Enhancing Flame-Retardant Properties of Polyurethane Aerogels Doped with Silica-Based Particles
Abstract
Highlights
Abstract
1. Introduction
2. Results and Discussion
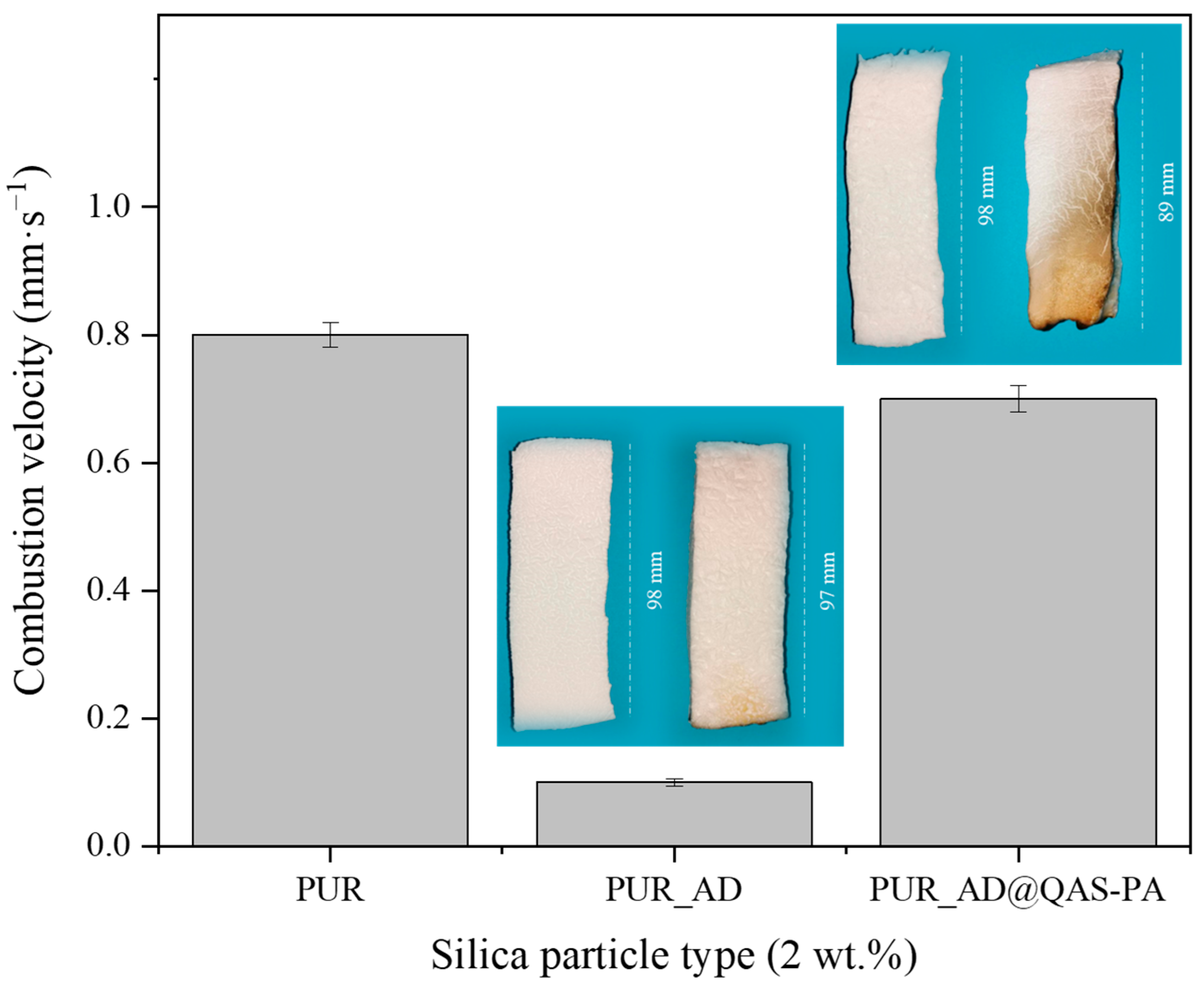
2.1. Impact of Silica Particles’ Nature as Dopant of PUR Aerogels
2.2. Effect of the Content of Acid-Extracted SiO2 Particles in PUR Aerogels
2.3. Effect of the Modification of SiO2 Particles with a Flame-Retardant Agent
3. Conclusions
4. Materials and Methods
4.1. Reagents and Materials
4.2. Synthesis Procedures
4.2.1. Extraction of SiO2 Particles
- -
- Acidic digestion: HNO3 (0.5 L) was added to 0.5 kg of RH (previously milled to 0.5 mm), and the digestion occurred for 2 h at 60 °C. The digested husk was then filtered (mesh 85 µm) and washed with distilled water until pH ca.7. The neutralized husk was then dried at 110 °C for 12 h. The SiO2 particles (SiO2_AD) were recovered after a calcination step (the temperature was raised to 700 °C at 4.5 °C/min and maintained at 700 °C for 3.5 h).
