Lubricating Polymer Gels/Coatings: Syntheses and Measurement Strategies
Abstract
1. Introduction
2. Measurement Strategies for Lubrication
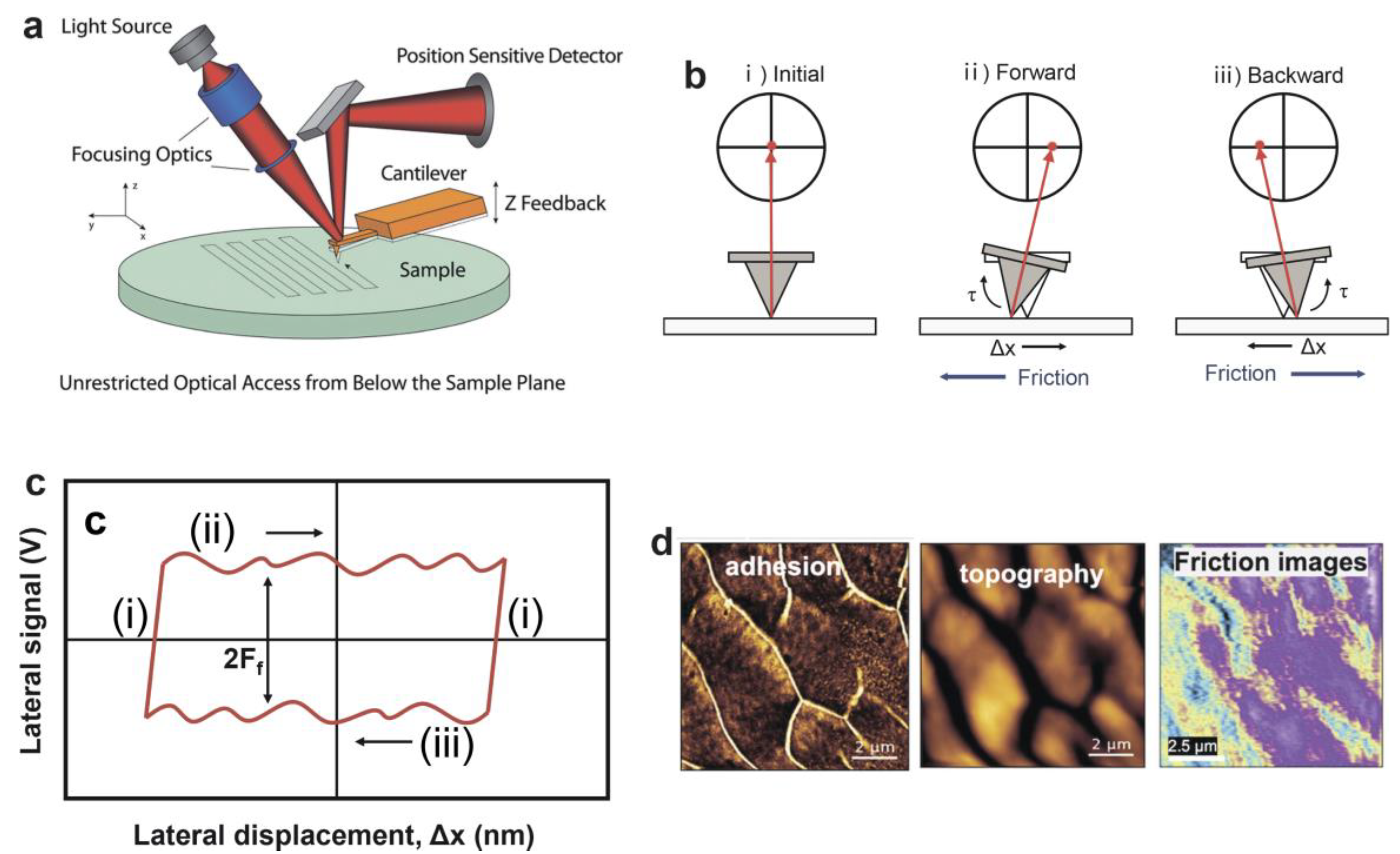
2.1. Surface Force Balance (SFB)
2.2. Atomic Force Microscopy (AFM)
2.3. Tribometer
3. Chemical Processes Utilized to Synthesize Lubricating Polymeric Materials
| Polymer-Based Lubricants | Synthesis Methods | Measurements | µ | Ref. |
|---|---|---|---|---|
| Homo-oligomeric phosphocholinated micelles (OMDPC) | FRP | SFB | 0.002 ± 0.001 | [36] |
| PC lipids/pHEMA hydrogels | FRP | Tribometer | ≈0.01 | [35] |
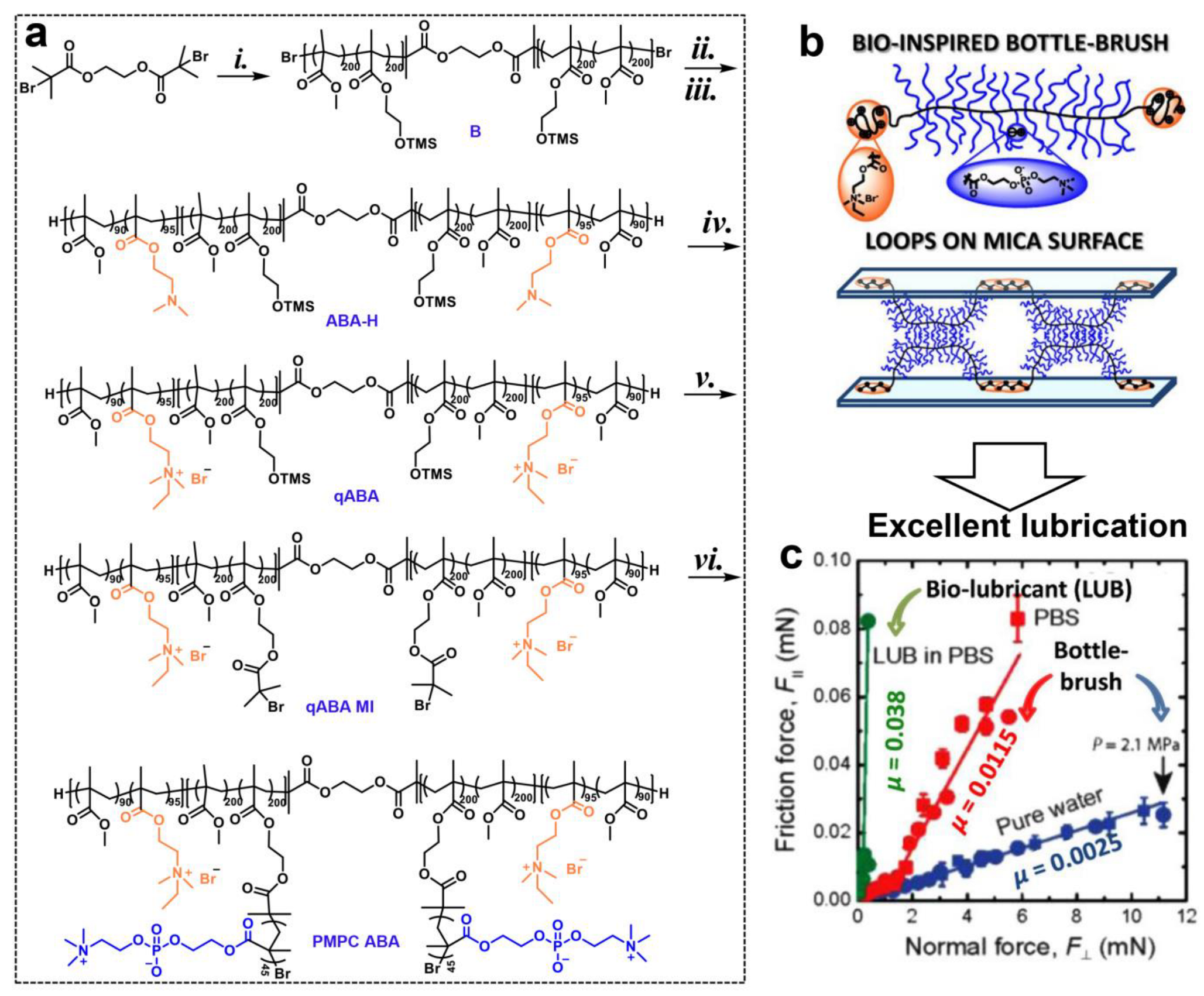
| Lubricin mimicking ABA bottle-brush polymer | ATRP | SFB | 0.0025 in pure water and 0.0115 in PBS | [37] |
| Agarose/poly(acrylamide-co-acrylic acid) | FRP | AFM | ~0.01 to 0.02 | [32] |
| Bottle-brush polymer (BPHEMA) | RAFT | Tribometer | ~0.3 | [38] |
| P(EO-co-AGE)-b-PEO-b-P(EO-co-AGE) triblock copolymers | Click chemistry | SFB | 0.002 ± 0.001 | [39] |
| Silk fibroin/PAAm/PVA hydrogel | Supramolecular assembly | Tribometer | ~0.08 | [40] |
| β-CD-PMPC polymer | Supramolecular assembly | Tribometer | 0.024–0.028 | [41] |
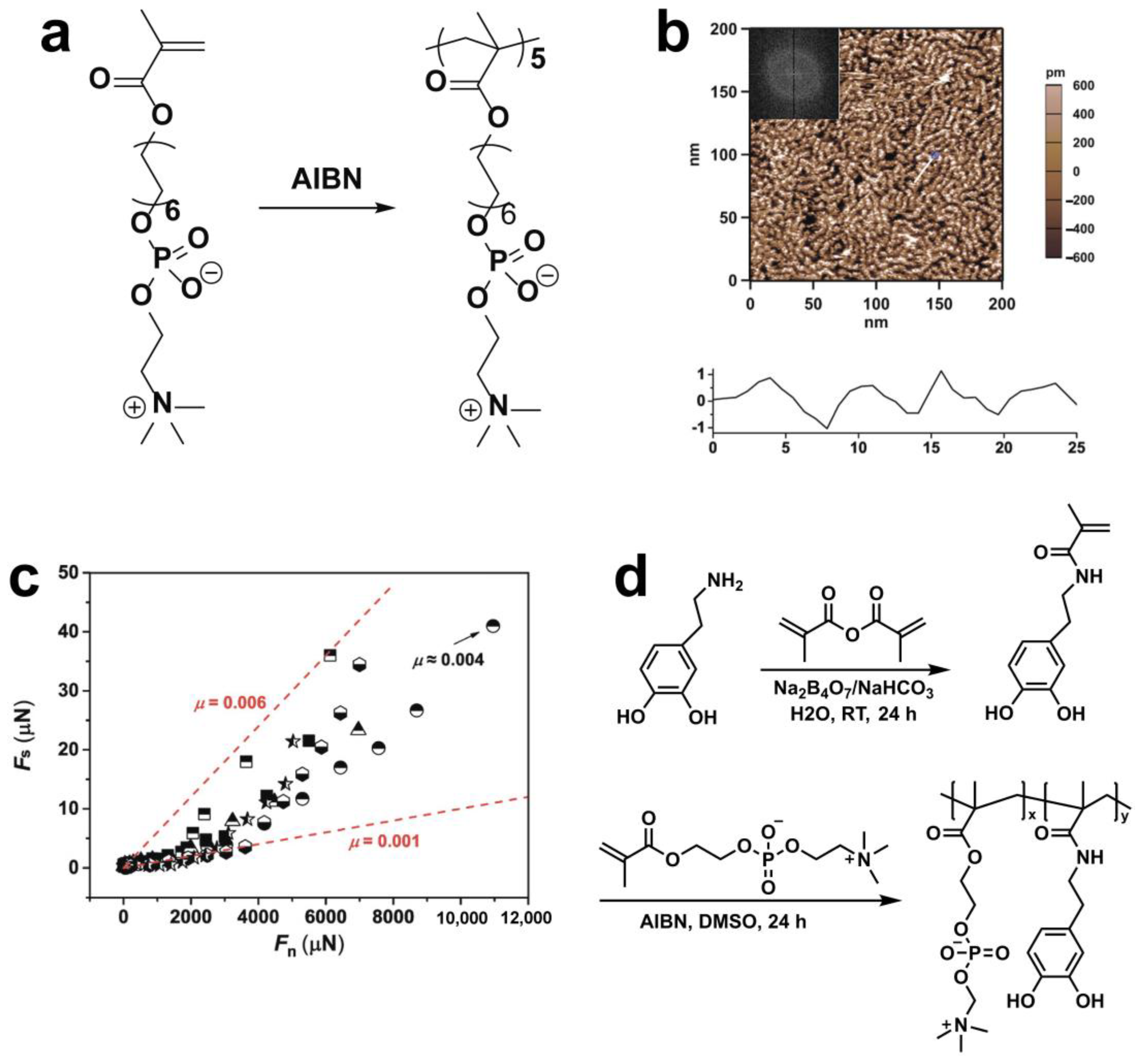
3.1. Free Radical Polymerization (FRP)
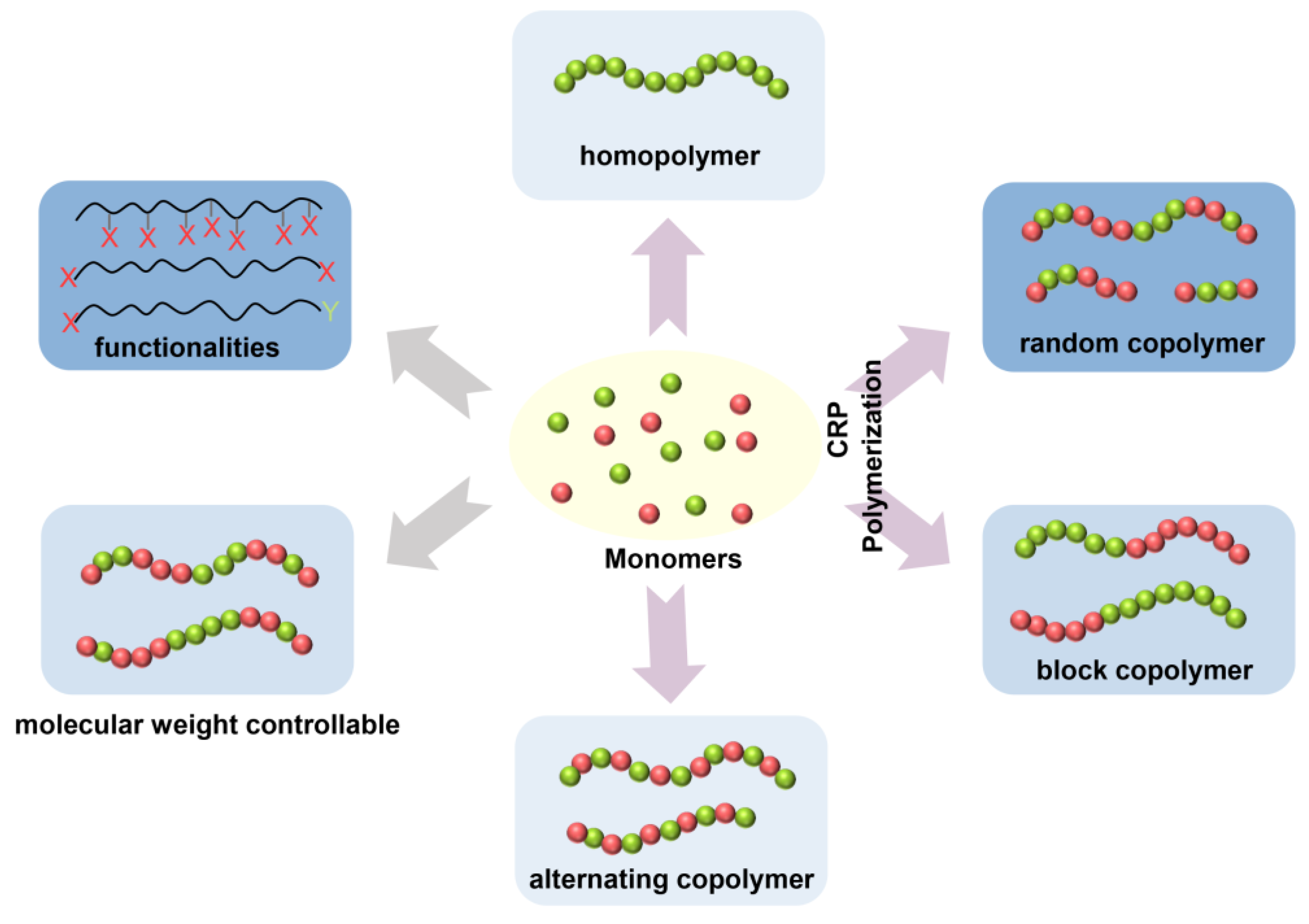
3.2. Controlled/Living Radical Polymerization (CRP)
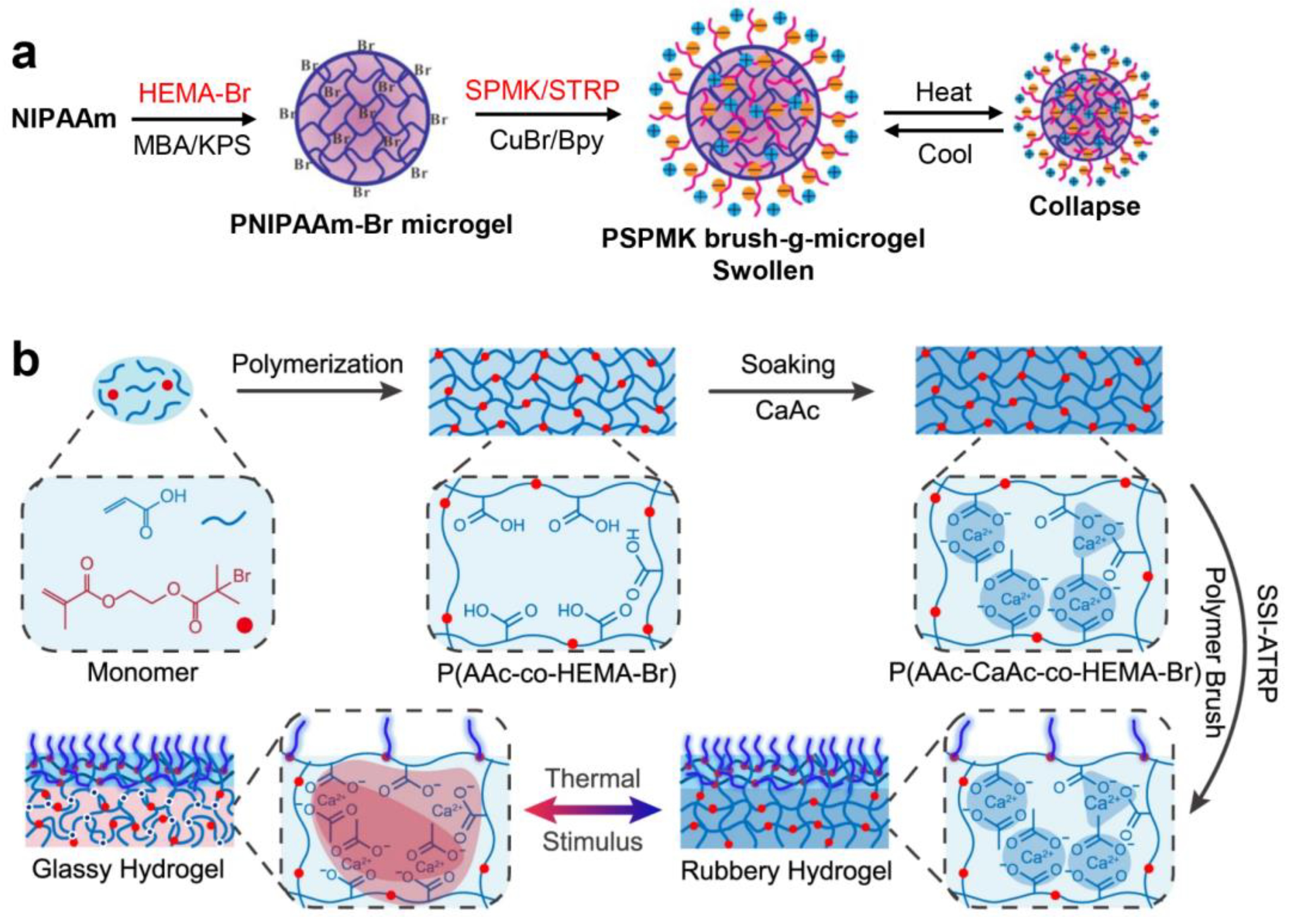
3.2.1. Atom Transfer Radical Polymerization (ATRP)
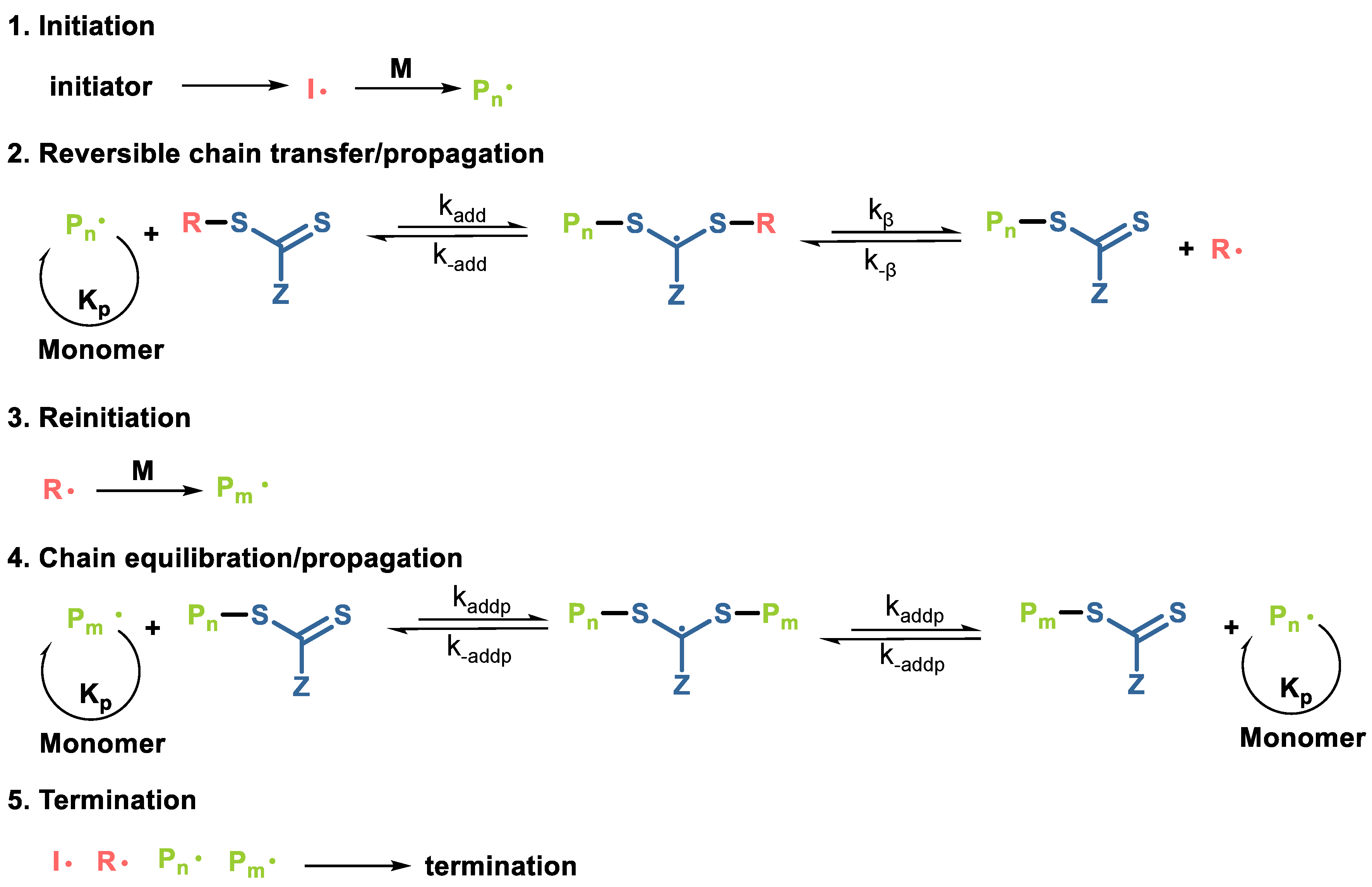
3.2.2. Reversible Addition-Fragmentation Chain-Transfer (RAFT)
3.3. Click Chemistry
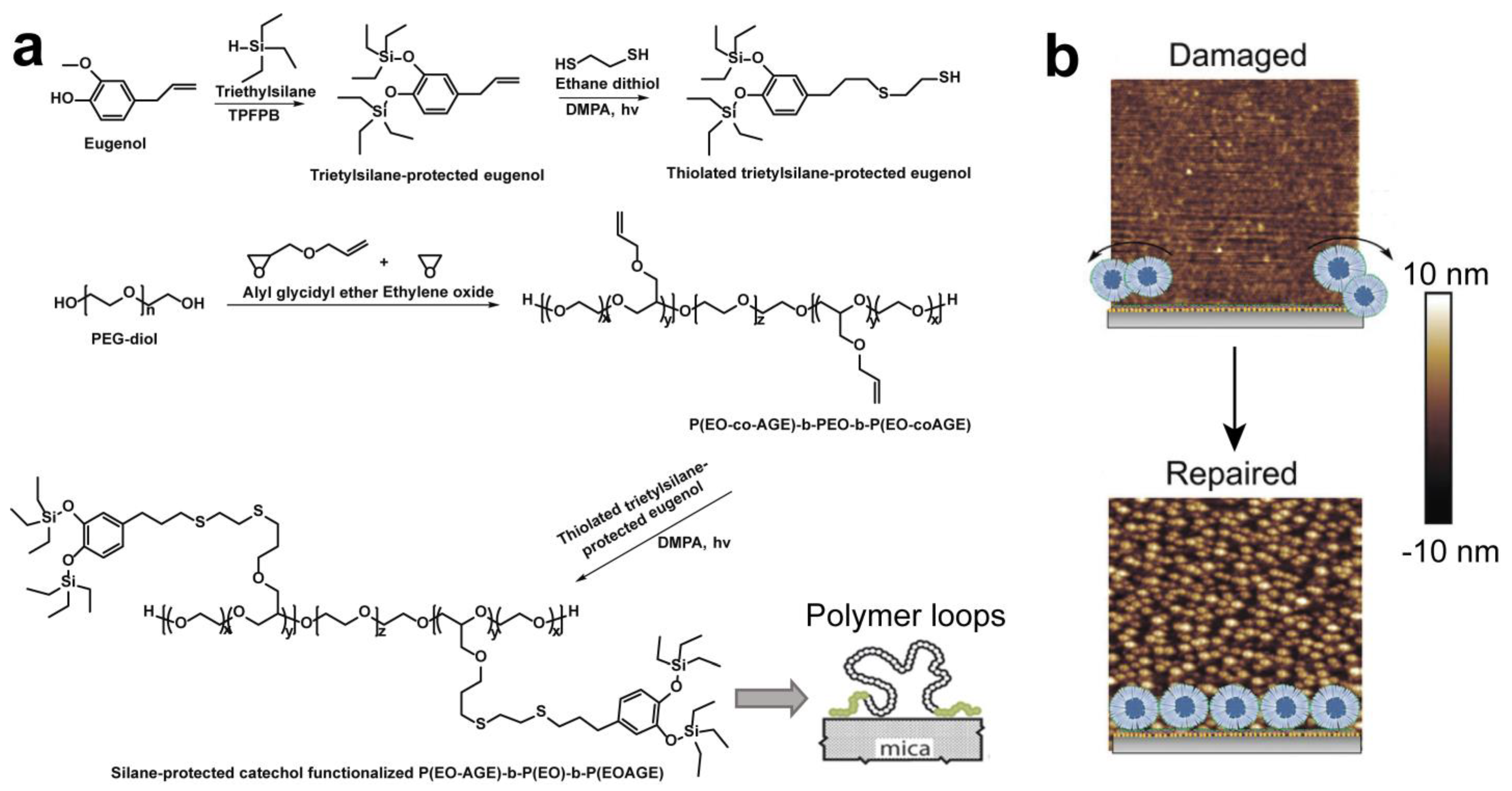
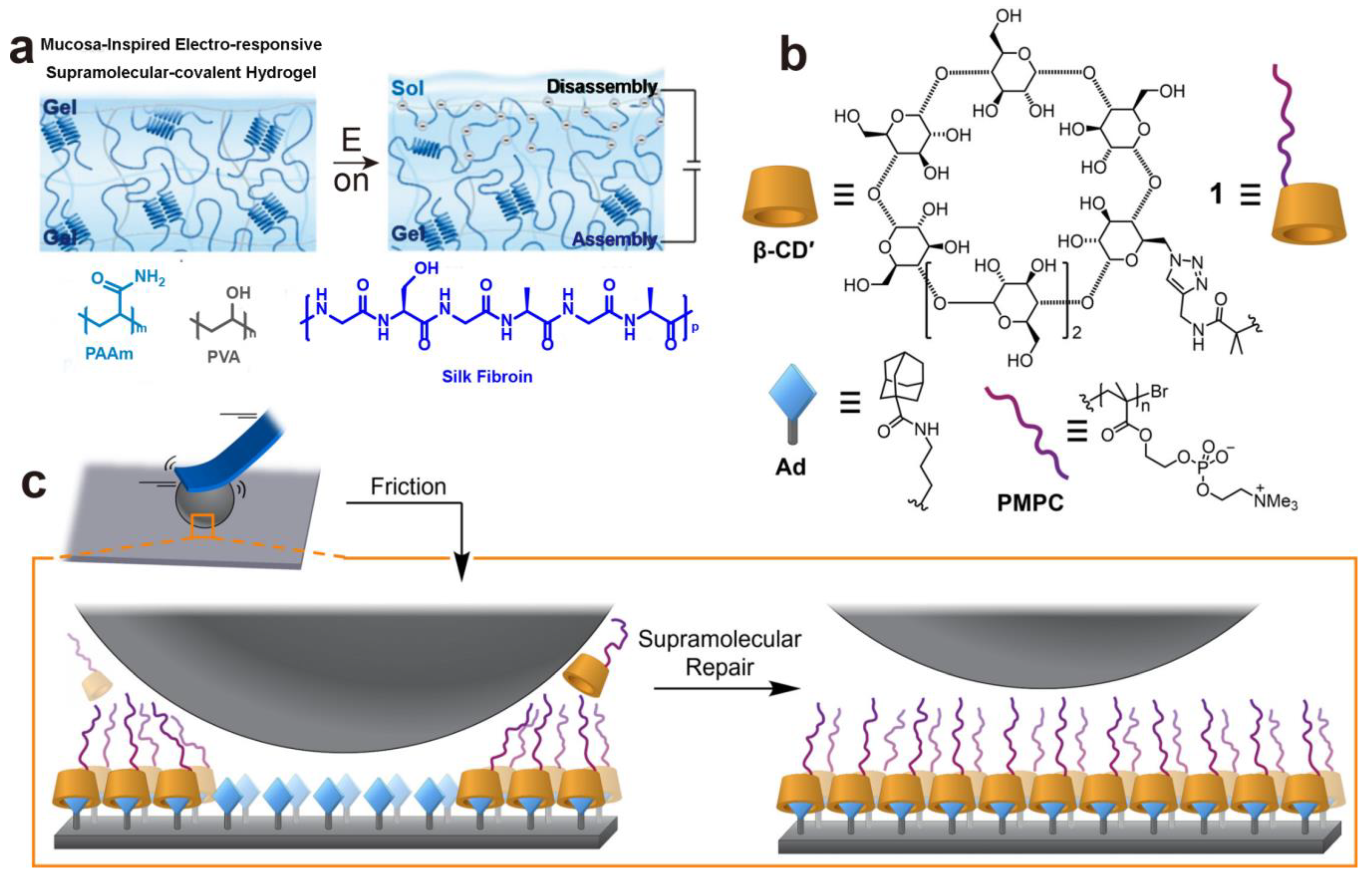
4. Supramolecular Assembly
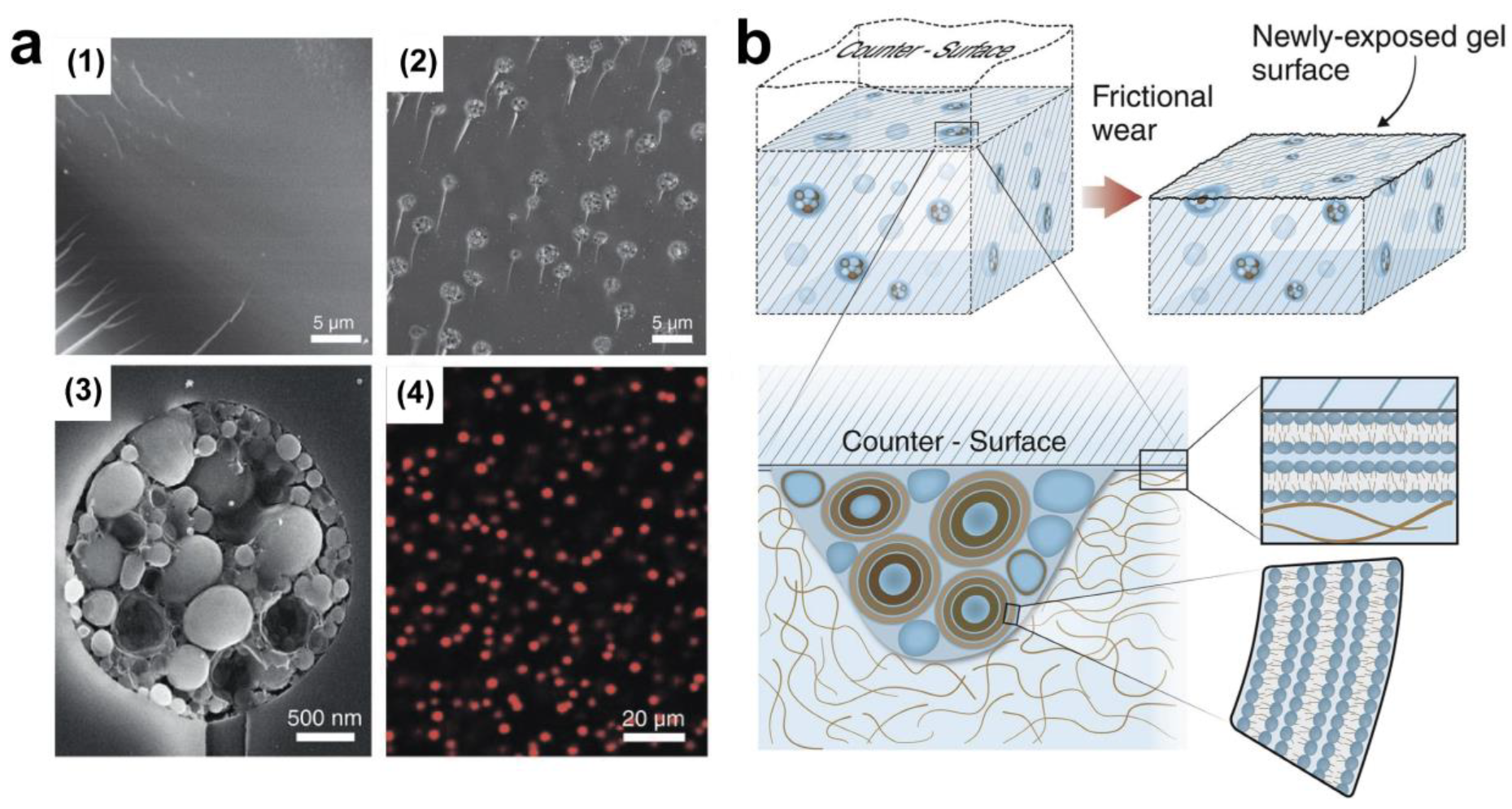
5. Lubrication Mechanism of Polymer-Based Lubricants
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, W.; Klein, J. Hydration Lubrication in Biomedical Applications: From Cartilage to Hydrogels. Acc. Mater. Res. 2022, 3, 213–223. [Google Scholar] [CrossRef]
- Seror, J.; Zhu, L.; Goldberg, R.; Day, A.J.; Klein, J. Supramolecular synergy in the boundary lubrication of synovial joints. Nat. Commun. 2015, 6, 6497. [Google Scholar] [CrossRef]
- Wu, W.; Liu, J.; Li, Z.; Zhao, X.; Liu, G.; Liu, S.; Ma, S.; Li, W.; Liu, W. Surface-functionalized nanoMOFs in oil for friction and wear reduction and antioxidation. Chem. Eng. J. 2021, 410, 128306. [Google Scholar] [CrossRef]
