Ketoprofen Associated with Hyaluronic Acid Hydrogel for Temporomandibular Disorder Treatment: An In Vitro Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (FTIR)
2.2. Thermogravimetric Analysis (TGA)
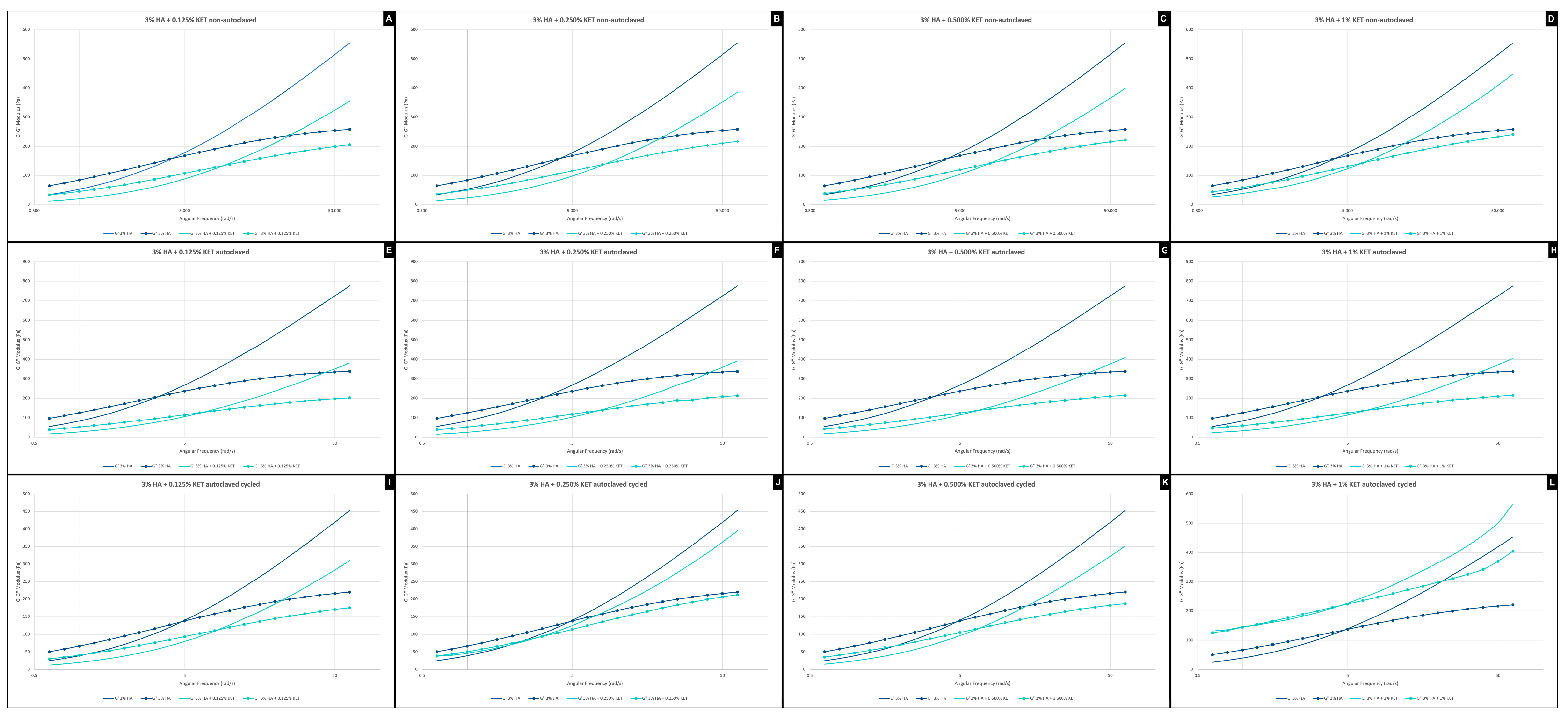
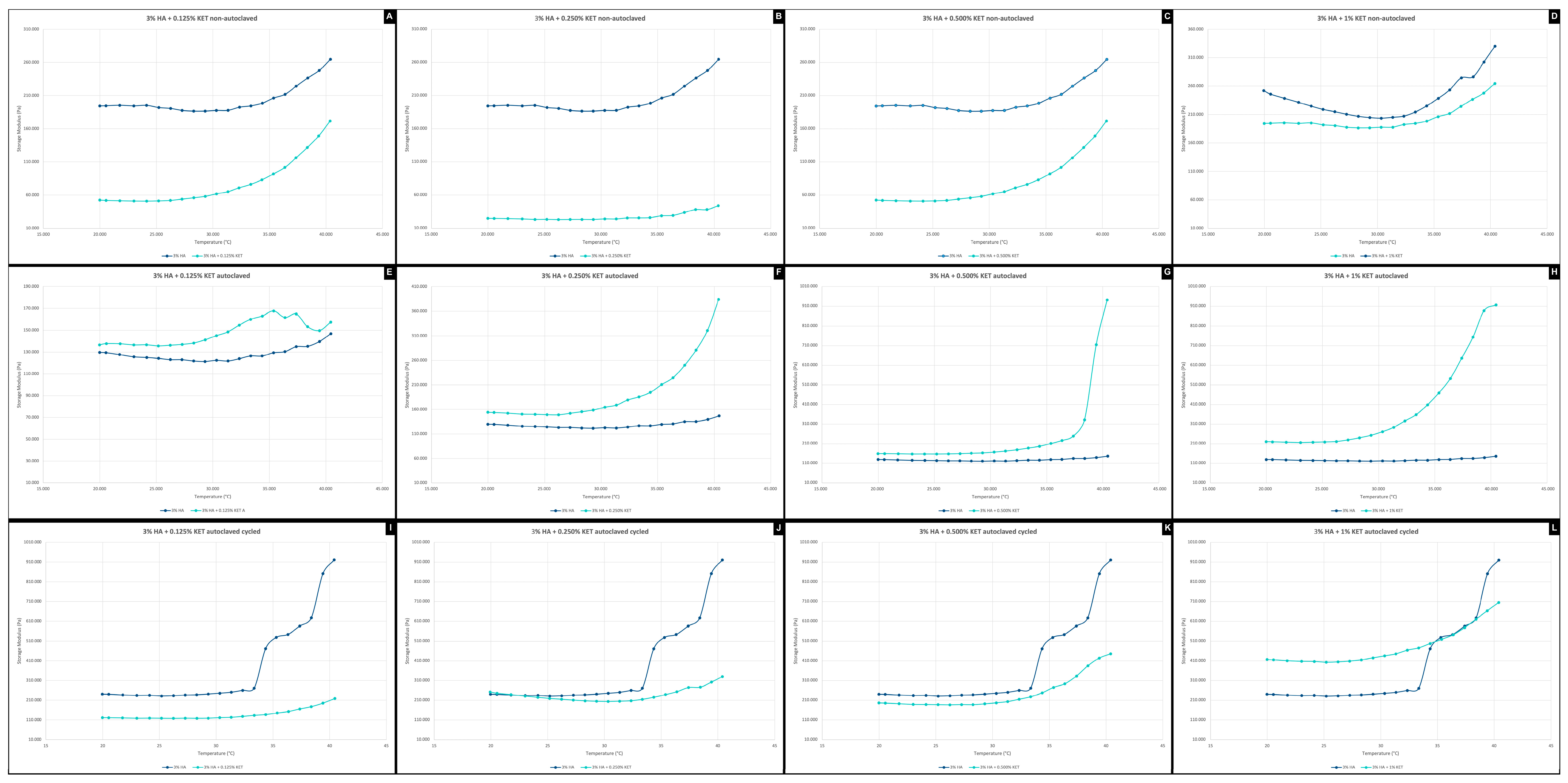
2.3. Rheology Assay
2.3.1. Frequency Sweep
2.3.2. Amplitude Sweep
2.3.3. Temperature Ramp
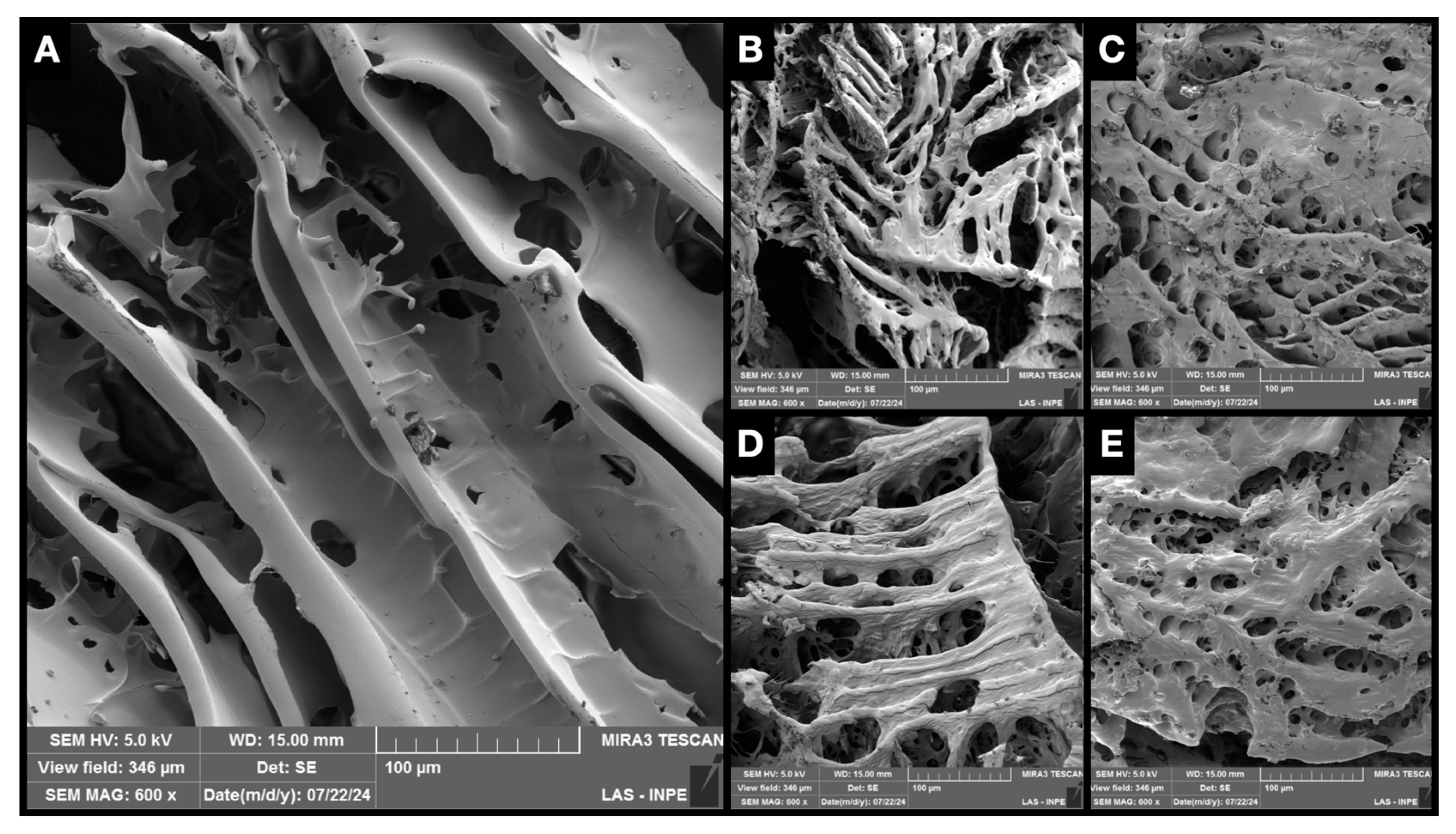
2.4. Scanning Electron Microscopy with Field Emission Gun (SEM-FEG)
2.5. Cell Viability by Resazurin Essay
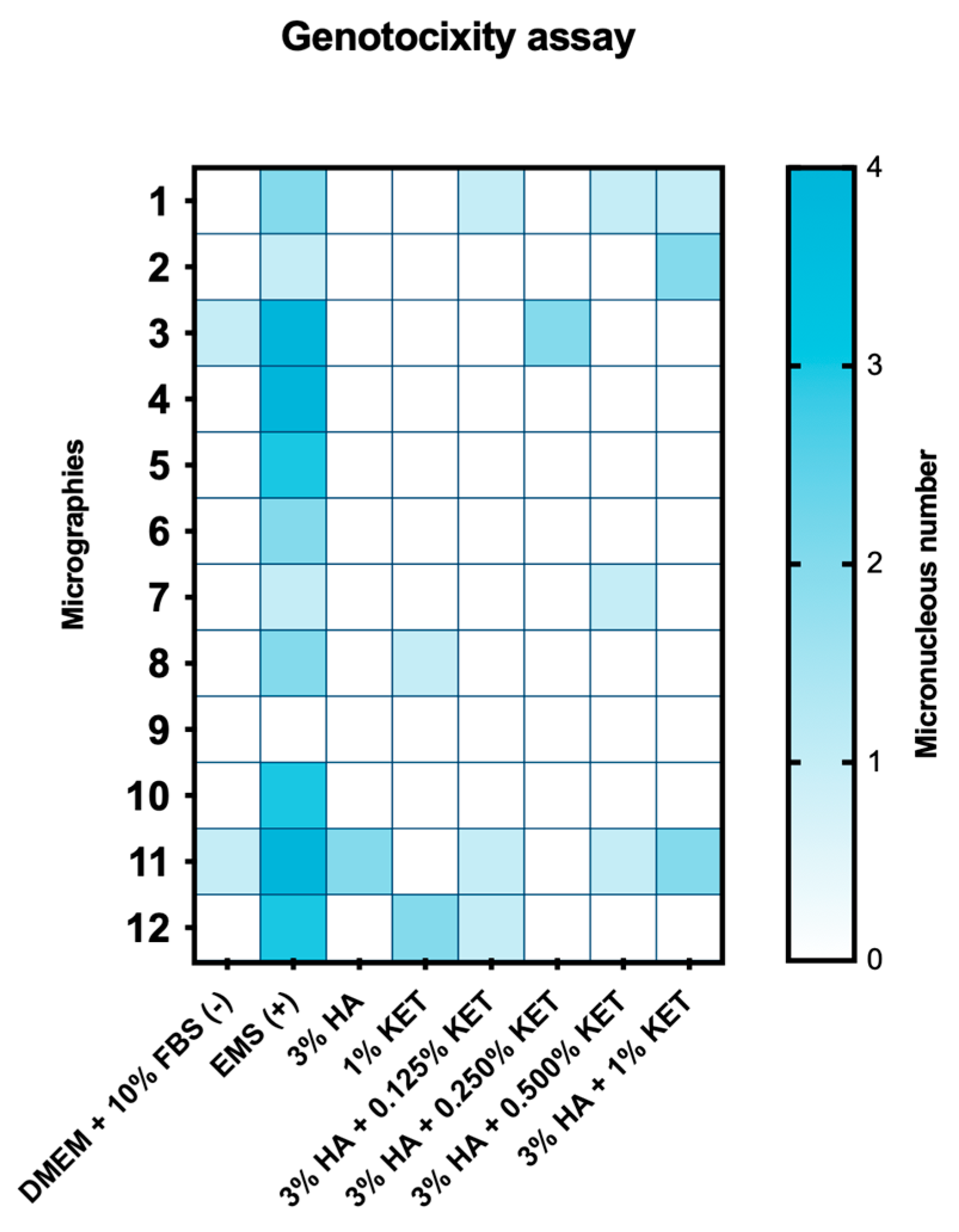
2.6. Genotoxicity Assay by Micronucleus
3. Conclusions
4. Materials and Methods
4.1. Chemical Reagents
4.2. Equipments
4.3. Hydrogels Preparation
4.4. Sterilization and Thermal Fatigue
4.5. Freeze-Drying
4.6. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (FTIR)
4.7. Thermogravimetric Analysis (TGA)
4.8. Rheology Assay
4.8.1. Frequency Sweep
4.8.2. Amplitude Sweep
4.8.3. Temperature Ramp
4.9. Scanning Electron Microscopy with Field Emission Gun (SEM-FEG)
4.10. Biological Performance on In Vitro Cell Cultures
4.11. Treatment via Transwell
4.12. Cell Viability by Resazurin Essay
4.13. Genotoxicity Assay by Micronucleus
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phillips, D.J.; Gelb, M.; Brown, C.R.; Kinderknecht, K.E.; Neff, P.A.; Kirk, W.S.; Schellhas, K.P.; Biggs, J.H.; Williams, B. Guide to Evaluation of Permanent Impairment of the Temporomandibular Joint. CRANIO® 1997, 15, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Valesan, L.F.; Da-Cas, C.D.; Réus, J.C.; Denardin, A.C.S.; Garanhani, R.R.; Bonotto, D.; Januzzi, E.; De Souza, B.D.M. Prevalence of Temporomandibular Joint Disorders: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2021, 25, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, Z.; Chen, Y.; Chen, Y.; Pan, Z.; Gu, Y. Temporomandibular Disorders among Medical Students in China: Prevalence, Biological and Psychological Risk Factors. BMC Oral Health 2021, 21, 549. [Google Scholar] [CrossRef] [PubMed]
- Cardoneanu, A.; Macovei, L.A.; Burlui, A.M.; Mihai, I.R.; Bratoiu, I.; Rezus, I.I.; Richter, P.; Tamba, B.-I.; Rezus, E. Temporomandibular Joint Osteoarthritis: Pathogenic Mechanisms Involving the Cartilage and Subchondral Bone, and Potential Therapeutic Strategies for Joint Regeneration. Int. J. Mol. Sci. 2022, 24, 171. [Google Scholar] [CrossRef]