- -
- Alkaline extraction: RH was calcined at 700 °C (4.5 °C/min up to 700 °C and maintained at 700 °C for 3.5 h). Then, 10 g of RH ash was added to 0.2 L of NaOH aqueous solution (2.5 M) and refluxed at 90 °C for 3 h. The mixture was then filtered, and the supernatant was recovered and neutralized with HCl 10%. After 24 h, the obtained gel was centrifuged at 9000 rpm for 10 min and washed with water. The washing and centrifugation steps were repeated 3 times, and the obtained SiO2 particles (SiO2_SG) were dried at 150 °C for 2 h.
4.2.2. Synthesis of SiO2-Doped Polyurethane Aerogels
4.3. Characterization Techniques
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garay, R.; Henriquez, M. Fire performance of boards and pine radiata wood with and without flame retardant paint. Maderas-Cienc. Tecnol. 2010, 12, 11–24. [Google Scholar]
- Yu, Z.L.; Yang, N.; Apostolopoulou-Kalkavoura, V.; Qin, B.; Ma, Z.Y.; Xing, W.Y.; Qiao, C.; Bergström, L.; Antonietti, M.; Yu, S.H. Fire-retardant and thermally insulating phenolic-silica aerogels. Angew. Chem. 2018, 57, 4538–4542. [Google Scholar] [CrossRef] [PubMed]
- Troitzsch, J.; Becker, W.; Haim, J. Plastics Flammability Handbook: Principles, Regulations, Testing, and Approval, 4th ed.; Hanser Publications: Munich, Germany, 2021. [Google Scholar]
- Horrocks, A.R.; Price, D. Fire Retardant Materials, 1st ed.; Woodhead Publishing: Cambridge, UK, 2001. [Google Scholar]
- Messersmith, P.B.; Giannelis, E.P. Synthesis and characterization of layered silicate-epoxy nanocomposites. Chem. Mater. 1994, 6, 1719–1725. [Google Scholar] [CrossRef]
- IDTechEx. Aerogels 2021–2031: Technologies, Markets and Players. Available online: https://www.idtechex.com/en/research-report/aerogels-2021-2031 (accessed on 2 April 2024).
- Simón-Herrero, C.; Chen, X.Y.; Ortiz, M.L.; Romero, A.; Valverde, J.L.; Sánchez-Silva, L. Linear and crosslinked polyimide aerogels: Synthesis and characterization. J. Mater. Res. Technol. 2019, 8, 2638–2648. [Google Scholar] [CrossRef]
- Sanchez, C.; Lebeau, B.; Ribot, F.; In, M. Molecular design of sol-gel derived hybrid organic-inorganic nanocomposites. J. Solgel Sci. Technol. 2000, 19, 31–38. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Maldonado-Hódar, F.J. Carbon aerogels for catalysis applications: An overview. Carbon 2005, 43, 455–465. [Google Scholar] [CrossRef]
