An Efficient and Economic Approach for Producing Nanocellulose-Based Aerogel from Kapok Fiber
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Cellulose Nanofibers (NFs)
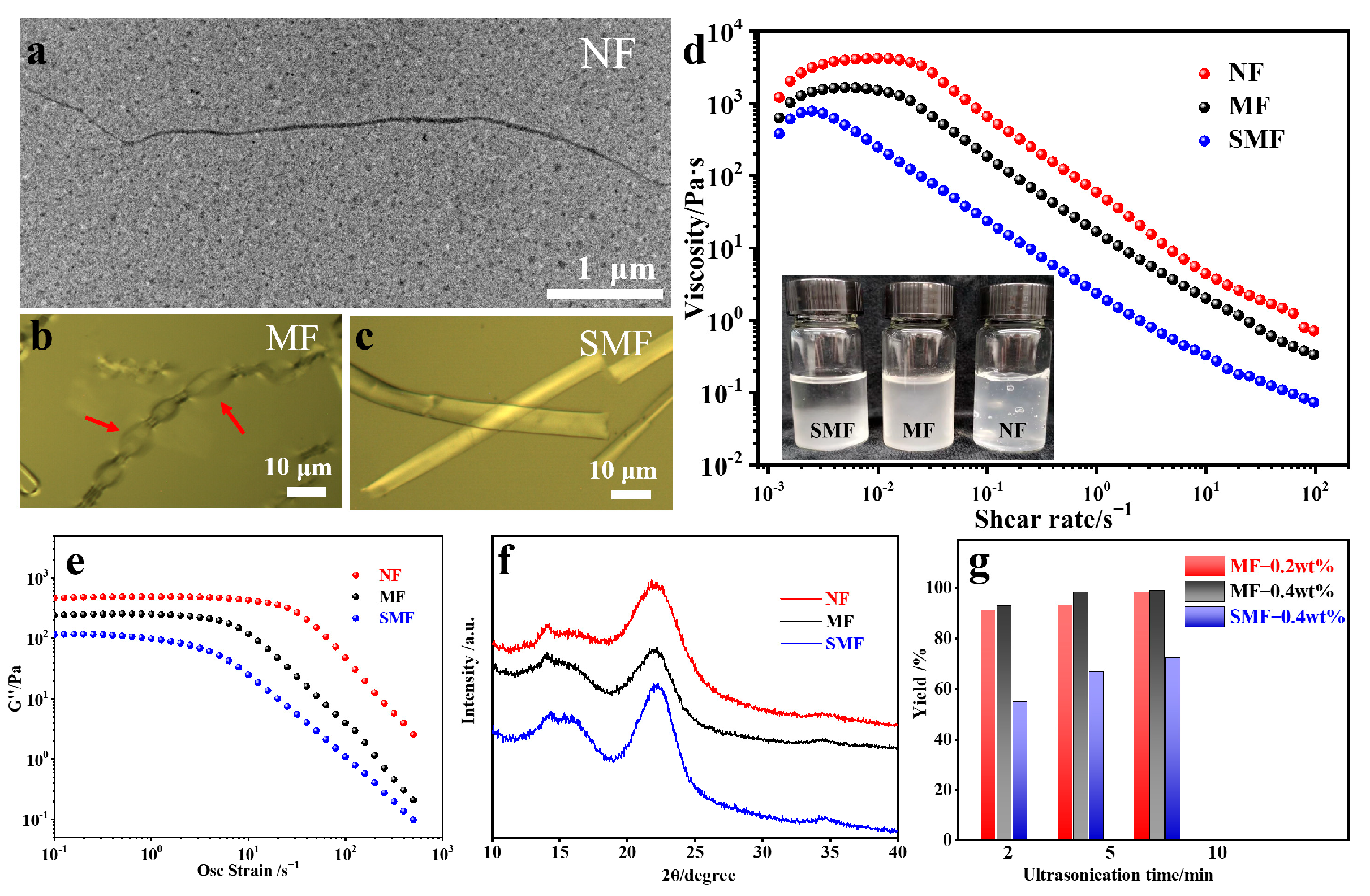
2.2. Characterizations of NF
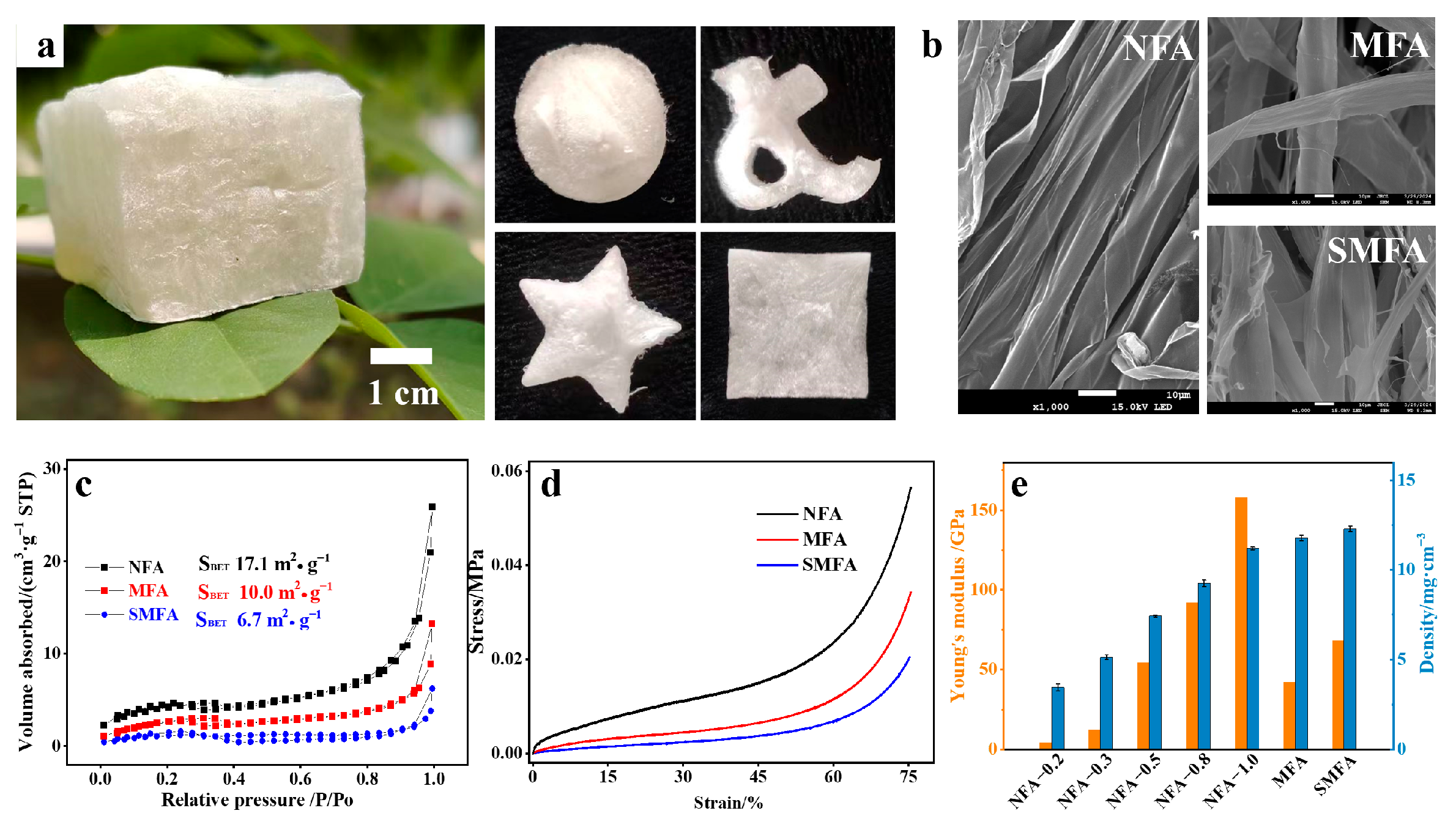
2.3. Mechanical Performance of NFA Aerogels
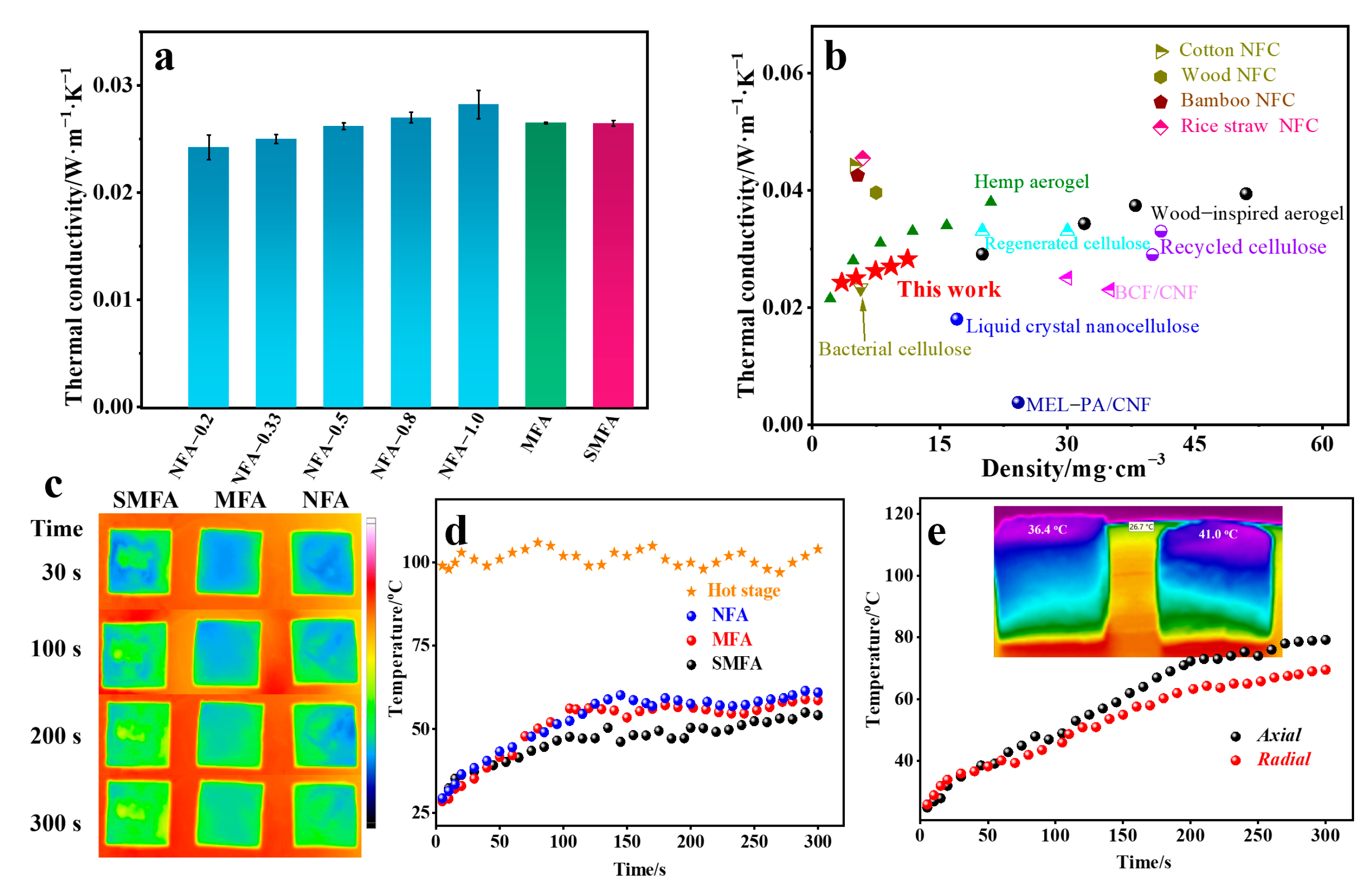
2.4. Thermal Insulation Performance of the NFA Aerogel
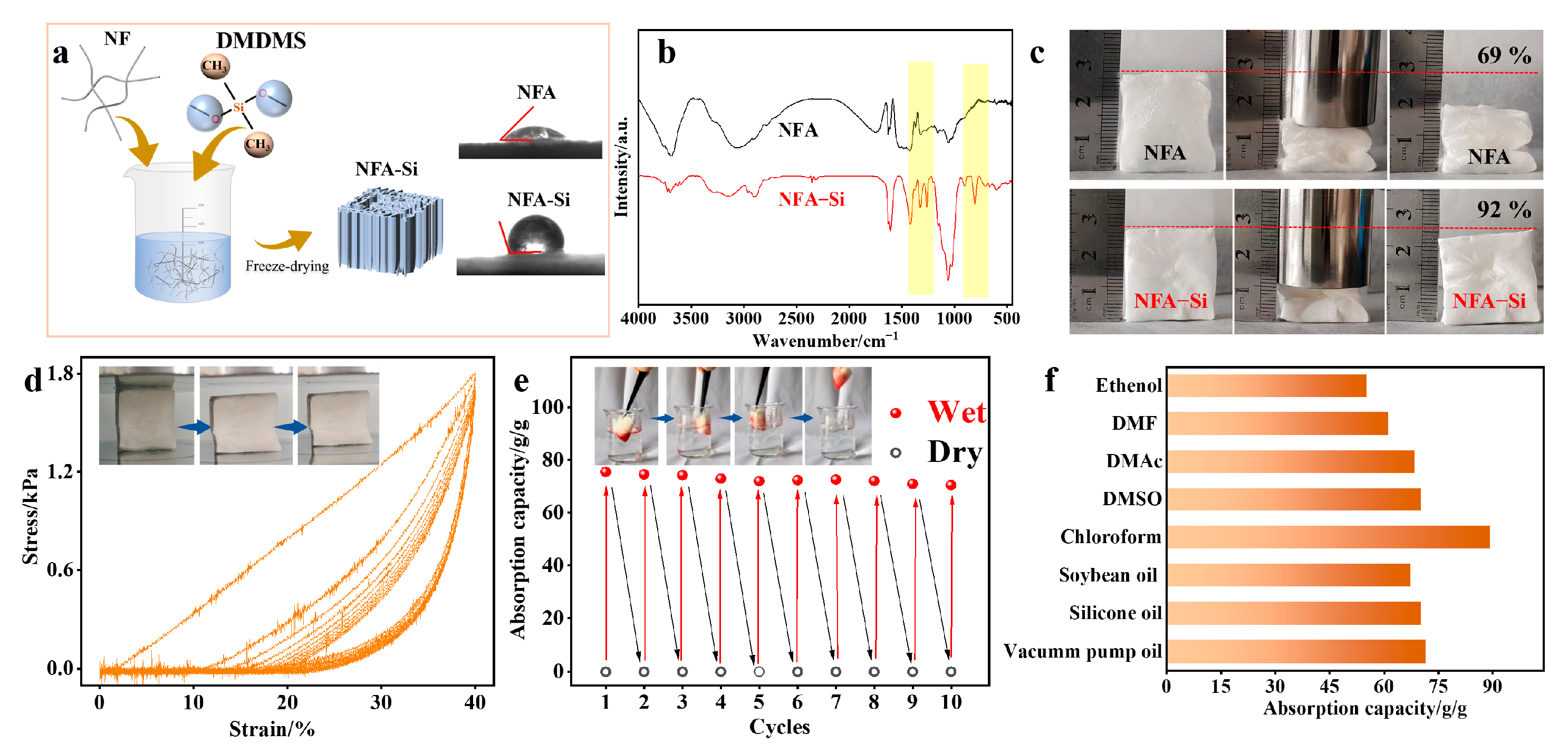
2.5. Preparation of NFA-Si Aerogel and the Absorption Performance
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Micro Kapok Fibers (MFs) and Cellulose Nanofibers (NFs)
4.3. Preparation of MFA and NFA Aerogels
4.4. Preparation of NFA-Si Aerogel
4.5. Liquid Adsorption Experiments
4.6. Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dorcheh, A.S.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Stergar, J.; Maver, U. Review of aerogel-based materials in biomedical applications. J. Sol-Gel Sci. Technol. 2016, 77, 738–752. [Google Scholar] [CrossRef]
- Riffat, S.B.; Qiu, G. A review of state-of-the-art aerogel applications in buildings. Int. J. Low-Carbon Technol. 2013, 8, 1–6. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Aerogel insulation for building applications: A state-of-the-art review. Energy Build. 2011, 43, 761–769. