Double Encapsulation of Resveratrol and Doxorubicin in Composite Nanogel—An Opportunity to Reduce Cardio- and Neurotoxicity of Doxorubicin
Abstract
1. Introduction
2. Results and Discussion
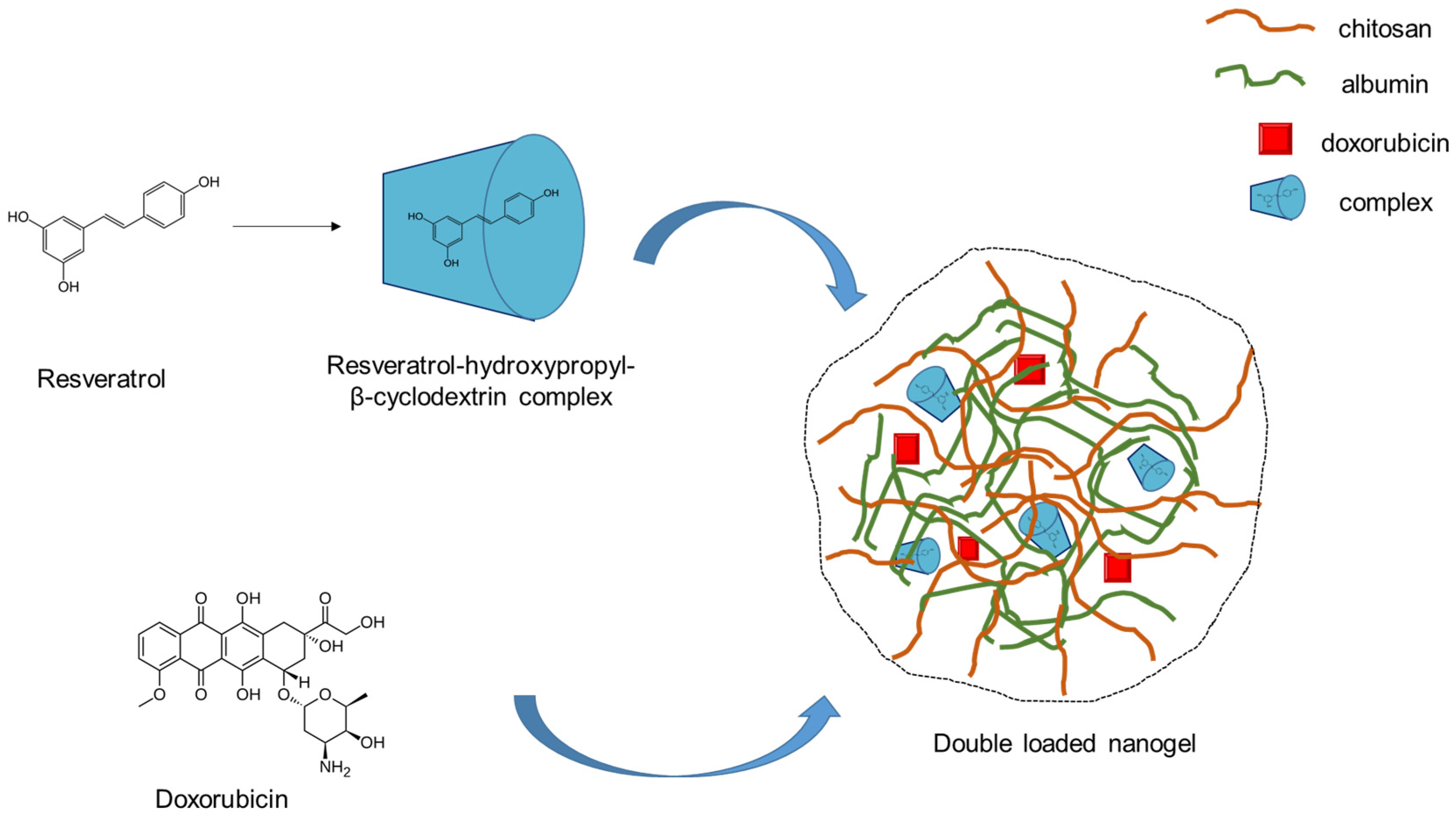
2.1. Preparation of Double-Loaded with Doxorubicin and Resveratrol Complex Composite Chitosan-Albumin Nanogels
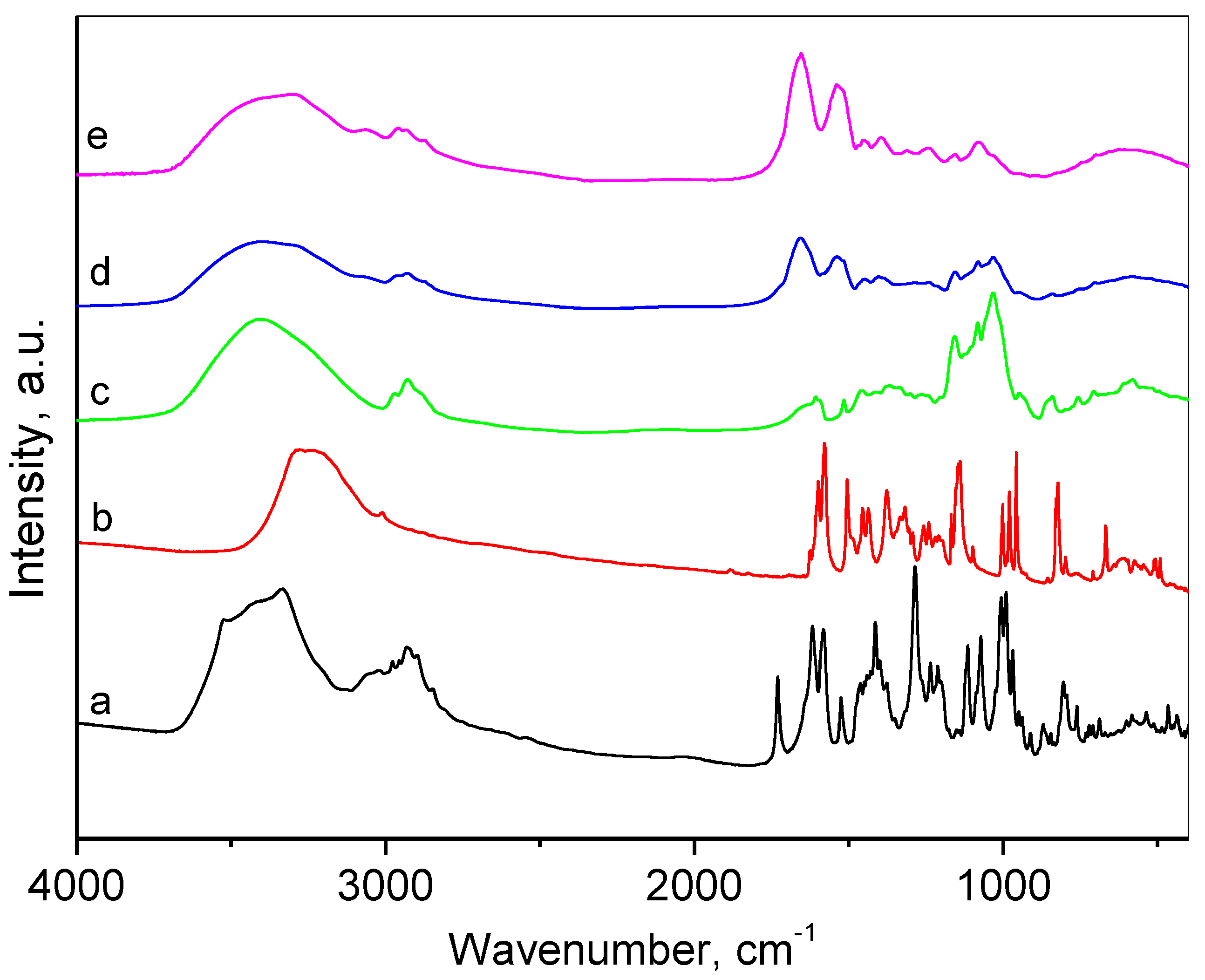
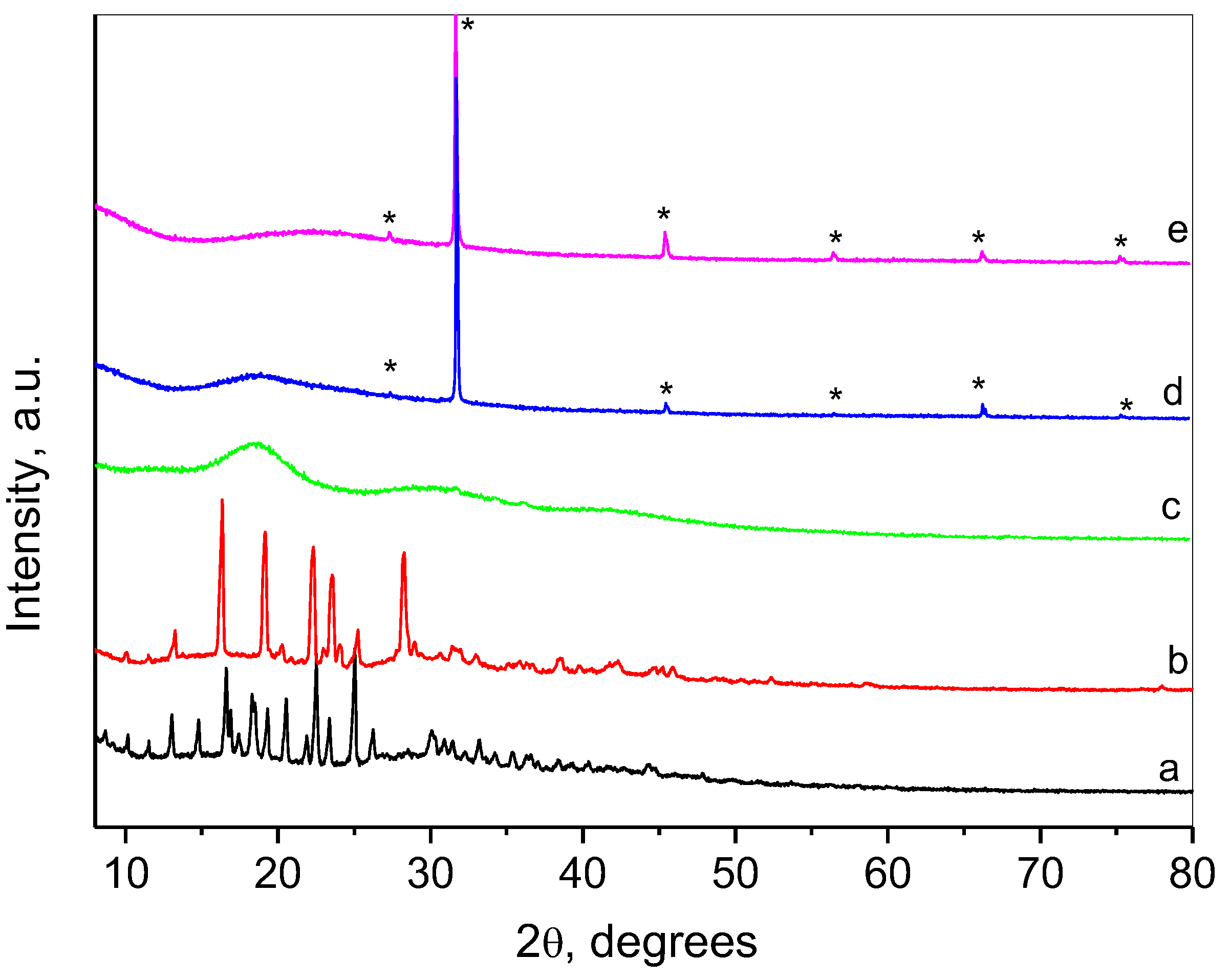
2.2. Characterization of the Composite Nanogel
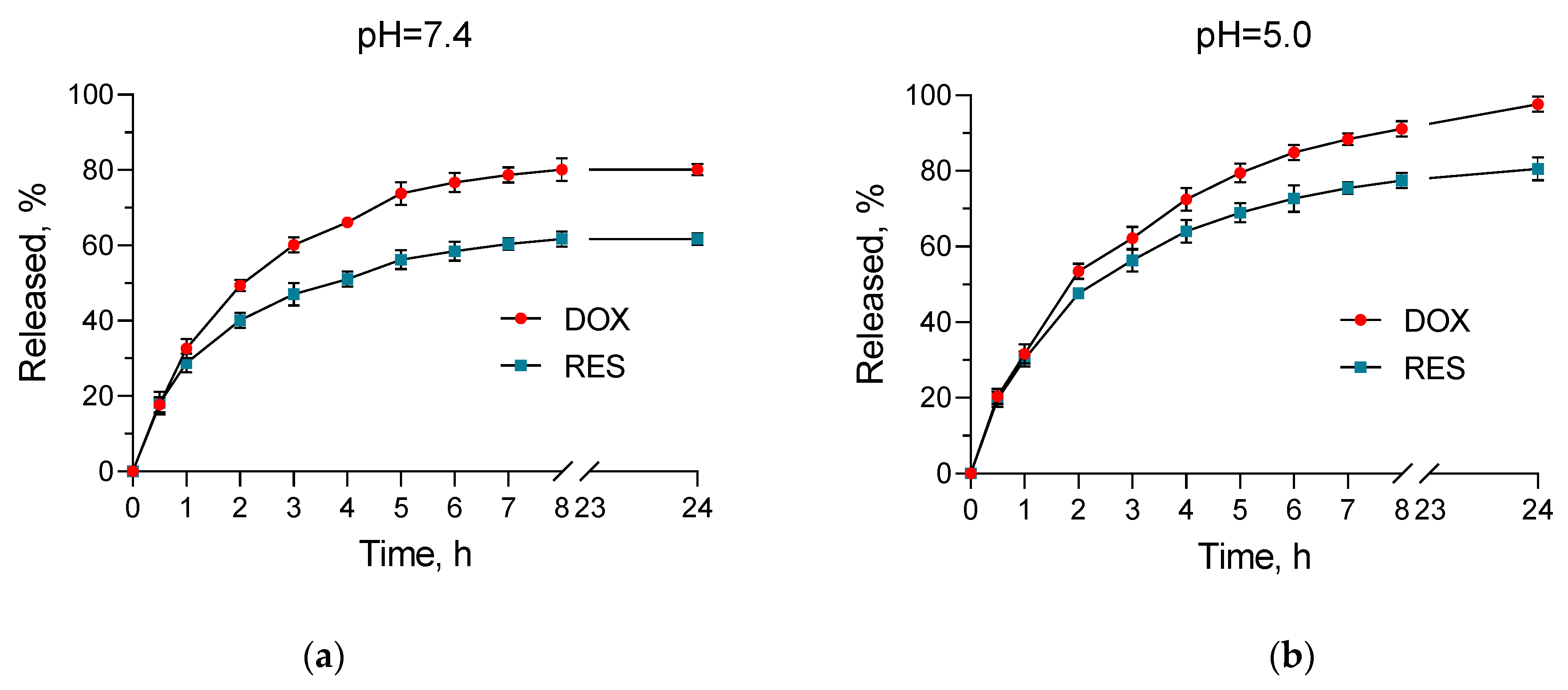
2.3. In Vitro Release Studies
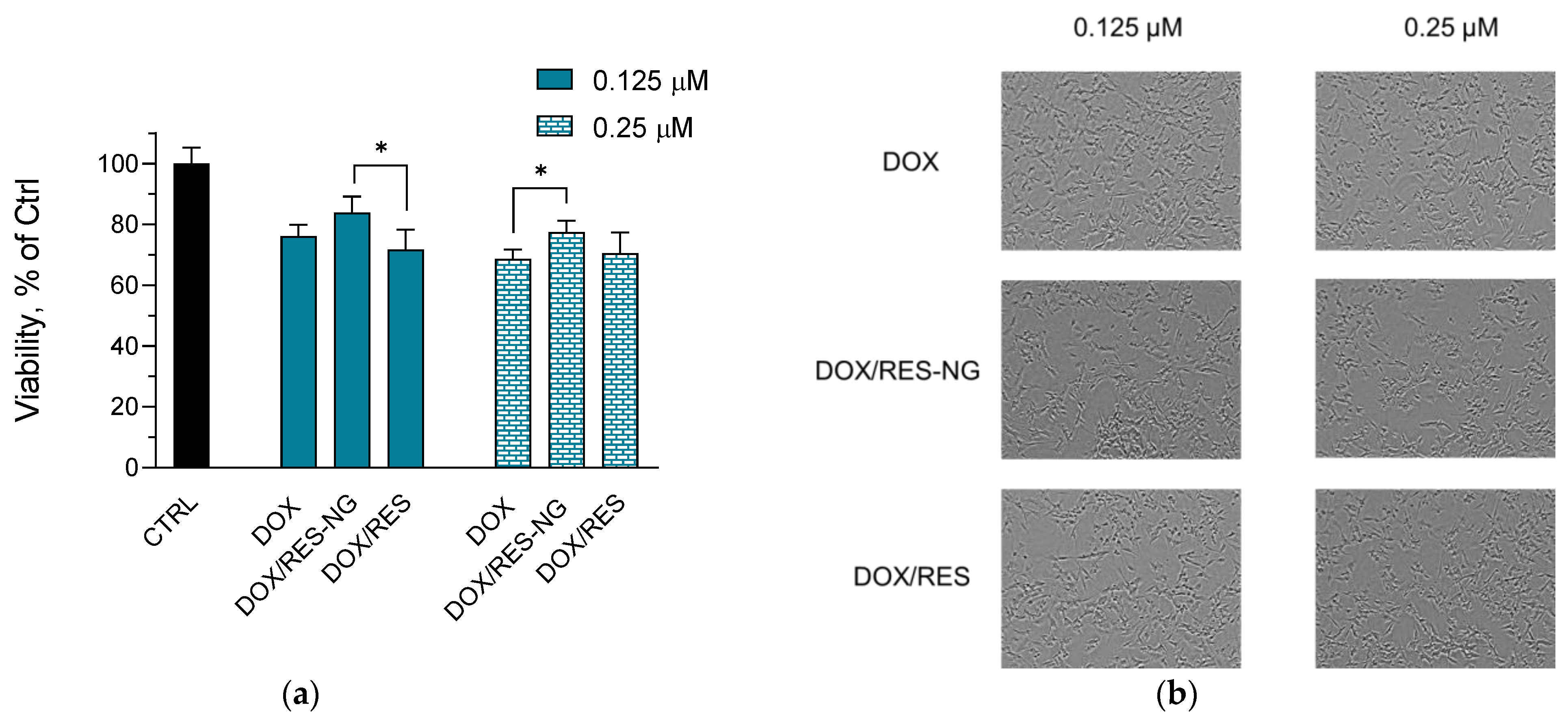
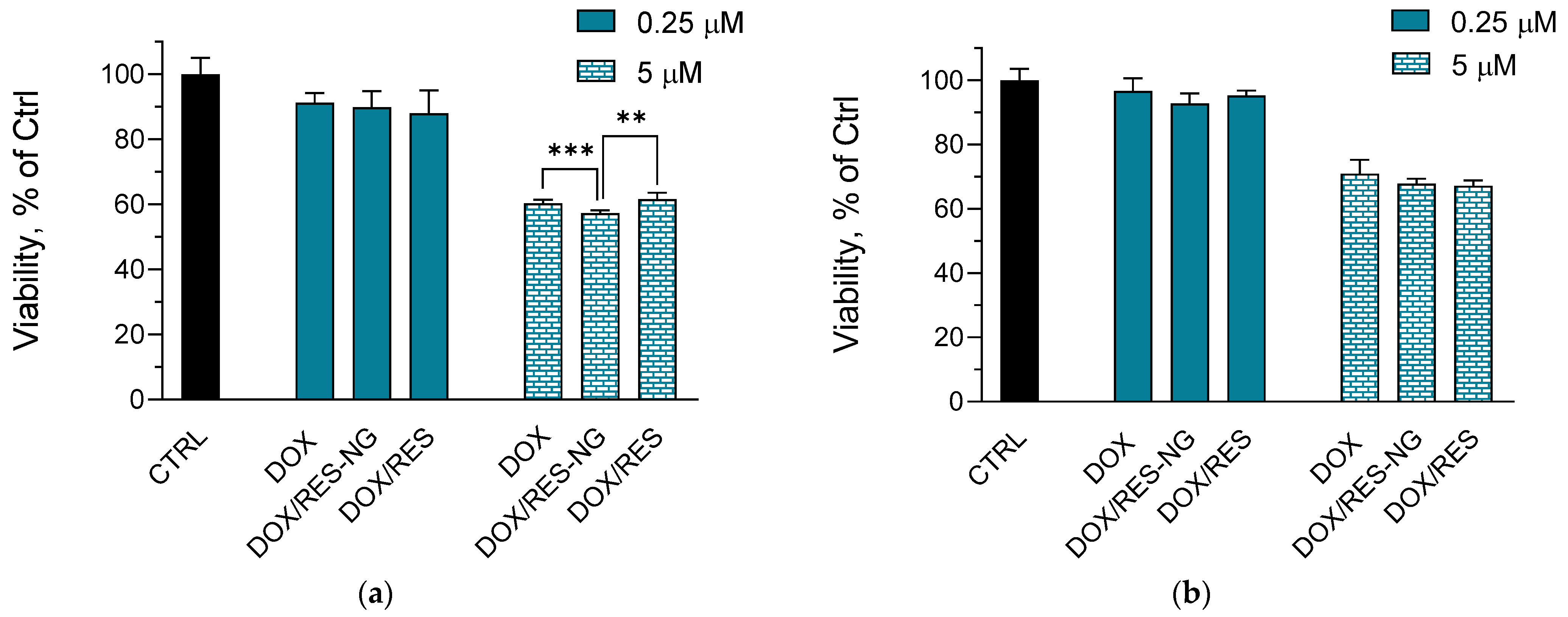
2.4. Cytotoxicity Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Double-Loaded with Doxorubicin and Resveratrol Complex Composite Chitosan-Albumin Nanogel
4.3. Physicochemical Characterization of the Loaded Nanogels
4.4. In Vitro Release Studies
4.5. Cell Cytotoxicity Studies
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor Vascular Permeability and the EPR Effect in Macromolecular Therapeutics: A Review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Al-Othman, A.; Al-Sayah, M.H. Multifunctional Stimuli-Responsive Hybrid Nanogels for Cancer Therapy: Current Status and Challenges. J. Control. Release 2022, 351, 476–503. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, F.; Ferracin, F.; Perale, G.; Rossi, F. Chapter Two—Synthesis and Applications of Nanogels via Covalent Cross-Linking Strategies. In Advances in Chemical Engineering; Mauri, E., Zhang, Z.J., Eds.; Soft Particles; Academic Press: Cambridge, MA, USA, 2023; Volume 62, pp. 35–58. [Google Scholar]
- Yin, Y.; Hu, B.; Yuan, X.; Cai, L.; Gao, H.; Yang, Q. Nanogel: A Versatile Nano-Delivery System for Biomedical Applications. Pharmaceutics 2020, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- Suhail, M.; Rosenholm, J.M.; Minhas, M.U.; Badshah, S.F.; Naeem, A.; Khan, K.U.; Fahad, M. Nanogels as Drug-Delivery Systems: A Comprehensive Overview. Ther. Deliv. 2019, 10, 697–717. [Google Scholar] [CrossRef]
- Keskin, D.; Zu, G.; Forson, A.M.; Tromp, L.; Sjollema, J.; van Rijn, P. Nanogels: A Novel Approach in Antimicrobial Delivery Systems and Antimicrobial Coatings. Bioact. Mater. 2021, 6, 3634–3657. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Wu, J. pH-Sensitive Nanogels for Drug Delivery in Cancer Therapy. Biomater. Sci. 2021, 9, 574–589. [Google Scholar] [CrossRef]
