Organogels of FmocFF: Exploring the Solvent-Dependent Gelmorphic Behavior
Abstract
:1. Introduction
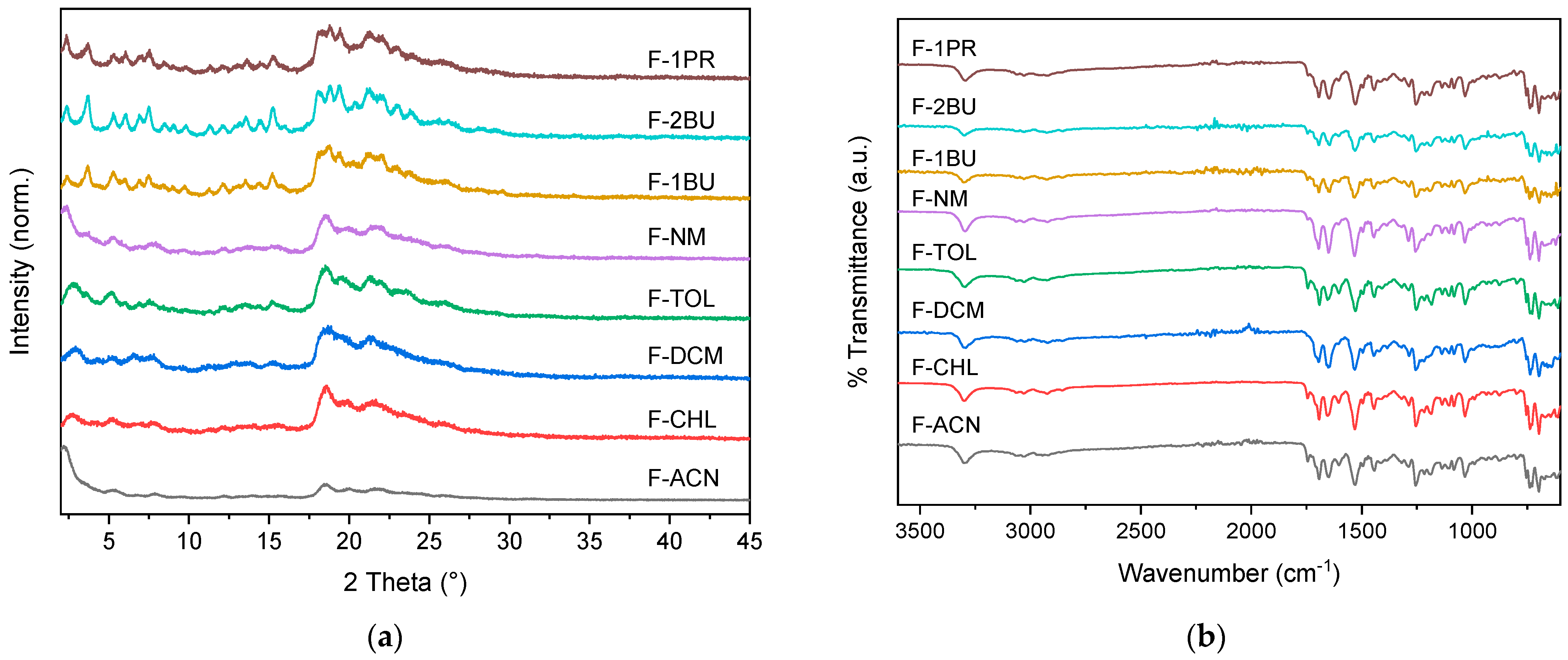
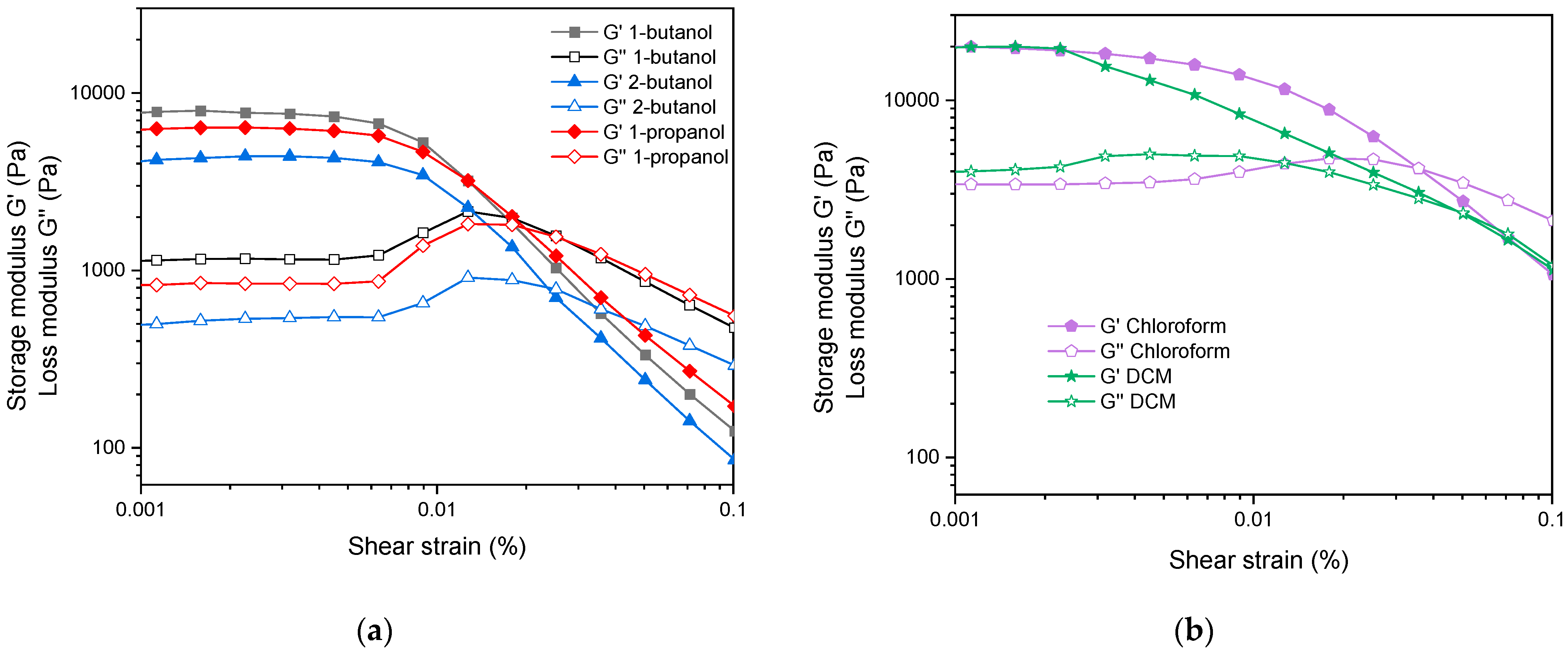
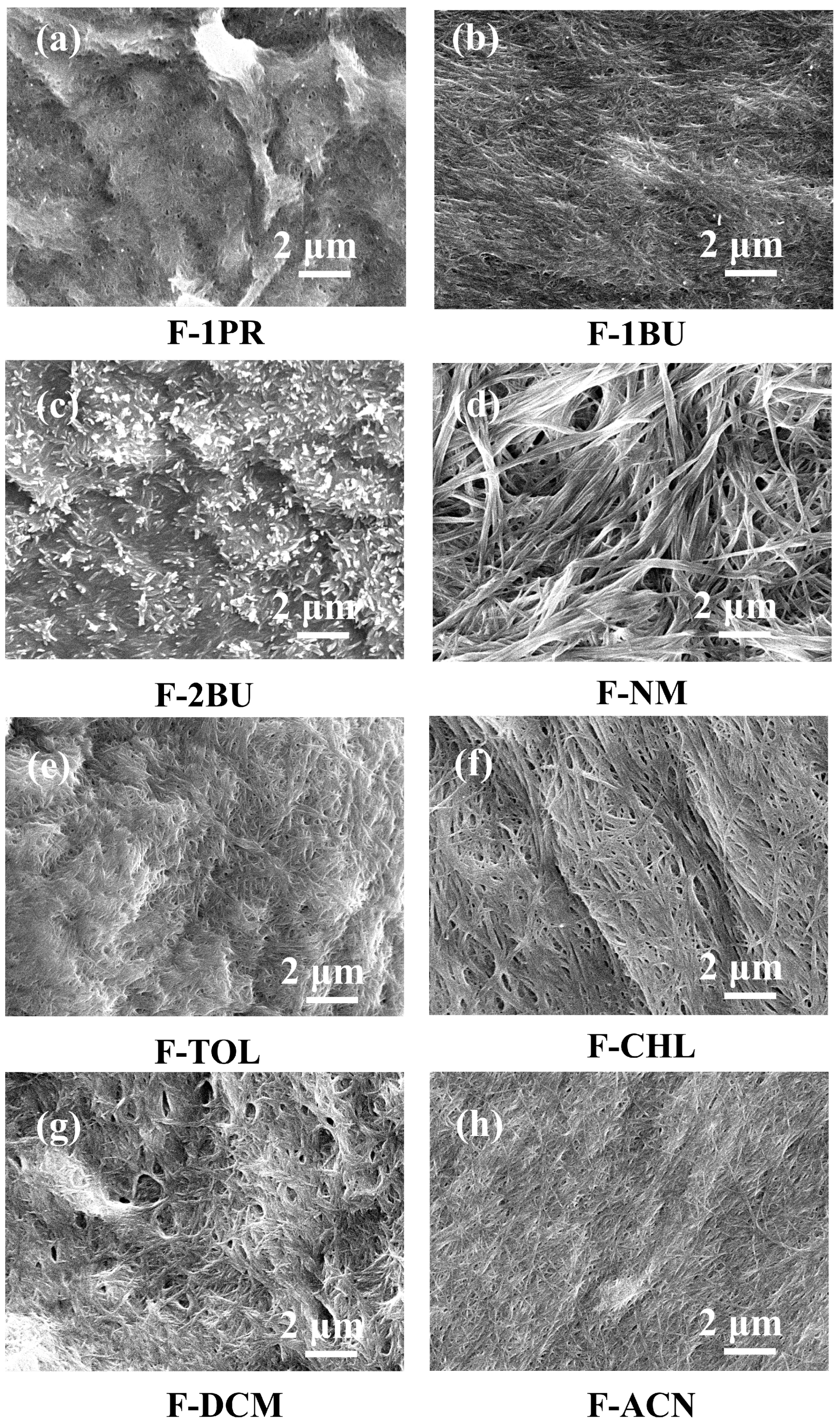
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Gel Preparation
4.3. Characterizations
4.3.1. Rheology
4.3.2. Scanning Electron Microscopy (SEM)
4.3.3. X-Ray Powder Diffraction (XRPD)
4.3.4. Attenuated Total Reflectance–Fourier Transform Infrared (ATR–FTIR) Spectroscopy
4.3.5. Second Harmonic Generation Microscopy (SHG Microscopy)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdallah, D.J.; Weiss, R.G. Organogels and Low Molecular Mass Organic Gelators. Adv. Mater. 2000, 12, 1237–1247. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef]
- Weiss, R.G. Molecular Gels, Monographs in Supramolecular Chemistry; Royal Society of Chemistry: Cambridge, UK, 2018. [Google Scholar]
- Steed, J.W. Supramolecular Gel Chemistry: Developments over the Last Decade. Chem. Commun. 2011, 47, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Terech, P.; Weiss, R.G. Low Molecular Mass Gelators of Organic Liquids and the Properties of Their Gels. Chem. Rev. 1997, 97, 3133–3159. [Google Scholar] [CrossRef]
- Raeburn, J.; Cardoso, A.Z.; Adams, D.J. The Importance of the Self-Assembly Process to Control Mechanical Properties of Low Molecular Weight Hydrogels. Chem. Soc. Rev. 2013, 42, 5143–5156. [Google Scholar] [CrossRef] [PubMed]
- Panja, S.; Adams, D.J. Stimuli Responsive Dynamic Transformations in Supramolecular Gels. Chem. Soc. Rev. 2021, 50, 5165–5200. [Google Scholar] [CrossRef]
- Hirst, A.R.; Smith, D.K. Solvent Effects on Supramolecular Gel-Phase Materials: Two-Component Dendritic Gel. Langmuir 2004, 20, 10851–10857. [Google Scholar] [CrossRef]
