Adsorption Capacity of Activated Carbon-Encapsulated Hollow-Type Spherical Bacterial Cellulose Gels for Uremic Toxins in a Simulated Human Gastrointestinal Environment
Abstract
:1. Introduction
2. Results and Discussion
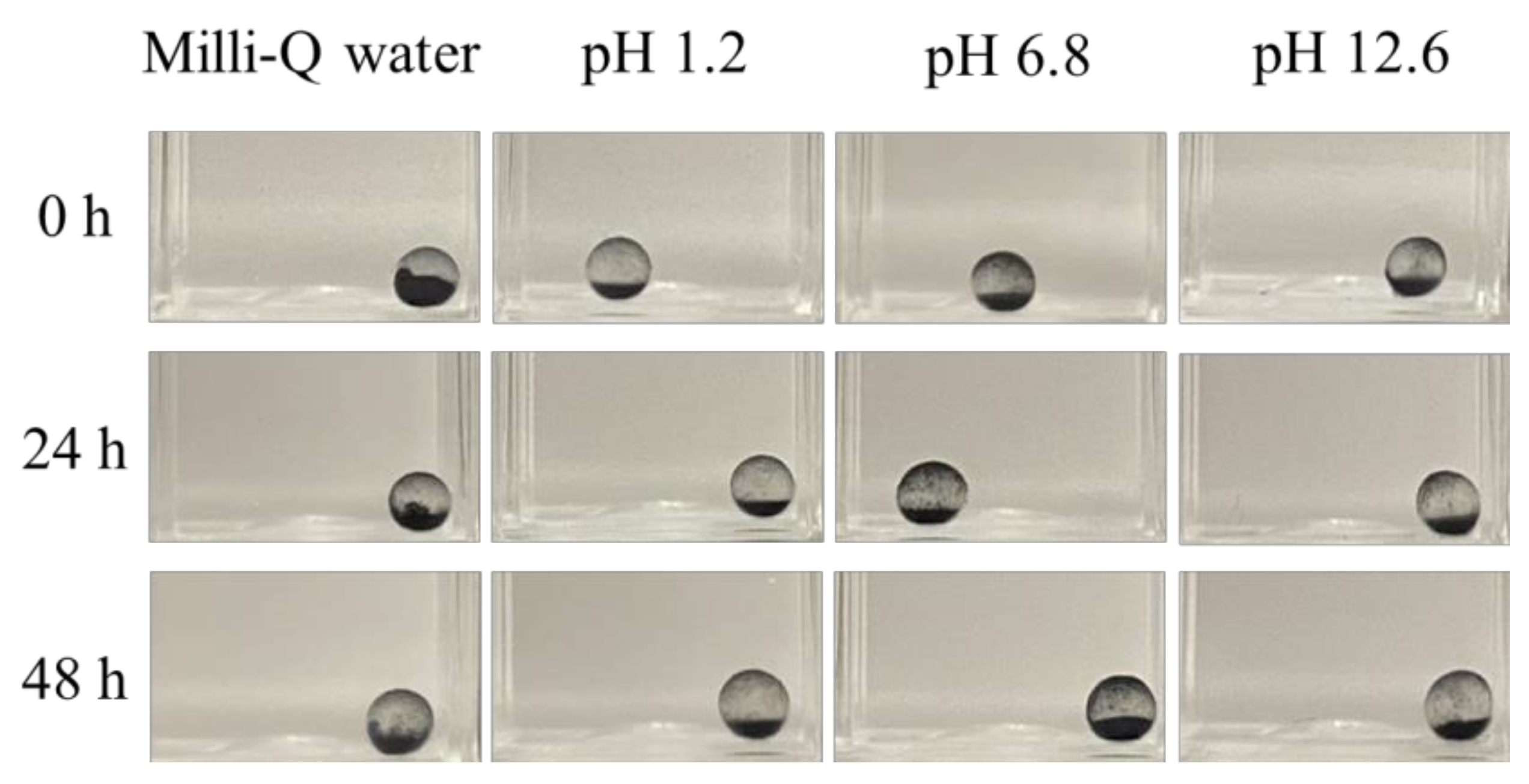
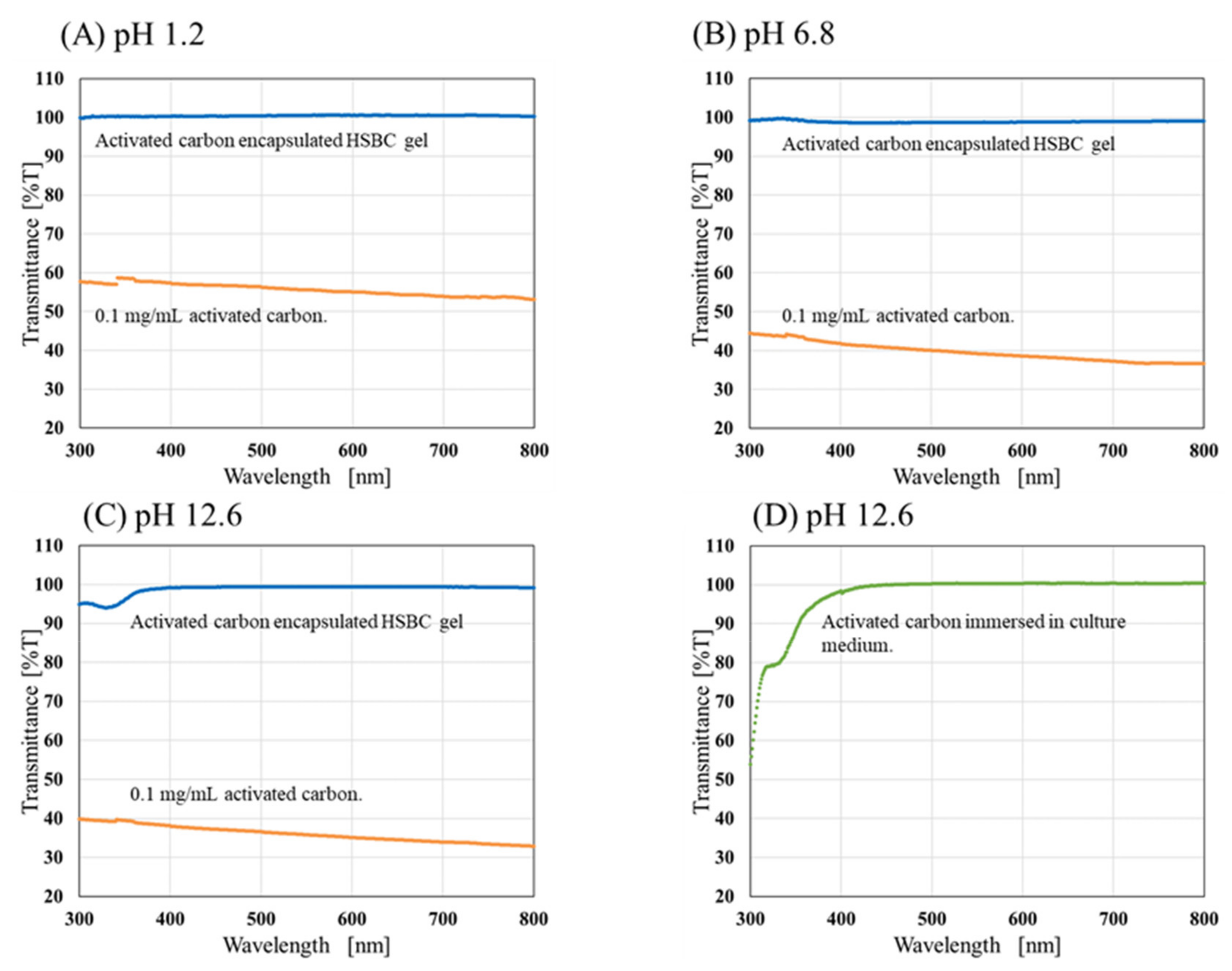
2.1. Stability Evaluation of Activated-Carbon-Encapsulating HSBC Gels in Static Conditions
2.2. Stability Evaluation of ACEGs under Agitation Conditions
2.3. Evaluation of Adsorption Capacity of Uremic Toxins in the Presence of Coexisting Ions
3. Conclusions
4. Materials and Methods
4.1. Materials
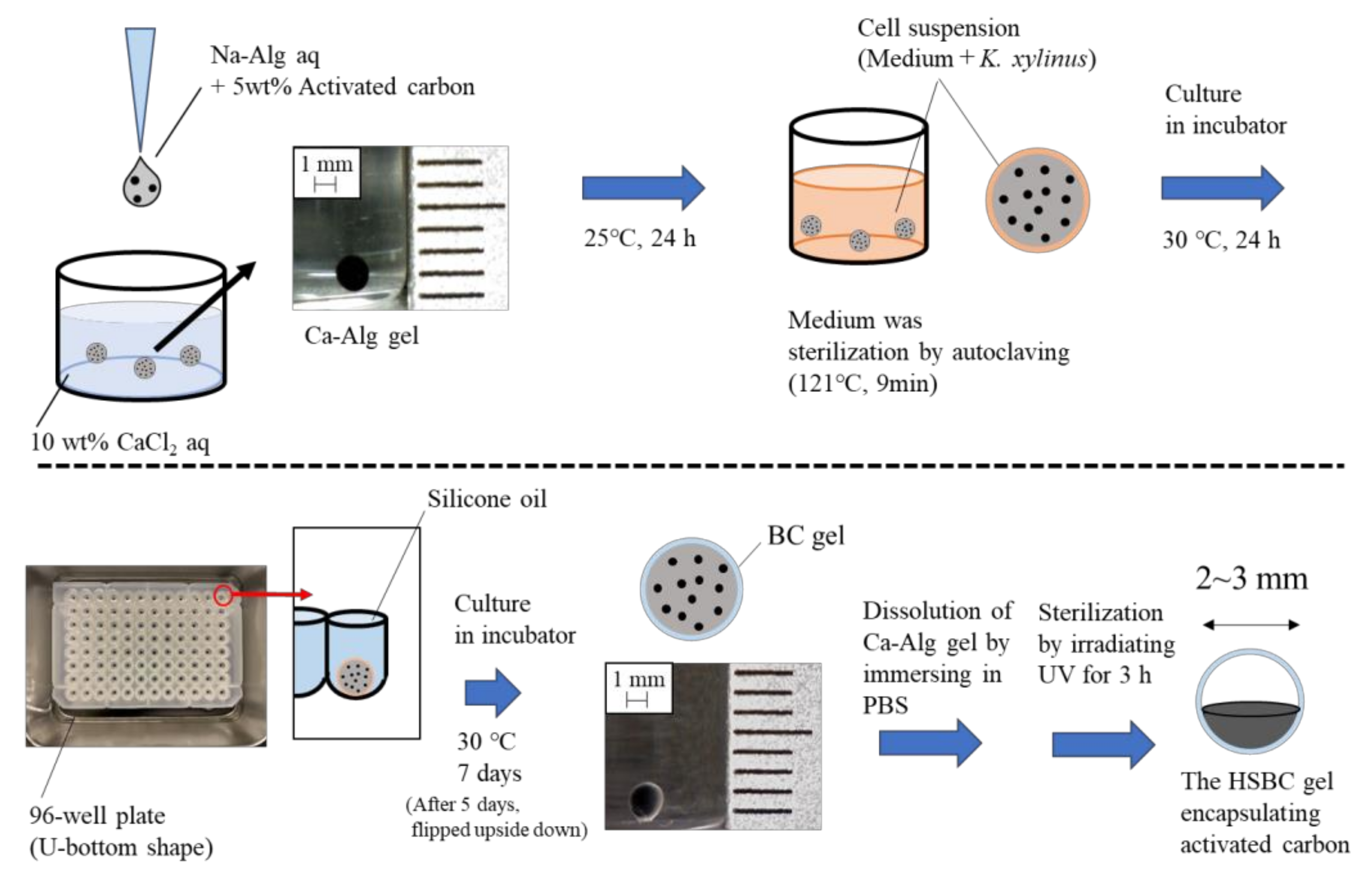
4.2. Preparation of the Activated-Carbon-Encapsulating HSBC Gel
