TiO2–Alginate–Chitosan-Based Composites for Skin Tissue Engineering Applications
Abstract
:1. Introduction
2. Results and Discussion
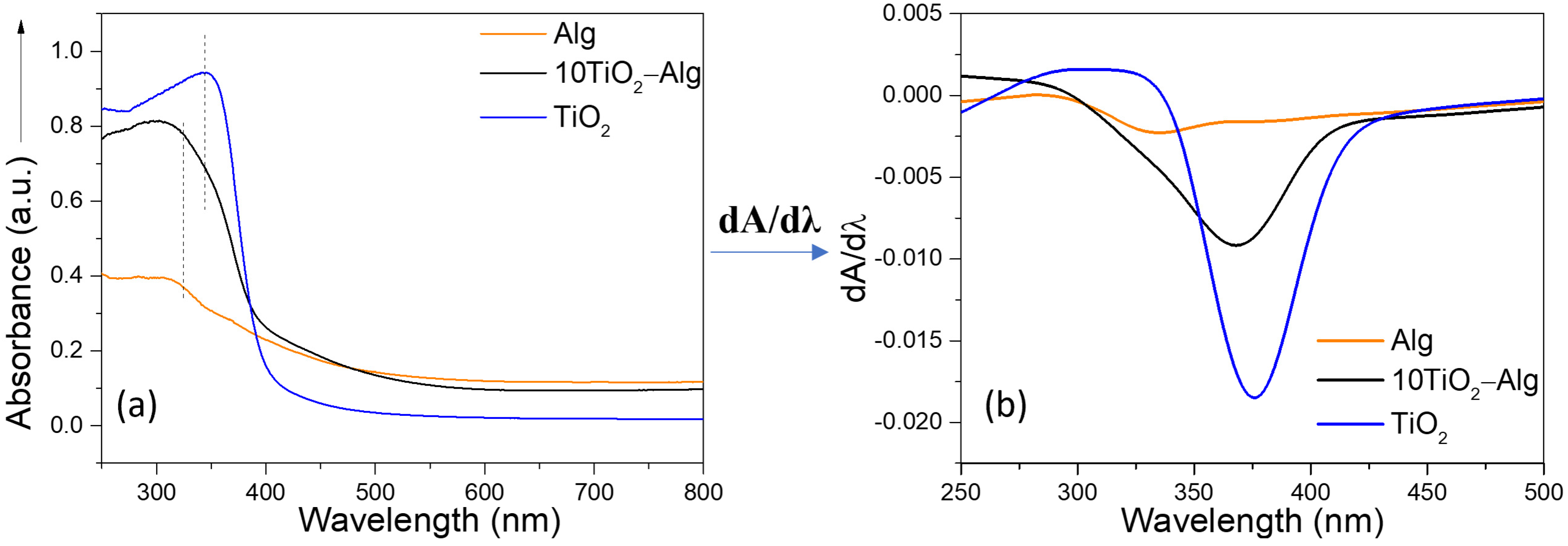
2.1. TiO2 Particle Characteristics
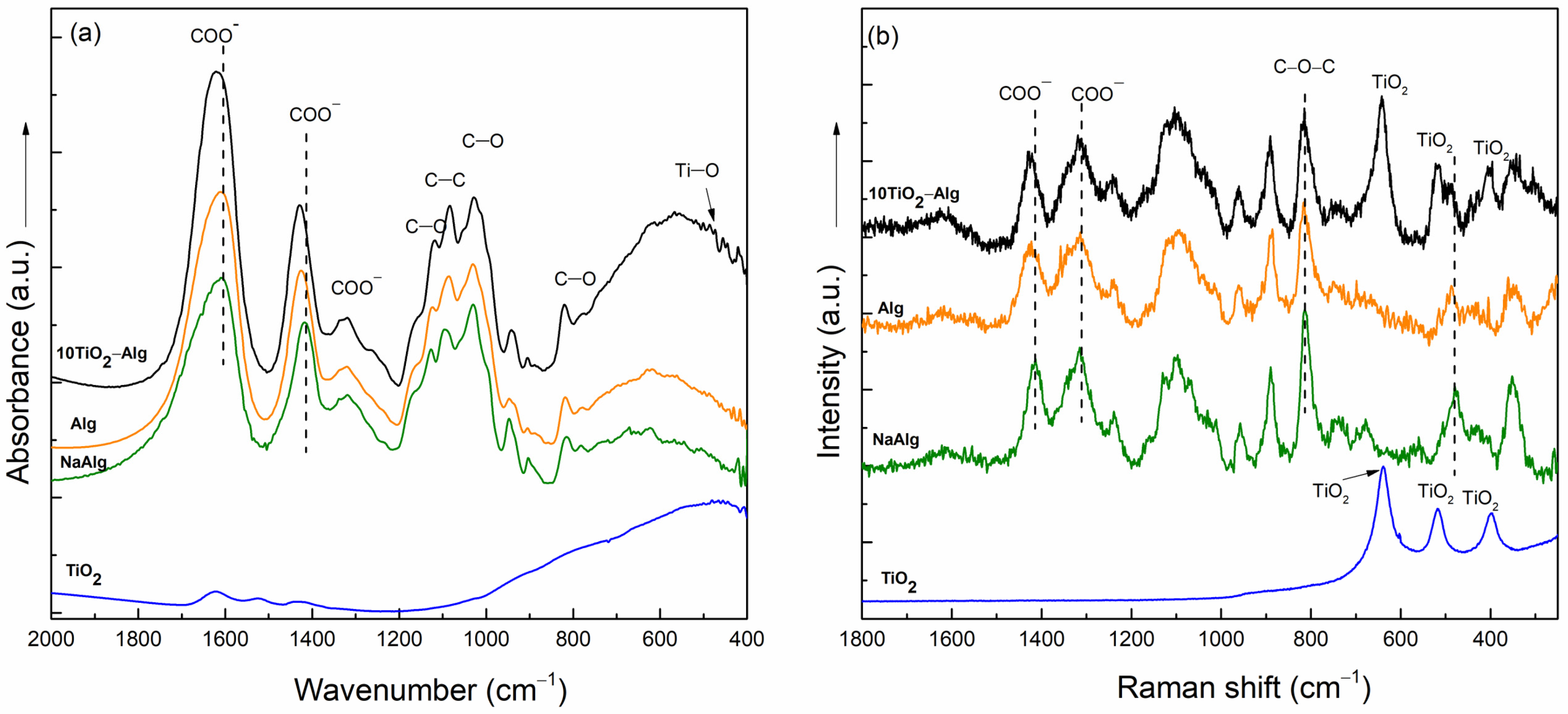
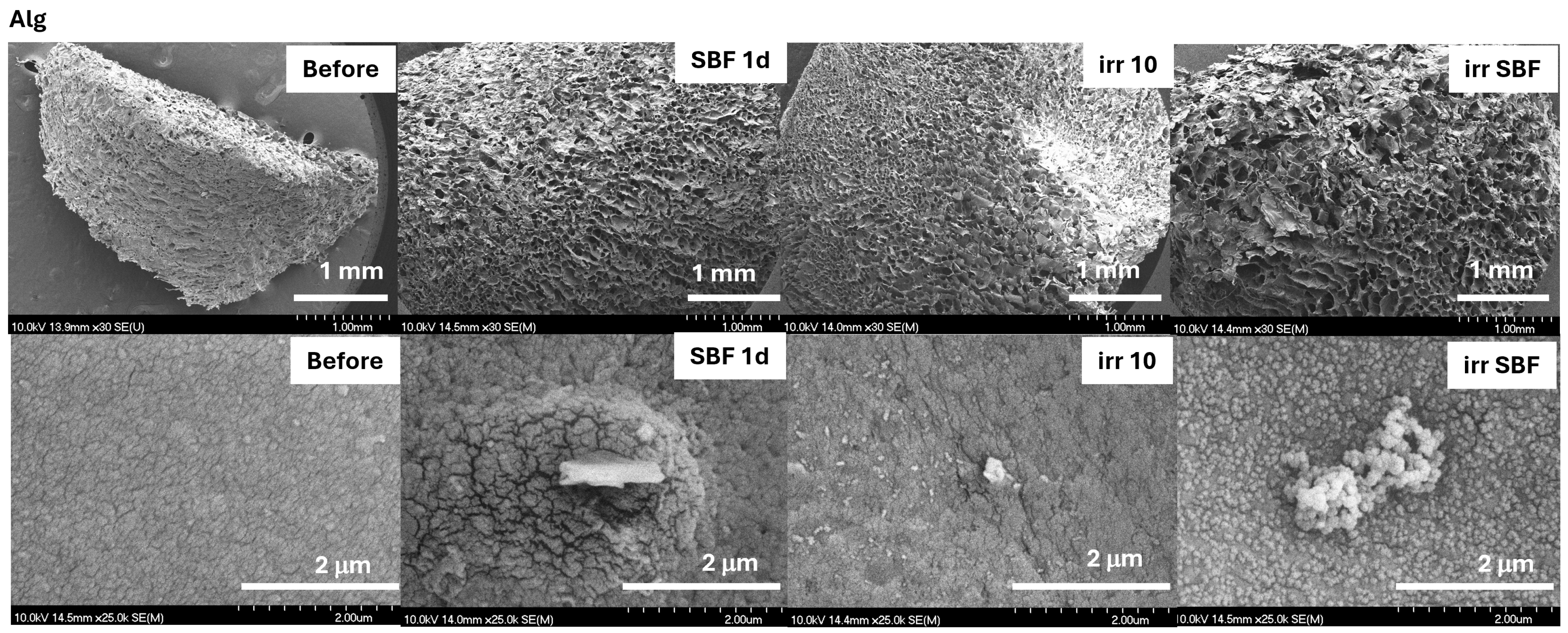
2.2. Evaluation of the TiO2–Alginate Composite Behavior
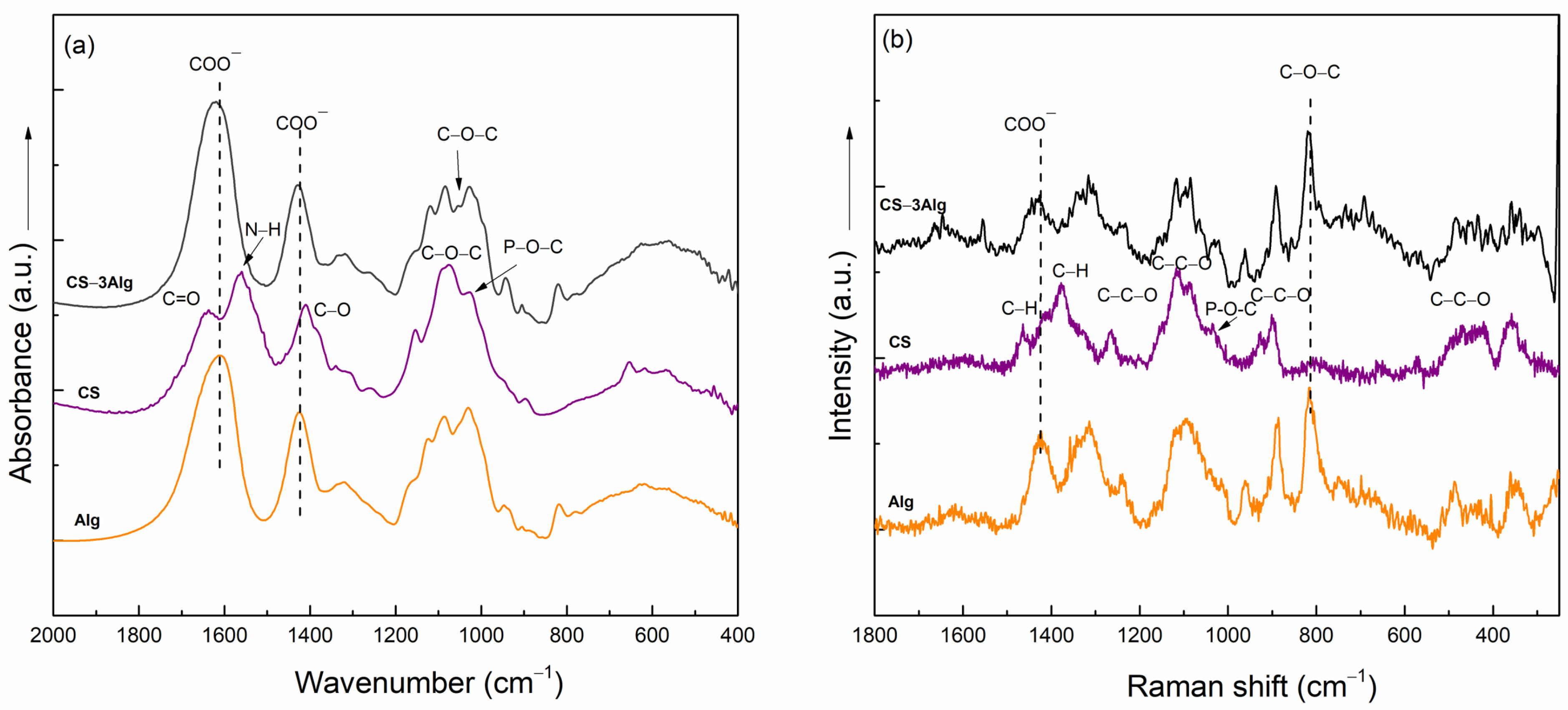
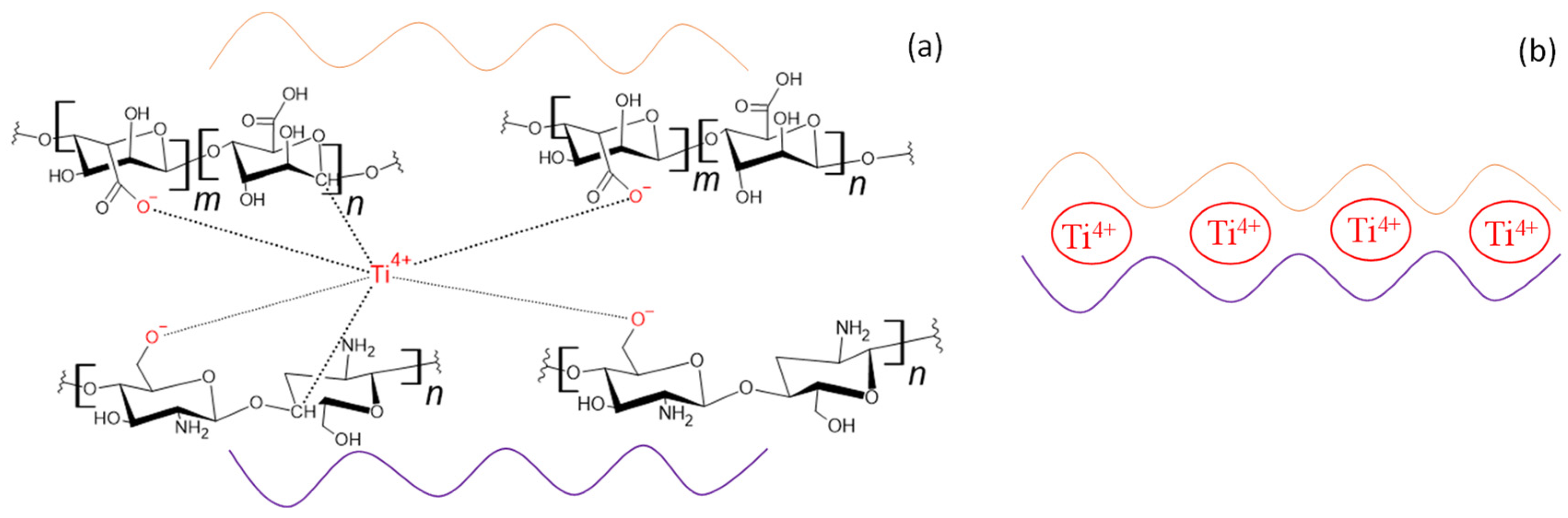
2.2.1. Cross-Linking of the TiO2–Alginate Composites
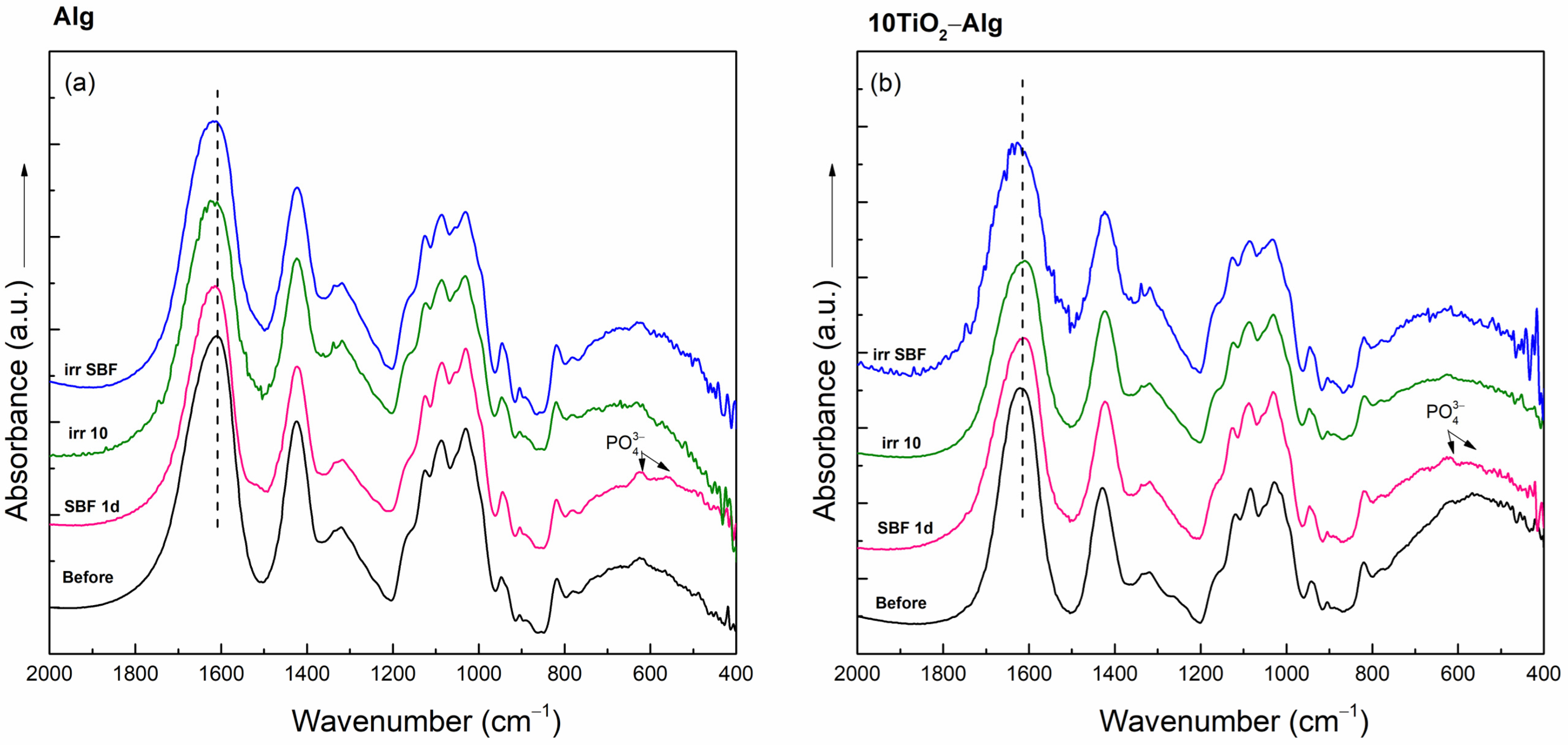
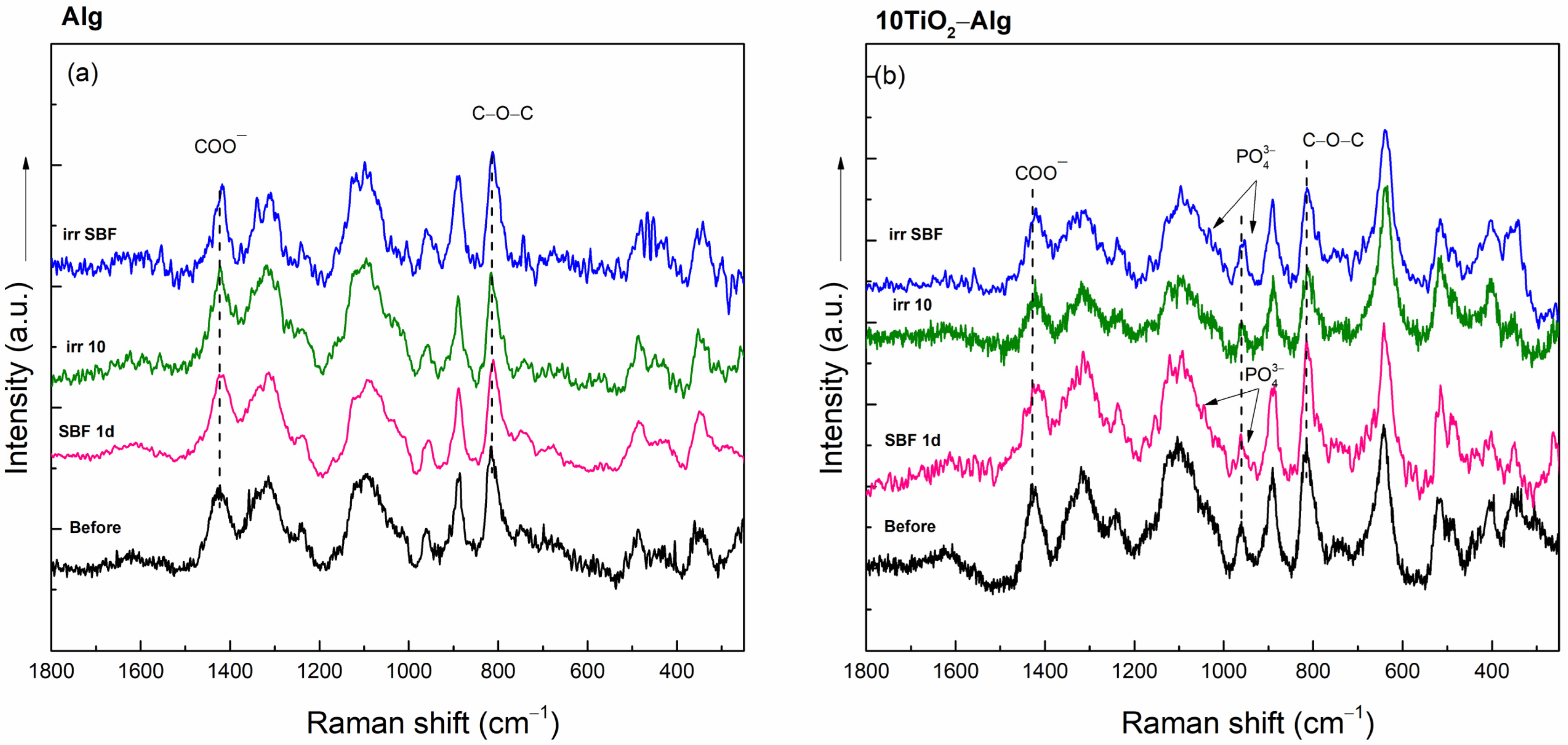
2.2.2. In Vitro Assays of the TiO2–Alginate Composites
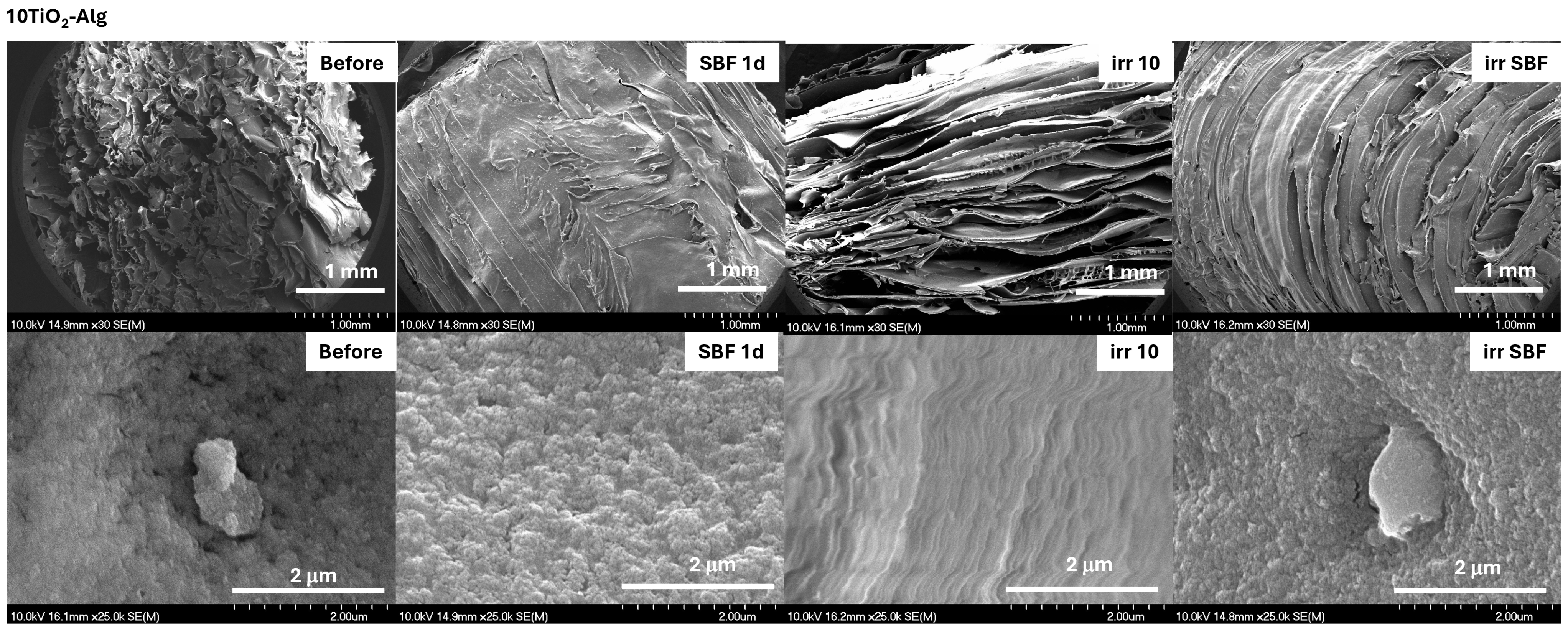
2.3. TiO2–Chitosan–Alginate Composites