- Edwards, W.; Lagadec, C.A.; Smith, D.K. Solvent–Gelator Interactions—Using Empirical Solvent Parameters to Better Understand the Self-Assembly of Gel-Phase Materials. Soft Matter 2010, 7, 110–117. [Google Scholar] [CrossRef]
- Suzuki, M.; Nakajima, Y.; Yumoto, M.; Kimura, M.; Shirai, H.; Hanabusa, K. Effects of Hydrogen Bonding and van Der Waals Interactions on Organogelation Using Designed Low-Molecular-Weight Gelators and Gel Formation at Room Temperature. Langmuir 2003, 19, 8622–8624. [Google Scholar] [CrossRef]
- Pinault, T.; Isare, B.; Bouteiller, L. Solvents with Similar Bulk Properties Induce Distinct Supramolecular Architectures. ChemPhysChem 2006, 7, 816–819. [Google Scholar] [CrossRef]
- Smith, D.K. Supramolecular Gels—A Panorama of Low-Molecular-Weight Gelators from Ancient Origins to next-Generation Technologies. Soft Matter 2023, 20, 10–70. [Google Scholar] [CrossRef] [PubMed]
- Mahler, A.; Reches, M.; Rechter, M.; Cohen, S.; Gazit, E. Rigid, Self-Assembled Hydrogel Composed of a Modified Aromatic Dipeptide. Adv. Mater. 2006, 18, 1365–1370. [Google Scholar] [CrossRef]
- Raeburn, J.; Pont, G.; Chen, L.; Cesbron, Y.; Lévy, R.; Adams, D.J. Fmoc-Diphenylalanine Hydrogels: Understanding the Variability in Reported Mechanical Properties. Soft Matter 2012, 8, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Raeburn, J.; Mendoza-Cuenca, C.; Cattoz, B.N.; Little, M.A.; Terry, A.E.; Zamith Cardoso, A.; Griffiths, P.C.; Adams, D.J. The Effect of Solvent Choice on the Gelation and Final Hydrogel Properties of Fmoc–Diphenylalanine. Soft Matter 2015, 11, 927–935. [Google Scholar] [CrossRef]
- Singh, V.; Snigdha, K.; Singh, C.; Sinha, N.; Thakur, A.K. Understanding the Self-Assembly of Fmoc–Phenylalanine to Hydrogel Formation. Soft Matter 2015, 11, 5353–5364. [Google Scholar] [CrossRef]
