Abstract
In this work, electrochromic devices were prepared using the redox couple ethyl viologen diperchlorate and 1,1′-diethyl ferrocene in propylene carbonate as an aprotic solvent to facilitate ions separation and diffusion inside the devices. Electrochromic devices were made using electrochromic gel mixtures at the concentrations of 55%, 60% and 65% with respect to the bisphenol A polymer. In particular, two sets of gels were made: one set contained the bisphenol A not-polymerized while and the second one contained the polymerized polymer. Different techniques, such as cyclic voltammetry, UV-vis-NIR, and Raman spectroscopy, were used to study such systems to understand the differences in terms of performances between the different sets of electrochromic devices. Cyclic voltammetry confirmed that the oxidation process of the 1,1′-diethyl ferrocene and the reduction of the ethyl viologen diperchlorate occurred at about 0.4 V. Interesting variations in the transmittances were found between the two groups of samples. The best values of CE were provided by the electrochromic devices based on the polymerized electrochromic gel mixture at a concentration of 60% (EM60). The EM60 device result was CE = 92.82 C/cm2 in the visible region and CE = 80.38 C/cm2 in the near–infrared region, confirming that these devices can be used for energy-saving applications. A structural characterization of the materials used in the two sets of electrochromic devices was made using Raman spectroscopy, and the analysis supports the electrochemical models used to explain the processes involved during operation of the electrochromic systems.
1. Introduction
Electrochromism is the phenomenon linked to a reversible change in the color of a device following a redox reaction, which occurs after the application of an external voltage to the device. Interest in electrochromic materials is, recently, increased significantly because of their possible applications in smart windows, displays [1] and in building energy-saving applications [2,3,4]. The color change occurs because of the different electronic structures of the species involved in the reversible redox reaction. In the gel, the polymer or even the monomers before the polymerization assist the ions separation that occurs during the redox process. Different kinds of polymeric gel electrolytes have been deeply studied and characterized [3,4].
All solid electrochromic devices have a typical structure: two transparent substrates covered by electronic conductor layers (i.e., indium tin oxide, ITO), an electrochromic active material (working electrode), an ion conductive layer, and an ion storage layer (counter electrode), which can also be made using a complementary electrochromic material.
The electrochromic devices can be classified into three large families: inorganic, organic, and polymeric. The inorganic electrochromic materials, such as transition metal oxides (WO3 or V2O5) [5], are usually deposited on the two electrodes in the electrochromic device as a redox couple: i.e., while WO3 is reducing, the V2O5 oxidation occurs. Therefore, when the WO3 is in its reduced form, the device becomes blue [6].
Instead, the use of polymeric electrochromic materials, such as polyaniline (PANI) or polypyrrole (PPy) is very promising, for example, in the field of supercapacitors [7] or to improve the performance of the electrochromic devices in combination with metal oxides [8].
Anyway, today, organic compounds are the most used materials to prepare electrochromic devices; in particular, the 1,1′-disubstituted-4.4′-bipyridinum salts bipyridinium systems, also known as Viologens, are of great interest nowadays [9]. They can be defined as 1,1′-disubstituted-4.4′-bipyridinum salts and exist in dicationic form and can be reduced two times: the most stable reduced form is the radical one [9]. Ethyl viologen diperchlorate has been used to make electrochromic devices studied in the present work [10,11]. As stated above, the viologen can be involved in two reduction reactions: the first one leads to the formation of a radical species, while the second one can entirely reduce the viologen leading to a neutral species. It is well-known that the radical species of viologen is very stable and it shows a very intense blue coloration because of the radical electron delocalization of the π-system of the bipyridyl rings, while the more unstable neutral species has lower intensity coloration [12]. Viologen-based electrochromic devices could be very interesting to obtain flexible electrochromic devices.
Cyclic voltammetry is the main technique used to characterize the electrochemical properties of electrochromic devices [13,14]. In order to study the transmittance and the absorbance of the materials, the principal technique used has been UV-vis-NIR spectroscopy [15,16]. Raman spectroscopy has been used to characterize the materials used in the samples because it is well known that it is a powerful tool for molecular analysis and structural phases of many materials such as: graphene [17,18], polymers [19], thin films [20,21], cultural heritage [22], bio-systems [23] and so on [24,25,26].
The aim of this work is to investigate the performances of the electrochromic devices made with ethyl viologen diperchlorate, 1,1′-diethyl ferrocene (as a redox couple) and bisphenol A, at different concentrations of the electrochromic gel mixture and as a function of the polymerization of bisphenol A glycerolate (1 glycerol/phenol) diacrylate.
2. Results and Discussion
2.1. Cyclic Voltammetry Measurements
Cyclic voltammetry measurements have been performed to study the electrochemical properties of two different series of electrochromic devices based on two series of electrochromic gel polymer mixture: the first series is based on three percentages of the electrochromic gel mixture set at 55%, 60% and 65% and indicated as EM55, EM60 and EM65, containing the not-polymerized bisphenol A. The second set of samples was made using the same electrochromic gel mixture at the stated percentage concentrations, but the bisphenol A was polymerized.
The cyclic voltammograms (CVs) were collected on the devices with a scanning speed of 50 mV/s in the voltage range between −1.5 V and 1.5 V. The estimation of the working voltages of each device, the identification of the redox peaks and the study of the effect of polymerization of the bisphenol A on the electrochemical performances of the device were obtained.
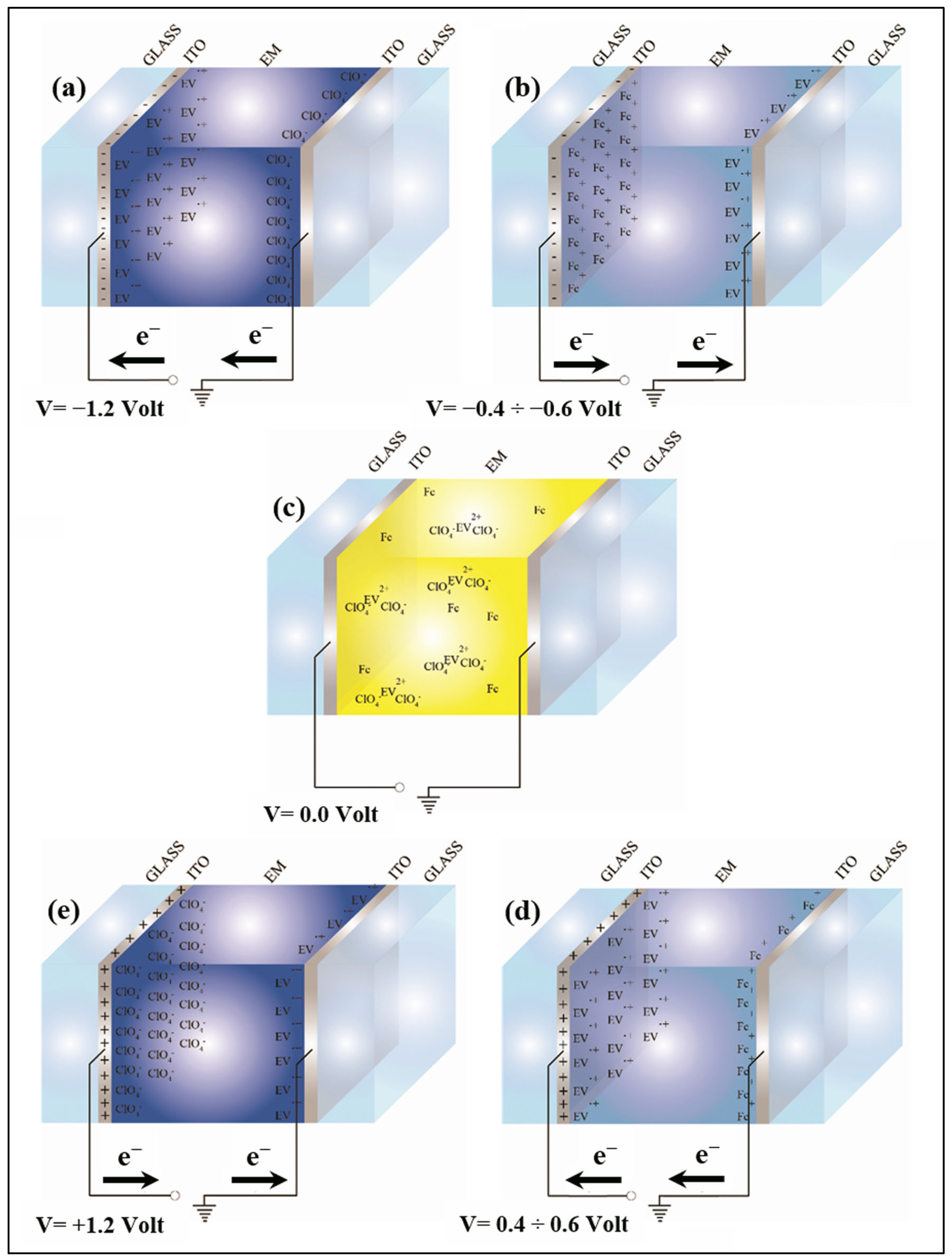
Figure 1 represents an electrochromic device when different voltages are applied: 1.2 V, between −0.4 V and −0.6 V, 0 V, between 0.4 V and 0.6 V and 1.2 V. The species involved in the redox process between the two electrodes are also indicated.
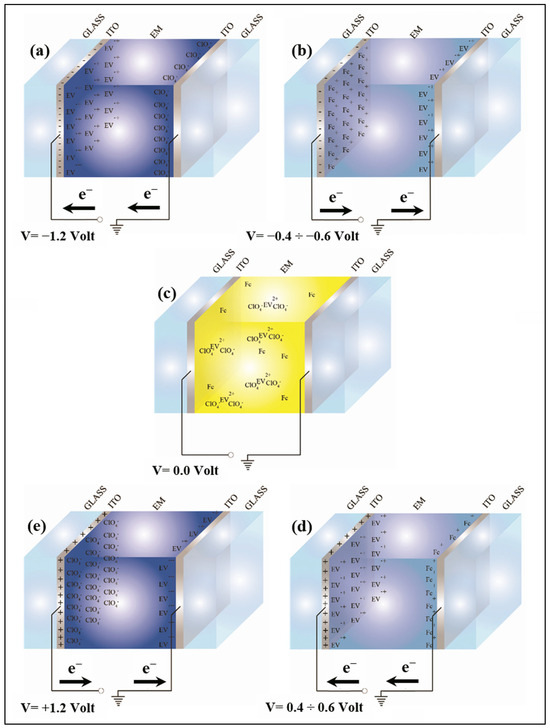
Figure 1.
Schematic representation of electrochromic device at different applied voltages: −1.2 V (a), between −0.4 and −0.6 V (b), 0 V (c), between 0.4 V and 0.6 V (d) and 1.2 V (e) (Fc indicates 1,1′-diethyl ferrocene, EV is ethyl viologen).
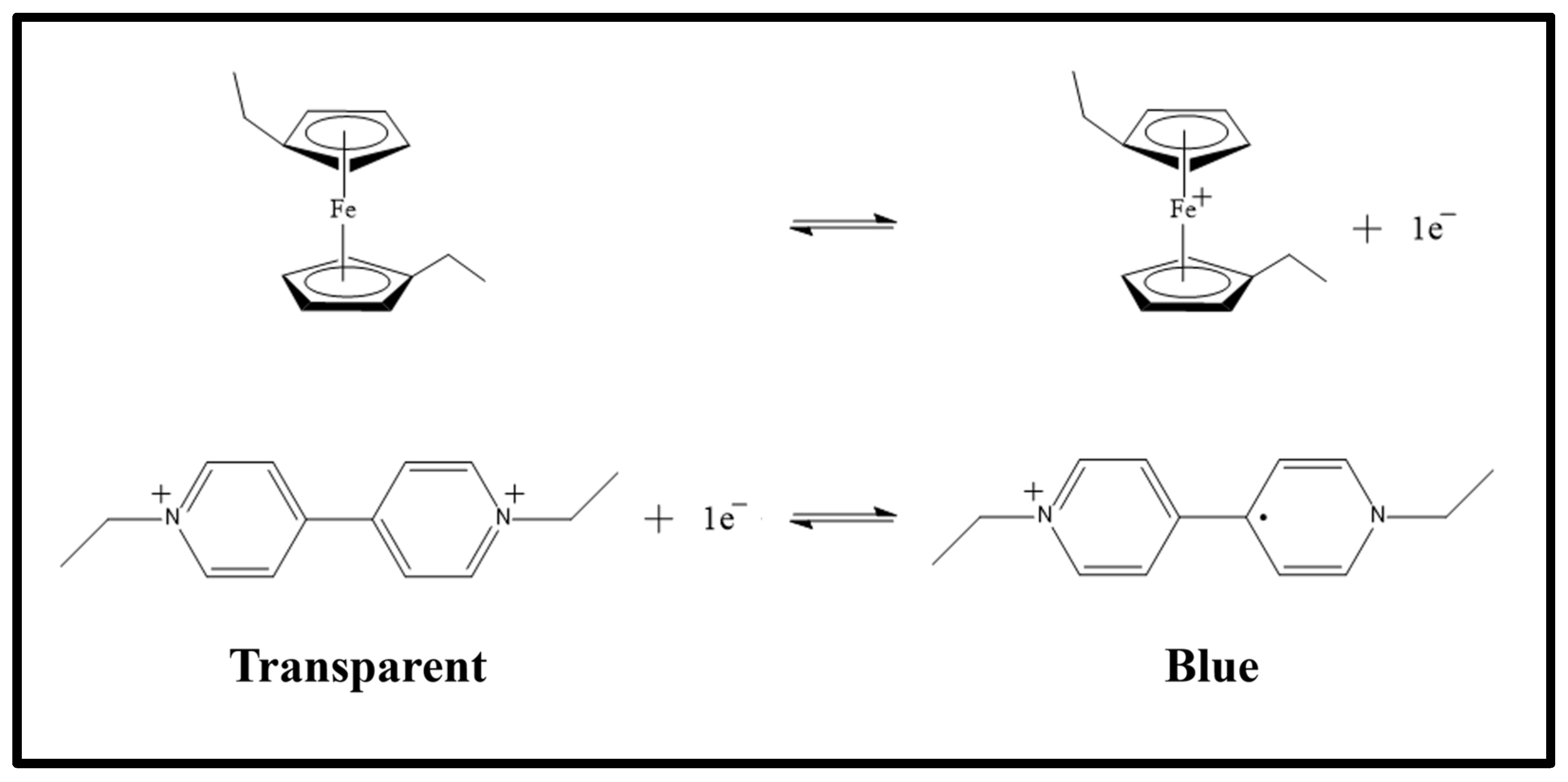
To better understand the schemes of Figure 1, it may be useful to look at Scheme 1, where the electrochromic reaction is drawn. In Scheme 1, the two semi-reactions are shown. In particular, at the top of the scheme, the oxidation of 1,1′-diethyl ferrocene is shown, which frees an electron that is used by the ethyl viologen diperchlorate in its dicationic form during the reduction reaction, at the bottom, which leads to the ethyl viologen diperchlorate radicalic monocationic form.
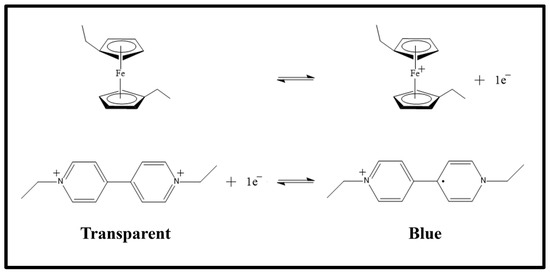
Scheme 1.
Schematic view of the redox reaction that occurs during the electrochromic process. In this scheme the two semi-reactions are shown: at the top the oxidation of the 1,1′-diethyl ferrocene and the reduction of the ethyl viologen diperchlorate. When the species are in the state shown on the left of the reactions, the electrochromic device is transparent; otherwise, when they are in the state on the right of the reactions, the devices becomes blue.
Figure 1a and Figure 1b show the same electrochromic conditions seen in Figure 1d and Figure 1e, respectively. The only difference is a change in the polarizability of the applied voltage: the electrode that before was positive becomes negative and vice versa. The charged species present in the gel move from one electrode to the other but no one reaction occurs. In Figure 1c the electrochromic species are dissolved in the gel and this situation is represented on the left side of Scheme 1, where the electrochromic device is transparent. When a voltage is applied to the device, then, depending on the sign of the applied voltage, one of the situations at the top or at the bottom of Figure 1 occurs, and the reactions are shifted to the right part of the scheme making the device blue.
When the voltage is removed, then, the device returns, spontaneously, to the transparent state (left side of the scheme).
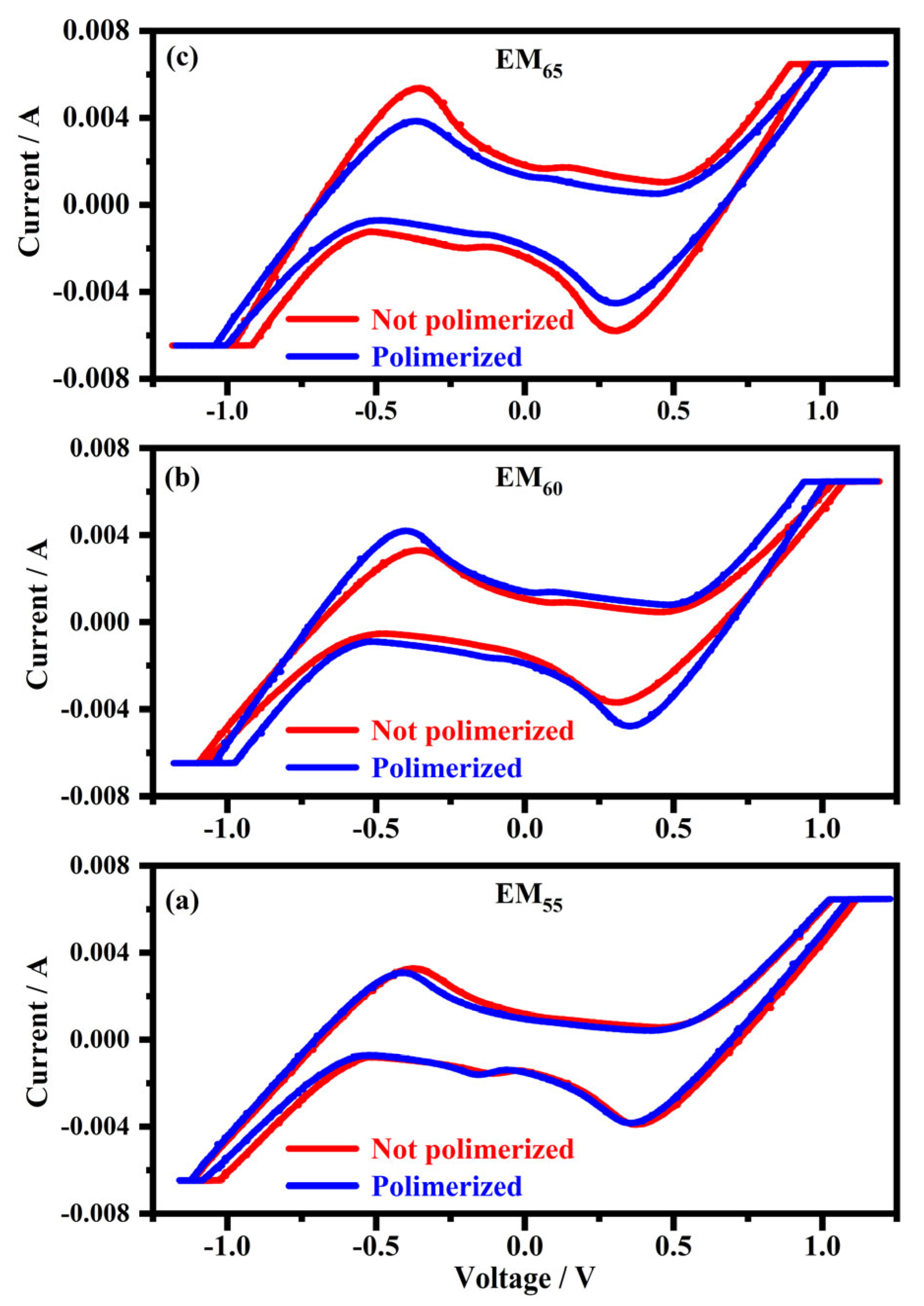
Figure 2 shows the cyclic voltammograms collected on the devices filled with the electrochromic gel mixtures in concentrations of 55%, 60% and 65%, labelled as EM55, EM60 and EM65, containing the bisphenol A polymerized and not-polymerized.