2.3.1. Cross-Linking of the TiO2–Chitosan–Alginate Composites
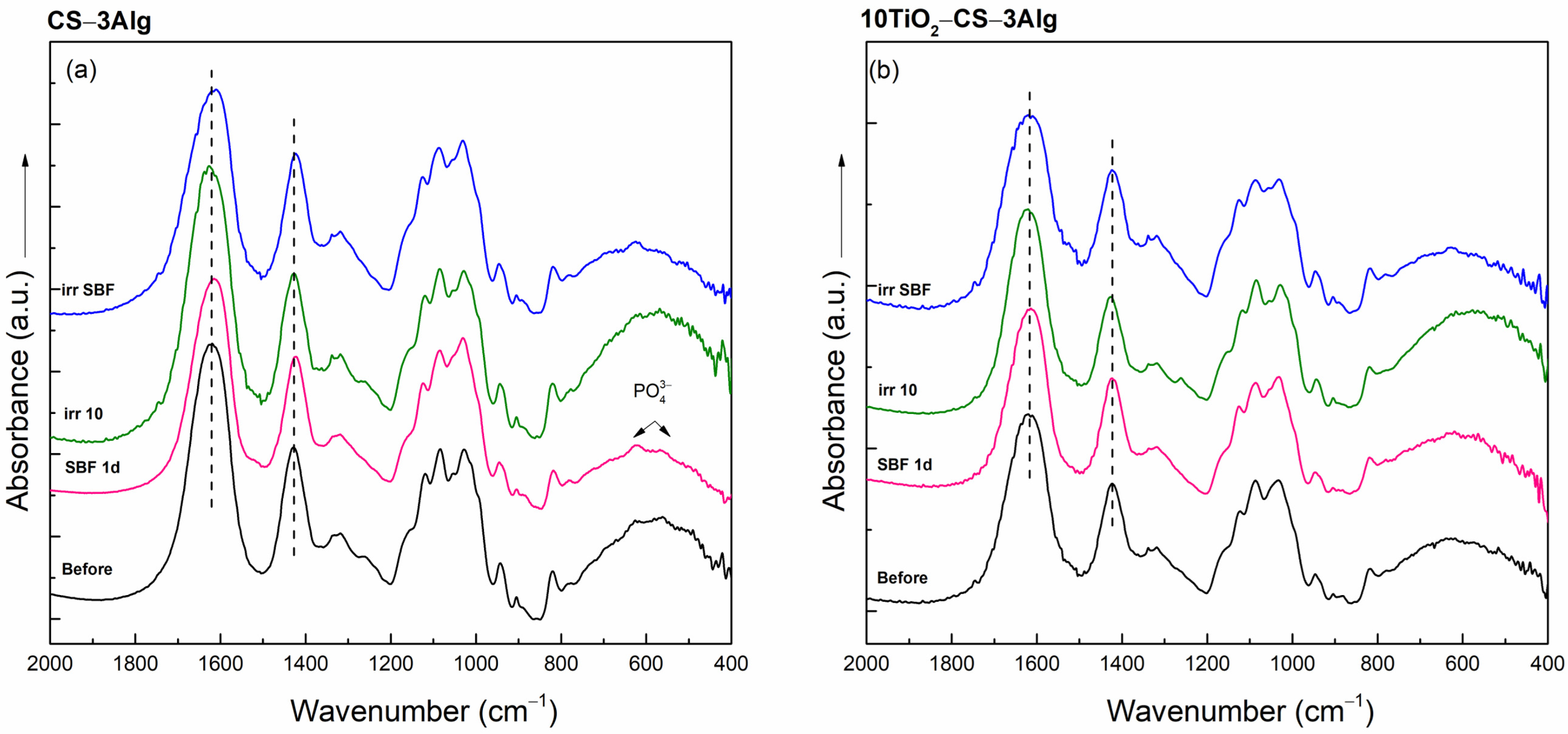
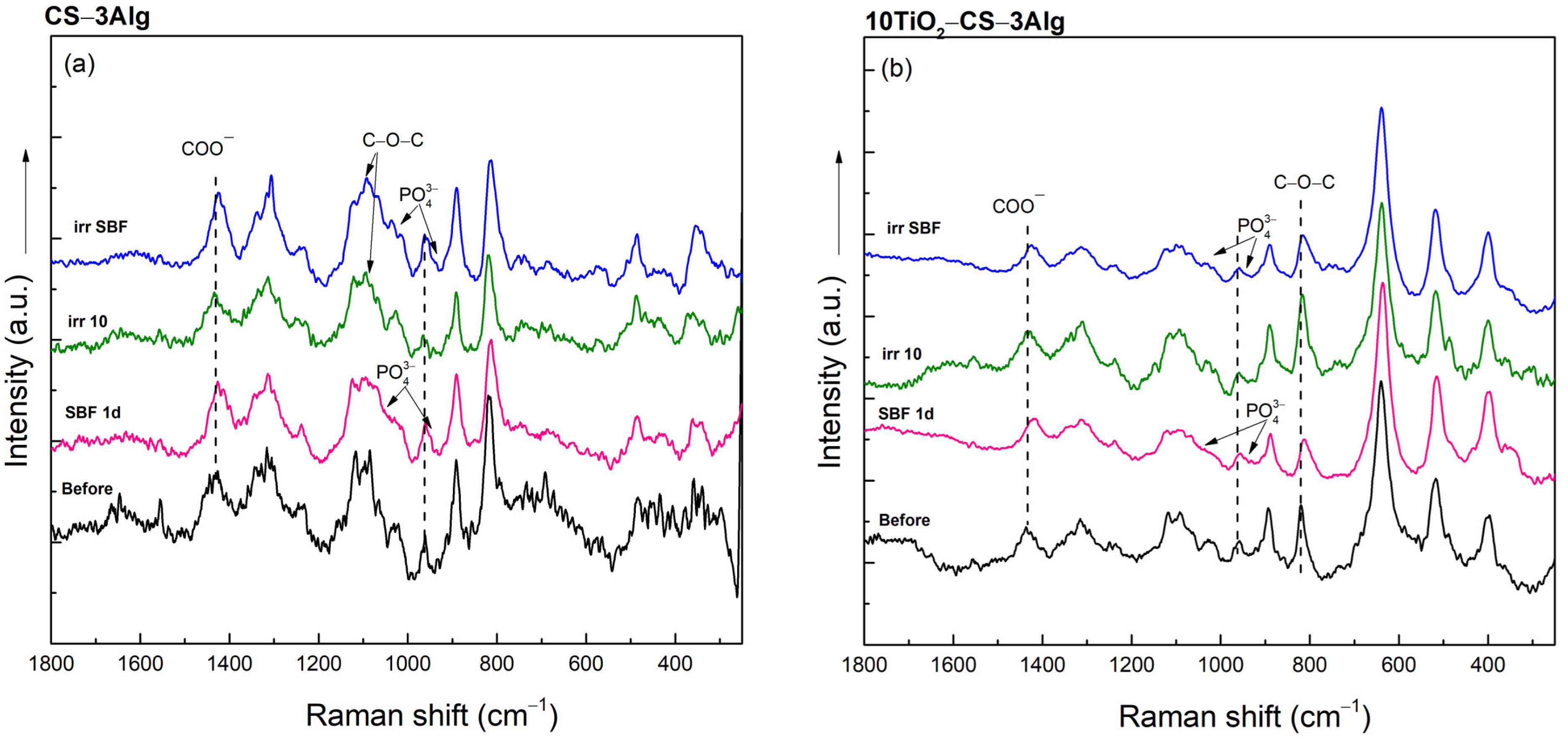
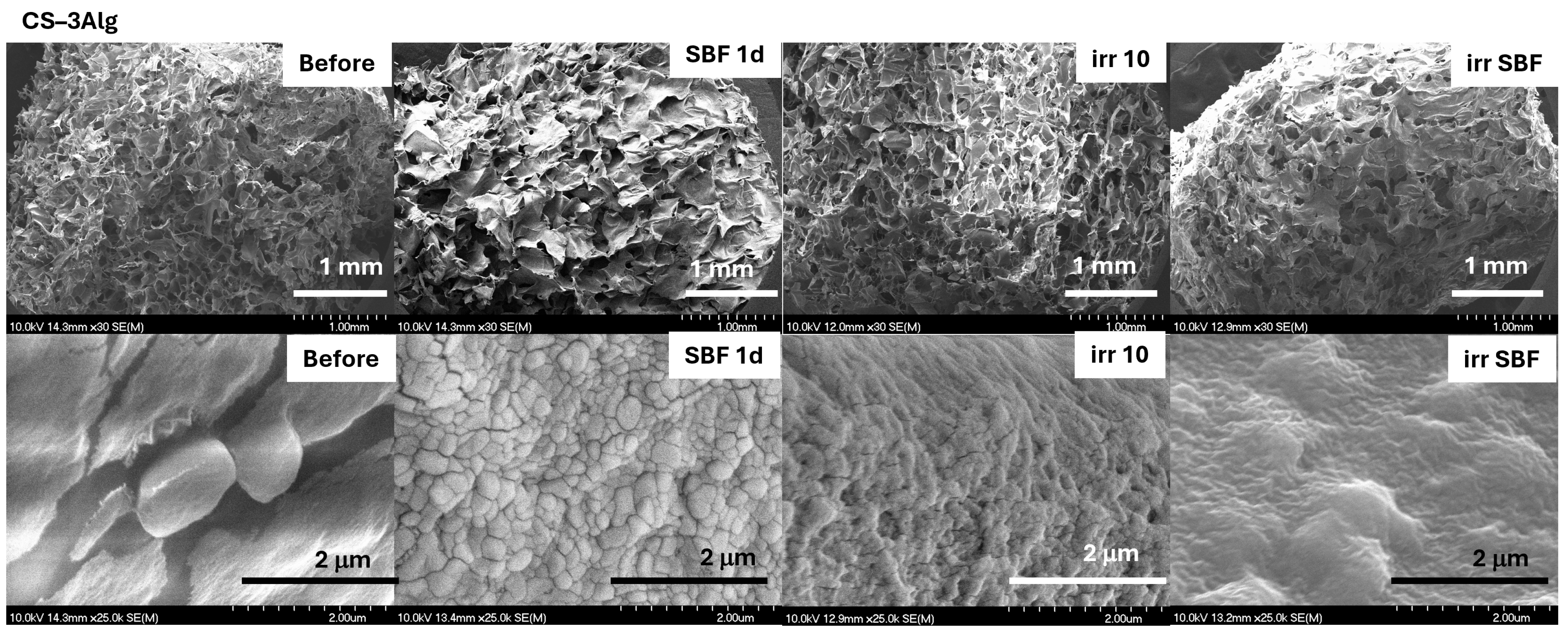
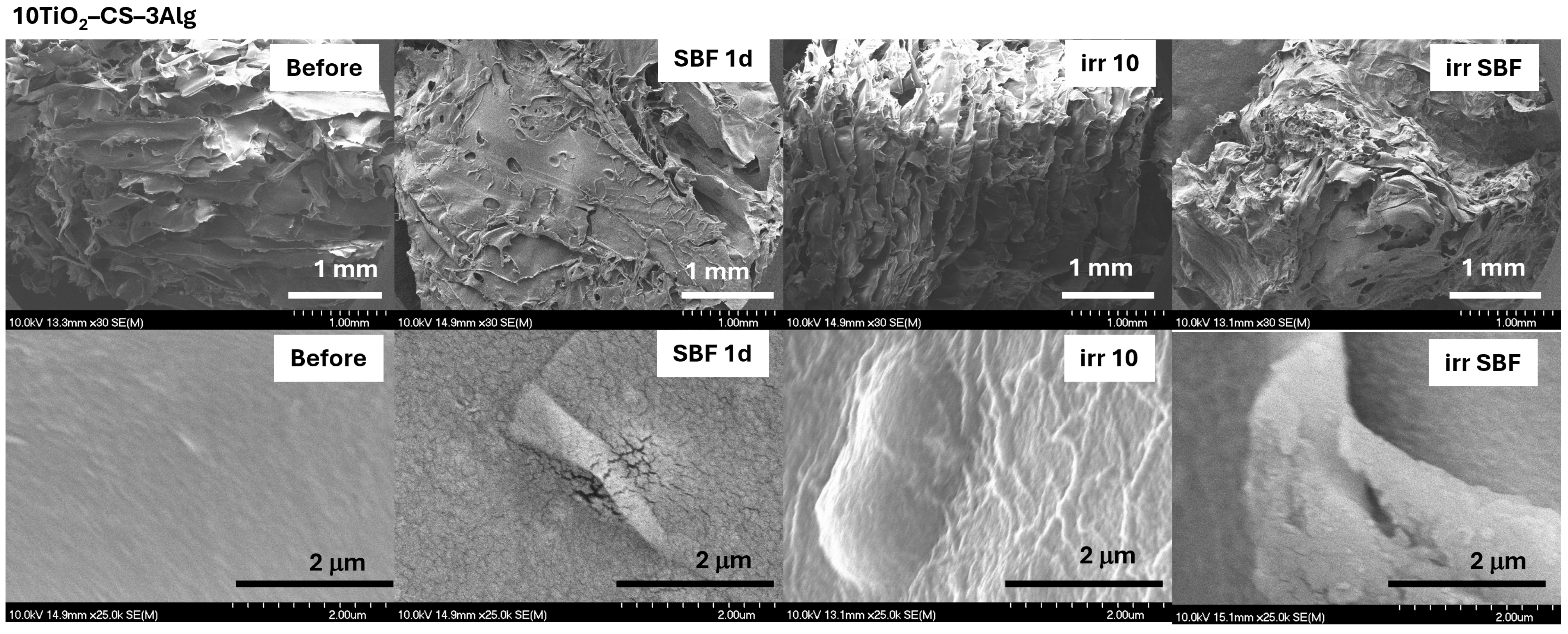
2.3.2. In Vitro Assays of the TiO2–Chitosan–Alginate Composites
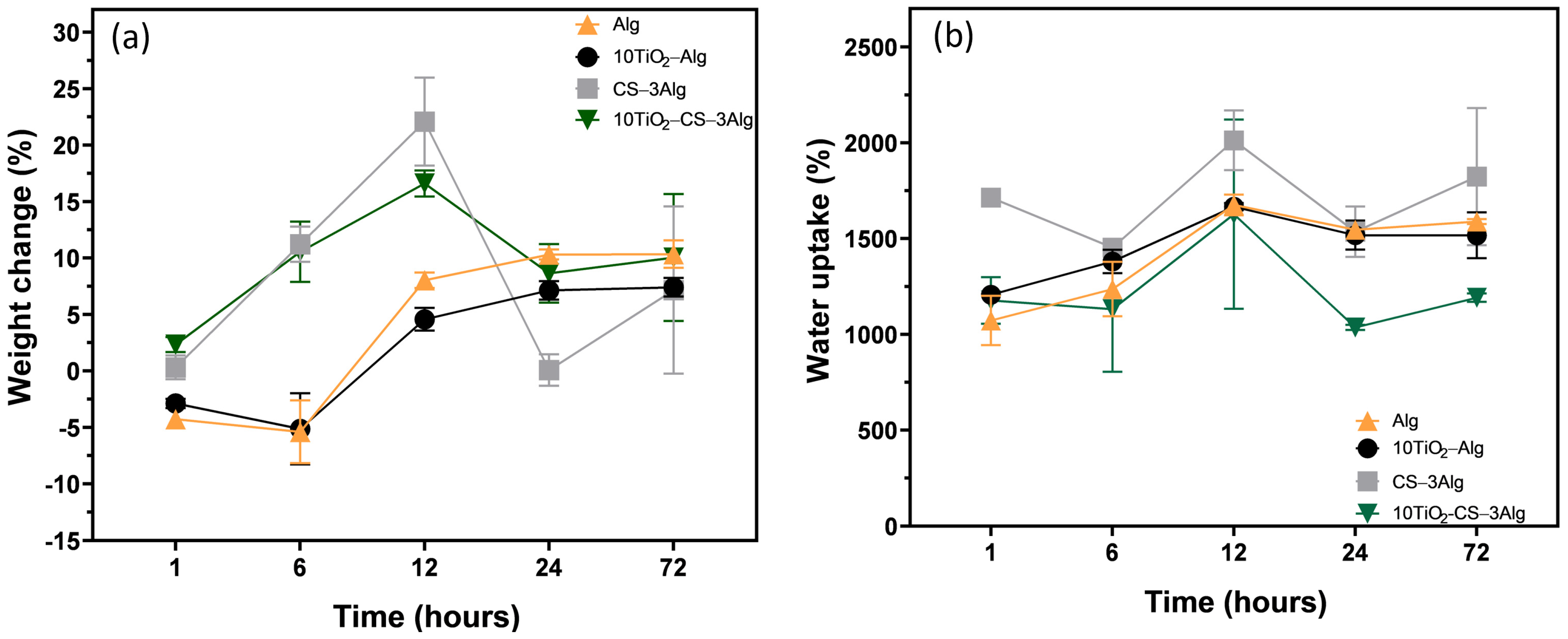
2.4. Porosity, Biodegradation, and Swelling Ratio of the Composites
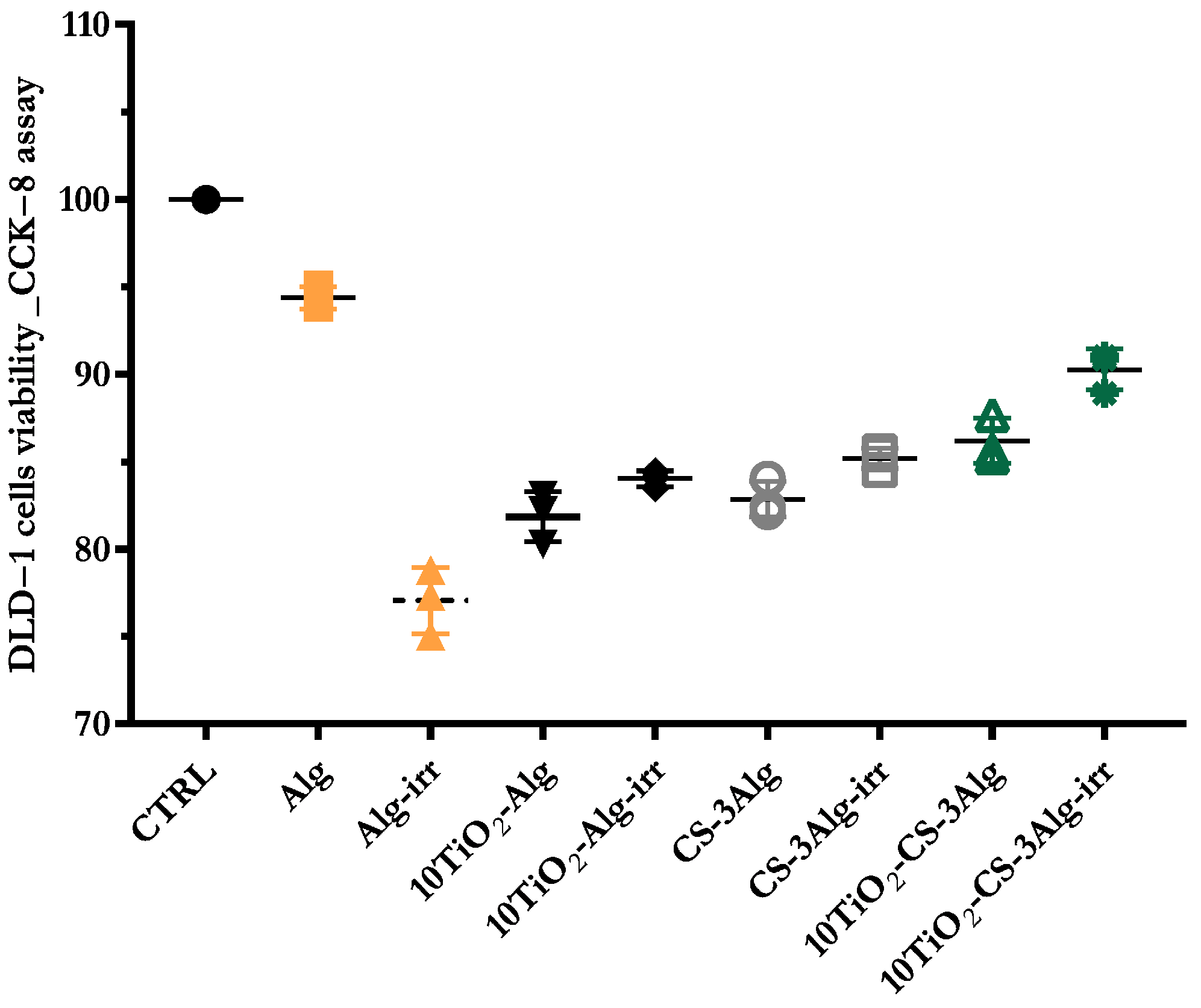
2.5. Cell Viability Evaluation of the Composites
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Composite Synthesis
4.2.1. Synthesis of the TiO2 Particles
4.2.2. Synthesis of the Composites
4.3. Investigation Methods
4.4. Porosity, Biodegradation, and Swelling Ratio Measurements
4.5. In Vitro Assays