- Vitale, M.; Ligorio, C.; McAvan, B.; Hodson, N.W.; Allan, C.; Richardson, S.M.; Hoyland, J.A.; Bella, J. Hydroxyapatite-Decorated Fmoc-Hydrogel as a Bone-Mimicking Substrate for Osteoclast Differentiation and Culture. Acta Biomater. 2022, 138, 144–154. [Google Scholar] [CrossRef]
- Diaferia, C.; Morelli, G.; Accardo, A. Fmoc-Diphenylalanine as a Suitable Building Block for the Preparation of Hybrid Materials and Their Potential Applications. J. Mater. Chem. B 2019, 7, 5142–5155. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, L.; Zhang, M.; Yi, T. Low-Molecular-Mass Gels Responding to Ultrasound and Mechanical Stress: Towards Self-Healing Materials. Chem. Soc. Rev. 2014, 43, 5346–5371. [Google Scholar] [CrossRef]
- Babu, S.S.; Prasanthkumar, S.; Ajayaghosh, A. Self-Assembled Gelators for Organic Electronics. Angew. Chem. Int. Ed. 2012, 51, 1766–1776. [Google Scholar] [CrossRef]
- Kuzina, M.A.; Kartsev, D.D.; Stratonovich, A.V.; Levkin, P.A.; Kuzina, M.A.; Levkin, P.A.; Kartsev, D.D.; Stratonovich, A.V. Organogels versus Hydrogels: Advantages, Challenges, and Applications. Adv. Funct. Mater. 2023, 33, 2301421. [Google Scholar] [CrossRef]
- Galindo, J.M.; Tardío, C.; Saikia, B.; Van Cleuvenbergen, S.; Torres-Moya, I. Recent Insights about the Role of Gels in Organic Photonics and Electronics. Gels 2023, 9, 875. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, P.; Kyarikwal, R.; Munjal, R.; Nag, P.; Vennapusa, S.R.; Mukhopadhyay, S. Enhancing Gel Strength and Catalytic Performance of a Nanocomposite Catalyst by Incorporating a Reinforcing Hydrogen-Bonding Component. ACS Appl. Eng. Mater. 2024, 2, 1503–1514. [Google Scholar] [CrossRef]
- Dawn, A.; Andrew, K.S.; Yufit, D.S.; Hong, Y.X.; Reddy, J.P.; Jones, C.D.; Aguilar, J.A.; Steed, J.W. Supramolecular Gel Control of Cisplatin Crystallization: Identification of a New Solvate Form Using a Cisplatin-Mimetic Gelator. Cryst. Growth Des. 2015, 15, 4591–4599. [Google Scholar] [CrossRef]