- Wang, T.; He, B.; Xue, S.; Chen, X.; Liu, S.; Ye, Q.; Zhou, F.; Liu, W. Supramolecular gelator functionalized liquid metal nanodroplets as lubricant additive for friction reduction and anti-wear. J. Colloid Interface Sci. 2024, 653, 258–266. [Google Scholar] [CrossRef]
- Rapoport, L.; Bilik, Y.; Feldman, Y.; Homyonfer, M.; Cohen, S.R.; Tenne, R. Hollow nanoparticles of WS2 aspotential solid-state lubricants. Nature 1997, 387, 791–793. [Google Scholar] [CrossRef]
- Torres, O.; Andablo-Reyes, E.; Murray, B.S.; Sarkar, A. Emulsion Microgel Particles as High-Performance Bio-Lubricants. ACS Appl. Mater. Interfaces 2018, 10, 26893–26905. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Z.; Li, N.; Wang, X.; Zhou, F.; Liu, W. Hairy polyelectrolyte brushes-grafted thermosensitive microgels as artificial synovial fluid for simultaneous biomimetic lubrication and arthritis treatment. ACS Appl. Mater. Interfaces 2014, 6, 20452–20463. [Google Scholar] [CrossRef]
- Stolte, S.; Steudte, S.; Areitioaurtena, O.; Pagano, F.; Thoming, J.; Stepnowski, P.; Igartua, A. Ionic liquids as lubricants or lubrication additives: An ecotoxicity and biodegradability assessment. Chemosphere 2012, 89, 1135–1141. [Google Scholar] [CrossRef]
- Qu, M.; Liu, H.; Yan, C.; Ma, S.; Cai, M.; Ma, Z.; Zhou, F. Layered Hydrogel with Controllable Surface Dissociation for Durable Lubrication. Chem. Mater. 2020, 32, 7805–7813. [Google Scholar] [CrossRef]
- Yan, F.; Hu, L.; Ji, Z.; Lyu, Y.; Chen, S.; Xu, L.; Hao, J. Highly Interfacial Active Gemini Surfactants as Simple and Versatile Emulsifiers for Stabilizing, Lubricating and Structuring Liquids. Angew. Chem. Int. Ed. 2024, 63, e202318926. [Google Scholar] [CrossRef]
- Yu, Y.; Yuk, H.; Parada, G.A.; Wu, Y.; Liu, X.; Nabzdyk, C.S.; Youcef-Toumi, K.; Zang, J.; Zhao, X. Multifunctional “Hydrogel Skins” on Diverse Polymers with Arbitrary Shapes. Adv. Mater. 2019, 31, e1807101. [Google Scholar] [CrossRef]
- Faivre, J.; Montembault, A.; Sudre, G.; Shrestha, B.R.; Xie, G.; Matyjaszewski, K.; Benayoun, S.; Banquy, X.; Delair, T.; David, L. Lubrication and Wear Protection of Micro-Structured Hydrogels Using Bioinspired Fluids. Biomacromolecules 2019, 20, 326–335. [Google Scholar] [CrossRef]
