Development and Applications of PLGA Hydrogels for Sustained Delivery of Therapeutic Agents
Abstract
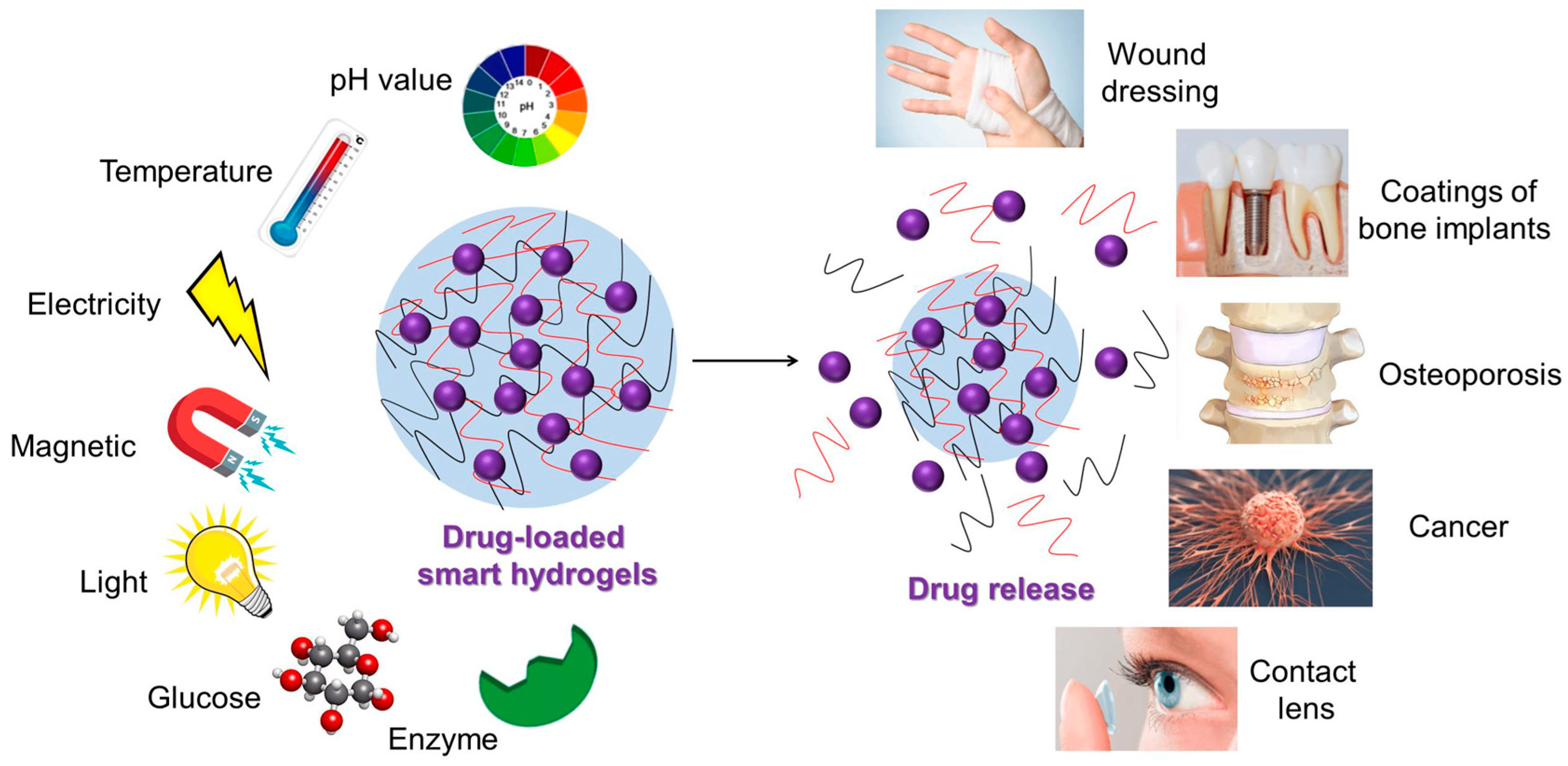
:1. Introduction
Definition and Composition of PLGA Hydrogels
- m represents the number of lactic acid units.
- n represents the number of glycolic acid units.
2. Synthesis Methods of PLGA Hydrogels
2.1. Chemical Crosslinking
2.2. Physical Crosslinking
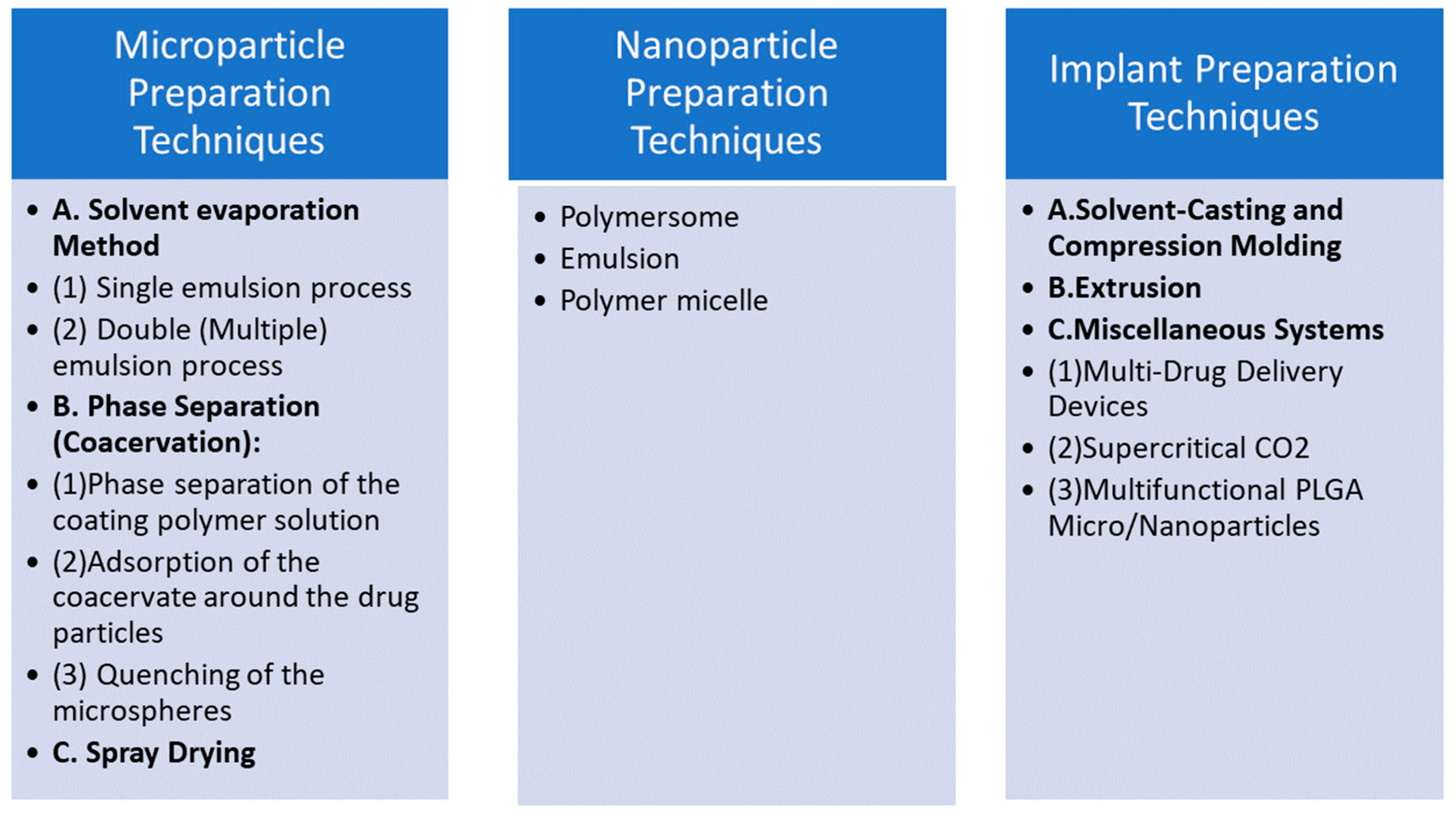
2.3. Fabrication Techniques for PLGA Hydrogels
2.4. Emulsion Solvent Evaporation Technique
2.5. Solvent Casting
2.6. Electrospinning
2.7. Thermal Gelation
2.8. Photopolymerization
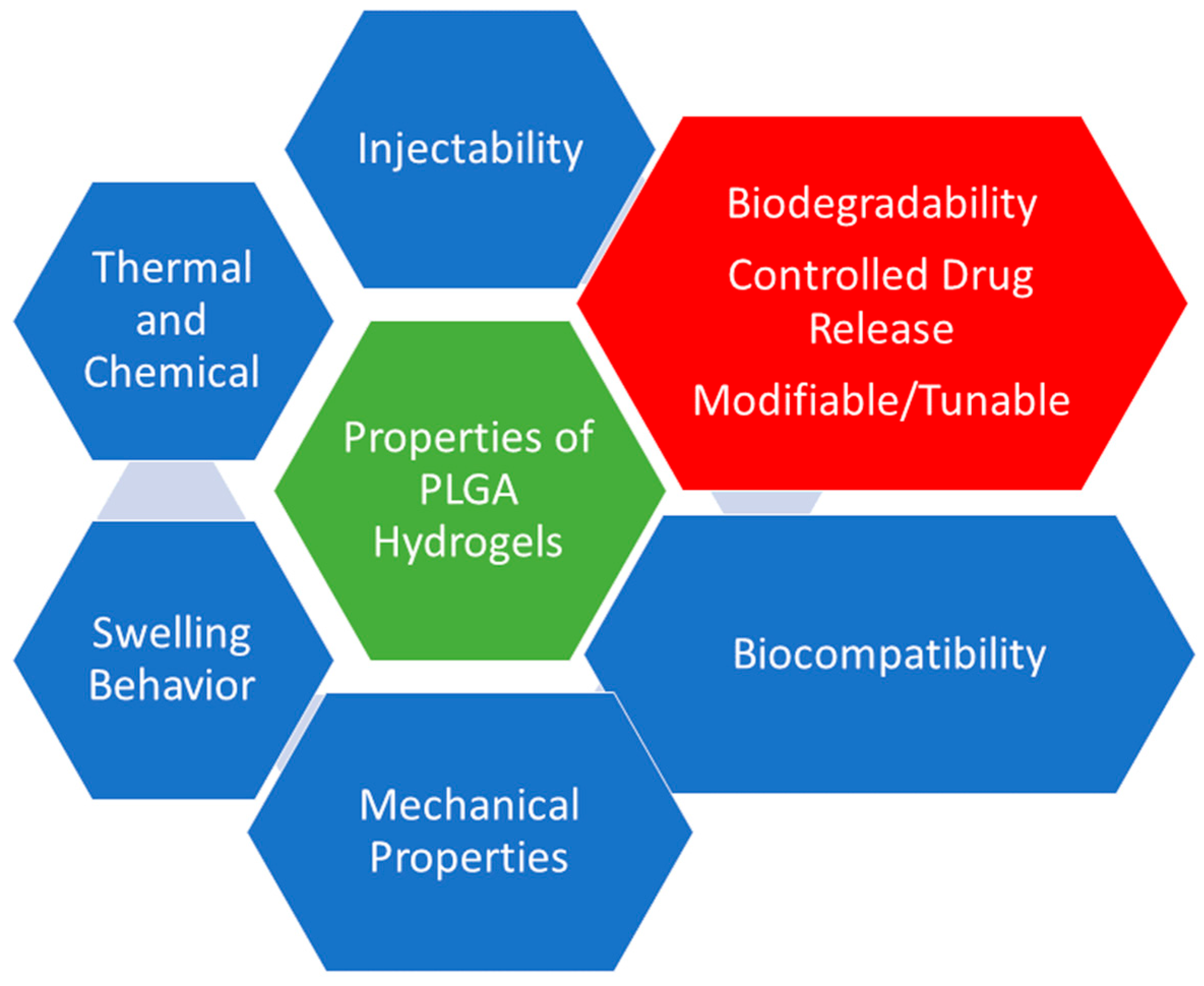
3. Properties of PLGA Hydrogels
3.1. Biodegradability
3.2. Biocompatibility
3.3. Mechanical Properties
3.4. Controlled Drug Release
3.5. Swelling Behavior
3.6. Thermal and Chemical Stability
3.7. Modifiable/Tunable Surface Properties
3.8. Tunable Degradation Kinetics
3.9. Injectability
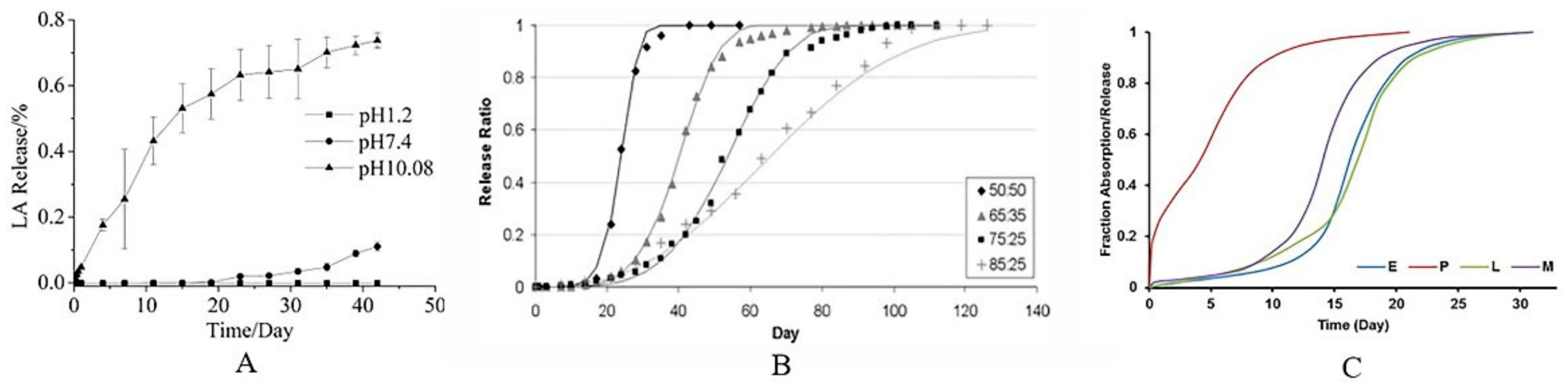
4. Sustained Release Mechanisms of PLGA Hydrogels
4.1. Diffusion-Controlled Drug Release System
4.1.1. Degradation-Controlled Release System
4.1.2. Swelling-Controlled Release System
4.1.3. Combined Release Mechanisms
4.2. Types of Kinetics of Drug Release Mechanisms
4.2.1. Zero-Order Kinetics
4.2.2. First-Order Kinetics
4.3. Higuchi Model
4.4. Peppas Model
5. Applications of PLGA Hydrogels in Drug Delivery
5.1. Cancer Therapeutics
5.2. Antibiotics and Antimicrobials
6. Challenges and Future Perspectives in PLGA Hydrogel Research
Enhanced Drug Loading and Release Control
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahmani, F.; Atabaki, R.; Behrouzi, S.; Mohamadpour, F.; Kamali, H. The recent advancement in the PLGA-based thermo-sensitive hydrogel for smart drug delivery. Int. J. Pharm. 2023, 631, 122484. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.; Jose, V.K.; Lee, J.M. Hydrogels for medical and environmental applications. Small Methods 2020, 4, 1900735. [Google Scholar] [CrossRef]
- Liu, X.; Gao, M.; Chen, J.; Guo, S.; Zhu, W.; Bai, L.; Zhai, W.; Du, H.; Wu, H.; Yan, C. Recent advances in stimuli-responsive shape-morphing hydrogels. Adv. Funct. Mater. 2022, 32, 2203323. [Google Scholar] [CrossRef]
- Ding, M.; Jing, L.; Yang, H.; Machnicki, C.; Fu, X.; Li, K.; Wong, I.; Chen, P.-Y. Multifunctional soft machines based on stimuli-responsive hydrogels: From freestanding hydrogels to smart integrated systems. Mater. Today Adv. 2020, 8, 100088. [Google Scholar] [CrossRef]
- Gholamali, I. Stimuli-responsive polysaccharide hydrogels for biomedical applications: A review. Regen. Eng. Transl. Med. 2021, 7, 91–114. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Smart hydrogels for advanced drug delivery systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef]
- Liu, X.; Nakamura, K.; Lowman, A. Composite hydrogels for sustained release of therapeutic agents. Soft Mater. 2003, 1, 393–408. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Z.; Guo, C.; Huang, Z.; Zhang, W.; Ma, F.; Wang, Z.; Kong, Q.; Wang, Y. Application and development of hydrogel biomaterials for the treatment of intervertebral disc degeneration: A literature review. Front. Cell Dev. Biol. 2023, 11, 1286223. [Google Scholar] [CrossRef]
- Cisneros, K.; Chowdhury, N.; Coleman, E.; Ferdous, T.; Su, H.; Jennings, J.A.; Bumgardner, J.D.; Fujiwara, T. Long-Term Controlled Release of Simvastatin from Photoprinted Triple-Networked Hydrogels Composed of Modified Chitosan and PLA–PEG Micelles. Macromol. Biosci. 2021, 21, 2100123. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Alharbi, F.D.; Alhibs, A.S.; Alanazi, N.B.; Alshehri, B.Y.; Saleh, M.A.; Alshehri, F.S.; Algarni, M.A.; Almugaiteeb, T.; Uddin, M.N. PLGA-based nanomedicine: History of advancement and development in clinical applications of multiple diseases. Pharmaceutics 2022, 14, 2728. [Google Scholar] [CrossRef]
- Goncalves, A.D.; Balestri, W.; Reinwald, Y. Biomedical implants for regenerative therapies. In Biomaterials; IntechOpen: Rijeka, Croatia, 2020; pp. 1–36. [Google Scholar]
- Chopra, H.; Singh, I.; Kumar, S.; Bhattacharya, T.; Rahman, M.H.; Akter, R.; Kabir, M.T. A comprehensive review on hydrogels. Curr. Drug Deliv. 2022, 19, 658–675. [Google Scholar]
- Huang, J.; Ali, S. PLGA—A versatile copolymer for design and development of nanoparticles for drug delivery. J. Anal. Pharm. Res. 2023, 12, 72–78. [Google Scholar]
- Ahn, W.; Lee, J.-H.; Kim, S.R.; Lee, J.; Lee, E.J. Designed protein-and peptide-based hydrogels for biomedical sciences. J. Mater. Chem. B 2021, 9, 1919–1940. [Google Scholar] [CrossRef]
