High-Temperature Behavior of Pd/MgO Catalysts Prepared via Various Sol–Gel Approaches
Abstract
:1. Introduction
2. Results and Discussion
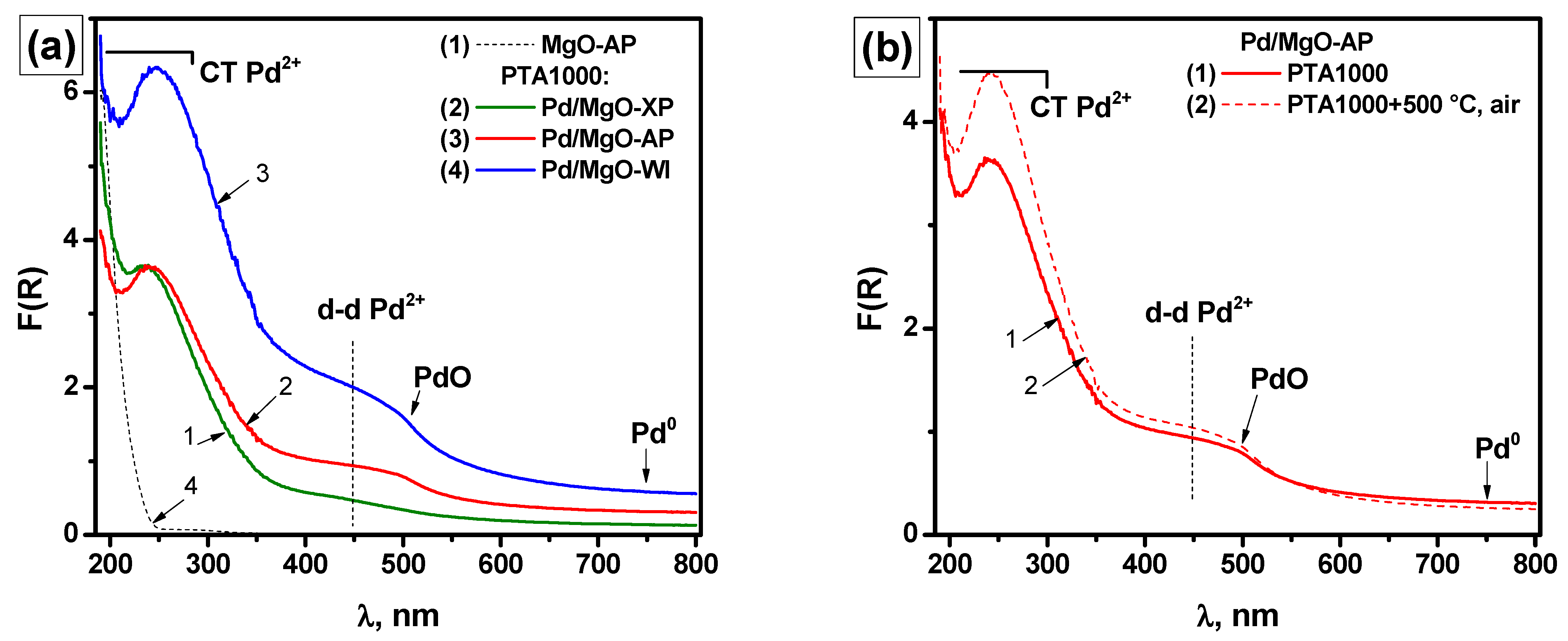
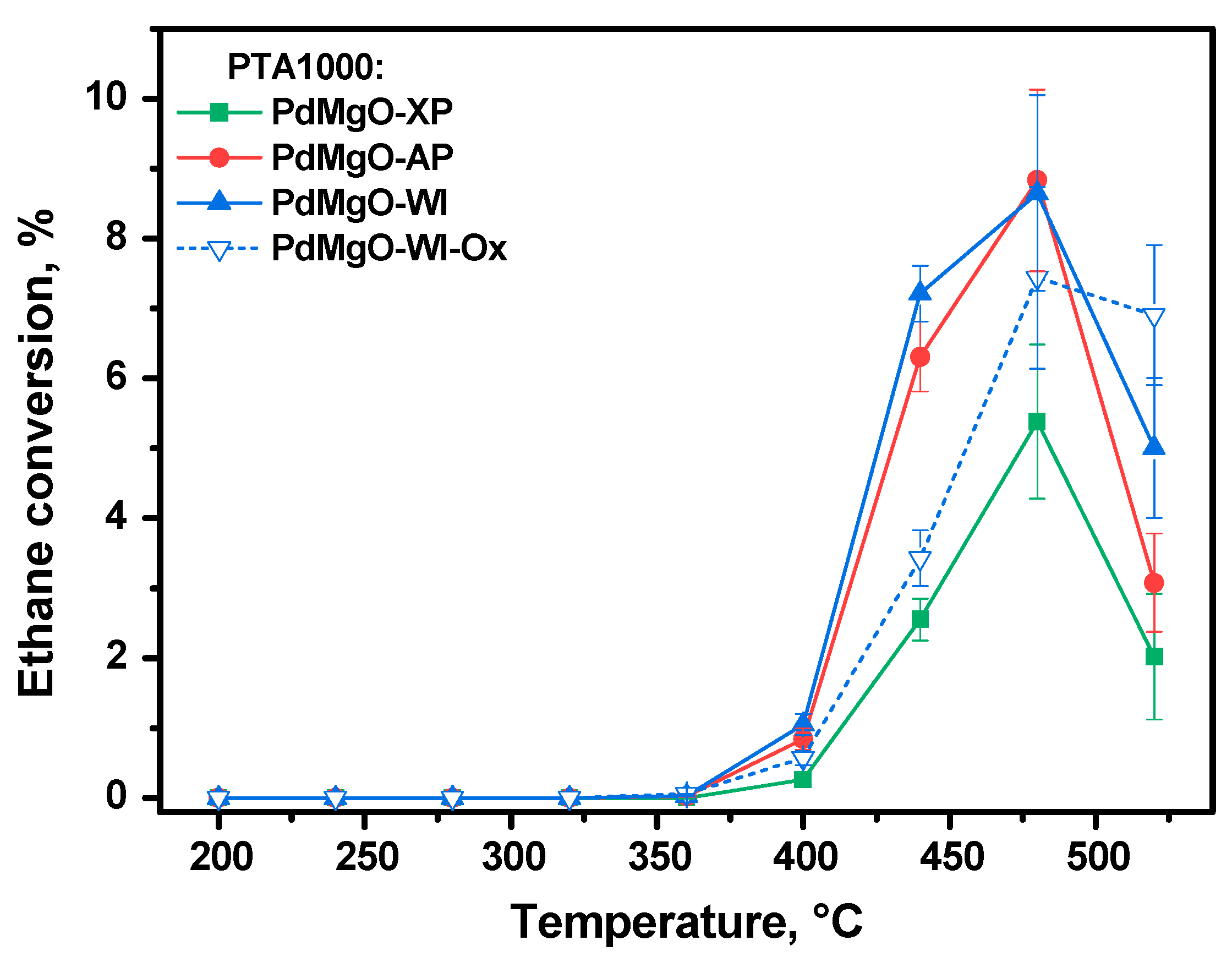
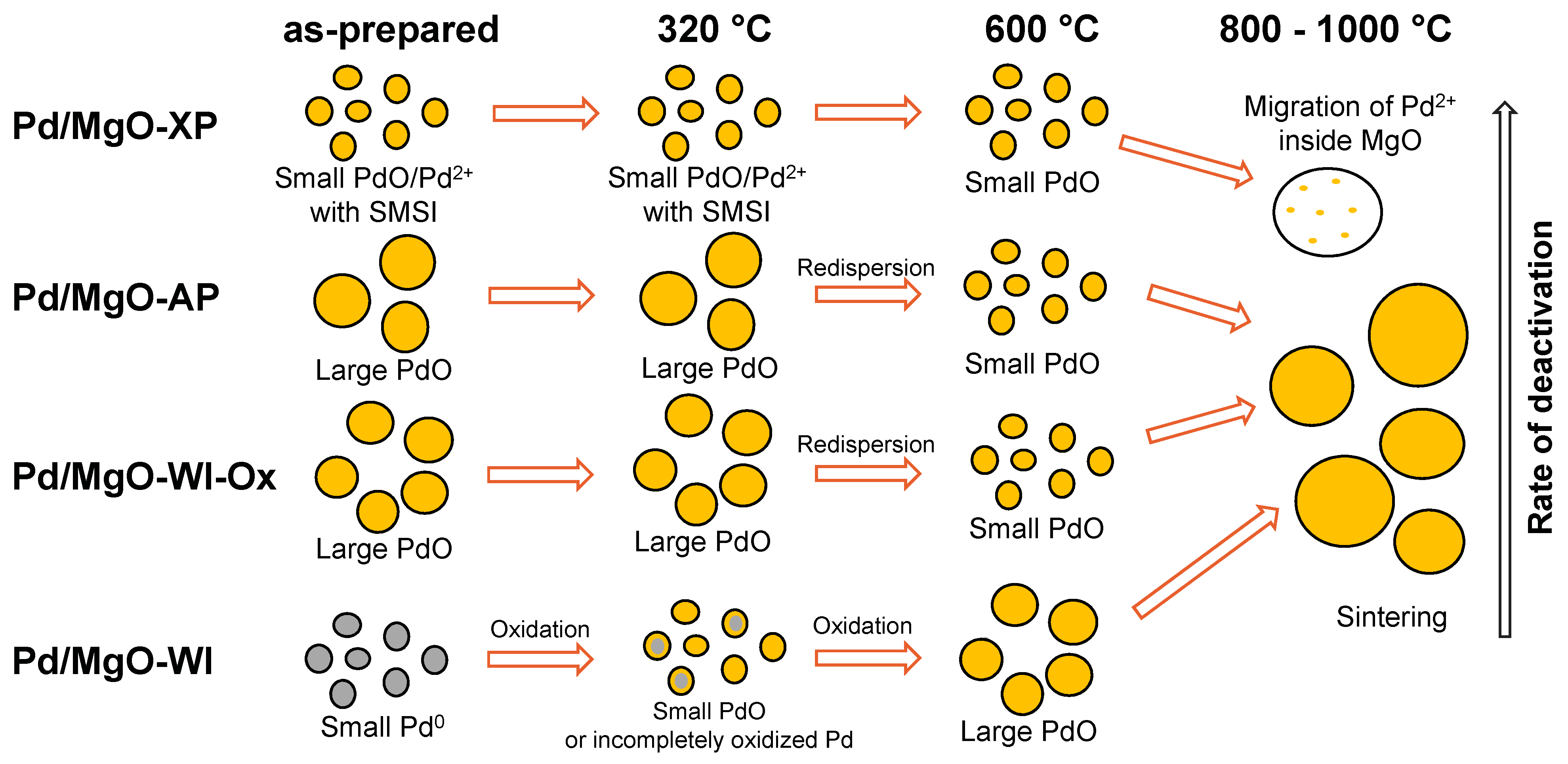
2.1. High-Temperature Behavior of Pd/MgO Catalysts Under PTA Conditions
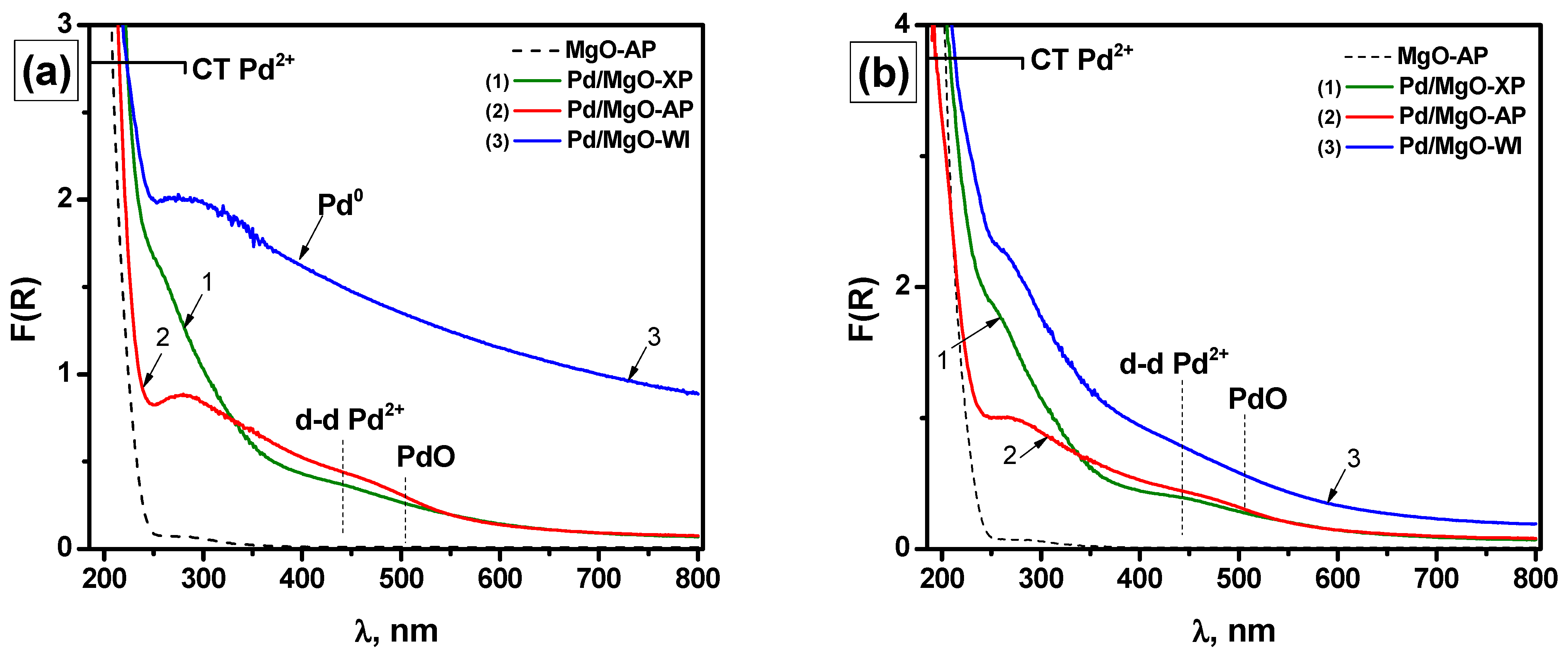
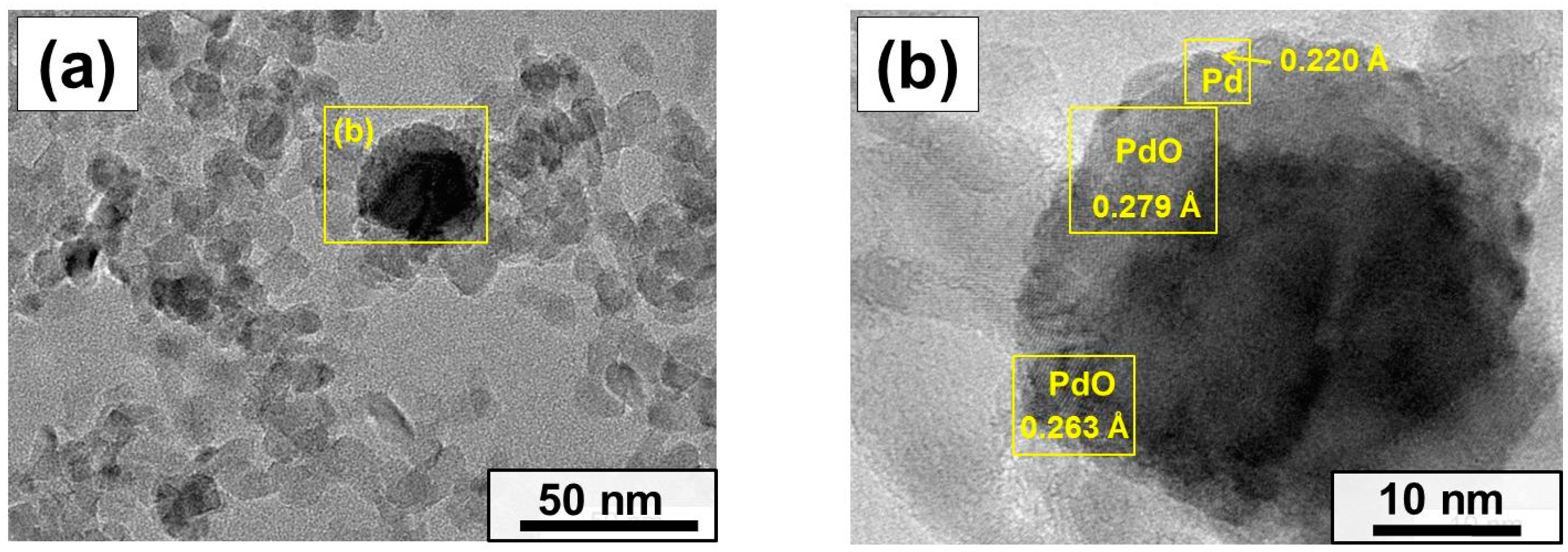
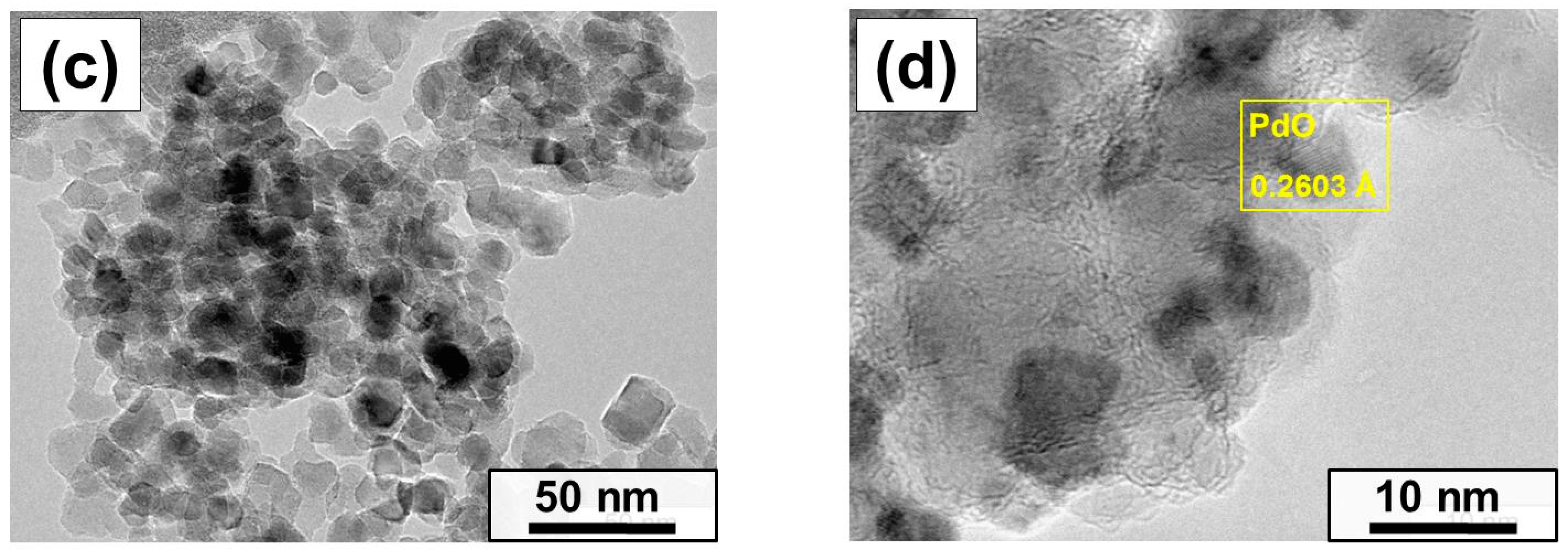
2.2. Characterization of the As-Prepared and Aged Pd/MgO Catalysts
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Materials
4.3. Physicochemical Characterization Methods
4.4. Testing the Catalytic Performance at Prompt Thermal Aging Conditions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giordano, L.; Pacchioni, G. Pd nanoclusters at the MgO(100) surface. Surf. Sci. 2005, 575, 197–209. [Google Scholar] [CrossRef]
- Goniakowski, J.; Noguera, C. Characteristics of Pd deposition on the MgO(111) surface. Phys. Rev. B 1999, 60, 16120–16128. [Google Scholar] [CrossRef]
- Zuo, Z.; Liu, S.; Wang, Z.; Liu, C.; Huang, W.; Huang, J.; Liu, P. Dry reforming of methane on single-site Ni/MgO catalysts: Importance of site confinement. ACS Catal. 2018, 8, 9821–9835. [Google Scholar] [CrossRef]
- Kaskow, I.; Sobczak, I.; Ziolek, M.; Corberán, V.C. The effect of support properties on n-octanol oxidation performed on gold–silver catalysts supported on MgO, ZnO and Nb2O5. Mol. Catal. 2020, 482, 110674. [Google Scholar] [CrossRef]
- Ju, X.; Liu, L.; Zhang, X.; Feng, J.; He, T.; Chen, P. Highly efficient Ru/MgO catalyst with surface-enriched basic sites for production of hydrogen from ammonia decomposition. ChemCatChem 2019, 11, 4161–4170. [Google Scholar] [CrossRef]
- Ju, X.; Feng, J.; Wang, J.; Guo, J.; Liu, L. One-step synthesis of cesium decorated Ru nanoparticles on MgO as efficient catalyst for ammonia synthesis. Catal. Lett. 2023, 153, 1615–1624. [Google Scholar] [CrossRef]