- Manzanares-Guevara, L.A.; Licea-Claverie, A.; Oroz-Parra, I.; Bernaldez-Sarabia, J.; Diaz-Castillo, F.; Licea-Navarro, A.F. Smart Nanoformulation Based on Stimuli-Responsive Nanogels and Curcumin: Promising Therapy against Colon Cancer. ACS Omega 2020, 5, 9171–9184. [Google Scholar] [CrossRef]
- Verma, G.; Gajipara, A.; Shelar, S.B.; Priyadarsini, K.I.; Hassan, P.A. Development of Water-Dispersible Gelatin Stabilized Hydroxyapatite Nanoformulation for Curcumin Delivery. J. Drug Deliv. Sci. Technol. 2021, 66, 102769. [Google Scholar] [CrossRef]
- Setayesh, A.; Bagheri, F.; Boddohi, S. Self-Assembled Formation of Chondroitin Sulfate-Based Micellar Nanogel for Curcumin Delivery to Breast Cancer Cells. Int. J. Biol. Macromol. 2020, 161, 771–778. [Google Scholar] [CrossRef]
- Radeva, L.; Zaharieva, M.M.; Spassova, I.; Kovacheva, D.; Pencheva-El Tibi, I.; Najdenski, H.; Yoncheva, K. Biopolymeric Nanogel as a Drug Delivery System for Doxorubicin—Improved Drug Stability and Enhanced Antineoplastic Activity in Skin Cancer Cells. Pharmaceuticals 2024, 17, 186. [Google Scholar] [CrossRef]
- Kamenova, K.; Radeva, L.; Yoncheva, K.; Ublekov, F.; Ravutsov, M.A.; Marinova, M.K.; Simeonov, S.P.; Forys, A.; Trzebicka, B.; Petrov, P.D. Functional Nanogel from Natural Substances for Delivery of Doxorubicin. Polymers 2022, 14, 3694. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, M.; Abbaspour-Ravasjani, S.; Ghorbani, M.; Babazadeh, A.; Soltanfam, T.; Santos, A.C.; Hamishehkar, H.; Hamblin, M.R. Nanovehicles for Co-Delivery of Anticancer Agents. Drug Discov. Today 2020, 25, 1416–1430. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, J.; Pu, G.; Zhang, F.; Liu, H.; Zhang, Y. Co-Delivery of Baicalein and Doxorubicin by Hyaluronic Acid Decorated Nanostructured Lipid Carriers for Breast Cancer Therapy. Drug Deliv. 2016, 23, 1364–1368. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, S.; Hamishehkar, H.; Ghorbani, M. A Novel Smart PEGylated Gelatin Nanoparticle for Co-Delivery of Doxorubicin and Betanin: A Strategy for Enhancing the Therapeutic Efficacy of Chemotherapy. Mater. Sci. Eng. C 2019, 97, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhao, Z.; Chen, D.; Qiao, M.; Wan, F.; Cun, D.; Sun, Y.; Yang, M. Co-Delivery of Resveratrol and Docetaxel via Polymeric Micelles to Improve the Treatment of Drug-Resistant Tumors. Asian J. Pharm. Sci. 2019, 14, 78–85. [Google Scholar] [CrossRef]