- Wall, V.; Nguyen, T.-H.; Nguyen, N.; Tran, P.A. Controlling antibiotic release from polymethylmethacrylate bone cement. Biomedicines 2021, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-based composites for various biomedical applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef]
- Bhushan, S.; Singh, S.; Maiti, T.K.; Sharma, C.; Dutt, D.; Sharma, S.; Li, C.; Tag Eldin, E.M. Scaffold fabrication techniques of biomaterials for bone tissue engineering: A critical review. Bioengineering 2022, 9, 728. [Google Scholar] [CrossRef]
- Silvestri, T.; Grumetto, L.; Neri, I.; De Falco, M.; Graziano, S.F.; Damiano, S.; Giaquinto, D.; Maruccio, L.; de Girolamo, P.; Villapiano, F. Investigating the effect of surface hydrophilicity on the destiny of PLGA-poloxamer nanoparticles in an in vivo animal model. Int. J. Mol. Sci. 2023, 24, 14523. [Google Scholar] [CrossRef]
- Zhuang, Y.; Shen, H.; Yang, F.; Wang, X.; Wu, D. Synthesis and characterization of PLGA nanoparticle/4-arm-PEG hybrid hydrogels with controlled porous structures. RSC Adv. 2016, 6, 53804–53812. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Zhou, J.-E.; Tan, J.; Li, M.; Xu, N.; Qu, F.; Chen, J.; Li, J.; Wang, J. A photopolymerized semi-interpenetrating polymer networks-based hydrogel incorporated with nanoparticle for local chemotherapy of tumors. Pharm. Res. 2021, 38, 669–680. [Google Scholar] [CrossRef]
- Hsu, X.-L.; Wu, L.-C.; Hsieh, J.-Y.; Huang, Y.-Y. Nanoparticle-hydrogel composite drug delivery system for potential ocular applications. Polymers 2021, 13, 642. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, B.; Tu, J.; Xiang, J.; Xiong, H.; Wu, Y.; Ding, S.; Zhu, D.; Zhu, D.; Liu, F. PLGA nanoparticles loaded with sorafenib combined with thermosensitive hydrogel system and microwave hyperthermia for multiple sensitized radiotherapy. Pharmaceutics 2023, 15, 487. [Google Scholar] [CrossRef]
- Kong, X.; Houzong, R.; Fu, J.; Shao, K.; Wang, L.; Ma, Y.; Shi, J. Application of a novel thermo-sensitive injectable hydrogel in therapy in situ for drug accurate controlled release. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 3200–3216. [Google Scholar] [CrossRef]
- Folle, C.; Díaz-Garrido, N.; Mallandrich, M.; Suñer-Carbó, J.; Sánchez-López, E.; Halbaut, L.; Marqués, A.M.; Espina, M.; Badia, J.; Baldoma, L. Hydrogel of thyme-Oil-PLGA nanoparticles designed for skin inflammation treatment. Gels 2024, 10, 149. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 1997, 28, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly (lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- El-Hammadi, M.M.; Arias, J.L. Recent advances in the surface functionalization of PLGA-based nanomedicines. Nanomaterials 2022, 12, 354. [Google Scholar] [CrossRef]
- Jemison, M.; Olabisi, R. Biomaterials for human space exploration: A review of their untapped potential. Acta Biomater. 2021, 128, 77–99. [Google Scholar] [CrossRef]
- Kheder, N.A.; Mabkhot, Y.N. Synthesis and Antimicrobial Studies of Some Novel Bis-[1,3,4] thiadiazole and Bis-thiazole Pendant to Thieno [2,3-b] thiophene Moiety. Int. J. Mol. Sci. 2012, 13, 3661–3670. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced materials and processing for drug delivery: The past and the future. Adv. Drug Deliv. Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Veld, M.A.; Palmans, A.R. Hydrolases part I: Enzyme mechanism, selectivity and control in the synthesis of well-defined polymers. Enzym. Polym. 2011, 237, 55–78. [Google Scholar]
- Ngwuluka, N.C.; Choonara, Y.E.; Kumar, P.; Du Toit, L.C.; Khan, R.A.; Pillay, V. A novel pH-responsive interpolyelectrolyte hydrogel complex for the oral delivery of levodopa. Part I. IPEC modeling and synthesis. J. Biomed. Mater. Res. Part A 2015, 103, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Yu, M.-Z.; Wang, J.-C.; Hou, W.-J.; Gao, L.-Y.; Ma, X.-F.; Pei, X.-W.; Niu, Y.-J.; Liu, X.-Y.; Qiu, C. Synergistic inhibition of breast cancer by co-delivery of VEGF siRNA and paclitaxel via vapreotide-modified core–shell nanoparticles. Biomaterials 2014, 35, 5028–5038. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, D.; Pannala, A.; Sandeman, S.; Lloyd, A. Sustained and targeted delivery of hydrophilic drug compounds: A review of existing and novel technologies from bench to bedside. J. Drug Deliv. Sci. Technol. 2022, 78, 103936. [Google Scholar] [CrossRef]
- Jin, S.; Xia, X.; Huang, J.; Yuan, C.; Zuo, Y.; Li, Y.; Li, J. Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomater. 2021, 127, 56–79. [Google Scholar] [CrossRef] [PubMed]
