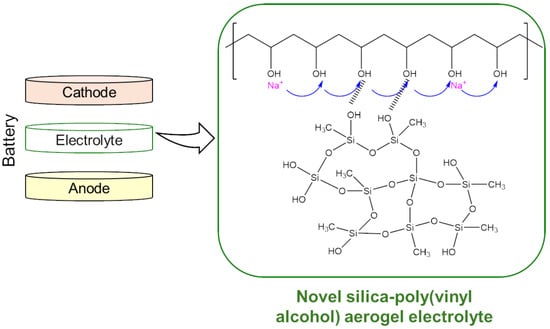
Silica-Poly(Vinyl Alcohol) Composite Aerogel: A Promising Electrolyte for Solid-State Sodium Batteries
Abstract
:1. Introduction
2. Results and Discussion
2.1. Properties of Aerogel Electrolytes
2.2. Sodium Ion Conduction
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of PVA–Silica Aerogel Electrolytes (AEs)
4.3. Characterization
4.4. Electrochemical Testing
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

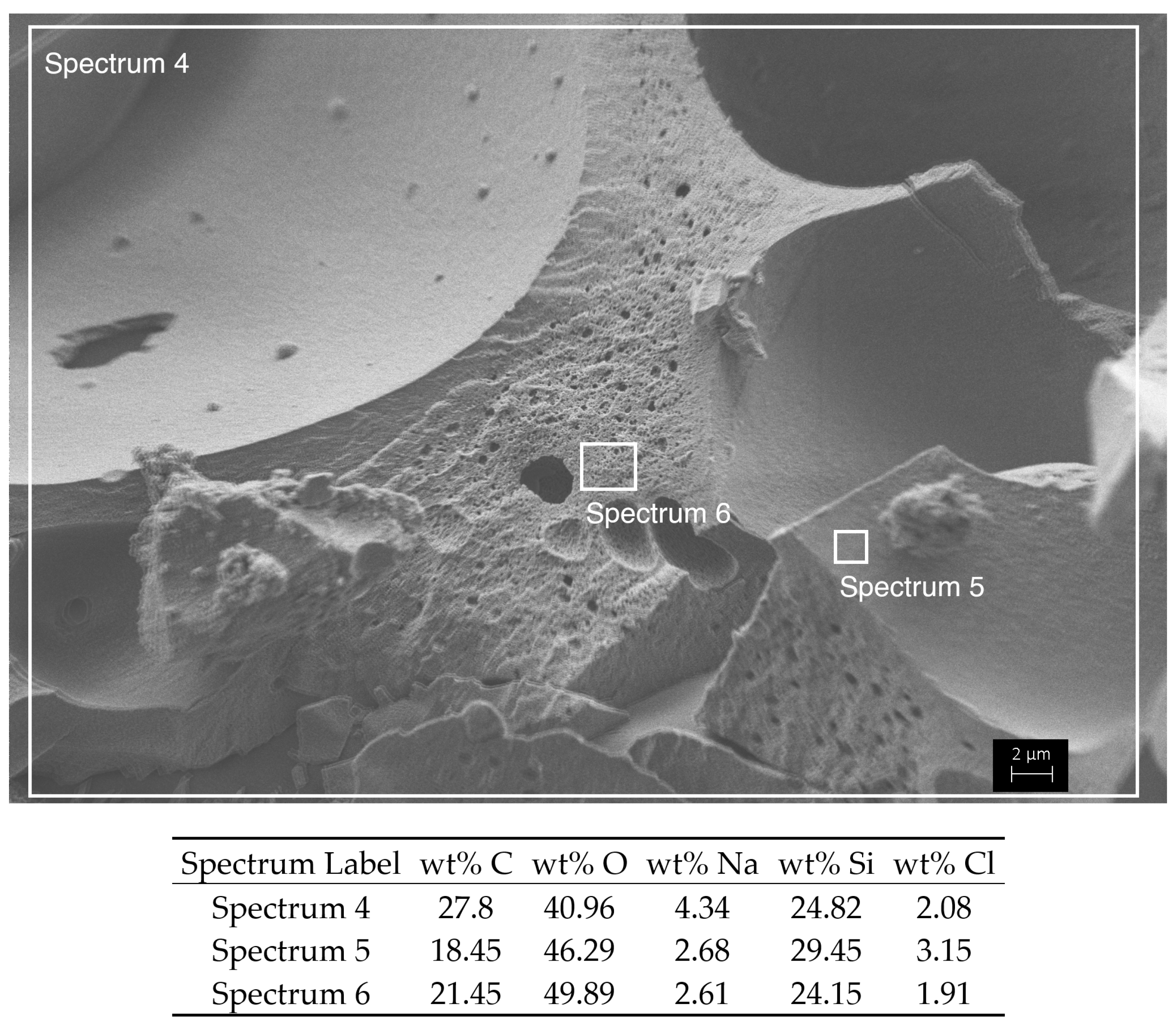
Appendix B

Appendix C
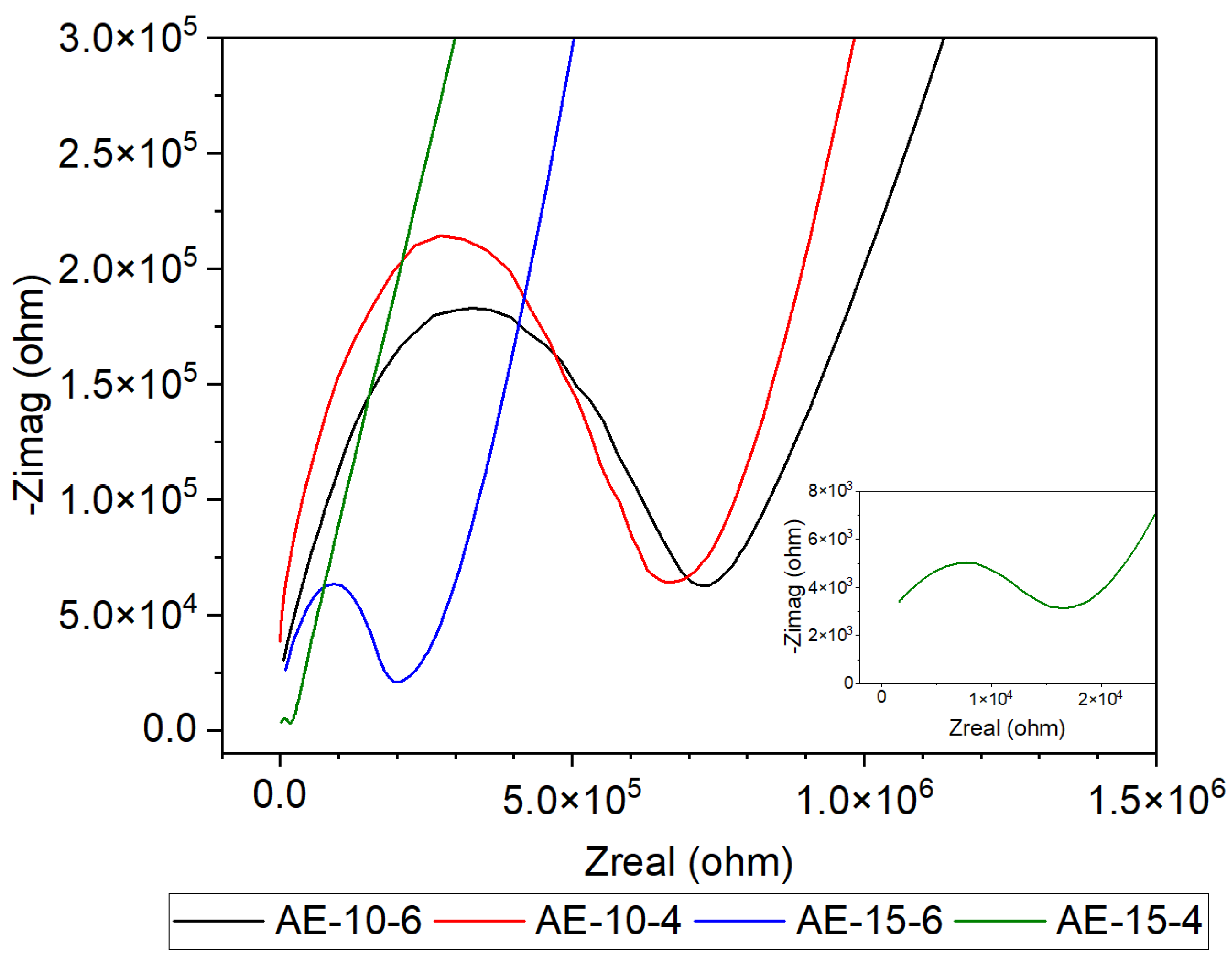
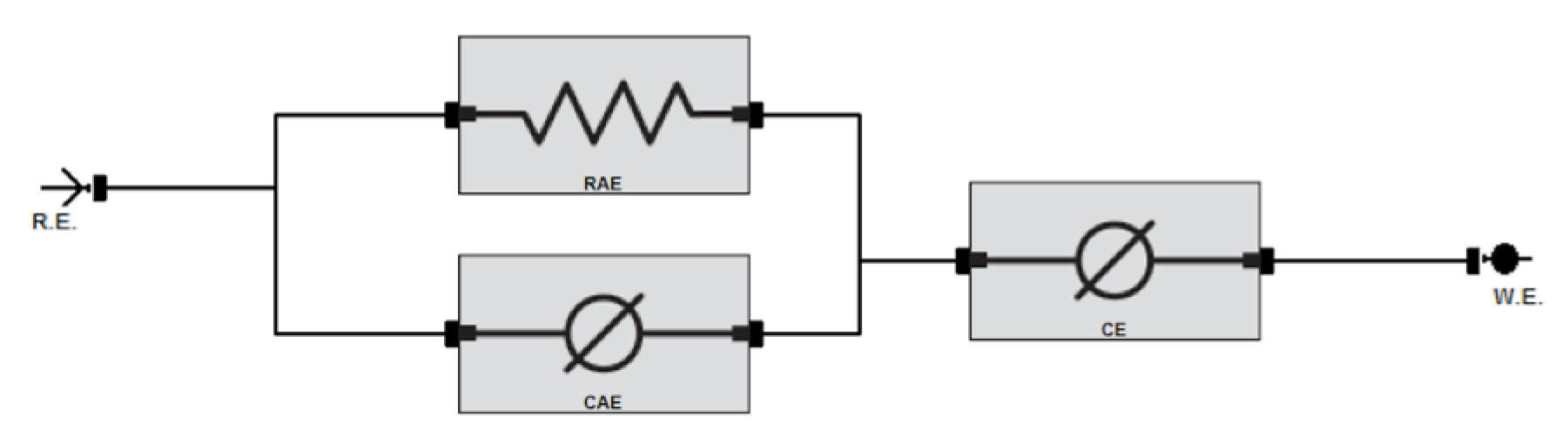
| Sample | RAE (Ω) | CAE | Ce | Goodness of Fit 1 | ||
|---|---|---|---|---|---|---|
| Q (S sα) | α | Q (S sα) | α | |||
| AE-10-6 | 6.5 × 105 | 3.5 × 10−10 | 0.70 | 8.1 × 10−7 | 0.39 | 5.6 × 10−3 |
| AE-10-4 | 6.1 × 105 | 2.0 × 10−11 | 0.86 | 2.5 × 10−7 | 0.48 | 7.5 × 10−3 |
| AE-15-6 | 1.3 × 105 | 2.2 × 10−10 | 0.74 | 1.4 × 10−6 | 0.39 | 3.7 × 10−3 |
| AE-15-4 | 1.8 × 104 | 1.4 × 10−8 | 0.54 | 1.6× 10−6 | 0.50 | 1.5 × 10−3 |
References
- Vadhva, P.; Hu, J.; Johnson, M.J.; Stocker, R.; Braglia, M.; Brett, D.J.L.; Rettie, A.J.E. Electrochemical Impedance Spectroscopy for All-Solid-State Batteries: Theory, Methods and Future Outlook. ChemElectroChem 2021, 8, 1930–1947. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Lu, G.; Li, W.; Tao, Q.; Shi, C.; Jin, H.; Chen, G.; Wang, S. Fundamentals of Electrolytes for Solid-State Batteries: Challenges and Perspectives. Front. Mater. 2020, 7, 111. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, L.; Qi, X.; Lu, Y.; Wu, F.; Zhao, J.; Yu, Y.; Hu, Y.; Chen, L. Solid-State Sodium Batteries. Adv. Energy Mater. 2018, 8, 1703012. [Google Scholar] [CrossRef]
- Takada, K. Progress in solid electrolytes toward realizing solid-state lithium batteries. J. Power Sources 2018, 394, 74–85. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Kim, J.G.; Son, B.; Mukherjee, S.; Schuppert, N.; Bates, A.; Kwon, O.; Choi, M.J.; Chung, H.Y.; Park, S. A review of lithium and non-lithium based solid state batteries. J. Power Sources 2015, 282, 299–322. [Google Scholar] [CrossRef]
