Nanoclay-Composite Hydrogels for Bone Tissue Engineering
Abstract
:1. Introduction
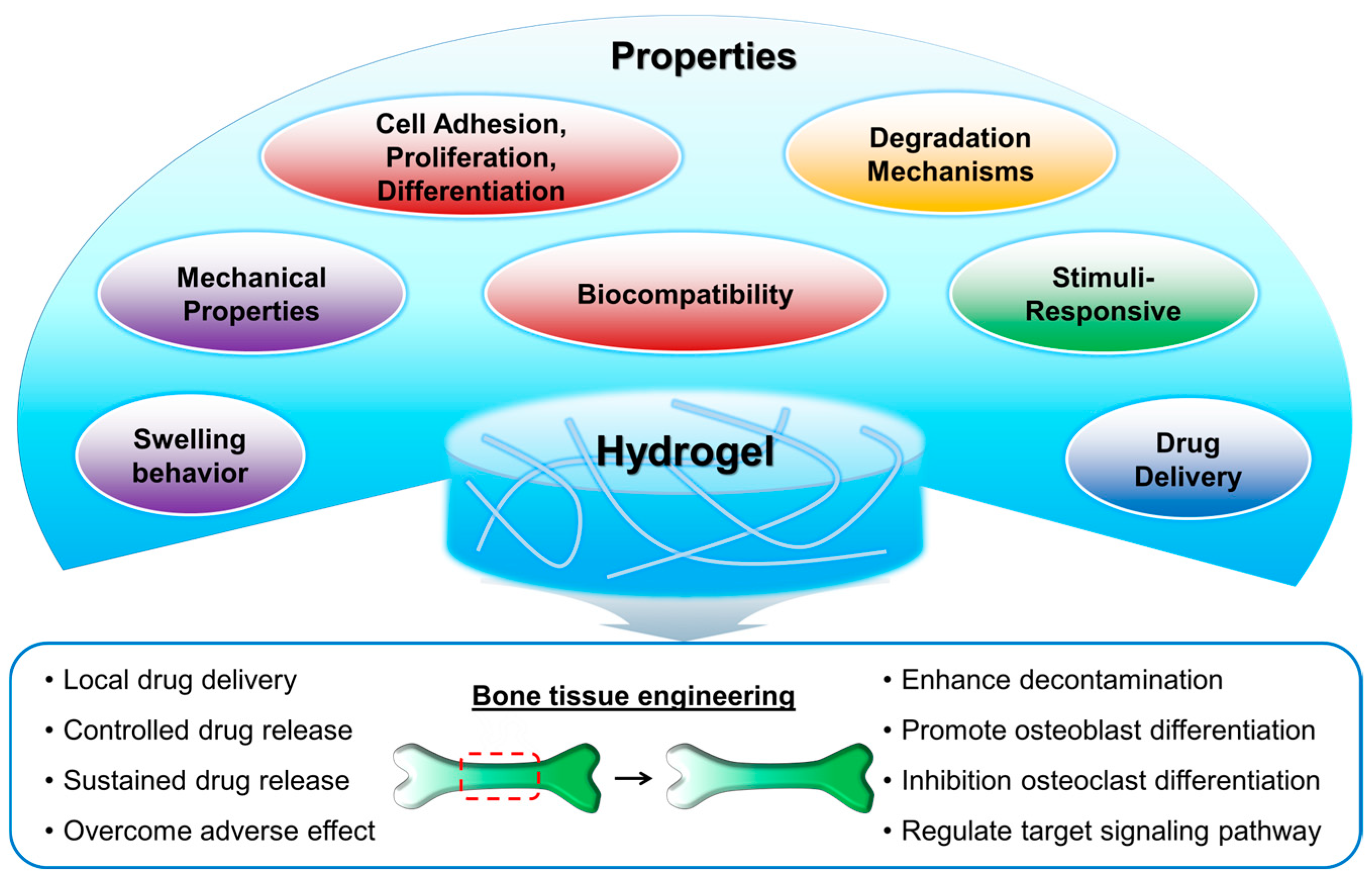
2. Hydrogels in Bone Tissue Engineering
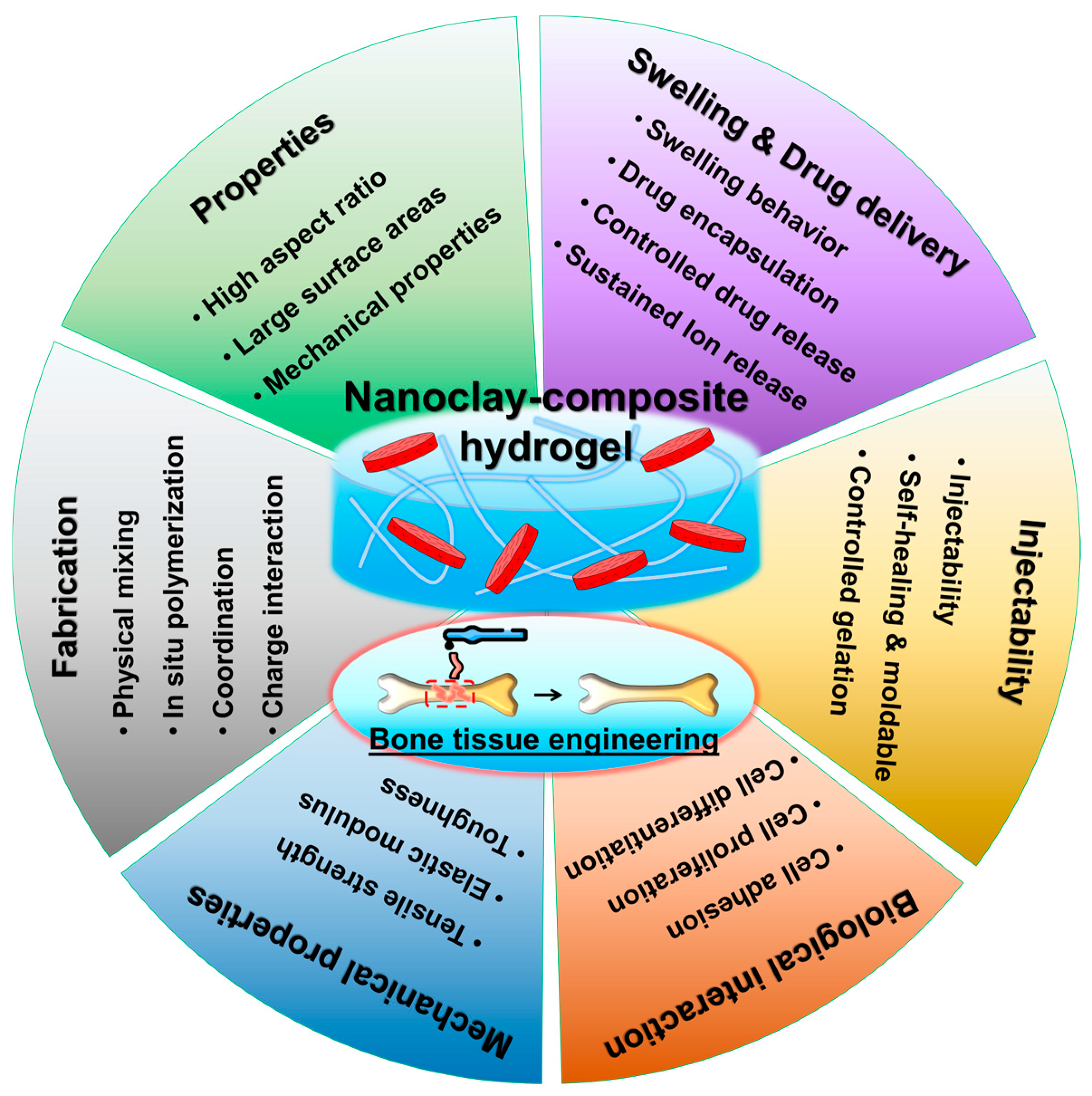
3. Nanoclay Reinforcement of Hydrogels
4. Recent Innovations and Applications in Bone Tissue Engineering
| Type of Nanoclay | Type of Hydrogel | Bioactive Agent | Features | Ref. |
|---|---|---|---|---|
| Laponite | Guanidine modified glycol chitosan | Demineralized bone matrix | Self-healing (injectable) and pro-osteogenic property, malleable carrier for the demineralized bone matrix (DBM), easy to handling, enhanced cell adhesion, activation of Wnt/β-catenin signaling pathway, and robust in vivo bone regeneration in a mouse calvarial defect model | [21,88] |
| Laponite | Catechol-modified glycol chitosan | Smoothened agonist (SAG) | Phytochemical-conjugated chitosan and nanoclay composite hydrogel via coordination and oxidation, porous structure by addition of nanoclays, self-healing and moldable properties, antibacterial activities against Gram-negative and Gram-positive bacteria, antioxidant activities against hydrogen peroxide and 2,2-diphenyl-1-picrylhydrazyl (DPPH), osteogenic agent delivery of nanoclays, and In vitro osteogenic activities and in vivo bone healing by Wnt/β-catenin and Hedgehog signaling pathway | [22] |
| Laponite | Polyethylene-glycol diacrylates | None | Approximately ~0.05 MPa of Young’s modulus, sustained release of magnesium ions and silicon ions from the nanoclay-composite hydrogel, and on vivo new bone formation in a rat tibia defect model | [101] |
| Laponite | Gelatin methacryloyl, 45S5 Bioglass, Polycaprolactone | Deferoxamine (DFO, an iron chelator and hypoxia mimicking agent) | Sustained releasing DFO, which in turn stimulates VEGF expression in stem cells and promotes osteogenesis of stem cells in vitro and cranial bone formation in vivo with the combination of nanoclay and Bioglass | [96] |
| Laponite | Gelatin methacryloyl | None | Nanocomposite hydrogel consisting of 15% gelatin methacryloyl and 8% Laponite improved the hydrogel’s degradation stability and mechanical properties by composite of nanoclay, excellent 3D-printability at room temperature due to its shear-thinning behavior, and in vitro good biocompatibility and osteogenic activities | [87] |
| Laponite | N-acryloyl glycinamide | None | Nanocomposite hydrogel as a hybrid bioink for 3D printing using N-acryloyl glycinamide and nanoclay enhanced mechanical properties and swelling stability of the hydrogels by combination of hydrogen bonding and physical cross-linking with nanoclay, sustained release of Mg2+ and Si4+ ions from hydrogels that can promote osteogenic differentiation of primary rat osteoblast cells and facilitate new bone regeneration in rat tibia defects | [102] |
| Laponite | 4-acryloylmorpholine | None | Superior mechanical properties with maximum tensile strength of 0.513 MPa and Young’s modulus of 0.138 MPa from the hydrogen bonding between the poly(4-acryloylmorpholine) chains and the physical cross-linking provided by the nanoclay, excellent biocompatibility, and controlled release of Mg2+ and Si4+ ions from the hydrogel that enhances its ability to support the osteogenic differentiation of primary rat osteoblasts and in vivo new bone formation of rat tibia defects | [64] |
| Laponite | Methacrylated hyaluronic acid, alginate, | None | High 3D shape accuracy and mechanical strength, magnetically responsive hydrogel for 3D printing applications, biocompatible for the growth of stem cells, and in vivo calvarial defect repair | [97] |
| Laponite | Gelatin methacryloyl | Extracellular vesicles (EV)-derived from TSA *-treated osteoblasts | Promotes osteoinductive potency with engineered osteoblast-derived EVs, improves the retention and control delivery of bioactive factors, Laponite nanoclay dose-dependent increase in hydrogel compressive modulus and shear-thinning properties, and enhanced proliferation (1.09-fold), migration (1.83-fold), histone acetylation (1.32-fold), mineralization (1.87-fold), and extracellular matrix collagen production (≥1.3-fold) | [103] |
| Laponite | Sodium alginate, gelatin | None | Enhances 3D printability and mechanical strength of the hydrogel bioink after incorporating nanoclays, promotes osteogenesis in encapsulated rat bone marrow stromal cells without additional osteoinductive factors, excellent bone healing properties without any adverse effects in vivo | [104] |