- Gebreel, R.M.; Edris, N.A.; Elmofty, H.M.; Tadros, M.I.; El-Nabarawi, M.A.; Hassan, D.H. Development and characterization of PLGA nanoparticle-laden hydrogels for sustained ocular delivery of norfloxacin in the treatment of pseudomonas keratitis: An experimental study. Drug Des. Dev. Ther. 2021, 15, 399–418. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric nanoparticles for delivery of natural bioactive agents: Recent advances and challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef]
- Cellesi, F. Thermoresponsive hydrogels for cellular delivery. Ther. Deliv. 2012, 3, 1395–1407. [Google Scholar] [CrossRef]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly (lactide-co-glycolide)(PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Li, Y.; Wei, R.; Ji, S.; Meng, F.; Zhong, Z. Reversibly crosslinked poly (vinyl alcohol) nanoparticles for triggered release of doxorubicin. J. Control. Release Off. J. Control. Release Soc. 2011, 152, e54–e55. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef]
- Lamba, J.K.; Lin, Y.S.; Schuetz, E.G.; Thummel, K.E. Genetic contribution to variable human CYP3A-mediated metabolism. Adv. Drug Deliv. Rev. 2002, 54, 1271–1294. [Google Scholar] [CrossRef]
- Burdick, J.A.; Anseth, K.S. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials 2002, 23, 4315–4323. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, S.; Kumar, S.P.; Jayakrishnan, A. Bacterial adhesion onto azidated poly (vinyl chloride) surfaces. J. Biomed. Mater. Res. 2002, 61, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Chiarantini, L.; Cerasi, A.; Fraternale, A.; Millo, E.; Benatti, U.; Sparnacci, K.; Laus, M.; Ballestri, M.; Tondelli, L. Comparison of novel delivery systems for antisense peptide nucleic acids. J. Control. Release 2005, 109, 24–36. [Google Scholar] [CrossRef]
- Jeong, B.; Bae, Y.H.; Lee, D.S.; Kim, S.W. Biodegradable block copolymers as injectable drug-delivery systems. Nature 1997, 388, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Fouassier, J.P.; Ruhlmann, D.; Erddalane, A. Photoinduced polymerization reactions in the presence of light stabilizers: Reactivity of the photoinitiator in solution and in bulk. Macromolecules 1993, 26, 721–728. [Google Scholar] [CrossRef]
- Akhter, A.; Hayashi, Y.; Sakurai, Y.; Ohga, N.; Hida, K.; Harashima, H. Ligand density at the surface of a nanoparticle and different uptake mechanism: Two important factors for successful siRNA delivery to liver endothelial cells. Int. J. Pharm. 2014, 475, 227–237. [Google Scholar] [CrossRef]
- Pondi, S.B. Controlled-Release of Curcumin from Poly (Lactide-Co-Glycolide) Acid/Albumin/Curcumin and Silica/Albumin/Curcumin Drug-Delivery Systems. Ph.D. Thesis, Universiti Teknologi Malaysia, Johor Bharu, Malaysia, 2017. [Google Scholar]
- Astete, C.E.; Sabliov, C.M. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 2006, 17, 247–289. [Google Scholar] [CrossRef]
- Feng, S.-S.; Chien, S. Chemotherapeutic engineering: Application and further development of chemical engineering principles for chemotherapy of cancer and other diseases. Chem. Eng. Sci. 2003, 58, 4087–4114. [Google Scholar] [CrossRef]
- Berkland, C.; King, M.; Cox, A.; Kim, K.K.; Pack, D.W. Precise control of PLG microsphere size provides enhanced control of drug release rate. J. Control. Release 2002, 82, 137–147. [Google Scholar] [CrossRef]
- Freiberg, S.; Zhu, X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef]
- Cleek, R.L.; Ting, K.C.; Eskin, S.G.; Mikos, A.G. Microparticles of poly (DL-lactic-co-glycolic acid)/poly (ethylene glycol) blends for controlled drug delivery. J. Control. Release 1997, 48, 259–268. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of collagen nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef]
- Zhang, Y.; Lim, C.T.; Ramakrishna, S.; Huang, Z.-M. Recent development of polymer nanofibers for biomedical and biotechnological applications. J. Mater. Sci. Mater. Med. 2005, 16, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.R.; Bowlin, G.L.; Mansfield, K.; Layman, J.; Sanders, E.; Simpson, D.G.; Wnek, G.E. Release of tetracycline hydrochloride from electrospun polymers. J. Control. Release 2002, 81, 57–64. [Google Scholar] [CrossRef]
- Knight, P.T.; Kirk, J.T.; Anderson, J.M.; Mather, P.T. In vivo kinetic degradation analysis and biocompatibility of aliphatic polyester polyurethanes. J. Biomed. Mater. Res. Part A 2010, 94, 333–343. [Google Scholar] [CrossRef]
- Peng, L.; Eltgroth, M.L.; LaTempa, T.J.; Grimes, C.A.; Desai, T.A. The effect of TiO2 nanotubes on endothelial function and smooth muscle proliferation. Biomaterials 2009, 30, 1268–1272. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Chung, T.-S.; Bai, X.-L.; Chan, W.-K. Effect of preparation conditions on morphology and release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion method. Chem. Eng. Sci. 2000, 55, 2223–2236. [Google Scholar] [CrossRef]