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Zheng, Y.; Xu, Y.; Zheng, Z.; Chen, X.; Liu, W. Versatile aerogels for sensors. Small 2019, 15, 1902826. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Maldonado-Hódar, F.J. Carbon aerogels for catalysis applications: An overview. Carbon 2005, 43, 455–465. [Google Scholar] [CrossRef]
- Yu, C.; Kim, H.; Youn, J.R.; Song, Y.S. Enhancement of structural stability of graphene aerogel for thermal energy harvesting. ACS Appl. Energy Mater. 2021, 4, 11666–11674. [Google Scholar] [CrossRef]
- Peng, H.; Xiong, W.; Yang, Z.; Xu, Z.; Cao, J.; Jia, M.; Xiang, Y. Advanced MOFs@ aerogel composites: Construction and application towards environmental remediation. J. Hazard. Mater. 2022, 432, 128684. [Google Scholar] [CrossRef] [PubMed]
- Rechberger, F.; Niederberger, M. Synthesis of aerogels: From molecular routes to 3-dimensional nanoparticle assembly. Nanoscale Horiz. 2017, 2, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Long, L.Y.; Weng, Y.X.; Wang, Y.Z. Cellulose aerogels: Synthesis, applications, and prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Bhagat, S.D.; Hirashima, H.; Pajonk, G.M. Synthesis of flexible silica aerogels using methyltrimethoxysilane (MTMS) precursor. J. Colloid Interface Sci. 2006, 300, 279–285. [Google Scholar] [PubMed]