- Han, L.; Xiang, L.; Zhang, J.; Chen, J.; Liu, J.; Yan, B.; Zeng, H. Biomimetic Lubrication and Surface Interactions of Dopamine-Assisted Zwitterionic Polyelectrolyte Coatings. Langmuir 2018, 34, 11593–11601. [Google Scholar] [CrossRef]
- Adibnia, V.; Mirbagheri, M.; Faivre, J.; Robert, J.; Lee, J.; Matyjaszewski, K.; Lee, D.W.; Banquy, X. Bioinspired polymers for lubrication and wear resistance. Prog. Polym. Sci. 2020, 110, 101298. [Google Scholar] [CrossRef]
- Murad Bhayo, A.; Yang, Y.; He, X. Polymer brushes: Synthesis, characterization, properties and applications. Prog. Mater Sci. 2022, 130, 101000. [Google Scholar] [CrossRef]
- Palivan, C.G.; Goers, R.; Najer, A.; Zhang, X.; Car, A.; Meier, W. Bioinspired polymer vesicles and membranes for biological and medical applications. Chem. Soc. Rev. 2016, 45, 377–411. [Google Scholar] [CrossRef]
- Singh, A.; Corvelli, M.; Unterman, S.A.; Wepasnick, K.A.; McDonnell, P.; Elisseeff, J.H. Enhanced lubrication on tissue and biomaterial surfaces through peptide-mediated binding of hyaluronic acid. Nat. Mater. 2014, 13, 988–995. [Google Scholar] [CrossRef]
- Lin, W.; Klein, J. Recent Progress in Cartilage Lubrication. Adv. Mater. 2021, 33, e2005513. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, X.; Liao, J.; Shen, J.; Li, Y.; Cai, Z.; Hu, N.; Luo, X.; Cui, W.; Huang, W. Shear-responsive boundary-lubricated hydrogels attenuate osteoarthritis. Bioact. Mater. 2022, 16, 472–484. [Google Scholar] [CrossRef]
- Jacobson, B. The Stribeck memorial lecture. Tribol. Int. 2003, 36, 781–789. [Google Scholar] [CrossRef]
- Hersey, M.D. The laws of lubrication of horizontal journal bearings. J. Wash. Acad. Sci. 1914, 4, 542–552. [Google Scholar]
- Hsu, S.M.; Gates, R.S. Boundary lubricating films: Formation and lubrication mechanism. Tribol. Int. 2005, 38, 305–312. [Google Scholar] [CrossRef]
- Sorkin, R.; Kampf, N.; Zhu, L.; Klein, J. Hydration lubrication and shear-induced self-healing of lipid bilayer boundary lubricants in phosphatidylcholine dispersions. Soft Matter 2016, 12, 2773–2784. [Google Scholar] [CrossRef]
- Ma, L.; Gaisinskaya-Kipnis, A.; Kampf, N.; Klein, J. Origins of hydration lubrication. Nat. Commun. 2015, 6, 6060. [Google Scholar] [CrossRef]
- Klein, J. Hydration lubrication. Friction 2013, 1, 1–23. [Google Scholar] [CrossRef]