- Peng, S.-Y.; Xu, Z.-N.; Chen, Q.-S.; Wang, Z.-Q.; Chen, Y.; Lv, D.-M.; Lu, G.; Guo, G.-C. MgO: An excellent catalyst support for CO oxidative coupling to dimethyl oxalate. Catal. Sci. Technol. 2014, 4, 1925–1930. [Google Scholar] [CrossRef]
- Ilyina, E.V.; Yurpalova, D.V.; Shlyapin, D.A.; Veselov, G.B.; Shivtsov, D.M.; Stoyanovskii, V.O.; Bukhtiyarov, A.V.; Vedyagin, A.A. Effect of preparation conditions of nanocrystalline Pd/MgO catalysts on their performance in selective hydrogenation of acetylene. Mol. Catal. 2024, 560, 114151. [Google Scholar] [CrossRef]
- Tao, X.; Nan, B.; Li, Y.; Du, M.; Guo, L.-L.; Tian, C.; Jiang, L.; Shen, L.; Sun, N.; Li, L.-N. Highly active isolated single-atom Pd catalyst supported on layered MgO for semihydrogenation of acetylene. ACS Appl. Energy Mater. 2022, 5, 10385–10390. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Mishakov, I.V.; Ilyina, E.V. A step forward in the preparation of V–Mg–O catalysts for oxidative dehydrogenation of propane. J. Sol-Gel Sci. Technol. 2021, 97, 117–125. [Google Scholar] [CrossRef]
- Loder, A.; Siebenhofer, M.; Lux, S. The reaction kinetics of CO2 methanation on a bifunctional Ni/MgO catalyst. J. Ind. Eng. Chem. 2020, 85, 196–207. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Wang, X.; Fang, X.; Wang, H.; Xu, X. New insights into CO2 methanation mechanisms on Ni/MgO catalysts by DFT calculations: Elucidating Ni and MgO roles and support effects. J. CO2 Util. 2019, 33, 55–63. [Google Scholar] [CrossRef]
- Li, P.; Chen, R.; Lin, Y.; Li, W. General approach to facile synthesis of MgO-based porous ultrathin nanosheets enabling high-efficiency CO2 capture. Chem. Eng. J. 2021, 404, 126459. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, C.; Sun, J.; Li, W.; Zhang, J.; Zhao, C. Biomass ash stabilized MgO adsorbents for CO2 capture application. Fuel 2020, 259, 116298. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, Y.; Sun, J.; Li, H.; Liu, W. Progress in MgO sorbents for cyclic CO2 capture: A comprehensive review. J. Mater. Chem. A 2019, 7, 20103–20120. [Google Scholar] [CrossRef]
