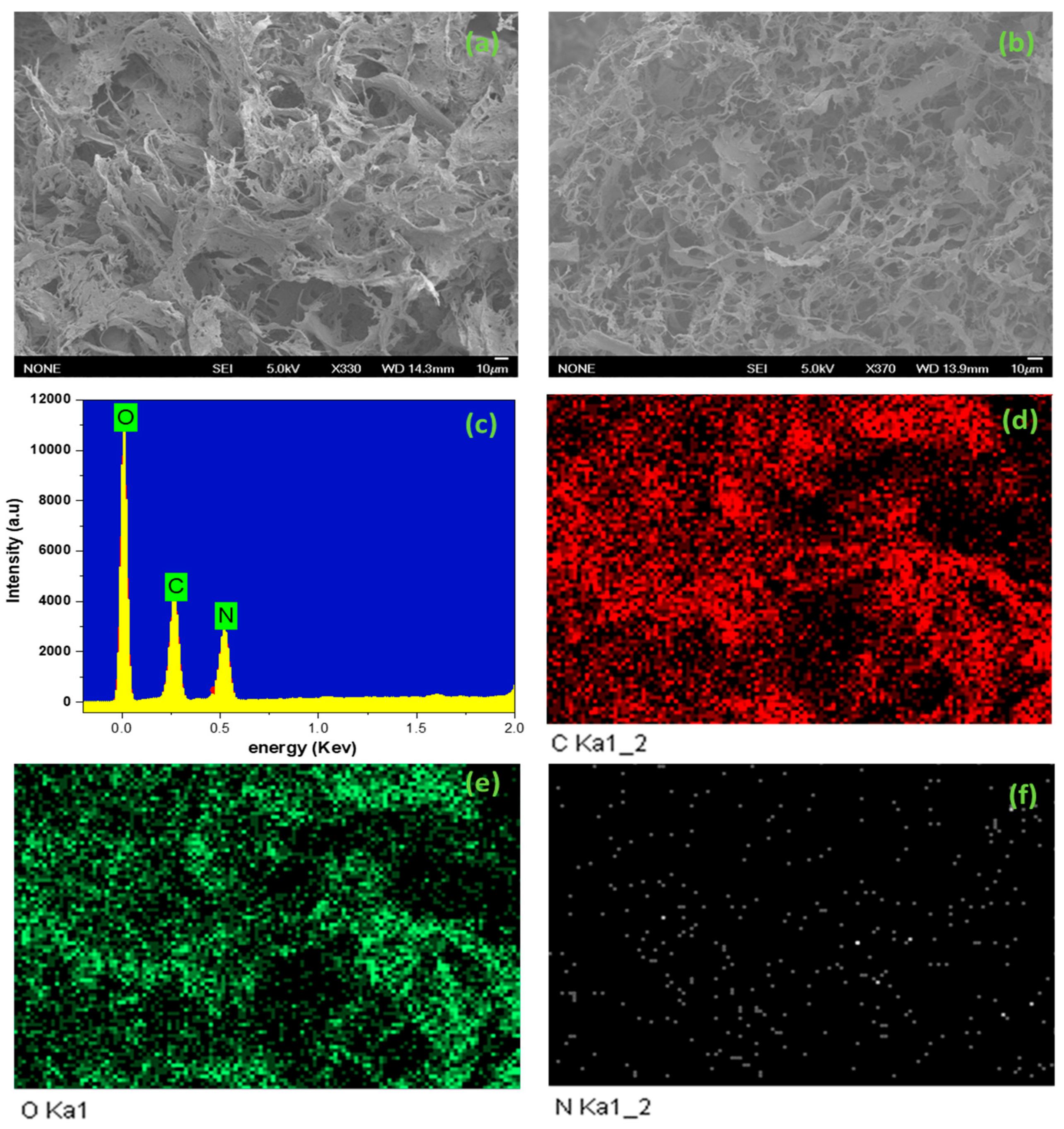
| Sample | Elemental Analysis | Surface Area (m2/g) | |||
|---|---|---|---|---|---|
| %C | %H | %N | %R | ||
| Am/TEMPO-CNF | 41.54 | 6.38 | 1.26 | 50.82 | 20.75 |
| Am-CNF | 40.06 | 7.93 | 2.93 | 49.09 | 9.18 |
| CNF | 41.89 | 6.02 | 0.00 | 52.09 | 2.82 |
| Models | Co (mg/L) | OTC | CAP | ||||
|---|---|---|---|---|---|---|---|
| 40 | 80 | 140 | 30 | 60 | 100 | ||
| Pseudo-first order | K1 (1/min) | 0.00304 | 0.0046 | 0.00422 | 0.01291 | 0.02182 | 0.02167 |
| qe (mg/g) | 21.598 | 29.216 | 41.412 | 11.578 | 18.36 | 16.030 | |
| R2 | 0.47208 | 0.50764 | 0.33198 | 0.06063 | 0.33219 | 0.22756 | |
| Pseudo-second order | K2 (g/mg/min) | 0.00025 | 0.00014 | 0.00010 | 0.00537 | 0.00102 | 0.00154 |
| qe (mg/g) | 39.761 | 79.051 | 131.578 | 27.012 | 58.411 | 89.928 | |
| R2 | 0.995 | 0.996 | 0.997 | 0.998 | 0.987 | 0.997 | |
| Models | Temp (°C) | OTC | CAP | ||||
|---|---|---|---|---|---|---|---|
| 15 | 30 | 40 | 15 | 30 | 40 | ||
| Langmuir | K1 (L/mg) | 0.00002 | 0.2727 | 0.1218 | 0.0700 | 0.00002 | 0.0969 |
| qmax (mg/g) | 130.71 | 136.05 | 153.13 | 116.14 | 128.20 | 150.15 | |
| R2 | 0.986 | 0.992 | 0.993 | 0.991 | 0.997 | 0.996 | |
| Freundlich | KF ([mg g−1(Lmg−1)1/n]) | 20.675 | 31.668 | 51.90 | 10.729 | 14.884 | 24.635 |
| n | 1.735 | 2.11 | 2.74 | 1.460 | 1.630 | 1.686 | |
| R2 | 0.984 | 0.967 | 0.974 | 0.984 | 0.981 | 0.981 | |
| Adsorbent | Adsorbate | pH | qmax (mg/g) | Reference |
|---|---|---|---|---|
| Am/TEMPO-CNF | CAP | 7.0 | 150.15 | This study |
| corn stalk biomass fiber | 6.5 | 50.17 | [41] | |
| sodium bicarbonate (NaHCO3)-impregnated coconut husk-activated carbon (CHAC) | - | 4.0 | [42] | |
| AC from eucalyptus wood | 4–4.5 | 21 | [43] | |
| Novel magnetic molecularly imprinted polymers (MMIPs) | - | 52.6 | [44] | |
| Am/TEMPO-CNF | OTC | 8 | 153.1 | This study |
| kaolinite | 5.5 | 15.2 | [45] | |
| magnetic layered double hydroxide (MLDH) | 7 | 127.7 | [46] | |
| Graphene oxide functionalized magnetic particles | - | 45.0 | [47] | |