| Laponite | Silk fibroin | None | Improve hydrogel’s mechanical properties and hydrophilicity with the addition of nanoclays, facilitate osteogenic and chondrogenic differentiation of stem cells, and support regeneration of both cartilage and subchondral bone in vivo | [105] |
| Laponite | Gelatin, calcium phosphate bone cement (CPC) | None | Designed for bone regeneration-adapted degradability to match the bone regeneration rate, good osteoinduction, osteoconduction, and angiogenesis, capable of complete transformation from implant to new bone, induction of ectopic bone regeneration, and promote ligament graft osseointegration in vivo | [106] |
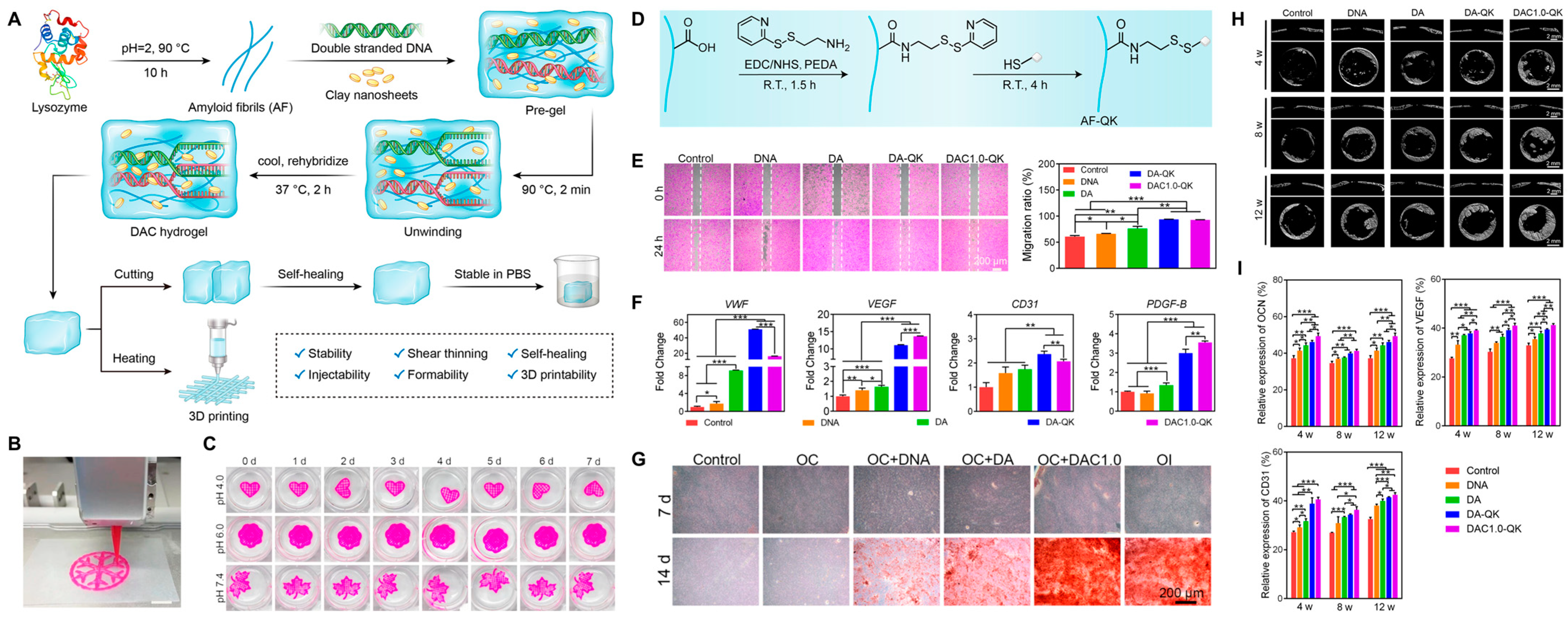
| Laponite | Double stranded DNA, QK peptide-conjugated amyloid fibrils | QK peptide | Nanocomposite hydrogel can be created without complex molecular synthesis, with strength and stability boosted by amyloid fibrils and nanoclays, showing shear-thinning, injectability, self-healing, self-supporting, 3D printable properties. It can be chemically grafted onto hydrogels for controlled release of QK peptide, stimulating endothelial cell functions, promoting stem cell differentiation through ion release of nanoclays, and improving vascularized bone regeneration in a rat cranial bone defect model | [98] |
| Laponite | Alginate, hydroxyapatite | None | Tuned hydrogel’s physical properties by Laponite concentration control, excellent osteoinductive ability and bone-enhancing properties, and possible to inject into the sub-periosteum for bone augmentation | [107] |
| Laponite | Gelatin methacryloyl | SDF-1α | Incorporation of nanoclay and SDF-1α can boost osteogenic capacity and MSC homing with easy injection capability, sustained release of SDF-1α, and good ability to stimulate bone formation both in vitro and in vivo | [108] |
| Type of Nanoclay | Type of Hydrogel | Bioactive Agent | Features | Ref. |
|---|---|---|---|---|
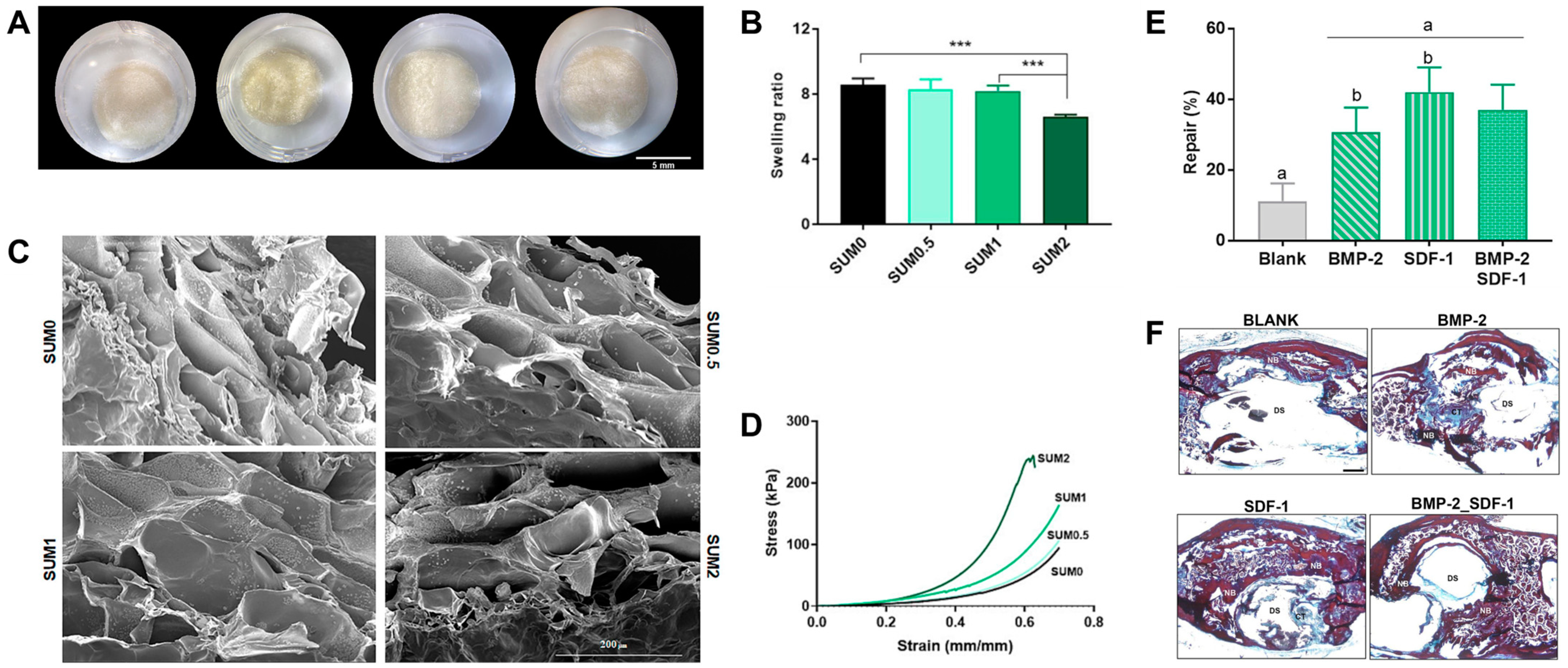
| Sumecton | Gelatin (bovine skin-derived, Type B) | SDF-1, bone morphogenetic protein-2 (BMP-2) | Enhanced mechanical properties of gelatin-based scaffolds by Sumecton nanoclay incorporation, osteoconductive properties in vitro, and ability of constructs to act as platforms for the release of growth factors in vivo | [114] |
| Montmorillonite | Thiol-modified hyaluronic acid, 8-arm PEGacrylate, alginate | Stromal cell-derived factor 1 (SDF-1) | Reinforce biocompatibility and osteogenic ability with nanoclay-composite, boost mineralization even in differentiation-free media, potential of hydrogels to mend bone and act as cell-carriers and delivery platforms for SDF-1, and in vivo enhanced capabilities of bone regeneration as well as of angiogenesis with SDF-1 delivery | [111] |
| Montmorillonite | Methacrylated glycol chitosan | None | Nanoclays can increase the Young’s modulus and slow down the degradation rate of hydrogels, promote proliferation, attachment, and differentiation of encapsulated mesenchymal stem cells, and enhance healing without additional therapeutic agents or stem cells in a critical-sized mouse calvarial defect model | [20] |
| Montmorillonite | Polycaprolactone, gelatin (bovine skin-derived, Type B), nanohydroxyapatite | None | Excellent mechanical properties but limited by hydrophobicity and long-term degradation, enhanced hydrophilicity, strength, adhesiveness, biocompatibility, biodegradability, and osteoconductivity by adding nanoclays, 3D printed structures with rectangular interconnected pores and well-distributed nanoclays, improved wettability, compressive strength, water uptake rate, biodegradability, and bioactivity, and enhanced cell proliferation, viability, and adherence | [115] |
| Type of Nanoclay | Type of Hydrogel | Bioactive Agent | Features | Ref. |
|---|---|---|---|---|
| Halloysite nanotubes | Gelatin methacryloyl | None | Improve mechanical properties due to the incorporation of HNTs, maintain good cytocompatibility in vitro, upregulate expression of osteogenic genes and proteins in human dental pulp stem cells, and facilitate bone regeneration in rat calvarial defects | [118] |
| Halloysite nanotubes | Chitosan, glycerophosphate | Icariin | Increase mechanical strength by incorporating nanoclays into hydrogel, improve stem cell proliferation with nanoclay loading, enhance differentiation of stem cells into bone tissue, and sustain release of Icariin for a synergistic bone differentiation effect | [119] |
