Conductive Polymer-Based Hydrogels for Wearable Electrochemical Biosensors
Abstract
:1. Introduction
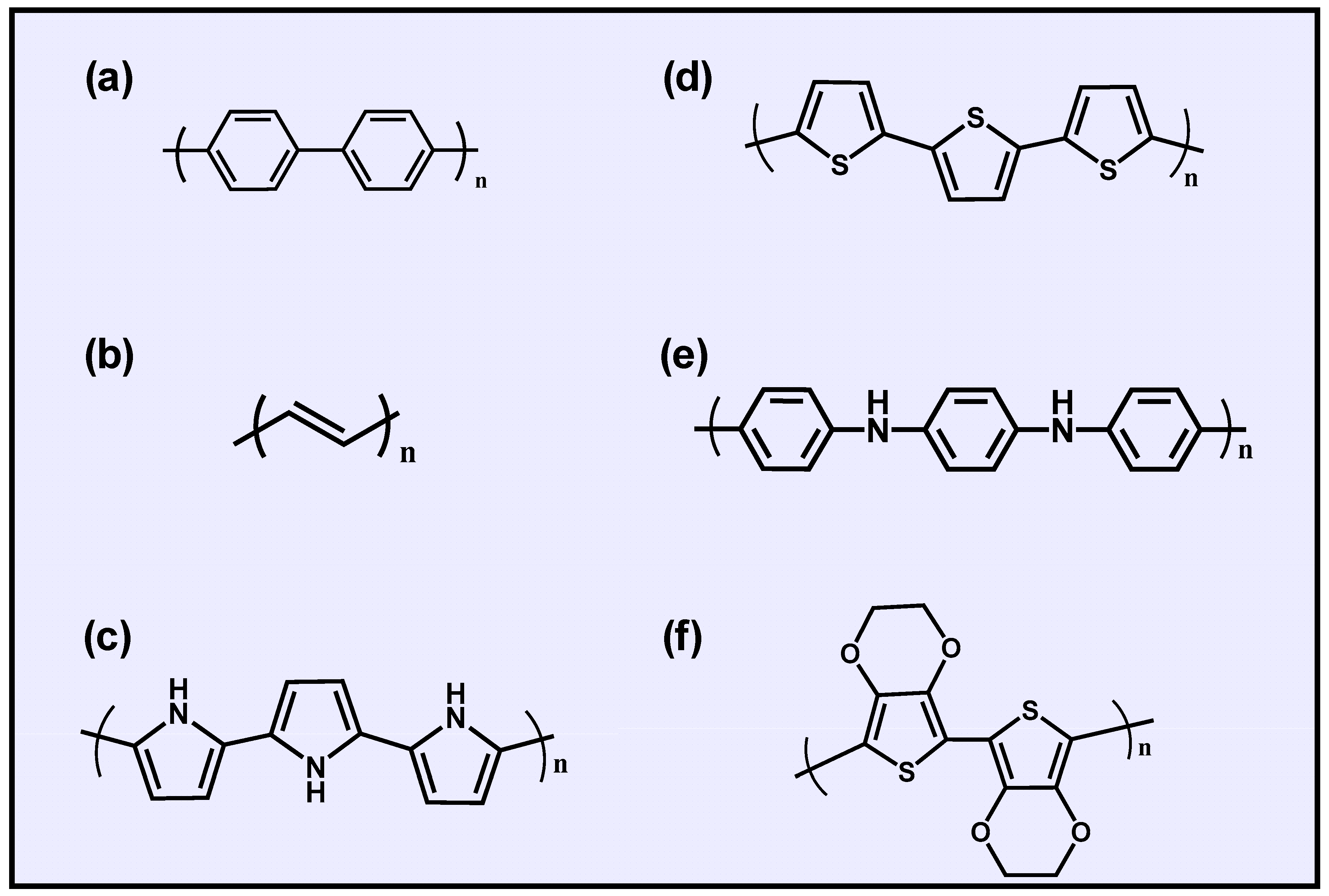
2. Conductive Polymer Hydrogels
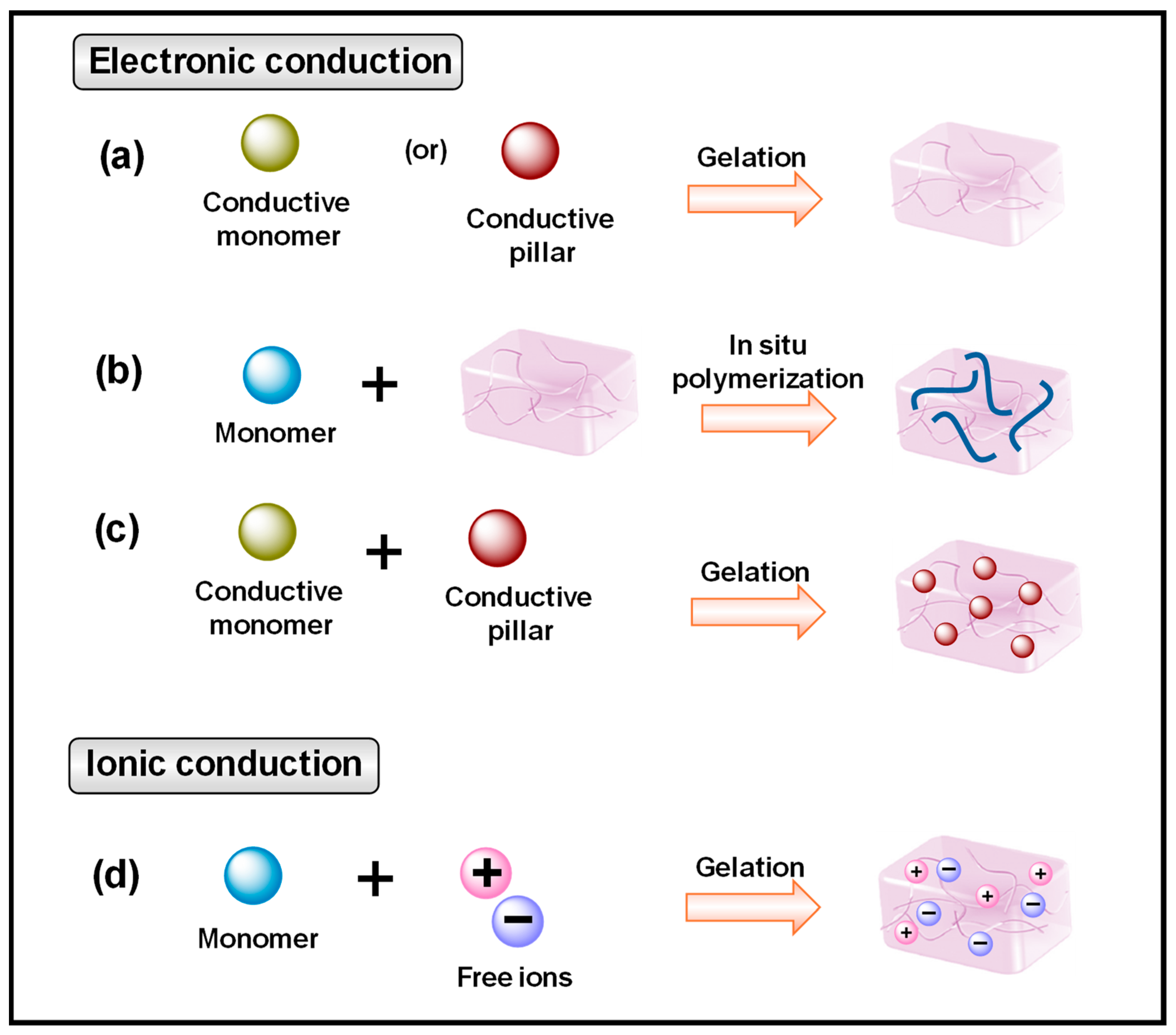
2.1. Conductive Mechanism
2.2. Fabrication of CP HGs
2.2.1. General Synthesis Approach
2.2.2. Copolymerization Techniques
2.2.3. Blending/Doping Method
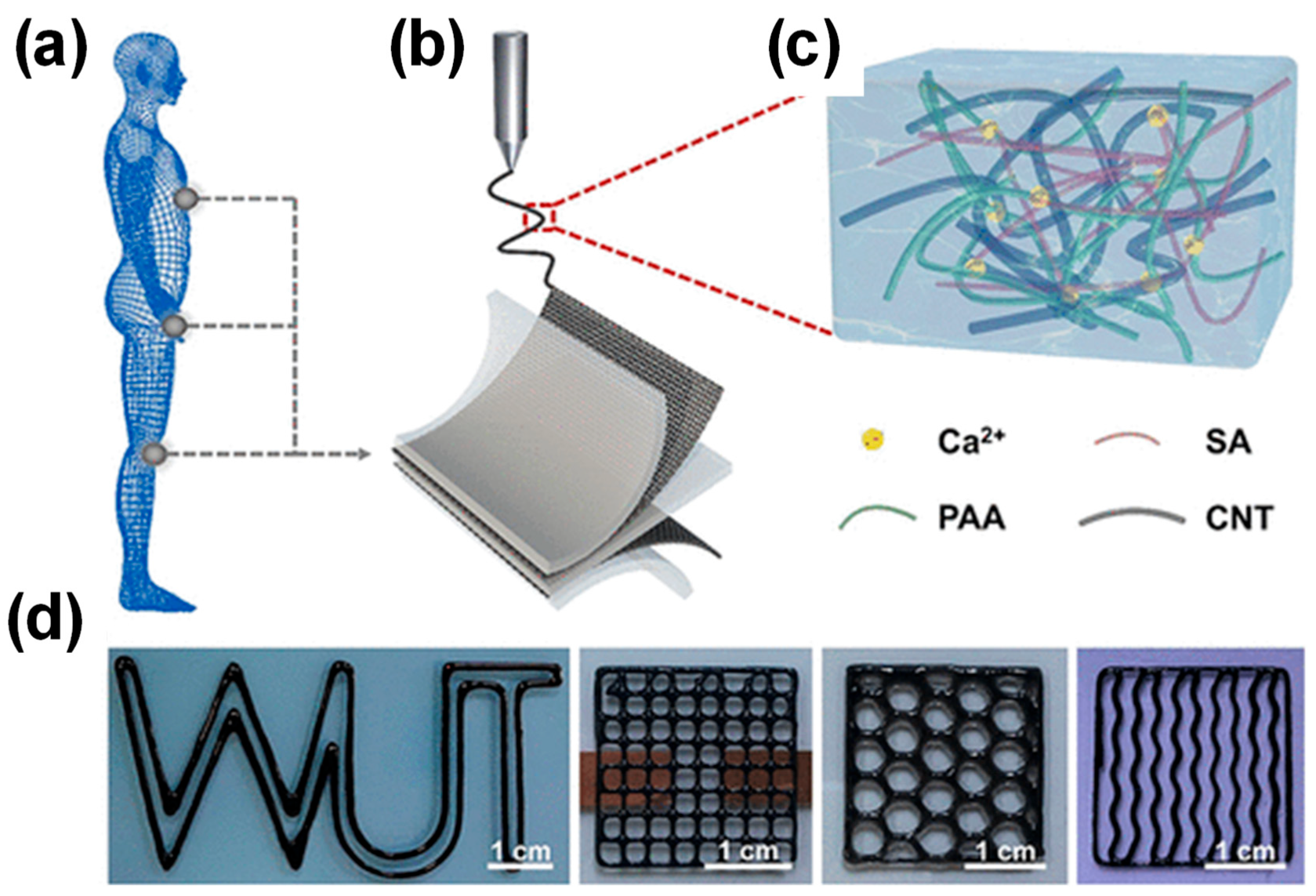
2.2.4. Advanced Techniques for the Development of CP HGs
2.3. Properties of CP HGs
2.3.1. Electrical Conductivity
2.3.2. Mechanical Properties
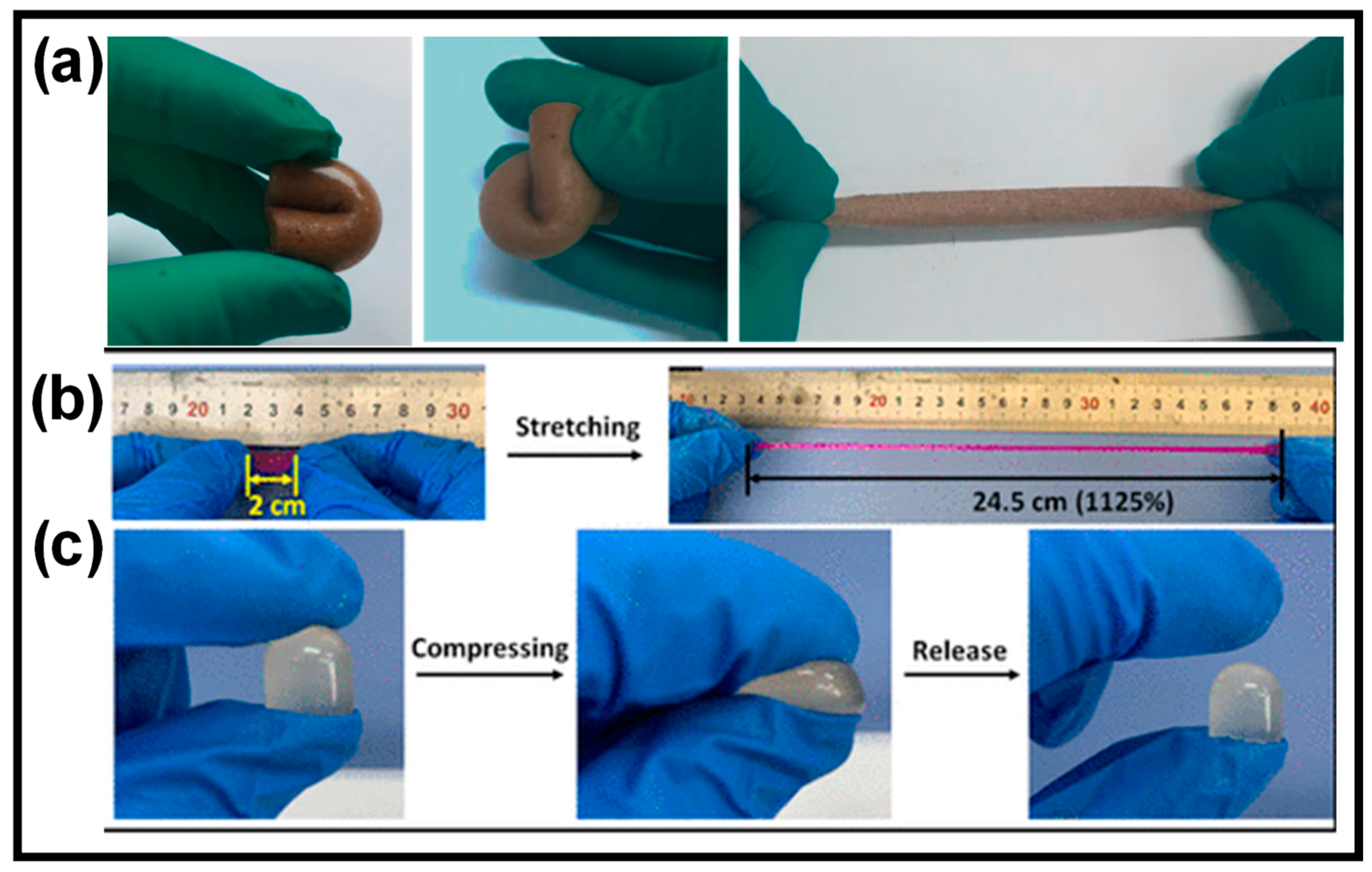
2.3.3. Self-Healing Property
2.3.4. Adhesion Property
2.3.5. Biocompatibility
3. Applications of Wearable Electrochemical Biosensors (WEBSs)
3.1. WEBSs for Glucose Monitoring
3.2. WEBSs for Lactate Detection
3.3. WEBSs for Other Biomarkers
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ates, H.C.; Nguyen, P.Q.; Gonzalez-Macia, L.; Morales-Narváez, E.; Güder, F.; Collins, J.J.; Dincer, C. End-to-end design of wearable sensors. Nat. Rev. Mater. 2022, 7, 887–907. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Ye, Z.; Liu, W.; Liu, X.; Wang, X. Conductive hydrogels for bioelectronics: Molecular structures, design principles, and operation mechanisms. J. Mater. Chem. C 2023, 11, 10785–10808. [Google Scholar] [CrossRef]
- Ye, S.; Feng, S.; Huang, L.; Bian, S. Recent progress in wearable biosensors: From healthcare monitoring to sports analytics. Biosensors 2020, 10, 205. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Y.; Dong, X.; Armacki, M.; Sitti, M. In situ sensing physiological properties of biological tissues using wireless miniature soft robots. Sci. Adv. 2023, 9, eadg3988. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xia, K.; Hou, Y.; Zhang, Q.; Ye, Z.; Lu, J. Designing flexible, smart and self-sustainable supercapacitors for portable/wearable electronics: From conductive polymers. Chem. Soc. Rev. 2021, 50, 12702–12743. [Google Scholar] [CrossRef] [PubMed]
- Zamani, F.G.; Moulahoum, H.; Ak, M.; Demirkol, D.O.; Timur, S. Current trends in the development of conducting polymers-based biosensors. Trends Anal. Chem. 2019, 118, 264–276. [Google Scholar] [CrossRef]
- Hackett, A.J.; Malmström, J.; Travas-Sejdic, J. Functionalization of conducting polymers for biointerface applications. Prog. Polym. Sci. 2017, 70, 18–33. [Google Scholar] [CrossRef]
- He, H.; Zhang, L.; Guan, X.; Cheng, H.; Liu, X.; Yu, S.; Wei, J.; Ouyang, J. Biocompatible conductive polymers with high conductivity and high stretchability. ACS Appl. Mater. Interfaces 2019, 11, 26185–26193. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Völlmecke, K.; Afroz, R.; Bierbach, S.; Brenker, L.J.; Frücht, S.; Glass, A.; Giebelhaus, R.; Hoppe, A.; Kanemaru, K.; Lazarek, M.; et al. Hydrogel-based biosensors. Gels 2022, 8, 768. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, K. Advances in Hydrogel-Based Drug Delivery Systems. Gels 2024, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Wang, S.; Zhao, Y.; Huang, H.; Wang, Q.; Shao, J.; Wang, W.; Dong, X. Skin-inspired highly stretchable, tough and adhesive hydrogels for tissue-attached sensor. Chem. Eng. J. 2021, 425, 131523. [Google Scholar] [CrossRef]
- Guiseppi-Elie, A.; Wilson, A.M.; Sujdak, A.S. Electroconductive gels for controlled electrorelease of bioactive peptides. In Tailored Polymeric Materials for Controlled Delivery Systems; ACS Symposium Series; ACS Publications: Washington, DC, USA, 1998; pp. 185–202. [Google Scholar]
- Small, C.; Too, C.; Wallace, G. Responsive conducting polymer-hydrogel composites. Polym. Gels Networks 1997, 5, 251–265. [Google Scholar] [CrossRef]
- Fu, L.; Yu, A.; Lai, G. Conductive hydrogel-based electrochemical sensor: A soft platform for capturing analyte. Chemosensors 2021, 9, 282. [Google Scholar] [CrossRef]
- Peng, Q.; Chen, J.; Wang, T.; Peng, X.; Liu, J.; Wang, X.; Wang, J.; Zeng, H. Recent advances in designing conductive hydrogels for flexible electronics. InfoMat 2020, 2, 843–865. [Google Scholar] [CrossRef]
- Park, J.; Jeon, N.; Lee, S.; Choe, G.; Lee, E.; Lee, J.Y. Conductive hydrogel constructs with three-dimensionally connected graphene networks for biomedical applications. Chem. Eng. J. 2022, 446, 137344. [Google Scholar] [CrossRef]
- Kougkolos, G.; Golzio, M.; Laudebat, L.; Valdez-Nava, Z.; Flahaut, E. Hydrogels with electrically conductive nanomaterials for biomedical applications. J. Mater. Chem. B 2023, 11, 2036–2062. [Google Scholar] [CrossRef]