- Wu, C.; Lou, J.; Zhang, J.; Chen, Z.; Kakar, A.; Emley, B.; Ai, Q.; Guo, H.; Liang, Y.; Lou, J.; et al. Current status and future directions of all-solid-state batteries with lithium metal anodes, sulfide electrolytes, and layered transition metal oxide cathodes. Nano Energy 2021, 87, 106081. [Google Scholar] [CrossRef]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Zheng, M.; Li, X.; Sun, J.; Wang, X.; Liu, G.; Yu, W.; Dong, X.; Wang, J. Research progress on chloride solid electrolytes for all-solid-state batteries. J. Power Sources 2024, 595, 234051. [Google Scholar] [CrossRef]
- Mishra, A.K.; Chaliyawala, H.A.; Patel, R.; Paneliya, S.; Vanpariya, A.; Patel, P.; Ray, A.; Pati, R.; Mukhopadhyay, I. Review—Inorganic Solid State Electrolytes: Insights on Current and Future Scope. J. Electrochem. Soc. 2021, 168, 080536. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Gonçalves, R.; Costa, C.M.; Lanceros-Méndez, S. Toward Sustainable Solid Polymer Electrolytes for Lithium-Ion Batteries. ACS Omega 2022, 7, 14457–14464. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lakraychi, A.E.; Chen, Z.; Liang, Y.; Yao, Y. Roadmap of Solid-State Lithium-Organic Batteries toward 500 Wh kg−1. ACS Energy Lett. 2021, 6, 3287–3306. [Google Scholar] [CrossRef]
- Mousa, E.; Hu, X.; Ånnhagen, L.; Ye, G.; Cornelio, A.; Fahimi, A.; Bontempi, E.; Frontera, P.; Badenhorst, C.; Santos, A.C.; et al. Characterization and Thermal Treatment of the Black Mass from Spent Lithium-Ion Batteries. Sustainability 2023, 15, 15. [Google Scholar] [CrossRef]
- Liao, Y.; Xu, X.; Luo, X.; Ji, S.; Zhao, J.; Liu, J.; Huo, Y. Recent Progress in Flame-Retardant Polymer Electrolytes for Solid-State Lithium Metal Batteries. Batteries 2023, 9, 439. [Google Scholar] [CrossRef]
- Mossali, E.; Picone, N.; Gentilini, L.; Rodrìguez, O.; Pérez, J.M.; Colledani, M. Lithium-ion batteries towards circular economy: A literature review of opportunities and issues of recycling treatments. J. Environ. Manag. 2020, 264, 110500. [Google Scholar] [CrossRef]
- Nair, J.R.; Imholt, L.; Brunklaus, G.; Winter, M. Lithium Metal Polymer Electrolyte Batteries: Opportunities and Challenges. Electrochem. Soc. Interface 2019, 28, 55–61. [Google Scholar] [CrossRef]