- Sharma, H.; Kalita, B.K.; Pathak, D.; Sarma, B. Low Molecular Weight Supramolecular Gels as a Crystallization Matrix. Cryst. Growth Des. 2024, 24, 17–37. [Google Scholar] [CrossRef]
- Buendía, J.; Matesanz, E.; Smith, D.K.; Sánchez, L. Multi-component supramolecular gels for the controlled crystallization of drugs: Synergistic and antagonistic effects. CrystEngComm 2015, 17, 8146–8152. [Google Scholar] [CrossRef]
- Kumar, D.K.; Steed, J.W. Supramolecular gel phase crystallization: Orthogonal self-assembly under non-equilibrium conditions. Chem. Soc. Rev. 2014, 43, 2080–2088. [Google Scholar] [CrossRef]
- Dastidar, P. Supramolecular Gelling Agents: Can They Be Designed? Chem. Soc. Rev. 2008, 37, 2699–2715. [Google Scholar] [CrossRef] [PubMed]
- Saikia, B.; Mulvee, M.T.; Torres-Moya, I.; Sarma, B.; Steed, J.W. Drug Mimetic Organogelators for the Control of Concomitant Crystallization of Barbital and Thalidomide. Cryst. Growth Des. 2020, 20, 7989–7996. [Google Scholar] [CrossRef]
- Contreras-Montoya, R.; Smith, J.P.; Boothroyd, S.C.; Aguilar, J.A.; Mirzamani, M.; Screen, M.A.; Yufit, D.S.; Robertson, M.; He, L.; Qian, S.; et al. Pathway Complexity in Fibre Assembly: From Liquid Crystals to Hyper-Helical Gelmorphs. Chem. Sci. 2023, 14, 11389–11401. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Li, Y. Supramolecular Gels. In Gel Chemistry. Lecture Notes in Chemistry; Springer: Singapore, 2018; Volume 96, pp. 5–59. [Google Scholar]
- Donley, G.J.; Singh, P.K.; Shetty, A.; Rogers, S.A. Elucidating the G? Overshoot in Soft Materials with a Yield Transition via a Time-Resolved Experimental Strain Decomposition. Proc. Natl. Acad. Sci. USA 2020, 117, 21945–21952. [Google Scholar] [CrossRef]