- Xiao, B.; Si, X.; Han, M.K.; Viennois, E.; Zhang, M.; Merlin, D. Co-Delivery of Camptothecin and Curcumin by Cationic Polymeric Nanoparticles for Synergistic Colon Cancer Combination Chemotherapy. J. Mater. Chem. B 2015, 3, 7724–7733. [Google Scholar] [CrossRef]
- Lages, E.B.; Fernandes, R.S.; Silva, J.d.O.; de Souza, Â.M.; Cassali, G.D.; de Barros, A.L.B.; Miranda Ferreira, L.A. Co-Delivery of Doxorubicin, Docosahexaenoic Acid, and α-Tocopherol Succinate by Nanostructured Lipid Carriers Has a Synergistic Effect to Enhance Antitumor Activity and Reduce Toxicity. Biomed. Pharmacother. 2020, 132, 110876. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Wang, Y.; Li, Z.; Zhu, C. Co-Delivery Doxorubicin and Silybin for Anti-Hepatoma via Enhanced Oral Hepatic-Targeted Efficiency. Int. J. Nanomed. 2019, 14, 301–315. [Google Scholar] [CrossRef]
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R.F. Hydrogels for Hydrophobic Drug Delivery. Classification, Synthesis and Applications. J. Funct. Biomater. 2018, 9, 13. [Google Scholar] [CrossRef]
- McKenzie, M.; Betts, D.; Suh, A.; Bui, K.; Kim, L.D.; Cho, H. Hydrogel-Based Drug Delivery Systems for Poorly Water-Soluble Drugs. Molecules 2015, 20, 20397–20408. [Google Scholar] [CrossRef]
- Yang, Z.; McClements, D.J.; Peng, X.; Xu, Z.; Meng, M.; Chen, L.; Jin, Z. Fabrication of Zein–Carboxymethyl Cellulose Nanoparticles for Co-Delivery of Quercetin and Resveratrol. J. Food Eng. 2023, 341, 111322. [Google Scholar] [CrossRef]
- Chen, X.; Song, L.; Li, X.; Zhang, L.; Li, L.; Zhang, X.; Wang, C. Co-Delivery of Hydrophilic/Hydrophobic Drugs by Multifunctional Yolk-Shell Nanoparticles for Hepatocellular Carcinoma Theranostics. Chem. Eng. J. 2020, 389, 124416. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin Pathways: Pharmacodynamics and Adverse Effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, A.; Li, J.; Liu, X.; Wu, S.; Wang, B.; Wang, Y.; Jia, H. Doxorubicin-Induced Cognitive Impairment: The Mechanistic Insights. Front. Oncol. 2021, 11, 673340. [Google Scholar] [CrossRef] [PubMed]