- Zhang, W.; Tu, Z.; Qian, J.; Choudhury, S.; Archer, L.A.; Lu, Y. Design Principles of Functional Polymer Separators for High-Energy, Metal-Based Batteries. Small 2018, 14, 1703001. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, H.; Zhou, Q.; Qu, H.; Dong, T.; Zhang, M.; Tang, B.; Zhang, J.; Cui, G. Safety-Enhanced Polymer Electrolytes for Sodium Batteries: Recent Progress and Perspectives. ACS Appl. Mater. Interfaces 2019, 11, 17109–17127. [Google Scholar] [CrossRef]
- Su, Y.; Xu, F.; Zhang, X.; Qiu, Y.; Wang, H. Rational Design of High-Performance PEO/Ceramic Composite Solid Electrolytes for Lithium Metal Batteries. Nano-Micro Lett. 2023, 15, 82. [Google Scholar] [CrossRef]
- Fan, L.-Z.; He, H.; Nan, C.-W. Tailoring inorganic–polymer composites for the mass production of solid-state batteries. Nat. Rev. Mater. 2021, 6, 1003–1019. [Google Scholar] [CrossRef]
- Saikia, D.; Chen-Yang, Y.; Chen, Y.; Li, Y.; Lin, S. Investigation of ionic conductivity of composite gel polymer electrolyte membranes based on P(VDF-HFP), LiClO4 and silica aerogel for lithium ion battery. Desalination 2008, 234, 24–32. [Google Scholar] [CrossRef]
- Chen-Yang, Y.; Wang, Y.; Chen, Y.; Li, Y.; Chen, H.; Chiu, H. Influence of silica aerogel on the properties of polyethylene oxide-based nanocomposite polymer electrolytes for lithium battery. J. Power Sources 2008, 182, 340–348. [Google Scholar] [CrossRef]
- Yoon, M.Y.; Hong, S.K.; Hwang, H.J. Fabrication of Li-polymer/silica aerogel nanocomposite electrolyte for an all-solid-state lithium battery. Ceram. Int. 2013, 39, 9659–9663. [Google Scholar] [CrossRef]
- Chen, Y.; Chuang, Y.; Su, J.; Yu, H.; Chen-Yang, Y. High discharge capacity solid composite polymer electrolyte lithium battery. J. Power Sources 2011, 196, 2802–2809. [Google Scholar] [CrossRef]
- Lim, Y.S.; Jung, H.-A.; Hwang, H. Fabrication of PEO-PMMA-LiClO4-Based Solid Polymer Electrolytes Containing Silica Aerogel Particles for All-Solid-State Lithium Batteries. Energies 2018, 11, 2559. [Google Scholar] [CrossRef]
- Li, M.; Qi, S.; Li, S.; Du, L. Realizing Scalable Nano-SiO2-Aerogel-Reinforced Composite Polymer Electrolytes with High Ionic Conductivity via Rheology-Tuning UV Polymerization. Molecules 2023, 28, 756. [Google Scholar] [CrossRef] [PubMed]
- Saikia, D.; Chen-Yang, Y.; Chen, Y.; Li, Y.; Lin, S. 7Li NMR spectroscopy and ion conduction mechanism of composite gel polymer electrolyte: A comparative study with variation of salt and plasticizer with filler. Electrochim. Acta 2009, 54, 1218–1227. [Google Scholar] [CrossRef]