- Gheonea, R.; Crasmareanu, E.C.; Plesu, N.; Sauca, S.; Simulescu, V.; Ilia, G. New Hybrid Materials Synthesized with Different Dyes by Sol-Gel Method. Adv. Mater. Sci. Eng. 2017, 2017, 4537039–4537046. [Google Scholar] [CrossRef]
- Biesmans, G.; Randall, D.; Francais, E.; Perrut, M. Polyurethane-based organic aerogels’ thermal performance. J. Non Cryst. Solids 1988, 225, 36–40. [Google Scholar] [CrossRef]
- Rigacci, A.; Marechal, J.C.; Repoux, M.; Moreno, M.; Achard, P. Preparation of polyurethane-based aerogels and xerogels for thermal superinsulation. J. Non Cryst. Solids 2004, 350, 372–378. [Google Scholar] [CrossRef]
- Diascorn, N.; Calas, S.; Sallée, H.; Achard, P.; Rigacci, A. Polyurethane aerogels synthesis for thermal insulation–textural, thermal and mechanical properties. J. Supercrit. Fluids 2015, 106, 76–84. [Google Scholar] [CrossRef]
- Liu, W.B.; Zhao, H.B.; Wang, Y.Z. Advanced flame-retardant methods for polymeric materials. Adv. Mater. 2022, 34, 2107905–2107940. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Yang, W.; Yuen, A.C.Y.; Xie, C.; Xie, J.; Lu, H.; Yeoh, G.H. Study on flame retarded flexible polyurethane foam/alumina aerogel composites with improved fire safety. J. Chem. Eng. 2017, 311, 310–317. [Google Scholar] [CrossRef]
- Huang, P.; Fan, M. Development of facture free clay-based aerogel: Formulation and architectural mechanisms. Compos. B Eng. 2016, 91, 169–175. [Google Scholar] [CrossRef]
- Zhang, D.; Zeng, J.; Liu, W.; Qiu, X.; Qian, Y.; Zhang, H.; Yang, Y.; Liu, M.; Yang, D. Pristine lignin as a flame retardant in flexible PU foam. Green Chem. 2021, 23, 5972–5980. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, F.; Liu, X.; Wu, N.; Che, S.; Li, Y. Phosphorus doped nickel-molybdenum aerogel for efficient overall water splitting. Appl. Catal. B 2021, 298, 120494–120503. [Google Scholar] [CrossRef]
- Cimavilla-Román, P.; Pérez-Tamarit, S.; Santiago-Calvo, M.; Rodríguez-Pérez, M.A. Influence of silica aerogel particles on the foaming process and cellular structure of rigid polyurethane foams. Eur. Polym. J. 2020, 135, 109884–109894. [Google Scholar] [CrossRef]
- Brook, M.A. Silicon in Organic, Organometallic, and Polymer Chemistry, 1st ed.; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Saint-Michel, F.; Chazeau, L.; Cavaillé, J.Y. Mechanical properties of high-density polyurethane foams: II Effect of the filler size. Compos. Sci. Technol. 2006, 66, 2709–2718. [Google Scholar] [CrossRef]