- Español, L.; Larrea, A.; Andreu, V.; Mendoza, G.; Arruebo, M.; Sebastian, V.; Aurora-Prado, M.S.; Kedor-Hackmann, E.R.M.; Santoro, M.I.R.M.; Santamaria, J. Dual Encapsulation of Hydrophobic and Hydrophilic Drugs in PLGA Nanoparticles by a Single-Step Method: Drug Delivery and Cytotoxicity Assays. RSC Adv. 2016, 6, 111060–111069. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Y.; Xu, J.; Song, X.; Wan, Z.; Du, Y.; Ma, W.; Li, X.; Zhang, L.; Li, S. High Loading of Hydrophobic and Hydrophilic Agents via Small Immunostimulatory Carrier for Enhanced Tumor Penetration and Combinational Therapy. Theranostics 2020, 10, 1136–1150. [Google Scholar] [CrossRef]
- Hueppe, N.; Wurm, F.R.; Landfester, K. Nanocarriers with Multiple Cargo Load—A Comprehensive Preparation Guideline Using Orthogonal Strategies. Macromol. Rapid Commun. 2023, 44, 2200611. [Google Scholar] [CrossRef]
- Sun, M.; Gao, M.; Bi, J.; Zhao, Y.; Gong, J. Highly Efficient Hydrogel Encapsulation of Hydrophobic Drugs via an Organic Solvent-Free Process Based on Oiling-Out Crystallization and a Mechanism Study. ACS Sustain. Chem. Eng. 2024, 12, 4813–4824. [Google Scholar] [CrossRef]
- Robinson, K.; Mock, C.; Liang, D. Pre-Formulation Studies of Resveratrol. Drug Dev. Ind. Pharm. 2015, 41, 1464–1469. [Google Scholar] [CrossRef]
- Radeva, L.; Yordanov, Y.; Spassova, I.; Kovacheva, D.; Tibi, I.P.-E.; Zaharieva, M.M.; Kaleva, M.; Najdenski, H.; Petrov, P.D.; Tzankova, V.; et al. Incorporation of Resveratrol-Hydroxypropyl-β-Cyclodextrin Complexes into Hydrogel Formulation for Wound Treatment. Gels 2024, 10, 346. [Google Scholar] [CrossRef]
- Saralkar, P.; Dash, A.K. Alginate Nanoparticles Containing Curcumin and Resveratrol: Preparation, Characterization, and In Vitro Evaluation Against DU145 Prostate Cancer Cell Line. AAPS PharmSciTech 2017, 18, 2814–2823. [Google Scholar] [CrossRef] [PubMed]