- Iugai, I.A.; Steksova, Y.P.; Vedyagin, A.A.; Mishakov, I.V.; Bauman, Y.I.; Belyy, V.A.; Danilovich, D.P.; Krivoshapkina, E.F.; Krivoshapkin, P.V. MgO/carbon nanofibers composite coatings on porous ceramic surface for CO2 capture. Surf. Coat. Technol. 2020, 400, 126208. [Google Scholar] [CrossRef]
- Song, D.H.; Jung, U.H.; Kim, Y.E.; Im, H.B.; Lee, T.H.; Lee, K.B.; Koo, K.Y. Influence of supports on the catalytic activity and coke resistance of Ni catalyst in dry reforming of methane. Catalysts 2022, 12, 216. [Google Scholar] [CrossRef]
- Gendy, T.S.; El-Salamony, R.A.; El-Temtamy, S.A.; Ghoneim, S.A.; El-Hafiz, D.R.A.; Ebiad, M.A.; Naggar, A.M.E. Optimization of dry reforming of methane over a Ni/MgO catalyst using response surface methodology. Chem. Eng. Technol. 2022, 45, 1087–1099. [Google Scholar] [CrossRef]
- Zanganeh, R.; Rezaei, M.; Zamaniyan, A. Preparation of nanocrystalline NiO–MgO solid solution powders as catalyst for methane reforming with carbon dioxide: Effect of preparation conditions. Adv. Powder Technol. 2014, 25, 1111–1117. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Liu, H.-M.; Xu, B.-Q. Durable Ni/MgO catalysts for CO2 reforming of methane: Activity and metal–support interaction. J. Mol. Catal. A Chem. 2009, 299, 44–52. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Hu, Y.H. Carbon dioxide reforming of methane over nickel/alkaline earth metal oxide catalysts. Appl. Catal. A Gen. 1995, 133, 149–161. [Google Scholar] [CrossRef]
- Jin, B.; Li, S.; Liang, X. Enhanced activity and stability of MgO-promoted Ni/Al2O3 catalyst for dry reforming of methane: Role of MgO. Fuel 2021, 284, 119082. [Google Scholar] [CrossRef]