- Sheng, N.; Boyce, M.C.; Parks, D.M.; Rutledge, G.C.; Abes, J.I.; Cohen, R.E. Multiscale micromechanical modeling of polymer/clay nanocomposites and the effective clay particle. Polymer 2004, 45, 487–506. [Google Scholar] [CrossRef]
- Francés, A.B.; Bañón, M.V.N. Effect of silica nanoparticles on polyurethane foaming process and foam properties. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2014; Volume 64, pp. 012020–012026. [Google Scholar] [CrossRef]
- Navidfar, A.; Sancak, A.; Yildirim, K.B.; Trabzon, L. A study on polyurethane hybrid nanocomposite foams reinforced with multiwalled carbon nanotubes and silica nanoparticles. Polym.-Plast. Technol. Eng. 2018, 57, 1463–1473. [Google Scholar] [CrossRef]
- Verdolotti, L.; Lavorgna, M.; Lamanna, R.; Di Maio, E.; Iannace, S. Polyurethane-silica hybrid foam by sol–gel approach: Chemical and functional properties. Polymer 2015, 56, 20–28. [Google Scholar] [CrossRef]
- Somdee, P.; Lassú -Kuknyó, T.; Kónya, C.; Szabó, T.; Marossy, K. Thermal analysis of polyurethane elastomers matrix with different chain extender contents for thermal conductive application. J. Therm. Anal. Calorim. 2019, 138, 1003–1010. [Google Scholar] [CrossRef]
- Trovati, G.; Sanches, E.A.; Neto, S.C.; Mascarenhas, Y.P.; Chierice, G.O. Characterization of polyurethane resins by FTIR, TGA, and XRD. J. Appl. Polym. Sci. 2010, 115, 263–268. [Google Scholar] [CrossRef]
- Claro, C.J.M.; Neto, S.; Chierice, G.O.; Santos, A.M. Study of the biodegradation of a polymer derived from castor oil by scanning electron microscopy, thermogravimetry and infrared spectroscopy. Polímeros 2006, 16, 129–135. [Google Scholar] [CrossRef][Green Version]
- Fernandes, I.J.; Calheiro, D.; Sánchez, F.A.; Camacho, A.L.D.; Rocha, T.L.A.D.; Moraes, C.A.M.; Sousa, V.C.D. Characterization of silica produced from rice husk ash: Comparison of purification and processing methods. J. Mater. Res. 2017, 20, 512–518. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Mu, M.; Winey, K.; Cipriano, B.; Raghavan, S.R.; Pack, S.; Rafailovich, M.; Yang, Y.; Grulke, E.; Shields, J.; et al. Relation between the viscoelastic and flammability properties of polymer nanocomposites. Polymer 2008, 49, 4358–4368. [Google Scholar] [CrossRef]