- Suktham, K.; Koobkokkruad, T.; Wutikhun, T.; Surassmo, S. Efficiency of Resveratrol-Loaded Sericin Nanoparticles: Promising Bionanocarriers for Drug Delivery. Int. J. Pharm. 2018, 537, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Gan, L.-C.; Guo, X.-Q.; Chen, F.-Z.; Song, Q.; Zhao, Q.; Gou, X.-J.; Hou, S.-X.; Yao, Q. Trans-Resveratrol Loaded Chitosan Nanoparticles Modified with Biotin and Avidin to Target Hepatic Carcinoma. Int. J. Pharm. 2013, 452, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Paul, B.K.; Chattopadhyay, N. Interaction of Cyclodextrins with Human and Bovine Serum Albumins: A Combined Spectroscopic and Computational Investigation. J. Chem. Sci. 2014, 126, 931–944. [Google Scholar] [CrossRef]
- Németh, Z.; Csóka, I.; Semnani Jazani, R.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by Design-Driven Zeta Potential Optimisation Study of Liposomes with Charge Imparting Membrane Additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef]
- Yoncheva, K.; Tzankov, B.; Yordanov, Y.; Spassova, I.; Kovacheva, D.; Frosini, M.; Valoti, M.; Tzankova, V. Encapsulation of Doxorubicin in Chitosan-Alginate Nanoparticles Improves Its Stability and Cytotoxicity in Resistant Lymphoma L5178 MDR Cells. J. Drug Deliv. Sci. Technol. 2020, 59, 101870. [Google Scholar] [CrossRef]
- Moyano-Mendez, J.R.; Fabbrocini, G.; De Stefano, D.; Mazzella, C.; Mayol, L.; Scognamiglio, I.; Carnuccio, R.; Ayala, F.; La Rotonda, M.I.; De Rosa, G. Enhanced Antioxidant Effect of Trans-Resveratrol: Potential of Binary Systems with Polyethylene Glycol and Cyclodextrin. Drug Dev. Ind. Pharm. 2014, 40, 1300–1307. [Google Scholar] [CrossRef]
- Shi, G.; Rao, L.; Yu, H.; Xiang, H.; Yang, H.; Ji, R. Stabilization and Encapsulation of Photosensitive Resveratrol within Yeast Cell. Int. J. Pharm. 2008, 349, 83–93. [Google Scholar] [CrossRef]
- Song, C.; Yu, H.; Zhang, M.; Yang, Y.; Zhang, G. Physicochemical Properties and Antioxidant Activity of Chitosan from the Blowfly Chrysomya megacephala Larvae. Int. J. Biol. Macromol. 2013, 60, 347–354. [Google Scholar] [CrossRef]
- Militello, V.; Casarino, C.; Emanuele, A.; Giostra, A.; Pullara, F.; Leone, M. Aggregation Kinetics of Bovine Serum Albumin Studied by FTIR Spectroscopy and Light Scattering. Biophys. Chem. 2004, 107, 175–187. [Google Scholar] [CrossRef]
- Bezzon, V.D.N.; Caturello, N.A.M.D.S.; Dalpian, G.M.; Ferreira, F.F. Crystal Structure Determination and DFT Analysis of Doxorubicin Hydrochloride for Controlled-Release Drug Formulations. J. Mol. Struct. 2023, 1294, 136412. [Google Scholar] [CrossRef]