- Chen, W.; Yu, H.; Li, Q.; Liu, Y.; Li, J. Ultralight and highly flexible aerogels with long cellulose I nanofibers. Soft Matter 2011, 7, 10360–10368. [Google Scholar] [CrossRef]
- Hayase, G.; Kanamori, K.; Nakanishi, K. New flexible aerogels and xerogels derived from methyltrimethoxysilane/dimethyldimethoxysilane co-precursors. J. Mater. Chem. 2011, 21, 17077–17079. [Google Scholar] [CrossRef]
- Qua, E.H.; Hornsby, P.R.; Sharma HS, S.; Lyons, G. Preparation and characterisation of cellulose nanofibres. J. Mater. Sci. 2011, 46, 6029–6045. [Google Scholar] [CrossRef]
- Isogai, A.; Bergström, L. Preparation of cellulose nanofibers using green and sustainable chemistry. Curr. Opin. Green Sustain. Chem. 2018, 12, 15–21. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.L. Super water absorbing and shape memory nanocellulose aerogels from TEMPO-oxidized cellulose nanofibrils via cyclic freezing–thawing. J. Mater. Chem. A 2014, 2, 350–359. [Google Scholar] [CrossRef]
- Qi, J.; Xie, Y.; Liang, H.; Wang, Y.; Ge, T.; Song, Y.; Wang, M.; Li, Q.; Yu, H.; Fan, Z.; et al. Lightweight, flexible, thermally-stable, and thermally-insulating aerogels derived from cotton nanofibrillated cellulose. ACS Sustain. Chem. Eng. 2019, 7, 9202–9210. [Google Scholar] [CrossRef]