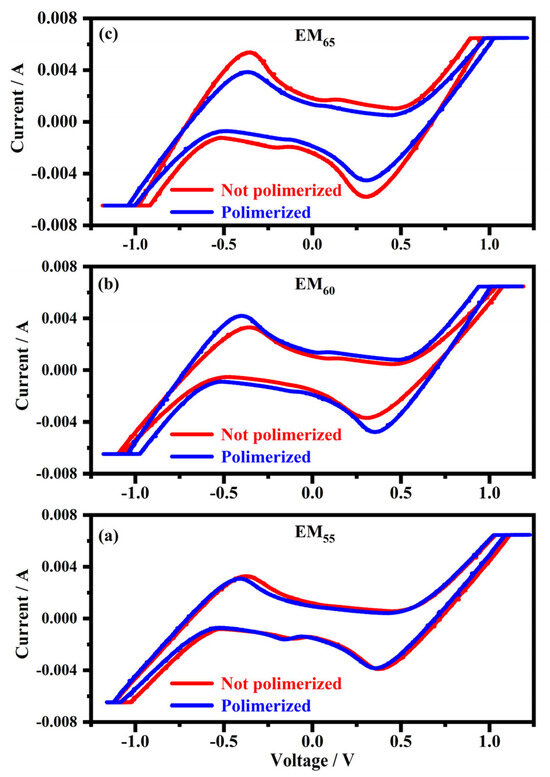
Figure 2.
Cyclic voltammograms collected on the samples filled with the electrochromic gel mixtures at the weight ratios of 55% (a), 60% (b) and 65% (c), indicated as EM55, EM60 and EM65 with the bisphenol A not-polymerized (red line) and polymerized (blue line).
As exposed in Figure 2, cyclic voltammograms show the current maximum value, expressed at 6.5 × 10−3 A (in absolute value), inside the devices, while the switching potentials are comprised between 1.1 V and 1.2 V (in absolute value) to obtain the full coloration of the devices. The cyclic voltammograms recorded for all the mentioned electrochromic devices seem to be symmetric.
A first noticeable redox peak occurs at 0.4 V (in absolute value), related to 1,1′-diethyl ferrocene oxidation [12] and to the viologen reduction, while a peak comprised between 1.1 V and 1.2 V can be related to the viologen compound charge separation. It was reported that at specific potentials, the ionic bond between perchlorate ions and viologen (in its oxidized form) could be broken, causing an accumulation of charges on the electrodes and reducing the viologen [27]. Since ferrocene-based compounds are blue in the oxidized state and a radical form of a viologen compound with short alkyl substituents (such as ethyls) promotes a dark blue form [28,29], the blue color of the devices can be due to both the reactions: the oxidation of 1,1′-diethyl ferrocene and the reduction of the viologen species. Although the coloration process starts at voltages of about 0.4 V and becomes more intense at voltages between 1.1 V and 1.2 V, it does not end with the formation of radical species of viologen compound but with the formation of its neutral species. Nevertheless, the literature states that the double reduction reactions of viologens are irreversible [30]. At the same time, the system described here shows that reversibility and a dark blue, typical of a single reduced viologen, occurs. A lower intense peak can be noted at 0.2 volt potentials (in absolute value); a redox potential of 0.16 V was reported for reactions involving methyl viologen diperchlorate dimers [31].
As it can be seen in Figure 2, the switching potentials seem to become lower, passing from 55% to 65%. On the contrary, the current intensities shown by the different devices become higher, and the composition in the electrochromic solution passes from 55% to 65%. The effect of the polymerization is negligible in the sample with EM55. In the case of EM60, the not-polymerized samples show switching potentials higher than the polymerized samples, while in the case of EM65, the not-polymerized samples show a higher current than the polymerized ones.
2.2. UV-Vis-NIR Spectroscopy
Dynamic transmittance measurements were performed using UV-Vis-NIR spectroscopy to study the optical properties of the electrochromic devices filled with EM55, EM60 and EM65 with the polymerized and not-polymerized bisphenol A in the OFF/ON states. The transmittance curves were collected from 100 nm to 1310 nm, when the system was under different constant applied voltage, starting from 0.1 V to 1.2 V and increasing in steps of 0.1 V.
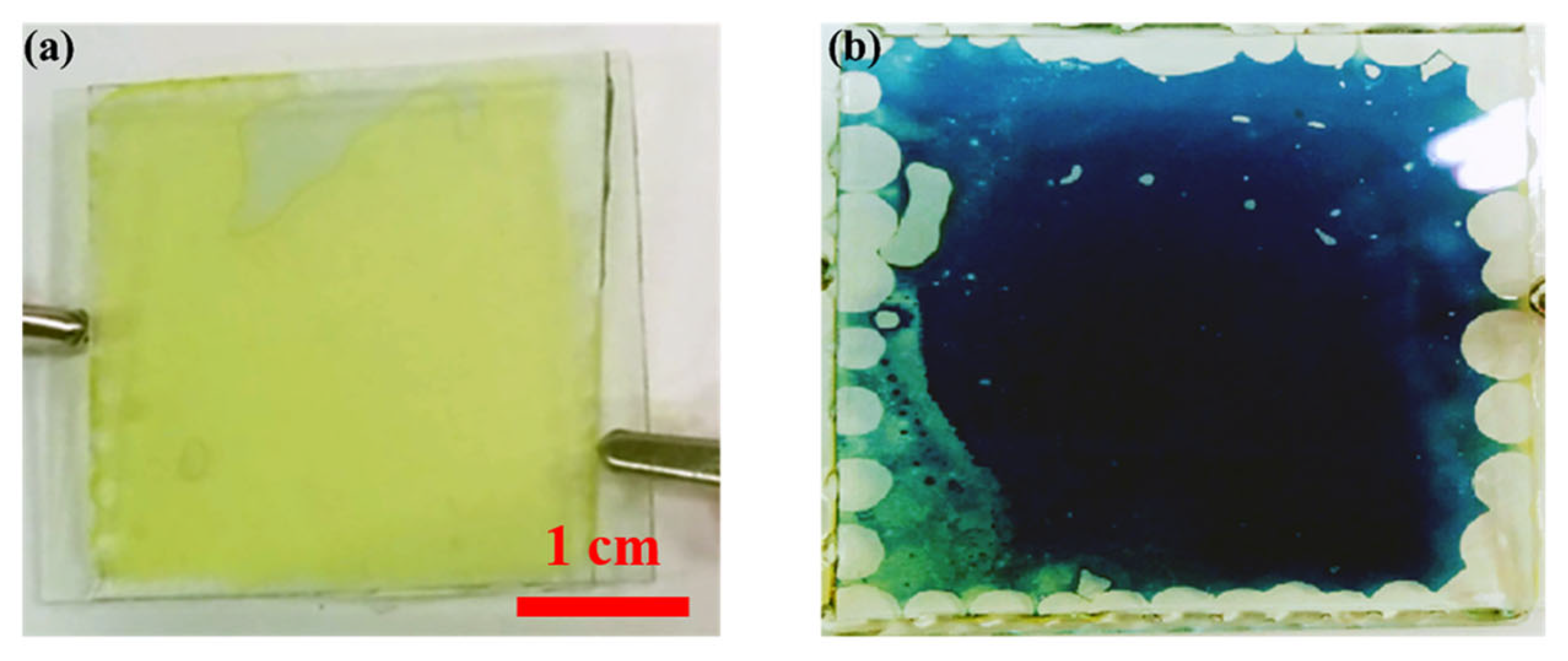
Figure 3 shows photographs of the electrochromic devices in their OFF state (bleached state) and ON state (blue-colored state) under the application of an external voltage.
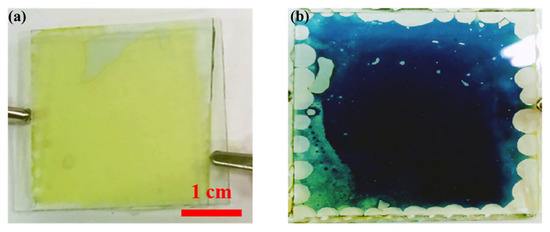
Figure 3.
The electrochromic device in the OFF state (a) and ON state (b).
In the presented electrochromic device, the transparent signs at the edges are due to the epoxy glue used to seal the device in the sandwich configuration. We are developing an automatic glue dispenser that controls the quantity of glue used at each point to avoid this effect. The glue will be placed on the inner face of one electrode, and the other electrode will be placed on top using automatic mechanical arms, to avoid any accidental signs of the glue.
It is possible to see in Figure 3 the presence of some bubbles at the edge of the device, which are due to the glue used to attach the electrodes of the device. It is planned to develop an automatic glue dispenser that will control the amount of the glue used during each point in order to avoid such an effect.
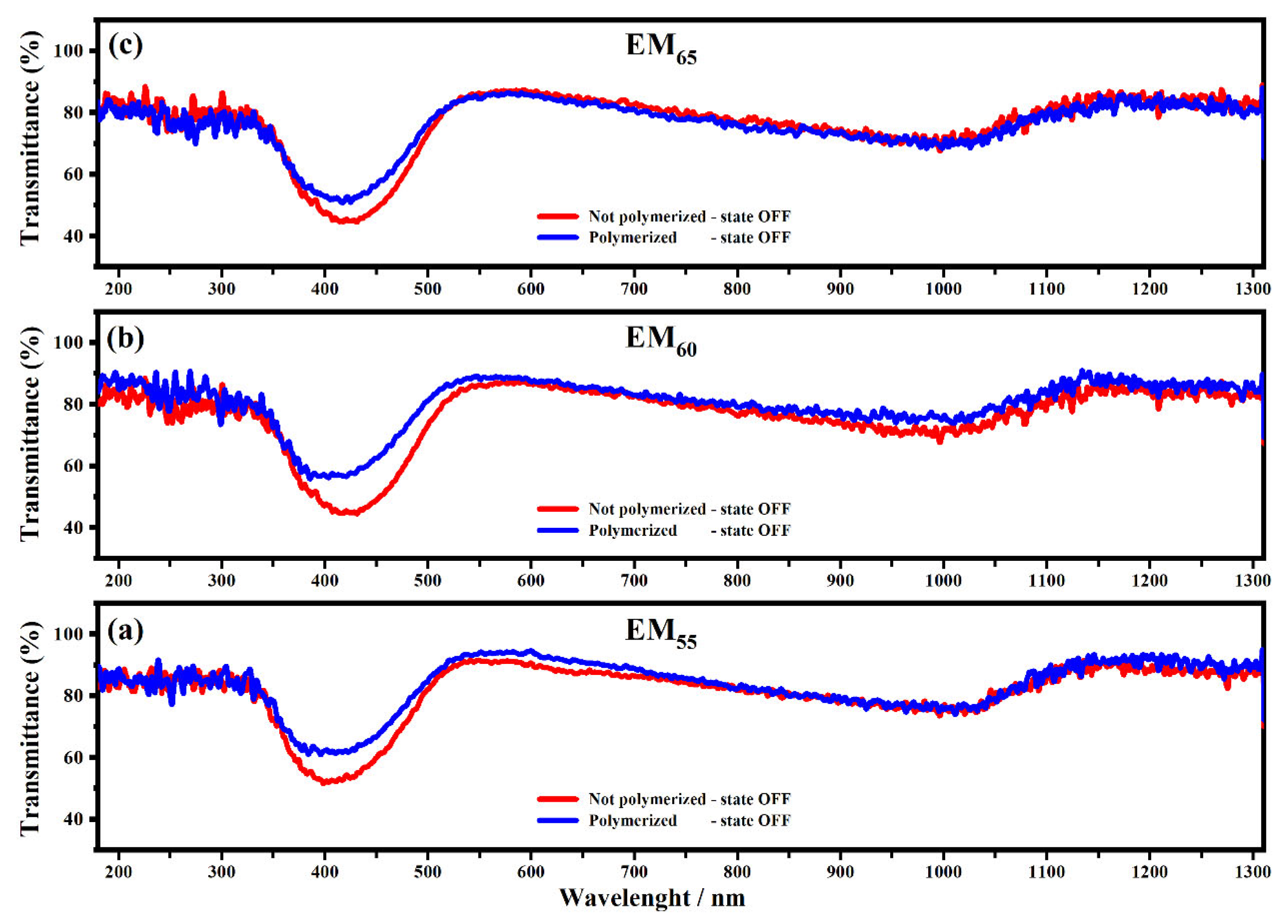
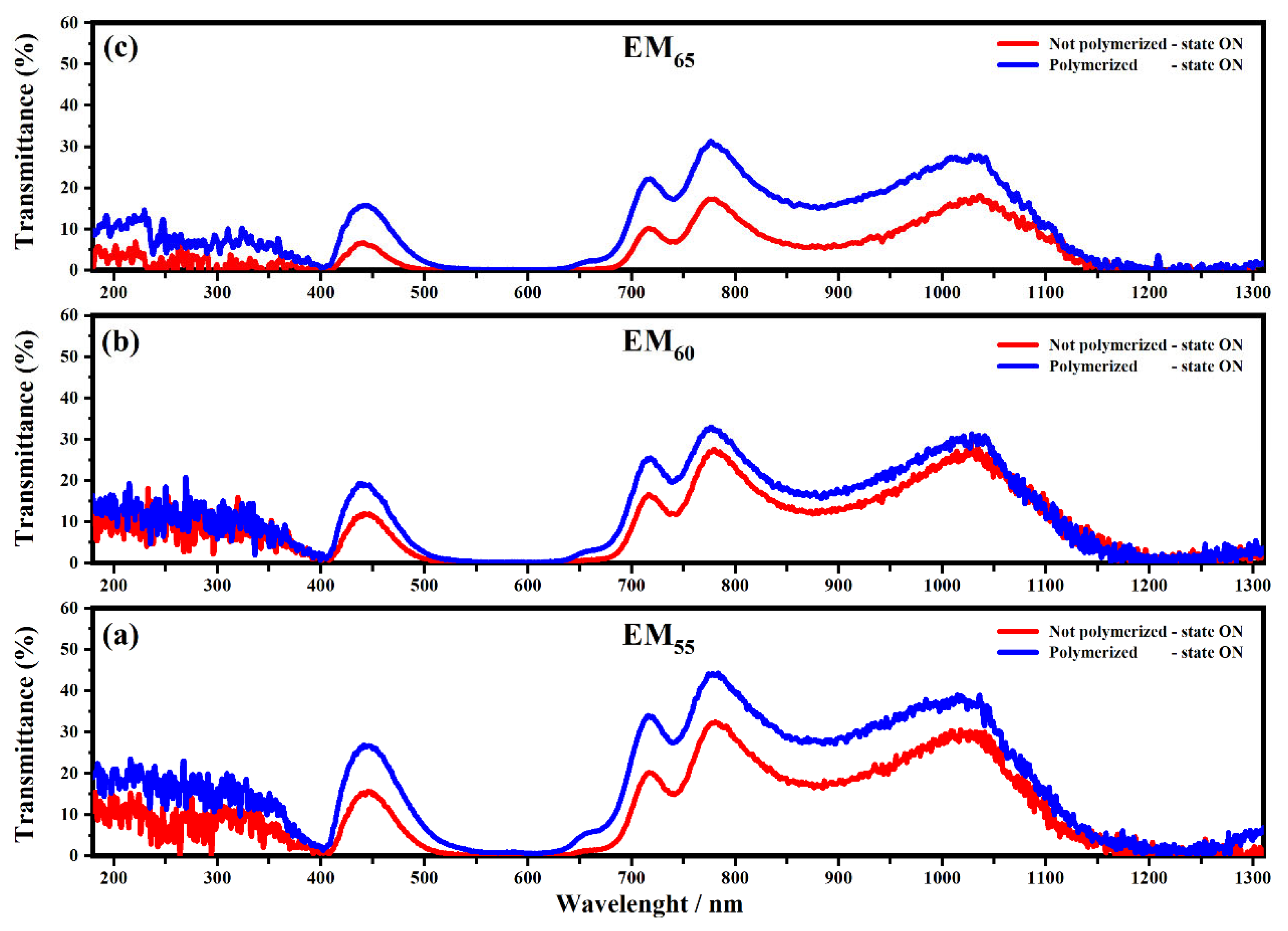
Figure 4 and Figure 5 show the transmittance spectra collected on the devices filled with EM55, EM60 and EM65 in the OFF state (0 V) and in the ON state (1.2 V).
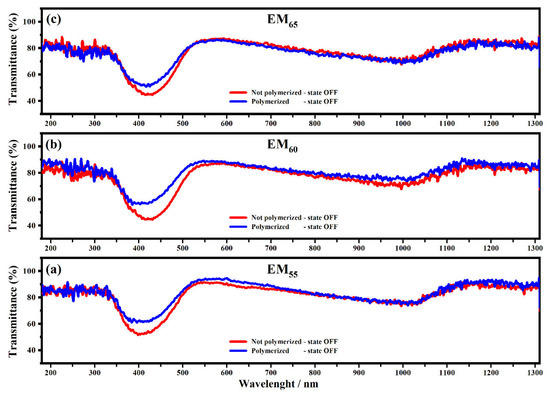
Figure 4.