- Ibi, M. Inflammation and Temporomandibular Joint Derangement. Biol. Pharm. Bull. 2019, 42, 538–542. [Google Scholar] [CrossRef]
- Almășan, O.; Hedeșiu, M.; Băciuț, M.; Buduru, S.; Dinu, C. Psoriatic Arthritis of the Temporomandibular Joint: A Systematic Review. J. Oral Rehabil. 2023, 50, 243–255. [Google Scholar] [CrossRef]
- Campos, D.E.S.; De Araújo Ferreira Muniz, I.; De Souza Villarim, N.L.; Ribeiro, I.L.A.; Batista, A.U.D.; Bonan, P.R.F.; De Sales, M.A.O. Is There an Association between Rheumatoid Arthritis and Bone Changes in the Temporomandibular Joint Diagnosed by Cone-Beam Computed Tomography? A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2021, 25, 2449–2459. [Google Scholar] [CrossRef]
- Xu, X.; Sui, B.; Liu, X.; Sun, J. A Bioinspired and High-Strengthed Hydrogel for Regeneration of Perforated Temporomandibular Joint Disc: Construction and Pleiotropic Immunomodulatory Effects. Bioact. Mater. 2023, 25, 701–715. [Google Scholar] [CrossRef]
- Sebastiani, A.M.; Dos Santos, K.M.; Cavalcante, R.C.; Pivetta Petinati, M.F.; Signorini, L.; Antunes, L.A.A.; Rebellato, N.L.B.; Küchler, E.C.; Scariot, R. Depression, Temporomandibular Disorders, and Genetic Polymorphisms in IL6 Impact on Oral Health-Related Quality of Life in Patients Requiring Orthognathic Surgery. Qual. Life Res. 2020, 29, 3315–3323. [Google Scholar] [CrossRef]
- Sprangers, M.A.G.; Thong, M.S.Y.; Bartels, M.; Barsevick, A.; Ordoñana, J.; Shi, Q.; Wang, X.S.; Klepstad, P.; Wierenga, E.A.; Singh, J.A.; et al. Biological Pathways, Candidate Genes, and Molecular Markers Associated with Quality-of-Life Domains: An Update. Qual. Life Res. 2014, 23, 1997–2013. [Google Scholar] [CrossRef]
- Ranawat, A.; Guo, K.; Phillips, M.; Guo, A.; Niazi, F.; Bhandari, M.; Waterman, B. Health Economic Assessments of Hyaluronic Acid Treatments for Knee Osteoarthritis: A Systematic Review. Adv. Ther. 2024, 41, 65–81. [Google Scholar] [CrossRef]
- Tsitadze, T.; Puturidze, S.; Lomidze, T.; Margvelashvili, V.; Kalandadze, M. Prevalence and risk-factors of bruxism in children and adolescent population and its impact on queality of life (review). Georgian Med. News 2021, 310, 36–39. [Google Scholar]
