Highlights
Aerogels doped with SiO2 particles derived from rice husks are obtained by a freeze-drying method.
SiO2 particles provide a reduced-flammability advantage over undoped PUR aerogels.
Increasing SiO2 content significantly enhances flame-retardancy in PUR aerogels.
Abstract
In this work, polyurethane (PUR) aerogels doped with different SiO2 particles, derived from a renewable source, were successfully synthesized, and the effects of SiO2 content on the properties of PUR aerogels were investigated. Specifically, three types of SiO2-based particles obtained from rice husk through different procedures were evaluated to enhance the thermal stability of the composites with special attention given to flame-retardant properties. With the optimal SiO2 particles, obtained through acid digestion, the influence of their content between 0.5 and 3 wt.% on the physicochemical characteristics of the synthesized aerogels was thoroughly examined. The results showed that increasing the doping agent content improved the lightness, thermal stability, and flame-retardant properties of the resulting PUR aerogels, with the best performance observed at a 2 wt.% doping level. The doped aerogel samples with non-modified SiO2 particles significantly enhanced the fire safety performance of the material, exhibiting up to an eightfold increase in flame retardancy. However, modification of the SiO2 particles with phytic acid did not slow down the combustion velocity when filling the aerogels. This research highlights the promising potential of doped PUR/SiO2 aerogels in advancing materials science and engineering applications for withstanding high temperatures and improving fire safety.
1. Introduction
Making buildings more efficient in terms of fire resistance in construction materials is essential to minimize fire spread and extend evacuation time. Incorporating fire-resistant materials, such as insulation panels and coatings, is key in slowing down flame propagation and reducing the emission of harmful toxic smoke [1].
Conventional insulation materials, such as foams (colloidal systems like sols, gels, and aerogels), are widely used for insulation and have advantages such as being widely available, affordable, and easy to install. However, they also have disadvantages such as low fire resistance (requiring the incorporation of flame-retardant additives), potential toxic emissions, and limited energy efficiency. In this sense, it is important to note that combustible insulation materials are responsible for most fatal fires. The widespread use of these types of commercial materials has generated an urgent need to develop safer and more efficient alternatives, such as new fire-resistant insulation materials for buildings [2]. These materials are essential for preventing the spread of fire and protecting the lives of building occupants. Ongoing technological advancements and research into new materials are leading to the development of safer and more efficient options in terms of fire resistance, providing enhanced protection in case of emergencies. Depending on their nature, flame-retardant systems can act either physically (by cooling, forming a protective layer, or diluting fuel) or chemically (through reaction in the condensed or gas phase). They can interfere with the various processes involved in polymer combustion (heating, pyrolysis, ignition, and propagation of thermal degradation) [3,4].
Aerogels, highly porous materials with low density (sometimes likened to solid foams due to their structural similarities), are renowned for their exceptional thermal insulation properties [5]. Their historical and commercial significance spans nearly a century, during which they have undergone complex development but demonstrated remarkable potential across diverse applications [6,7]. Most aerogels are synthesized using the sol–gel method, a green synthesis technique that is applied under mild conditions and with eco-friendly solvents such as water and alcohols [8,9,10]. Recent focus has been on organic aerogels, particularly those primarily composed of polyurethane polymer chains (PUR aerogels), aimed at producing materials with exceptionally low conductivity and density [11,12,13]. Key attributes of organic aerogels include high insulation capacity, stemming from their low thermal conductivity effectively reducing heat transfer. Additionally, they boast low density and high porosity, affording a substantial surface area that enhances insulation performance. Furthermore, aerogels exhibit flexibility, enabling them to conform to various shapes and surfaces for convenient installation. However, the high flammability of most organic polymeric materials presents a significant challenge, resulting in the generation of substantial smoke, toxic gases, heat, and melting drips during combustion. Consequently, these factors contribute to considerable damage to human lives and properties annually, underscoring safety concerns across various fields of application [14].