- Hyun, K.; Wilhelm, M.; Klein, C.O.; Cho, K.S.; Nam, J.G.; Ahn, K.H.; Lee, S.J.; Ewoldt, R.H.; McKinley, G.H. A Review of Nonlinear Oscillatory Shear Tests: Analysis and Application of Large Amplitude Oscillatory Shear (LAOS). Prog. Polym. Sci. 2011, 36, 1697–1753. [Google Scholar] [CrossRef]
- McFetridge, M.L.; Kulkarni, K.; Hilsenstein, V.; Del Borgo, M.P.; Aguilar, M.I.; Ricardo, S.D. A Comparison of Fixation Methods for SEM Analysis of Self-Assembling Peptide Hydrogel Nanoarchitecture. Nanoscale 2023, 15, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Paterson, S.M.; Casadio, Y.S.; Brown, D.H.; Shaw, J.A.; Chirila, T.V.; Baker, M.V. Laser Scanning Confocal Microscopy versus Scanning Electron Microscopy for Characterization of Polymer Morphology: Sample Preparation Drastically Distorts Morphologies of Poly(2-Hydroxyethyl Methacrylate)-Based Hydrogels. J. Appl. Polym. Sci. 2013, 127, 4296–4304. [Google Scholar] [CrossRef]
- Dok, A.R.; Legat, T.; de Coene, Y.; van der Veen, M.A.; Verbiest, T.; Van Cleuvenbergen, S. Nonlinear Optical Probes of Nucleation and Crystal Growth: Recent Progress and Future Prospects. J. Mater. Chem. C Mater. 2021, 9, 11553–11568. [Google Scholar] [CrossRef]
- Boyd, R.W. Nonlinear Optics, 4th ed.; Academic Press: New York, NY, USA, 2020. [Google Scholar]
- Verbiest, T.; Clays, K.; Rodriguez, V. Second-Order Nonlinear Optical. Characterization Techniques: An Introduction; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Yokota, H.; Kaneshiro, J.; Uesu, Y. Optical Second Harmonic Generation Microscopy as a Tool of Material Diagnosis. Phys. Res. Int. 2012, 2012, 704634. [Google Scholar] [CrossRef]