- Ástvaldsdóttir, Á.; Boström, A.; Davidson, T.; Gabre, P.; Gahnberg, L.; Sandborgh Englund, G.; Skott, P.; Ståhlnacke, K.; Tranæus, S.; Wilhelmsson, H.; et al. Oral Health and Dental Care of Older Persons—A Systematic Map of Systematic Reviews. Gerodontology 2018, 35, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Gilheaney, Ó.; Stassen, L.F.; Walshe, M. The Epidemiology, Nature, and Impact of Eating and Swallowing Problems in Adults Presenting with Temporomandibular Disorders. CRANIO® 2022, 40, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Andre, A.; Kang, J.; Dym, H. Pharmacologic Treatment for Temporomandibular and Temporomandibular Joint Disorders. Oral Maxillofac. Surg. Clin. N. Am. 2022, 34, 49–59. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.; Tang, Y.; Tang, Y.; Fei, W.; Liang, X. Multiple Treatment Meta-Analysis of Intra-Articular Injection for Temporomandibular Osteoarthritis. J. Oral Maxillofac. Surg. 2020, 78, 373.e1–373.e18. [Google Scholar] [CrossRef]
- Thorpe, A.R.D.S.; Haddad, Y.; Hsu, J. A Systematic Review and Meta-Analysis of Randomized Controlled Trials Comparing Arthrocentesis with Conservative Management for Painful Temporomandibular Joint Disorder. Int. J. Oral Maxillofac. Surg. 2023, 52, 889–896. [Google Scholar] [CrossRef]
- Tsui, H.C.; Lam, C.M.; Leung, Y.Y.; Li, K.Y.; Wong, N.S.M.; Li, D.T.S. Lavage Volume of Arthrocentesis in the Management of Temporomandibular Disorders: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 2622. [Google Scholar] [CrossRef]
- Soni, A. Arthrocentesis of Temporomandibular Joint- Bridging the Gap between Non-Surgical and Surgical Treatment. Ann. Maxillofac. Surg. 2019, 9, 158. [Google Scholar] [CrossRef]
- Webner, D.; Huang, Y.; Hummer, C.D. Intraarticular Hyaluronic Acid Preparations for Knee Osteoarthritis: Are Some Better Than Others? CARTILAGE 2021, 13, 1619S–1636S. [Google Scholar] [CrossRef]
- Jantzen, C.; Ebskov, L.B.; Andersen, K.H.; Benyahia, M.; Rasmussen, P.B.; Johansen, J.K. The Effect of a Single Hyaluronic Acid Injection in Ankle Arthritis: A Prospective Cohort Study. J. Foot Ankle Surg. 2020, 59, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, M.; Manjoo, A.; Shaw, P.; Niazi, F.; Rosen, J. A Comparison Between Rheological Properties of Intra-Articular Hyaluronic Acid Preparations and Reported Human Synovial Fluid. Adv. Ther. 2018, 35, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, M.; Manjoo, A.; Shaw, P.; Niazi, F.; Rosen, J. Rheological Properties of Commercially Available Hyaluronic Acid Products in the United States for the Treatment of Osteoarthritis Knee Pain. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2018, 11, 117954411775162. [Google Scholar] [CrossRef]