4.3. Stability Evaluation of ACEGs in Different pH Environments
4.3.1. Stability Evaluation of ACEGs in Static Conditions
4.3.2. Stability Evaluation of ACEGs in Agitation Conditions
4.4. Evaluation of Adsorption Capacity of Uremic Toxins in the Presence of Coexisting Ions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nitta, K.; Goto, S.; Masakane, I.; Hanafusa, N.; Taniguchi, M.; Hasegawa, T.; Nakai, S.; Wada, A.; Hamano, T.; Hoshino, J.; et al. Annual dialysis data report for 2018, JSDT Renal Data Registry: Survey methods, facility data, incidence, prevalence, and mortality. Ren. Replace. Ther. 2020, 6, 41. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Sadykhov, N.K.; Kartuesov, A.G.; Borisov, E.E.; Sukhorukov, V.N.; Orekhov, A.N. Atherosclerosis Specific Features in Chronic Kidney Disease (CKD). Biomedicines 2022, 10, 2094. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.K.; Bush, K.T. Uraemic syndrome of chronic kidney disease: Altered remote sensing and signalling. Nat. Rev. Nephrol. 2019, 15, 301–316. [Google Scholar] [CrossRef]
- Wei, J.; Li, R.; Zhang, P.; Jin, H.; Zhang, Z.; Li, Y.; Chen, Y. Efficient selective removal of uremic toxin precursor by olefin-linked covalent organic frameworks for nephropathy treatment. Nat. Commun. 2023, 14, 2805. [Google Scholar] [CrossRef] [PubMed]
- Summers, C.S.; Quimby, M.J.; Isaiah, A.; Suchodolski, S.J.; Lunghofer, J.P.; Gustafson, L.D. The fecal microbiome and serum concentrations of indoxyl sulfate and p-cresol sulfate in cats with chronic kidney disease. J. Vet. Intern. Med. 2019, 33, 662–669. [Google Scholar] [CrossRef]
- Ribeiro, A.; Liu, F.; Srebrzynski, M.; Rother, S.; Adamowicz, K.; Wadowska, M.; Steiger, S.; Anders, H.-J.; Schmaderer, C.; Koziel, J.; et al. Uremic Toxin Indoxyl Sulfate Promotes Macrophage-Associated Low-Grade Inflammation and Epithelial Cell Senescence. Int. J. Mol. Sci. 2023, 24, 8031. [Google Scholar] [CrossRef]
- Ethical Drugs: KREMEZIN®. Available online: https://www.kegg.jp/medicus-bin/japic_med?japic_code=00067063 (accessed on 3 June 2024).