To improve the fire-retardant behavior of PUR aerogels (and other hybrids synthesized via sol–gel in environmentally friendly conditions) and to reduce the release of plentiful heat and toxic smoke, different flame-retardant fillers can be incorporated into the aerogel matrix. These flame-retardant dopants should not negatively affect the synthesis process. Some additives that improve flame resistance and suppress smoke include carbon nanotubes [15], clays [16], lignin [17], phosphorus [18], and silica (SiO2) particles [19]. Among them, SiO2 particles stand out due to their small size and high surface area, which allow them to interact with the PUR matrix and enhance its structure and properties [20].
During the synthesis of SiO2-doped PUR aerogels, the concentration and dispersion of SiO2 particles, as well as the manufacturing process, can significantly influence the extent of property enhancement [21,22]. Generally, an increase in SiO2 content leads to enhanced mechanical strength, thermal stability, and fire resistance of the aerogel [19,23,24]. However, an excess of SiO2 particles can negatively affect the porosity and density of the aerogel, which can reduce its thermal insulation performance. Therefore, it is important to establish an appropriate balance in the content of SiO2 particles to achieve the best final properties of PUR aerogels [21,25].
The objective of this study was to investigate the influence of three types of SiO2 particles as doping agents in PUR aerogels, with a particular focus on flame-retardant properties. A novel aspect of this research is the use of SiO2 additives derived from natural rice husk (RH) as dopants, which are incorporated into the polymer matrix. This innovative approach not only reduces the environmental footprint by using a low-cost and widely available crop residue, but also enhances the sustainability of SiO2 production, contributing to the circular economy. The study details the effect of particle content on the physicochemical properties and performance of the synthesized aerogels.
2. Results and Discussion
2.1. Impact of Silica Particles’ Nature as Dopant of PUR Aerogels
The incorporation of SiO2 particles of a different nature depending on the extraction procedure (AD and SG at 2 wt.%) into the polymeric structure of PUR aerogels was evaluated in detail. Table 1 summarizes the SiO2-doped aerogels’ characterization results obtained in terms of density, thermal conductivity, Young’s modulus, and glass transition temperature (Tg). The “PUR” sample corresponds to the aerogel sample that is free of SiO2 particles, commonly known as a blank sample.

Table 1.
Characterization results of the SiO2-doped PUR aerogels.
As can be seen, the densities of the doped samples increase such that PUR_AD2 < PUR_SG2, while the low thermal conductivity values obtained for the doped PUR aerogels remain practically constant regardless of the type of particles incorporated. On the other hand, the incorporation of SiO2_AD2 particles lightly increases the surface area and total pore volume of the resulting aerogel compared with the undoped one, contrary to what was observed when incorporating the SiO2_SG2 particles, which resulted in aerogels with a very low surface area. As a result, higher density in the doped samples and lower surface area correspond to increased sample hardness, as indicated by Young’s modulus. The Tg results for all samples at around −50 °C are consistent with the typical PUR structure [26].
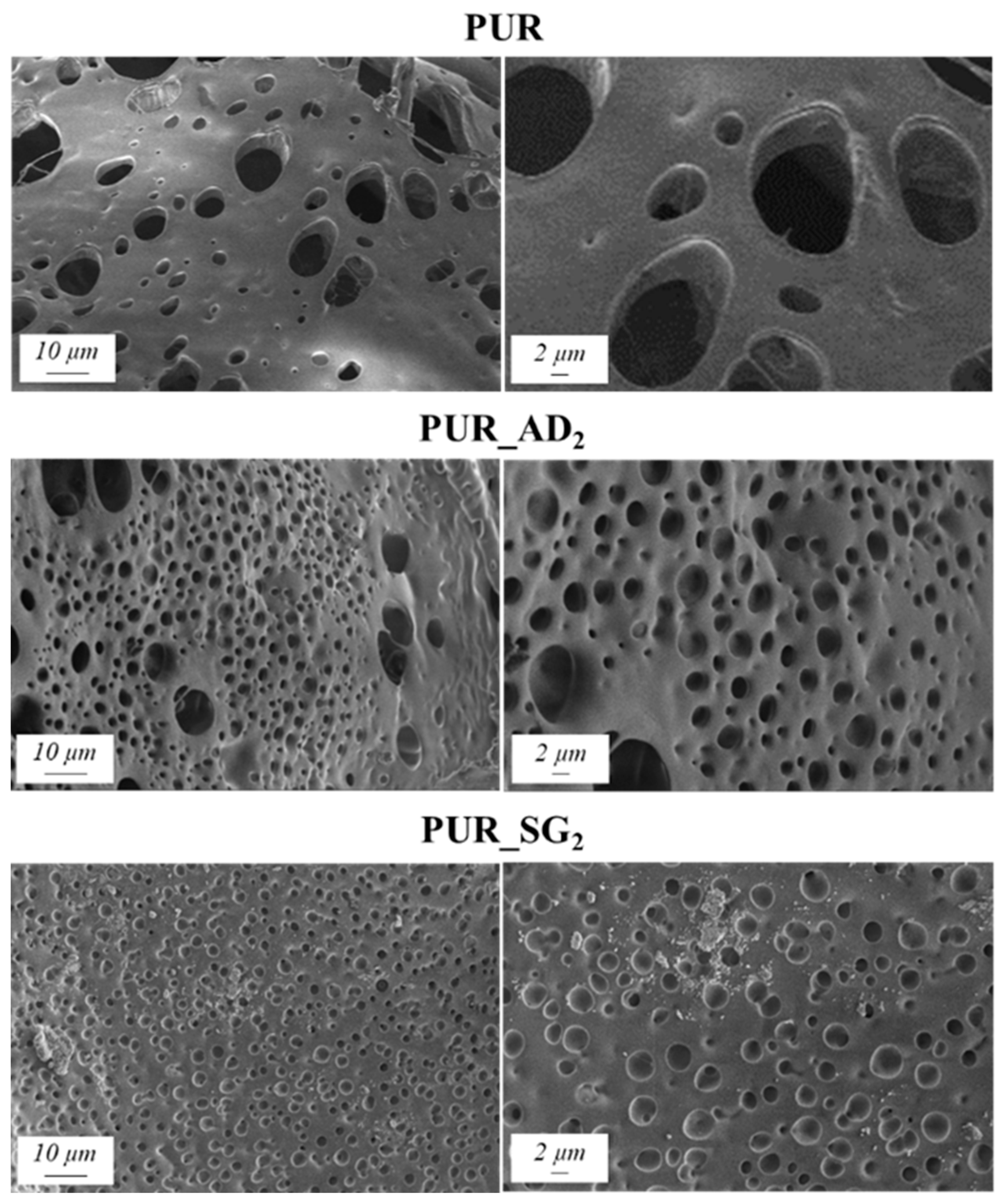
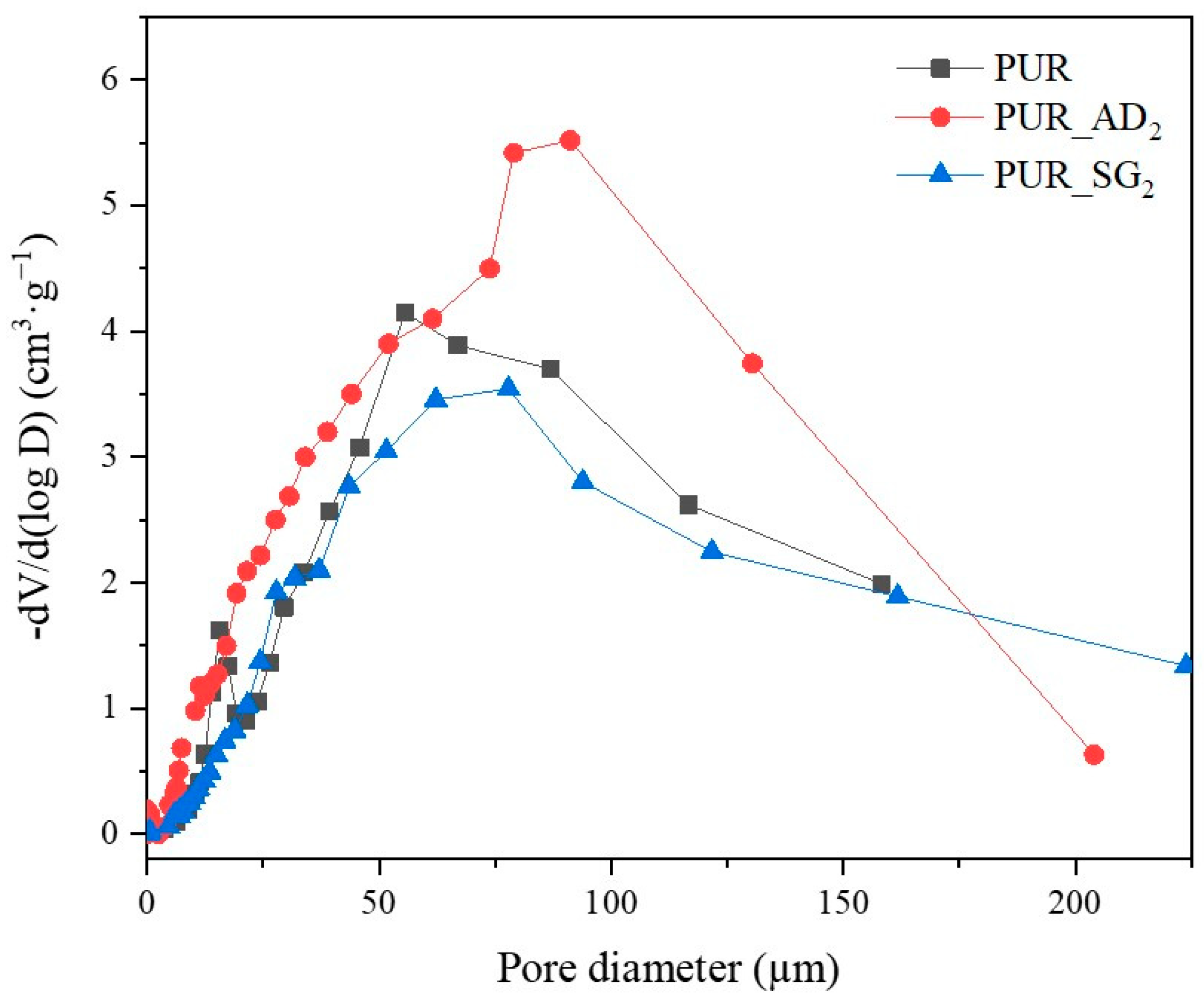
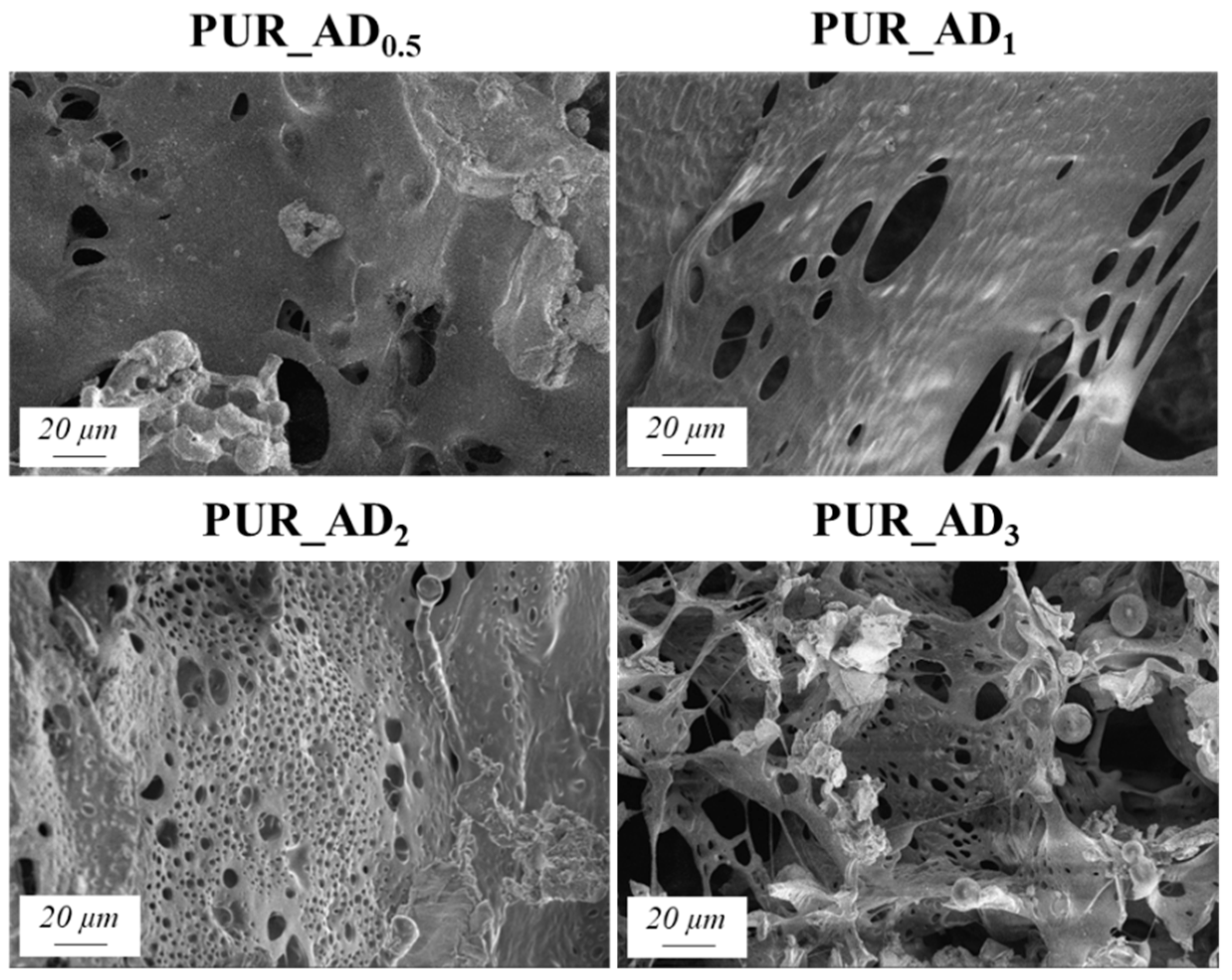
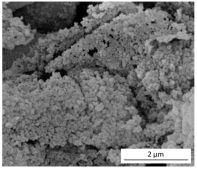
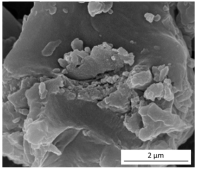
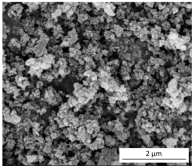
The SEM results depicted in Figure 1 confirm the earlier findings. The porous structure of the PUR_SG2 aerogels is observed to be closing, resulting in a more compact appearance consistent with the surface area, pore volume, and density values already mentioned. This fact is further supported by the pore size distribution (PSD) obtained by a Hg porosimeter, which indicated pore sizes ranging from 3 to 300 µm for both samples, as illustrated in Figure 2. Moreover, as an example, the elemental mapping and EDS analysis of the PUR_AD2 sample are presented in Figure S1, showcasing the well-dispersed SiO2 particles throughout the polymer.
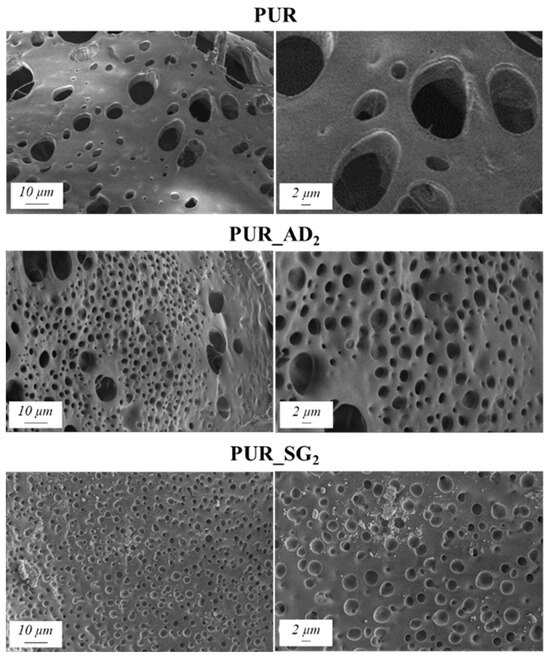
Figure 1.
SEM micrographs corresponding to the synthesized SiO2-doped PUR aerogels.
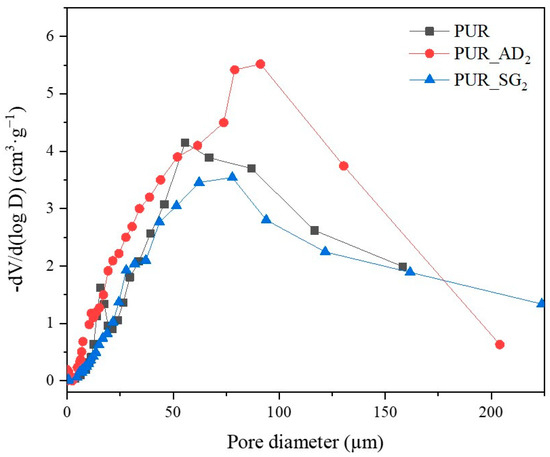
Figure 2.
Pore size distribution of the synthesized SiO2-doped PUR aerogels.