- Yoshioka, K.; Kisukeda, T.; Zuinen, R.; Yasuda, Y.; Miyamoto, K. Pharmacological Effects of N-[2-[[2-[2-[(2,6-Dichlorophenyl)Amino]Phenyl]Acetyl]Oxy]Ethyl]Hyaluronamide (Diclofenac Etalhyaluronate, SI-613), a Novel Sodium Hyaluronate Derivative Chemically Linked with Diclofenac. BMC Musculoskelet. Disord. 2018, 19, 157. [Google Scholar] [CrossRef]
- Derwich, M.; Mitus-Kenig, M.; Pawlowska, E. Mechanisms of Action and Efficacy of Hyaluronic Acid, Corticosteroids and Platelet-Rich Plasma in the Treatment of Temporomandibular Joint Osteoarthritis—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7405. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic Acid—Based Wound Dressings: A Review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic Acid, a Promising Skin Rejuvenating Biomedicine: A Review of Recent Updates and Pre-Clinical and Clinical Investigations on Cosmetic and Nutricosmetic Effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef] [PubMed]
- Mortada, H.; Seraj, H.; Barasain, O.; Bamakhrama, B.; Alhindi, N.I.; Arab, K. Ocular Complications Post-Cosmetic Periocular Hyaluronic Acid Injections: A Systematic Review. Aesth. Plast. Surg. 2022, 46, 760–773. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, G.; Liu, H.; Han, J.; Liu, Z.; Ma, H. Molecular Weight Impact on the Mechanical Forces between Hyaluronan and Its Receptor. Carbohydr. Polym. 2018, 197, 326–336. [Google Scholar] [CrossRef]
- Qiu, Y.; Ma, Y.; Huang, Y.; Li, S.; Xu, H.; Su, E. Current Advances in the Biosynthesis of Hyaluronic Acid with Variable Molecular Weights. Carbohydr. Polym. 2021, 269, 118320. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Xavier, A.A.; Da Rosa, P.P.; De Brum Mackmill, L.; Roll, V.F.B. An Assessment of the Effectiveness of Hyaluronic Acid and Polyacrylamide Hydrogel in Horses with Osteoarthritis: Systematic Review and Network Meta-Analysis. Res. Vet. Sci. 2021, 134, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic Acid: A Review on Its Biology, Aspects of Drug Delivery, Route of Administrations and a Special Emphasis on Its Approved Marketed Products and Recent Clinical Studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef]