- Ma, Z.; Shi, W.; Yan, K.; Pan, L.; Yu, G. Doping engineering of conductive polymer hydrogels and their application in advanced sensor technologies. Chem. Sci. 2019, 10, 6232–6244. [Google Scholar] [CrossRef]
- Tomczykowa, M.; Plonska-Brzezinska, M.E. Conducting polymers, hydrogels and their composites: Preparation, properties and bioapplications. Polymers 2019, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xiao, G.; Wang, Y.; Chen, X.; Duan, G.; Wu, Y.; Gong, X.; Wang, H. Design and fabrication of conductive polymer hydrogels and their applications in flexible supercapacitors. J. Mater. Chem. A 2020, 8, 23059–23095. [Google Scholar] [CrossRef]
- Guo, B.; Ma, Z.; Pan, L.; Shi, Y. Properties of conductive polymer hydrogels and their application in sensors. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 1606–1621. [Google Scholar] [CrossRef]
- Liu, D.; Huyan, C.; Wang, Z.; Guo, Z.; Zhang, X.; Torun, H.; Mulvihill, D.; Xu, B.B.; Chen, F. Conductive polymer based hydrogels and their application in wearable sensors: A review. Mater. Horiz. 2023, 10, 2800–2823. [Google Scholar] [CrossRef]
- Samwang, T.; Watanabe, N.M.; Okamoto, Y.; Srinives, S.; Umakoshi, H. Study of Chemical Polymerization of Polypyrrole with SDS Soft Template: Physical, Chemical, and Electrical Properties. ACS Omega 2023, 8, 48946–48957. [Google Scholar] [CrossRef]
- Alam, S.; Chowdhury, M.A.; Shahid, A.; Alam, R.; Rahim, A. Synthesis of emerging two-dimensional (2D) materials–Advances, challenges and prospects. FlatChem 2021, 30, 100305. [Google Scholar] [CrossRef]
- Awang, N.; Nasir, A.; Yajid, M.; Jaafar, J. A review on advancement and future perspective of 3D hierarchical porous aerogels based on electrospun polymer nanofibers for electrochemical energy storage application. J. Environ. Chem. Eng. 2021, 9, 105437. [Google Scholar] [CrossRef]
- Li, L.; Meng, J.; Zhang, M.; Liu, T.; Zhang, C. Recent advances in conductive polymer hydrogel composites and nanocomposites for flexible electrochemical supercapacitors. Chem. Commun. 2022, 58, 185–207. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Pan, L.; Ding, Y.; Zhao, Y.; Li, Y.; Shi, Y.; Yu, G. Dopant-enabled supramolecular approach for controlled synthesis of nanostructured conductive polymer hydrogels. Nano Lett. 2015, 15, 7736–7741. [Google Scholar] [CrossRef]
- Zhou, L.; Fan, L.; Yi, X.; Zhou, Z.; Liu, C.; Fu, R.; Dai, C.; Wang, Z.; Chen, X.; Yu, P. Soft conducting polymer hydrogels cross-linked and doped by tannic acid for spinal cord injury repair. ACS Nano 2018, 12, 10957–10967. [Google Scholar] [CrossRef]
- Jordan, R.S.; Frye, J.; Hernandez, V.; Prado, I.; Giglio, A.; Abbasizadeh, N.; Flores-Martinez, M.; Shirzad, K.; Xu, B.; Hill, I.M. 3D printed architected conducting polymer hydrogels. J. Mater. Chem. B 2021, 9, 7258–7270. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhu, Y. Printing conductive nanomaterials for flexible and stretchable electronics: A review of materials, processes, and applications. Adv. Mater. Technol. 2019, 4, 1800546. [Google Scholar] [CrossRef]
- Distler, T.; Boccaccini, A.R. 3D printing of electrically conductive hydrogels for tissue engineering and biosensors–A review. Acta Biomater. 2020, 101, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Wadnap, S.; Xu, C.; Ahsan, F. Digital light processing (DLP) 3D-printing technology and photoreactive polymers in fabrication of modified-release tablets. Eur. J. Pharm. Sci. 2019, 135, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, M.; Roppolo, I.; Chiappone, A.; Larush, L.; Pirri, C.F.; Magdassi, S. 3D-printed self-healing hydrogels via Digital Light Processing. Nat. Commun. 2021, 12, 2462. [Google Scholar] [CrossRef]
- Wei, J.; Xie, J.; Zhang, P.; Zou, Z.; Ping, H.; Wang, W.; Xie, H.; Shen, J.Z.; Lei, L.; Fu, Z. Bioinspired 3D printable, self-healable, and stretchable hydrogels with multiple conductivities for skin-like wearable strain sensors. ACS Appl. Mater. Interfaces 2021, 13, 2952–2960. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Qian, X.; Si, Y.; Liu, G.; Shi, C. Recent advances in the synthesis and application of three-dimensional graphene-based aerogels. Molecules 2022, 27, 924. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Feng, J. Self-assembled three-dimensional hierarchical graphene/polypyrrole nanotube hybrid aerogel and its application for supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 9671–9679. [Google Scholar] [CrossRef]