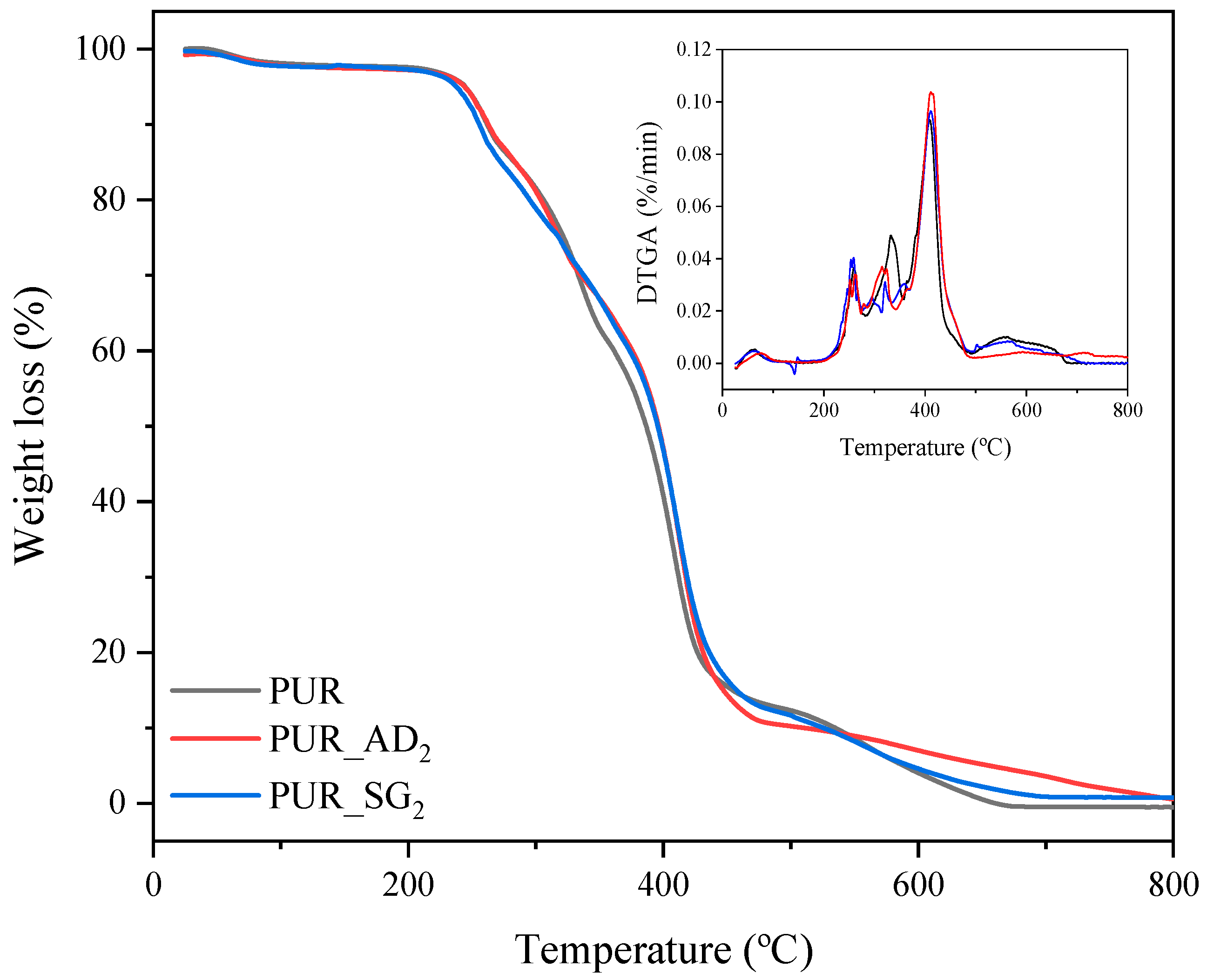
The influence of the nature of the SiO2 particles incorporated into the structure of PUR aerogels on their thermal degradation is illustrated in Figure 3 (TGA curves corresponding to the synthesized SiO2-doped aerogel samples). As can be observed, similar profiles are found for the different aerogels, including the undoped one. PUR aerogel samples exhibit thermal stability of up to 230 °C, wherein they undergo complete loss of any water or residual solvents. Subsequently, between 230 and 440 °C, the most significant mass loss (>90 wt.%) occurs due to the decomposition of a PUR network. Finally, from 440 to 700 °C, the decomposition of ester groups takes place, leaving approximately 1 wt.% remaining [27,28].
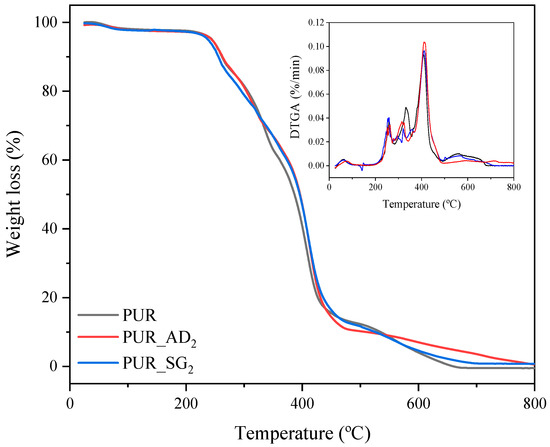
Figure 3.
TGA and DTGA curves of the different SiO2-doped PUR aerogels.
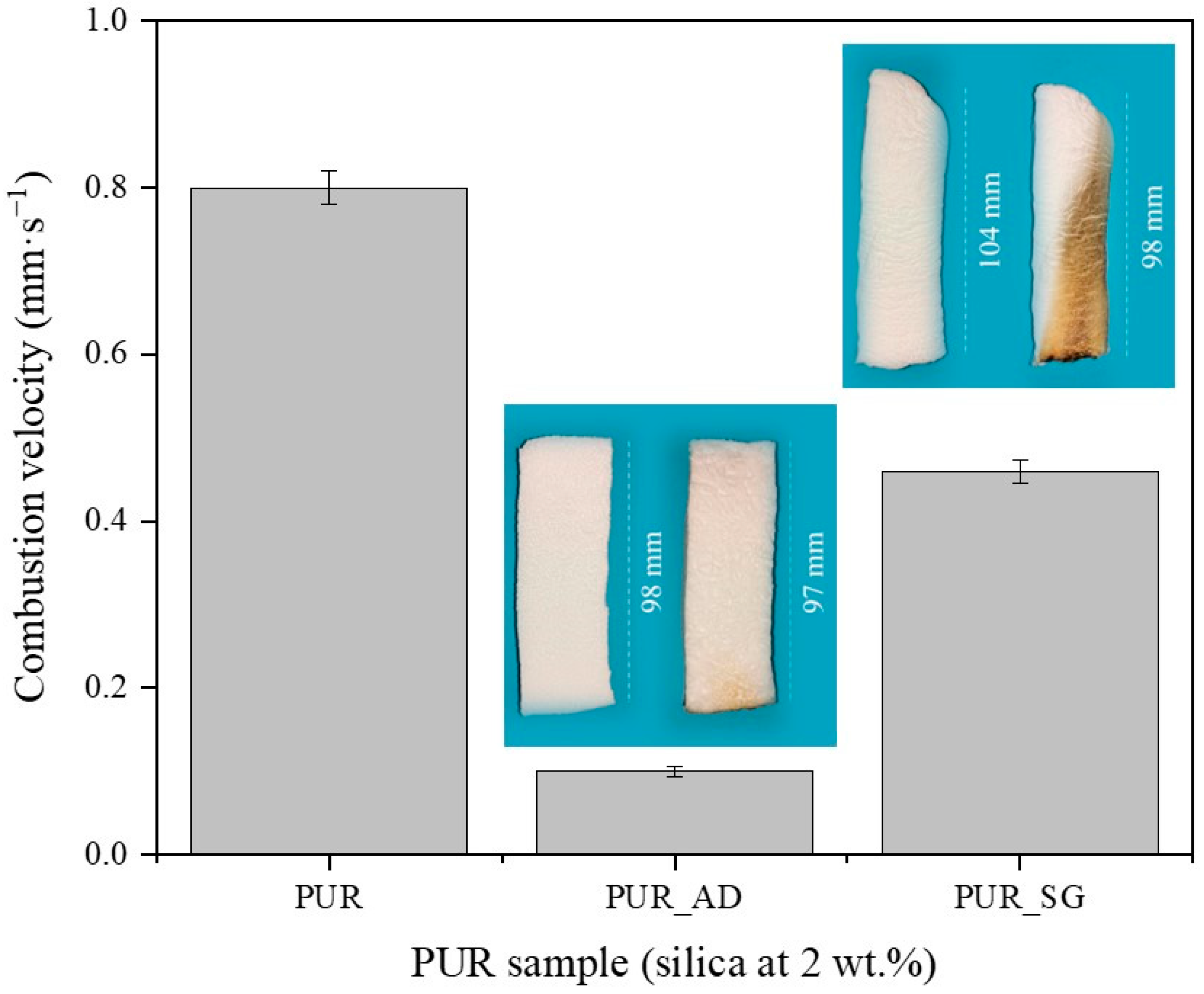
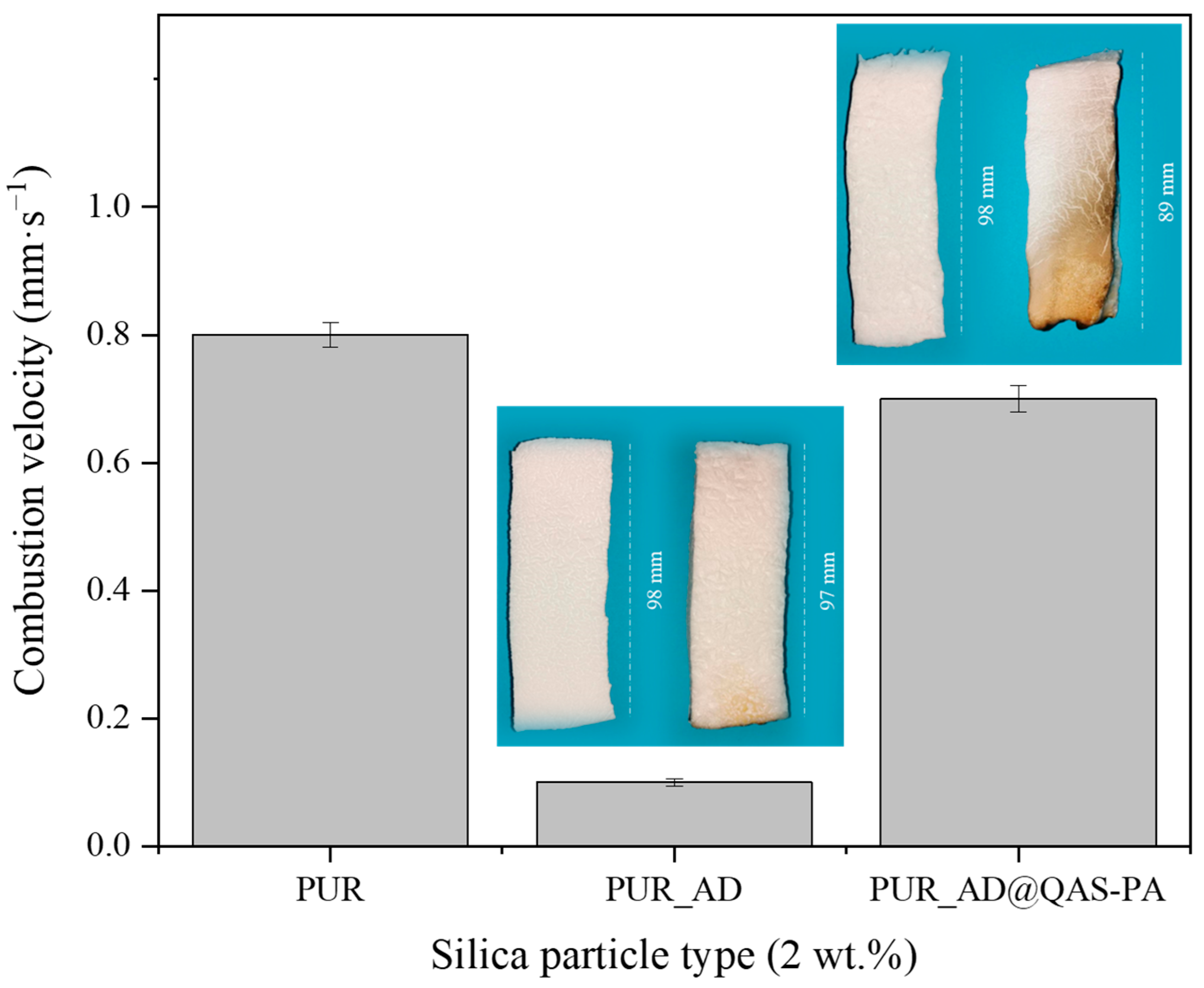
To explore the use of synthesized SiO2-doped aerogels as fireproof materials in the construction sector, their flame-retardant capability was studied. The combustion velocity was calculated after the total time, ignition, and propagation (13 s) (Figure 4). As can be observed, both doped aerogels with AD and SG at 2 wt.% reduced the combustion velocity compared with the undoped aerogel. Interestingly, the presence of AD2 particles in the polymeric matrix significantly mitigated the combustion velocity of the aerogel, and it does so up to more than six times compared with PUR_SG2, resulting in a velocity as low as 0.07 ± 0.01 mm·s−1. Therefore, these results strongly suggest that SiO2 particles obtained through AD of RH and subsequent calcination (PUR_AD2) demonstrates superior performance of flame retardancy when acting as a doping agent for PUR compared with the sample obtained through alkaline extraction of the RH ash, followed by sol–gel precipitation (PUR_SG2). Considering the physicochemical characterization of the particles (Table 4), the results could be attributed to the fact that the alkaline extraction and sol–gel precipitation process generates SiO2 particles with the presence of metallic impurities, particularly alkaline oxides [29], which could be related to the formation of more compact and, therefore, more dense aerogels, as demonstrated previously. Nevertheless, it is noteworthy that acid leaching effectively removed most inorganic impurities, resulting in highly pure SiO2 (see FTIR results; Figure S2). In any case, the obtained flame-retardant results are in perfect agreement with the thermal stability observed from the thermogravimetric analyses conducted on each of the SiO2 particles (Figure S3). The combustion of polymeric materials initiates upon the application of heat, leading to thermal degradation. The resultant degradation byproducts become superheated and congregate to create bubbles. One strategy for flame retardancy involves reducing the rate of bubbling. In this sense, SiO2 particles reduce the flammability of polymer materials by suppressing the intense bubbling phenomenon that occurs during the degradation process in combustion [30,31].