- Gigante, A.; Tagarro, I. Non-Steroidal Anti-Inflammatory Drugs and Gastroprotection with Proton Pump Inhibitors: A Focus on Ketoprofen/Omeprazole. Clin. Drug Investig. 2012, 32, 221–233. [Google Scholar] [CrossRef]
- Schumacher, H.R. Ketoprofen Extended-Release Capsules: A New Formulation for the Treatment of Osteoarthritis and Rheumatoid Arthritis. Clin. Ther. 1994, 16, 145–159. [Google Scholar]
- Sarzi-Puttini, P.; Atzeni, F.; Lanata, L.; Bagnasco, M.; Colombo, M.; Fischer, F.; D’Imporzano, M. Pain and Ketoprofen: What Is Its Role in Clinical Practice? Reumatismo 2011, 62, 172–188. [Google Scholar] [CrossRef]
- Chawla, G.; Ranjan, C.; Kumar, J.; Siddiqui, A.A. Chemical Modifications of Ketoprofen (NSAID) in Search of Better Lead Compounds: A Review of Literature from 2004–2016. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2017, 15, 154–177. [Google Scholar] [CrossRef] [PubMed]
- Yakushin, S.; Polyakova, S.; Shvarts, Y.; Kastanayan, A.; Krechikova, D.; Ershova, O.; Nikulenkova, N.; Vinogradova, I.; Hyun, B.J.; Cha, J.E. Comparison of the Efficacy and Safety of Ketoprofen Plaster and Diclofenac Plaster for Osteoarthritis-Related Knee Pain: A Multicenter, Randomized, Active-Controlled, Open-Label, Parallel-Group, Phase III Clinical Trial. Clin. Ther. 2021, 43, 1720–1734. [Google Scholar] [CrossRef]
- Atzeni, F.; Masala, I.F.; Bagnasco, M.; Lanata, L.; Mantelli, F.; Sarzi-Puttini, P. Comparison of Efficacy of Ketoprofen and Ibuprofen in Treating Pain in Patients with Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Pain Ther. 2021, 10, 577–588. [Google Scholar] [CrossRef]
- Alcântara, L.O.; De Sousa, J.R.; Andrade, F.K.; Teixeira, E.H.; Cerqueira, M.Â.; Da Silva, A.L.C.; Souza Filho, M.D.S.M.; De Souza, B.W.S. Extraction and Characterization of Hyaluronic Acid from the Eyeball of Nile Tilapia (Oreochromis Niloticus). Int. J. Biol. Macromol. 2023, 226, 172–183. [Google Scholar] [CrossRef]