Transmittance spectra collected on the devices filled with the electrochromic gel mixtures: EM55 (a), EM60 (b) and EM65 (c), not-polymerized (red line) and polymerized (blue line), in the state OFF and in the range from 100 nm to 1310 nm at 0 V.
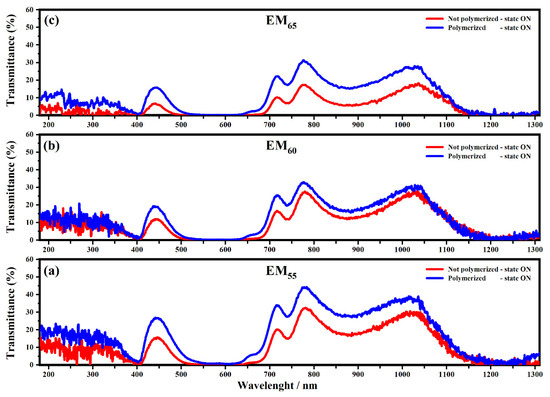
Figure 5.
Transmittance spectra collected on the devices filled with the electrochromic gel mixtures: EM55 (a), EM60 (b) and EM65 (c), not-polymerized (red line) and polymerized (blue line), in the state ON and in the range from 100 nm to 1310 nm at 1.2 V.
All spectra showed interesting variations of the transmittances in both OFF/ON states. As shown in Figure 4, the electrochromic devices are characterized by the first appreciable decrease of the transmittance, which falls at about 400 nm, and it is due to the presence of ethyl viologen radicals [29]. For the devices based on the not-polymerized EM55, the transmittance in the OFF state is equal to 50%, while in the case of the polymerized EM55, the transmittance is equal to 60%. For the devices based on the not-polymerized EM60, in the OFF state, the transmittance is equal to 45%, while in the case of the polymerized EM60, the transmittance is equal to 60%. The electrochromic devices containing the not-polymerized EM65 exhibit a transmittance of 50%, while the devices prepared with the polymerized EM65 exhibit a transmittance of 60%. No appreciable variations of transmittance were found at the wavelengths of 550 nm, 880 nm and 1200 nm. In Figure 5, at the wavelengths of 550 nm, 880 nm and 1200 nm, a decrease of the transmittances can be observed for all kinds of electrochromic devices, and this behavior is justified by the formation of the radical species of the ethyl viologen, after which the redox process occurred. In the ON state, all the devices made using the not-polymerized and polymerized gel mixtures EM55, EM60 and EM65 are characterized by values of transmittances at about 0%. At 880 nm, the transmittance of devices with the not-polymerized EM55 is about 20%, while for the polymerized EM55, it is equal to 30%. At 880 nm, the transmittance of the devices containing the not-polymerized EM60 is about 15%, while the devices with the polymerized EM60 show a decrease of the transmittance of 20%. The devices composed by the not-polymerized EM65 show a value of transmittance of 10% in the ON state, and with the polymerized EM65, the transmittance is equal to 20%, compared to the OFF state. Since there is no evidence of the oxidized ferrocene compound’s characteristic green coloration (when they are in a ferrocene-based solution) [31], one can conclude that 1,1′-diethyl ferrocene oxidation and ethyl viologen diperchlorate reduction occur at the same potential.
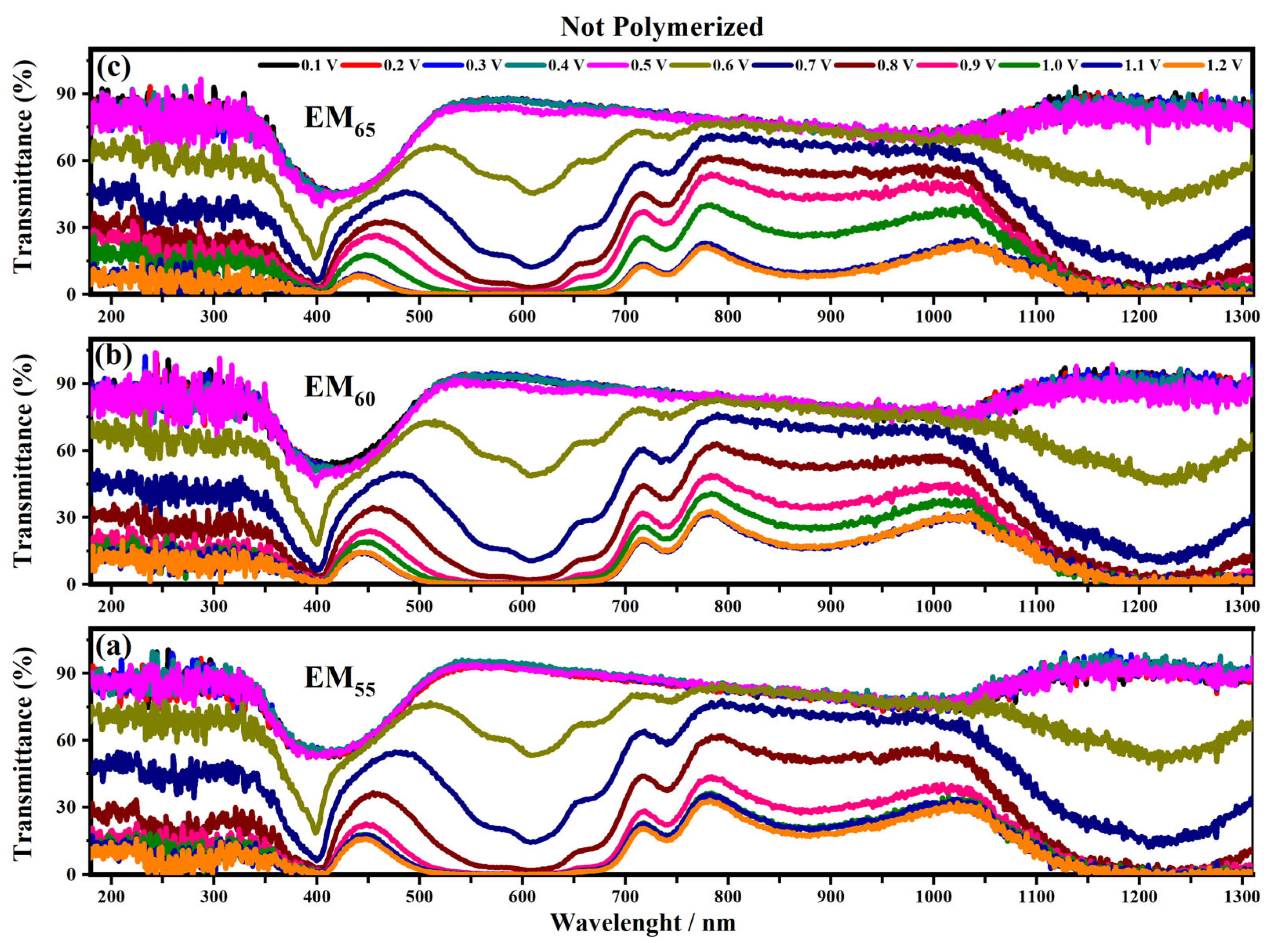
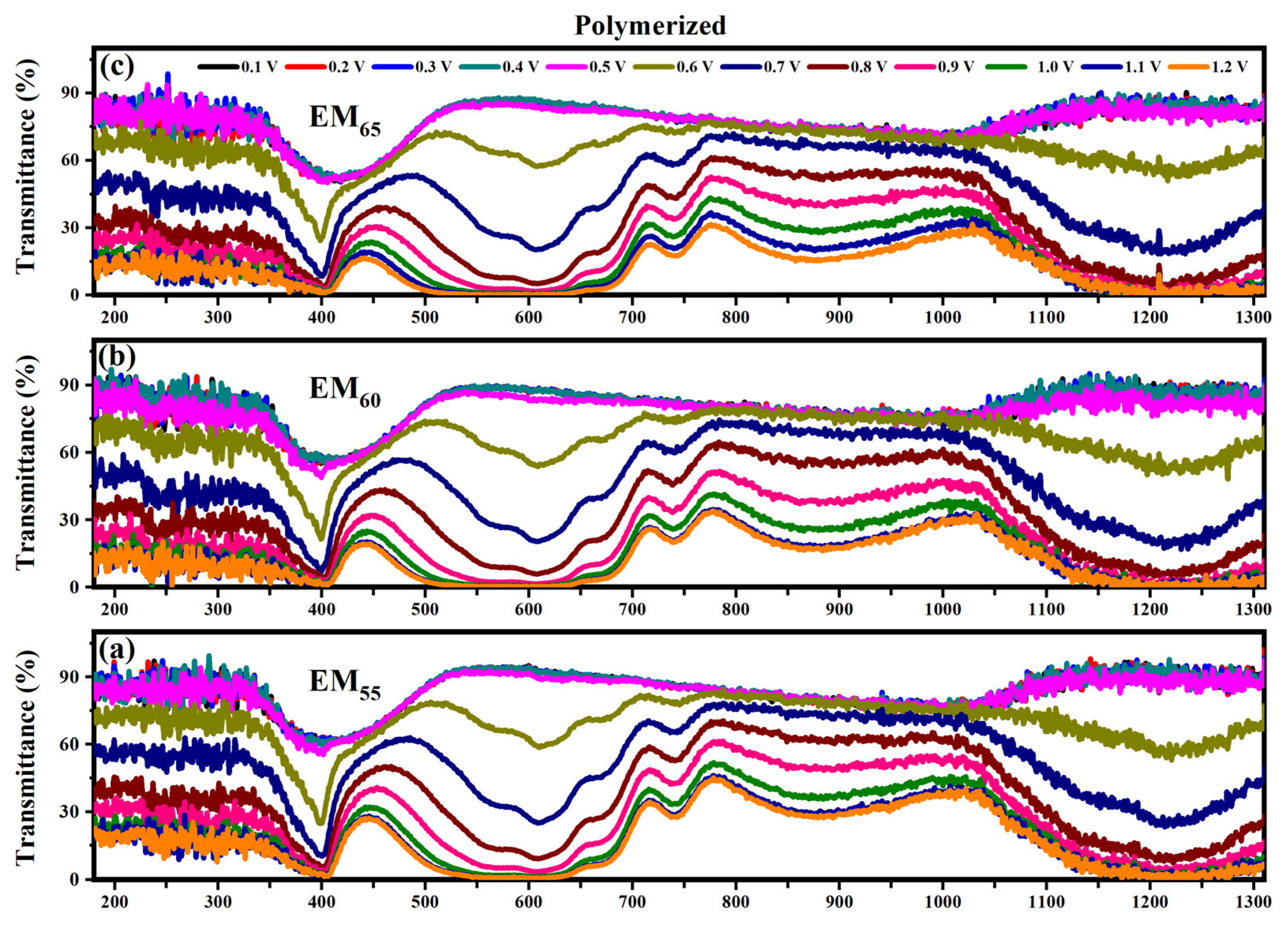
Figure 6 and Figure 7 show the transmittance spectra of the electrochromic devices made using EM55, EM60 and EM65 (containing the not-polymerized and polymerized bisphenol A gels) when each system was in its ON state under an applied voltage from 0.1 V to 1.2 V.
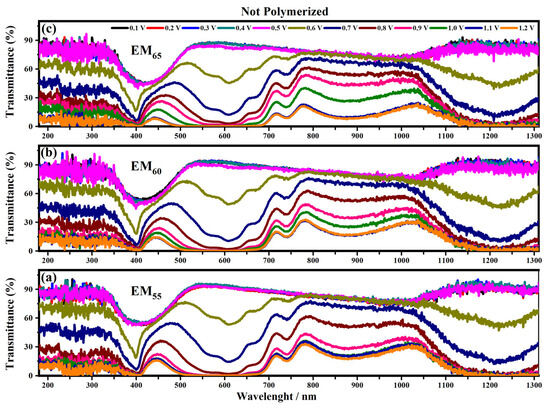
Figure 6.
Transmittance spectra collected on the devices prepared with the not-polymerized electrochromic gel mixtures, EM55 (a), EM60 (b) and EM65 (c), at different applied voltages comprised between 0.1 V and 1.2 V.
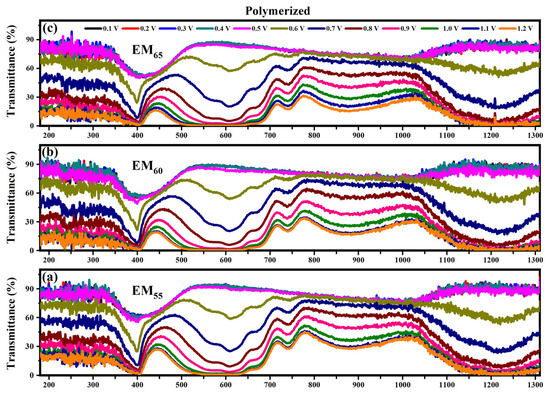
Figure 7.
Transmittance spectra collected on the devices prepared with the polymerized electrochromic gel mixtures, EM55 (a), EM60 (b) and EM65 (c), at different applied voltages comprised between 0.1 V and 1.2 V.
A noticeable change of transmittance in the visible and infrared regions can be seen for voltages between 0.5 V and 0.6 V. This behavior can be associated with the starting of the redox process: i.e., the oxidation of the 1,1′-diethyl ferrocene and the reduction of the ethyl viologen diperchlorate. In particular, for the devices containing the not-polymerized EM55 gel the first appreciable transmittance variation is found under the application of 0.6 V. The transmittance of these kind of devices is about 45% until 0.5 V, then the transmittance varies from 15% (at 0.6 V) to 0% (at 1.2 V) at 400 nm. At 550 nm, the not-polymerized EM55 devices show 90% of transmittance until 0.5 V, then a decrease of up to 60% is found at 0.6 V and a value of 0% at 1.2 V. At 880 nm, the not-polymerized EM55 devices show 75% of transmittance until 0.5 V, then a decrease up to 15% is found at 1.2 V. At 1200 nm, the transmittance values for the not-polymerized EM55 devices vary from 90% (until 0.5 V) to 60% (at 0.6 V) and to 0% (1.2 V).
For the devices based on the not-polymerized EM60 gel devices at 400 nm, the transmittance changes from 45% (until 0.5 V) to 15% (at 0.6 V) and then to 0% at 1.2 V. The same kind of devices at 550 nm, exhibit a variation of the transmittances from 90% (until 0.5 V) to 45% (at 0.6 V) and to 0% (at 1.2 V), while at 880 nm, the transmittance changes from 80% at 0.6 V to 15% at 1.2 V. At 1200 nm, the transmittance is stable at 90% until 0.5 V, and then it decreases to 45% at 0.6 V and to 0% at 1.2 V. The systems containing the not-polymerized EM65 gel at 400 nm exhibit a variation of the transmittances from 60% (until 0.5 V) to 20% at 0.6 V and to 0% at 1.2 V. At 550 nm, these systems are characterized by 90% of transmittance up to 0.5 V, and then a decrease up to 50% is found at 0.6 V. At 880 nm, the transmittance of these devices varies from 75% (until 0.6 V) to 15% at 1.2 V, and at 1200 nm, the devices show 90% of transmittance until 0.5 V, and then a decrease up to 45% under the application of 0.6 V and about 0% at 1.2 V were observed.
The electrochromic devices based on the polymerized EM55, EM60 and EM65 gel mixtures show a variation of the transmittance at 400 nm from 60% (until 0.5 V) to 30% (at 0.6 V) and to 0% at 1.2 V. At 550 nm, the transmittance changes from 90% (until 0.5 V) to 60% (at 0.6 V) and to 0% at 1.2 V. At 880 nm, for EM55, the transmittance changes from 90% (until 0.5 V) to 15% (at 1.2 V), and for EM60 and EM65, the transmittances vary from 90% (until 0.5 V) to 15% (1.2 V). At 1200 nm, the transmittances vary from 90% up to 0.5 V, and then after the application of 0.6 V, a decrease at around 60% is reached, and at 1.2 V, the devices show transmittances of about 0%.
This agrees with cyclic voltammetry measurements, which confirmed that the maximum current is achieved at 1.2 V, when perchlorate ions and viologen dications separate and diffuse to the electrodes.
Focusing on the highest value of voltage (1.2 V), the time required for the switching ON of the devices and, consequently, the evaluation of the memory effect of the devices have been studied.