- Liu, L.; Cai, Y.; Zhao, Z.; Ma, C.; Li, C.; Mu, D. A succinonitrile-infiltrated silica aerogel synergistically-reinforced hybrid solid electrolyte for durable solid-state lithium metal batteries. Mater. Chem. Front. 2022, 6, 430–439. [Google Scholar] [CrossRef]
- Kumar, D.; Suleman, M.; Hashmi, S. Studies on poly(vinylidene fluoride-co-hexafluoropropylene) based gel electrolyte nanocomposite for sodium–sulfur batteries. Solid State Ion. 2011, 202, 45–53. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, S.Y.; Lee, J.Y.; Nam, J.; Lee, W.B.; Kim, S.; Hyun, K. Effects of ionic liquids and silica nanoparticles on the ionic conductivities, mechanical properties, and rheological properties of sodium-containing solid polymer electrolytes. J. Power Sources 2021, 518, 230748. [Google Scholar] [CrossRef]
- Lin, Y.; Li, X.; Zheng, W.; Gang, Y.; Liu, L.; Cui, X.; Dan, Y.; Chen, L.; Cheng, X. Effect of SiO2 microstructure on ionic transport behavior of self-healing composite electrolytes for sodium metal batteries. J. Membr. Sci. 2023, 672, 121442. [Google Scholar] [CrossRef]
- Praveen, D.; Bhat, S.V.; Damle, R. Role of silica nanoparticles in conductivity enhancement of nanocomposite solid polymer electrolytes: (PEGx NaBr): YSiO2. Ionics 2013, 19, 1375–1379. [Google Scholar] [CrossRef]
- Das, A.; Melepurakkal, A.; Sreeram, P.; Gireesh, K.; Balakrishnan, N.T.; Fatima, M.J.; Pullanchiyodan, A.; Ahn, J.-H.; Shelke, M.V.; Raghavan, P. Exceptional cyclability of thermally stable PVdF-co-HFP/SiO2 nanocomposite polymer electrolytes for sodium ion batteries. J. Energy Storage 2023, 73, 109026. [Google Scholar] [CrossRef]
- Kwon, D.-S.; Jeong, D.; Kang, H.B.; Chang, W.; Bang, J.; Shim, J. Tailoring composite gel polymer electrolytes with regularly arranged pores and silica particles for sodium metal batteries via breath-figure self-assembly. J. Mater. Chem. A 2023, 12, 1465–1475. [Google Scholar] [CrossRef]
- Zhai, Y.; Hou, W.; Chen, Z.; Zeng, Z.; Wu, Y.; Tian, W.; Liang, X.; Paoprasert, P.; Wen, Z.; Hu, N.; et al. A hybrid solid electrolyte for high-energy solid-state sodium metal batteries. Appl. Phys. Lett. 2022, 120, 253902. [Google Scholar] [CrossRef]