- Shan, G.; Jin, W.; Chen, H.; Zhao, M.; Surampalli, R.; Ramakrishnan, A.; Zhang, T.; Tyagi, R.D. Flame-retardant polymer nanocomposites and their heat-release rates. J. Hazard. Toxic Radioact. Waste 2015, 19, 04015006–04015020. [Google Scholar] [CrossRef]
- Babiarczuk, B.; Lewandowski, D.; Kierzek, K.; Detyna, J.; Jones, W.; Kaleta, J.; Krzak, J. Mechanical properties of silica aerogels controlled by synthesis parameters. J. Non Cryst. Solids 2023, 606, 122171–122180. [Google Scholar] [CrossRef]
- Lin, X.C.; Li, S.L.; Li, W.X.; Wang, Z.H.; Zhang, J.Y.; Liu, B.W.; Fu, T.; Zhao, H.B.; Wang, Y.Z. Thermo-responsive self-Ceramifiable robust aerogel with exceptional strengthening and thermal insulating performance at ultrahigh temperatures. Adv. Funct. Mater. 2023, 33, 2214913–2214923. [Google Scholar] [CrossRef]
- Cao, M.; Liu, B.W.; Zhang, L.; Peng, Z.C.; Zhang, Y.Y.; Wang, H.; Zhao, H.B.; Wang, Y.Z. Fully biomass-based aerogels with ultrahigh mechanical modulus, enhanced flame retardancy, and great thermal insulation applications. Compos. B Eng. 2021, 25, 109309–109320. [Google Scholar] [CrossRef]
- Shi, B.; Xie, L.; Ma, B.; Zhou, Z.; Xu, B.; Qu, L. Preparation and properties of highly transparent SiO2 aerogels for thermal insulation. Gels 2022, 8, 744–756. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Yadav, A.; de Souza, F.M.; Dawsey, T.; Gupta, R.K. Recent advancements in flame-retardant polyurethane foams: A review. Ind. Eng. Chem. Res. 2022, 61, 15046–15065. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Wang, X. Influence of nano-silica on the flame retardancy and smoke suppression properties of transparent intumescent fire-retardant coatings. Prog. Org. Coat. 2017, 112, 319–329. [Google Scholar] [CrossRef]
- Sykam, K.; Försth, M.; Sas, G.; Restás, Á.; Das, O. Phytic acid: A bio-based flame retardant for cotton and wool fabrics. Ind. Crops Prod. 2021, 64, 113349–113364. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Sadiku, E.R.; Ray, S.S.; Mochane, M.J.; Matabola, K.P.; Motloung, M. Flame retardancy efficacy of phytic acid: An overview. J. Appl. Polym. Sci. 2022, 39, 52495–52530. [Google Scholar] [CrossRef]
- Nzereogu, P.; Omah, A.; Ezema, F.; Iwuoha, E.; Nwanya, A. Silica extraction from rice husk: Comprehensive review and applications. Hybrid Adv. 2023, 4, 100111–100126. [Google Scholar] [CrossRef]