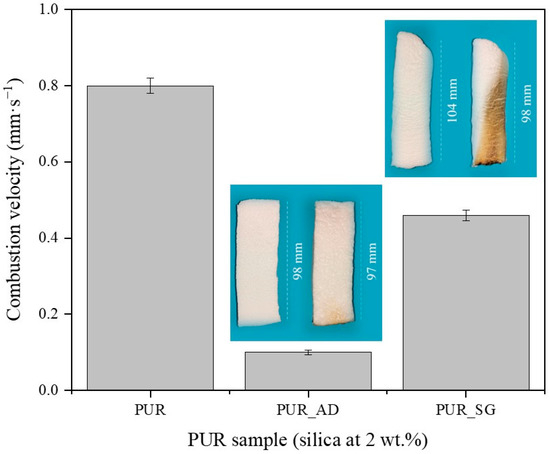
Figure 4.
Combustion velocity of the synthesized SiO2-doped PUR aerogels (PUR_AD2 and PUR_SG2) and the corresponding pictures of PUR aerogels before and after the burning process.
Based on the achieved results, the SiO2_AD2 particle type was selected as a dopant for PUR aerogels with the aim of enhancing the flame-retardant properties of the resulting materials.
2.2. Effect of the Content of Acid-Extracted SiO2 Particles in PUR Aerogels
After selecting AD particles as the best SiO2 doping agent, the impact of their concentration in the aerogels was evaluated within the range of 0.5 to 3 wt.%. The findings related to density, thermal conductivity, and Young’s modulus are outlined in Table 2.

Table 2.
Characterization results of the SiO2-doped PUR aerogels with different particle contents.
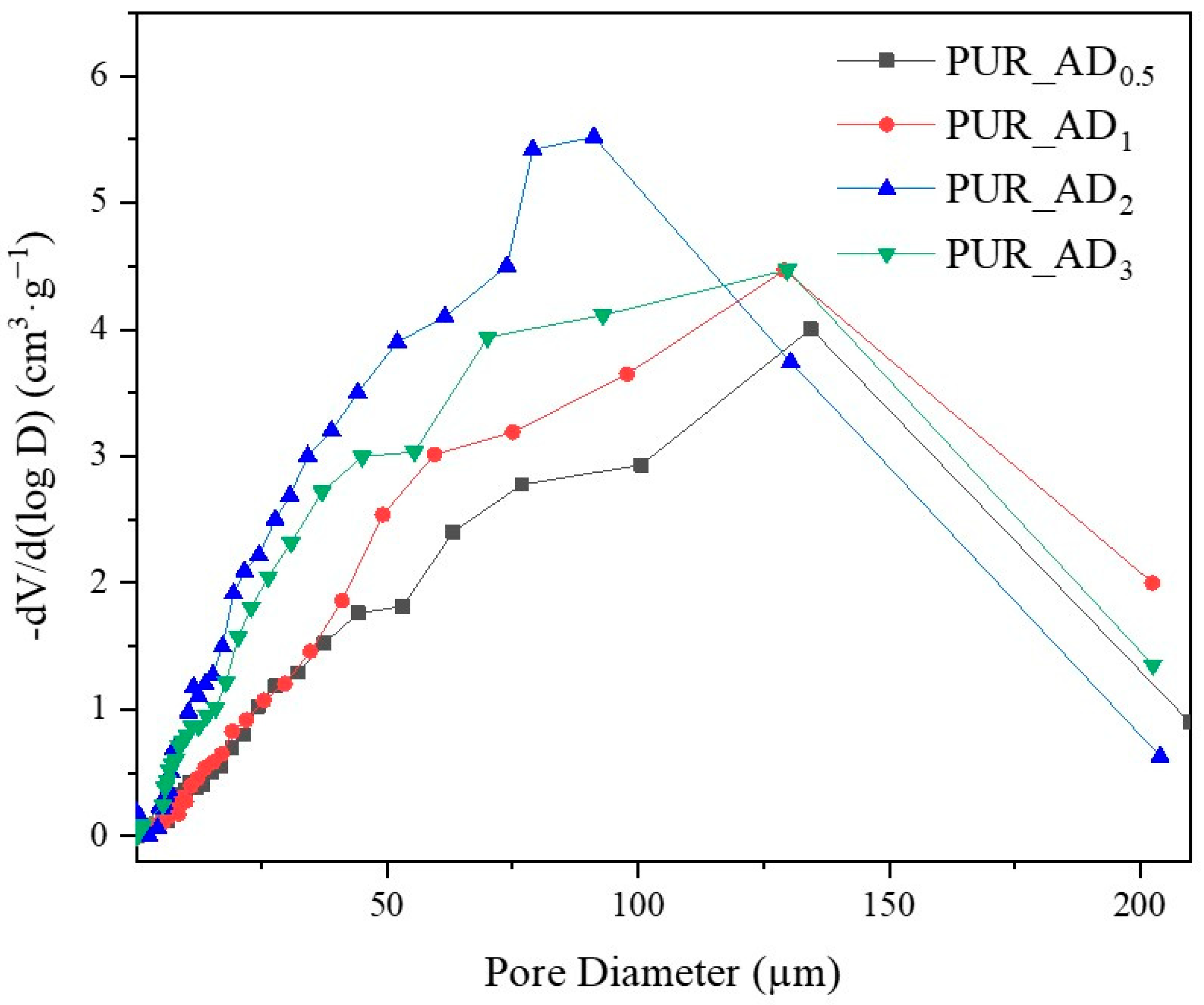
The correlation between SiO2 content and density values is evident, supported by observations from SEM micrographs (Figure 5). These images reveal increased porosity at a higher dopant content, leading to a more porous structure in samples with over 0.5% SiO2 content. This contrasts with the denser nature of the 0.5 wt.% doped sample, as confirmed by density results. As anticipated, lighter samples (PUR_AD2 and PUR_AD3) indicate a higher surface area and greater total intruded pore volume. However, it is remarkable that the PUR_AD2 sample contains a higher number of small-sized pores compared with the PUR_AD3 aerogel, which has an open surface and lacks large-sized pores. This fact can be corroborated in Figure 6, where the pore size distributions (PSDs) of PUR aerogels doped with SiO2_AD particles at different percentages are represented. The PSD shows how the peak of the Gaussian representation shifts to lower values as the metallic content increases, until it reaches approximately 2%.
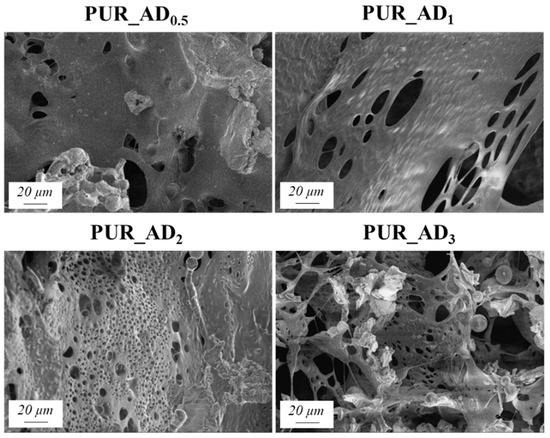
Figure 5.
SEM micrographs corresponding to the different PUR_AD aerogels.
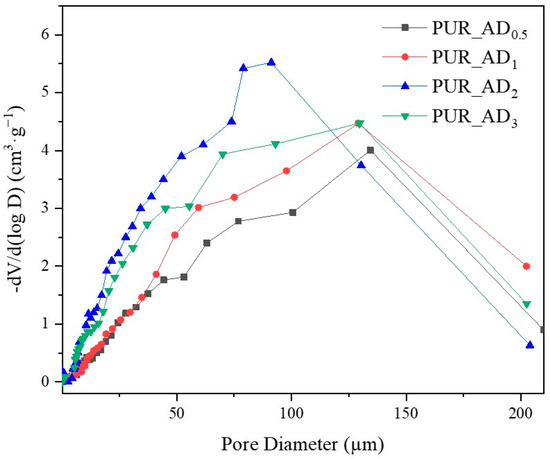
Figure 6.
Pore size distribution corresponding to the different PUR_AD aerogels.