- Qin, B.; Yu, Z.L.; Huang, J.; Meng, Y.F.; Chen, R.; Chen, Z.; Yu, S.H. A Petrochemical-Free Route to Superelastic Hierarchical Cellulose Aerogel. Angew. Chem. 2023, 135, e202214809. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, Y.; Jiang, J.; Wang, L.; Song, M.; Bi, R.; Zhu, P.; Jiang, F. Multifunctional superelastic cellulose nanofibrils aerogel by dual ice-templating assembly. Adv. Funct. Mater. 2021, 31, 2106269. [Google Scholar] [CrossRef]
- Eriksen, Ø.; Syverud, K.; Gregersen, Ø. The use of microfibrillated cellulose produced from kraft pulp as strength enhancer in TMP paper. Nord. Pulp Pap. Res. J. 2008, 23, 299–304. [Google Scholar] [CrossRef]
- Kargupta, W.; Stevenson, T.; Sharman, S.; Tanner, J.; Batchelor, W. Sustainable production of nanocellulose: Technoeconomic assessment, energy savings and scalability. J. Clean. Prod. 2023, 425, 138748. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for extraction of nanocellulose from various sources. Handb. Nanocellul. Cellul. Nanocompos. 2017, 1, 1–49. [Google Scholar]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wu, Z.; An, B.; Ma, C.; Xu, L.; Zhang, Z.; Luo, S.; Li, W.; Liu, S. Thermal-insulating, flame-retardant and mechanically resistant aerogel based on bio-inspired tubular cellulose. Compos. Part B Eng. 2021, 220, 108997. [Google Scholar] [CrossRef]