- Al-Baqmaa, Y.A.; Al-Fatesh, A.S.; Ibrahim, A.A.; Bagabas, A.A.; Almubadde, F.S.; Alromaeh, A.I.; Abu-Dahrieh, J.K.; Abasaeed, A.E.; Fakeeha, A.H. Effects of MgO on Ni/Al2O3 catalysts for CO2 reforming of methane to syngas. Res. Chem. Intermed. 2023, 49, 5015–5028. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, X.; Huang, S.; Wu, H.; Jiang, L.; Zhou, Y.; Song, Y.; Diao, Y. Effects of MgO doping in Pd/γ-Al2O3 catalysts forthe hydrogenation of perfluoro olefin. Mol. Catal. 2024, 552, 113652. [Google Scholar] [CrossRef]
- Bayazed, M.O.; Al-Fatesh, A.S.; Fakeeha, A.H.; Ibrahim, A.A.; Abasaeed, A.E.; Alromaeh, A.I.; Frusteri, F.; Abu Dahrieh, J.K. Role of MgO in Al2O3-supported Fe catalysts for hydrogen and carbon nanotubes formation during catalytic methane decomposition. Energy Sci. Eng. 2024, 1–14. [Google Scholar] [CrossRef]
- Ranjbar, A.; Aghamiri, S.F.; Irankhah, A. Effect of MgO/Al2O3 ratio in the support of mesoporous Ni/MgO–Al2O3 catalysts for CO2 utilization via reverse water gas shift reaction. Int. J. Hydrog. Energy 2023, 48, 19115–19125. [Google Scholar] [CrossRef]
- Yang, L.; Pan, Z.; Wang, D.; Wang, S.; Wang, X.; Ma, H.; Liu, H.; Wang, C.; Qu, W.; Tian, Z. Highly effective Pd/MgO/γ-Al2O3 catalysts for CO oxidative coupling to dimethyl oxalate: The effect of MgO coating on γ-Al2O3. ACS Appl. Mater. Interfaces 2021, 13, 28064–28071. [Google Scholar] [CrossRef]
- Barzegari, F.; Kazemeini, M.; Rezaei, M.; Farhadi, F.; Keshavarz, A. Propane steam reforming on mesoporous NiO–MgO–SiO2 catalysts for syngas production: Effect of the MgO/SiO2 molar ratio. Int. J. Hydrog. Energy 2020, 45, 24840–24858. [Google Scholar] [CrossRef]
- Sinha Majumdar, S.; Moses-DeBusk, M.; Deka, D.J.; Kidder, M.K.; Thomas, C.R.; Pihl, J.A. Impact of Mg on Pd-based methane oxidation catalysts for lean-burn natural gas emissions control. Appl. Catal. B Environ. 2024, 341, 123253. [Google Scholar] [CrossRef]
- Zhao, G.; Pan, X.; Zhang, Z.; Liu, Y.; Lu, Y. A thin-felt Pd–MgO–Al2O3/Al-fiber catalyst for catalytic combustion of methane with resistance to water-vapor poisoning. J. Catal. 2020, 384, 122–135. [Google Scholar] [CrossRef]