- Van Cleuvenbergen, S.; Hennrich, G.; Willot, P.; Koeckelberghs, G.; Clays, K.; Verbiest, T.; Van Der Veen, M.A. All Optical Determination of Microscopic and Macroscopic Structure of Chiral, Polar Microcrystals from Achiral, Nonpolar Molecules. J. Phys. Chem. C 2012, 116, 12219–12225. [Google Scholar] [CrossRef]
- van Cleuvenbergen, S.; Depotter, G.; Clays, K.; Kędziora, P. Second-Order NLO Response in Chiral Ferroelectric Liquid Crystals: Molecular and Bulk Consideration. J. Mol. Liq. 2021, 326, 115328. [Google Scholar] [CrossRef]
- Markey, K.; Putzeys, T.; Horcajada, P.; Devic, T.; Guillou, N.; Wübbenhorst, M.; Van Cleuvenbergen, S.; Verbiest, T.; De Vos, D.E.; Van Der Veen, M.A. Second Harmonic Generation Microscopy Reveals Hidden Polar Organization in Fluoride Doped MIL-53(Fe). Dalton Trans. 2016, 45, 4401–4406. [Google Scholar] [CrossRef]
- Moris, M.; Van Den Eede, M.P.; Koeckelberghs, G.; Deschaume, O.; Bartic, C.; Clays, K.; Van Cleuvenbergen, S.; Verbiest, T. Solvent Role in the Self-Assembly of Poly(3-Alkylthiophene): A Harmonic Light Scattering Study. Macromolecules 2021, 54, 2477–2484. [Google Scholar] [CrossRef]
- Marco, A.B.; Aparicio, F.; Faour, L.; Iliopoulos, K.; Morille, Y.; Allain, M.; Franco, S.; Andreu, R.; Sahraoui, B.; Gindre, D.; et al. Promoting Spontaneous Second Harmonic Generation through Organogelation. J. Am. Chem. Soc. 2016, 138, 9025–9028. [Google Scholar] [CrossRef]
- Aparicio, F.; Faour, L.; Gindre, D.; Canevet, D.; Sallé, M. Supramolecular Control over the Structural Organization of a Second-Order NLO-Active Organogelator. Soft Matter 2016, 12, 8480–8484. [Google Scholar] [CrossRef] [PubMed]
- Belén Marco, A.; Gindre, D.; Iliopoulos, K.; Franco, S.; Andreu, R.; Canevet, D.; Sallé, M. (Super)Gelators Derived from Push–Pull Chromophores: Synthesis, Gelling Properties and Second Harmonic Generation. Org. Biomol. Chem. 2018, 16, 2470–2478. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Lin, N.; Yu, W.; Liu, X.Y. Crystal Networks in Supramolecular Gels: Formation Kinetics and Mesoscopic Engineering Principles. CrystEngComm 2015, 17, 7986–8010. [Google Scholar] [CrossRef]