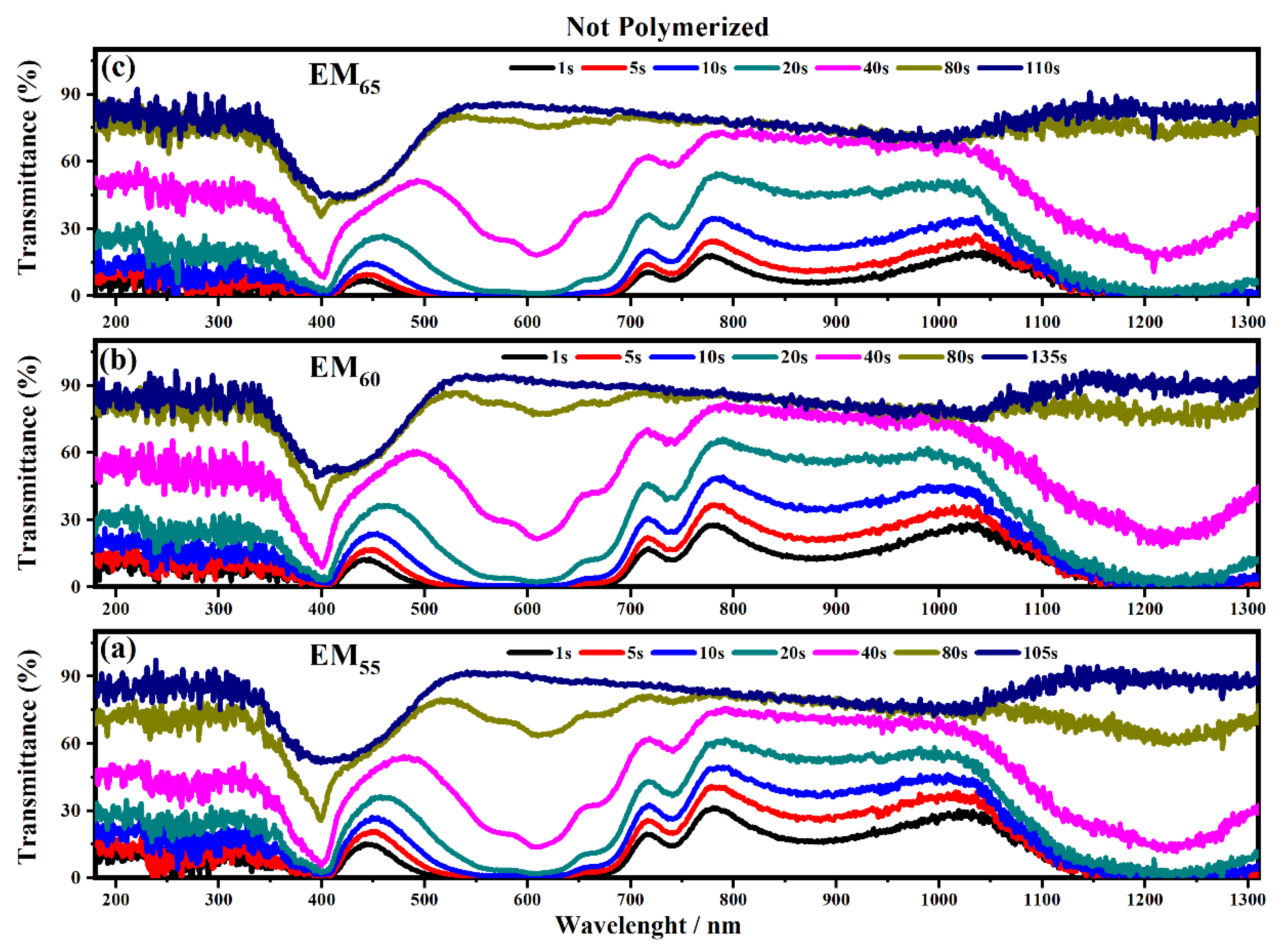
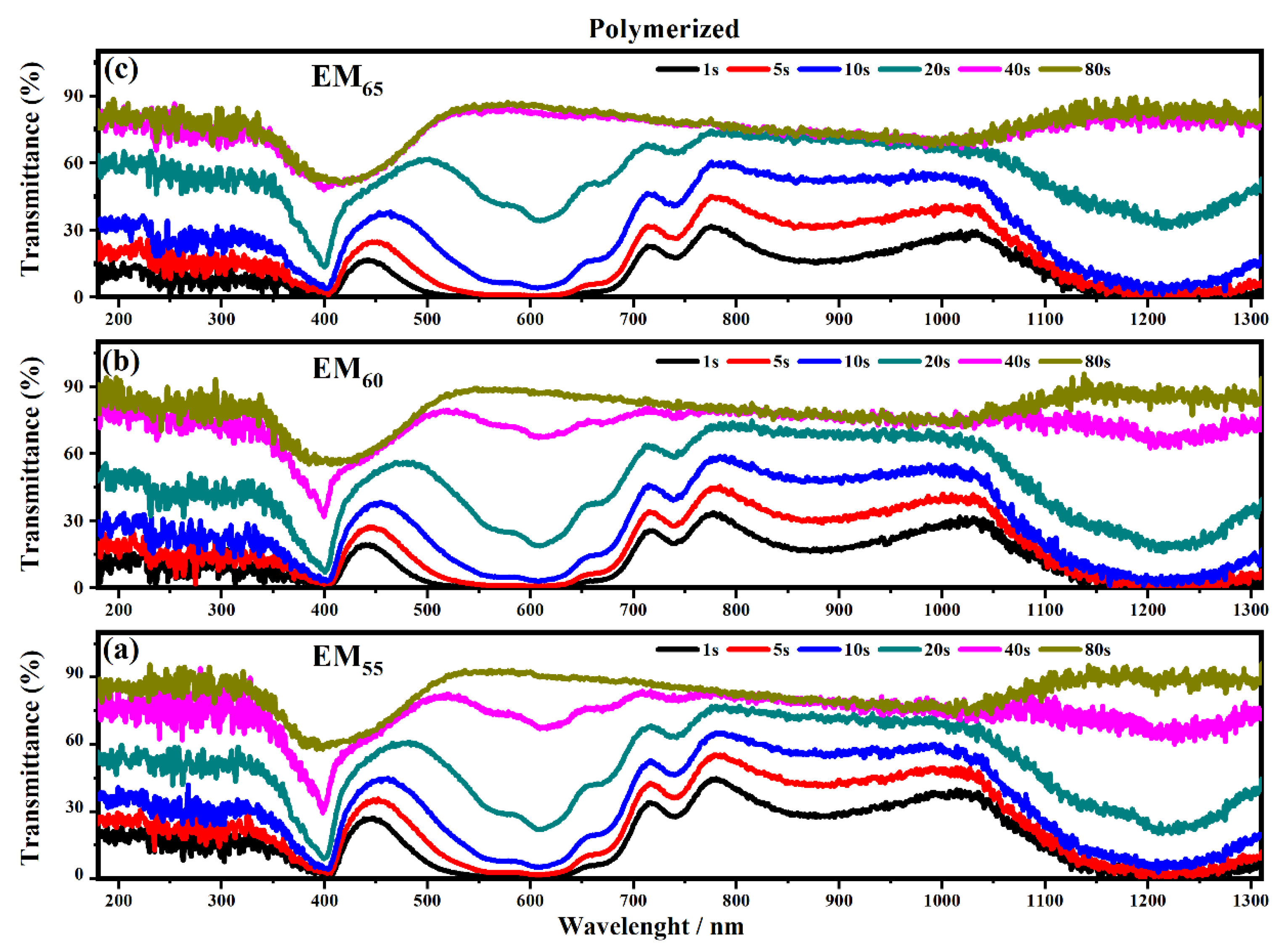
Figure 8 and Figure 9 show the transmittance spectra of the electrochromic devices containing the not-polymerized and polymerized EM55, EM60 and EM65 gels when the devices are switched from the OFF to the ON state.
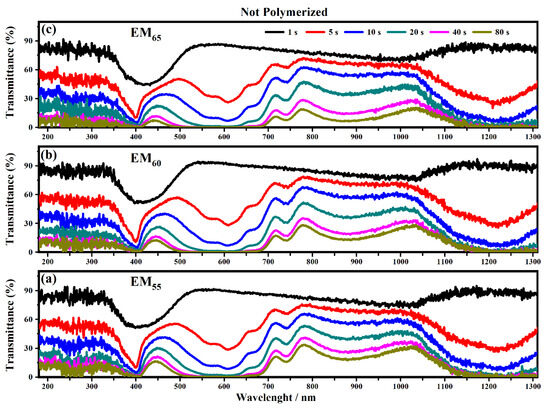
Figure 8.
Transmittance spectra collected on the electrochromic devices filled with the not polymerize electrochromic gel mixtures, EM55 (a), EM60 (b) and EM65 (c), under the application of a voltage of 1.2 V to switch ON the devices.
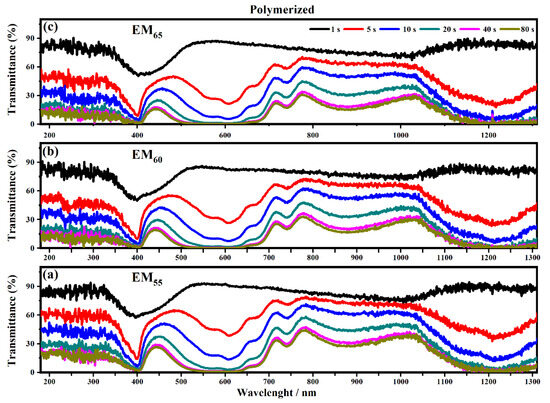
Figure 9.
Transmittance spectra collected on the electrochromic devices filled with the polymerized electrochromic gel mixtures, EM55 (a), EM60 (b) and EM65 (c), under the application of a voltage of 1.2 V to switch ON the devices.
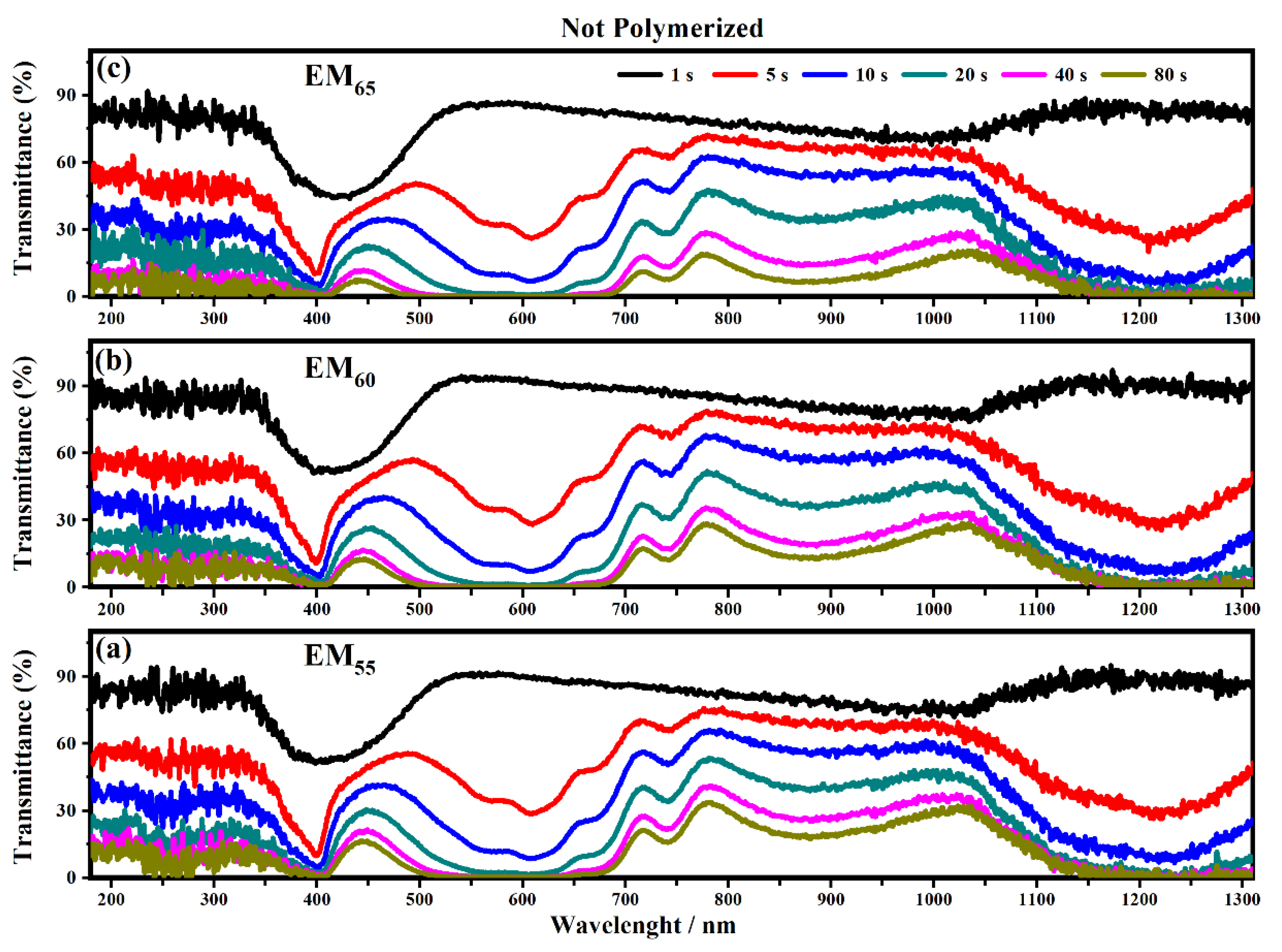
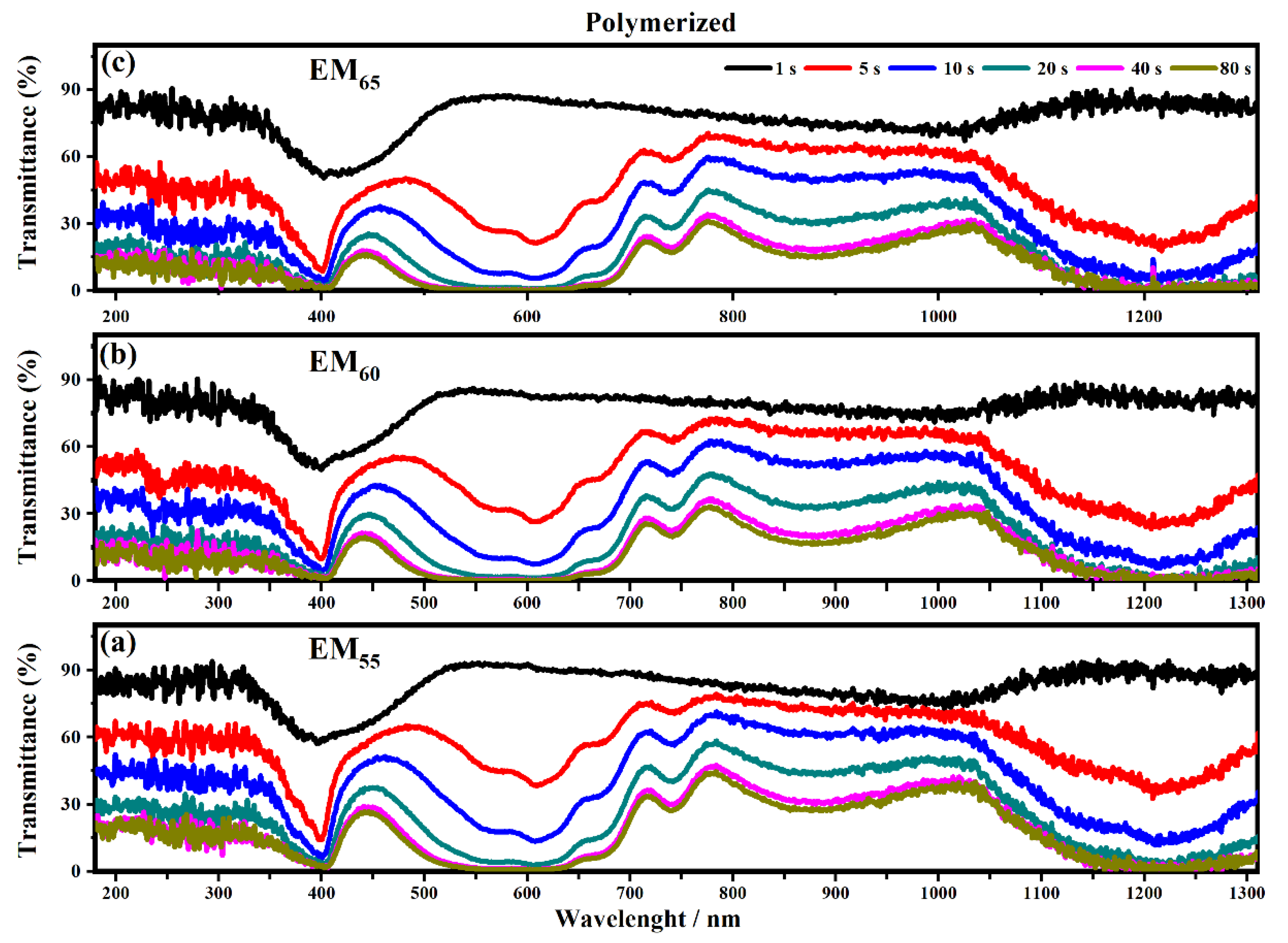
As shown in Figure 8 all the devices based on the not-polymerized electrochromic gel mixtures (EM55, EM60 and EM65) need 5 s to switch from the OFF to the ON state. The same time of 5 s was found for electrochromic devices containing the polymerized electrochromic gel mixtures EM55, EM60, and EM65 to obtain the switching between the OFF/ON states under the maximum values of applied potential (1.2 V). In both series of electrochromic devices, the highest decreases of transmittance were found after 80 s.
Figure 10 and Figure 11 show the transmittance spectra collected on the electrochromic devices, based on the not-polymerized and polymerized electrochromic gel mixtures (EM55, EM60 and EM65) when the devices are switching from the ON to the OFF state.
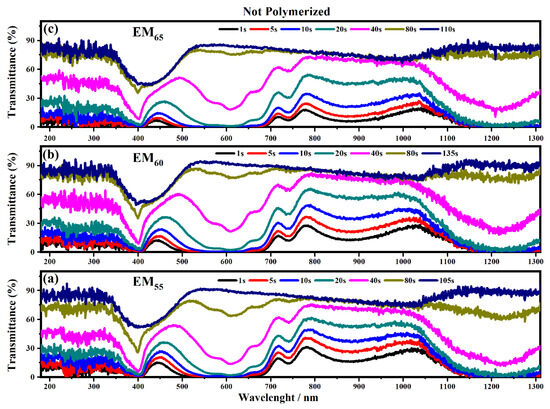
Figure 10.
Transmittance spectra collected on the electrochromic devices filled with EM55 (a), EM60 (b) and EM65 (c) not-polymerized gels under a voltage of 1.2 V to switch OFF the devices.
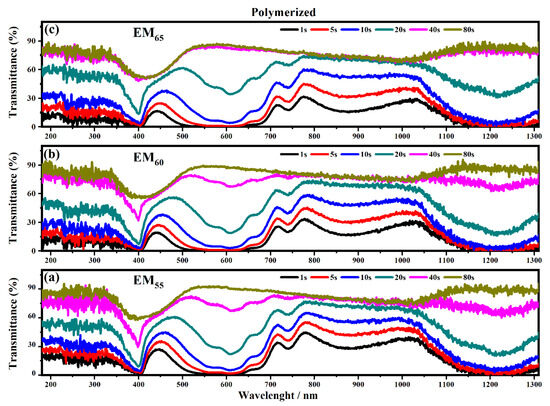
Figure 11.
Transmittance spectra collected on the electrochromic devices filled with EM55 (a), EM60 (b) and EM65 (c) polymerized gels under a voltage of 1.2 V to switch OFF the devices.
The polymerization process of the bisphenol A seems to improve the switching of the devices between the ON/OFF states. As shown in Figure 10, the electrochromic devices containing the not-polymerized bisphenol A took 40 s in order to switch from the ON to the OFF state, and at the end, the devices are completely switched OFF after a time of between 80 and 135 s.
In particular, the device with EM55 needs 105 s to switch OFF, while the devices based on EM60 and EM65 need a time of 135 s and 110 s, respectively. After the polymerization process of bisphenol A, all the devices need 80 s to switch OFF.