- Kozlov, S.M.; Aleksandrov, H.A.; Goniakowski, J.; Neyman, K.M. Effect of MgO(100) support on structure and properties of Pd and Pt nanoparticles with 49-155 atoms. J. Chem. Phys. 2013, 139, 084701. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Huai, L.; Chen, G.; Zhao, X.; Chen, C.; Zhou, S.; Zhang, J. Selective switching hydrogenation products of 5-hydroxymethylfurfural at high substrate concentrations by regulating Pd-MgO interactions. Appl. Catal. B Environ. Energy 2025, 361, 124578. [Google Scholar] [CrossRef]
- Tanabe, T.; Nagai, Y.; Dohmae, K.; Sobukawa, H.; Shinjoh, H. Sintering and redispersion behavior of Pt on Pt/MgO. J. Catal. 2008, 257, 117–124. [Google Scholar] [CrossRef]
- Sarma, B.B.; Agostini, G.; Farpón, M.G.; Marini, C.; Pfänder, N.; Prieto, G. Bottom-up assembly of bimetallic nanocluster catalysts from oxide-supported single-atom precursors. J. Mater. Chem. A 2021, 9, 8401–8415. [Google Scholar] [CrossRef]
- Okumura, K.; Hoshi, H.; Iiyoshi, H.; Takaba, H. Formation of a Pt–MgO solid solution: Analysis by X-ray absorption fine structure spectroscopy. ACS Omega 2022, 7, 27458–27468. [Google Scholar] [CrossRef]
- Okumura, K.; Morita, S.; Iiyoshi, H.; Takaba, H. Formation and segregation of a Pd–MgO solid solution studied by X-ray absorption spectroscopy. ACS Omega 2023, 8, 7507–7516. [Google Scholar] [CrossRef]
- Lupescu, J.A.; Schwank, J.W.; Fisher, G.B.; Chen, X.; Peczonczyk, S.L.; Drews, A.R. Pd model catalysts: Effect of aging duration on lean redispersion. Appl. Catal. B Environ. 2016, 185, 189–202. [Google Scholar] [CrossRef]
- Wanke, S.E. Sintering and redispersion of conventional supported metal catalysts in hydrogen and oxygen atmospheres. In Materials Science Research: Volume 16 Sintering and Heterogeneous Catalysis; Kuczynski, G.C., Miller, A.E., Sargent, G.A., Eds.; Springer: Boston, MA, USA, 1984; pp. 223–242. [Google Scholar] [CrossRef]