Moreover, although the thermal conductivity values seem to remain relatively unchanged across the doped samples, all of them exhibit enhanced insulating properties compared with the undoped sample. Furthermore, an increase in SiO2 content corresponds to a rise in Young’s modulus, which was determined from the linear region of the stress–strain curves, indicating an improvement in the mechanical properties of the samples [32].
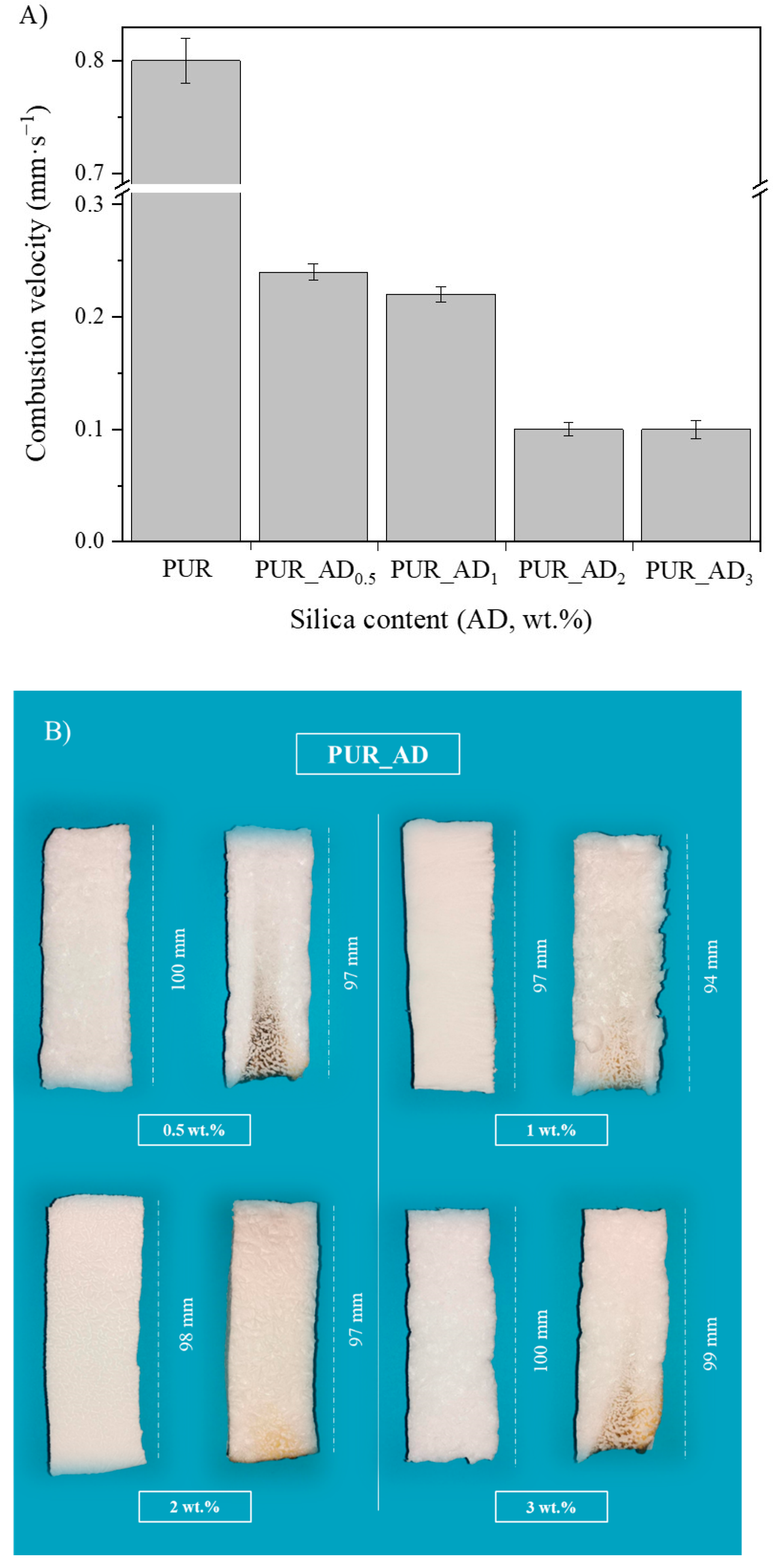
The study aimed to evaluate the influence of SiO2 content on fire safety enhancement. Specifically, the impact of SiO2 content on PUR aerogels was examined in relation to combustion velocity (Figure 7A). Experimental images of the residual char post-sample combustion are presented in Figure 7B. The results indicate an inverse relationship between SiO2 content and combustion rate within the studied range. However, a SiO2 content exceeding 2 wt.% does not result in a reduction in combustion velocity. According to the results, it seems that the improvement in the flame-retardant properties of the synthesized aerogels is closely connected to the porosity of the samples. According to the literature for other fillers [33], aerogels doped with SiO2 fillers exhibited smaller sizes and more uniform pores (see Figure 1 and Figure 5, PUR undoped vs. PUR_AD2). This was attributed to the addition of fillers increasing the viscosity of the precursor solution, acting as nucleation agents. This contributed to an ordered structure, resulting in enhanced mechanical and thermal properties, thereby making flame propagation more challenging. Therefore, the comparable combustion speeds of the PUR_AD2 and PUR_AD3 samples are attributed to their similar surface area and pore volume, as previously mentioned, despite their differing morphologies. Therefore, a SiO2 content of 2 wt.% of SiO2_AD is deemed the optimal particle concentration for incorporation into the polymer structure to minimize the flammability of aerogel samples. Following common practice in the literature for this type of materials [34,35], different images were recorded with a thermal camera where the sample emits an amount of infrared radiation as a function of its temperature (Figure S4). The images reveal the notable difference in combustion when the samples contain a SiO2-based additive (2 wt.%) extracted by AD from RH, showing the char residues generated in each case. PUR undergoes thermal degradation through a well-known mechanism [36,37] that involves its dissociation into primary amines, olefins, and carbon dioxide. This process typically occurs when PUR is exposed to elevated temperatures, leading to the breakdown of its polymeric structure (Figure S5). With the addition of a SiO2-based additive, the samples tend to form an intumescent char layer after combustion. The high quality of a SiO2 layer form would act as a good physical barrier to resist the transfer of heat and combustible gases, resulting in the enhancement of flame-retardant performance. However, as other authors have observed, SiO2 excess could suppress the intumescent process of the carbonization layer, resulting in a decrease in the synergistic effect on the flame retardancy and smoke suppression properties of the coatings [38].
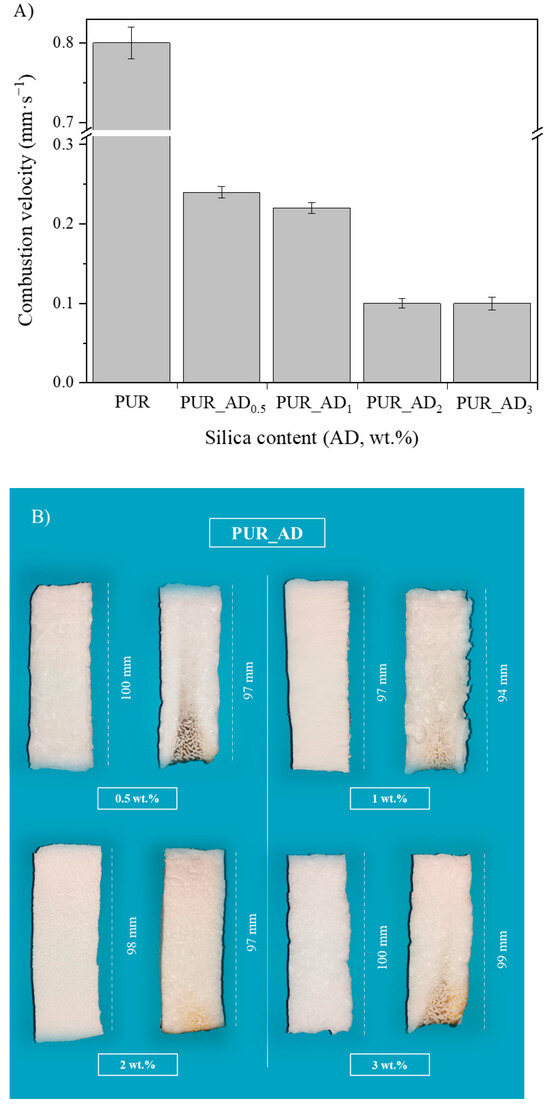
Figure 7.
(A) Combustion velocity corresponding to the aerogel synthesized using different wt.% of AD_SiO2 particles; (B) pictures of polyurethane aerogels before (left) and after (right) the burning process.
The combustion study suggests that enhancing the fire resistance of samples can be feasibly achieved by incorporating additives derived from natural and cost-effective sources, such as SiO2 particles obtained from RH, as opposed to their respective counterparts.
2.3. Effect of the Modification of SiO2 Particles with a Flame-Retardant Agent
It is widely described in the literature that PA is a bio-based and environmentally friendly compound that is used as a flame-retardant agent in different matrices [39,40]. By using QAS as a crosslinker, an effective anchoring of PA to SiO2_AD particles was achieved (as demonstrated by FTIR analysis; see Figure S2).