- Caruso, F.; Tanski, J.; Villegas-Estrada, A.; Rossi, M. Structural Basis for Antioxidant Activity of Trans-Resveratrol: Ab Initio Calculations and Crystal and Molecular Structure. J. Agric. Food Chem. 2004, 52, 7279–7285. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Sun, X.; Zhu, H.; Xie, L.; Wang, X.; Jiang, N.; Fu, P.; Sang, M. Hydroxypropyl-β-Cyclodextrin-Complexed Resveratrol Enhanced Antitumor Activity in a Cervical Cancer Model: In Vivo Analysis. Front. Pharmacol. 2021, 12, 573909. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.F.R.; Monteiro, M.; Resende, D.; Braga, S.S.; Coimbra, M.A.; Silva, A.M.S.; Cardoso, S.M. Inclusion Complex of Resveratrol with γ-Cyclodextrin as a Functional Ingredient for Lemon Juices. Foods 2021, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.; Meehan, J.; Gray, M.E.; Murray, A.F.; Argyle, D.J.; Kunkler, I.H.; Langdon, S.P. The Impact of Tumour pH on Cancer Progression: Strategies for Clinical Intervention. Explor. Target. Anti-Tumor Ther. 2020, 1, 71–100. [Google Scholar] [CrossRef]
- Vivek, R.; Thangam, R.; Nipunbabu, V.; Ponraj, T.; Kannan, S. Oxaliplatin-Chitosan Nanoparticles Induced Intrinsic Apoptotic Signaling Pathway: A “Smart” Drug Delivery System to Breast Cancer Cell Therapy. Int. J. Biol. Macromol. 2014, 65, 289–297. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-Solubility of Chitosan and Its Antimicrobial Activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Vivek, R.; Nipun Babu, V.; Thangam, R.; Subramanian, K.S.; Kannan, S. pH-Responsive Drug Delivery of Chitosan Nanoparticles as Tamoxifen Carriers for Effective Anti-Tumor Activity in Breast Cancer Cells. Colloids Surf. B Biointerfaces 2013, 111, 117–123. [Google Scholar] [CrossRef]
- Matshetshe, K.I.; Parani, S.; Manki, S.M.; Oluwafemi, O.S. Preparation, Characterization and in Vitro Release Study of β-Cyclodextrin/Chitosan Nanoparticles Loaded Cinnamomum zeylanicum Essential Oil. Int. J. Biol. Macromol. 2018, 118, 676–682. [Google Scholar] [CrossRef]
- Nair, R.S.; Morris, A.; Billa, N.; Leong, C.-O. An Evaluation of Curcumin-Encapsulated Chitosan Nanoparticles for Transdermal Delivery. AAPS PharmSciTech 2019, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P. Drug Carriers: A Review on the Most Used Mathematical Models for Drug Release. Processes 2022, 10, 1094. [Google Scholar] [CrossRef]