- Wang, Q.; Song, H.; Li, W.; Wang, S.; Liu, L.; Li, T.; Han, Y. Facile synthesis of polypyrrole/graphene composite aerogel with Alizarin Red S as reactive dopant for high-performance flexible supercapacitor. J. Power Source 2022, 517, 230737. [Google Scholar] [CrossRef]
- Yang, Y.; Xi, Y.; Li, J.; Wei, G.; Klyui, N.; Han, W. Flexible supercapacitors based on polyaniline arrays coated graphene aerogel electrodes. Nanoscale Res. Lett. 2017, 12, 394. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, J.; Zhang, W.; Zhang, P.; He, W.; Chen, J.; Sun, Z. A multidimensional nanostructural design towards electrochemically stable and mechanically strong hydrogel electrodes. Nanoscale 2020, 12, 6637–6643. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, F.; Su, H.; Xiong, J.; Yang, L.; Xia, J.; Liang, Y. Advanced nanocomposite hydrogels for cartilage tissue engineering. Gels 2022, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Madani, S.Z.M.; Safaee, M.M.; Gravely, M.; Silva, C.; Kennedy, S.; Bothun, G.D.; Roxbury, D. Carbon nanotube–liposome complexes in hydrogels for controlled drug delivery via near-infrared laser stimulation. ACS Appl. Nano Mater. 2020, 4, 331–342. [Google Scholar] [CrossRef]
- Hong, Y.; Lin, Z.; Yang, Y.; Jiang, T.; Shang, J.; Luo, Z. Biocompatible conductive hydrogels: Applications in the field of biomedicine. Int. J. Mol. Sci. 2022, 23, 4578. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Herrmann, A.-K.; Bigall, N.C.; Rodriguez, P.; Wen, D.; Oezaslan, M.; Schmidt, T.J.; Gaponik, N.; Eychmüller, A. Noble Metal Aerogels Synthesis, Characterization, and Application as Electrocatalysts. Acc. Chem. Res. 2015, 48, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Clasky, A.J.; Watchorn, J.D.; Chen, P.Z.; Gu, F.X. From prevention to diagnosis and treatment: Biomedical applications of metal nanoparticle-hydrogel composites. Acta Biomater. 2021, 122, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Wang, Z.; Shen, Y.; Tang, L.; Zhang, P.; Wang, Y.; Chen, Y.; Huang, B.; Lu, B. Natural skin-inspired versatile cellulose biomimetic hydrogels. J. Mater. Chem. A 2019, 7, 26442–26455. [Google Scholar] [CrossRef]
- Jing, X.; Wang, X.-Y.; Mi, H.-Y.; Turng, L.-S. Stretchable gelatin/silver nanowires composite hydrogels for detecting human motion. Mater. Lett. 2019, 237, 53–56. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Liu, M. Conductive hydrogels as smart materials for flexible electronic devices. Chem. Eur. J. 2018, 24, 16930–16943. [Google Scholar] [CrossRef]
- Alam, A.; Zhang, Y.; Kuan, H.-C.; Lee, S.-H.; Ma, J. Polymer composite hydrogels containing carbon nanomaterials—Morphology and mechanical and functional performance. Prog. Polym. Sci. 2018, 77, 1–18. [Google Scholar] [CrossRef]
- Anjali, J.; Jose, V.K.; Lee, J.-M. Carbon-based hydrogels: Synthesis and their recent energy applications. J. Mater. Chem. A 2019, 7, 15491–15518. [Google Scholar] [CrossRef]
- Xia, S.; Song, S.; Jia, F.; Gao, G. A flexible, adhesive and self-healable hydrogel-based wearable strain sensor for human motion and physiological signal monitoring. J. Mater. Chem. B 2019, 7, 4638–4648. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lu, X.; Wang, M.; Gan, D.; Deng, W.; Wang, K.; Fang, L.; Liu, K.; Chan, C.W.; Tang, Y. A mussel-inspired conductive, self-adhesive, and self-healable tough hydrogel as cell stimulators and implantable bioelectronics. Small 2017, 13, 1601916. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Zhao, X.-J.; Jia, S.-R.; Bai, H.; Zhong, C. Nanocomposite hydrogels as multifunctional systems for biomedical applications: Current state and perspectives. Compos. Part B 2020, 200, 108208. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Dammu, S.A.; Canter, J.M.; Wu, C.-J.; Schmidt, G. Highly extensible, tough, and elastomeric nanocomposite hydrogels from poly (ethylene glycol) and hydroxyapatite nanoparticles. Biomacromolecules 2011, 12, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Karchoubi, F.; Ghotli, R.A.; Pahlevani, H.; Salehi, M.B. New insights into nanocomposite hydrogels; a review on recent advances in characteristics and applications. Adv. Ind. Eng. Polym. Res. 2023, 7, 54–78. [Google Scholar] [CrossRef]
- Murata, K.; Haraguchi, K. Optical anisotropy in polymer–clay nanocomposite hydrogel and its change on uniaxial deformation. J. Mater. Chem. 2007, 17, 3385–3388. [Google Scholar] [CrossRef]