- Morgan, K.; Goguet, A.; Hardacre, C. Metal redispersion strategies for recycling of supported metal catalysts: A perspective. ACS Catal. 2015, 5, 3430–3445. [Google Scholar] [CrossRef]
- Kang, S.B.; Lim, J.B.; Jo, D.; Nam, I.-S.; Cho, B.K.; Hong, S.B.; Kim, C.H.; Oh, S.H. Ostwald-ripening sintering kinetics of Pd-based three-way catalyst: Importance of initial particle size of Pd. Chem. Eng. J. 2017, 316, 631–644. [Google Scholar] [CrossRef]
- Chen, Y.; Rana, R.; Zhang, Y.; Hoffman, A.S.; Huang, Z.; Yang, B.; Vila, F.D.; Perez-Aguilar, J.E.; Hong, J.; Li, X.; et al. Dynamic structural evolution of MgO-supported palladium catalysts: From metal to metal oxide nanoparticles to surface then subsurface atomically dispersed cations. Chem. Sci. 2024, 15, 6454–6464. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Veselov, G.B.; Karnaukhov, T.M.; Stoyanovskii, V.O.; Vedyagin, A.A. Preparation of the nanostructured Ni-Mg-O oxide system by a sol–gel technique at varied pH. Nanomaterials 2022, 12, 952. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.B.; Dieguez, L.C. Dispersion stability and methylcyclopentane hydrogenolysis in Pd/Al2O3 catalysts. Appl. Catal. A Gen. 2000, 201, 241–251. [Google Scholar] [CrossRef]
- Tessier, D.; Rakai, A.; Bozon-Verduraz, F. Spectroscopic study of the interaction of carbon monoxide with cationic and metallic palladium in palladium–alumina catalysts. J. Chem. Soc. Faraday Trans. 1992, 88, 741–749. [Google Scholar] [CrossRef]
- Okamoto, H.; Asô, T. Formation of thin films of PdO and their electric properties. Jpn. J. Appl. Phys. 1967, 6, 779. [Google Scholar] [CrossRef]
- Nilsson, P.O. Optical properties of PdO in the range of 0.5-5.4 eV. J. Phys. C Solid State Phys. 1979, 12, 1423. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Volodin, A.M.; Stoyanovskii, V.O.; Kenzhin, R.M.; Plyusnin, P.E.; Shubin, Y.V.; Mishakov, I.V. Effect of alumina phase transformation on stability of low-loaded Pd-Rh catalysts for CO oxidation. Top. Catal. 2017, 60, 152–161. [Google Scholar] [CrossRef]