- Green, J.P.; McCauley, W. Bowel perforation after single-dose activated charcoal. Can. J. Emerg. Med. 2006, 8, 358–360. [Google Scholar] [CrossRef]
- Goulbourne, K.B.; Cisek, J.E. Small-Bowel Obstruction Secondary to Activated Charcoal and Adhesions. Ann. Emerg. Med. 1994, 24, 108–110. [Google Scholar] [CrossRef]
- Dorrington, C.L.; Johnson, D.W.; Brant, R.; Multiple Dose Activated Charcoal Complication Study Group. The frequency of complications associated with the use of multiple-dose activated charcoal. Ann. Emerg. Med. 2003, 41, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Abe, I. Multifunctional Materials: Activated Carbon, Charcoal. J. Jpn. Soc. Colour Mater. 1999, 72, 388–396. [Google Scholar] [CrossRef]
- Cao, L.; Li, N. Activated-carbon-filled agarose hydrogel as a natural medium for seed germination and seedling growth. Int. J. Biol. Macromol. 2021, 177, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Han, J.; Choi, Y.-K.; Park, S.; Lee, S.H. Reswellable alginate/activated carbon/carboxymethyl cellulose hydrogel beads for ibuprofen adsorption from aqueous solutions. Int. J. Biol. Macromol. 2023, 249, 126053. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, T.; Suzuki, M.; Ishikawa, M.; Endo, M.; Aoyagi, T. Encapsulation of Micro- and Milli-Sized Particles with a Hollow-Type Spherical Bacterial Cellulose Gel via Particle-Preloaded Droplet Cultivation. Int. J. Mol. Sci. 2019, 20, 4919. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.-C.; Hsiao, Y.-S.; Ke, J.-W.; Syu, W.-L.; Liu, T.-Y.; Liu, S.-H.; Juang, R.-S. Adsorptive removal of p-cresol and creatinine from simulated serum using porous polyethersulfone mixed-matrix membranes. Sep. Purif. Technol. 2020, 245, 116884. [Google Scholar] [CrossRef]
- Juang, R.-S.; Li, Y.-M.; Hsiao, Y.-S.; Fu, C.-C.; Liu, S.-H. Synergistic adsorption of creatinine and p-cresol from simulated serum using zeolites in electrospun fibrous mixed-matrix membranes. Sep. Purif. Technol. 2024, 335, 126186. [Google Scholar] [CrossRef]
- Cheng, Y.-C.; Fu, C.-C.; Hsiao, Y.-S.; Chien, C.-C.; Juang, R.-S. Clearance of low molecular-weight uremic toxins p-cresol, creatinine, and urea from simulated serum by adsorption. J. Mol. Liq. 2018, 252, 203–210. [Google Scholar] [CrossRef]
- Hsiao, Y.-S.; Tran, H.N.; Ke, J.-W.; Fu, C.-C.; Syu, W.-L.; Liu, S.-H.; Juang, R.-S. Porous cellulose acetate mixed-matrix membrane adsorbents for efficient clearance of p-cresol and creatinine from synthetic serum. J. Taiwan Inst. Chem. Eng. 2022, 133, 104199. [Google Scholar] [CrossRef]
- Stiapis, C.S.; Skouras, E.D.; Pavlenko, D.; Stamatialis, D.; Burganos, V.N. Evaluation of the Toxin-to-Protein Binding Rates during Hemodialysis Using Sorbent-Loaded Mixed-Matrix Membranes. Appl. Sci. 2018, 8, 536. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Li, K.; Liu, Y.; Xie, D.; Shan, S.; He, L.; Mei, Y. Adsorption of uremic toxins using biochar for dialysate regeneration. Biomass Convers. Biorefinery 2023, 13, 11499–11511. [Google Scholar] [CrossRef]
- Guangle, Q.; Gan, Z.; Dapeng, C.; Jingjie, S. Adsorption of uremic toxins by modified activated carbon of different mesh with sulfuric acid. Adsorption 2024. [Google Scholar] [CrossRef]
- Adler, T.K.; Albert, A. The Biological and Physical Properties of the Azaindoles. J. Med. Chem. 1963, 6, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Lide, D.R. Handbook of Chemistry and Physics, 85th ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 7-1–7-3. [Google Scholar]