4.5.1. In Vitro Bioactivity Assay
4.5.2. In Vitro Stability under Sunlight Irradiation
4.5.3. In Vitro Cell Viability Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kolarsick, P.A.J.; Kolarsick, M.A.; Goodwin, C. Anatomy and Physiology of the Skin. J. Dermatol. Nurses Assoc. 2011, 3, 203–213. [Google Scholar] [CrossRef]
- Wang, P.-W.; Hung, Y.-C.; Lin, T.-Y.; Fang, J.-Y.; Yang, P.-M.; Chen, M.-H.; Pan, T.-L. Comparison of the Biological Impact of UVA and UVB upon the Skin with Functional Proteomics and Immunohistochemistry. Antioxidants 2019, 8, 569. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Mead, M.N. Benefits of Sunlight: A Bright Spot for Human Health. Env. Health Perspect. 2008, 116, 160–167. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; la Mora, Z.V.-D.; Ruvalcaba-Gómez, J.M.; Romero-Toledo, R.; Sandoval-Contreras, T.; Aguilera-Aguirre, S.; Montalvo-González, E.; Pérez-Larios, A. Use of Titanium Dioxide (TiO2) Nanoparticles as Reinforcement Agent of Polysaccharide-Based Materials. Processes 2020, 8, 1395. [Google Scholar] [CrossRef]
- Lin, C.-C.; Lin, W.-J. Sun protection factor analysis of sunscreens containing titanium dioxide nanoparticles. J. Food Drug Anal. 2011, 19, 5. [Google Scholar] [CrossRef]
- Pulvin, S. Toxicity Associated with the Photo Catalytic and Photo Stable Forms of Titanium Dioxide Nanoparticles Used in Sunscreen lotion. MOJ Toxicol. 2015, 1, 1–18. [Google Scholar] [CrossRef]