- Cantero, D.; Pinilla-Peñalver, E.; Romero, A.; Sánchez-Silva, L. Synthesis of waterborne polyurethane aerogels-like materials via freeze-drying: An innovative approach. J. Mater. Sci. 2023, 58, 9087–9102. [Google Scholar] [CrossRef]
- Pinilla-Peñalver, E.; Cantero, D.; Romero, A.; Sánchez-Silva, L. Exploring the impact of the synthesis variables involved in the polyurethane aerogels-like materials design. Gels 2024, 10, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Simón-Herrero, C.; Romero, A.; Valverde, J.L.; Sánchez-Silva, L. Hydroxyethyl cellulose/alumina-based aerogels as lightweight insulating materials with high mechanical strength. J. Mater. Sci. 2018, 53, 1556–1567. [Google Scholar] [CrossRef]
- UNE-EN 3844-3:2019; Material Aeroespacial. Inflamabilidad de los Materiales No Metálicos. Parte 3: Ensayo de Pequeño Quemador, 45º. Determinación de la Resistencia del Material a la Propagación de la Llama y de la Incandescencia y a la Penetración de la Llama (Ratificada por la Asociación Española de Normalización en Octubre de 2019). Asociacion Espanola de Normalizacion: Madrid, Spain, 2019.
- Gong, S.Q.; Epasinghe, D.J.; Zhang, W.; Zhou, B.; Niu, L.N.; Ryou, H.; Eid, A.A.; Frassetto, A.; Yiu, C.K.Y.; Arola, D.D.; et al. Synthesis of antimicrobial silsesquioxane–silica hybrids by hydrolytic co-condensation of alkoxysilanes. Polym. Chem. 2014, 5, 454–462. [Google Scholar] [CrossRef]
- Li, K.M.; Jiang, J.G.; Tian, S.C.; Chen, X.J.; Yan, F. Influence of silica types on synthesis and performance of amine–silica hybrid materials used for CO2 capture. J. Phys. Chem. C 2014, 118, 2454–2462. [Google Scholar] [CrossRef]
- Ullah, R.; Deb, B.K.; Mollah, M.Y.A. Synthesis and characterization of silica coated iron-oxide composites of different ratios. Int. J. Compos. Mater. 2014, 4, 135–145. [Google Scholar] [CrossRef]
- Comite, A. Preparation of silica membranes by sol-gel method. In Current Trends and Future Developments on (Bio-)Membranes, 1st ed.; Basile, A., Favvas, E.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–23. [Google Scholar]
- Zhang, R.; Cai, S.; Xu, G.; Zhao, H.; Li, Y.; Wang, X.; Huang, K.; Wu, X. Crack self-healing of phytic acid conversion coating on AZ31 magnesium alloy by heat treatment and the corrosion resistance. Appl. Surf. Sci. 2014, 313, 896–904. [Google Scholar] [CrossRef]
- Yudovin-Farber, I.; Beyth, N.; Weiss, E.I.; Domb, A.J. Antibacterial effect of composite resins containing quaternary ammonium polyethyleneimine nanoparticles. J. Nanopart. Res. 2010, 12, 591–603. [Google Scholar] [CrossRef]