| Halloysite nanotubes | Gelatin methacryloyl | Nanosilver | Exhibit good biocompatibility with human periodontal ligament stem cells and macrophages, modulate inflammatory cytokines released by macrophages, enhance osteogenic differentiation of stem cells in an inflammatory environment, inhibit the growth of Gram-positive and Gram-negative bacteria, and better in vivo modulation of the osteoimmune microenvironment in the presence of nanosilver and effectively eliminate bacterial infection | [120] |
| Halloysite nanotubes | Chitosan, collagen type I (rat tail) | Alkaline phosphatase (ALP) | Significantly increase swelling in hydrogels with 30 wt% of nanoclay-ALP, increase scaffold porosity with composite of collagen and nanoclay-ALP, improve mechanical properties with nanoclays, reduce storage modulus with 20% collagen, and slow degradation in physiological pH | [121] |
| Halloysite nanotubes | Sodium alginate | None | Improve molding performance and good formability for 3D printing, good shape fidelity, printability, and mechanical properties after 3D printing, can be converted to a rigid ceramic scaffold at 1200 °C with good biocompatibility and osteogenic activity, and good rat calvarial bone repair abilities in vivo | [122] |
| Halloysite nanotubes | Polycaprolactone–polyethylene glycol-polycaprolactone, gelatin, nanohydroxyapatite (nHA), iron oxide nanoparticle (Fe3O4) | None | Increase mechanical performance by incorporating 3% HNT into hydrogels, enhance osteogenic activity with nanoclay, nHA, and Fe3O4 | [123] |
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mistry, A.S.; Mikos, A.G. Tissue engineering strategies for bone regeneration. Adv. Biochem. Eng. Biotechnol. 2005, 94, 1–22. [Google Scholar]
- Hwang, H.S.; Lee, C.-S. Recent progress in hyaluronic-acid-based hydrogels for bone tissue engineering. Gels 2023, 9, 588. [Google Scholar] [CrossRef]
- Lee, C.-S.; Singh, R.K.; Hwang, H.S.; Lee, N.-H.; Kurian, A.G.; Lee, J.-H.; Kim, H.S.; Lee, M.; Kim, H.-W. Materials-based nanotherapeutics for injured and diseased bone. Prog. Mater. Sci. 2023, 135, 101087. [Google Scholar] [CrossRef]
- Lee, C.-S.; Hwang, H.S. Starch-Based Hydrogels as a Drug Delivery System in Biomedical Applications. Gels 2023, 9, 951. [Google Scholar] [CrossRef]
- Flores, M.J.; Brown, K.E.; O’Marr, J.M.; Adejuyigbe, B.; Rodarte, P.; Gomez-Alvarado, F.; Nwachuku, K.; Urva, M.; Shearer, D. The economic impact of infection and/or nonunion on long-bone shaft fractures: A systematic review. OTA Int. 2024, 7, e337. [Google Scholar] [CrossRef]
- Abbas, S.; Chokotho, L.; Nyamulani, N.; Oliver, V.L. The burden of long bone fracture and health system response in Malawi: A scoping review. Injury 2023, 55, 111243. [Google Scholar] [CrossRef]
- Moayyeri, A.; Warden, J.; Han, S.; Suh, H.; Pinedo-Villanueva, R.; Harvey, N.; Curtis, J.; Silverman, S.; Multani, J.; Yeh, E. Estimating the economic burden of osteoporotic fractures in a multinational study: A real-world data perspective. Osteoporos. Int. 2023, 34, 2121–2132. [Google Scholar] [CrossRef]
- Muschitz, C.; Hummer, M.; Grillari, J.; Hlava, A.; Birner, A.; Hemetsberger, M.; Dimai, H. Epidemiology and economic burden of fragility fractures in Austria. Osteoporos. Int. 2022, 33, 637–647. [Google Scholar] [CrossRef]
- Metsemakers, W.-J.; Moriarty, T.F.; Morgenstern, M.; Marais, L.; Onsea, J.; O’Toole, R.V.; Depypere, M.; Obremskey, W.T.; Verhofstad, M.H.; McNally, M. The global burden of fracture-related infection: Can we do better? Lancet Infect. Dis. 2024, 24, e386–e393. [Google Scholar] [CrossRef]
- Kang, M.; Lee, C.-S.; Lee, M. Bioactive scaffolds integrated with liposomal or extracellular vesicles for bone regeneration. Bioengineering 2021, 8, 137. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, allograft, and bone graft substitutes: Clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Wang, K.; Hao, Y.; Wang, Y.; Chen, J.; Mao, L.; Deng, Y.; Chen, J.; Yuan, S.; Zhang, T.; Ren, J. Functional hydrogels and their application in drug delivery, biosensors, and tissue engineering. Int. J. Polym. Sci. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, L.-Q.; Nugraha, B.; Gao, Y.; Leo, H. Current hydrogel solutions for repairing and regeneration of complex tissues. Curr. Med. Chem. 2014, 21, 2480–2496. [Google Scholar] [CrossRef]
- Hof, K.S.; Bastings, M.M. Programmable control in extracellular matrix-mimicking polymer hydrogels. Chimia 2017, 71, 342–348. [Google Scholar] [CrossRef]
- Taghipour, Y.D.; Hokmabad, V.R.; Bakhshayesh, D.; Rahmani, A.; Asadi, N.; Salehi, R.; Nasrabadi, H.T. The application of hydrogels based on natural polymers for tissue engineering. Curr. Med. Chem. 2020, 27, 2658–2680. [Google Scholar] [CrossRef]