- Schnieders, J.; Gbureck, U.; Thull, R.; Kissel, T. Controlled release of gentamicin from calcium phosphate—Poly (lactic acid-co-glycolic acid) composite bone cement. Biomaterials 2006, 27, 4239–4249. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Sacks, M.S.; Beckman, E.J.; Wagner, W.R. Biodegradable poly (ether ester urethane) urea elastomers based on poly (ether ester) triblock copolymers and putrescine: Synthesis, characterization and cytocompatibility. Biomaterials 2004, 25, 85–96. [Google Scholar] [CrossRef]
- Zhang, K.; Xue, K.; Loh, X.J. Thermo-responsive hydrogels: From recent progress to biomedical applications. Gels 2021, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T. Structures and applications of thermoresponsive hydrogels and nanocomposite-hydrogels based on copolymers with poly (Ethylene glycol) and poly (lactide-co-glycolide) blocks. Bioengineering 2019, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Qu, Y.; Shi, K.; Zhou, K.; He, X.; Chu, B.; Qian, Z. Advances in the application of injectable thermosensitive hydrogel systems for cancer therapy. J. Biomed. Nanotechnol. 2020, 16, 1427–1453. [Google Scholar] [CrossRef] [PubMed]
- Zawani, M.; Fauzi, M.B. Injectable hydrogels for chronic skin wound management: A concise review. Biomedicines 2021, 9, 527. [Google Scholar] [CrossRef]
- Mantripragada, V.P.; Jayasuriya, A.C. Injectable chitosan microparticles incorporating bone morphogenetic protein-7 for bone tissue regeneration. J. Biomed. Mater. Res. Part A 2014, 102, 4276–4289. [Google Scholar] [CrossRef]
- Giacca, M.; Zacchigna, S. Virus-mediated gene delivery for human gene therapy. J. Control. Release 2012, 161, 377–388. [Google Scholar] [CrossRef]
- Elisseeff, J.; Anseth, K.; Sims, D.; McIntosh, W.; Randolph, M.; Langer, R. Transdermal photopolymerization for minimally invasive implantation. Proc. Natl. Acad. Sci. USA 1999, 96, 3104–3107. [Google Scholar] [CrossRef] [PubMed]
- Elomaa, L.; Teixeira, S.; Hakala, R.; Korhonen, H.; Grijpma, D.W.; Seppälä, J.V. Preparation of poly (ε-caprolactone)-based tissue engineering scaffolds by stereolithography. Acta Biomater. 2011, 7, 3850–3856. [Google Scholar] [CrossRef]
- Ratzinger, G.; Agrawal, P.; Körner, W.; Lonkai, J.; Sanders, H.M.; Terreno, E.; Wirth, M.; Strijkers, G.J.; Nicolay, K.; Gabor, F. Surface modification of PLGA nanospheres with Gd-DTPA and Gd-DOTA for high-relaxivity MRI contrast agents. Biomaterials 2010, 31, 8716–8723. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.-Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Guo, R.; Zheng, F.; Liu, H.; Yu, J.; Shi, X. Controlled release and antibacterial activity of antibiotic-loaded electrospun halloysite/poly (lactic-co-glycolic acid) composite nanofibers. Colloids Surf. B Biointerfaces 2013, 110, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, K.E.; Demadis, K.D. Polymeric matrices for the controlled release of phosphonate active agents for medicinal applications. In Handbook of Polymers for Pharmaceutical Technologies: Bioactive and Compatible Synthetic/Hybrid Polymers; Wiley: Hoboken, NJ, USA, 2015; Volume 4, pp. 89–124. [Google Scholar]
- Burke, M.D.; Park, J.O.; Srinivasarao, M.; Khan, S.A. A novel enzymatic technique for limiting drug mobility in a hydrogel matrix. J. Control. Release 2005, 104, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Weigel, T.; Schinkel, G.; Lendlein, A. Design and preparation of polymeric scaffolds for tissue engineering. Expert Rev. Med. Devices 2006, 3, 835–851. [Google Scholar] [CrossRef] [PubMed]
- Aksungur, P.; Demirbilek, M.; Denkbaş, E.B.; Vandervoort, J.; Ludwig, A.; Ünlü, N. Development and characterization of Cyclosporine A loaded nanoparticles for ocular drug delivery: Cellular toxicity, uptake, and kinetic studies. J. Control. Release 2011, 151, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Ye, M.; Park, K. Biodegradable polymers for microencapsulation of drugs. Molecules 2005, 10, 146–161. [Google Scholar] [CrossRef]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Sousa Lobo, J.M. Applications of poloxamers in ophthalmic pharmaceutical formulations: An overview. Expert Opin. Drug Deliv. 2013, 10, 1223–1237. [Google Scholar] [CrossRef]
- Verma, M.S.; Rogowski, J.L.; Jones, L.; Gu, F.X. Colorimetric biosensing of pathogens using gold nanoparticles. Biotechnol. Adv. 2015, 33, 666–680. [Google Scholar] [CrossRef]
- Wassif, R.K.; Elkayal, M.; Shamma, R.N.; Elkheshen, S.A. Recent advances in the local antibiotics delivery systems for management of osteomyelitis. Drug Deliv. 2021, 28, 2392–2414. [Google Scholar] [CrossRef]