- Dang, N.T.T.; Nguyen, T.T.A.; Phan, T.D.; Tran, H.; Van Dang, P.; Nguyen, H.Q. Synthesis of silica nanoparticles from rice husk ash. Sci. Technol. Develop. J. 2017, 20, 50–54. [Google Scholar] [CrossRef]
- Li, Y.; Lan, J.Y.; Liu, J.; Yu, J.; Luo, Z.; Wang, W.; Sun, L. Synthesis of gold nanoparticles on rice husk silica for catalysis applications. Ind. Eng. Chem. Res. 2015, 54, 5656–5663. [Google Scholar] [CrossRef]
- Wang, X.; Shi, L.; Zhang, J.; Cheng, J.; Wang, X. Self-assembly fabrication, microstructures and antibacterial performance of layer-structured montmorillonite nanocomposites with cationic silica nanoparticles. RSC Adv. 2017, 7, 31502–31511. [Google Scholar] [CrossRef]
- Galimberti, M.; Martino, M.; Guenzi, M.; Leonardi, G.; Citterio, A. Thermal stability of ammonium salts as compatibilizers in polymer/layered silicate nanocomposites. e-Polymers 2009, 9, 56–69. [Google Scholar] [CrossRef]
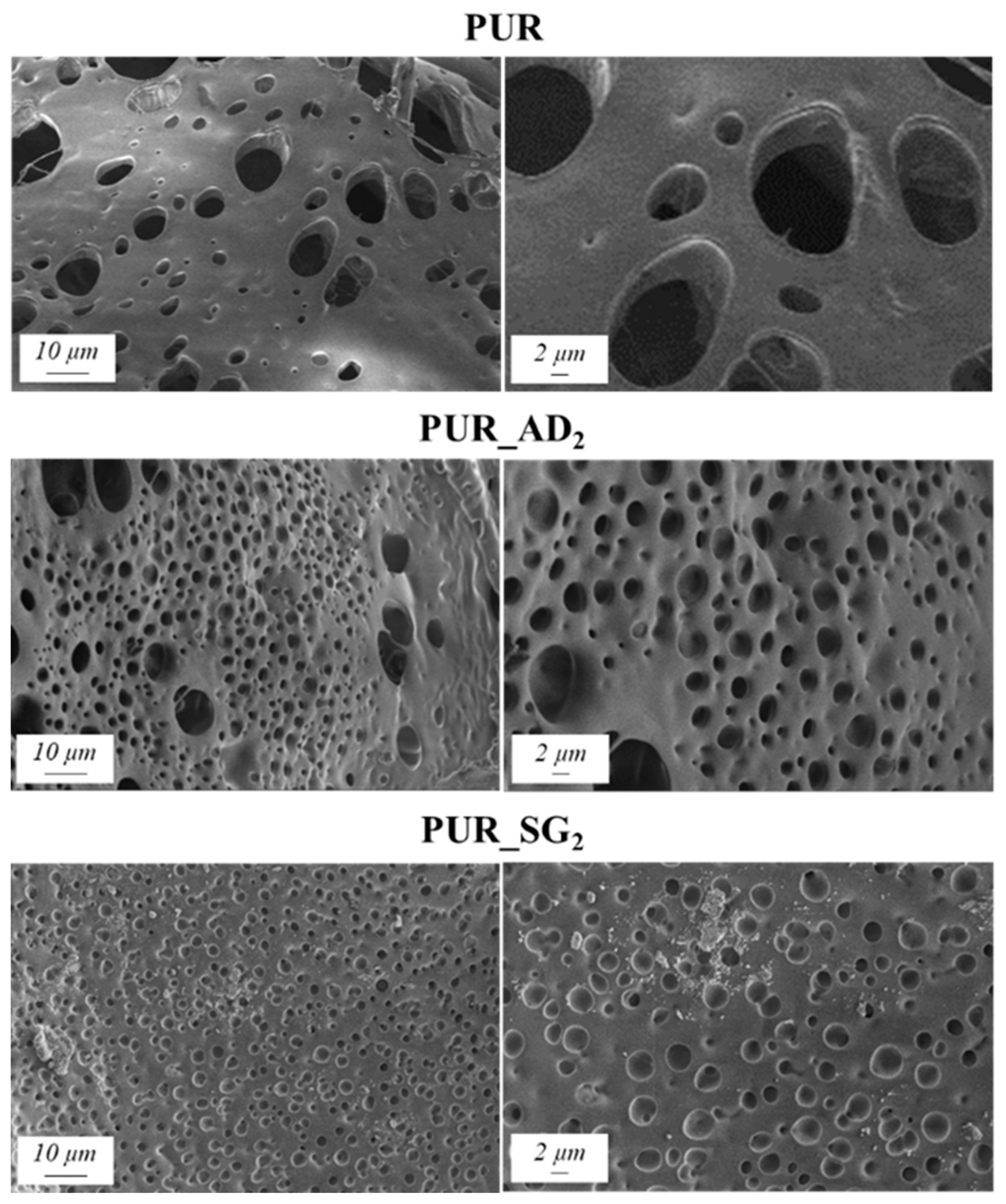
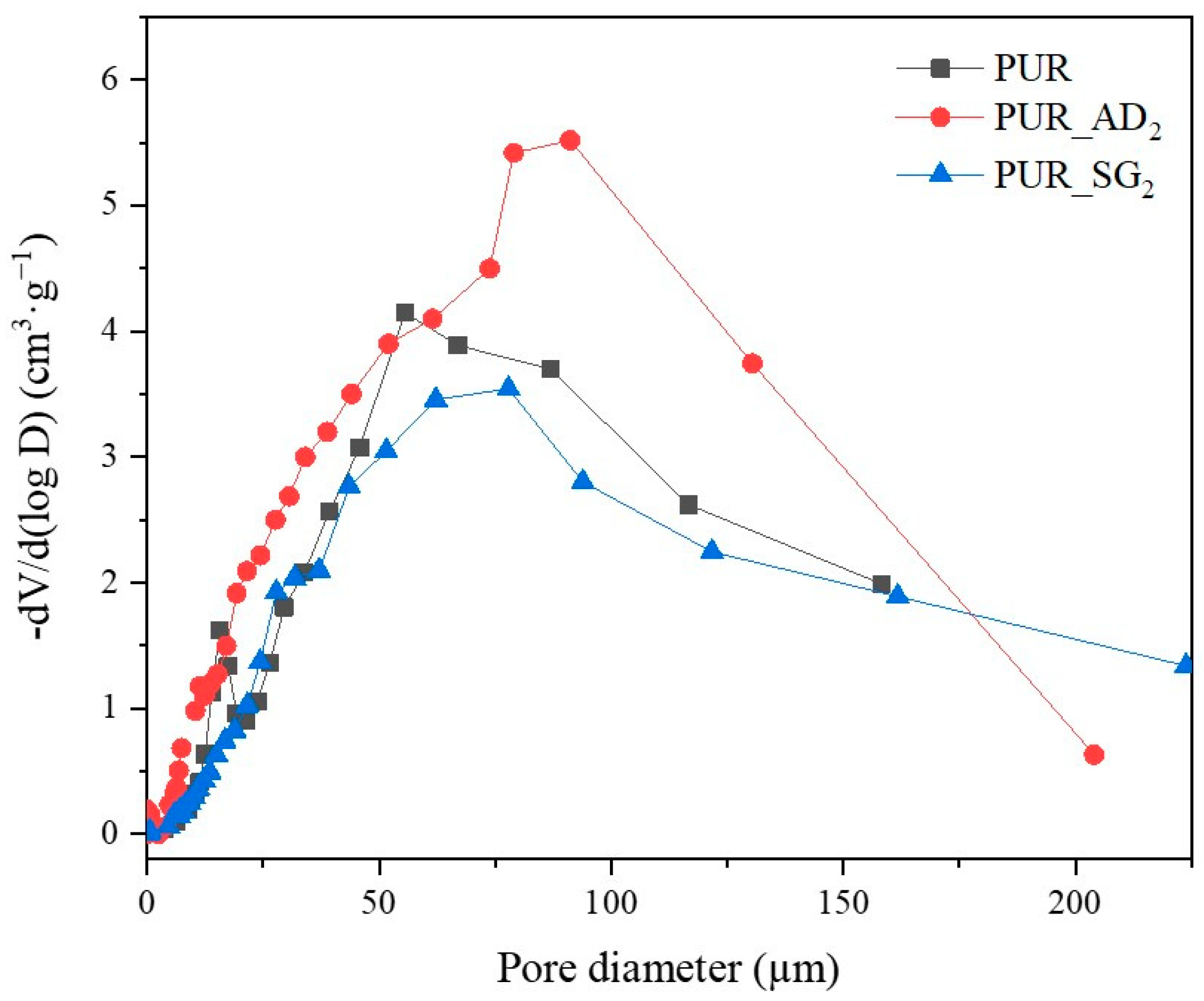


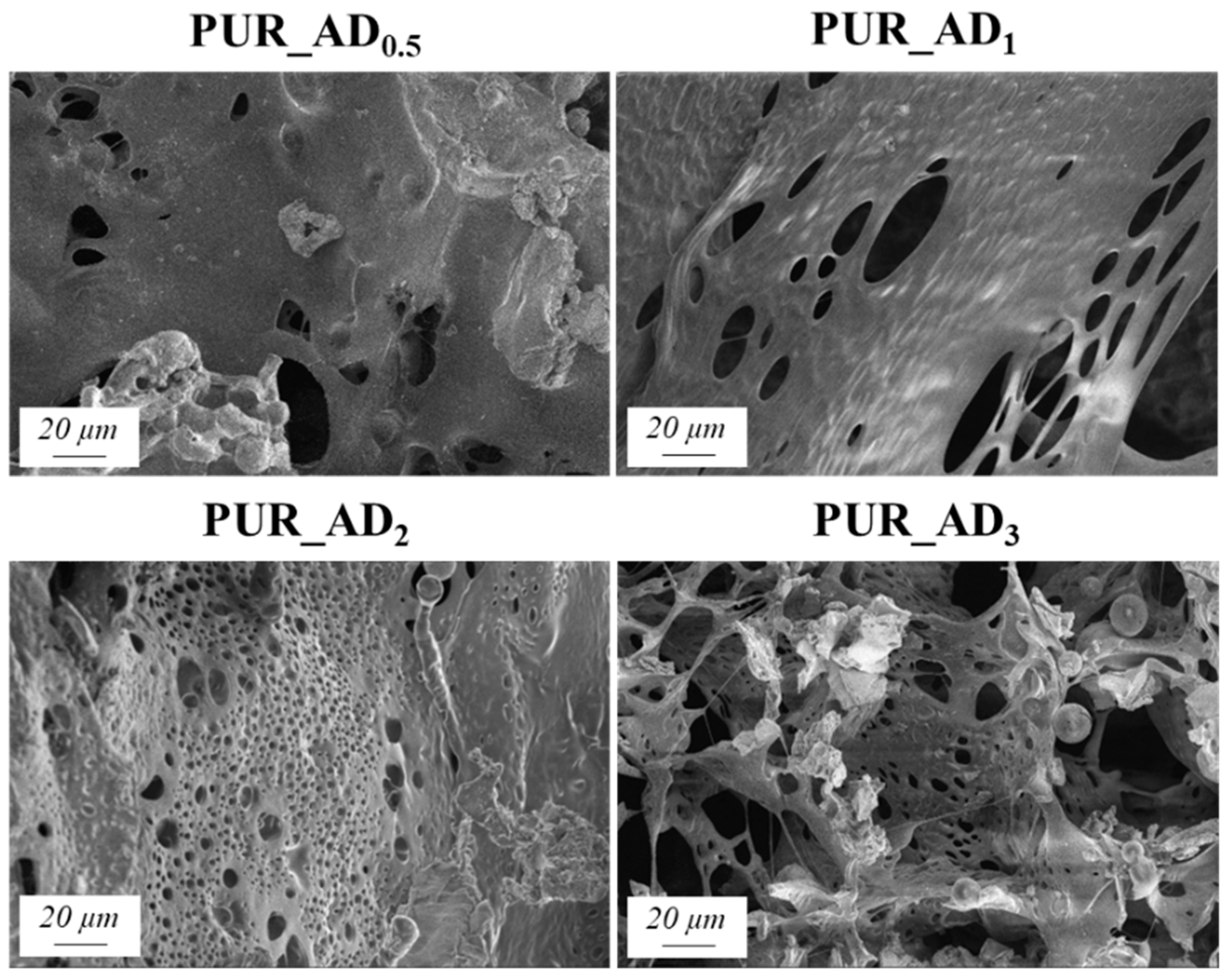
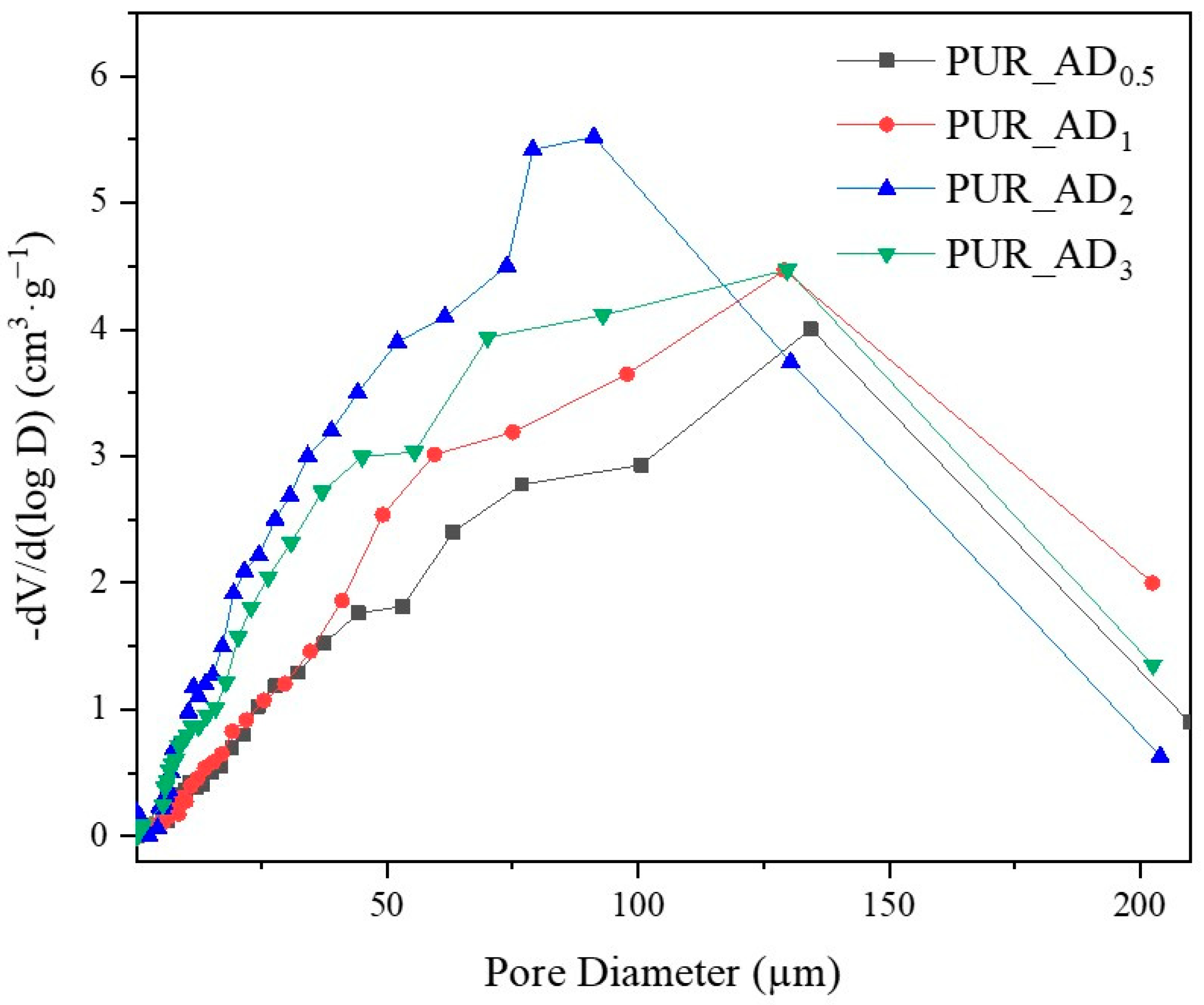


| Sample | SiO2 Particles Density (g·cm−3) | Surface Area (m2·g−1) * | Total Intruded Pore Volume (cc·g−1) * | Density (±0.002, g·cm−3) | Thermal Conductivity (±0.0010, W·m−1·K−1) | Young’s Modulus (±0.0005, MPa) | Tg (°C) |
|---|---|---|---|---|---|---|---|
| PUR | - | 11.67 | 3.17 | 0.051 | 0.0387 | 0.001 | −49.8 |
| PUR_AD2 | 0.30 | 13.48 | 4.71 | 0.058 | 0.0346 | 0.005 | −48.9 |
| PUR_SG2 | 0.32 | 0.36 | 2.92 | 0.068 | 0.0346 | 0.013 | −47.2 |
| Sample | Surface Area (m2·g−1) * | Total Intruded Pore Volume (cc·g−1) * | Density (±0.002, g·cm−3) | Thermal Conductivity (±0.0010, W·m−1·K−1) | Young’s Modulus (±0.0005, MPa) |
|---|---|---|---|---|---|
| PUR_AD0.5 | 0.42 | 2.92 | 0.064 | 0.0363 | 0.001 |
| PUR_AD1 | 9.68 | 3.14 | 0.063 | 0.0338 | 0.002 |
| PUR_AD2 | 13.48 | 4.71 | 0.058 | 0.0346 | 0.005 |
| PUR_AD3 | 11.14 | 4.07 | 0.056 | 0.0357 | 0.008 |
| Sample | SiO2 Particle Density (g·cm−3) | Surface Area (m2·g−1) * | Total Intruded Pore Volume (cc·g−1) * | Density (±0.002, g·cm−3) | Thermal Conductivity (W·m−1·K−1) | Young’s Modulus (±0.0005, MPa) | Tg (°C) |
|---|---|---|---|---|---|---|---|
| PUR_AD2 | 0.30 | 13.48 | 4.71 | 0.058 | 0.0346 | 0.005 | −48.9 |
| PUR_AD@QAS-PA2 | 0.53 | 0.36 | 2.06 | 0.065 | 0.0335 | 0.010 | −47.5 |