- Kulshrestha, N.; Chatterjee, B.; Gupta, P.N. Structural, thermal, electrical, and dielectric properties of synthesized nanocomposite solid polymer electrolytes. High Perform. Polym. 2014, 26, 677–688. [Google Scholar] [CrossRef]
- Chandra, A.; Chandra, A.; Thakur, K. Synthesis and ion conduction mechanism on hot-pressed sodium ion conducting nano composite polymer electrolytes. Arab. J. Chem. 2016, 9, 400–407. [Google Scholar] [CrossRef]
- Villaluenga, I.; Bogle, X.; Greenbaum, S.; Gil de Muro, I.; Rojo, T.; Armand, M. Cation only conduction in new polymer–SiO2 nanohybrids: Na+ electrolytes. J. Mater. Chem. A 2013, 1, 8348–8352. [Google Scholar] [CrossRef]
- Song, S.; Kotobuki, M.; Zheng, F.; Xu, C.; Savilov, S.V.; Hu, N.; Lu, L.; Wang, Y.; Li, W.D.Z. A hybrid polymer/oxide/ionic-liquid solid electrolyte for Na-metal batteries. J. Mater. Chem. A 2017, 5, 6424–6431. [Google Scholar] [CrossRef]
- Kumar, D.; Hashmi, S. Ion transport and ion–filler-polymer interaction in poly(methyl methacrylate)-based, sodium ion conducting, gel polymer electrolytes dispersed with silica nanoparticles. J. Power Sources 2010, 195, 5101–5108. [Google Scholar] [CrossRef]
- Lin, D.; Yuen, P.Y.; Liu, Y.; Liu, W.; Liu, N.; Dauskardt, R.H.; Cui, Y. A Silica-Aerogel-Reinforced Composite Polymer Electrolyte with High Ionic Conductivity and High Modulus. Adv. Mater. 2018, 30, e1802661. [Google Scholar] [CrossRef] [PubMed]
- Lu, K. Porous and high surface area silicon oxycarbide-based materials—A review. Mater. Sci. Eng. R Rep. 2015, 97, 23–49. [Google Scholar] [CrossRef]
- Vareda, J.P.; Lamy-Mendes, A.; Durães, L. A reconsideration on the definition of the term aerogel based on current drying trends. Microporous Mesoporous Mater. 2018, 258, 211–216. [Google Scholar] [CrossRef]
- Durães, L.; Maleki, H.; Vareda, J.P.; Lamy-Mendes, A.; Portugal, A. Exploring the Versatile Surface Chemistry of Silica Aerogels for Multipurpose Application. MRS Adv. 2017, 2, 3511–3519. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, G.; Li, X.; Cui, S.; Ying, S.; Feng, X.; Mi, L.; Chen, W. Synergism of surface group transfer and in-situ growth of silica-aerogel induced high-performance modified polyacrylonitrile separator for lithium/sodium-ion batteries. J. Membr. Sci. 2019, 577, 137–144. [Google Scholar] [CrossRef]