- Fedotova, M.V.; Kruchinin, S.E. Ion-binding of glycine zwitterion with inorganic ions in biologically relevant aqueous electrolyte solutions. Biophys. Chem. 2014, 190–191, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Fedotova, M.V.; Dmitrieva, O.A. Ion-selective interactions of biologically relevant inorganic ions with alanine zwitterion: A 3D-RISM study. Amino Acids 2015, 47, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Urano, K.; Nishimura, Y.; Yanaga, Y. Adsorption of polyethylene glycol of different molecular weights on activated carbon in water. J. Chem. Soc. Jpn. 1975, 1975, 1444–1445. [Google Scholar]
- Lorenc-Grabowska, E. Effect of micropore size distribution on phenol adsorption on steam activated carbons. Adsorption 2016, 22, 599–607. [Google Scholar] [CrossRef]
- Jiang, X.-Y.; Li, L.; Yan, J.-N.; Wang, C.; Lai, B.; Wu, H.-T. Binary hydrogels constructed from lotus rhizome starch and different types of carrageenan for dysphagia management: Nonlinear rheological behaviors and structural characteristics. Food Chem. X 2024, 22, 101466. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Londhe, V. Oral jelly of metformin hydrochloride—Formulation development using Design of Experiments and characterization. J. Drug Deliv. Sci. Technol. 2021, 63, 102519. [Google Scholar] [CrossRef]
- Choi, S.M.; Rao, K.M.; Zo, S.M.; Shin, E.J.; Han, S.S. Bacterial Cellulose and Its Applications. Polymers 2022, 14, 1080. [Google Scholar] [CrossRef]
- Roberts, E.L.; Abdollahi, S.; Oustadi, F.; Stephens, E.D.; Badv, M. Bacterial-Nanocellulose-Based Biointerfaces and Biomimetic Constructs for Blood-Contacting Medical Applications. ACS Mater. Au 2023, 3, 418–441. [Google Scholar] [CrossRef]
- Hoshi, T.; Endo, M.; Hirai, A.; Suzuki, M.; Aoyagi, T. Encapsulation of Activated Carbon into a Hollow-Type Spherical Bacterial Cellulose Gel and Its Indole-Adsorption Ability Aimed at Kidney Failure Treatment. Pharmaceutics 2020, 12, 1076. [Google Scholar] [CrossRef] [PubMed]


| Adsorbent | Target Molecule | Solvent | Qm[µmol/mg] | K | R2 | |
|---|---|---|---|---|---|---|
| Activated carbon powder | Indole | Milli-Q | 5.06 | 7.38 | 0.99 | |
| Activated carbon encapsulated HSBC gel | Indole | Milli-Q | 3.23 | 2.73 | 0.99 | Figure 4a |
| Indole | Disintegration test solution 2 | 2.62 | 4.38 | 0.99 | Figure 4b | |
| Tryptophan | Milli-Q | 3.60 | 18.64 | 0.99 | Figure 4c | |
| Tryptophan | Disintegration test solution 2 | 1.32 | 6.86 | 0.97 | Figure 4d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirai, A.; Suzuki, M.; Sato, K.; Hoshi, T.; Aoyagi, T. Adsorption Capacity of Activated Carbon-Encapsulated Hollow-Type Spherical Bacterial Cellulose Gels for Uremic Toxins in a Simulated Human Gastrointestinal Environment. Gels 2024, 10, 417. https://doi.org/10.3390/gels10070417
Hirai A, Suzuki M, Sato K, Hoshi T, Aoyagi T. Adsorption Capacity of Activated Carbon-Encapsulated Hollow-Type Spherical Bacterial Cellulose Gels for Uremic Toxins in a Simulated Human Gastrointestinal Environment. Gels. 2024; 10(7):417. https://doi.org/10.3390/gels10070417
Chicago/Turabian StyleHirai, Aya, Masashige Suzuki, Kaito Sato, Toru Hoshi, and Takao Aoyagi. 2024. "Adsorption Capacity of Activated Carbon-Encapsulated Hollow-Type Spherical Bacterial Cellulose Gels for Uremic Toxins in a Simulated Human Gastrointestinal Environment" Gels 10, no. 7: 417. https://doi.org/10.3390/gels10070417