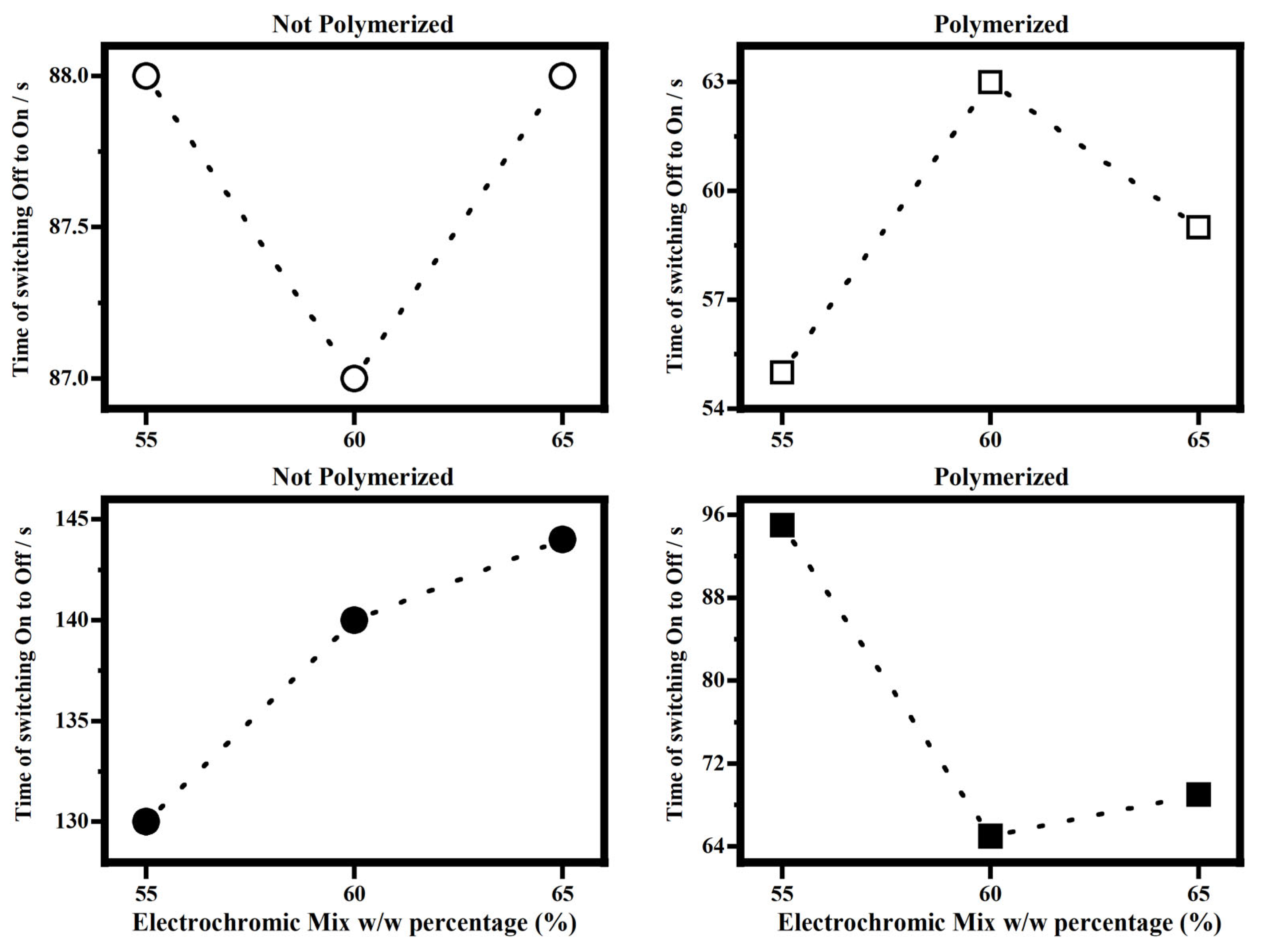
Figure 12 shows the times of the switching starting by the state OFF to the state ON and starting to state On to the state OFF, for the electrochromic devices filled with the polymerized and not-polymerized EM55, EM60 and EM65 gels to compare the effect of the polymerization on such response times.
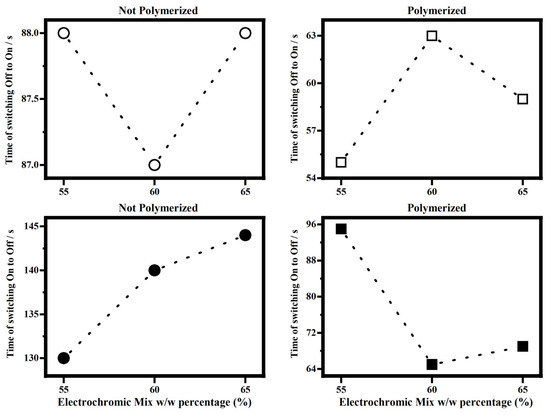
Figure 12.
Time of switching OFF/ON and ON/OFF vs. percentage w/w mix of electrochromic/bisphenol A, EM55, EM60 and EM65 gels of the electrochromic devices not-polymerized (on the left) and polymerized (on the right).
As it can be seen in Figure 12, once a voltage of 1.2 V is applied, the times for the switching OFF/ON of the electrochromic devices filled with not-polymerized EM55, EM60 and EM65 gels are about 88 s, while for the polymerized samples, the switching time falls in the range between 55 s and 63 s. As stated above, the memory times in not-polymerized devices are higher than those in polymerized. In fact, for the not-polymerized devices, the memory times fall in the range between 130 s and 145 s, while for the polymerized ones, they fall in the range between 64 s and 96 s. To reach full coloration, electrochromic devices filled with EM55, EM60, and EM65 polymerized gels are faster than the respective not-polymerized devices (Figure 12). Since the effect of the polymerization is to decrease the resistance to the ions diffusion inside the devices, it affects the memory times, which are lower for polymerized systems than for not-polymerized.
In both the systems, polymerized and not-polymerized, the spectra collected in the devices with the highest concentration of the electrochromic materials show a lower transmittance, at each wavelength, in both the states OFF and ON. Of course, it can be assigned to the number of the color centers that increase, as well as the concentration of the electrochromic materials.
The coloration efficiency (CE) at a given wavelength is an important parameter to establish how good a device is (Equation (1)):
In the previous formula, ΔA is the absorbance difference between ON and OFF states, and Q is the number of charges injected per unit area ([C]/[cm]2).
The values of CE at 580 nm and at 1200 nm for the devices filled with the polymerized and not-polymerized electrochromic gel mixtures (EM55, EM60, and EM65) are reported in Table 1.

Table 1.
Coloration efficiency at 580 nm and 1200 nm of the electrochromic devices filled with EM55, EM60 and EM65.
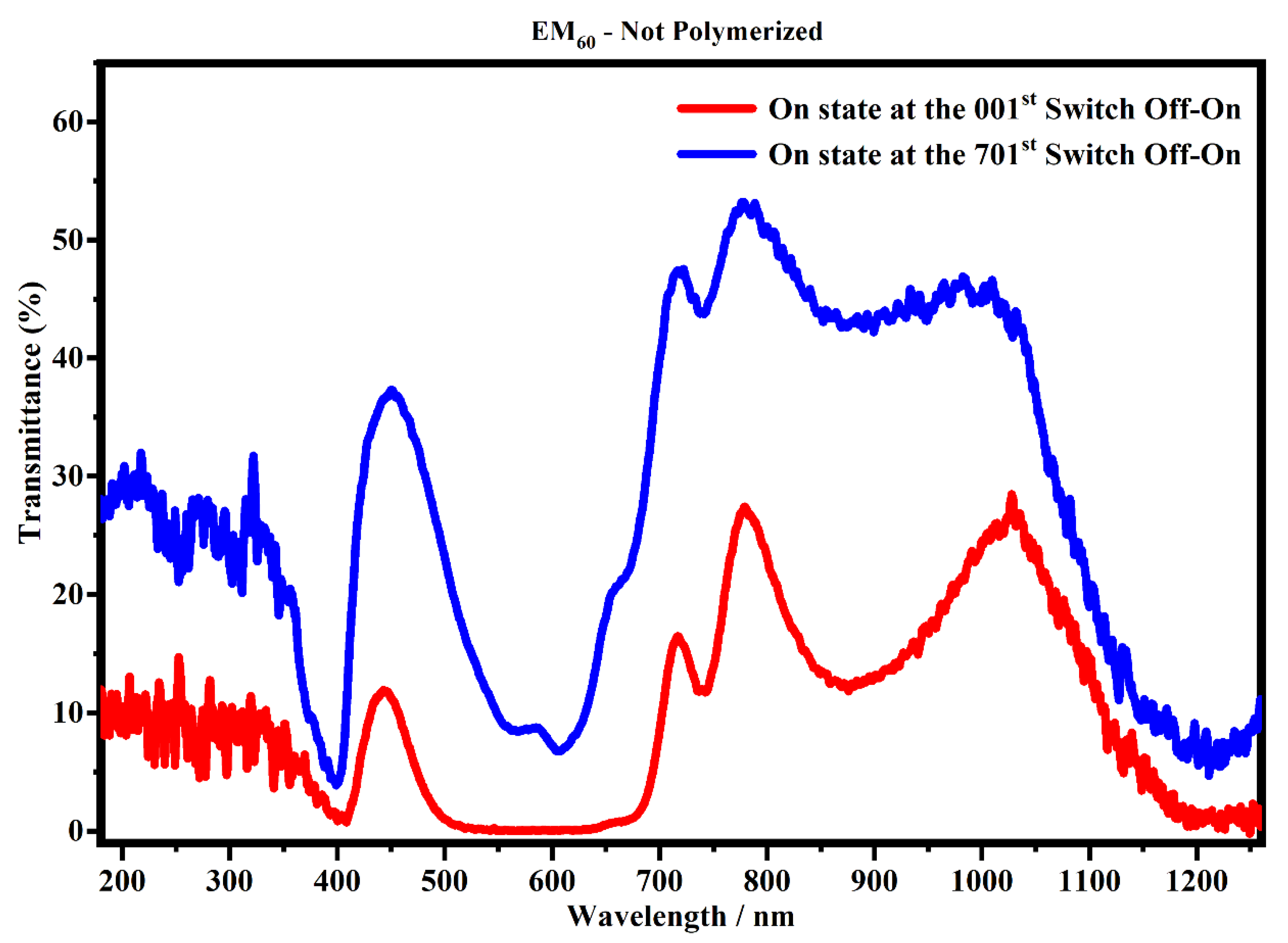
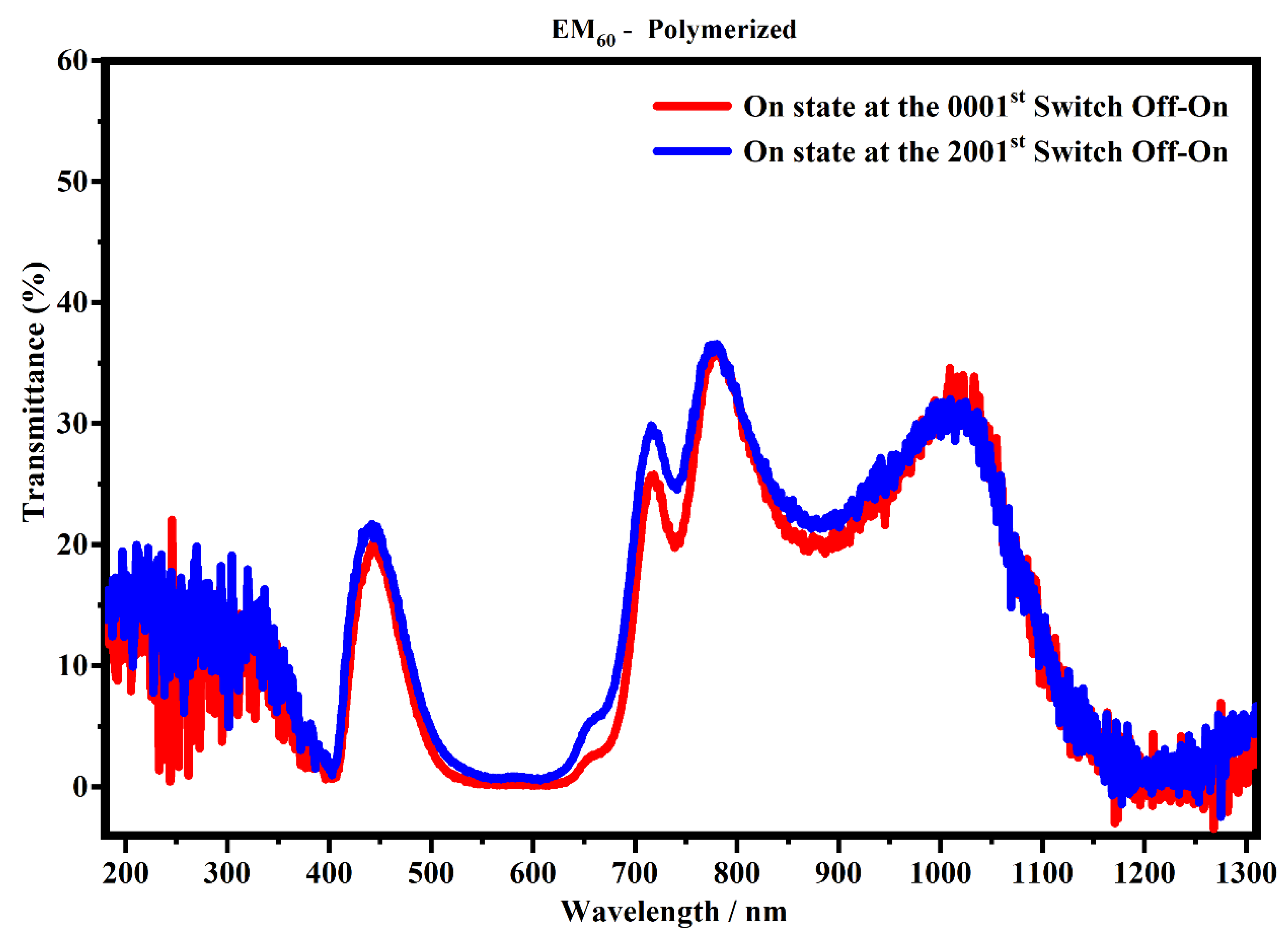
Focusing on the devices made of the electrochromic gel mixture at a concentration of 60% (EM60), containing the not-polymerized and the polymerized bisphenol A, Figure 13 and Figure 14 show the transmittances recorded after the first cycle and after 700 cycles (one cycle correspond to the two processes: switch OFF/ON and switch ON/OFF).
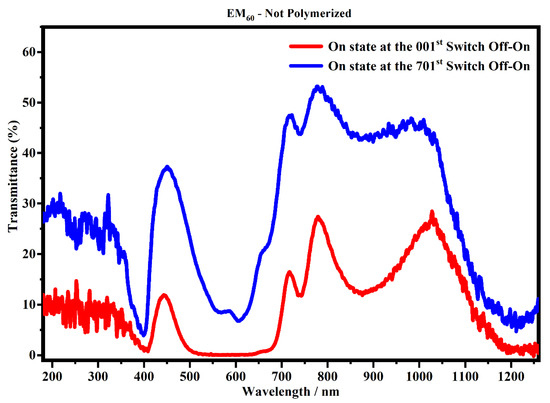
Figure 13.
Transmittance spectra, in the state ON, of the devices filled with the EM60 not-polymerized gel at the 1st (red line) and 701st cycle (blue line).
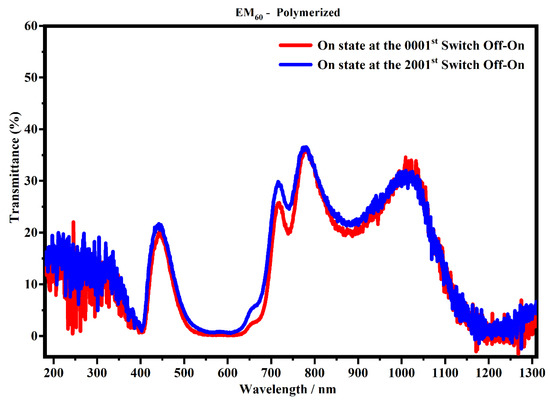
Figure 14.
Transmittance spectra, in the state ON, of the devices filled with the EM60 polymerized gel at the 1st (red line) and 2001st cycle (blue line).
Figure 13 shows the transmittance spectra collected on the devices filled with EM60, not-polymerized gel after the first cycle and after 700 cycles (switch OFF/ON and switch ON/OFF).
Figure 13 shows that the spectra collected after 700 cycles have a lower transmitance than the spectra collected after one cycle, and in particular, ΔT% at 400 nm, 600 nm and 1205 nm is 3.3%, 6.6% and 6.3%, respectively. This means that the sample could suffer a possible degradation of the gel mixture subjected to an external applied voltage. On the contrary, the system which contains the polymerized EM60 is still stable up to 2000 cycles, as shown in Figure 14. In particular, it can be noticed that the polymerized devices do not exhibit appreciable variation of transmittance in the considered range. The polymerization process and the combination of the gel polymer mixture play a key role in enhancing the life of electrochromic devices.
The novelty of these devices is using a polymeric gel mixture made using a viologen and ferrocene couple, and bisphenol A can assist in the involved redox process. An essential feature of the electrochromic device is the high transparency and the possibility of using this unique feature to fabricate smart windows compared to other devices studied in the literature [32,33,34]. In addition, the fabricated ECDs revealed interesting optical properties, as confirmed via UV-vis spectroscopy and independent color-switching reversible coloration and bleaching cycles, making them useful for coloration efficiency and promoting energy-saving buildings.
2.3. Raman Spectroscopy
Raman spectroscopy was performed on the electrochromic devices filled with the polymerized and not-polymerized electrochromic gel mixtures (EM55, EM60 and EM65).
Raman spectra are shown in two ranges: from 310 cm–1 to 2000 cm−1 and from 2000 cm−1 to 3510 cm−1. In Figure 15, Figure 16, Figure 17, Figure 18, Figure 19 and Figure 20, the Raman spectra collected on the electrochromic devices are shown.