- Moulder, J.F.; Chastain, J. Handbook of X-Ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics Division, Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Che, L.; Guo, Y.; Liao, C.; Sheng, X.; Zeng, Z.; Liu, Z.; Cai, C. One-step fabrication of effective mesoporous layer consisted of self-assembled MgO/TiO2 core/shell nanoparticles for mesostructured perovskite solar cells. Mater. Res. Exp. 2019, 6, 086440. [Google Scholar] [CrossRef]
- Khudorozhkov, A.K.; Chetyrin, I.A.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Bukhtiyarov, V.I. Propane oxidation over Pd/Al2O3: Kinetic and in situ XPS study. Top. Catal. 2017, 60, 190–197. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Volodin, A.M.; Stoyanovskii, V.O.; Mishakov, I.V.; Medvedev, D.A.; Noskov, A.S. Characterization of active sites of Pd/Al2O3 model catalysts with low Pd content by luminescence, EPR and ethane hydrogenolysis. Appl. Catal. B Environ. 2011, 103, 397–403. [Google Scholar] [CrossRef]
- Shivtsov, D.M.; Koskin, A.P.; Stepanenko, S.A.; Ilyina, E.V.; Ayupov, A.B.; Bedilo, A.F.; Yakovlev, V.A. Hydrogen production by N-heterocycle dehydrogenation over Pd supported on aerogel-prepared Mg-Al oxides. Catalysts 2023, 13, 334. [Google Scholar] [CrossRef]
- Alikin, E.A.; Baksheev, E.O.; Veselov, G.B.; Kenzhin, R.M.; Stoyanovskii, V.O.; Plyusnin, P.E.; Shubin, Y.V.; Vedyagin, A.A. Advantages and disadvantages of barium oxide addition to bimetallic Pd-Rh three-way catalysts supported on zirconia-doped alumina. J. Environ. Chem. Eng. 2024, 12, 112945. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 2002, 60, 309–319. [Google Scholar] [CrossRef]
- Boehm, H.-P.; Knözinger, H. Nature and estimation of functional groups on solid surfaces. In Catalysis: Science and Technology; Anderson, J.R., Boudart, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 39–207. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