- Raviv, U.; Giasson, S.; Kampf, N.; Gohy, J.F.; Jérôme, R.; Klein, J. Lubrication by charged polymers. Nature 2003, 425, 163–165. [Google Scholar] [CrossRef]
- Klein, J.; Kumacheva, E.; Mahalu, D.; Perahla, D.; Fetterst, L.J. Reduction of frictional forces between solid surfaces bearing polymer brushes. Nature 1994, 370, 634–636. [Google Scholar] [CrossRef]
- Klein, J.; Perahia, D.; Warburg, S. Forces between polymer-bearing surfaces undergoing shear. Nature 1991, 352, 143–145. [Google Scholar] [CrossRef]
- Lin, W.; Klein, J. Direct measurement of surface forces: Recent advances and insights. Appl. Phys. Rev. 2021, 8, 031316. [Google Scholar] [CrossRef]
- Roa, J.J.; Oncins, G.; Díaz, J.; Capdevila, X.G.; Sanz, F.; Segarra, M. Study of the friction, adhesion and mechanical properties of single crystals, ceramics and ceramic coatings by AFM. J. Eur. Ceram. Soc. 2011, 31, 429–449. [Google Scholar] [CrossRef]
- Sader, J.E.; Green, C.P. In-plane deformation of cantilever plates with applications to lateral force microscopy. Rev. Sci. Instrum. 2004, 75, 878–883. [Google Scholar] [CrossRef]
- Lee, M.J.; Espinosa-Marzal, R.M. Intrinsic and Extrinsic Tunability of Double-Network Hydrogel Strength and Lubricity. ACS Appl. Mater. Interfaces 2023, 15, 20495–20507. [Google Scholar] [CrossRef]
- Sader, J.E.; Chon, J.W.M.; Mulvaney, P. Calibration of rectangular atomic force microscope cantilevers. Rev. Sci. Instrum. 1999, 70, 3967–3969. [Google Scholar] [CrossRef]
- Geisse, N.A. AFM and combined optical techniques. Mater. Today 2009, 12, 40–45. [Google Scholar] [CrossRef]
- Lin, W.; Kluzek, M.; Iuster, N.; Shimoni, E.; Kampf, N.; Goldberg, R.; Klein, J. Cartilage-inspired, lipid-based boundary-lubricated hydrogels. Science 2020, 370, 335–338. [Google Scholar] [CrossRef]
- Lin, W.; Kampf, N.; Klein, J. Designer Nanoparticles as Robust Superlubrication Vectors. ACS Nano 2020, 14, 7008–7017. [Google Scholar] [CrossRef]
- Banquy, X.; Burdynska, J.; Lee, D.W.; Matyjaszewski, K.; Israelachvili, J. Bioinspired bottle-brush polymer exhibits low friction and Amontons-like behavior. J. Am. Chem. Soc. 2014, 136, 6199–6202. [Google Scholar] [CrossRef]
- Moon, H.H.; Choi, E.J.; Yun, S.H.; Kim, Y.C.; Premkumar, T.; Song, C. Aqueous lubrication and wear properties of nonionic bottle-brush polymers. RSC Adv. 2022, 12, 17740–17746. [Google Scholar] [CrossRef]