- Kim, S.; Lee, M. Rational design of hydrogels to enhance osteogenic potential. Chem. Mater. 2020, 32, 9508–9530. [Google Scholar] [CrossRef]
- Wang, A.; Wang, W. Nanomaterials from Clay Minerals: A New Approach to Green Functional Materials; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Khatoon, N.; Chu, M.Q.; Zhou, C.H. Nanoclay-based drug delivery systems and their therapeutic potentials. J. Mater. Chem. B 2020, 8, 7335–7351. [Google Scholar] [CrossRef]
- Naumenko, E.; Fakhrullin, R. Halloysite nanoclay/biopolymers composite materials in tissue engineering. Biotechnol. J. 2019, 14, 1900055. [Google Scholar] [CrossRef]
- Cui, Z.-K.; Kim, S.; Baljon, J.J.; Wu, B.M.; Aghaloo, T.; Lee, M. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat. Commun. 2019, 10, 3523. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, J.; Lee, C.-S.; Kim, S.; Chen, C.; Lee, M. Supramolecular hydrogels based on nanoclay and guanidine-rich chitosan: Injectable and moldable osteoinductive carriers. ACS Appl. Mater. Interfaces 2020, 12, 16088–16096. [Google Scholar] [CrossRef]
- Lee, C.S.; Hwang, H.S.; Kim, S.; Fan, J.; Aghaloo, T.; Lee, M. Inspired by nature: Facile design of nanoclay–organic hydrogel bone sealant with multifunctional properties for robust bone regeneration. Adv. Funct. Mater. 2020, 30, 2003717. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, J.; Chen, C.; Aghaloo, T.; Lee, M. Co-delivery of simvastatin and demineralized bone matrix hierarchically from nanosheet-based supramolecular hydrogels for osteogenesis. J. Mater. Chem. B 2021, 9, 7741–7750. [Google Scholar] [CrossRef]
- Fakhrullin, R.F.; Lvov, Y.M. Halloysite clay nanotubes for tissue engineering. Nanomedicine 2016, 11, 2243–2246. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Deng, Y.; Su, J. Recent advances in design of functional biocompatible hydrogels for bone tissue engineering. Adv. Funct. Mater. 2021, 31, 2009432. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Guan, J.; Mao, Y.; Zhou, P. Hydrogel: A potential therapeutic material for bone tissue engineering. AIP Adv. 2021, 11, 010701. [Google Scholar] [CrossRef]
- Lei, K.; Chen, M.; Guo, P.; Fang, J.; Zhang, J.; Liu, X.; Wang, W.; Li, Y.; Hu, Z.; Ma, Y. Environmentally Adaptive Polymer Hydrogels: Maintaining Wet-Soft Features in Extreme Conditions. Adv. Funct. Mater. 2023, 33, 2303511. [Google Scholar] [CrossRef]
- Tipa, C.; Cidade, M.T.; Borges, J.P.; Costa, L.C.; Silva, J.C.; Soares, P.I. Clay-based nanocomposite hydrogels for biomedical applications: A review. Nanomaterials 2022, 12, 3308. [Google Scholar] [CrossRef]
- Sohail, K.; Khan, I.U.; Shahzad, Y.; Hussain, T.; Ranjha, N.M. pH-sensitive polyvinylpyrrolidone-acrylic acid hydrogels: Impact of material parameters on swelling and drug release. Braz. J. Pharm. Sci. 2014, 50, 173–184. [Google Scholar] [CrossRef]
- Gehrke, S.H. Synthesis, equilibrium swelling, kinetics, permeability and applications of environmentally responsive gels. Adv. Polym. Sci. 1993, 110, 81–144. [Google Scholar]
- Goycoolea, F.M.; Heras, A.; Aranaz, I.; Galed, G.; Fernández-Valle, M.E.; Argüelles-Monal, W. Effect of chemical crosslinking on the swelling and shrinking properties of thermal and pH-responsive chitosan hydrogels. Macromol. Biosci. 2003, 3, 612–619. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and applications in biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chu, G.; Wang, H.; Chen, S.; Li, B.; Han, F. Effects of matrix stiffness on the differentiation of multipotent stem cells. Curr. Stem Cell Res. Ther. 2020, 15, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Griebel, M.E.; Uroz, M.; Bubli, S.Y.; Gagnon, K.A.; Trappmann, B.; Baker, B.M.; Eyckmans, J.; Chen, C.S. A Protein-Adsorbent Hydrogel with Tunable Stiffness for Tissue Culture Demonstrates Matrix-Dependent Stiffness Responses. Adv. Funct. Mater. 2024, 34, 2309567. [Google Scholar] [CrossRef] [PubMed]
- Chimene, D.; Kaunas, R.; Gaharwar, A.K. Hydrogel bioink reinforcement for additive manufacturing: A focused review of emerging strategies. Adv. Mater. 2020, 32, 1902026. [Google Scholar] [CrossRef] [PubMed]
- Maranchi, J.P.; Trexler, M.M.; Guo, Q.; Elisseeff, J.H. Fibre-reinforced hydrogels with high optical transparency. Int. Mater. Rev. 2014, 59, 264–296. [Google Scholar] [CrossRef]
- Awasthi, S.; Gaur, J.K.; Bobji, M.; Srivastava, C. Nanoparticle-reinforced polyacrylamide hydrogel composites for clinical applications: A review. J. Mater. Sci. 2022, 57, 8041–8063. [Google Scholar] [CrossRef]