| Posidonia oceanica | 6 | 11.8 | [34] |
| Adsorbate | T (°C) | Qe (mg/g) | ΔG° (KJ·mol−1) | ΔH° (KJ·mol−1) | ΔS° (J·mol−1·K−1) | ||
|---|---|---|---|---|---|---|---|
| OTC | 15 | 39.26 | 2.56597 | 0.94233 | −2.25753 | 29.98155 | 112.1152 |
| 30 | 22.9 | 5.19816 | 1.64830 | −4.15437 | |||
| 40 | 15.0 | 8.32711 | 2.11951 | −5.60633 | |||
| CAP | 15 | 20.78 | 3.81231 | 1.33823 | −3.20431 | 32.06048 | 122.2296 |
| 30 | 12.43 | 6.96460 | 1.94084 | −4.88925 | |||
| 40 | 6.9 | 13.4927 | 2.60215 | −6.87970 |
| Model | Equation |
|---|---|
| Langmuir linear | |
| Langmuir non-linear | |
| Freundlich linear | |
| Freundlich non-linear | |
| Pseudo-first order non-linear | |
| Pseudo-first order linear | |
| Pseudo-second order non-linear | |
| Pseudo-second order linear |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amen, R.; Elsayed, I.; Schueneman, G.T.; Hassan, E.B. Self-Assembled Aminated and TEMPO Cellulose Nanofibers (Am/TEMPO-CNF) Aerogel for Adsorptive Removal of Oxytetracycline and Chloramphenicol Antibiotics from Water. Gels 2024, 10, 77. https://doi.org/10.3390/gels10010077
Amen R, Elsayed I, Schueneman GT, Hassan EB. Self-Assembled Aminated and TEMPO Cellulose Nanofibers (Am/TEMPO-CNF) Aerogel for Adsorptive Removal of Oxytetracycline and Chloramphenicol Antibiotics from Water. Gels. 2024; 10(1):77. https://doi.org/10.3390/gels10010077
Chicago/Turabian StyleAmen, Rabia, Islam Elsayed, Gregory T. Schueneman, and El Barbary Hassan. 2024. "Self-Assembled Aminated and TEMPO Cellulose Nanofibers (Am/TEMPO-CNF) Aerogel for Adsorptive Removal of Oxytetracycline and Chloramphenicol Antibiotics from Water" Gels 10, no. 1: 77. https://doi.org/10.3390/gels10010077
APA StyleAmen, R., Elsayed, I., Schueneman, G. T., & Hassan, E. B. (2024). Self-Assembled Aminated and TEMPO Cellulose Nanofibers (Am/TEMPO-CNF) Aerogel for Adsorptive Removal of Oxytetracycline and Chloramphenicol Antibiotics from Water. Gels, 10(1), 77. https://doi.org/10.3390/gels10010077