- Shichor, I.; Shomron, N.; Lawlor, M.W.; Bae, S.A.; Zoldan, J.; Langer, R.; Kohane, D.S. Toxicogenomic analysis of a sustained release local anesthetic delivery system. Biomaterials 2012, 33, 3586–3593. [Google Scholar] [CrossRef]
- Zheng, Z.; Yin, W.; Zara, J.N.; Li, W.; Kwak, J.; Mamidi, R.; Lee, M.; Siu, R.K.; Ngo, R.; Wang, J. The use of BMP-2 coupled–Nanosilver-PLGA composite grafts to induce bone repair in grossly infected segmental defects. Biomaterials 2010, 31, 9293–9300. [Google Scholar] [CrossRef]
- Sorkin, R.; Kampf, N.; Dror, Y.; Shimoni, E.; Klein, J. Origins of extreme boundary lubrication by phosphatidylcholine liposomes. Biomaterials 2013, 34, 5465–5475. [Google Scholar] [CrossRef]
- Kohane, D.S.; Smith, S.E.; Louis, D.N.; Colombo, G.; Ghoroghchian, P.; Hunfeld, N.G.; Berde, C.B.; Langer, R. Prolonged duration local anesthesia from tetrodotoxin-enhanced local anesthetic microspheres. Pain 2003, 104, 415–421. [Google Scholar] [CrossRef]
- Gref, R.; Minamitake, Y.; Peracchia, M.T.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable long-circulating polymeric nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef]
- Subramanian, B.; Das, P.; Biswas, S.; Roy, A.; Basak, P. Polymers for Additive Manufacturing and 4D-Printing for Tissue Regenerative Applications. In Advances in Biomedical Polymers and Composites; Elsevier: Amsterdam, The Netherlands, 2023; pp. 159–182. [Google Scholar]
- Duvvuri, S.; Janoria, K.G.; Mitra, A.K. Development of a novel formulation containing poly (d,l-lactide-co-glycolide) microspheres dispersed in PLGA–PEG–PLGA gel for sustained delivery of ganciclovir. J. Control. Release 2005, 108, 282–293. [Google Scholar] [CrossRef]
- Lu, Y.; Park, K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, H.; Gu, H.; Li, J.; Wang, Y.; Wei, M. Development of lateral flow immunoassay system based on superparamagnetic nanobeads as labels for rapid quantitative detection of cardiac troponin I. Mater. Sci. Eng. C 2009, 29, 702–707. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, X.; Xu, Y.; Zhang, W.; Liu, C.-H.; Wang, X. Controlled release of growth factors for regenerative medicine. Curr. Pharm. Des. 2015, 21, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.-B.; Kwon, Y.; Kim, J.; Hong, K.H.; Kim, S.-E.; Song, H.-R.; Kim, Y.-M.; Song, S.-C. Injectable polymeric nanoparticle hydrogel system for long-term anti-inflammatory effect to treat osteoarthritis. Bioact. Mater. 2022, 7, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, J.B.; Hoare, T.R.; Iwata, N.G.; Behlau, I.; Dohlman, C.H.; Langer, R.; Kohane, D.S. A drug-eluting contact lens. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3346–3352. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.K.; Lee, D.S. Injectable biodegradable hydrogels. Macromol. Biosci. 2010, 10, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Gu, L.; Ren, L.; Chen, J.; Li, T.; Wang, X.; Yang, J.; Chen, C.; Sun, L. Intra-articular injection of etoricoxib-loaded PLGA-PEG-PLGA triblock copolymeric nanoparticles attenuates osteoarthritis progression. Am. J. Transl. Res. 2019, 11, 6775. [Google Scholar] [PubMed]
- Jaiswal, L.; Limayem, A.; Shankar, S. Polysaccharide-based nanomaterials. In Food, Medical, and Environmental Applications of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 95–111. [Google Scholar]
- Gaudana, R.; Jwala, J.; Boddu, S.H.; Mitra, A.K. Recent perspectives in ocular drug delivery. Pharm. Res. 2009, 26, 1197–1216. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Schwendeman, S.P. Pore closing and opening in biodegradable polymers and their effect on the controlled release of proteins. Mol. Pharm. 2007, 4, 104–118. [Google Scholar] [CrossRef]
- Park, T.G. Degradation of poly (lactic-co-glycolic acid) microspheres: Effect of copolymer composition. Biomaterials 1995, 16, 1123–1130. [Google Scholar] [CrossRef]
- Singh, M.; O’Hagan, D. Advances in vaccine adjuvants. Nat. Biotechnol. 1999, 17, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Saroja, C.; Lakshmi, P.; Bhaskaran, S. Recent trends in vaccine delivery systems: A review. Int. J. Pharm. Investig. 2011, 1, 64. [Google Scholar] [PubMed]
- Matichescu, A.; Ardelean, L.C.; Rusu, L.-C.; Craciun, D.; Bratu, E.A.; Babucea, M.; Leretter, M. Advanced biomaterials and techniques for oral tissue engineering and regeneration—A review. Materials 2020, 13, 5303. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Song, B.; Ao, Y.; Nowak, A.P.; Abelowitz, R.B.; Korsak, R.A.; Havton, L.A.; Deming, T.J.; Sofroniew, M.V. Biocompatibility of amphiphilic diblock copolypeptide hydrogels in the central nervous system. Biomaterials 2009, 30, 2881–2898. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Mauck, R.L. Biomaterials for Tissue Engineering Applications: A Review of the Past and Future Trends; Springer: Vienna, Austria, 2010. [Google Scholar]