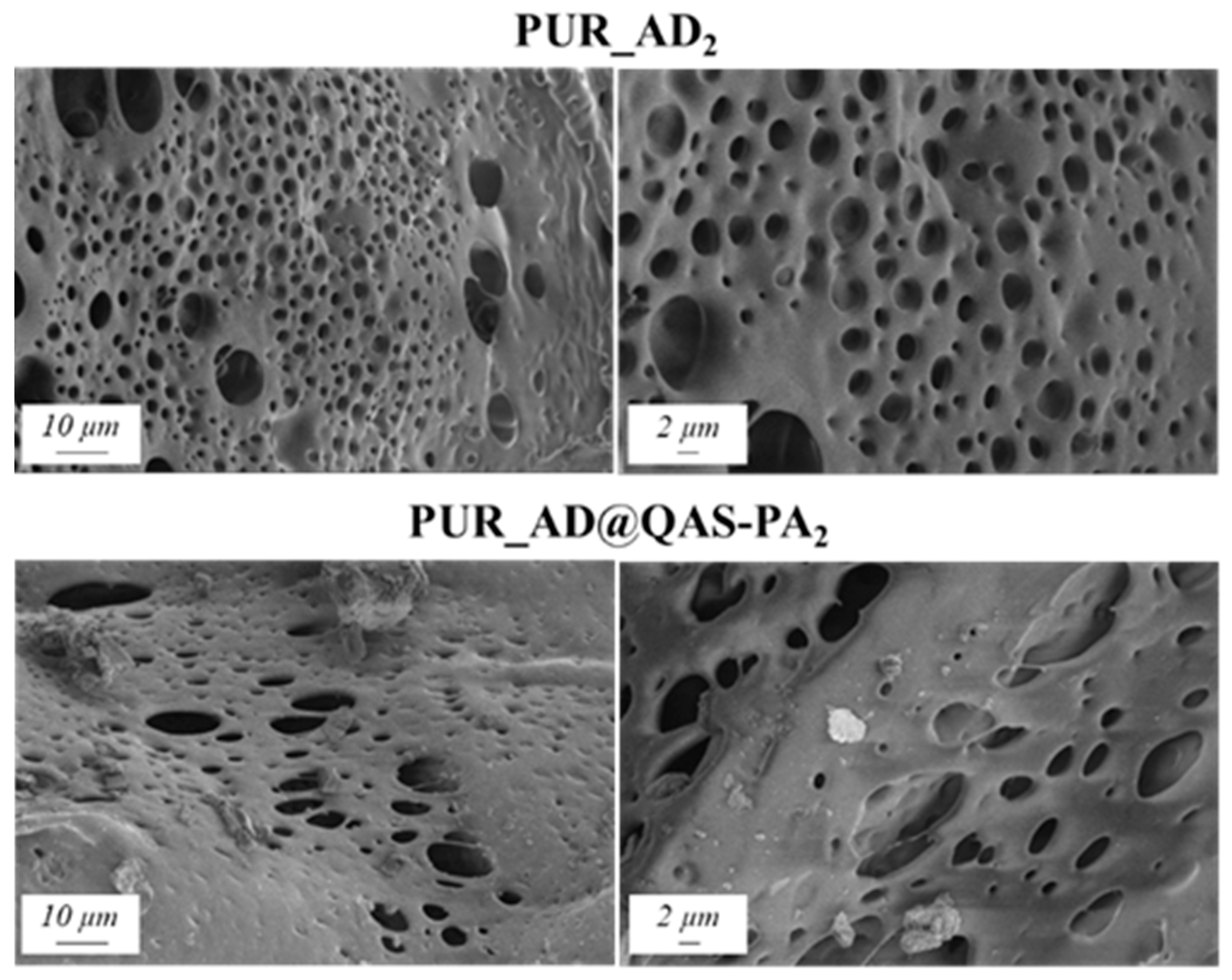
In agreement with the findings reported in the previous subsection, the selected content of AD@QAS-PA for the preparation of PUR samples was 2 wt.%. Physicochemical properties (Table 3) and the SEM results (Figure 8) show that the incorporation of SiO2_AD@QAS-PA2 particles resulted in aerogels with a very low surface area and high density. As a result, this increased the aerogel hardness, as indicated by Young’s modulus. The porous structure of the PUR_AD@QAS-PA2 aerogels is observed to be closing, resulting in a more compact appearance consistent with the surface area, pore volume, and density values already mentioned.

Table 3.
Characterization results of the SiO2-doped PUR aerogels with particles derived from acidic extraction.
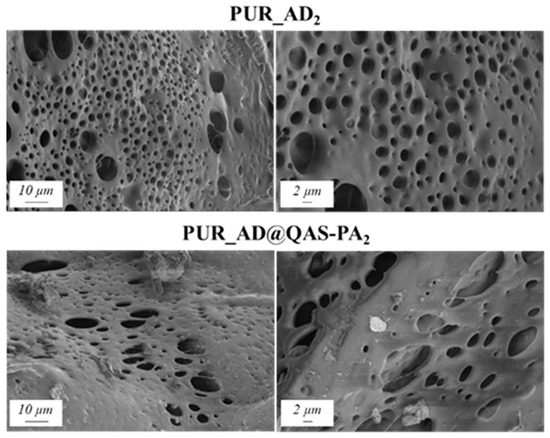
Figure 8.
SEM micrographs corresponding to PUR aerogels doped with SiO2 particles derived from acidic extraction.
Figure 9 shows the calculated combustion velocity of the doped aerogels with AD and AD@QAS-PA particles. Unexpectedly, the incorporation of AD@QAS-PA particles into the polymeric matrix practically does not significantly slow down the combustion velocity of the sample, showing slightly lower values but very close to those of the undoped aerogel (PUR). This observation contradicts the expected improvement of the AD particles modification in the flame-retardant behavior according to other authors [39,40]. This discrepancy likely arises from the density of the modified particles. Specifically, since the modified particles are denser than their non-modified counterparts (as indicated in Table 1 and Table 3) due to the long alkyl silane chain of QAS, a smaller quantity of AD@QAS-PA particles fills the matrix when compared with the same mass of SiO2_AD. Consequently, this minimizes the potential flame-retardant effect.
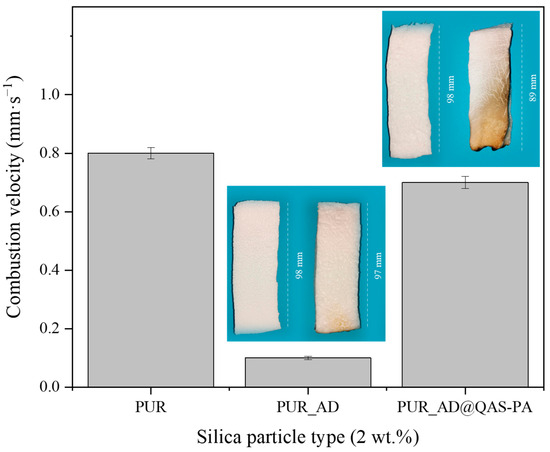
Figure 9.
Combustion velocity of the synthesized SiO2-doped PUR aerogels (PUR_AD2 and PUR_AD@QAS-PA2) and the corresponding pictures of PUR aerogels before and after the burning process.
To gain further insights into the flame-retardant behavior of doped aerogels, additional studies—particularly those considering an addition of SiO2 dopants in a weight/volume ratio instead of a weight/weight ratio—should be conducted to explore the effective impact of using the modified biosilica in the polymeric matrix. In any case, this study suggests that enhancing the fire resistance of samples can be feasibly achieved by incorporating additives derived from natural and cost-effective sources, such as SiO2 particles obtained from RH, as opposed to their respective counterparts.
3. Conclusions
A novel method is proposed to produce polyurethane/silica (PUR/SiO2) aerogel composites with evenly dispersed SiO2 particles. This innovation utilizes SiO2 particles obtained from sustainable sources like rice husk through different procedures, introducing them as doping agents into the polymer structure of PUR aerogels. The physicochemical properties of both undoped and doped aerogels with different types of SiO2 particles (AD and SG) were analyzed, resulting in mechanically strengthened materials across the board. Additionally, the flammability of aerogels was investigated, showing a notable reduction in the burning rate, especially for aerogels doped with SiO2 particles extracted from acid digestion (AD). The influence of particle content on the combustion rate was confirmed, with samples containing over 2 wt.% displaying similar thermal conductivity, density, and porosity results without further enhancement. However, the functionalization of the AD particles with PA did not delay flame propagation. In summary, the doped aerogel samples significantly enhance the fire safety performance of the material, which is crucial in various sectors such as construction and automotive, with up to an eightfold increase in fire resistance when only doped with 2 wt.% of AD particles. Future work could focus on optimizing the doping process and exploring other sustainable sources for SiO2 particles, as well as investigating additional functionalization techniques to further improve the flame-retardant properties and overall performance of the aerogels.
4. Materials and Methods
4.1. Reagents and Materials
For hydrogel preparation: 4,4′-methylenebis(cyclohexyl isocyanate) [HMDI, 90% mixture of isomers, Sigma-Aldrich, St. Louis, MO, USA]; polyethylene glycol [PEG, number-average molecular weight (Mn) = 2000 g·mol−1, Sigma-Aldrich]; 2,2-bis(hydroxymethyl)propionic acid [DMPA, 98%; Aldrich Chemicals]; 1-methyl-2-pyrrolidinone anhydrous [NMP, 99.5%, Sigma-Aldrich)]; dibutyltin dilaurate [DBTDL, 95%, Aldrich Chemicals, Milwaukee, WI, USA]; triethylamine [TEA, ≥99%, Sigma-Aldrich]; ethylenediamine [EDA, ≥99%, Sigma-Aldrich]; and ethyl acetate anhydrous [EtOAc, 99.8%, PanReac AppliChem, Barcelona, Spain]. Water was purified by distillation, followed by deionization using ion-exchange resins (resistivity 18.2 MΩ·cm). PEG and DMPA were dried overnight at 60 °C under vacuum before use to remove residual water, while other chemicals were used as acquired.
For the SiO2 particles extraction from rice husk: rice husk [RH, Arrozeiras Mundiarroz, S.A., Coruche, Portugal], nitric acid [69%, PanReac AppliChem, Chicago, IL, USA], sodium hydroxide [Pronalab, Lisbon, Portugal], and hydrochloric acid [37% v/v, Fischer Scientific, Madrid, Spain] were used.
For the modification of SiO2 particles: dimethyloctadecyl(3-(trimethylsilyl)propyl ammonium chloride solution [QAS, 42 wt.%, Sigma-Aldrich], phytic acid [PA, 50 wt.%, Fischer Scientific], and absolute ethanol [EtOH, Aga].
4.2. Synthesis Procedures
4.2.1. Extraction of SiO2 Particles
The SiO2 particles were obtained by acidic digestion of RH or by alkaline extraction of the husk ash, following procedures widely described in the literature [41]. Both the acidic and alkaline processes yielded approximately 10% of SiO2. The extraction was performed as follows:
- -
- Acidic digestion: HNO3 (0.5 L) was added to 0.5 kg of RH (previously milled to 0.5 mm), and the digestion occurred for 2 h at 60 °C. The digested husk was then filtered (mesh 85 µm) and washed with distilled water until pH ca.7. The neutralized husk was then dried at 110 °C for 12 h. The SiO2 particles (SiO2_AD) were recovered after a calcination step (the temperature was raised to 700 °C at 4.5 °C/min and maintained at 700 °C for 3.5 h).
- -
- Alkaline extraction: RH was calcined at 700 °C (4.5 °C/min up to 700 °C and maintained at 700 °C for 3.5 h). Then, 10 g of RH ash was added to 0.2 L of NaOH aqueous solution (2.5 M) and refluxed at 90 °C for 3 h. The mixture was then filtered, and the supernatant was recovered and neutralized with HCl 10%. After 24 h, the obtained gel was centrifuged at 9000 rpm for 10 min and washed with water. The washing and centrifugation steps were repeated 3 times, and the obtained SiO2 particles (SiO2_SG) were dried at 150 °C for 2 h.
The SiO2 particles obtained by acidic extraction were modified with QAS and PA. In brief, a dispersion (1.5 wt.%) of SiO2_RH_AD particles in EtOH:H2O (1:1) was prepared. PA was added to the mixture, and after homogenization, QAS was added dropwise (molar ratio SiO2_RH_AD:PA:QAS was 1:5:1.3). The reaction was kept at 23 °C for 3 h, and the as-obtained solid was centrifuged (10 min at 9000 rpm) and washed 3 times with water. After this process, the agglomerates of particles were dried at 40 °C and ground to obtain the particles as a powder (SiO2_AD@QAS-PA).
The main features of the synthesized SiO2 particles are summarized in Table 4.

Table 4.
Main characteristics of the extracted SiO2 particles from rice husk.
4.2.2. Synthesis of SiO2-Doped Polyurethane Aerogels
SiO2-doped PUR aerogels were prepared at a pilot-plant scale (using a freeze-drying unit with a production of 2 m2 of aerogel panels per batch), following our previous reported works [42,43]. The main modification involved the incorporation of a doping agent (SiO2 particles). Table 5 lists the prepared aerogel samples.