| Solvents | Pure Solvent | Mixed with Water (φorganic solvent ≈ 0.1) | ||
|---|---|---|---|---|
| Gelation Possibility | Concentration (mg/mL) | Gelation Possibility | Concentration (mg/mL) | |
| Methanol | S | x | G | ≥10 |
| Acetonitrile | G | ≥10 | G | ≥5 |
| DMF | S | x | G | ≥10 |
| 1-Propanol | G | ≥9 | G | ≥10 |
| 2-Propanol | PG | ≥20 | G | ≥5 |
| 1-Butanol | G | ≥12 | x | |
| 2-Butanol | G | ≥19 | x | |
| Octanol | G | ≥7 | x | |
| Ethyl acetate | S | x | x | |
| γ-Butyrolactone | S | x | G | ≥5 |
| Toluene | G | ≥10 | x | |
| Dichloromethane | G | ≥8 | x | |
| Chloroform | G | ≥9 | x | |
| Cyclohexanone | S | x | x | |
| 1,4-Dioxane | S | x | x | |
| n-Hexane | S | x | x | |
| Nitromethane | G | ≥10 | x | |
| Tetrahydrofuran | x | x | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saikia, B.; Chen, D.; de Coene, Y.; Van Cleuvenbergen, S. Organogels of FmocFF: Exploring the Solvent-Dependent Gelmorphic Behavior. Gels 2024, 10, 749. https://doi.org/10.3390/gels10110749
Saikia B, Chen D, de Coene Y, Van Cleuvenbergen S. Organogels of FmocFF: Exploring the Solvent-Dependent Gelmorphic Behavior. Gels. 2024; 10(11):749. https://doi.org/10.3390/gels10110749
Chicago/Turabian StyleSaikia, Basanta, Dong Chen, Yovan de Coene, and Stijn Van Cleuvenbergen. 2024. "Organogels of FmocFF: Exploring the Solvent-Dependent Gelmorphic Behavior" Gels 10, no. 11: 749. https://doi.org/10.3390/gels10110749
APA StyleSaikia, B., Chen, D., de Coene, Y., & Van Cleuvenbergen, S. (2024). Organogels of FmocFF: Exploring the Solvent-Dependent Gelmorphic Behavior. Gels, 10(11), 749. https://doi.org/10.3390/gels10110749