- Elhiss, S.; Hamdi, A.; Chahed, L.; Boisson-Vidal, C.; Majdoub, H.; Bouchemal, N.; Laschet, J.; Kraiem, J.; Le Cerf, D.; Maaroufi, R.M.; et al. Hyaluronic Acid from Bluefin Tuna by-Product: Structural Analysis and Pharmacological Activities. Int. J. Biol. Macromol. 2024, 264, 130424. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Yang, Y.; Shen, W.; Ren, L.; Wang, W.; Zhu, P. Hyaluronic Acid-Conjugated Methotrexate and 5-Fluorouracil for Targeted Drug Delivery. Int. J. Biol. Macromol. 2024, 273, 132671. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.G.A.; Simonca, A.G.; Rau, I.; Coman, A.E.; Marin, M.M.; Popa, L.; Trusca, R.; Dinu-Pirvu, C.-E.; Ghica, M.V. Topical Biocomposites Based on Collagen, Hyaluronic Acid and Metronidazole as Periodontitis Treatment. Pharmaceuticals 2024, 17, 1336. [Google Scholar] [CrossRef]
- Abou-Taleb, H.A.; Shoman, M.E.; Makram, T.S.; Abdel-Aleem, J.A.; Abdelkader, H. Exploration of the Safety and Solubilization, Dissolution, Analgesic Effects of Common Basic Excipients on the NSAID Drug Ketoprofen. Pharmaceutics 2023, 15, 713. [Google Scholar] [CrossRef]
- Rajamohan, R.; Kamaraj, E.; Muthuraja, P.; Murugavel, K.; Govindasamy, C.; Prabakaran, D.S.; Malik, T.; Lee, Y.R. Enhancing Ketoprofen’s Solubility and Anti-Inflammatory Efficacy with Safe Methyl-β-Cyclodextrin Complexation. Sci. Rep. 2024, 14, 21516. [Google Scholar] [CrossRef]
- Zhang, F.; Li, L.; Zhang, X.; Yang, H.; Fan, Y.; Zhang, J.; Fang, T.; Liu, Y.; Nie, Z.; Wang, D. Ionic Liquid Transdermal Patches of Two Active Ingredients Based on Semi-Ionic Hydrogen Bonding for Rheumatoid Arthritis Treatment. Pharmaceutics 2024, 16, 480. [Google Scholar] [CrossRef]
- De Oliveira Junior, H.; Borges, B.A.; Barbosa, T.W.L.; Batista, A.; Braga, M.T.L.; De Araújo, M.B.; Bonfilio, R. A New Crystalline Ketoprofen Sodium Salt: Solid-State Characterization, Solubility, and Stability. J. Pharm. Sci. 2022, 111, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Raffaini, G.; Catauro, M. Surface Interactions between Ketoprofen and Silica-Based Biomaterials as Drug Delivery System Synthesized via Sol–Gel: A Molecular Dynamics Study. Materials 2022, 15, 2759. [Google Scholar] [CrossRef]
- Miranda, D.G.; Malmonge, S.M.; Campos, D.M.; Attik, N.G.; Grosgogeat, B.; Gritsch, K. A Chitosan-hyaluronic Acid Hydrogel Scaffold for Periodontal Tissue Engineering. J. Biomed. Mater. Res. 2016, 104, 1691–1702. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y.; Xia, X.; Cao, Z.; Deng, Y.; Tang, B. Sr/PTA Metal Organic Framework as A Drug Delivery System for Osteoarthritis Treatment. Sci. Rep. 2019, 9, 17570. [Google Scholar] [CrossRef]