- Ma, P.; Wang, Z.; Jiang, Y.; Huang, Z.; Xia, L.; Jiang, J.; Yuan, F.; Xia, H.; Zhang, Y. Clay-based nanocomposite hydrogels with microstructures and sustained ozone release for antibacterial activity. Colloids Surf. A 2022, 641, 128497. [Google Scholar] [CrossRef]
- Yang, W.; Yamamoto, S.; Sueyoshi, K.; Inadomi, T.; Kato, R.; Miyamoto, N. Perovskite nanosheet hydrogels with mechanochromic structural color. Angew. Chem. 2021, 133, 8547–8552. [Google Scholar] [CrossRef]
- Ohm, Y.; Pan, C.; Ford, M.J.; Huang, X.; Liao, J.; Majidi, C. An electrically conductive silver–polyacrylamide–alginate hydrogel composite for soft electronics. Nat. Electron. 2021, 4, 185–192. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Wang, F.; Zhang, J.H.; Zhang, J.; Shi, Y.; Pan, L. Application of conductive polymer hydrogels in flexible electronics. J. Polym. Sci. 2022, 60, 2635–2662. [Google Scholar] [CrossRef]
- He, H.; Li, H.; Pu, A.; Li, W.; Ban, K.; Xu, L. Hybrid assembly of polymeric nanofiber network for robust and electronically conductive hydrogels. Nat. Commun. 2023, 14, 759. [Google Scholar] [CrossRef]
- Ciarleglio, G.; Toto, E.; Santonicola, M.G. Conductive and thermo-responsive composite hydrogels with poly (N-isopropylacrylamide) and carbon nanotubes fabricated by two-step photopolymerization. Polymers 2023, 15, 1022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, Z.; Zhao, Y.; Hu, J.; Wang, H. Highly stretchable porous composite hydrogels with stable conductivity for strain sensing. Compos. Sci. Technol. 2021, 213, 108968. [Google Scholar] [CrossRef]
- Di, X.; Ma, Q.; Xu, Y.; Yang, M.; Wu, G.; Sun, P. High-performance ionic conductive poly (vinyl alcohol) hydrogels for flexible strain sensors based on a universal soaking strategy. Mater. Chem. Front. 2021, 5, 315–323. [Google Scholar] [CrossRef]
- Gao, Y.; Duan, L.; Guan, S.; Gao, G.; Cheng, Y.; Ren, X.; Wang, Y. The effect of hydrophobic alkyl chain length on the mechanical properties of latex particle hydrogels. RSC Adv. 2017, 7, 44673–44679. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.; Jiang, W.; Zhang, Q.; Liu, N.; Wang, Z.; Li, Z.; Zhang, D. Double-network hydrogels for biomaterials: Structure-property relationships and drug delivery. Eur. Polym. J. 2023, 185, 111807. [Google Scholar] [CrossRef]
- Lee, H.R.; Kim, C.C.; Sun, J.Y. Stretchable ionics–a promising candidate for upcoming wearable devices. Adv. Mater. 2018, 30, 1704403. [Google Scholar] [CrossRef]
- Zhao, L.; Ke, T.; Ling, Q.; Liu, J.; Li, Z.; Gu, H. Multifunctional ionic conductive double-network hydrogel as a long-term flexible strain sensor. ACS Appl. Polym. Mater. 2021, 3, 5494–5508. [Google Scholar] [CrossRef]
- Li, P.; Li, Z.; Chen, H.; Zhu, Y.; Yang, D.; Hou, Y. Graphene-Based Flexible Strain Sensor Based on PDMS for Strain Detection of Steel Wire Core Conveyor Belt Joints. Sensors 2023, 23, 7473. [Google Scholar] [CrossRef]
- Fu, H.; Wang, B.; Li, J.; Cao, D.; Zhang, W.; Xu, J.; Li, J.; Zeng, J.; Gao, W.; Chen, K. Ultra-strong, nonfreezing, and flexible strain sensors enabled by biomass-based hydrogels through triple dynamic bond design. Mater. Horiz. 2024, 11, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Li, X.; Wang, S.; Yuan, L.; Ge, L.; Li, D.; Mu, C. Facile fabrication of biocompatible gelatin-based self-healing hydrogels. ACS Appl. Polym. Mater. 2019, 1, 1350–1358. [Google Scholar] [CrossRef]
- Darabi, M.A.; Khosrozadeh, A.; Mbeleck, R.; Liu, Y.; Chang, Q.; Jiang, J.; Cai, J.; Wang, Q.; Luo, G.; Xing, M. Skin-inspired multifunctional autonomic-intrinsic conductive self-healing hydrogels with pressure sensitivity, stretchability, and 3D printability. Adv. Mater. 2017, 29, 1700533. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Cheng, Y.; Huang, C.; Chen, R.; Wang, Z.; Wei, J. Self-healing conductive hydrogels based on alginate, gelatin and polypyrrole serve as a repairable circuit and a mechanical sensor. J. Mater. Chem. B 2019, 7, 5704–5712. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Shao, B.; Liu, T.; Zhang, Y.; Huang, R.; Chen, F.; Fu, Q. Robust and mechanically and electrically self-healing hydrogel for efficient electromagnetic interference shielding. ACS Appl. Mater. Interfaces 2018, 10, 8245–8257. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Han, Y.; Lv, S. Design of self-healing and electrically conductive silk fibroin-based hydrogels. ACS Appl. Mater. Interfaces 2019, 11, 20394–20403. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Su, Q.; Zhang, J.; Dong, A.; Lin, C.; Zhang, J. Facile access to multisensitive and self-healing hydrogels with reversible and dynamic boronic ester and disulfide linkages. Biomacromolecules 2017, 18, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Kong, X.; Xiong, Y.; Zhang, X.; Chen, H.; Jiang, W.; Niu, Y.; Xu, W.; Ren, C. An overview of dynamic covalent bonds in polymer material and their applications. Eur. Polym. J. 2020, 141, 110094. [Google Scholar] [CrossRef]