- Kang, T.; Banquy, X.; Heo, J.; Lim, C.; Lynd, N.A.; Lundberg, P.; Oh, D.X.; Lee, H.K.; Hong, Y.K.; Hwang, D.S.; et al. Mussel-Inspired Anchoring of Polymer Loops That Provide Superior Surface Lubrication and Antifouling Properties. ACS Nano 2016, 10, 930–937. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, X.; Yang, X.; Yang, X.; Wang, S.; Song, W. Mucosa-Inspired Electro-Responsive Lubricating Supramolecular-Covalent Hydrogel. Adv. Mater. 2023, 35, e2307705. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Avestro, A.-J.; McGonigal, P.R.; Zhang, H. Supramolecular repair of hydration lubrication surfaces. Chem 2022, 8, 480–493. [Google Scholar] [CrossRef]
- Colombani, D. Chain-growth control in free radical polymerization. Prog. Polym. Sci. 1997, 22, 1649–1720. [Google Scholar]
- Bui, H.L.; Su, Y.H.; Yang, C.J.; Huang, C.J.; Lai, J.Y. Mucoadhesive, antioxidant, and lubricant catechol-functionalized poly(phosphobetaine) as biomaterial nanotherapeutics for treating ocular dryness. J. Nanobiotechnol. 2024, 22, 160–181. [Google Scholar] [CrossRef]
- Shoaib, T.; Espinosa-Marzal, R.M. Influence of Loading Conditions and Temperature on Static Friction and Contact Aging of Hydrogels with Modulated Microstructures. ACS Appl. Mater. Interfaces 2019, 11, 42722–42733. [Google Scholar] [CrossRef]
- Gombert, Y.; Simič, R.; Roncoroni, F.; Dübner, M.; Geue, T.; Spencer, N.D. Structuring Hydrogel Surfaces for Tribology. Adv. Mater. Interfaces 2019, 6, 1901320. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Liu, Y.; Luo, J. Synthesis and characterizations of zwitterionic copolymer hydrogels with excellent lubrication behavior. Tribol. Int. 2020, 143, 106026. [Google Scholar] [CrossRef]
- Ma, S.; Rong, M.; Lin, P.; Bao, M.; Xie, J.; Wang, X.; Huck, W.T.S.; Zhou, F.; Liu, W. Fabrication of 3D Tubular Hydrogel Materials through On-Site Surface Free Radical Polymerization. Chem. Mater. 2018, 30, 6756–6768. [Google Scholar] [CrossRef]