- Koç, F.; Gizli, N. Synergistic effect of ionic liquid and organo-functional silane on the preparation of silica based hybrid ionogels as solid-state electrolyte for Li-ion batteries. Ceram. Int. 2021, 47, 25398–25407. [Google Scholar] [CrossRef]
- Kim, S.; Jung, H.K.; Handayani, P.L.; Kim, T.; Jung, B.M.; Choi, U.H. Fast Li+ Transport via Silica Network-Driven Nanochannels in Ionomer-in-Framework for Lithium Metal Batteries. Adv. Funct. Mater. 2023, 33, 2210916. [Google Scholar] [CrossRef]
- Mercken, J.; De Sloovere, D.; Joos, B.; Calvi, L.; Mangione, G.; Pitet, L.; Derveaux, E.; Adriaensens, P.; Van Bael, M.K.; Hardy, A. Altering Mechanical Properties to Improve Electrode Contacts by Organic Modification of Silica-Based Ionogel Electrolytes for Sodium-Ion Batteries. Small 2023, 19, e2301862. [Google Scholar] [CrossRef]
- DeBlock, R.H.; Wei, Q.; Ashby, D.S.; Butts, D.M.; Whang, G.J.; Choi, C.S.; Dunn, B.S. Siloxane-Modified, Silica-Based Ionogel as a Pseudosolid Electrolyte for Sodium-Ion Batteries. ACS Appl. Energy Mater. 2020, 4, 154–163. [Google Scholar] [CrossRef]
- DeBlock, R.H.; Lai, C.-H.; Butts, D.M.; Dunn, B.S. Sodium-ion conducting pseudosolid electrolyte for energy-dense, sodium-metal batteries. J. Power Sources 2023, 554, 232305. [Google Scholar] [CrossRef]
- Vareda, J.P.; García-González, C.A.; Valente, A.J.M.; Simón-Vázquez, R.; Stipetic, M.; Durães, L. Insights on toxicity, safe handling and disposal of silica aerogels and amorphous nanoparticles. Environ. Sci. Nano 2021, 8, 1177–1195. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R″Si(OR′)3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Bishop, J.L.; Quinn, R.; Dyar, M.D. What Lurks in the Martian Rocks and Soil? Investigations of Sulfates, Phosphates, and Perchlorates. Spectral and thermal properties of perchlorate salts and implications for Mars. Am. Mineral. 2014, 99, 1580–1592. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, M.M.; Hugenschmidt, C.; Dickmann, M.; Abdel-Hady, E.E.; Mohamed, H.F.M.; Abdel-Hamed, M.O. Crosslinked PVA/SSA proton exchange membranes: Correlation between physiochemical properties and free volume determined by positron annihilation spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 28287–28299. [Google Scholar] [CrossRef]