- Han, X.; Meng, X.; Wu, Z.; Wu, Z.; Qi, X. Dynamic imine bond cross-linked self-healing thermosensitive hydrogels for sustained anticancer therapy via intratumoral injection. Mater. Sci. Eng. C 2018, 93, 1064–1072. [Google Scholar] [CrossRef]
- Xu, P.; Liu, J.; Wang, S.; Chen, J.; Han, B.; Meng, Y.; Yang, S.; Xie, L.; Yang, M.; Jia, R. Dynamic covalent polymer engineering for stable and self-healing perovskite solar cells. Mater. Horiz. 2023, 10, 5223–5234. [Google Scholar] [CrossRef]
- Miki, R.; Yamaki, T.; Uchida, M.; Natsume, H. Phenylboronate-salicylate ester cross-linked self-healing hydrogel composed of modified hyaluronan at physiological pH. Soft Matter 2024, 20, 2926–2936. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, F.; Lv, M.; Chen, F.; Gao, F.; Xiong, Z.; Chen, X.; Shen, L.; Lin, F.; Gao, X. Self-healable covalently adaptable networks based on disulfide exchange. Polymers 2022, 14, 3953. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Xin, Y.; Wu, X.; Ao, Q. Mechanism of self-healing hydrogels and application in tissue engineering. Polymers 2022, 14, 2184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, P.; Zeng, J.; Li, J.; Wang, B.; Gao, W.; Xu, J.; Chen, K. Dynamic covalent bond enabled strong bio-based polyimide materials with thermally-driven adaptivity, healability and recycling. Chem. Eng. J. 2023, 465, 143017. [Google Scholar] [CrossRef]
- Inoue, A.; Yuk, H.; Lu, B.; Zhao, X. Strong adhesion of wet conducting polymers on diverse substrates. Sci. Adv. 2020, 6, eaay5394. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Burni, F.A.; Raghavan, S.R. Reversibly Sticking Metals and Graphite to Hydrogels and Tissues. ACS Cent. Sci. 2024, 10, 695–707. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.; Zhang, Y.; Yang, F.; Liu, Y.; Wang, X.; Yang, J.; Gong, X.; Zheng, J. Highly stretchable, self-adhesive, biocompatible, conductive hydrogels as fully polymeric strain sensors. J. Mater. Chem. A 2020, 8, 20474–20485. [Google Scholar] [CrossRef]
- Sun, F.; Huang, X.; Wang, X.; Liu, H.; Wu, Y.; Du, F.; Zhang, Y. Highly transparent, adhesive, stretchable and conductive PEDOT: PSS/polyacrylamide hydrogels for flexible strain sensors. Colloids Surf. A 2021, 625, 126897. [Google Scholar] [CrossRef]
- Zhou, Z.; Yuan, W.; Xie, X. A stretchable and adhesive composite hydrogel containing PEDOT: PSS for wide-range and precise motion sensing and electromagnetic interference shielding and as a triboelectric nanogenerator. Mater. Chem. Front. 2022, 6, 3359–3368. [Google Scholar] [CrossRef]
- Ma, X.; Wang, C.; Yuan, W.; Xie, X.; Song, Y. Highly adhesive, conductive, and self-healing hydrogel with triple cross-linking inspired by mussel and DNA for wound adhesion and human motion sensing. ACS Appl. Polym. Mater. 2021, 3, 6586–6597. [Google Scholar] [CrossRef]
- Fan, H.; Wang, J.; Jin, Z. Tough, swelling-resistant, self-healing, and adhesive dual-cross-linked hydrogels based on polymer–tannic acid multiple hydrogen bonds. Macromolecules 2018, 51, 1696–1705. [Google Scholar] [CrossRef]
- Mo, J.; Dai, Y.; Zhang, C.; Zhou, Y.; Li, W.; Song, Y.; Wu, C.; Wang, Z. Design of ultra-stretchable, highly adhesive and self-healable hydrogels via tannic acid-enabled dynamic interactions. Mater. Horiz. 2021, 8, 3409–3416. [Google Scholar] [CrossRef] [PubMed]
- Li, C. Towards conductive hydrogels in e-skins: A review on rational design and recent developments. RSC Adv. 2021, 11, 33835–33848. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Gan, D.; Han, L.; Wang, M.; Xing, W.; Xu, T.; Zhang, H.; Wang, K.; Fang, L.; Lu, X. Conductive and tough hydrogels based on biopolymer molecular templates for controlling in situ formation of polypyrrole nanorods. ACS Appl. Mater. Interfaces 2018, 10, 36218–36228. [Google Scholar] [CrossRef] [PubMed]
- Memic, A.; Colombani, T.; Eggermont, L.J.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Navare, K.J.; Mohammed, H.S.; Bencherif, S.A. Latest advances in cryogel technology for biomedical applications. Adv. Therap. 2019, 2, 1800114. [Google Scholar] [CrossRef]