- Kanczler, J.; Oreffo, R. Osteogenesis and angiogenesis: The potential for engineering bone. Eur. Cell Mater 2008, 15, 100–114. [Google Scholar] [CrossRef]
- Lu, Y.; Cheng, D.; Niu, B.; Wang, X.; Wu, X.; Wang, A. Properties of poly (lactic-co-glycolic acid) and progress of poly (lactic-co-glycolic acid)-based biodegradable materials in biomedical research. Pharmaceuticals 2023, 16, 454. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, G.; Ding, F. The effect of pH on the polymer degradation and drug release from PLGA-mPEG microparticles. J. Appl. Polym. Sci. 2008, 109, 475–482. [Google Scholar] [CrossRef]
- Amann, L.C.; Gandal, M.J.; Lin, R.; Liang, Y.; Siegel, S.J. In vitro–in vivo correlations of scalable PLGA-risperidone implants for the treatment of schizophrenia. Pharm. Res. 2010, 27, 1730–1737. [Google Scholar] [CrossRef]
- Wan, B.; Bao, Q.; Burgess, D.J. In vitro-in vivo correlation of PLGA microspheres: Effect of polymer source variation and temperature. J. Control. Release 2022, 347, 347–355. [Google Scholar] [CrossRef]
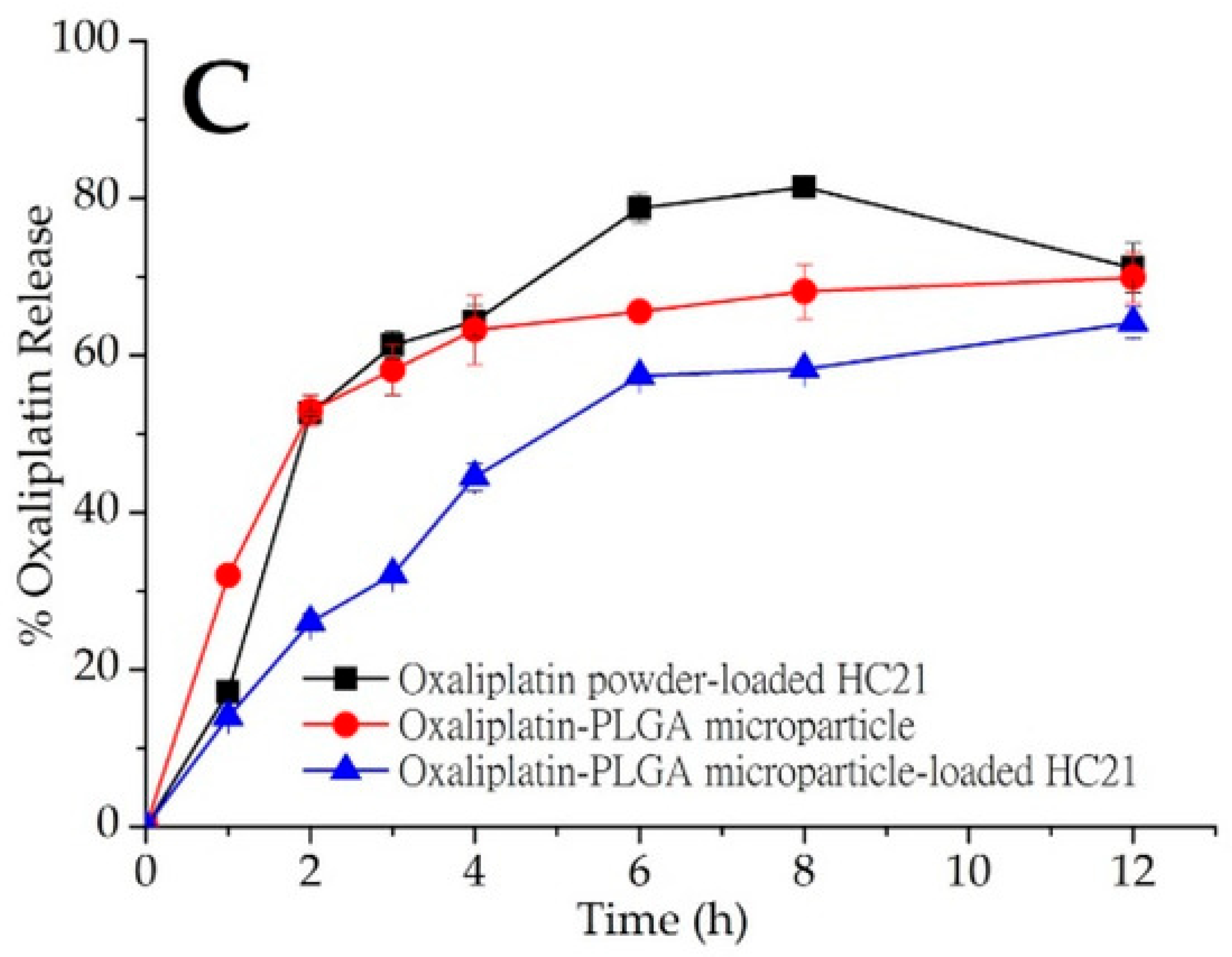
- Abuzar, S.M.; Ahn, J.-H.; Park, K.S.; Park, E.J.; Baik, S.H.; Hwang, S.-J. Pharmacokinetic profile and anti-adhesive effect of oxaliplatin-PLGA microparticle-loaded hydrogels in rats for colorectal cancer treatment. Pharmaceutics 2019, 11, 392. [Google Scholar] [CrossRef]
- Göpferich, A. Mechanisms of polymer degradation and erosion. In The Biomaterials: Silver Jubilee Compendium; Elsevier Science: Oxford, UK, 1996; pp. 117–128. [Google Scholar]
- Bermejo, M.; Sanchez-Dengra, B.; Gonzalez-Alvarez, M.; Gonzalez-Alvarez, I. Oral controlled release dosage forms: Dissolution versus diffusion. Expert Opin. Drug Deliv. 2020, 17, 791–803. [Google Scholar] [CrossRef]
- Dhal, C.; Mishra, R. In vitro and in vivo evaluation of gentamicin sulphate-loaded PLGA nanoparticle-based film for the treatment of surgical site infection. Drug Deliv. Transl. Res. 2020, 10, 1032–1043. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, M.; Wang, Z.; Zhu, X.; Guan, Y.; Zhang, Y. A sustained zero-order release carrier for long-acting, peakless basal insulin therapy. J. Mater. Chem. B 2020, 8, 1952–1959. [Google Scholar] [CrossRef] [PubMed]
- Mariz, M.; Murta, J.; Gil, M.; Ferreira, P. An ocular insert with zero-order extended delivery: Release kinetics and mathematical models. Eur. J. Pharm. Biopharm. 2022, 181, 79–87. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Siepmann, J.; Göpferich, A. Mathematical modeling of bioerodible, polymeric drug delivery systems. Adv. Drug Deliv. Rev. 2001, 48, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Vasir, J.K.; Tambwekar, K.; Garg, S. Bioadhesive microspheres as a controlled drug delivery system. Int. J. Pharm. 2003, 255, 13–32. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Noyes, A.A.; Whitney, W.R. The rate of solution of solid substances in their own solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef]
- Mehryab, F.; Rabbani, S.; Shahhosseini, S.; Shekari, F.; Fatahi, Y.; Baharvand, H.; Haeri, A. Exosomes as a next-generation drug delivery system: An update on drug loading approaches, characterization, and clinical application challenges. Acta Biomater. 2020, 113, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.T.; Irwan, R.M.; Li, Z.; Goh, K. Quantifying how drug-polymer interaction and volume phase transition modulate the drug release kinetics from core-shell microgels. Int. J. Pharm. 2022, 622, 121838. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as drug delivery systems: A review of current characterization and evaluation techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Heredia, N.S.; Vizuete, K.; Flores-Calero, M.; Pazmiño, V.K.; Pilaquinga, F.; Kumar, B.; Debut, A. Comparative statistical analysis of the release kinetics models for nanoprecipitated drug delivery systems based on poly (lactic-co-glycolic acid). PLoS ONE 2022, 17, e0264825. [Google Scholar] [CrossRef] [PubMed]
- Askarizadeh, M.; Esfandiari, N.; Honarvar, B.; Sajadian, S.A.; Azdarpour, A. Kinetic modeling to explain the release of medicine from drug delivery systems. ChemBioEng Rev. 2023, 10, 1006–1049. [Google Scholar] [CrossRef]
- Peppas, N.A.; Korsmeyer, R.W. Dynamically swelling hydrogels in controlled release applications. Hydrogels Med. Pharm. 1987, 3, 109–136. [Google Scholar]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Harish, N.; Kiran, A.; Rathnanand, M.; Shirwaikar, A.; Shenoy, K. Sustained-release matrix tablets of terbutaline sulphate: Formulation and in vitro evaluation. Indian Drugs 2007, 44, 233–235. [Google Scholar]