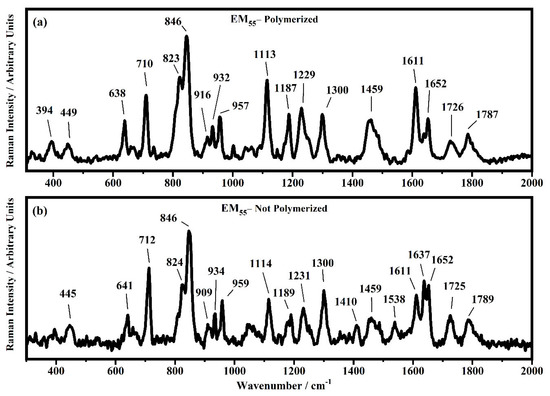
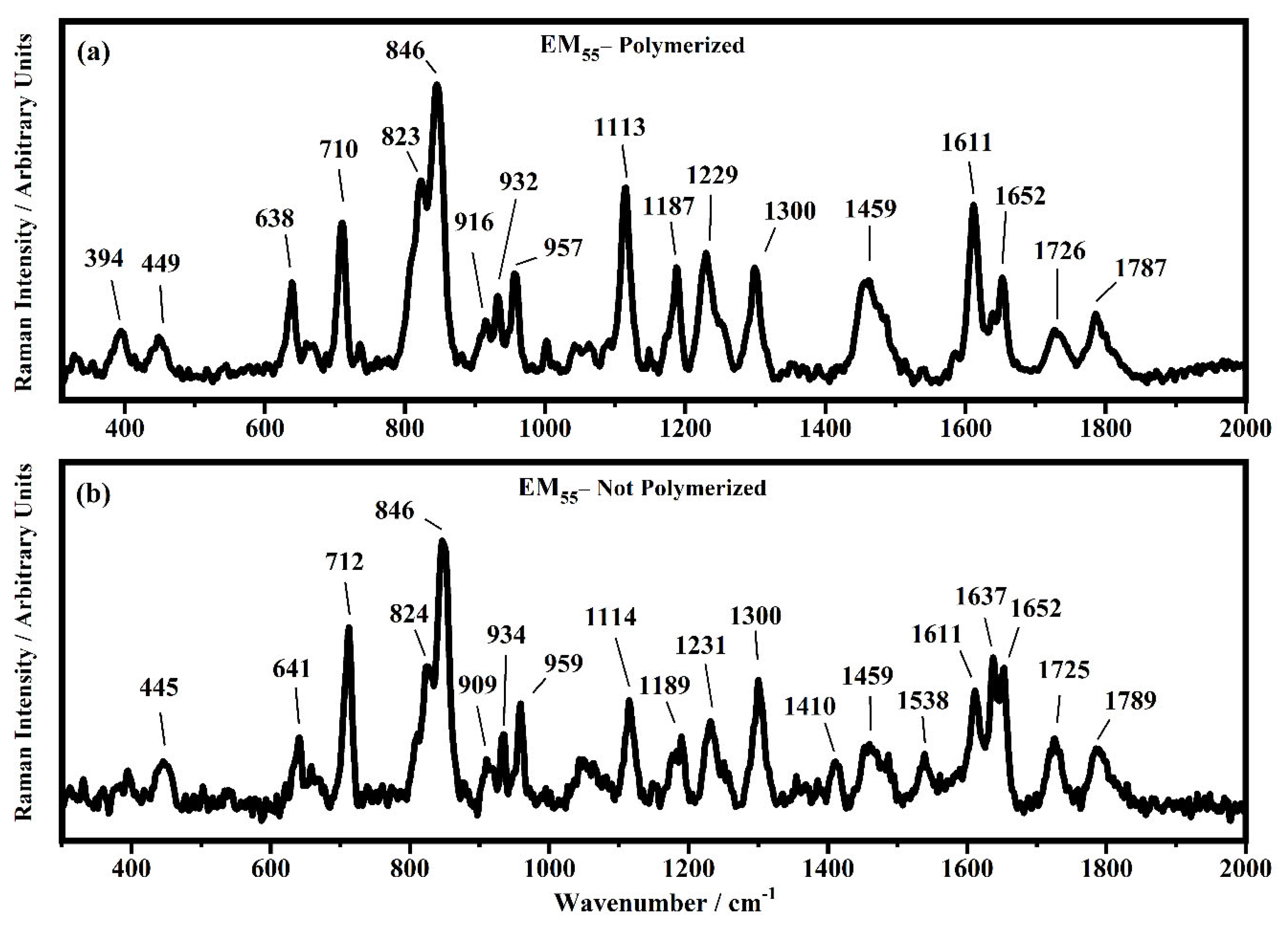
Figure 15.
Raman spectra collected in the range between 310 and 2000 cm−1 on the samples EM55 polymerized (a) and not-polymerized (b).
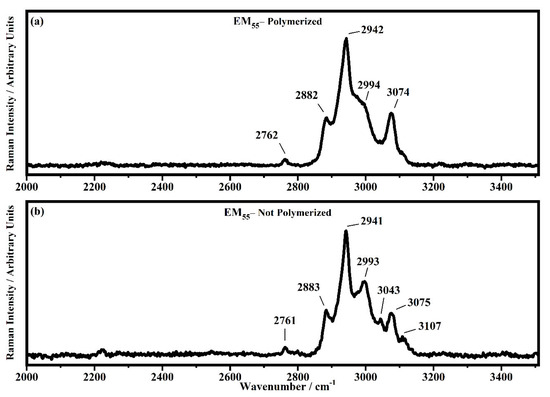
Figure 16.
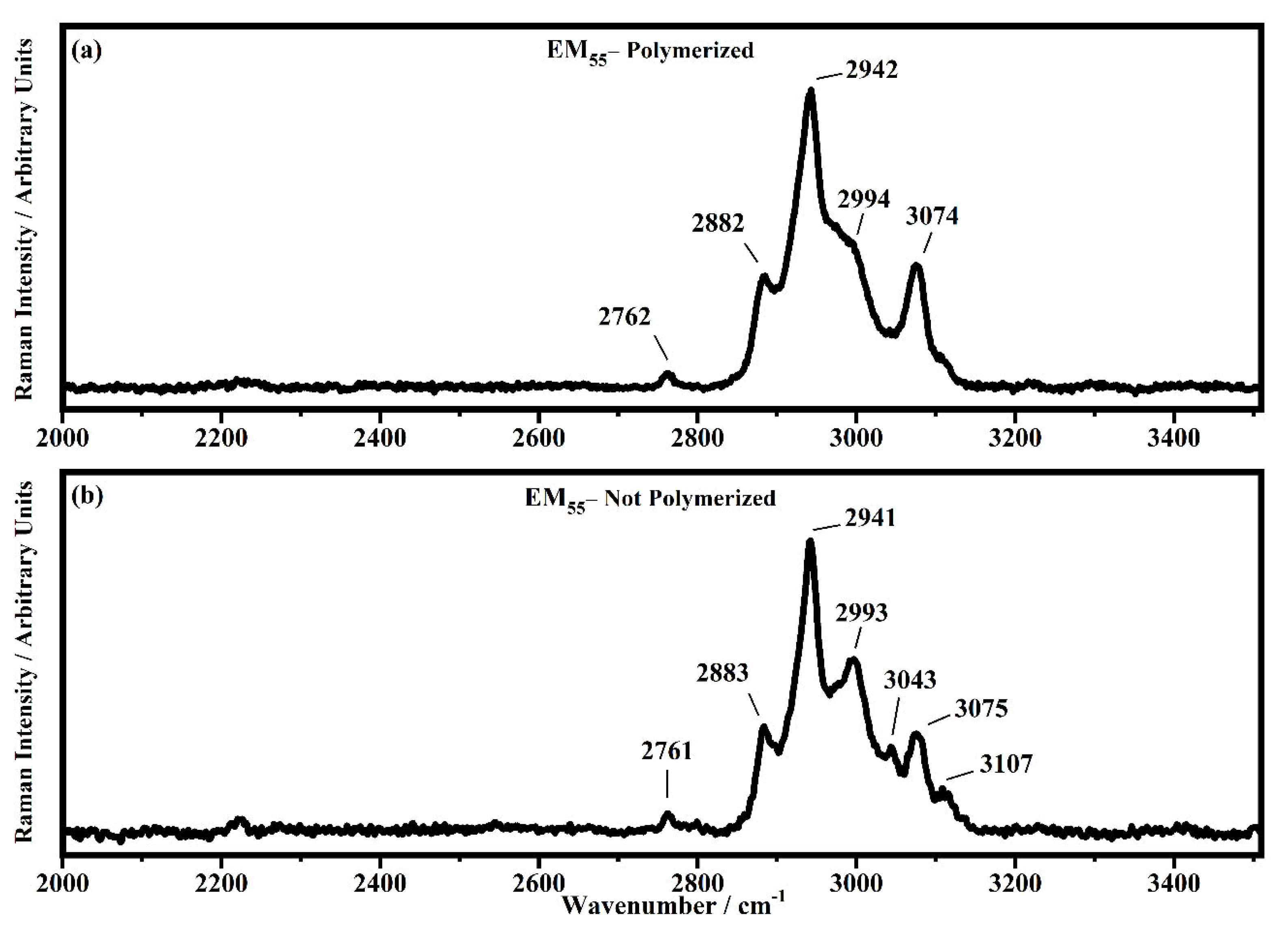
Raman spectra collected in the range between 2000 and 3510 cm−1 on the samples EM55 polymerized (a) and not-polymerized (b).
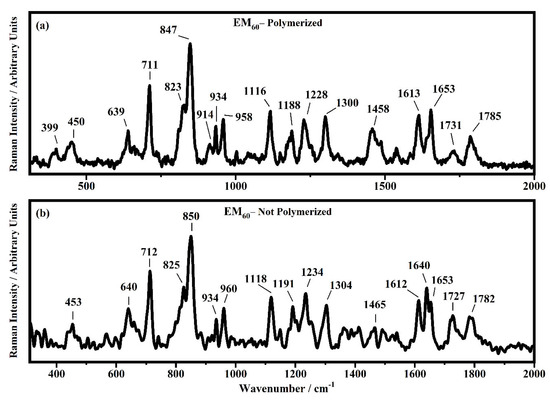
Figure 17.
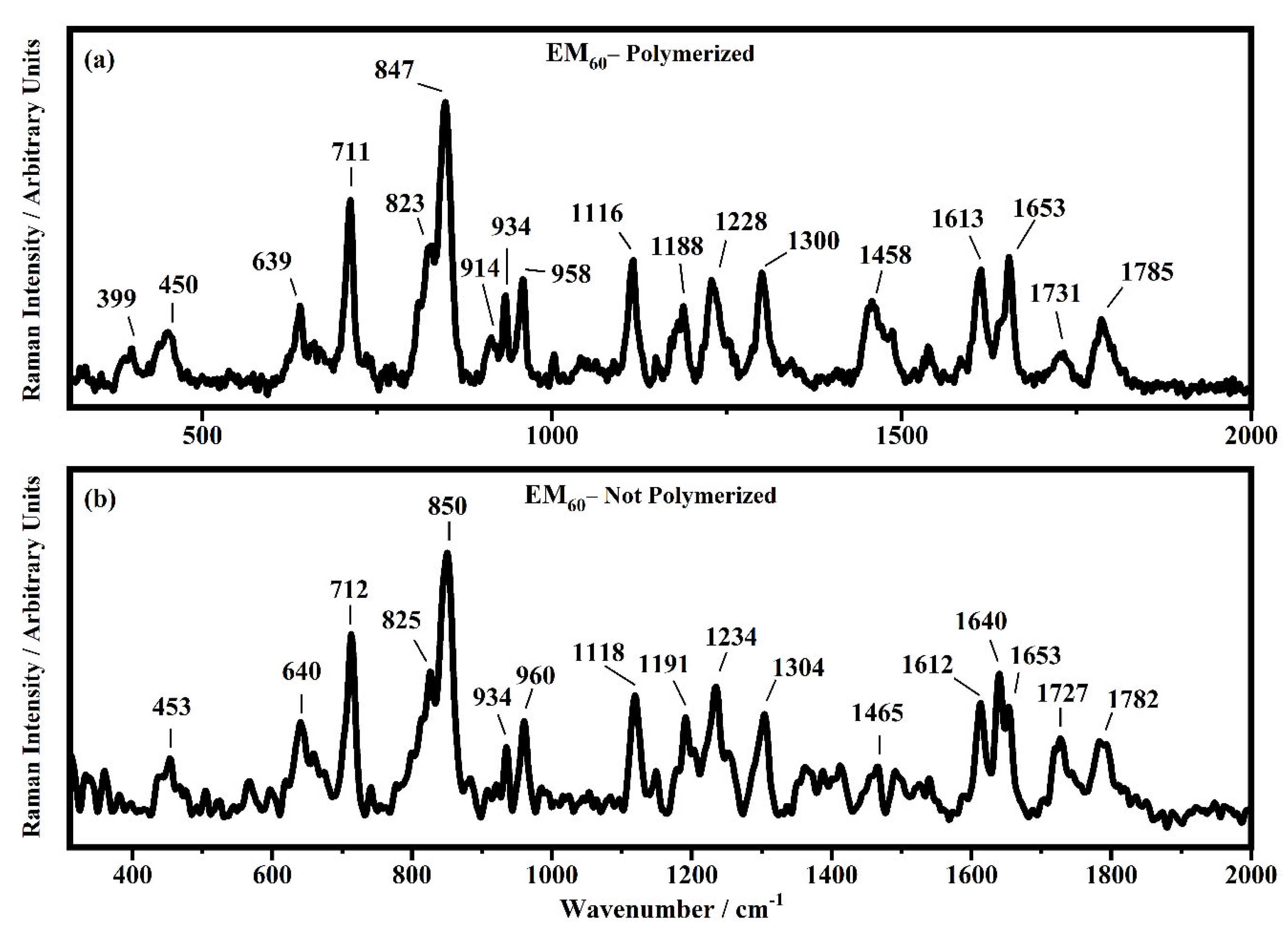
Raman spectra collected in the range between 310 and 2000 cm−1 on the samples EM60 polymerized (a) and not-polymerized (b).
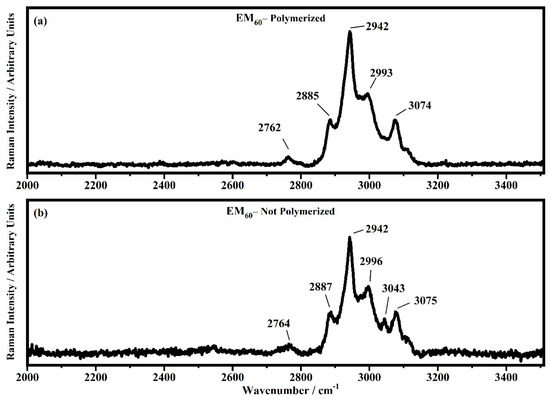
Figure 18.
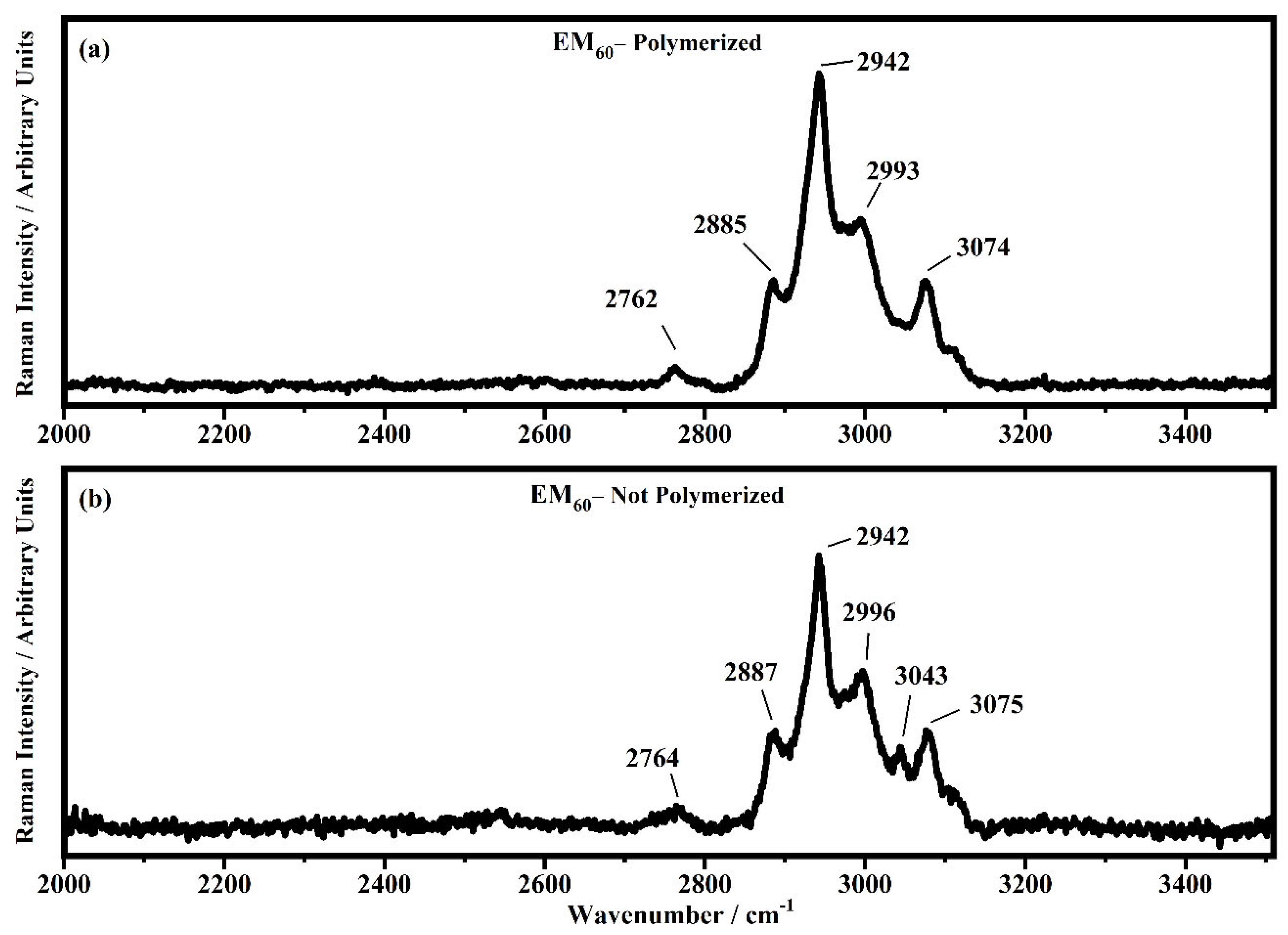
Raman spectra collected in the range between 2000 and 3510 cm−1 on the samples EM60 polymerized (a) and not-polymerized (b).
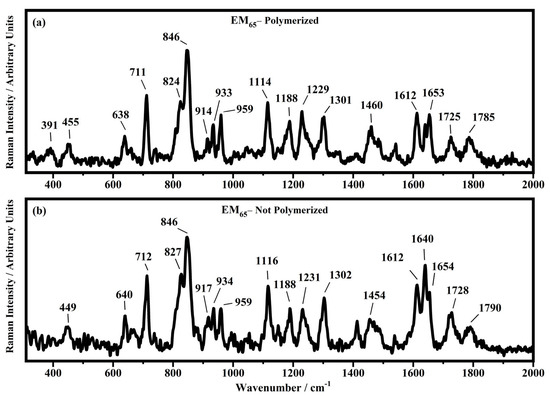
Figure 19.