Table 5.
SiO2 nature and content of the final doped PUR aerogels.
During the synthesis, the different types of silica particles extracted from RH were doped in the PUR aerogels. PEG (polyol, 5.23·10−2–5.54·10−2 mol) and DMPA (emulsifier agent, 0.17 mol) that were previously conditioned for dehydration are used. SiO2 particles of specific types (SiO2_AD, SiO2_SG, SiO2_AD@QAS-PA) were added at a concentration of 2 wt.% for all types except for SiO2_AD particles, which ranged from 0.5 to 3.0 wt.%.
PEG and DMPA were melted together with SiO2 particles under vigorous mechanical stirring in a jacketed vessel at 80 °C. The amount of PEG was adjusted for each experiment based on the amount of doping agent added. Once melted, HMDI (isocyanate, 0.34 mol), NMP (anhydrous solvent, 0.17 mol), and DBTDL (catalyst, 8.86·10−4 mol) were added, and the reaction proceeded for 3 h at 450 rpm, maintaining the same temperature. Subsequently, the temperature was lowered to 40 °C before adding EtOAc (organic solvent, 4.04 mol). The mixture was stirred for an additional hour before adding TEA (neutralizer agent, 0.20 mol) to neutralize the DMPA acidic functionalities. This neutralization step took 30 min to achieve complete neutralization of carboxylic groups. All previous steps were carried out under a N2 stream to create an inert atmosphere. In the next step, polymer chains were extended using a slight excess of the chain extender EDA (0.07 mol), adding another 30 min to the process. The EDA/HMDI ratio was 0.20. In the final stage of the synthesis method, water content was added, and the polymer dispersion was completed by mechanical stirring (900 rpm) for approximately 30 min. The added water content affected the solid content of the samples, fixed at 3.7 wt.%. Finally, the obtained wet gel was transferred into the freeze-drying trays with dimensions of 43.5 cm (long) × 34 cm (wide) × 4 cm (high) and subjected to a freeze-drying process involving freezing for 6 h at −40 °C, followed by 60 h at 25 °C and 200 µbar of primary drying and, finally, 10 h at 40 °C of secondary drying.
4.3. Characterization Techniques
Thermogravimetric analyses of SiO2 particles and SiO2-doped polyurethane aerogels (TGA, 2STARe system, Mettler Toledo, Madrid, Spain) were conducted to assess their thermal stability. Additionally, the glass transition temperature of PUR aerogels (Tg) was determined through differential scanning calorimetry (DSC, 2STARe system, Mettler Toledo, Madrid, Spain) at a heating rate of 20 K·min−1, spanning from −80 to 80 °C. Data acquisition for both TGA and DSC experiments was performed using 2STARe V16.30 software.
The chemical structure of the samples was determined by assigning the functional groups based on vibrations obtained with an infrared spectrometer (Fourier transform infrared–attenuated total reflectance (FTIR–ATR), Spectrum Two, PerkinElmer, Waltham, MA, US) in the 500–4000 cm−1 range.
Density values of the synthesized aerogels were determined using a 3D scanner (REXCAN DS3 Silver, eQuality Tech Inc., Rochester Hills, MI, USA). The instrument generated a three-dimensional image of the sample with ezScan v 3.26 software, and the volume of the aerogel was subsequently calculated using Geometric Wrap v2021.2.2 software. Specifically, three square-shaped samples were extracted from different regions of each synthesized aerogel. The samples were previously weighted to calculate their density (ρ, g·cm−3) by dividing their mass by the volume obtained from the scanner.
Moreover, the thermal conductivity of the synthesized aerogels was assessed through heat transfer measurements between two parallel plates (HFM 300, Linseis, Selb, Germany). To conduct these assessments, samples with dimensions of 30 × 30 cm were used. These analyses were carried out at five intermediate temperatures, between 0 and 40 °C.
The morphological structure of the samples was examined through high-resolution scanning electron microscopy (HRSEM, GeminiSEM 500, ZEISS, Jena, Germany) using an 80 mm2 energy dispersive spectroscopy (EDS) sensor and another electron backscatter diffraction (EBSD) sensor. Thus, SEM images, elemental mapping, and EDS spectrum were obtained.
To analyze the pore size distribution (PSD) of the aerogels, a PoreMaster mercury-intrusion apparatus (Quantachrome Instruments, Boynton Beach, FL, USA) was employed. The method relies on the infiltration of mercury into the pores of the samples under controlled pressure, enabling penetration. Subsequently, the registration of mercury volume, correlated with increasing pressure, allows the determination of the pore size distribution [44].
Finally, the flame-retardancy behavior of the synthesized aerogels was directly assessed in the absence and presence of the different SiO2 particles (AD, SG, and AD@QAS-PA). The flame spread across vertically oriented aerogels (ca. 10 × 3 cm) was evaluated according to [45]. The ignition source was applied to the aerogel surface for 3 s, followed by a waiting period of 10 s to measure the subsequent flame spread. Each analysis was carried out with triplicate samples (n = 3).
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/gels10070465/s1. Figure S1: EDS analysis and elemental mapping of PUR_AD2 sample; Figure S2: FTIR spectra corresponding to the different silica particles; Figure S3: TGA curves relating to the three types of SiO2 particles; Figure S4: Combustion process of reference aerogel (undoped) and aerogel doped with 2% AD_SiO2 particles. Inset: shows the images obtained using a thermal camera; Figure S5: (a) Main reactions and (b) mechanism for the thermal degradation of polyurethane (PUR). References [46,47,48,49,50,51,52,53,54,55] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, E.P.-P., A.R., and L.S.-S.; methodology, E.P.-P., Ó.d.F., A.R., A.M., F.G., M.O. (Marcelo Oliveira), and M.O. (Mariana Ornelas); validation, E.P.-P., D.C., A.R., A.M., F.G., and M.O. (Marcelo Oliveira); formal analysis, E.P.-P., A.R., A.M., F.G., and M.O. (Marcelo Oliveira); investigation, E.P.-P., Ó.d.F., D.C., A.M., F.G., F.M., and M.O. (Marcelo Oliveira); data curation, E.P.-P., Ó.d.F., A.M., F.G., F.M., and M.O. (Marcelo Oliveira); writing—original draft preparation, E.P.-P. and F.G.; writing—review and editing, E.P.-P., A.R., L.S.-S., F.G., and M.O. (Mariana Ornelas); visualization, E.P.-P., F.G., and M.O. (Mariana Ornelas); supervision, A.R., L.S.-S., and M.O. (Mariana Ornelas); project administration, L.S-S. and M.O. (Mariana Ornelas); funding acquisition, L.S.-S. and M.O. (Mariana Ornelas). All authors have read and agreed to the published version of the manuscript.
Funding
European Union’s Horizon 2020 research and innovation program under Grant Agreement No. 953270.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in article.
Acknowledgments
This work was performed within the framework of the BIOMAT project. This project has received funding from the European Union’s Horizon 2020 research and innovation program under Grant Agreement No. 953270.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Garay, R.; Henriquez, M. Fire performance of boards and pine radiata wood with and without flame retardant paint. Maderas-Cienc. Tecnol. 2010, 12, 11–24. [Google Scholar]
- Yu, Z.L.; Yang, N.; Apostolopoulou-Kalkavoura, V.; Qin, B.; Ma, Z.Y.; Xing, W.Y.; Qiao, C.; Bergström, L.; Antonietti, M.; Yu, S.H. Fire-retardant and thermally insulating phenolic-silica aerogels. Angew. Chem. 2018, 57, 4538–4542. [Google Scholar] [CrossRef] [PubMed]
- Troitzsch, J.; Becker, W.; Haim, J. Plastics Flammability Handbook: Principles, Regulations, Testing, and Approval, 4th ed.; Hanser Publications: Munich, Germany, 2021. [Google Scholar]
- Horrocks, A.R.; Price, D. Fire Retardant Materials, 1st ed.; Woodhead Publishing: Cambridge, UK, 2001. [Google Scholar]
- Messersmith, P.B.; Giannelis, E.P. Synthesis and characterization of layered silicate-epoxy nanocomposites. Chem. Mater. 1994, 6, 1719–1725. [Google Scholar] [CrossRef]
- IDTechEx. Aerogels 2021–2031: Technologies, Markets and Players. Available online: https://www.idtechex.com/en/research-report/aerogels-2021-2031 (accessed on 2 April 2024).