- Rong, M.; Liu, H.; Scaraggi, M.; Bai, Y.; Bao, L.; Ma, S.; Ma, Z.; Cai, M.; Dini, D.; Zhou, F. High Lubricity Meets Load Capacity: Cartilage Mimicking Bilayer Structure by Brushing Up Stiff Hydrogels from Subsurface. Adv. Funct. Mater. 2020, 30, 2004062. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Lalevée, J.; Yagci, Y. Photoinduced free radical promoted cationic polymerization 40 years after its discovery. Polym. Chem. 2020, 11, 1111–1121. [Google Scholar] [CrossRef]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated Polymerization: Advances, Challenges, and Opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Y.; Yu, B.; Ma, S.; Yang, H.; Liu, L.; Yu, J.; Pei, X.; Cai, M.; Zhou, F. Vertical and Horizontal Double Gradient Design for Super-Slippery and High-Bearing Hydrogel Skeleton. Adv. Funct. Mater. 2024, 2400360. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Yu, W.J.; Yu, Y.; Li, R.Y.; Gao, Q.; Ren, K.F.; Ji, J. A Bioinspired Hydrogel-Elastomer Hybrid Surface for Enhanced Mechanical Properties and Lubrication. ACS Appl. Mater. Interfaces 2021, 13, 50461–50469. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, Z.; Zhang, D.; Song, D. Tough, Injectable Calcium Phosphate Cement Based Composite Hydrogels to Promote Osteogenesis. Gels 2023, 9, 302. [Google Scholar] [CrossRef]
- Huang, J.; Tang, Y.; Wang, P.; Zhou, H.; Li, H.; Cheng, Z.; Wu, Y.; Xie, Z.; Cai, Z.; Wu, D.; et al. One-Pot Construction of Articular Cartilage-Like Hydrogel Coating for Durable Aqueous Lubrication. Adv. Mater. 2024, 36, 2309141. [Google Scholar] [CrossRef]
- Lyu, J.; Li, Y.; Li, Z.; Polanowski, P.; Jeszka, J.K.; Matyjaszewski, K.; Wang, W. Modelling Development in Radical (Co)Polymerization of Multivinyl Monomers. Angew. Chem. Int. Ed. Engl. 2022, 62, e202212235. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Spanswick, J. Controlled/living radical polymerization. Mater. Today 2005, 8, 26–33. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; Zhang, Z.; Xu, T. Atom transfer radical polymerization (ATRP): A versatile and forceful tool for functional membranes. Prog. Polym. Sci. 2014, 39, 124–144. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom Transfer Radical Polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef]
- Jay, G.D.; Waller, K.A. The biology of lubricin: Near frictionless joint motion. Matrix Biol. 2014, 39, 17–24. [Google Scholar] [CrossRef]
- Chen, M.; Briscoe, W.H.; Armes, S.P.; Klein, J. Lubrication at Physiological Pressures by Polyzwitterionic Brushes. Science 2009, 323, 1698–1701. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, W.; Ma, S.; Liu, H.; Wang, X.; Zhao, X.; Yu, B.; Cai, M.; Zhou, F. Modulus adaptive lubricating prototype inspired by instant muscle hardening mechanism of catfish skin. Nat. Commun. 2022, 13, 377–387. [Google Scholar] [CrossRef]