- Humpolíček, P.; Radaszkiewicz, K.A.; Capáková, Z.; Pacherník, J.; Bober, P.; Kašpárková, V.; Rejmontová, P.; Lehocký, M.; Ponížil, P.; Stejskal, J. Polyaniline cryogels: Biocompatibility of novel conducting macroporous material. Sci. Rep. 2018, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Carreiro, S.; Wittbold, K.; Indic, P.; Fang, H.; Zhang, J.; Boyer, E.W. Wearable biosensors to detect physiologic change during opioid use. J. Med. Toxicol. 2016, 12, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Chatterjee, S.; Kaswan, K.; Chung, J.-H.; Fan, K.-P.; Lin, Z.-H. Recent advancements in sampling, power management strategies and development in applications for non-invasive wearable electrochemical sensors. J. Electroanal. Chem. 2022, 907, 116064. [Google Scholar] [CrossRef]
- Chenani, H.; Saeidi, M.; Rastkhiz, M.A.; Bolghanabadi, N.; Aghaii, A.H.; Orouji, M.; Hatamie, A.; Simchi, A. Challenges and Advances of Hydrogel-Based Wearable Electrochemical Biosensors for Real-Time Monitoring of Biofluids: From Lab to Market. A Review. Anal. Chem. 2024, 96, 8160–8183. [Google Scholar] [CrossRef]
- Saha, T.; Del Caño, R.; Mahato, K.; De la Paz, E.; Chen, C.; Ding, S.; Yin, L.; Wang, J. Wearable electrochemical glucose sensors in diabetes management: A comprehensive review. Chem. Rev. 2023, 123, 7854–7889. [Google Scholar] [CrossRef]
- Zhu, X.; Ju, Y.; Chen, J.; Liu, D.; Liu, H. Nonenzymatic wearable sensor for electrochemical analysis of perspiration glucose. ACS Sens. 2018, 3, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Bae, C.W.; Toi, P.T.; Kim, B.Y.; Lee, W.I.; Lee, H.B.; Hanif, A.; Lee, E.H.; Lee, N.-E. Fully stretchable capillary microfluidics-integrated nanoporous gold electrochemical sensor for wearable continuous glucose monitoring. ACS Appl. Mater. Interfaces 2019, 11, 14567–14575. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Liu, Y.; Zhang, C. A sample-to-answer, wearable cloth-based electrochemical sensor (WCECS) for point-of-care detection of glucose in sweat. Sens. Actuators B 2021, 343, 130131. [Google Scholar] [CrossRef]
- Li, Q.-F.; Chen, X.; Wang, H.; Liu, M.; Peng, H.-L. Pt/MXene-based flexible wearable non-enzymatic electrochemical sensor for continuous glucose detection in sweat. ACS Appl. Mater. Interfaces 2023, 15, 13290–13298. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Su, T.; Lu, Q.; Shang, Z.; Xu, Q.; Hu, X. Highly stretchable wearable electrochemical sensor based on Ni-Co MOF nanosheet-decorated Ag/rGO/PU fiber for continuous sweat glucose detection. Anal. Chem. 2021, 93, 16222–16230. [Google Scholar] [CrossRef]
- Shu, Y.; Shang, Z.; Su, T.; Zhang, S.; Lu, Q.; Xu, Q.; Hu, X. A highly flexible Ni–Co MOF nanosheet coated Au/PDMS film based wearable electrochemical sensor for continuous human sweat glucose monitoring. Analyst 2022, 147, 1440–1448. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Rinawati, M.; Chang, L.-Y.; Wang, Y.-X.; Wu, Y.-T.; Yen, Y.-H.; Chen, K.-J.; Ho, K.-C.; Yeh, M.-H. A non-invasive wearable sweat biosensor with a flexible N-GQDs/PANI nanocomposite layer for glucose monitoring. Sens. Actuators B 2023, 383, 133617. [Google Scholar] [CrossRef]
- Lin, P.-H.; Sheu, S.-C.; Chen, C.-W.; Huang, S.-C.; Li, B.-R. Wearable hydrogel patch with noninvasive, electrochemical glucose sensor for natural sweat detection. Talanta 2022, 241, 123187. [Google Scholar] [CrossRef]
- Aycan, D.; Karaca, F.; Alemdar, N. Development of hyaluronic acid-based electroconductive hydrogel as a sensitive non-enzymatic glucose sensor. Mater. Today Commun. 2023, 35, 105745. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, J.; Wu, C.; Hu, X.; Lu, Y.; Wang, G.; Yu, F.; Zhang, X.; Wang, Y. Flexible and self-healing electrochemical hydrogel sensor with high efficiency toward glucose monitoring. Biosens. Bioelectron. 2020, 155, 112105. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, J.; Liu, H.; Ran, R.; Zhang, T.; Li, Z. Enzyme-chitosan hydrogels for high sensitivity flexible silk-based electrochemical glucose sensor. Microchem. J. 2024, 200, 110306. [Google Scholar] [CrossRef]
- Kanokpaka, P.; Chang, Y.-H.; Chang, C.-C.; Rinawati, M.; Wang, P.-C.; Chang, L.-Y.; Yeh, M.-H. Enabling glucose adaptive self-healing hydrogel based triboelectric biosensor for tracking a human perspiration. Nano Energy 2023, 112, 108513. [Google Scholar] [CrossRef]