- Jafari, S.; Soleimani, M.; Badinezhad, M. Application of different mathematical models for further investigation of in vitro drug release mechanisms based on magnetic nano-composite. Polym. Bull. 2022, 79, 1021–1038. [Google Scholar] [CrossRef]
- Sulttan, S.; Rohani, S. Controlled drug release of smart magnetic self-assembled micelle, kinetics and transport mechanisms. J. Pharm. Sci. 2022, 111, 2378–2388. [Google Scholar] [CrossRef] [PubMed]
- Damodharan, N. Mathematical modelling of dissolution kinetics in dosage forms. Res. J. Pharm. Technol. 2020, 13, 1339–1345. [Google Scholar]
- Ling, Y.; Chen, L.; Huang, M.; Zhou, C.; Yang, L.; Niu, H.; Su, L.; Yang, Y.; Pirraco, R.P.; Reis, R.L. A novel method for the preparation of poly (acrylamide-co-acrylonitrile) upper critical solution temperature thermosensitive hydrogel by the partial dehydration of acrylamide grafted polypropylene sheets. Gels 2022, 8, 345. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y. Rational design of smart hydrogels for biomedical applications. Front. Chem. 2021, 8, 615665. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart hydrogels in tissue engineering and regenerative medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [PubMed]
- Onaciu, A.; Munteanu, R.A.; Moldovan, A.I.; Moldovan, C.S.; Berindan-Neagoe, I. Hydrogels based drug delivery synthesis, characterization and administration. Pharmaceutics 2019, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Alvi, M.; Yaqoob, A.; Rehman, K.; Shoaib, S.M.; Akash, M.S.H. PLGA-based nanoparticles for the treatment of cancer: Current strategies and perspectives. AAPS Open 2022, 8, 12. [Google Scholar] [CrossRef]
- Omidi, Y.; Omidian, H. Transformative dynamism in pharmaceutical and biomedical research: Complexity of integration of innovative R & D hubs. BioImpacts BI 2021, 11, 227–233. [Google Scholar]
- Pandey, M.; Choudhury, H.; Abdul-Aziz, A.; Bhattamisra, S.K.; Gorain, B.; Su, J.S.T.; Tan, C.L.; Chin, W.Y.; Yip, K.Y. Advancement on sustained antiviral ocular drug delivery for herpes simplex virus keratitis: Recent update on potential investigation. Pharmaceutics 2020, 13, 1. [Google Scholar] [CrossRef]
- Zhao, Y.; Alakhova, D.Y.; Zhao, X.; Band, V.; Batrakova, E.V.; Kabanov, A.V. Eradication of cancer stem cells in triple negative breast cancer using doxorubicin/pluronic polymeric micelles. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102124. [Google Scholar] [CrossRef] [PubMed]
- Aloss, K.; Hamar, P. Recent preclinical and clinical progress in liposomal doxorubicin. Pharmaceutics 2023, 15, 893. [Google Scholar] [CrossRef] [PubMed]
- Hiraiwa, M.; Ozaki, K.; Yamada, T.; Iezaki, T.; Park, G.; Fukasawa, K.; Horie, T.; Kamada, H.; Tokumura, K.; Motono, M. mTORC1 activation in osteoclasts prevents bone loss in a mouse model of osteoporosis. Front. Pharmacol. 2019, 10, 684. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, J.; Yang, Y.; Li, S.; Dong, Y.; Sun, Y. In vitro antibacterial effect of vancomycin hydrogel on methicillin-resistant Staphylococcus aureus. Am. J. Transl. Res. 2022, 14, 3964. [Google Scholar] [PubMed]
- Badea, I.-C.; Csaki, I.; Serban, B.-A.; Constantin, N.; Mitrica, D.; Burada, M.; Anasiei, I.; Olaru, M.T.; Ghita, A.-N.; Popescu, A.-M.J. Characterisation of a Novel Complex Concentrated Alloy for Marine Applications. Materials 2022, 15, 3345. [Google Scholar] [CrossRef] [PubMed]
- Handler, A.M.; Fallah, M.; Pedersen, A.J.; Pedersen, G.P.; Nielsen, K.T.; Janfelt, C. MALDI mass spectrometry imaging as a complementary analytical method for improved skin distribution analysis of drug molecule and excipients. Int. J. Pharm. 2020, 590, 119949. [Google Scholar] [CrossRef] [PubMed]
- Mulliez, M.A.; Schilling, C.; Grupp, T.M. Equivalent mechanical properties of X-ray and E-beam cross-linked vitamin E blended ultrahigh molecular weight polyethylene. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2131–2140. [Google Scholar] [CrossRef]
- Nazarkina, Z.K.; Chelobanov, B.P.; Kuznetsov, K.A.; Shutov, A.V.; Romanova, I.V.; Karpenko, A.A.; Laktionov, P.P. Influence of elongation of paclitaxel-eluting electrospun-produced stent coating on paclitaxel release and transport through the arterial wall after stenting. Polymers 2021, 13, 1165. [Google Scholar] [CrossRef]
- Jana, S.; Jana, S. Nanoengineering of Biomaterials: Drug Delivery & Biomedical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Podsiadły, B.; Skalski, A.; Słoma, M. Soldering of electronics components on 3D-printed conductive substrates. Materials 2021, 14, 3850. [Google Scholar] [CrossRef]