- Szczepaniak, G.; Fu, L.; Jafari, H.; Kapil, K.; Matyjaszewski, K. Making ATRP More Practical: Oxygen Tolerance. Acc. Chem. Res. 2021, 54, 1779–1790. [Google Scholar] [CrossRef]
- Pan, X.; Fantin, M.; Yuan, F.; Matyjaszewski, K. Externally controlled atom transfer radical polymerization. Chem. Soc. Rev. 2018, 47, 5457–5490. [Google Scholar] [CrossRef]
- Chong, B.Y.K.; Le, T.P.T.; Moad, G.; Rizzardo, E.; Thang, S.H. A More Versatile Route to Block Copolymers and Other Polymers of Complex Architecture by Living Radical Polymerization: The RAFT Process. Macromolecules 1999, 32, 2071–2074. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization—A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Sun, Z.; Feeney, E.; Guan, Y.; Cook, S.G.; Gourdon, D.; Bonassar, L.J.; Putnam, D. Boundary mode lubrication of articular cartilage with a biomimetic diblock copolymer. Proc. Natl. Acad. Sci. USA 2019, 116, 12437–12441. [Google Scholar] [CrossRef]
- Gleghorn, J.P.; Bonassar, L.J. Lubrication mode analysis of articular cartilage using Stribeck surfaces. J. Biomech. 2008, 41, 1910–1918. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Mushtaq, S.; Yun, S.J.; Jeon, J. Recent Advances in Bioorthogonal Click Chemistry for Efficient Synthesis of Radiotracers and Radiopharmaceuticals. Molecules 2019, 24, 3567. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. Polym. Chem. 2010, 1, 17–36. [Google Scholar] [CrossRef]
- Marco-Dufort, B.; Iten, R.; Tibbitt, M.W. Linking Molecular Behavior to Macroscopic Properties in Ideal Dynamic Covalent Networks. J. Am. Chem. Soc. 2020, 142, 15371–15385. [Google Scholar] [CrossRef]
- Xiang, L.; Zhang, J.; Wang, W.; Wei, Z.; Chen, Y.; Zeng, H. Targeted Repair of Super-Lubricating Surfaces via Pairing Click Chemistry. Adv. Funct. Mater. 2023, 33, 2301593. [Google Scholar] [CrossRef]
- Talreja, S.; Tiwari, S. Supramolecular Chemistry: Unveiling the Fascinating World of Non-Covalent Interactions and Complex Assemblies. J. Pharm. Pharmacol. Res. 2023, 7, 133–139. [Google Scholar] [CrossRef]