- Kurbasic, M.; Romano, C.; Garcia, A.; Kralj, S.; Marchesan, S. Assembly of a Tripeptide and Anti-Inflammatory Drugs into Supramolecular Hydrogels for Sustained Release. Gels 2017, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Fam., H.; Bryant, J.T.; Kontopoulou, M. Rheological properties of synovial fluids. Biorheology 2007, 44, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Otto, F.; Froelich, A. Microemulsion-Based Polymer Gels with Ketoprofen and Menthol: Physicochemical Properties and Drug Release Studies. Gels 2024, 10, 435. [Google Scholar] [CrossRef] [PubMed]
- García-Villegas, A.; Fernández-Ochoa, Á.; Rojas-García, A.; Alañón, M.E.; Arráez-Román, D.; Cádiz-Gurrea, M.D.L.L.; Segura-Carretero, A. The Potential of Mangifera Indica L. Peel Extract to Be Revalued in Cosmetic Applications. Antioxidants 2023, 12, 1892. [Google Scholar] [CrossRef]
- Sánchez-Téllez, D.A.; Rodríguez-Lorenzo, L.M.; Téllez-Jurado, L. Siloxane-Inorganic Chemical Crosslinking of Hyaluronic Acid—Based Hybrid Hydrogels: Structural Characterization. Carbohydr. Polym. 2020, 230, 115590. [Google Scholar] [CrossRef]
- Criado-Gonzalez, M.; Espinosa-Cano, E.; Rojo, L.; Boulmedais, F.; Aguilar, M.R.; Hernández, R. Injectable Tripeptide/Polymer Nanoparticles Supramolecular Hydrogel: A Candidate for the Treatment of Inflammatory Pathologies. ACS Appl. Mater. Interfaces 2022, 14, 10068–10080. [Google Scholar] [CrossRef]
- Moses, V.S.; Hardy, J.; Bertone, A.L.; Weisbrode, S.E. Effects of Anti-Inflammatory Drugs on Lipopolysaccharide-Challenged and -Unchallenged Equine Synovial Explants. Am. J. Vet. Res. 2001, 62, 54–60. [Google Scholar] [CrossRef]
- Wyns, H.; Meyer, E.; Plessers, E.; Watteyn, A.; Van Bergen, T.; Schauvliege, S.; De Baere, S.; Devreese, M.; De Backer, P.; Croubels, S. Modulation by Gamithromycin and Ketoprofen of in Vitro and in Vivo Porcine Lipopolysaccharide-Induced Inflammation. Vet. Immunol. Immunopathol. 2015, 168, 211–222. [Google Scholar] [CrossRef]
- Philipose, B.; Singh, R.; Khan, K.A.; Giri, A.K. Comparative Mutagenic and Genotoxic Effects of Three Propionic Acid Derivatives Ibuprofen, Ketoprofen and Naproxen. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 1997, 393, 123–131. [Google Scholar] [CrossRef]