| SiO2 Particle Type | |||
|---|---|---|---|
| AD | AD@QAS-PA | SG | |
| Method of production | Acidic extraction | Acidic extraction Functionalization with PA and QAS | Alkaline extraction Sol–gel precipitation |
| Inorganic residue (%)—evaluated by TGA analysis (800 °C) | 98.7 | 39.6 | 88.6 |
| Z potential (mV) | −20 ± 1 (water) | 41 ± 2 (ethanol) | −34 ± 1 (water) |
| Morphology (SEM images) | 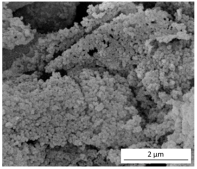 |  | 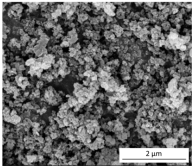 |
| Elemental composition | C (9 wt.%) O (44 wt.%) Si (47 wt.%) | C (54 wt.%) O (23 wt.%) Si (10 wt.%) P (8 wt.%) impurities (5 wt.%) | C (3 wt.%) O (59 wt.%) Si (32 wt.%) impurities (6 wt.%) |
| Density (g·cm−3) | 0.30 | 0.53 | 0.32 |
| Set | Experiment (*) | PUR Sample (**) | Doping Agent | SiO2 Content (wt.%) |
|---|---|---|---|---|
| 0 | PUR | None | - | |
| 1 | 1 | SiO2_AD2 | AD | 2 |
| 2 | SiO2_AD@QAS-PA2 | AD@QAS-PA | ||
| 3 | SiO2_SG2 | SG | ||
| 2 | 4 | SiO2_AD0.5 | AD | 0.5 |
| 5 | SiO2_AD1 | 1 | ||
| 6 | SiO2_AD2 | 2 | ||
| 7 | SiO2_AD3 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinilla-Peñalver, E.; del Fresno, Ó.; Cantero, D.; Moreira, A.; Gomes, F.; Miranda, F.; Oliveira, M.; Ornelas, M.; Sánchez-Silva, L.; Romero, A. Enhancing Flame-Retardant Properties of Polyurethane Aerogels Doped with Silica-Based Particles. Gels 2024, 10, 465. https://doi.org/10.3390/gels10070465
Pinilla-Peñalver E, del Fresno Ó, Cantero D, Moreira A, Gomes F, Miranda F, Oliveira M, Ornelas M, Sánchez-Silva L, Romero A. Enhancing Flame-Retardant Properties of Polyurethane Aerogels Doped with Silica-Based Particles. Gels. 2024; 10(7):465. https://doi.org/10.3390/gels10070465
Chicago/Turabian StylePinilla-Peñalver, Esther, Óscar del Fresno, Darío Cantero, Adriana Moreira, Filipa Gomes, Francisca Miranda, Marcelo Oliveira, Mariana Ornelas, Luz Sánchez-Silva, and Amaya Romero. 2024. "Enhancing Flame-Retardant Properties of Polyurethane Aerogels Doped with Silica-Based Particles" Gels 10, no. 7: 465. https://doi.org/10.3390/gels10070465
APA StylePinilla-Peñalver, E., del Fresno, Ó., Cantero, D., Moreira, A., Gomes, F., Miranda, F., Oliveira, M., Ornelas, M., Sánchez-Silva, L., & Romero, A. (2024). Enhancing Flame-Retardant Properties of Polyurethane Aerogels Doped with Silica-Based Particles. Gels, 10(7), 465. https://doi.org/10.3390/gels10070465