- Chen, T.; Hou, K.; Ren, Q.; Chen, G.; Wei, P.; Zhu, M. Nanoparticle–polymer synergies in nanocomposite hydrogels: From design to application. Macromol. Rapid Commun. 2018, 39, 1800337. [Google Scholar] [CrossRef] [PubMed]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef]
- Motealleh, A.; Kehr, N.S. Nanocomposite hydrogels and their applications in tissue engineering. Adv. Healthc. Mater. 2017, 6, 1600938. [Google Scholar] [CrossRef]
- Gyles, D.A.; Castro, L.D.; Silva Jr, J.O.C.; Ribeiro-Costa, R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Gonella, S.; Domingues, M.F.; Miguel, F.; Moura, C.S.; Rodrigues, C.A.; Ferreira, F.C.; Silva, J.C. Fabrication and Characterization of Porous PEGDA Hydrogels for Articular Cartilage Regeneration. Gels 2024, 10, 422. [Google Scholar] [CrossRef] [PubMed]
- Göpferich, A. Mechanisms of polymer degradation and erosion. Biomater. Silver Jubil. Compend. 1996, 17, 117–128. [Google Scholar]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef] [PubMed]
- Ashley, G.W.; Henise, J.; Reid, R.; Santi, D.V. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl. Acad. Sci. 2013, 110, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Kokol, V. Preparation, characterization, and in vitro enzymatic degradation of chitosan-gelatine hydrogel scaffolds as potential biomaterials. J. Biomed. Mater. Res. Part A 2012, 100, 1655–1667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; King, M.W. Biodegradable polymers as the pivotal player in the design of tissue engineering scaffolds. Adv. Healthc. Mater. 2020, 9, 1901358. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Alves, C.M.; Kasper, F.K.; Mikos, A.G.; Reis, R.L. Responsive and in situ-forming chitosan scaffolds for bone tissue engineering applications: An overview of the last decade. J. Mater. Chem. 2010, 20, 1638–1645. [Google Scholar] [CrossRef]
- Andrade, F.; Roca-Melendres, M.M.; Durán-Lara, E.F.; Rafael, D.; Schwartz Jr, S. Stimuli-responsive hydrogels for cancer treatment: The role of pH, light, ionic strength and magnetic field. Cancers 2021, 13, 1164. [Google Scholar] [CrossRef] [PubMed]
- Willner, I. Stimuli-controlled hydrogels and their applications. Acc. Chem. Res. 2017, 50, 657–658. [Google Scholar] [CrossRef]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016, 23, 748–770. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Lavrador, P.; Esteves, M.R.; Gaspar, V.M.; Mano, J.F. Stimuli-responsive nanocomposite hydrogels for biomedical applications. Adv. Funct. Mater. 2021, 31, 2005941. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, H.; Tang, D.; Li, Y.; Li, X.; Xu, F. Bioactuators based on stimulus-responsive hydrogels and their emerging biomedical applications. NPG Asia Mater. 2019, 11, 64. [Google Scholar] [CrossRef]
- Pele, K.G.; Amaveda, H.; Mora, M.; Marcuello, C.; Lostao, A.; Alamán-Díez, P.; Pérez-Huertas, S.; Ángeles Pérez, M.; García-Aznar, J.M.; García-Gareta, E. Hydrocolloids of egg white and gelatin as a platform for hydrogel-based tissue engineering. Gels 2023, 9, 505. [Google Scholar] [CrossRef]
- de Melo Barbosa, R.; Ferreira, M.A.; Meirelles, L.M.A.; Zorato, N.; Raffin, F.N. Nanoclays in drug delivery systems. In Clay Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 185–202. [Google Scholar]
- Jafarbeglou, M.; Abdouss, M.; Shoushtari, A.M.; Jafarbeglou, M. Clay nanocomposites as engineered drug delivery systems. RSC Adv. 2016, 6, 50002–50016. [Google Scholar] [CrossRef]
- Dong, J.; Cheng, Z.; Tan, S.; Zhu, Q. Clay nanoparticles as pharmaceutical carriers in drug delivery systems. Expert Opin. Drug Deliv. 2021, 18, 695–714. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Cross, L.M.; Peak, C.W.; Gold, K.; Carrow, J.K.; Brokesh, A.; Singh, K.A. 2D nanoclay for biomedical applications: Regenerative medicine, therapeutic delivery, and additive manufacturing. Adv. Mater. 2019, 31, 1900332. [Google Scholar] [CrossRef]
- Guo, F.; Aryana, S.; Han, Y.; Jiao, Y. A review of the synthesis and applications of polymer–nanoclay composites. Appl. Sci. 2018, 8, 1696. [Google Scholar] [CrossRef]
- Xing, W.; Tang, Y. On mechanical properties of nanocomposite hydrogels: Searching for superior properties. Nano Mater. Sci. 2022, 4, 83–96. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Rivera, C.P.; Wu, C.-J.; Schmidt, G. Transparent, elastomeric and tough hydrogels from poly (ethylene glycol) and silicate nanoparticles. Acta Biomater. 2011, 7, 4139–4148. [Google Scholar] [CrossRef]