- Xu, C.; Jiang, D.; Ge, Y.; Huang, L.; Xiao, Y.; Ren, X.; Liu, X.; Zhang, Q.; Wang, Y. A PEDOT: PSS conductive hydrogel incorporated with Prussian blue nanoparticles for wearable and noninvasive monitoring of glucose. Chem. Eng. J. 2022, 431, 134109. [Google Scholar] [CrossRef]
- Xia, Y.; Su, T.; Mi, Z.; Feng, Z.; Hong, Y.; Hu, X.; Shu, Y. Wearable electrochemical sensor based on bimetallic MOF coated CNT/PDMS film electrode via a dual-stamping method for real-time sweat glucose analysis. Anal. Chim. Acta 2023, 1278, 341754. [Google Scholar] [CrossRef]
- Alam, F.; RoyChoudhury, S.; Jalal, A.H.; Umasankar, Y.; Forouzanfar, S.; Akter, N.; Bhansali, S.; Pala, N. Lactate biosensing: The emerging point-of-care and personal health monitoring. Biosens. Bioelectron. 2018, 117, 818–829. [Google Scholar] [CrossRef]
- Pundir, C.S.; Narwal, V.; Batra, B. Determination of lactic acid with special emphasis on biosensing methods: A review. Biosens. Bioelectron. 2016, 86, 777–790. [Google Scholar] [CrossRef]
- Rasitanon, N.; Ittisoponpisan, S.; Kaewpradub, K.; Jeerapan, I. Wearable Electrodes for Lactate: Applications in Enzyme-Based Sensors and Energy Biodevices. Anal. Sens. 2023, 3, e202200066. [Google Scholar] [CrossRef]
- Wu, Z.-Q.; Cao, X.-Q.; Hua, Y.; Yu, C.-M. A Bifunctional Wearable Sensor Based on a Nanoporous Membrane for Simultaneous Detection of Sweat Lactate and Temperature. Anal. Chem. 2024, 96, 3087–3095. [Google Scholar] [CrossRef]
- Yao, C.-Y.; Qin, Y.; Fan, W.-T.; Yan, L.-P.; Chen, M.; Liu, Y.-L.; Huang, W.-H. A three-dimensional electrochemical biosensor integrated with hydrogel for cells culture and lactate release monitoring. J. Electroanal. Chem. 2022, 915, 116338. [Google Scholar] [CrossRef]
- Babeli, I.; Puiggalí-Jou, A.; Roa, J.J.; Ginebra, M.-P.; García-Torres, J.; Aleman, C. Hybrid conducting alginate-based hydrogel for hydrogen peroxide detection from enzymatic oxidation of lactate. Int. J. Biol. Macromol. 2021, 193, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ling, Z.; Liu, X.; Bai, L.; Wang, W.; Chen, H.; Yang, H.; Yang, L.; Wei, D. Nanocomposite hydrogels flexible sensors with functional cellulose nanocrystals for monitoring human motion and lactate in sweat. Chem. Eng. J. 2023, 466, 143306. [Google Scholar] [CrossRef]
- Saha, T.; Songkakul, T.; Knisely, C.T.; Yokus, M.A.; Daniele, M.A.; Dickey, M.D.; Bozkurt, A.; Velev, O.D. Wireless wearable electrochemical sensing platform with zero-power osmotic sweat extraction for continuous lactate monitoring. ACS Sens. 2022, 7, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Qiao, X.; Tao, R.; Li, Y.; Zhao, S.; Cai, Y.; Luo, X. A wearable sensor based on multifunctional conductive hydrogel for simultaneous accurate pH and tyrosine monitoring in sweat. Biosens. Bioelectron. 2023, 234, 115360. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Chung, S.; Chang, A.-Y.; Wang, J.; Hall, D.A. A non-invasive wearable stress patch for real-time cortisol monitoring using a pseudoknot-assisted aptamer. Biosens. Bioelectron. 2023, 227, 115097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dong, G.; Feng, H.; Shan, S.; Huang, L.; Yuan, F.; Bao, B.; Yan, L.; Xia, Z.; Lawson, T. Wearable corneal biosensors fabricated from PEDOT functionalized sulfur-doped graphene for use in the early detection of myopia. Adv. Mater. Technol. 2020, 5, 2000682. [Google Scholar] [CrossRef]
- San Nah, J.; Barman, S.C.; Zahed, M.A.; Sharifuzzaman, M.; Yoon, H.; Park, C.; Yoon, S.; Zhang, S.; Park, J.Y. A wearable microfluidics-integrated impedimetric immunosensor based on Ti3C2Tx MXene incorporated laser-burned graphene for noninvasive sweat cortisol detection. Sens. Actuators B 2021, 329, 129206. [Google Scholar] [CrossRef]
- Inukonda, S.M.; Panda, S. Bio-inspired wettability patterning for flexible and wearable electrochemical immunosensors utilizing PDMS-TiO2 coating. Microchem. J. 2024, 200, 110425. [Google Scholar] [CrossRef]
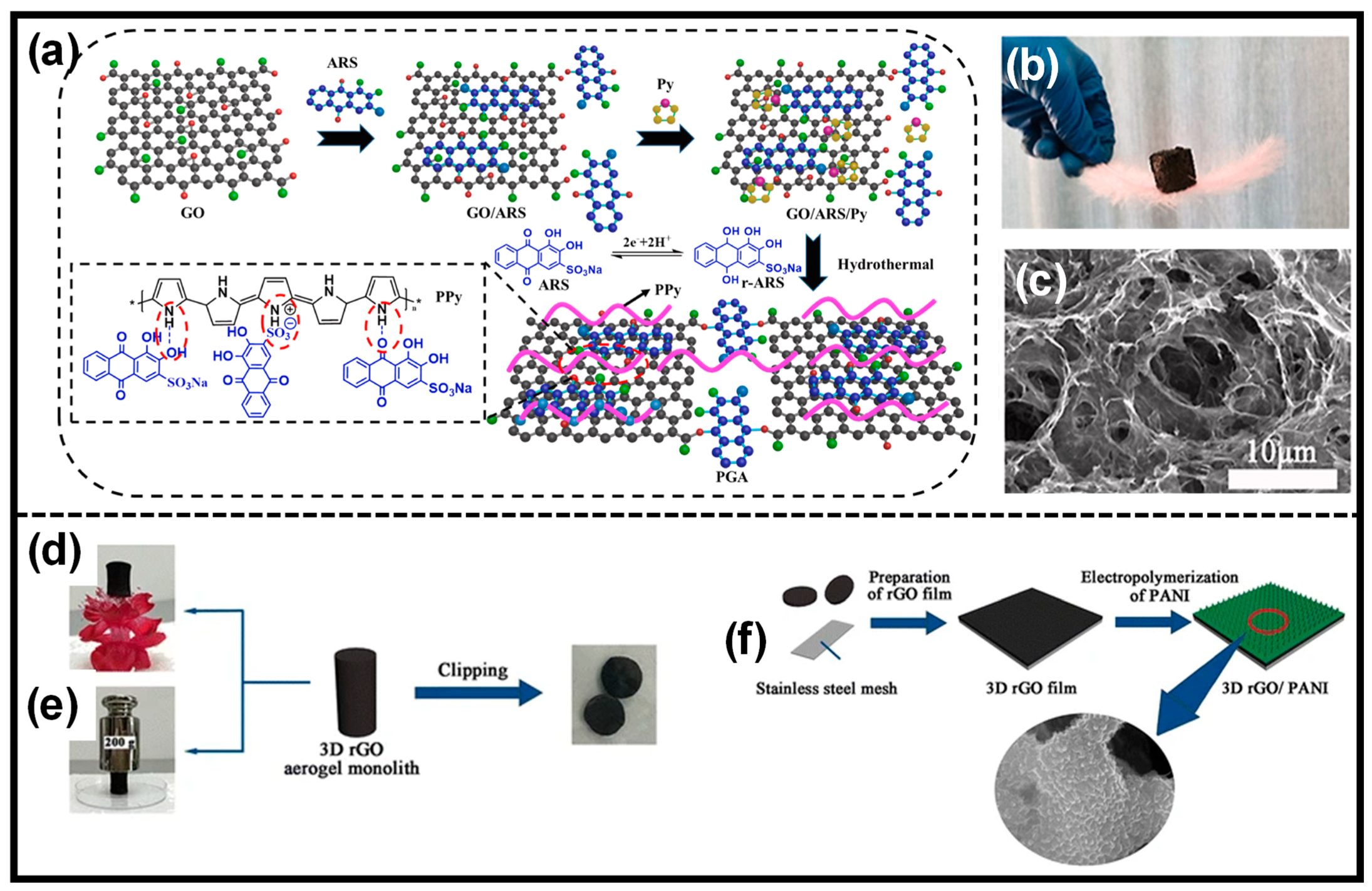

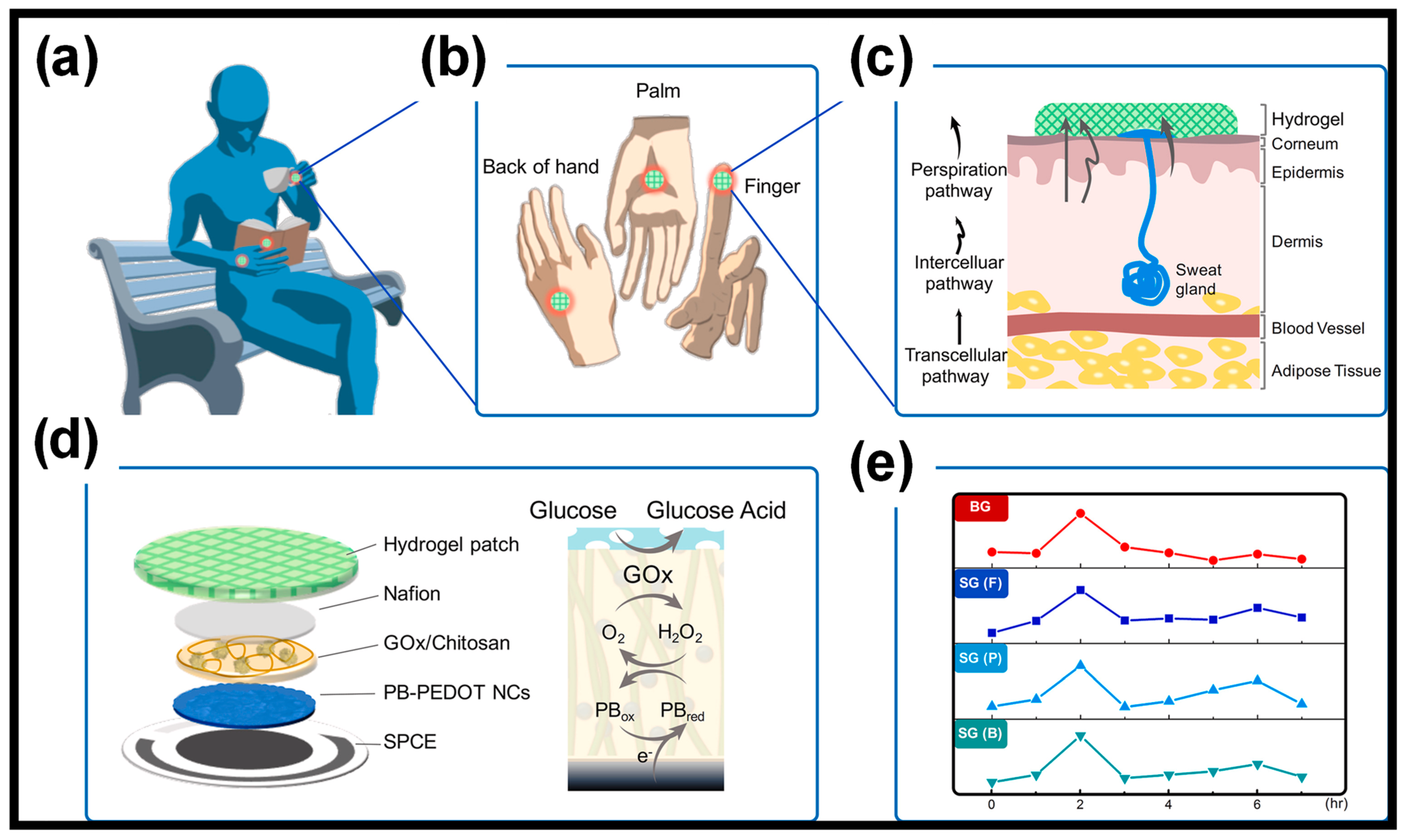
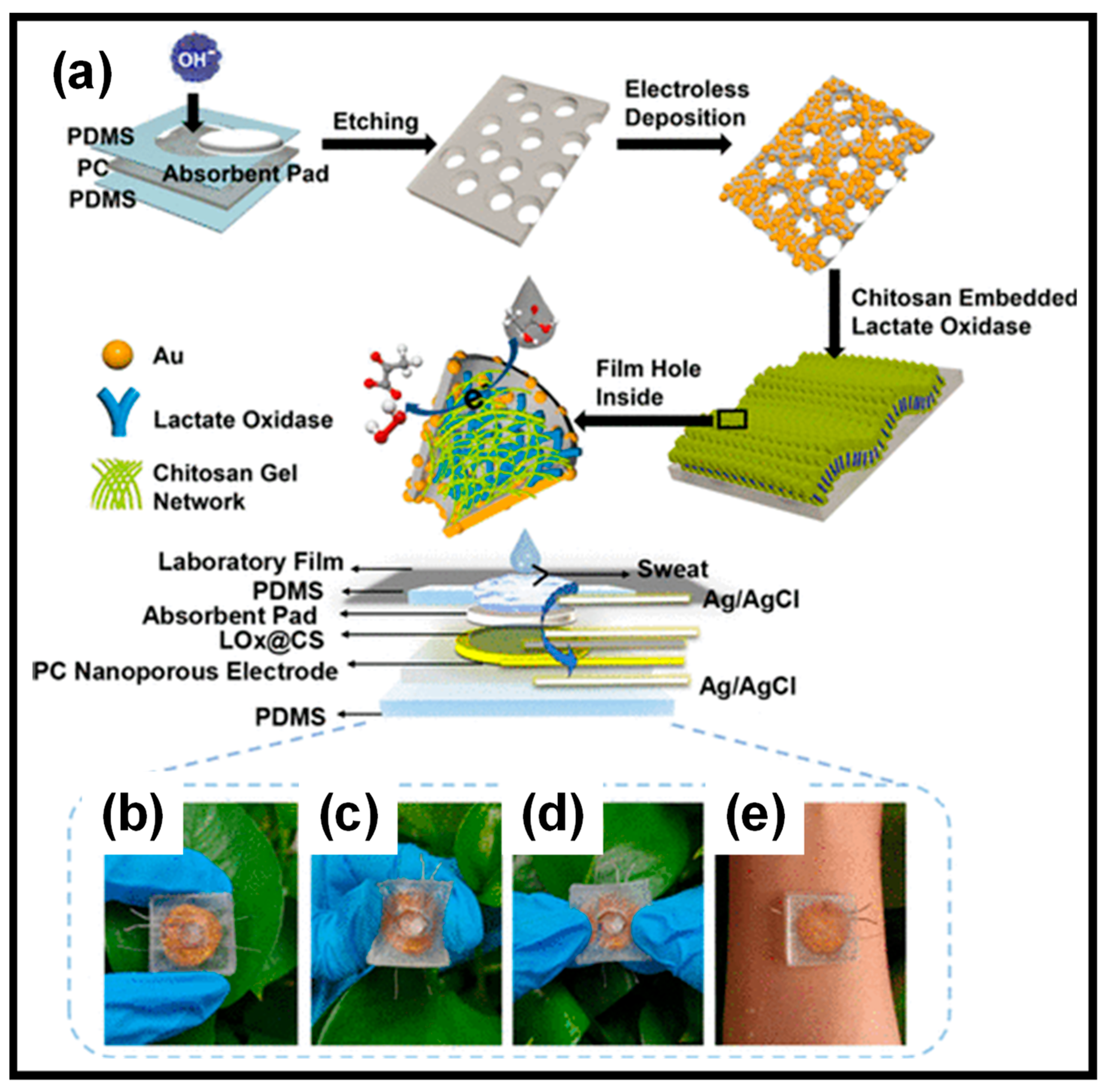
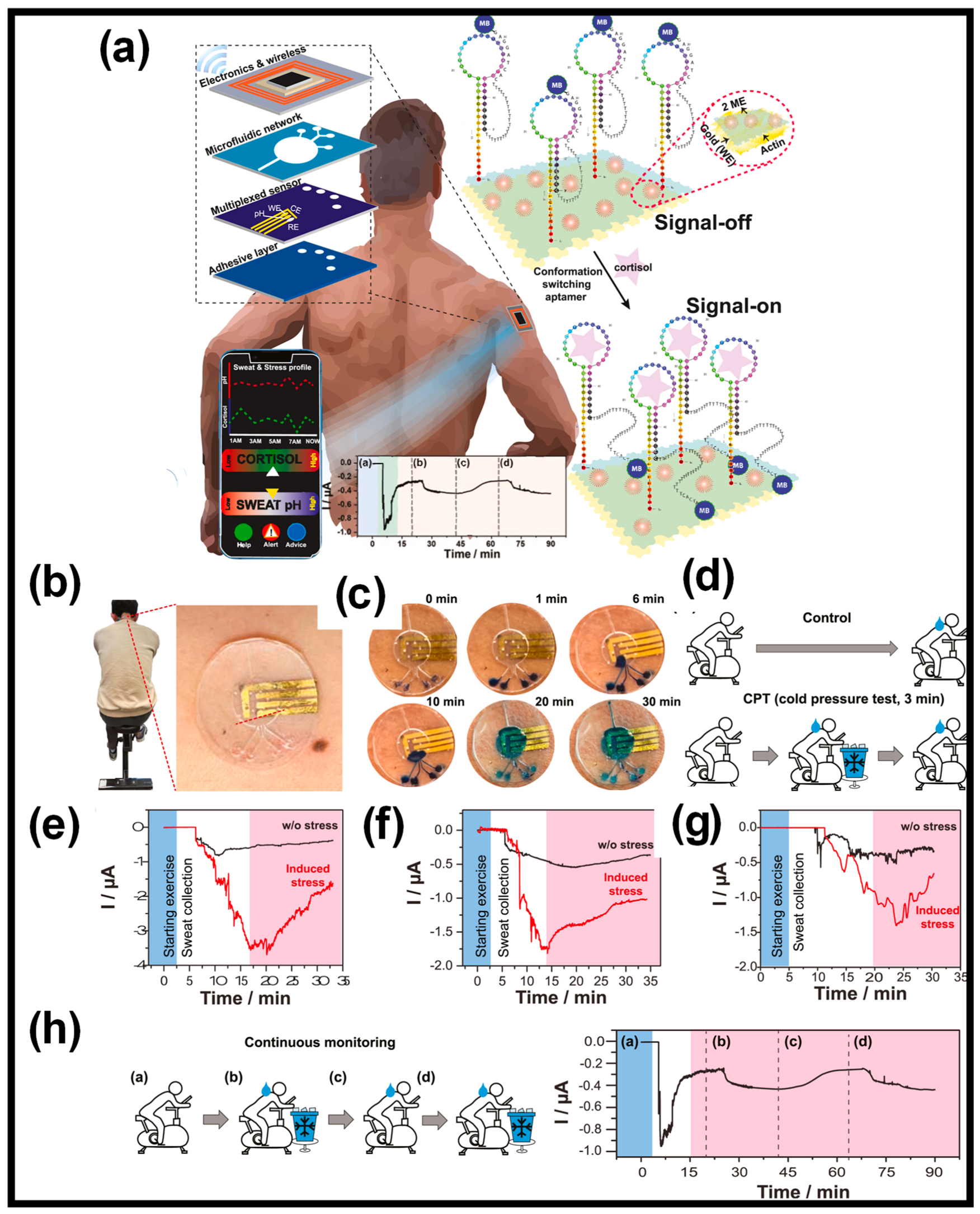
| CPs | Advantages | Disadvantages |
|---|---|---|
| PPy | High conductivity | Brittle |
| Ease of synthesis | Poor solubility | |
| Biocompatibility | Relatively expensive | |
| Stable in an oxidized form | Poor cycling stability | |
| Non-transparent | ||
| PANI | Low cost | Relatively poor conductivity |
| Large specific surface area | Lack of flexibility | |
| High stability | Non-biodegradable | |
| Ease of synthesis | Low processability | |
| PEDOT | Electrochemically stable | Poor solubility |