- Sallustio, B.C.; DeGraaf, Y.C.; Weekley, J.S.; Burcham, P.C. Bioactivation of Carboxylic Acid Compounds by UDP-Glucuronosyltransferases to DNA-Damaging Intermediates: Role of Glycoxidation and Oxidative Stress in Genotoxicity. Chem. Res. Toxicol. 2006, 19, 683–691. [Google Scholar] [CrossRef]
- Haridas, N.; Rosemary, M.J. Effect of Steam Sterilization and Biocompatibility Studies of Hyaluronic Acid Hydrogel for Viscosupplementation. Polym. Degrad. Stab. 2019, 163, 220–227. [Google Scholar] [CrossRef]
- Guess, P.C.; Vagkopoulou, T.; Zhang, Y.; Wolkewitz, M.; Strub, J.R. Marginal and Internal Fit of Heat Pressed versus CAD/CAM Fabricated All-Ceramic Onlays after Exposure to Thermo-Mechanical Fatigue. J. Dent. 2014, 42, 199–209. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, D.G.; Ramos, L.d.P.; Lopes, N.F.d.S.; Silva, N.V.D.H.F.; Soares, C.P.; Rodrigues, F.P.; Morais, V.d.P.; Sani-Taiariol, T.; Baldan, M.R.; Vasconcellos, L.M.R.d.; et al. Ketoprofen Associated with Hyaluronic Acid Hydrogel for Temporomandibular Disorder Treatment: An In Vitro Study. Gels 2024, 10, 811. https://doi.org/10.3390/gels10120811
Miranda DG, Ramos LdP, Lopes NFdS, Silva NVDHF, Soares CP, Rodrigues FP, Morais VdP, Sani-Taiariol T, Baldan MR, Vasconcellos LMRd, et al. Ketoprofen Associated with Hyaluronic Acid Hydrogel for Temporomandibular Disorder Treatment: An In Vitro Study. Gels. 2024; 10(12):811. https://doi.org/10.3390/gels10120811
Chicago/Turabian StyleMiranda, Diego Garcia, Lucas de Paula Ramos, Nicole Fernanda dos Santos Lopes, Nicole Van Der Heijde Fernandes Silva, Cristina Pacheco Soares, Flavia Pires Rodrigues, Vinicius de Paula Morais, Thalita Sani-Taiariol, Mauricio Ribeiro Baldan, Luana Marotta Reis de Vasconcellos, and et al. 2024. "Ketoprofen Associated with Hyaluronic Acid Hydrogel for Temporomandibular Disorder Treatment: An In Vitro Study" Gels 10, no. 12: 811. https://doi.org/10.3390/gels10120811
APA StyleMiranda, D. G., Ramos, L. d. P., Lopes, N. F. d. S., Silva, N. V. D. H. F., Soares, C. P., Rodrigues, F. P., Morais, V. d. P., Sani-Taiariol, T., Baldan, M. R., Vasconcellos, L. M. R. d., Borges, A. L. S., Grosgogeat, B., & Gritsch, K. (2024). Ketoprofen Associated with Hyaluronic Acid Hydrogel for Temporomandibular Disorder Treatment: An In Vitro Study. Gels, 10(12), 811. https://doi.org/10.3390/gels10120811