- Lin, X.; Li, Y.; Fang, Z.; Li, G.; Liu, Y.; Qiu, X. Strong Yet Tough Transparent Paper with Superb Foldability. Small 2024, 2400151. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, D.O.; Lindh, J.; Nyholm, L.; Strømme, M.; Mihranyan, A. Cooxidant-free TEMPO-mediated oxidation of highly crystalline nanocellulose in water. RSC Adv. 2014, 4, 52289–52298. [Google Scholar] [CrossRef]
- Tahiri, C.; Vignon, M.R. TEMPO-oxidation of cellulose: Synthesis and characterisation of polyglucuronans. Cellulose 2000, 7, 177–188. [Google Scholar] [CrossRef]
- Zhang, K.; Zhai, W.; Cao, Z.; Wang, Y.; Li, L.; Li, J.; Liu, J.; Xie, Y.; Gan, W. A sustainable pore wall strengthening for strong and fire-retardant nanopolymerised wood. Chem. Eng. J. 2024, 480, 147971. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, H.; Wang, H. Thermal conductive properties of solid-liquid-gas three-phase unsaturated concrete. Constr. Build. Mater. 2020, 232, 117242. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Y.; Ye, Y.; Qiu, Z.; Zhang, Y.; Yu, Z.; Sun, X.; Bressler, D.C.; Jiang, F. Superelastic and ultralight aerogel assembled from hemp microfibers. Adv. Funct. Mater. 2023, 33, 2300893. [Google Scholar] [CrossRef]
- Isogai, A. Emerging nanocellulose technologies: Recent developments. Adv. Mater. 2021, 33, 2000630. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]