- Jacobs, J.F.; van de Poel, I.; Osseweijer, P. Sunscreens with Titanium Dioxide (TiO2) Nano-Particles: A Societal Experiment. Nanoethics 2010, 4, 103–113. [Google Scholar] [CrossRef]
- Racovita, A.D. Titanium Dioxide: Structure, Impact, and Toxicity. Int. J. Environ. Res. Public Health 2022, 19, 5681. [Google Scholar] [CrossRef]
- Limsakul, S.; Mahatnirunkul, T.; Phromma, C.; Chomtong, T.; Cholnakasem, N.; Yimklan, S.; Ruankham, P.; Siyasukh, A.; Chimupala, Y. Novel physical sunscreen from one-dimensional TiO2 nanowire: Synthesis, characterization and the effects of morphologies and particle size for use as a physical sunscreen. Nano-Struct. Nano-Objects 2023, 35, 101027. [Google Scholar] [CrossRef]
- Aderibigbe, B.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Froelich, A.; Jakubowska, E.; Wojtyłko, M.; Jadach, B.; Gackowski, M.; Gadziński, P.; Napierała, O.; Ravliv, Y.; Osmałek, T. Alginate-Based Materials Loaded with Nanoparticles in Wound Healing. Pharmaceutics 2023, 15, 1142. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Chitosan: A Promising Multifunctional Cosmetic Ingredient for Skin and Hair Care. Cosmetics 2022, 9, 99. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Tan, Y.-M.; Allen, D.G.; Bell, S.; Brown, P.C.; Browning, L.; Ceger, P.; Gearhart, J.; Hakkinen, P.J.; Kabadi, S.V.; et al. IVIVE: Facilitating the Use of In Vitro Toxicity Data in Risk Assessment and Decision Making. Toxics 2022, 10, 232. [Google Scholar] [CrossRef]
- Goh, J.-Y.; Weaver, R.J.; Dixon, L.; Platt, N.J.; Roberts, R.A. Development and use of in vitro alternatives to animal testing by the pharmaceutical industry 1980–2013. Toxicol. Res. 2015, 4, 1297–1307. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Pekruhn, W.; Germer, R. Solar simulator. Sol. Energy 1980, 25, 381–383. [Google Scholar] [CrossRef]
- Bernauer, U.; Bodin, L.; Celleno, L.; Chaudhry, Q.; Coenraads, P.J.; Dusinska, M.; Galli, C.L.; Panderi, I.; Rousselle, C.; Stepnik, M.; et al. OPINION ON Titanium Dioxide (Nano Form) as UV-Filter in Dermally Applied Cosmetic Products 2016. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_202.pdf (accessed on 19 November 2023).