- Linders, A.N.; Dias, I.B.; López Fernández, T.; Tocchetti, C.G.; Bomer, N.; Van der Meer, P. A Review of the Pathophysiological Mechanisms of Doxorubicin-Induced Cardiotoxicity and Aging. npj Aging 2024, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Dulf, P.L.; Mocan, M.; Coadă, C.A.; Dulf, D.V.; Moldovan, R.; Baldea, I.; Farcas, A.-D.; Blendea, D.; Filip, A.G. Doxorubicin-Induced Acute Cardiotoxicity Is Associated with Increased Oxidative Stress, Autophagy, and Inflammation in a Murine Model. Naunyn. Schmiedebergs Arch. Pharmacol. 2023, 396, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.; Ker, D.F.E.; Su, H.; Kanade, T. Apoptosis Detection for Adherent Cell Populations in Time-Lapse Phase-Contrast Microscopy Images. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2012; Ayache, N., Delingette, H., Golland, P., Mori, K., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2012; Volume 7510, pp. 331–339. ISBN 978-3-642-33414-6. [Google Scholar]
- Ölander, M.; Handin, N.; Artursson, P. Image-Based Quantification of Cell Debris as a Measure of Apoptosis. Anal. Chem. 2019, 91, 5548–5552. [Google Scholar] [CrossRef]
- Kamińska, K.; Cudnoch-Jędrzejewska, A. A Review on the Neurotoxic Effects of Doxorubicin. Neurotox. Res. 2023, 41, 383–397. [Google Scholar] [CrossRef]
- Alhowail, A.H.; Bloemer, J.; Majrashi, M.; Pinky, P.D.; Bhattacharya, S.; Yongli, Z.; Bhattacharya, D.; Eggert, M.; Woodie, L.; Buabeid, M.A.; et al. Doxorubicin-Induced Neurotoxicity Is Associated with Acute Alterations in Synaptic Plasticity, Apoptosis, and Lipid Peroxidation. Toxicol. Mech. Methods 2019, 29, 457–466. [Google Scholar] [CrossRef]
- de la Lastra, C.A.; Villegas, I. Resveratrol as an Antioxidant and Pro-Oxidant Agent: Mechanisms and Clinical Implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef]
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An Overview of Its Anti-Cancer Mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- D’Souza, S. A Review of In Vitro Drug Release Test Methods for Nano-Sized Dosage Forms. Adv. Pharm. 2014, 2014, 304757. [Google Scholar] [CrossRef]