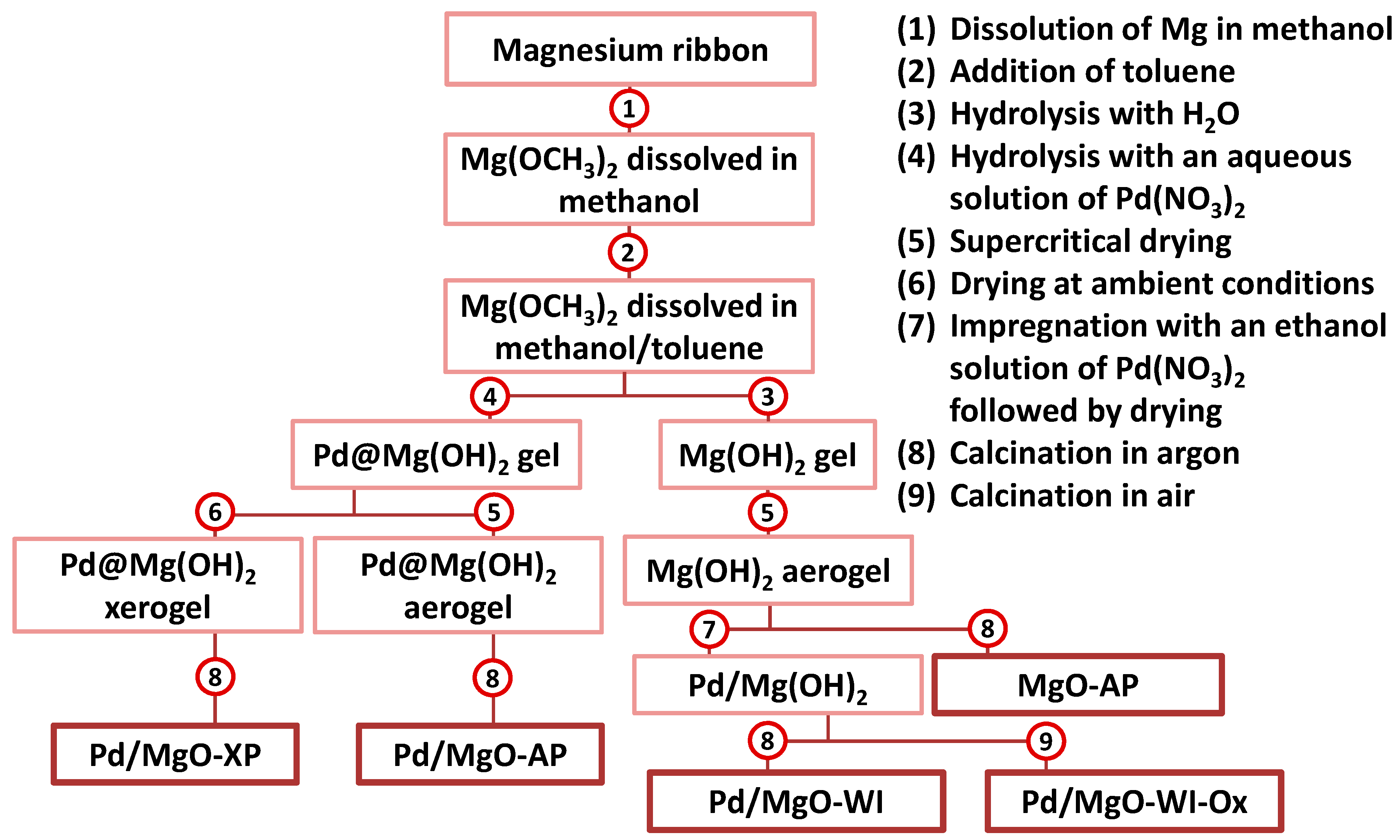
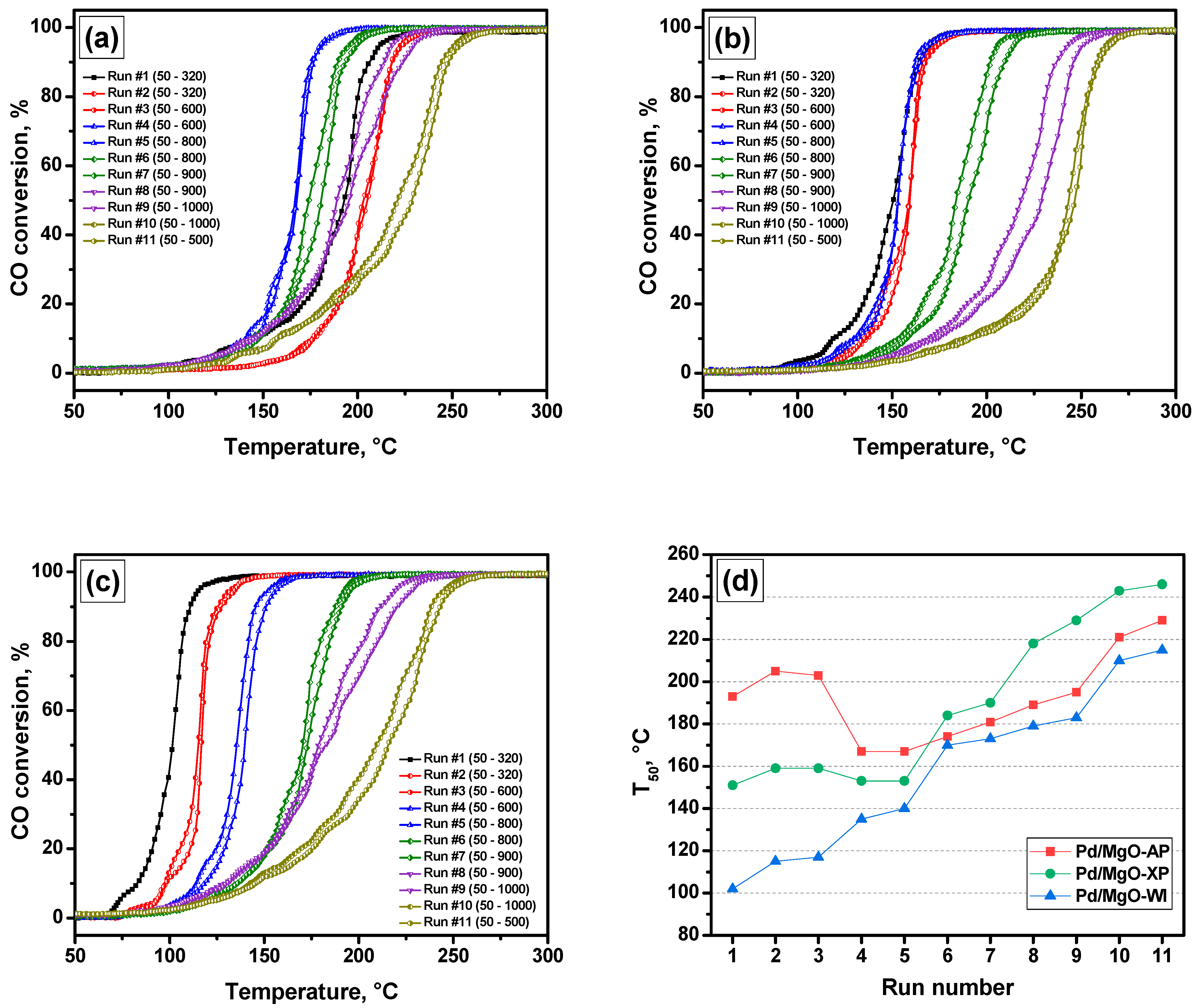
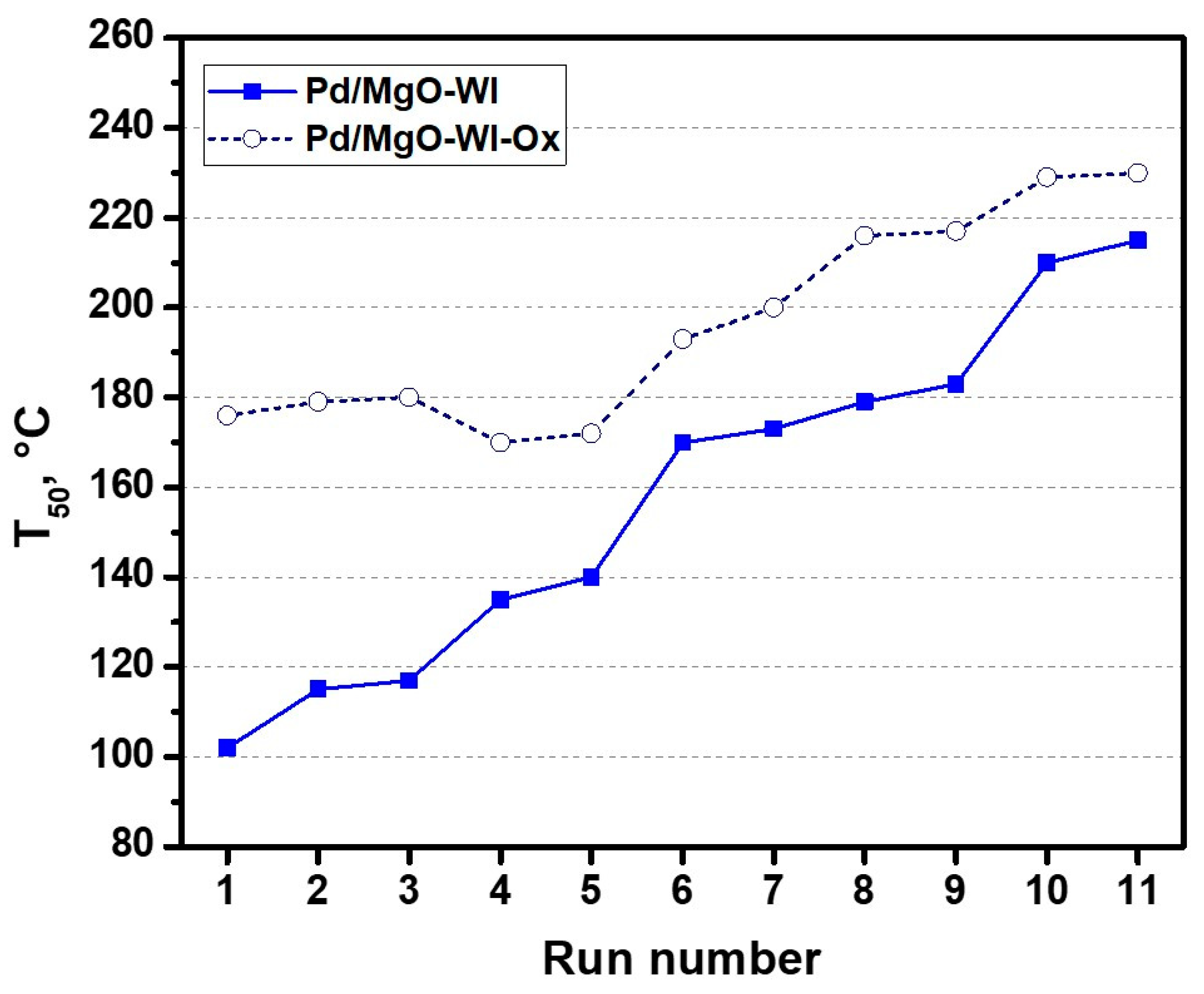
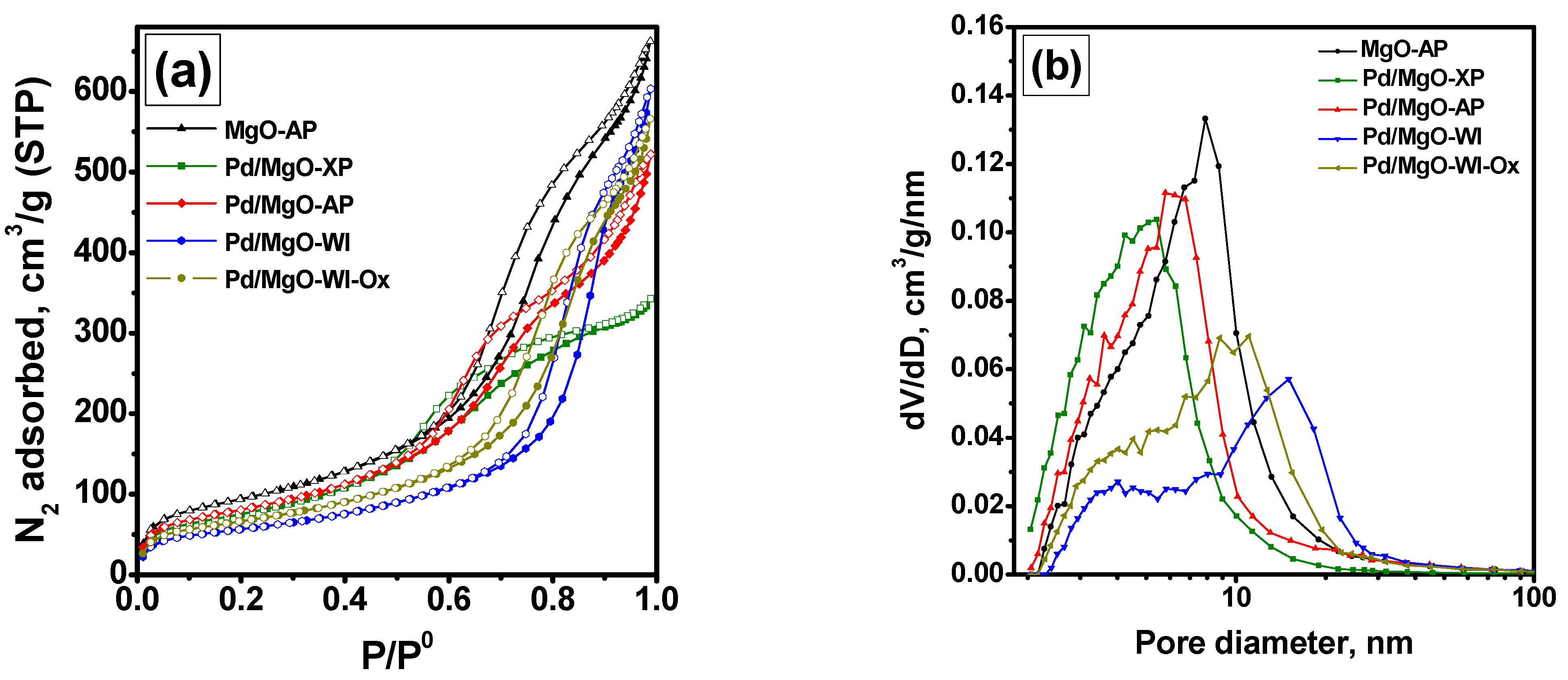


| Sample | SSA, m2/g | Pore Volume, cm3/g | Dpore *, nm |
|---|---|---|---|
| MgO-AP | 340 | 1.03 | 8 |
| Pd/MgO-AP | 295 | 0.81 | 7 |
| Pd/MgO-XP | 280 | 0.53 | 6 |
| Pd/MgO-WI | 205 | 0.93 | 15 |
| Pd/MgO-WI-Ox | 240 | 0.88 | 11 |
| Sample | Pd/Mg | O/Mg | C/Mg | Pd0/Pd2+ |
|---|---|---|---|---|
| Pd/MgO-XP | 0.0070 | 1.3 | 0.42 | 0.6 |
| Pd/MgO-AP | 0.0038 | 1.4 | 0.54 | - * |
| Pd/MgO-WI | 0.0040 | 1.4 | 0.19 | 5.5 |
| Pd/MgO-AP PTA600 | 0.0069 | 1.5 | 0.73 | - * |
| Pd/MgO-AP PTA1000 | 0.0047 | 1.6 | 1.04 | - * |
| Pd/MgO-XP PTA1000 | 0.0031 | 1.6 | 0.87 | - * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veselov, G.B.; Shivtsov, D.M.; Ilyina, E.V.; Stoyanovskii, V.O.; Bukhtiyarov, A.V.; Vedyagin, A.A. High-Temperature Behavior of Pd/MgO Catalysts Prepared via Various Sol–Gel Approaches. Gels 2024, 10, 698. https://doi.org/10.3390/gels10110698
Veselov GB, Shivtsov DM, Ilyina EV, Stoyanovskii VO, Bukhtiyarov AV, Vedyagin AA. High-Temperature Behavior of Pd/MgO Catalysts Prepared via Various Sol–Gel Approaches. Gels. 2024; 10(11):698. https://doi.org/10.3390/gels10110698
Chicago/Turabian StyleVeselov, Grigory B., Danil M. Shivtsov, Ekaterina V. Ilyina, Vladimir O. Stoyanovskii, Andrey V. Bukhtiyarov, and Aleksey A. Vedyagin. 2024. "High-Temperature Behavior of Pd/MgO Catalysts Prepared via Various Sol–Gel Approaches" Gels 10, no. 11: 698. https://doi.org/10.3390/gels10110698
APA StyleVeselov, G. B., Shivtsov, D. M., Ilyina, E. V., Stoyanovskii, V. O., Bukhtiyarov, A. V., & Vedyagin, A. A. (2024). High-Temperature Behavior of Pd/MgO Catalysts Prepared via Various Sol–Gel Approaches. Gels, 10(11), 698. https://doi.org/10.3390/gels10110698