- Hisaki, I.; Suzuki, Y.; Gomez, E.; Ji, Q.; Tohnai, N.; Nakamura, T.; Douhal, A. Acid Responsive Hydrogen-Bonded Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 2111–2121. [Google Scholar] [CrossRef]
- Yu, G.; Yan, X.; Han, C.; Huang, F. Characterization of supramolecular gels. Chem. Soc. Rev. 2013, 42, 6697–6722. [Google Scholar] [CrossRef]
- Wang, D.; Tong, G.; Dong, R.; Zhou, Y.; Shen, J.; Zhu, X. Self-assembly of supramolecularly engineered polymers and their biomedical applications. Chem. Commun. 2014, 50, 11994–12017. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Jin, H.; Wang, S.; Song, W. Bioinspired Supramolecular Lubricating Hydrogel Induced by Shear Force. J. Am. Chem. Soc. 2018, 140, 3186–3189. [Google Scholar] [CrossRef]
- Dong, R.; Zhou, Y.; Huang, X.; Zhu, X.; Lu, Y.; Shen, J. Functional supramolecular polymers for biomedical applications. Adv. Mater. 2015, 27, 498–526. [Google Scholar] [CrossRef]
- Wang, D.; Yang, J.; Guo, J.; Duan, Z.; Wang, D.; Xia, F.; Deng, F.; Deng, X. Liquid-like polymer lubricating surfaces: Mechanism and applications. Nano Res. 2023, 17, 476–491. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, D.; Tivony, R.; Kampf, N.; Klein, J. Cell-inspired, massive electromodulation of friction via transmembrane fields across lipid bilayers. Nat. Mater. 2024. accepted for publication. [Google Scholar]
- Rahimi, A.; García, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar] [CrossRef]
- Li, X.L.; Clarke, R.W.; An, H.Y.; Gowda, R.R.; Jiang, J.Y.; Xu, T.Q.; Chen, E.Y. Dual Recycling of Depolymerization Catalyst and Biodegradable Polyester that Markedly Outperforms Polyolefins. Angew. Chem. Int. Ed. Engl. 2023, 62, e202303791. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Klein, J. Lubricating Polymer Gels/Coatings: Syntheses and Measurement Strategies. Gels 2024, 10, 407. https://doi.org/10.3390/gels10060407
Zhao P, Klein J. Lubricating Polymer Gels/Coatings: Syntheses and Measurement Strategies. Gels. 2024; 10(6):407. https://doi.org/10.3390/gels10060407
Chicago/Turabian StyleZhao, Panpan, and Jacob Klein. 2024. "Lubricating Polymer Gels/Coatings: Syntheses and Measurement Strategies" Gels 10, no. 6: 407. https://doi.org/10.3390/gels10060407
APA StyleZhao, P., & Klein, J. (2024). Lubricating Polymer Gels/Coatings: Syntheses and Measurement Strategies. Gels, 10(6), 407. https://doi.org/10.3390/gels10060407