| Sample | Mechanical Crushing | NaClO Pretreatment | NaClO Added in TEMPO Oxidation (mmol) |
|---|---|---|---|
| MF-0 | No | Yes | 0 |
| MF-1 | No | Yes | 1 |
| MF-2 | No | Yes | 3 |
| MF-3 | No | Yes | 5 |
| MF-4 | No | Yes | 7 |
| SMF | Yes | Yes | 7 |
| NF * | No | Yes | 7 |
| Sample | Solid Concentration in Suspension (wt%) | Density (mg cm−3) |
|---|---|---|
| NFA-0.2 | 0.2 | 3.5 ± 0.2 |
| NFA-0.3 | 0.3 | 5.1 ± 0.1 |
| NFA-0.5 | 0.5 | 7.4 ± 0.1 |
| NFA-0.8 | 0.8 | 9.3 ± 0.2 |
| NFA-1.0 | 1.0 | 11.2 ± 0.1 |
| MFA | 1.0 | 11.8 ± 0.2 |
| SMFA | 1.0 | 12.3 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, M.; Wang, Q.; Wang, S.; Yang, Z.; Deng, X.; Zhao, H. An Efficient and Economic Approach for Producing Nanocellulose-Based Aerogel from Kapok Fiber. Gels 2024, 10, 490. https://doi.org/10.3390/gels10080490
Hou M, Wang Q, Wang S, Yang Z, Deng X, Zhao H. An Efficient and Economic Approach for Producing Nanocellulose-Based Aerogel from Kapok Fiber. Gels. 2024; 10(8):490. https://doi.org/10.3390/gels10080490
Chicago/Turabian StyleHou, Minjie, Qi Wang, Shunyu Wang, Zeze Yang, Xuefeng Deng, and Hailong Zhao. 2024. "An Efficient and Economic Approach for Producing Nanocellulose-Based Aerogel from Kapok Fiber" Gels 10, no. 8: 490. https://doi.org/10.3390/gels10080490