| Tunable conductivity | Limited flexibility | |
| Environmentally stable | Hard to process | |
| Biocompatibility |
| Composites | Linear Range (mM) | LOD (µM) | Sensitivity (μA mM−1 cm−2) | Merits | Demerits | Ref. |
|---|---|---|---|---|---|---|
| Pt/MXene CH * | 0−8 | 29.15 | 3.43 | Enhanced the stability Long-term monitoring | Sweat accumulation affects the patch | [106] |
| Ni-Co MOF/Ag/rGO/PU fiber | 0.01−0.66 | 3.28 | 425.9 | Good mechanical flexibility | Short linear range | [107] |
| Ni-Co MOF/Au/PDMS | 0.02−0.79 | 4.25 | 205.1 | Good mechanical flexibility Long-term monitoring | Short linear range | [108] |
| GOx/N-GQDs/PANI | 0.05−0.5 | 34 | 28.2 | Good mechanical flexibility Long-term monitoring | Short linear range | [109] |
| PB-PEDOT NC | 0.00625−0.8 | 4.0 | - | Natural sweating sample | Hydrogel patch used only for sweat collection Non-flexible sensor | [110] |
| HA/MA-rGO-PANI | 0.0003–0.005 | 0.3 | 421.42 | Good mechanical flexibility | Short linear range | [111] |
| PET/QCS-MOx-OD/GOx | 1.0–111 | 32.4 | 176 | Self-healing ability | Hydrogel removal on PET surface by simple press | [112] |
| GOD/PB/RGO/SF | 0.07–2.0 2.0–6.0 6.0–10 | 65.57 | 230.96 39.4 14.89 | Chitosan hydrogel provides biocompatibility for enzyme | Poor long-term stability | [113] |
| PVA/CA/β-CD/GOx | 0–0.5 | 98.84 | 8.762 V/mM | Triboelectric biosensors Self-healing ability Self-powered | Short linear range | [114] |
| PEDOT:PSS/DF/PB/GOx | 0.001–0.243 0.243–3.243 | 0.85 | 340.1184.3 | In vivo non-invasive monitoring of interstitial fluid glucose Reverse iontophoresis | Poor long-term storage stability | [115] |
| Ni-Co MOF/CNTs/MWCNTs/PDMS | 0.02–1.1 | 6.78 | 71.62 | Stamping-vacuum filtration dry transfer method Good mechanical flexibility | External sweat absorbent cloth | [116] |
| Composites | Linear Range (mM) | LOD (µM) | Sensitivity (μA mM−1 cm−2) | Merits | Demerits | Ref. |
|---|---|---|---|---|---|---|
| PC-AuNPs-LOx-CS * | Simultaneously sweat lactate and temperature detection | External sweat absorbent pad | [120] | |||
| 0.01−35 | 0.144 | 0.0824 μA mM–1 | ||||
| LOx/PCP | 0.02–1.0 | 0.75 | - | Real-time monitoring of lactate released from C6 glioma cells | Short linear range | [121] |
| Alg/PEDOT/GNP/LOx-h | Good mechanical flexibility | Poor reproducibility | [122] | |||
| 1.0−100 | 400 | 0.0216 | ||||
| PVA/CNCs@PDA-AuNPs/LOx | 0.5−30 | 310 | 0.098 μA mM–1 | Good mechanical flexibility Self-healing ability | High LOD | [123] |
| C/PB/GR/LOx | 0.0−15 | 0.35 | 9.0 | Zero-power sweat sampling patch Osmotic sweat extraction | Long-term stability not reported | [124] |
| Composites | Biomarker | Linear Range | LOD | Sensitivity | Merits | Demerits | Ref. |
|---|---|---|---|---|---|---|---|
| TA-Ag-CNT-PANI * | Tyrosine | 0.01−0.2 mM | 3.3 µM | - | Good mechanical elasticity Antibacterial property | Hydrogel lifetime is limited by water uptake | [125] |
| PDMS/Gold/Actin/2-ME | Cortisol | 1.0 pM–1.0 µM | 0.2 pM | 0.33 μA/mm2/pM | Pseudoknot-assisted aptamer | Expensive than other methods | [126] |
| PEDOT-G-TYR | Dopamine (DA) | 0.0–70 µM | 101 nM | 12.9 μA mM−1 cm−2 | DA in tears was linked to myopia Biocompatibility | 80% of the DA was lost from the eye during the in vivo test | [127] |
| Ti3C2Tx MXene/LBG | Cortisol | 0.01−100 nM | 3.88 pM | - | Low-cost immunosensor Non-invasive Rapid microfluidic analysis | Short linear range | [128] |
| PDMS-TiO2/PET | Model biomarker (Prostate-specific antigen) | 0.0001−100 ng mL−1 | 0.0001 ng mL−1 | 30.3 µA ng−1 mL | Low-volume (2 μL) point-of-care immunosensor | In vivo not studied | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thirumalai, D.; Santhamoorthy, M.; Kim, S.-C.; Lim, H.-R. Conductive Polymer-Based Hydrogels for Wearable Electrochemical Biosensors. Gels 2024, 10, 459. https://doi.org/10.3390/gels10070459
Thirumalai D, Santhamoorthy M, Kim S-C, Lim H-R. Conductive Polymer-Based Hydrogels for Wearable Electrochemical Biosensors. Gels. 2024; 10(7):459. https://doi.org/10.3390/gels10070459
Chicago/Turabian StyleThirumalai, Dinakaran, Madhappan Santhamoorthy, Seong-Cheol Kim, and Hyo-Ryoung Lim. 2024. "Conductive Polymer-Based Hydrogels for Wearable Electrochemical Biosensors" Gels 10, no. 7: 459. https://doi.org/10.3390/gels10070459
APA StyleThirumalai, D., Santhamoorthy, M., Kim, S.-C., & Lim, H.-R. (2024). Conductive Polymer-Based Hydrogels for Wearable Electrochemical Biosensors. Gels, 10(7), 459. https://doi.org/10.3390/gels10070459