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Xiao, Q.; Lu, K.; Tong, Q.; Liu, C. Barrier Properties and Microstructure of Pullulan-Alginate-Based Films. J. Food Process. Eng. 2015, 38, 155–161. [Google Scholar] [CrossRef]
- Dreanca, A.; Muresan-Pop, M.; Taulescu, M.; Toth, Z.-R.; Bogdan, S.; Pestean, C.; Oren, S.; Toma, C.; Popescu, A.; Pall, E.; et al. Bioactive glass-biopolymers-gold nanoparticle based composites for tissue engineering applications. Mater. Sci. Eng. C 2021, 123, 112006. [Google Scholar] [CrossRef] [PubMed]
- Schmid, T.; Messmer, A.; Yeo, B.-S.; Zhang, W.; Zenobi, R. Towards chemical analysis of nanostructures in biofilms II: Tip-enhanced Raman spectroscopy of alginates. Anal. Bioanal. Chem. 2008, 391, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- El-Deen, S.S.; Hashem, A.M.; Ghany, A.E.A.; Indris, S.; Ehrenberg, H.; Mauger, A.; Julien, C.M. Anatase TiO2 nanoparticles for lithium-ion batteries. Ionics 2018, 24, 2925–2934. [Google Scholar] [CrossRef]
- Urbanova, M.; Pavelkova, M.; Czernek, J.; Kubova, K.; Vyslouzil, J.; Pechova, A.; Molinkova, D.; Vyslouzil, J.; Vetchy, D.; Brus, J. Interaction Pathways and Structure–Chemical Transformations of Alginate Gels in Physiological Environments. Biomacromolecules 2019, 20, 4158–4170. [Google Scholar] [CrossRef] [PubMed]
- Magyari, K.; Dreancă, A.; Székely, I.; Popescu, A.; Feraru, A.; Páll, E.; Gyulavári, T.; Suciu, M.; Cenariu, M.; Bobu, E.; et al. How does the structure of pullulan alginate composites change in the biological environment? J. Mater. Sci. 2022, 57, 19050–19067. [Google Scholar] [CrossRef]
- Magyari, K.; One, R.; Tódor, I.; Baia, M.; Simon, V.; Simon, S.; Baia, L. Titania effect on the bioactivity of silicate bioactive glasses. J. Raman Spectrosc. 2016, 47, 1102–1108. [Google Scholar] [CrossRef]
- Stammeier, J.A.; Purgstaller, B.; Hippler, D.; Mavromatis, V.; Dietzel, M. In-situ Raman spectroscopy of amorphous calcium phosphate to crystalline hydroxyapatite transformation. MethodsX 2018, 5, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, K.; Wu, C.; Liu, X.; Chang, J. Preparation of hierarchical enamel-like structures from nano- to macro-scale, regulated by inorganic templates derived from enamel. J. Mater. Chem. B 2015, 3, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Magyari, K.; Baia, L.; Vulpoi, A.; Simon, S.; Popescu, O.; Simon, V. Bioactivity evolution of the surface functionalized bioactive glasses. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Mohammadi, N. Development of sodium alginate-pectin/TiO2 nanocomposites: Antibacterial and bioactivity investigations. Carbohydr. Polym. 2022, 285, 119226. [Google Scholar] [CrossRef]
- Shanmugam, B.K.; Rangaraj, S.; Subramani, K.; Srinivasan, S.; Aicher, W.K.; Venkatachalam, R. Biomimetic TiO2-chitosan/sodium alginate blended nanocomposite scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2020, 110, 110710. [Google Scholar] [CrossRef] [PubMed]
- Panyamao, P.; Ruksiriwanich, W.; Sirisa-ard, P.; Charumanee, S. Injectable Thermosensitive Chitosan/Pullulan-Based Hydrogels with Improved Mechanical Properties and Swelling Capacity. Polymers 2020, 12, 2514. [Google Scholar] [CrossRef] [PubMed]
- Scalzone, A.; Ferreira, A.M.; Tonda-Turo, C.; Ciardelli, G.; Dalgarno, K.; Gentile, P. The interplay between chondrocyte spheroids and mesenchymal stem cells boosts cartilage regeneration within a 3D natural-based hydrogel. Sci. Rep. 2019, 9, 14630. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.F.; Melo, K.; Sabry, D.; Sassaki, G.; Rocha, H. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2014, 13, 141–158. [Google Scholar] [CrossRef]
- Zając, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Meng, Y.; Luo, C.; Liu, Z.; Li, D.; Li, J. Ti4+-dopamine/sodium alginate multicomponent complex derived N-doped TiO2@carbon nanocomposites for efficient removal of methylene blue. Int. J. Biol. Macromol. 2023, 243, 125200. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobu, E.; Saszet, K.; Tóth, Z.-R.; Páll, E.; Gyulavári, T.; Baia, L.; Magyari, K.; Baia, M. TiO2–Alginate–Chitosan-Based Composites for Skin Tissue Engineering Applications. Gels 2024, 10, 358. https://doi.org/10.3390/gels10060358
Bobu E, Saszet K, Tóth Z-R, Páll E, Gyulavári T, Baia L, Magyari K, Baia M. TiO2–Alginate–Chitosan-Based Composites for Skin Tissue Engineering Applications. Gels. 2024; 10(6):358. https://doi.org/10.3390/gels10060358
Chicago/Turabian StyleBobu, Emma, Kata Saszet, Zsejke-Réka Tóth, Emőke Páll, Tamás Gyulavári, Lucian Baia, Klara Magyari, and Monica Baia. 2024. "TiO2–Alginate–Chitosan-Based Composites for Skin Tissue Engineering Applications" Gels 10, no. 6: 358. https://doi.org/10.3390/gels10060358