- Rafieian, S.; Mirzadeh, H.; Mahdavi, H.; Masoumi, M.E. A review on nanocomposite hydrogels and their biomedical applications. Sci. Eng. Compos. Mater. 2019, 26, 154–174. [Google Scholar] [CrossRef]
- Zhai, X.; Ruan, C.; Shen, J.; Zheng, C.; Zhao, X.; Pan, H.; Lu, W.W. Clay-based nanocomposite hydrogel with attractive mechanical properties and sustained bioactive ion release for bone defect repair. J. Mater. Chem. B 2021, 9, 2394–2406. [Google Scholar] [CrossRef] [PubMed]
- Kheirabadi, M.; Bagheri, R.; Kabiri, K. Swelling and mechanical behavior of nanoclay reinforced hydrogel: Single network vs. full interpenetrating polymer network. Polym. Bull. 2015, 72, 1663–1681. [Google Scholar] [CrossRef]
- Ghanaatian, E.; Entezam, M. Mechanical properties and drug release rate of poly (vinyl alcohol)/poly (ethylene glycol)/clay nanocomposite hydrogels: Correlation with structure and physical properties. J. Appl. Polym. Sci. 2019, 136, 47843. [Google Scholar] [CrossRef]
- Villalba-Rodríguez, A.M.; Martínez-González, S.; Sosa-Hernández, J.E.; Parra-Saldívar, R.; Bilal, M.; Iqbal, H.M. Nanoclay/polymer-based hydrogels and enzyme-loaded nanostructures for wound healing applications. Gels 2021, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Padil, V.V.; Kumar, K.A.; Murugesan, S.; Torres-Mendieta, R.; Wacławek, S.; Cheong, J.Y.; Černík, M.; Varma, R.S. Sustainable and safer nanoclay composites for multifaceted applications. Green Chem. 2022, 24, 3081–3114. [Google Scholar] [CrossRef]
- Ianchis, R.; Ninciuleanu, C.M.; Gifu, I.C.; Alexandrescu, E.; Nistor, C.L.; Nitu, S.; Petcu, C. Hydrogel-clay nanocomposites as carriers for controlled release. Curr. Med. Chem. 2020, 27, 919–954. [Google Scholar] [CrossRef] [PubMed]
- Vashist, A.; Ghosal, A.; Vashist, A.; Kaushik, A.; Gupta, Y.; Nair, M.; Ahmad, S. Impact of nanoclay on the pH-responsiveness and biodegradable behavior of biopolymer-based nanocomposite hydrogels. Gels 2019, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Gharekhani, A.; Hamishehkar, H.; Zakeri-Milani, P.; Gharekhani, H. Stomach-specific drug delivery of clarithromycin using a semi interpenetrating polymeric network hydrogel made of montmorillonite and chitosan: Synthesis, characterization and in vitro drug release study. Adv. Pharm. Bull. 2019, 9, 159. [Google Scholar] [CrossRef]
- Chaudhuri, S.D.; Mandal, A.; Dey, A.; Chakrabarty, D. Tuning the swelling and rheological attributes of bentonite clay modified starch grafted polyacrylic acid based hydrogel. Appl. Clay Sci. 2020, 185, 105405. [Google Scholar] [CrossRef]
- Huang, H.; Xu, J.; Wei, K.; Xu, Y.J.; Choi, C.K.K.; Zhu, M.; Bian, L. Bioactive nanocomposite poly (ethylene glycol) hydrogels crosslinked by multifunctional layered double hydroxides nanocrosslinkers. Macromol. Biosci. 2016, 16, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Jiang, L.; Chen, X.; Dong, J.; Shao, Z. Enhancing the gelation and bioactivity of injectable silk fibroin hydrogel with laponite nanoplatelets. ACS Appl. Mater. Interfaces 2016, 8, 9619–9628. [Google Scholar] [CrossRef] [PubMed]
- Cross, L.M.; Shah, K.; Palani, S.; Peak, C.W.; Gaharwar, A.K. Gradient nanocomposite hydrogels for interface tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Kerativitayanan, P.; Gaharwar, A.K. Elastomeric and mechanically stiff nanocomposites from poly (glycerol sebacate) and bioactive nanosilicates. Acta Biomater. 2015, 26, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Tipa, C.; Cidade, M.T.; Vieira, T.; Silva, J.C.; Soares, P.I.; Borges, J.P. A new long-term composite drug delivery system based on thermo-responsive hydrogel and nanoclay. Nanomaterials 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Palem, R.R.; Rao, K.M.; Shimoga, G.; Saratale, R.G.; Shinde, S.K.; Ghodake, G.S.; Lee, S.-H. Physicochemical characterization, drug release, and biocompatibility evaluation of carboxymethyl cellulose-based hydrogels reinforced with sepiolite nanoclay. Int. J. Biol. Macromol. 2021, 178, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Mauro, N.; Chiellini, F.; Bartoli, C.; Gazzarri, M.; Laus, M.; Antonioli, D.; Griffiths, P.; Manfredi, A.; Ranucci, E.; Ferruti, P. RGD-mimic polyamidoamine–montmorillonite composites with tunable stiffness as scaffolds for bone tissue-engineering applications. J. Tissue Eng. Regen. Med. 2017, 11, 2164–2175. [Google Scholar] [CrossRef] [PubMed]
- Pereira, K.A.; Aguiar, K.L.; Oliveira, P.F.; Vicente, B.M.; Pedroni, L.G.; Mansur, C.R. Synthesis of hydrogel nanocomposites based on partially hydrolyzed polyacrylamide, polyethyleneimine, and modified clay. ACS Omega 2020, 5, 4759–4769. [Google Scholar] [CrossRef]