- Subculture of Adherent Cell Lines. Available online: https://www.sigmaaldrich.com/BG/en/technical-documents/protocol/cell-culture-and-cell-culture-analysis/mammalian-cell-culture/subculture-of-adherent (accessed on 2 October 2024).
- Cell Culture Protocol 5: Subculture of Suspension Cell Lines. Available online: https://www.sigmaaldrich.com/BG/en/technical-documents/technical-article/cell-culture-and-cell-culture-analysis/mammalian-cell-culture/subculture-of-suspension (accessed on 2 October 2024).
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (Resazurin) Fluorescent Dye for the Assessment of Mammalian Cell Cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]


| Sample | Size, nm | PDI | ƺ-Potential, mV |
|---|---|---|---|
| NG | 51 ± 6 | 0.241 | +35.54 |
| DOX/RES-NG | 30 ± 4 | 0.188 | +51.23 |
| pH of the Medium | Zero Order | First Order | Higuchi Model |
|---|---|---|---|
| 7.4 | 0.8451 | 0.9557 | 0.9737 |
| 5.0 | 0.8824 | 0.9973 | 0.9867 |
| pH of the Medium | Zero Order | First Order | Higuchi Model |
|---|---|---|---|
| 7.4 | 0.8203 | 0.9707 | 0.9060 |
| 5.0 | 0.8824 | 0.9973 | 0.9867 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radeva, L.; Yordanov, Y.; Spassova, I.; Kovacheva, D.; Tzankova, V.; Yoncheva, K. Double Encapsulation of Resveratrol and Doxorubicin in Composite Nanogel—An Opportunity to Reduce Cardio- and Neurotoxicity of Doxorubicin. Gels 2024, 10, 699. https://doi.org/10.3390/gels10110699
Radeva L, Yordanov Y, Spassova I, Kovacheva D, Tzankova V, Yoncheva K. Double Encapsulation of Resveratrol and Doxorubicin in Composite Nanogel—An Opportunity to Reduce Cardio- and Neurotoxicity of Doxorubicin. Gels. 2024; 10(11):699. https://doi.org/10.3390/gels10110699
Chicago/Turabian StyleRadeva, Lyubomira, Yordan Yordanov, Ivanka Spassova, Daniela Kovacheva, Virginia Tzankova, and Krassimira Yoncheva. 2024. "Double Encapsulation of Resveratrol and Doxorubicin in Composite Nanogel—An Opportunity to Reduce Cardio- and Neurotoxicity of Doxorubicin" Gels 10, no. 11: 699. https://doi.org/10.3390/gels10110699
APA StyleRadeva, L., Yordanov, Y., Spassova, I., Kovacheva, D., Tzankova, V., & Yoncheva, K. (2024). Double Encapsulation of Resveratrol and Doxorubicin in Composite Nanogel—An Opportunity to Reduce Cardio- and Neurotoxicity of Doxorubicin. Gels, 10(11), 699. https://doi.org/10.3390/gels10110699