- Okada, H. One-and three-month release injectable microspheres of the LH-RH superagonist leuprorelin acetate. Adv. Drug Deliv. Rev. 1997, 28, 43–70. [Google Scholar] [CrossRef]
- Ullman, F.; Boutellier, R. A case study of lean drug discovery: From project driven research to innovation studios and process factories. Drug Discov. Today 2008, 13, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Gumz, B. Octreotide–a review of its use in treating neuroendocrine tumours. Eur. Endocrinol. 2014, 10, 70. [Google Scholar]
- Kane, J.M.; Eerdekens, M.; Lindenmayer, J.-P.; Keith, S.J.; Lesem, M.; Karcher, K. Long-acting injectable risperidone: Efficacy and safety of the first long-acting atypical antipsychotic. Am. J. Psychiatry 2003, 160, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Kong, H.; Ding, H.; Xu, Q.; Zeng, J.; Jiang, F.; Yu, M.; Zhang, Y. Improving UV resistance of aramid fibers by simultaneously synthesizing TiO2 on their surfaces and in the interfaces between fibrils/microfibrils using supercritical carbon dioxide. Polymers 2020, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, S.S.; Alves, L.; Sebastiao, M.; Medronho, B.; Almeida, Z.L.; Faria, T.Q.; Brito, R.M.; Moreno, M.J.; Antunes, F.E. Effect of ethyleneoxide groups of anionic surfactants on lipase activity. Biotechnol. Prog. 2016, 32, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.-H. Engineering and functionalization of gelatin biomaterials: From cell culture to medical applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.F.; Peltzer, M.A.; Wagner, J.R.; Salvay, A.G. Hydration and water vapour transport properties in yeast biomass based films: A study of plasticizer content and thickness effects. Eur. Polym. J. 2018, 99, 9–17. [Google Scholar] [CrossRef]
- Vaidya, B.; Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Damon, J.K.; Sarode, A.; Kanabar, D.; Garcia, J.V.; Mitragotri, S.; Muth, A. Cyclodextrin modified erlotinib loaded PLGA nanoparticles for improved therapeutic efficacy against non-small cell lung cancer. Int. J. Biol. Macromol. 2019, 122, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cheng, J.; Ran, L.; Yu, K.; Lu, B.; Lan, G.; Dai, F.; Lu, F. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr. Polym. 2018, 201, 522–531. [Google Scholar] [CrossRef]
- Scharber, M.C.; Sariciftci, N.S. Efficiency of bulk-heterojunction organic solar cells. Prog. Polym. Sci. 2013, 38, 1929–1940. [Google Scholar] [CrossRef]



| Synthesis Method | Advantages | Disadvantages | Case Example (Drug Released) | Ref. |
|---|---|---|---|---|
| Emulsion Solvent Evaporation | Good control over particle size; high drug loading | Requires use of organic solvents | Dexamethasone | [41] |
| Solvent Casting/Particulate Leaching | Creates porous structure; simple method | Potential residual solvents; limited to small-scale production | Vancomycin | [46] |
| Electrospinning | High surface area; controlled release profile | Complex setup; requires high voltage | Ciprofloxacin | [47] |
| Thermal Gelation | No need for organic solvents; temperature-controlled gelation | Limited to thermosensitive drugs; potential thermal degradation | BSA (Bovine Serum Albumin) | [48] |
| Photopolymerization | Precise control over gelation; spatial control | Requires photoinitiators; potential UV damage to drugs | Methotrexate | [49] |
| Commercial Product | Drug Release in PLGA Matrix | Benefit | Reference |
|---|---|---|---|
| Lupron Depot | Leuprolide acetate released over 1–6 months | Treats prostate cancer, endometriosis, and uterine fibroids with sustained release | [158] |
| Zoladex | Goserelin acetate released over 1–3 months | Provides hormone therapy for prostate and breast cancer with controlled release | [159] |
| Sandostatin LAR | Octreotide acetate released over 1 month | Treats acromegaly and severe diarrhea by sustained drug levels | [160] |
| Risperdal Consta | Risperidone released over 2 weeks | Treats schizophrenia with prolonged therapeutic effect | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visan, A.I.; Negut, I. Development and Applications of PLGA Hydrogels for Sustained Delivery of Therapeutic Agents. Gels 2024, 10, 497. https://doi.org/10.3390/gels10080497
Visan AI, Negut I. Development and Applications of PLGA Hydrogels for Sustained Delivery of Therapeutic Agents. Gels. 2024; 10(8):497. https://doi.org/10.3390/gels10080497
Chicago/Turabian StyleVisan, Anita Ioana, and Irina Negut. 2024. "Development and Applications of PLGA Hydrogels for Sustained Delivery of Therapeutic Agents" Gels 10, no. 8: 497. https://doi.org/10.3390/gels10080497