- Huang, B.; Liu, M.; Long, Z.; Shen, Y.; Zhou, C. Effects of halloysite nanotubes on physical properties and cytocompatibility of alginate composite hydrogels. Mater. Sci. Eng. C 2017, 70, 303–310. [Google Scholar] [CrossRef]
- Li, J.; Weber, E.; Guth-Gundel, S.; Schuleit, M.; Kuttler, A.; Halleux, C.; Accart, N.; Doelemeyer, A.; Basler, A.; Tigani, B. Tough composite hydrogels with high loading and local release of biological drugs. Adv. Healthc. Mater. 2018, 7, 1701393. [Google Scholar] [CrossRef]
- Hua, S.; Yang, H.; Wang, W.; Wang, A. Controlled release of ofloxacin from chitosan–montmorillonite hydrogel. Appl. Clay Sci. 2010, 50, 112–117. [Google Scholar] [CrossRef]
- Kim, S.; Cui, Z.-K.; Fan, J.; Fartash, A.; Aghaloo, T.L.; Lee, M. Photocrosslinkable chitosan hydrogels functionalized with the RGD peptide and phosphoserine to enhance osteogenesis. J. Mater. Chem. B 2016, 4, 5289–5298. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Fan, J.; Lee, C.-S.; Chen, C.; Bubukina, K.; Lee, M. Heparinized chitosan stabilizes the bioactivity of BMP-2 and potentiates the osteogenic efficacy of demineralized bone matrix. J. Biol. Eng. 2020, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Kishore, V.; Rivera, C.; Bullock, W.; Wu, C.J.; Akkus, O.; Schmidt, G. Physically crosslinked nanocomposites from silicate-crosslinked PEO: Mechanical properties and osteogenic differentiation of human mesenchymal stem cells. Macromol. Biosci. 2012, 12, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Bu, Z.; Xiong, Y.; Zhang, H.; Fang, J.; Hu, H.; Liu, Z.; Li, X. Facile extrusion 3D printing of gelatine methacrylate/Laponite nanocomposite hydrogel with high concentration nanoclay for bone tissue regeneration. Int. J. Biol. Macromol. 2021, 188, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, Z.; Xu, C.; Kang, M.; Lee, C.S.; Aghaloo, T.; Lee, M. Self-assembled Nanocomposite Hydrogels as Carriers for Demineralized Bone Matrix Particles and Enhanced Bone Repair. Adv. Healthc. Mater. 2024, 13, 2303592. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; He, H.; Li, B.; Hou, T. Hydrogel as a biomaterial for bone tissue engineering: A review. Nanomaterials 2020, 10, 1511. [Google Scholar] [CrossRef]
- Snigdha, S.; Jishma, P.; Nandakumar, K.; Sylas, V.; Thomas, S.; Radhakrishnan, E. Laponite® nanoclay gel based microenvironment for plant probiotic rhizobacterial delivery. Rhizosphere 2021, 18, 100346. [Google Scholar] [CrossRef]
- Au, P.-I.; Hassan, S.; Liu, J.; Leong, Y.-K. Behaviour of LAPONITE® gels: Rheology, ageing, pH effect and phase state in the presence of dispersant. Chem. Eng. Res. Des. 2015, 101, 65–73. [Google Scholar] [CrossRef]
- Cummins, H.Z. Liquid, glass, gel: The phases of colloidal Laponite. J. Non-Cryst. Solids 2007, 353, 3891–3905. [Google Scholar] [CrossRef]
- Govea-Alonso, D.O.; García-Soto, M.J.; Betancourt-Mendiola, L.; Padilla-Ortega, E.; Rosales-Mendoza, S.; González-Ortega, O. Nanoclays: Promising materials for vaccinology. Vaccines 2022, 10, 1549. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Mihaila, S.M.; Swami, A.; Patel, A.; Sant, S.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Bioactive silicate nanoplatelets for osteogenic differentiation of human mesenchymal stem cells. Adv. Mater. 2013, 25, 3329–3336. [Google Scholar] [CrossRef] [PubMed]
- Mihaila, S.M.; Gaharwar, A.K.; Reis, R.L.; Khademhosseini, A.; Marques, A.P.; Gomes, M.E. The osteogenic differentiation of SSEA-4 sub-population of human adipose derived stem cells using silicate nanoplatelets. Biomaterials 2014, 35, 9087–9099. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, X.; Wang, Y.; Liu, Y.; Pan, Y.; Li, Y.; Ji, M.; Zhao, X.; Huang, S.; Yao, Q. Hypoxia-mimicking 3D bioglass-nanoclay scaffolds promote endogenous bone regeneration. Bioact. Mater. 2021, 6, 3485–3495. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Dong, L.; Xia, J.; Mi, S.; Sun, W. 3D printing unique nanoclay-incorporated double-network hydrogels for construction of complex tissue engineering scaffolds. Adv. Healthc. Mater. 2021, 10, 2100036. [Google Scholar] [CrossRef]
- Yang, Q.; Miao, Y.; Luo, J.; Chen, Y.; Wang, Y. Amyloid Fibril and Clay Nanosheet Dual-Nanoengineered DNA Dynamic Hydrogel for Vascularized Bone Regeneration. ACS Nano 2023, 17, 17131–17147. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. Obsidio 510(k) Approval Letter. June 2022. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf21/K213385.pdf (accessed on 2 August 2024).