- Simón-Herrero, C.; Chen, X.Y.; Ortiz, M.L.; Romero, A.; Valverde, J.L.; Sánchez-Silva, L. Linear and crosslinked polyimide aerogels: Synthesis and characterization. J. Mater. Res. Technol. 2019, 8, 2638–2648. [Google Scholar] [CrossRef]
- Sanchez, C.; Lebeau, B.; Ribot, F.; In, M. Molecular design of sol-gel derived hybrid organic-inorganic nanocomposites. J. Solgel Sci. Technol. 2000, 19, 31–38. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Maldonado-Hódar, F.J. Carbon aerogels for catalysis applications: An overview. Carbon 2005, 43, 455–465. [Google Scholar] [CrossRef]
- Gheonea, R.; Crasmareanu, E.C.; Plesu, N.; Sauca, S.; Simulescu, V.; Ilia, G. New Hybrid Materials Synthesized with Different Dyes by Sol-Gel Method. Adv. Mater. Sci. Eng. 2017, 2017, 4537039–4537046. [Google Scholar] [CrossRef]
- Biesmans, G.; Randall, D.; Francais, E.; Perrut, M. Polyurethane-based organic aerogels’ thermal performance. J. Non Cryst. Solids 1988, 225, 36–40. [Google Scholar] [CrossRef]
- Rigacci, A.; Marechal, J.C.; Repoux, M.; Moreno, M.; Achard, P. Preparation of polyurethane-based aerogels and xerogels for thermal superinsulation. J. Non Cryst. Solids 2004, 350, 372–378. [Google Scholar] [CrossRef]
- Diascorn, N.; Calas, S.; Sallée, H.; Achard, P.; Rigacci, A. Polyurethane aerogels synthesis for thermal insulation–textural, thermal and mechanical properties. J. Supercrit. Fluids 2015, 106, 76–84. [Google Scholar] [CrossRef]
- Liu, W.B.; Zhao, H.B.; Wang, Y.Z. Advanced flame-retardant methods for polymeric materials. Adv. Mater. 2022, 34, 2107905–2107940. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Yang, W.; Yuen, A.C.Y.; Xie, C.; Xie, J.; Lu, H.; Yeoh, G.H. Study on flame retarded flexible polyurethane foam/alumina aerogel composites with improved fire safety. J. Chem. Eng. 2017, 311, 310–317. [Google Scholar] [CrossRef]
- Huang, P.; Fan, M. Development of facture free clay-based aerogel: Formulation and architectural mechanisms. Compos. B Eng. 2016, 91, 169–175. [Google Scholar] [CrossRef]
- Zhang, D.; Zeng, J.; Liu, W.; Qiu, X.; Qian, Y.; Zhang, H.; Yang, Y.; Liu, M.; Yang, D. Pristine lignin as a flame retardant in flexible PU foam. Green Chem. 2021, 23, 5972–5980. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, F.; Liu, X.; Wu, N.; Che, S.; Li, Y. Phosphorus doped nickel-molybdenum aerogel for efficient overall water splitting. Appl. Catal. B 2021, 298, 120494–120503. [Google Scholar] [CrossRef]
- Cimavilla-Román, P.; Pérez-Tamarit, S.; Santiago-Calvo, M.; Rodríguez-Pérez, M.A. Influence of silica aerogel particles on the foaming process and cellular structure of rigid polyurethane foams. Eur. Polym. J. 2020, 135, 109884–109894. [Google Scholar] [CrossRef]
- Brook, M.A. Silicon in Organic, Organometallic, and Polymer Chemistry, 1st ed.; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Saint-Michel, F.; Chazeau, L.; Cavaillé, J.Y. Mechanical properties of high-density polyurethane foams: II Effect of the filler size. Compos. Sci. Technol. 2006, 66, 2709–2718. [Google Scholar] [CrossRef]
- Sheng, N.; Boyce, M.C.; Parks, D.M.; Rutledge, G.C.; Abes, J.I.; Cohen, R.E. Multiscale micromechanical modeling of polymer/clay nanocomposites and the effective clay particle. Polymer 2004, 45, 487–506. [Google Scholar] [CrossRef]
- Francés, A.B.; Bañón, M.V.N. Effect of silica nanoparticles on polyurethane foaming process and foam properties. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2014; Volume 64, pp. 012020–012026. [Google Scholar] [CrossRef]
- Navidfar, A.; Sancak, A.; Yildirim, K.B.; Trabzon, L. A study on polyurethane hybrid nanocomposite foams reinforced with multiwalled carbon nanotubes and silica nanoparticles. Polym.-Plast. Technol. Eng. 2018, 57, 1463–1473. [Google Scholar] [CrossRef]
- Verdolotti, L.; Lavorgna, M.; Lamanna, R.; Di Maio, E.; Iannace, S. Polyurethane-silica hybrid foam by sol–gel approach: Chemical and functional properties. Polymer 2015, 56, 20–28. [Google Scholar] [CrossRef]
- Somdee, P.; Lassú -Kuknyó, T.; Kónya, C.; Szabó, T.; Marossy, K. Thermal analysis of polyurethane elastomers matrix with different chain extender contents for thermal conductive application. J. Therm. Anal. Calorim. 2019, 138, 1003–1010. [Google Scholar] [CrossRef]
- Trovati, G.; Sanches, E.A.; Neto, S.C.; Mascarenhas, Y.P.; Chierice, G.O. Characterization of polyurethane resins by FTIR, TGA, and XRD. J. Appl. Polym. Sci. 2010, 115, 263–268. [Google Scholar] [CrossRef]
- Claro, C.J.M.; Neto, S.; Chierice, G.O.; Santos, A.M. Study of the biodegradation of a polymer derived from castor oil by scanning electron microscopy, thermogravimetry and infrared spectroscopy. Polímeros 2006, 16, 129–135. [Google Scholar] [CrossRef][Green Version]
- Fernandes, I.J.; Calheiro, D.; Sánchez, F.A.; Camacho, A.L.D.; Rocha, T.L.A.D.; Moraes, C.A.M.; Sousa, V.C.D. Characterization of silica produced from rice husk ash: Comparison of purification and processing methods. J. Mater. Res. 2017, 20, 512–518. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Mu, M.; Winey, K.; Cipriano, B.; Raghavan, S.R.; Pack, S.; Rafailovich, M.; Yang, Y.; Grulke, E.; Shields, J.; et al. Relation between the viscoelastic and flammability properties of polymer nanocomposites. Polymer 2008, 49, 4358–4368. [Google Scholar] [CrossRef]
- Shan, G.; Jin, W.; Chen, H.; Zhao, M.; Surampalli, R.; Ramakrishnan, A.; Zhang, T.; Tyagi, R.D. Flame-retardant polymer nanocomposites and their heat-release rates. J. Hazard. Toxic Radioact. Waste 2015, 19, 04015006–04015020. [Google Scholar] [CrossRef]
- Babiarczuk, B.; Lewandowski, D.; Kierzek, K.; Detyna, J.; Jones, W.; Kaleta, J.; Krzak, J. Mechanical properties of silica aerogels controlled by synthesis parameters. J. Non Cryst. Solids 2023, 606, 122171–122180. [Google Scholar] [CrossRef]
- Lin, X.C.; Li, S.L.; Li, W.X.; Wang, Z.H.; Zhang, J.Y.; Liu, B.W.; Fu, T.; Zhao, H.B.; Wang, Y.Z. Thermo-responsive self-Ceramifiable robust aerogel with exceptional strengthening and thermal insulating performance at ultrahigh temperatures. Adv. Funct. Mater. 2023, 33, 2214913–2214923. [Google Scholar] [CrossRef]
- Cao, M.; Liu, B.W.; Zhang, L.; Peng, Z.C.; Zhang, Y.Y.; Wang, H.; Zhao, H.B.; Wang, Y.Z. Fully biomass-based aerogels with ultrahigh mechanical modulus, enhanced flame retardancy, and great thermal insulation applications. Compos. B Eng. 2021, 25, 109309–109320. [Google Scholar] [CrossRef]
- Shi, B.; Xie, L.; Ma, B.; Zhou, Z.; Xu, B.; Qu, L. Preparation and properties of highly transparent SiO2 aerogels for thermal insulation. Gels 2022, 8, 744–756. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Yadav, A.; de Souza, F.M.; Dawsey, T.; Gupta, R.K. Recent advancements in flame-retardant polyurethane foams: A review. Ind. Eng. Chem. Res. 2022, 61, 15046–15065. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Wang, X. Influence of nano-silica on the flame retardancy and smoke suppression properties of transparent intumescent fire-retardant coatings. Prog. Org. Coat. 2017, 112, 319–329. [Google Scholar] [CrossRef]
- Sykam, K.; Försth, M.; Sas, G.; Restás, Á.; Das, O. Phytic acid: A bio-based flame retardant for cotton and wool fabrics. Ind. Crops Prod. 2021, 64, 113349–113364. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Sadiku, E.R.; Ray, S.S.; Mochane, M.J.; Matabola, K.P.; Motloung, M. Flame retardancy efficacy of phytic acid: An overview. J. Appl. Polym. Sci. 2022, 39, 52495–52530. [Google Scholar] [CrossRef]
- Nzereogu, P.; Omah, A.; Ezema, F.; Iwuoha, E.; Nwanya, A. Silica extraction from rice husk: Comprehensive review and applications. Hybrid Adv. 2023, 4, 100111–100126. [Google Scholar] [CrossRef]
- Cantero, D.; Pinilla-Peñalver, E.; Romero, A.; Sánchez-Silva, L. Synthesis of waterborne polyurethane aerogels-like materials via freeze-drying: An innovative approach. J. Mater. Sci. 2023, 58, 9087–9102. [Google Scholar] [CrossRef]
- Pinilla-Peñalver, E.; Cantero, D.; Romero, A.; Sánchez-Silva, L. Exploring the impact of the synthesis variables involved in the polyurethane aerogels-like materials design. Gels 2024, 10, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Simón-Herrero, C.; Romero, A.; Valverde, J.L.; Sánchez-Silva, L. Hydroxyethyl cellulose/alumina-based aerogels as lightweight insulating materials with high mechanical strength. J. Mater. Sci. 2018, 53, 1556–1567. [Google Scholar] [CrossRef]
- UNE-EN 3844-3:2019; Material Aeroespacial. Inflamabilidad de los Materiales No Metálicos. Parte 3: Ensayo de Pequeño Quemador, 45º. Determinación de la Resistencia del Material a la Propagación de la Llama y de la Incandescencia y a la Penetración de la Llama (Ratificada por la Asociación Española de Normalización en Octubre de 2019). Asociacion Espanola de Normalizacion: Madrid, Spain, 2019.
- Gong, S.Q.; Epasinghe, D.J.; Zhang, W.; Zhou, B.; Niu, L.N.; Ryou, H.; Eid, A.A.; Frassetto, A.; Yiu, C.K.Y.; Arola, D.D.; et al. Synthesis of antimicrobial silsesquioxane–silica hybrids by hydrolytic co-condensation of alkoxysilanes. Polym. Chem. 2014, 5, 454–462. [Google Scholar] [CrossRef]
- Li, K.M.; Jiang, J.G.; Tian, S.C.; Chen, X.J.; Yan, F. Influence of silica types on synthesis and performance of amine–silica hybrid materials used for CO2 capture. J. Phys. Chem. C 2014, 118, 2454–2462. [Google Scholar] [CrossRef]
- Ullah, R.; Deb, B.K.; Mollah, M.Y.A. Synthesis and characterization of silica coated iron-oxide composites of different ratios. Int. J. Compos. Mater. 2014, 4, 135–145. [Google Scholar] [CrossRef]
- Comite, A. Preparation of silica membranes by sol-gel method. In Current Trends and Future Developments on (Bio-)Membranes, 1st ed.; Basile, A., Favvas, E.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–23. [Google Scholar]
- Zhang, R.; Cai, S.; Xu, G.; Zhao, H.; Li, Y.; Wang, X.; Huang, K.; Wu, X. Crack self-healing of phytic acid conversion coating on AZ31 magnesium alloy by heat treatment and the corrosion resistance. Appl. Surf. Sci. 2014, 313, 896–904. [Google Scholar] [CrossRef]
- Yudovin-Farber, I.; Beyth, N.; Weiss, E.I.; Domb, A.J. Antibacterial effect of composite resins containing quaternary ammonium polyethyleneimine nanoparticles. J. Nanopart. Res. 2010, 12, 591–603. [Google Scholar] [CrossRef]
- Dang, N.T.T.; Nguyen, T.T.A.; Phan, T.D.; Tran, H.; Van Dang, P.; Nguyen, H.Q. Synthesis of silica nanoparticles from rice husk ash. Sci. Technol. Develop. J. 2017, 20, 50–54. [Google Scholar] [CrossRef]
- Li, Y.; Lan, J.Y.; Liu, J.; Yu, J.; Luo, Z.; Wang, W.; Sun, L. Synthesis of gold nanoparticles on rice husk silica for catalysis applications. Ind. Eng. Chem. Res. 2015, 54, 5656–5663. [Google Scholar] [CrossRef]
- Wang, X.; Shi, L.; Zhang, J.; Cheng, J.; Wang, X. Self-assembly fabrication, microstructures and antibacterial performance of layer-structured montmorillonite nanocomposites with cationic silica nanoparticles. RSC Adv. 2017, 7, 31502–31511. [Google Scholar] [CrossRef]
- Galimberti, M.; Martino, M.; Guenzi, M.; Leonardi, G.; Citterio, A. Thermal stability of ammonium salts as compatibilizers in polymer/layered silicate nanocomposites. e-Polymers 2009, 9, 56–69. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).