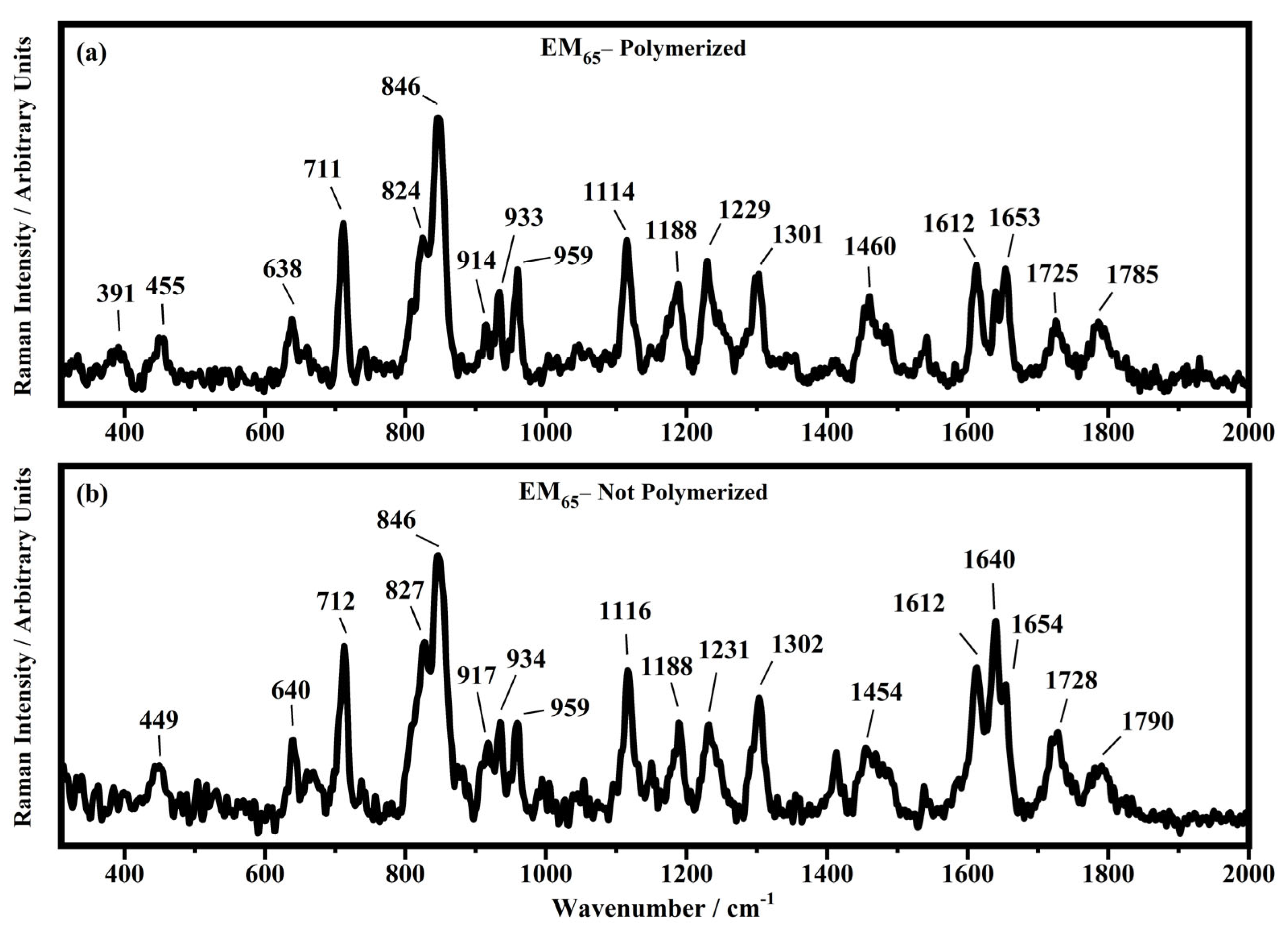
Raman spectra collected in the range between 300 and 2000 cm−1 on the samples EM65 polymerized (a) and not-polymerized (b).
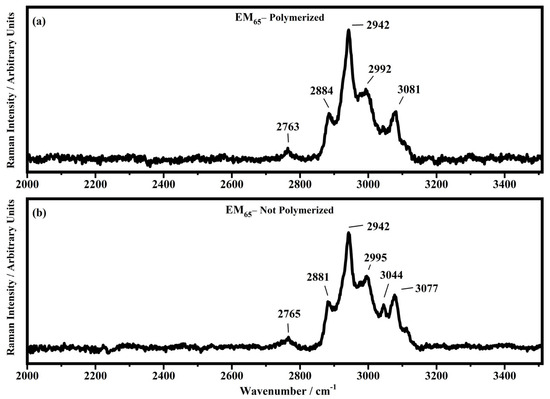
Figure 20.
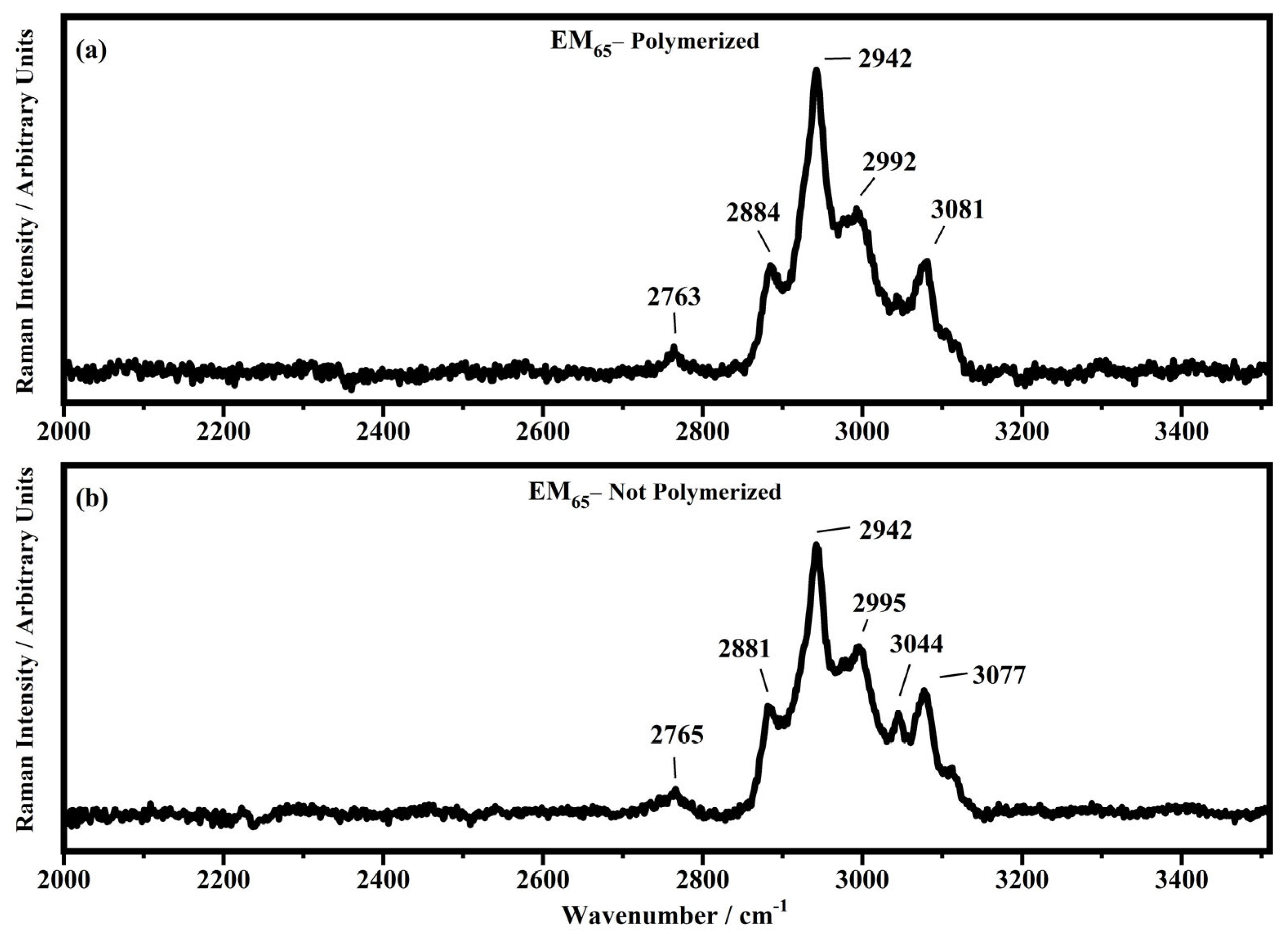
Raman spectra collected in the range between 2000 and 3510 cm−1 on the samples EM65 polymerized (a) and not-polymerized (b).
All the Raman spectra show similar peaks. Very low Raman peaks below 455 cm−1 can be assigned to 1,1′-diethyl ferrocene ring modes. The bands comprised between 822 cm−1 and 826 cm−1 can be assigned to the ring breathing mode of the ethyl viologen diperchlorate. The triplet centered around 935 cm−1 is related to the Cl–O stretching in the perchlorate ions when they are free, which interact with propylene carbonate or with each other [27,35]. Several signals in the spectra can be assigned to propylene carbonate [36]: the band at 455 cm−1 can be assigned to the propylene carbonate ring deformation, while the strong band at 712 cm−1 can be assigned to its symmetric ring deformation. The strong band at 849 cm−1 can be assigned to the C–H methyl group and to O–C–C (not comprising a carbon bond to the methyl group) bending of propylene carbonate. The band at 1119 cm−1 is related to the C–H methyl group and to the C–H stretching of carbon of the methyl group of propylene carbonate. A very low signal can be identified at about 1147 cm−1, corresponding to the stretching of the C–C bond and to the bending of the O–C–O group of the propylene carbonate.
Some Raman bands comprised between 1336 cm−1 and 1391 cm−1 could be assigned to the bending of the C-H group of the propylene carbonate. Signals with very low intensity can be identified between 1336 cm−1 and 1391 cm−1, related to the C–H methyl group bending of propylene carbonate. The vibrational mode of 1460 cm−1 can be assigned to the umbrella mode of the C–H methyl group in the propylene carbonate. The Raman bands at 1726 cm−1 and 1787 cm−1 are ascribed to the stretching of the C=O groups: the first one is assigned to the acrylate carbonyl group and the second one to the carbonyl of the propylene carbonate.
In the region comprised between 2000 cm−1 and 3500 cm−1, a change in the Raman spectra can be noticed between the spectra collected on the polymerized and not-polymerized devices. The signal at about 3043 cm−1 in the spectra of the polymerized samples is very weak or totally absent compared to the spectra of not-polymerized devices. Such behavior is similar to the peak at 1640 cm−1 because both are related to the C=C stretching of the acrylate group of bisphenol A. The bisphenol A polymerization occurs at the terminal C=C double bond; studies of the intensity variation of the 1640 cm−1 bands during polymerization have already been reported [36].
Signals at ~2882 cm−1, ~2940 cm−1 and ~2990 cm−1 can be assigned to the various CH2 and CH3 stretching modes of species in the solution. The band at 3073 cm−1 can be assigned to the C-H stretching of aromatic rings. Since no signals can be observed in the 3200 cm−1–3500 cm−1 range, condensation between different polymeric chains can be excluded (i.e., no water can be identified in the spectra).
3. Conclusions
In this work, a study of electrochromic devices based on ethyl viologen diperchlorate and 1,1′-diethyl ferrocene as a redox couple in propylene carbonate has been outlined. Electrochromic devices were made using viologen-based electrochromic gel mixtures at concentrations of 55%, 60% and 65%, with the first set of samples containing not-polymerized bisphenol A and the second set polymerized bisphenol A.
The effects of the different concentration ratios and of the state of the bisphenol A (not-polymerized or polymerized) on the electrochromic performances of the devices have been shown.
Several characterizations, such as cyclic voltammetry measurements, UV-vis and Raman spectroscopies, clarified that the devices based on the concentration of 60 w/w% of electrochromic solution revealed engaging performances useful for the implementation of electrochromic devices for energy-saving applications.
Cyclic voltammetry measurements confirmed the reversible electrochemical behavior of the electrochromic devices based on the investigated electrochromic gel mixtures.
The redox peak at about 0.4 V was assigned to the oxidation peak of the 1,1′-diethyl ferrocene oxidation and to the reduction of the ethyl viologen. The potentials needed to switch on the devices seems to be lower and the composition in the electrochromic solution passes from 55% to 65%. In comparison, the current intensities present in the different devices become higher and the composition in the electrochromic solution passes from 55% to 65%. The effect of the polymerization is negligible in the sample with EM55. In the case of EM60, the not-polymerized samples show switching potentials higher than the polymerized samples, while in the case of EM65, the not-polymerized samples show a higher current with respect to the polymerized ones.
At 550 nm, 880 nm and 1200 nm, decreases of the transmittances can be observed for all electrochromic devices, and the formation of the radical species of the ethyl viologen justifies this behavior. Focusing on the devices EM60, after the polymerization of the bisphenol A, the transmittance changed from 90% to 60% at 0.6 V and reached 0% at 1.2 V, at 550 nm. At 880 nm, the transmittances varied from 90% (until 0.5 V) to 15% (1.2 V), and at 1200 nm, the devices showed a variation from 90% until 0.5 V to 60% at 0.6 V and, at the end, to about 0% at the maximum voltage. At 1.2 V, the devices with not-polymerized bisphenol A and based on the mixture at a concentration of 60% (EM60) took a time of 63 s to switch OFF/ON and 60 s to switch ON/OFF.
The polymerization of bisphenol A helped to shorten the switching times and lengthen the cycle life of the investigated electrochromic devices (more than 2000 cycles), making them suitable devices for fabrication of smart windows.
EM60 devices can be used for energy-saving applications in buildings, with a promising CE of 92.82 C/cm2 in the visible region and 80.38 C/cm2 in the near infrared region.
4. Materials and Methods
An electrochromic solution (ES) based on the redox couple 1,1′-diethyl ferrocene and ethyl viologen diperchlorate (EV) was prepared in an aprotic solvent, propylene carbonate (PC, 99%), to assist the ions separation inside the electrochromic device. A second solution made of bisphenol A glycerolate (1 glycerol/phenol) diacrylate and the photo-initiator Irgacure (dimethoxy-phenylacetophenon 99%) was prepared and added to the first solution to obtain three electrochromic gel mixtures (EM) at the weight ratios of 55%, 60% and 65% (EM55, EM60 and EM65). Another series of the electrochromic gel mixtures was prepared at the same weight ratios of 55%, 60% and 65% (EM55, EM60 and EM65), after which the polymerization process of the bisphenol A occurred.
The electrochromic devices were fabricated using two transparent electrodes of Indium Tin Oxide (ITO)-coated glass, separated by silica sphere spacers with a diameter of 88 μm. The electrochromic devices were sealed using an epoxy resin and were exposed to the UV lamp light (from Jelosil, Milan, Italy, HG 200 L model, optical power per unit area 0.043 W/cm−2) for 1 min and 30 s. Each electrochromic device was filled with the electrochromic gel mixtures via capillarity.
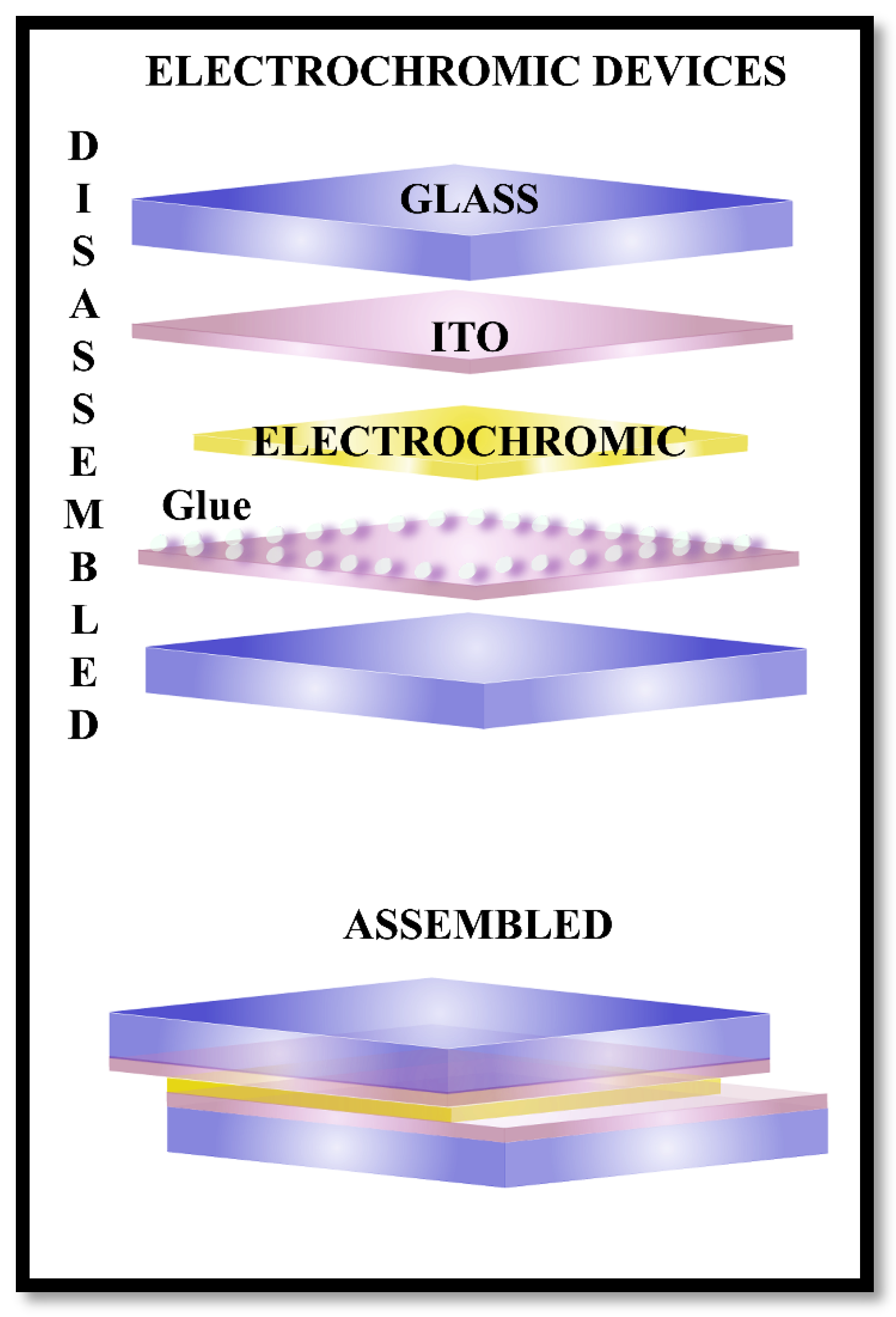
Figure 21 shows the main components of an electrochromic device and the final assembled device.
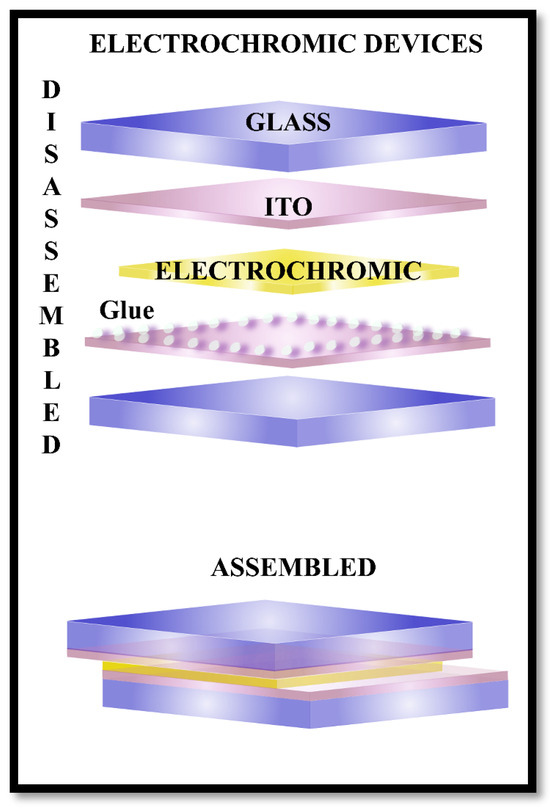
Figure 21.