- Gonzalez-Pujana, A.; Igartua, M.; Hernandez, R.M.; Santos-Vizcaino, E. Laponite nanoclays for the sustained delivery of therapeutic proteins. Eur. J. Pharm. Sci. 2024, 201, 106858. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Hou, C.; Pan, H.; Lu, W.W.; Liu, W.; Ruan, C. Nanoclay incorporated polyethylene-glycol nanocomposite hydrogels for stimulating in vitro and in vivo osteogenesis. J. Biomed. Nanotechnol. 2018, 14, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Ma, Y.; Hou, C.; Gao, F.; Zhang, Y.; Ruan, C.; Pan, H.; Lu, W.W.; Liu, W. 3D-printed high strength bioactive supramolecular polymer/clay nanocomposite hydrogel scaffold for bone regeneration. ACS Biomater. Sci. Eng. 2017, 3, 1109–1118. [Google Scholar] [CrossRef]
- Man, K.; Barroso, I.A.; Brunet, M.Y.; Peacock, B.; Federici, A.S.; Hoey, D.A.; Cox, S.C. Controlled release of epigenetically-enhanced extracellular vesicles from a GelMA/nanoclay composite hydrogel to promote bone repair. Int. J. Mol. Sci. 2022, 23, 832. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Lei, X.; Cheng, P.; Song, Y.; Gao, Y.; Hu, J.; Wang, C.; Zhang, S.; Li, D. 3D-bioprinted functional and biomimetic hydrogel scaffolds incorporated with nanosilicates to promote bone healing in rat calvarial defect model. Mater. Sci. Eng. C 2020, 112, 110905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Zhang, A.; Ling, C.; Sheng, R.; Li, X.; Yao, Q.; Chen, J. Enzymatically crosslinked silk-nanosilicate reinforced hydrogel with dual-lineage bioactivity for osteochondral tissue engineering. Mater. Sci. Eng. C 2021, 127, 112215. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, L.; Wei, D.; Liu, S.; Zhang, Z.; Lian, R.; Wang, L.; Chen, Y.; Jiang, J.; Xiao, Y. Injectable biomimetic hydrogel guided functional bone regeneration by adapting material degradation to tissue healing. Adv. Funct. Mater. 2023, 33, 2213047. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, D.; Wang, Z.; Meng, Y.; Liu, B.; Li, L.; Liu, R.; Dong, S.; Wei, F. Minimally invasive bone augmentation through subperiosteal injectable hydroxylapatite/laponite/alginate nanocomposite hydrogels. Int. J. Biol. Macromol. 2023, 231, 123232. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Xu, Y.; Mulatibieke, R.; Zhong, Q.; Pan, X.; Chen, Y.; Lian, Q.; Luo, X.; Shi, Z.; Zhu, Q. Nano-silicate-reinforced and SDF-1α-loaded gelatin-methacryloyl hydrogel for bone tissue engineering. Int. J. Nanomed. 2020, 15, 9337–9353. [Google Scholar] [CrossRef] [PubMed]
- Jayrajsinh, S.; Shankar, G.; Agrawal, Y.K.; Bakre, L. Montmorillonite nanoclay as a multifaceted drug-delivery carrier: A review. J. Drug Deliv. Sci. Technol. 2017, 39, 200–209. [Google Scholar] [CrossRef]
- Sharifzadeh, G.; Hezaveh, H.; Muhamad, I.I.; Hashim, S.; Khairuddin, N. Montmorillonite-based polyacrylamide hydrogel rings for controlled vaginal drug delivery. Mater. Sci. Eng. C 2020, 110, 110609. [Google Scholar] [CrossRef] [PubMed]
- Erezuma, I.; Lukin, I.; Pimenta-Lopes, C.; Ventura, F.; Garcia-Garcia, P.; Reyes, R.; Arnau, M.R.; Delgado, A.; Taebnia, N.; Kadumudi, F.B. Nanoclay-reinforced HA/alginate scaffolds as cell carriers and SDF-1 delivery-platforms for bone tissue engineering. Int. J. Pharm. 2022, 623, 121895. [Google Scholar] [CrossRef] [PubMed]
- Poyatos-Racionero, E.; Pérez-Esteve, É.; Medaglia, S.; Aznar, E.; Barat, J.M.; Martínez-Máñez, R.; Marcos, M.D.; Bernardos, A. Gated Organonanoclays for Large Biomolecules: Controlled Release Triggered by Surfactant Stimulus. Nanomaterials 2022, 12, 2694. [Google Scholar] [CrossRef]
- Yoshida, Y.; Shimada, T.; Ishida, T.; Takagi, S. Thermodynamic study of the adsorption of acridinium derivatives on the clay surface. RSC Adv. 2020, 10, 21360–21368. [Google Scholar] [CrossRef]
- Lukin, I.; Erezuma, I.; Garcia-Garcia, P.; Reyes, R.; Evora, C.; Kadumudi, F.B.; Dolatshahi-Pirouz, A.; Orive, G. Sumecton reinforced gelatin-based scaffolds for cell-free bone regeneration. Int. J. Biol. Macromol. 2023, 249, 126023. [Google Scholar] [CrossRef] [PubMed]
- Nazari, S.; Naeimi, M.; Rafienia, M.; Monajjemi, M. Fabrication and characterization of 3D nanostructured polycaprolactone-gelatin/nanohydroxyapatite-nanoclay scaffolds for bone tissue regeneration. J. Polym. Environ. 2024, 32, 94–110. [Google Scholar] [CrossRef]
- Satish, S.; Tharmavaram, M.; Rawtani, D. Halloysite nanotubes as a nature’s boon for biomedical applications. Nanobiomedicine 2019, 6, 1849543519863625. [Google Scholar] [CrossRef] [PubMed]
- Abdullayev, E.; Lvov, Y. Halloysite for controllable loading and release. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2016; Volume 7, pp. 554–605. [Google Scholar]
- Huang, K.; Ou, Q.; Xie, Y.; Chen, X.; Fang, Y.; Huang, C.; Wang, Y.; Gu, Z.; Wu, J. Halloysite nanotube based scaffold for enhanced bone regeneration. ACS Biomater. Sci. Eng. 2019, 5, 4037–4047. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Aghdam, F.; Jahed, V.; Dehghan-Niri, M.; Ganji, F.; Vasheghani-Farahani, E. Injectable chitosan hydrogel embedding modified halloysite nanotubes for bone tissue engineering. Carbohydr. Polym. 2021, 269, 118311. [Google Scholar] [CrossRef] [PubMed]
- Ou, Q.; Huang, K.; Fu, C.; Huang, C.; Fang, Y.; Gu, Z.; Wu, J.; Wang, Y. Nanosilver-incorporated halloysite nanotubes/gelatin methacrylate hybrid hydrogel with osteoimmunomodulatory and antibacterial activity for bone regeneration. Chem. Eng. J. 2020, 382, 123019. [Google Scholar] [CrossRef]
- Pietraszek, A.; Ledwójcik, G.; Lewandowska-Łańcucka, J.; Horak, W.; Lach, R.; Łatkiewicz, A.; Karewicz, A. Bioactive hydrogel scaffolds reinforced with alkaline-phosphatase containing halloysite nanotubes for bone repair applications. Int. J. Biol. Macromol. 2020, 163, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gao, X.; Zhao, M.; Li, L.; Liu, M. Three-dimensional printed sodium alginate clay nanotube composite scaffold for bone regeneration. Compos. Sci. Technol. 2024, 250, 110537. [Google Scholar] [CrossRef]
- Same, S.; Kadkhoda, J.; Navidi, G.; Abedi, F.; Aghazadeh, M.; Milani, M.; Akbarzadeh, A.; Davaran, S. The fabrication of halloysite nanotube-based multicomponent hydrogel scaffolds for bone healing. J. Appl. Biomater. Funct. Mater. 2022, 20, 22808000221111875. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, H.S.; Lee, C.-S. Nanoclay-Composite Hydrogels for Bone Tissue Engineering. Gels 2024, 10, 513. https://doi.org/10.3390/gels10080513
Hwang HS, Lee C-S. Nanoclay-Composite Hydrogels for Bone Tissue Engineering. Gels. 2024; 10(8):513. https://doi.org/10.3390/gels10080513
Chicago/Turabian StyleHwang, Hee Sook, and Chung-Sung Lee. 2024. "Nanoclay-Composite Hydrogels for Bone Tissue Engineering" Gels 10, no. 8: 513. https://doi.org/10.3390/gels10080513