- Devlin, D.J.; Herley, P.J. Thermal decomposition and dehydration of sodium perchlorate monohydrate. React. Solids 1987, 3, 75–84. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Girão, A.V.; Silva, R.F.; Durães, L. Polysilsesquioxane-based silica aerogel monoliths with embedded CNTs. Microporous Mesoporous Mater. 2019, 288, 109575. [Google Scholar] [CrossRef]
- Hashim, H.; El-Mekawey, F.; El-Kashef, H.; Ghazy, R. Determination of scattering parameters of polyvinyl alcohol by static laser scattering. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 203–208. [Google Scholar] [CrossRef]
- Torres, R.B.; Vareda, J.P.; Lamy-Mendes, A.; Durães, L. Effect of different silylation agents on the properties of ambient pressure dried and supercritically dried vinyl-modified silica aerogels. J. Supercrit. Fluids 2019, 147, 81–89. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Torres, R.B.; Vareda, J.P.; Lopes, D.; Ferreira, M.; Valente, V.; Girão, A.V.; Valente, A.J.M.; Durães, L. Amine Modification of Silica Aerogels/Xerogels for Removal of Relevant Environmental Pollutants. Molecules 2019, 24, 3701. [Google Scholar] [CrossRef] [PubMed]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Silica Aerogels/Xerogels Modified with Nitrogen-Containing Groups for Heavy Metal Adsorption. Molecules 2020, 25, 2788. [Google Scholar] [CrossRef] [PubMed]
- Vareda, J.P.; Matos, P.D.; Valente, A.J.M.; Durães, L. A New Schiff Base Organically Modified Silica Aerogel-Like Material for Metal Ion Adsorption with Ni Selectivity. Adsorpt. Sci. Technol. 2022, 2022, 8237403. [Google Scholar] [CrossRef]


| Sample | Bulk Density (kg m−3) | Linear Shrinkage (%) | Porosity (%) | SBET (m2 g−1) | Vpore (cm3 g−1) | Dpore (nm) |
|---|---|---|---|---|---|---|
| AE-10-6 | 176 ± 5 | 8.1 ± 0.9 | 86.4 ± 0.4 | 321 ± 10 | 4.9 ± 0.2 | 61 ± 3 |
| AE-10-4 | 196 ± 8 | 11.5 ± 0.9 | 84.9 ± 0.7 | 339 ± 5 | 4.3 ± 0.2 | 51 ± 3 |
| AE-15-6 | 194 ± 10 | 8 ± 2 | 85.1 ± 0.8 | 104 ± 1 | 4.4 ± 0.3 | 169 ± 10 |
| AE-15-4 | 213.6 ± 0.4 | 8.6 ± 0.7 | 83.6 ± 0.0 | 233 ± 5 | 3.9 ± 0.0 | 67 ± 1 |
| Sample | σ (S cm−1) |
|---|---|
| AE-10-6 | (2.4 ± 0.4) × 10−7 |
| AE-10-4 | (3.8 ± 0.9) × 10−7 |
| AE-15-6 | (1.6 ± 0.3) × 10−6 |
| AE-15-4 | (1.1 ± 0.3) × 10−5 |
| Polymer/Ionic Liquid | Silica Form | σ (S cm−1) | Reference |
|---|---|---|---|