Electrochromic device disassembled (on the top) and assembled (on the bottom).
All chemicals, shown in Figure 22, were purchased from Sigma−Aldrich Merk, Milan, Italy and used without any particular additional treatments, except for bisphenol A glycerolate (1 glycerol/phenol) diacrylate. Since this compound has too high a density even at room temperature, a heat treatment was necessary to manipulate it [3].
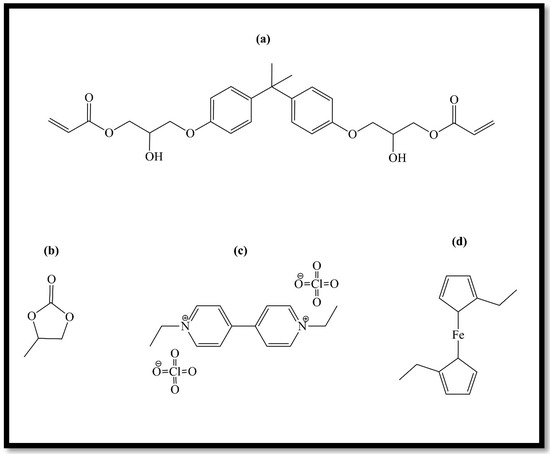
Figure 22.
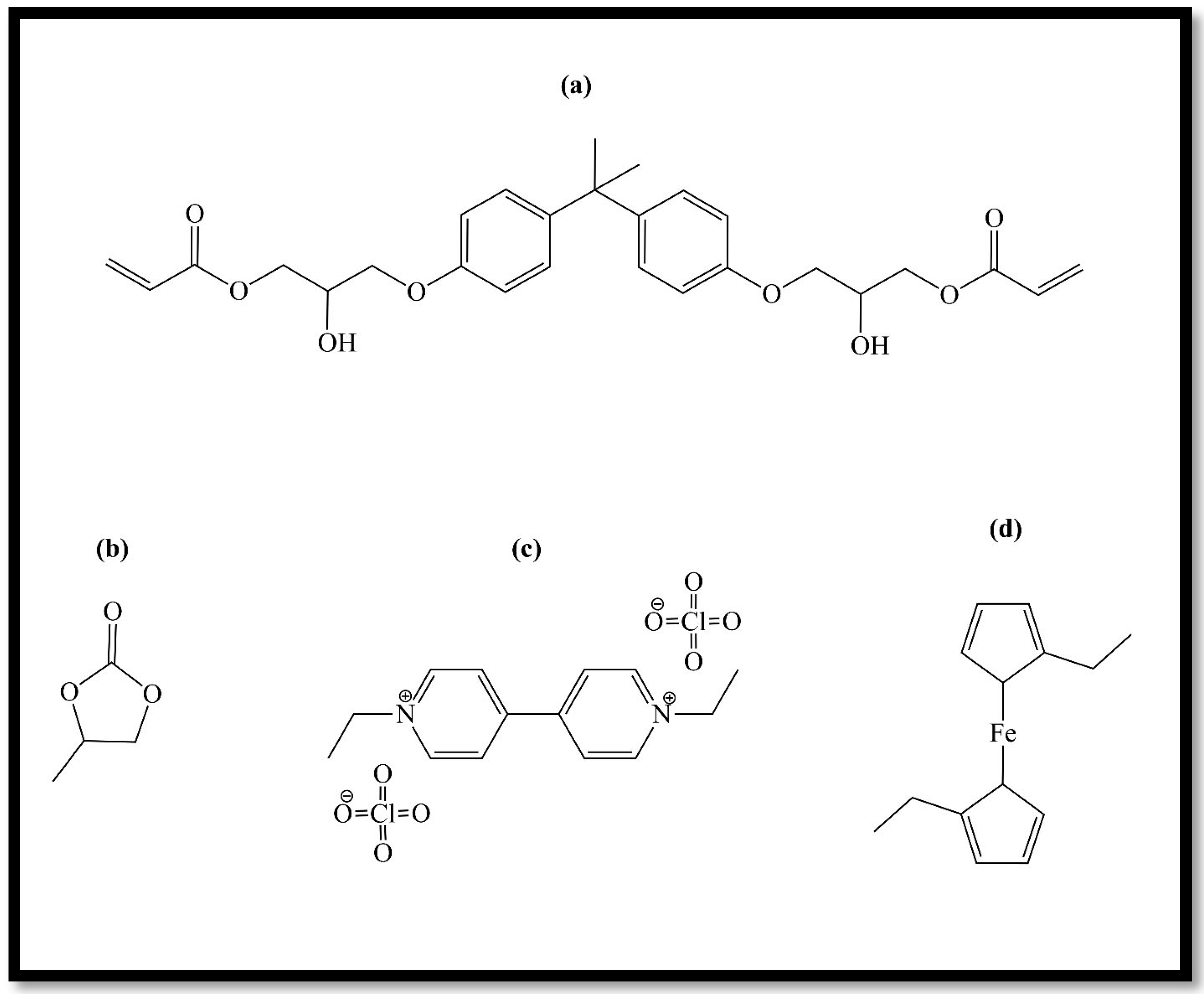
Electrochromic gel chemicals: (a) bisphenol A glycerolate (1 glycerol/phenol) diacrylate, (b) propylene carbonate; (c) ethyl viologen diperchlorate; (d) diethyl ferrocene.
Transmittance measurements were performed using a spectrophotometer (from AvaSpec supplied by Avantes, Apeldoorn Netherlands), 2048-Avantes equipped with a Deuterium-Halogen light source (DH-2000 UV-Vis-Nir Lightsource Avantes) and Agilent E3632A DC Power Supply (0-15V, 7A), Agilent Technologies, Palo Alto, CA, USA
Cyclic voltammetry measurements were performed using a potentiostat/galvanostat (model 2059 from Amel Instrument s.r.l. Perigny, France) equipped with the 7800 interface in order to manage measurements using Junior Assist software 2059.
Raman spectroscopy measurements were performed using a micro-Raman-LABRAM (from Horiba-Jobin Yvon Srl, Piscataway, NJ, USA) equipped with an optical microscope in order to collect Raman spectra from small regions of the sample, such as 2–5 µm. MicroRaman-LABRAM is equipped with a HeNe 17 mW laser (633 nm), a NdYAG 50 mW laser (doubled, 532 nm) and a CCD detector (Peltier cooled, 1024 × 256). The optical microscope is equipped with 10× NA 0.25, 50× NA 0.7 and 100× NA 0.9 objectives.
Author Contributions
Conceptualization, M.M.P., A.N. and M.C.; methodology, C.R. and I.B.C. and M.C.; software, A.N. and C.R.; validation, R.C.B., R.T. and M.C.; formal analysis, M.M.P., R.C.B. and M.C.; investigation, M.M.P. and A.N.; resources, M.C.; data curation, R.T. and M.C.; writing—original draft preparation, M.M.P., I.B.C., C.R. and M.C.; writing—review and editing, C.R., A.N., R.C.B. and M.C.; visualization, M.C.; supervision, R.T. and M.C.; project administration, R.C.B. and M.C.; funding acquisition, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors wish to thank: “Technologies for climate change adaptation and quality of life improvement”—Tech4You CUPB83C22003980006 and “Hybrid organic/inorganic microchip based on oligonucleotides and gold nanoparticles” CUP H53D23009110001 projects.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Capoferri, D.; Álvarez-Diduk, R.; Del Carlo, M.; Compagnone, D.; Merkoçi, A. Electrochromic Molecular Imprinting Sensor for Visual and Smartphone-Based Detections. Anal. Chem. 2018, 90, 5850–5856. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, A.; Simone, F. Performance Requirements for Electrochromic Smart Window. J. Build. Eng. 2015, 3, 94–103. [Google Scholar] [CrossRef]
- Rizzuto, C.; Barberi, R.C.; Castriota, M. Development of Electrochromic Devices, Based on Polymeric Gel, for Energy Saving Applications. Polymers 2023, 15, 3347. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, C.; Barberi, R.C.; Castriota, M. Tungsten and Titanium Oxide Thin Films Obtained by the Sol-Gel Process as Electrodes in Electrochromic Devices. Front. Mater. 2022, 9, 912013. [Google Scholar] [CrossRef]
- Barawi, M.; Veramonti, G.; Epifani, M.; Giannuzzi, R.; Sibillano, T.; Giannini, C.; Rougier, A.; Manca, M. A Dual Band Electrochromic Device Switchable across Four Distinct Optical Modes. J. Mater. Chem. A 2018, 6, 10201–10205. [Google Scholar] [CrossRef]
- Granqvist, C.G. Handbook of Inorganic Electrochromic Materials; Elsevier Science & Technology: Amsterdam, The Netherlands, 2005; ISBN 978-1-281-98511-8. [Google Scholar]
- Zhou, K.; Wang, H.; Jiu, J.; Liu, J.; Yan, H.; Suganuma, K. Polyaniline Films with Modified Nanostructure for Bifunctional Flexible Multicolor Electrochromic and Supercapacitor Applications. Chem. Eng. J. 2018, 345, 290–299. [Google Scholar] [CrossRef]
- Sonavane, A.C.; Inamdar, A.I.; Dalavi, D.S.; Deshmukh, H.P.; Patil, P.S. Simple and Rapid Synthesis of NiO/PPy Thin Films with Improved Electrochromic Performance. Electrochim. Acta 2010, 55, 2344–2351. [Google Scholar] [CrossRef]
- Chang, T.-H.; Lu, H.-C.; Lee, M.-H.; Kao, S.-Y.; Ho, K.-C. Multi-Color Electrochromic Devices Based on Phenyl and Heptyl Viologens Immobilized with UV-Cured Polymer Electrolyte. Sol. Energy Mater. Sol. Cells 2018, 177, 75–81. [Google Scholar] [CrossRef]
- Mishra, S.; Pandey, H.; Yogi, P.; Saxena, S.K.; Roy, S.; Sagdeo, P.R.; Kumar, R. Interfacial Redox Centers as Origin of Color Switching in Organic Electrochromic Device. Opt. Mater. 2017, 66, 65–71. [Google Scholar] [CrossRef]
- Danine, A.; Manceriu, L.; Fargues, A.; Rougier, A. Eco-Friendly Redox Mediator Gelatin-Electrolyte for Simplified TiO2-Viologen Based Electrochromic Devices. Electrochim. Acta 2017, 258, 200–207. [Google Scholar] [CrossRef]
- Groat, K.A.; Creager, S.E. Self-Assembled Monolayers in Organic Solvents: Electrochemistry at Alkanethiolate-Coated Gold in Propylene Carbonate. Langmuir 1993, 9, 3668–3675. [Google Scholar] [CrossRef]
- Vergaz, R.; Barrios, D.; Sánchez-Pena, J.-M.; Pozo-Gonzalo, C.; Salsamendi, M. Relating Cyclic Voltammetry and Impedance Analysis in a Viologen Electrochromic Device. Sol. Energy Mater. Sol. Cells 2009, 93, 2125–2132. [Google Scholar] [CrossRef]
- Wen, R.-T.; Granqvist, C.G.; Niklasson, G.A. Cyclic Voltammetry on Sputter-Deposited Films of Electrochromic Ni Oxide: Power-Law Decay of the Charge Density Exchange. Appl. Phys. Lett. 2014, 105, 163502. [Google Scholar] [CrossRef]
- Gadgil, B.; Damlin, P.; Dmitrieva, E.; Ääritalo, T.; Kvarnström, C. ESR/UV-Vis-NIR Spectroelectrochemical Study and Electrochromic Contrast Enhancement of a Polythiophene Derivative Bearing a Pendant Viologen. RSC Adv. 2015, 5, 42242–42249. [Google Scholar] [CrossRef]
- Wałęsa-Chorab, M.; Skene, W.G. Visible-to-NIR Electrochromic Device Prepared from a Thermally Polymerizable Electroactive Organic Monomer. ACS Appl. Mater. Interfaces 2017, 9, 21524–21531. [Google Scholar] [CrossRef]
- Politano, G.G.; Cazzanelli, E.; Versace, C.; Castriota, M.; Desiderio, G.; Davoli, M.; Vena, C.; Bartolino, R. Micro-Raman Investigation of Ag/Graphene Oxide/Au Sandwich Structure. Mater. Res. Express 2019, 6, 075605. [Google Scholar] [CrossRef]
- De Luca, O.; Grillo, R.; Castriota, M.; Policicchio, A.; De Santo, M.P.; Desiderio, G.; Fasanella, A.; Agostino, R.G.; Cazzanelli, E.; Giarola, M.; et al. Different Spectroscopic Behavior of Coupled and Freestanding Monolayer Graphene Deposited by CVD on Cu Foil. Appl. Surf. Sci. 2018, 458, 580–585. [Google Scholar] [CrossRef]
- Rizzuto, C.; Teeters, D.C.; Barberi, R.C.; Castriota, M. Plasticizers and Salt Concentrations Effects on Polymer Gel Electrolytes Based on Poly (Methyl Methacrylate) for Electrochemical Applications. Gels 2022, 8, 363. [Google Scholar] [CrossRef]
- Politano, G.G.; Castriota, M.; De Santo, M.P.; Pipita, M.M.; Desiderio, G.; Vena, C.; Versace, C. Variable Angle Spectroscopic Ellipsometry Characterization of Spin-Coated MoS2 Films. Vacuum 2021, 189, 110232. [Google Scholar] [CrossRef]
- Marino, S.; Castriota, M.; Bruno, V.; Cazzanelli, E.; Strangi, G.; Versace, C.; Scaramuzza, N. Changes of the Electro-Optic Response of Nematic Liquid Crystal Cells Due to Inserted Titania-Vanadia Films. J. Appl. Phys. 2005, 97, 013523. [Google Scholar] [CrossRef]
- Barba Castagnaro, I.; Nucera, A.; Barberi, R.C.; Castriota, M. Study and Micro-Raman Characterization of Pigments Present on Majolicas of Historical and Artistic Interest from Gerace, Italy. Herit. Sci. 2023, 11, 24. [Google Scholar] [CrossRef]
- Fasanella, A.; Cosentino, K.; Beneduci, A.; Chidichimo, G.; Cazzanelli, E.; Barberi, R.C.; Castriota, M. Thermal Structural Evolutions of DMPC-Water Biomimetic Systems Investigated by Raman Spectroscopy. Biochim. Biophys. Acta (BBA)—Biomembr. 2018, 1860, 1253–1258. [Google Scholar] [CrossRef]
- Algieri, V.; Tursi, A.; Costanzo, P.; Maiuolo, L.; De Nino, A.; Nucera, A.; Castriota, M.; De Luca, O.; Papagno, M.; Caruso, T.; et al. Thiol-Functionalized Cellulose for Mercury Polluted Water Remediation: Synthesis and Study of the Adsorption Properties. Chemosphere 2024, 355, 141891. [Google Scholar] [CrossRef] [PubMed]
- Nucera, A.; Grillo, R.; Rizzuto, C.; Barberi, R.C.; Castriota, M.; Bürgi, T.; Caputo, R.; Palermo, G. Effect of the Combination of Gold Nanoparticles and Polyelectrolyte Layers on SERS Measurements. Biosensors 2022, 12, 895. [Google Scholar] [CrossRef]
- Rafailov, P.M.; Maultzsch, J.; Thomsen, C.; Dettlaff-Weglikowska, U.; Roth, S. Kohn Anomaly and Electron−Phonon Interaction at the K-Derived Point of the Brillouin Zone of Metallic Nanotubes. Nano Lett. 2009, 9, 3343–3348. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Pandey, H.; Yogi, P.; Saxena, S.K.; Roy, S.; Sagdeo, P.R.; Kumar, R. Live Spectroscopy to Observe Electrochromism in Viologen Based Solid State Device. Solid State Commun. 2017, 261, 17–20. [Google Scholar] [CrossRef]
- Kamau, G.N.; Saccucci, T.M.; Gounili, G.; Nassar, A.-E.F.; Rusling, J.F. Films Formed by Oxidation of Ferrocene at Platinum Electrodes. Anal. Chem. 1994, 66, 994–1001. [Google Scholar] [CrossRef]
- Lu, H.-C.; Kao, S.-Y.; Yu, H.-F.; Chang, T.-H.; Kung, C.-W.; Ho, K.-C. Achieving Low-Energy Driven Viologens-Based Electrochromic Devices Utilizing Polymeric Ionic Liquids. ACS Appl. Mater. Interfaces 2016, 8, 30351–30361. [Google Scholar] [CrossRef]
- Raghavan, R.; Iwamoto, R.T. Chemical-Electrochemical Method for the Determination of the Positions of Coupling in the Dimeric One-Electron Reduction Products of Ring-Substitued 1-Alkylpyridinium Ions. J. Electroanal. Chem. Interfacial Electrochem. 1979, 102, 85–92. [Google Scholar] [CrossRef]
- Tahara, H.; Baba, R.; Iwanaga, K.; Sagara, T.; Murakami, H. Electrochromism of a Bipolar Reversible Redox-Active Ferrocene–Viologen Linked Ionic Liquid. Chem. Commun. 2017, 53, 2455–2458. [Google Scholar] [CrossRef]
- Luo, X.; Wan, R.; Zhang, Z.; Song, M.; Yan, L.; Xu, J.; Yang, H.; Lu, B. 3D-Printed Hydrogel-Based Flexible Electrochromic Device for Wearable Displays. Adv. Sci. 2024, 11, 2404679. [Google Scholar] [CrossRef] [PubMed]
- Pande, G.K.; Heo, J.S.; Choi, J.H.; Eom, Y.S.; Kim, J.; Park, S.K.; Park, J.S. RGB-to-Black Multicolor Electrochromic Devices Enabled with Viologen Functionalized Polyhedral Oligomeric Silsesquioxanes. Chem. Eng. J. 2021, 420, 130446. [Google Scholar] [CrossRef]
- Woodward, A.N.; Kolesar, J.M.; Hall, S.R.; Saleh, N.-A.; Jones, D.S.; Walter, M.G. Thiazolothiazole Fluorophores Exhibiting Strong Fluorescence and Viologen-Like Reversible Electrochromism. J. Am. Chem. Soc. 2017, 139, 8467–8473. [Google Scholar] [CrossRef] [PubMed]
- Battisti, D.; Nazri, G.A.; Klassen, B.; Aroca, R. Vibrational Studies of Lithium Perchlorate in Propylene Carbonate Solutions. J. Phys. Chem. 1993, 97, 5826–5830. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Misra, A.; Park, J.; Ye, Q.; Spencer, P. Diffusion Coefficients of Water and Leachables in Methacrylate-Based Crosslinked Polymers Using Absorption Experiments. J. Mater. Sci. Mater. Med. 2012, 23, 1157–1172. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).