| Poly(ethylene oxide) | NP | 2 × 10−5 | [38] |
| Poly(ethylene glycol) grafted silica | NP | 2 × 10−5 | [39] |
| Poly(ethylene oxide) | NP | 7 × 10−4 | [40] |
| Poly (ethylene glycol)-co-ureidopyrimidinone | NP | 2 × 10−5 | [32] |
| Poly(ethylene glycol) | NP | 9 × 10−5 | [33] |
| Poly(methyl methacrylate) | NP | 3 × 10−3 | [41] |
| Poly(vinylidene fluoride-co-hexafluoropropylene) | NP | 4 × 10−3 | [30] |
| Poly(vinylidene fluoride-co-hexafluoropropylene) | NP | 7 × 10−4 | [34] |
| Poly(vinylidene fluoride-co-hexafluoropropylene) | NP | 8 × 10−4 | [35] |
| Poly(vinyl alcohol) | NP | 4 × 10−3 | [37] |
| Poly(ethylene oxide) + 1-butyl-3-methyl-imidazolium thiocyanate | NP | 1 × 10−4 | [31] |
| 1-ethyl-3-methylimidazolium bis(fluorosulfonyl)imide | aerogel | 5 × 10−3 | [50] |
| 1-Butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide | aerogel | 7 × 10−4 | [51] |
| Poly(vinylidene fluoride-co-hexafluoropropylene) | aerogel | 9 × 10−4 | [52] |
| 1-ethyl-3-methylimidazolium bis(fluorosulfonyl)imide + Poly(ethylene oxide) 1 | aerogel | 2 × 10−3 | [36] |
| Sample | Silica Mixture | Polymer Mass 1 (%) | Polymer:Na-Salt 2 |
|---|---|---|---|
| AE-10-6 | 85% MTES 15% TEOS | 10 | 6:1 |
| AE-10-4 | 10 | 4:1 | |
| AE-15-6 | 15 | 6:1 | |
| AE-15-4 | 15 | 4:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vareda, J.P.; Fonseca, A.C.; Ribeiro, A.C.F.; Pontinha, A.D.R. Silica-Poly(Vinyl Alcohol) Composite Aerogel: A Promising Electrolyte for Solid-State Sodium Batteries. Gels 2024, 10, 293. https://doi.org/10.3390/gels10050293
Vareda JP, Fonseca AC, Ribeiro ACF, Pontinha ADR. Silica-Poly(Vinyl Alcohol) Composite Aerogel: A Promising Electrolyte for Solid-State Sodium Batteries. Gels. 2024; 10(5):293. https://doi.org/10.3390/gels10050293
Chicago/Turabian StyleVareda, João Pedro, Ana Clotilde Fonseca, Ana Cristina Faria Ribeiro, and Ana Dora Rodrigues Pontinha. 2024. "Silica-Poly(Vinyl Alcohol) Composite Aerogel: A Promising Electrolyte for Solid-State Sodium Batteries" Gels 10, no. 5: 293. https://doi.org/10.3390/gels10050293
APA StyleVareda, J. P., Fonseca, A. C., Ribeiro, A. C. F., & Pontinha, A. D. R. (2024). Silica-Poly(Vinyl Alcohol) Composite Aerogel: A Promising Electrolyte for Solid-State Sodium Batteries. Gels, 10(5), 293. https://doi.org/10.3390/gels10050293