Comparative Study of TPGS and Soluplus Polymeric Micelles Embedded in Poloxamer 407 In Situ Gels for Intranasal Administration
Abstract
1. Introduction
2. Results and Discussion
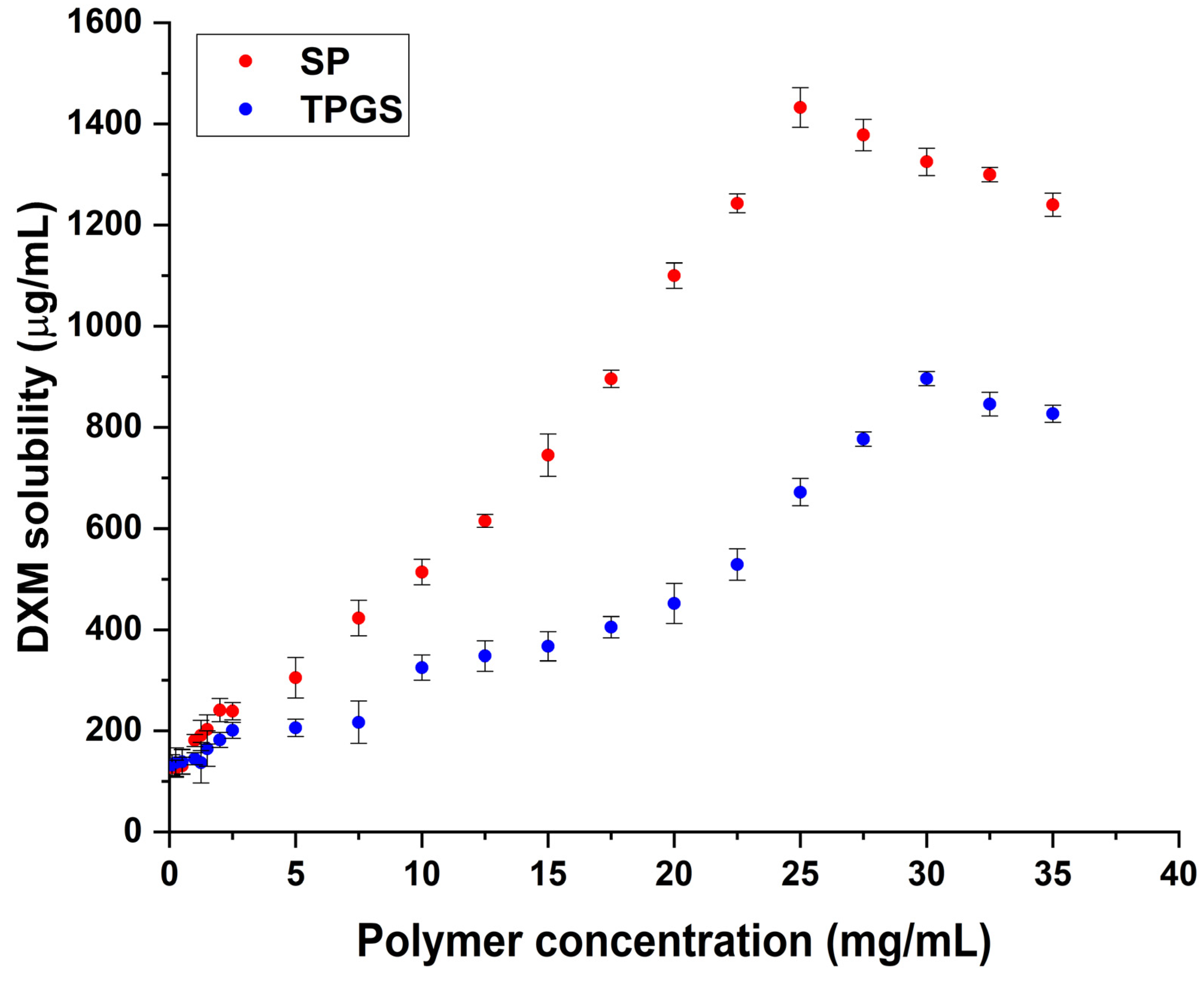
2.1. Finding the Optimal Polymer Concentrations via Phase Solubility Study
2.2. Micelle Size, Micelle Size Distribution, and Zeta Potential Measurements
2.3. Determination of Encapsulation Efficiency and Thermodynamic Solubility
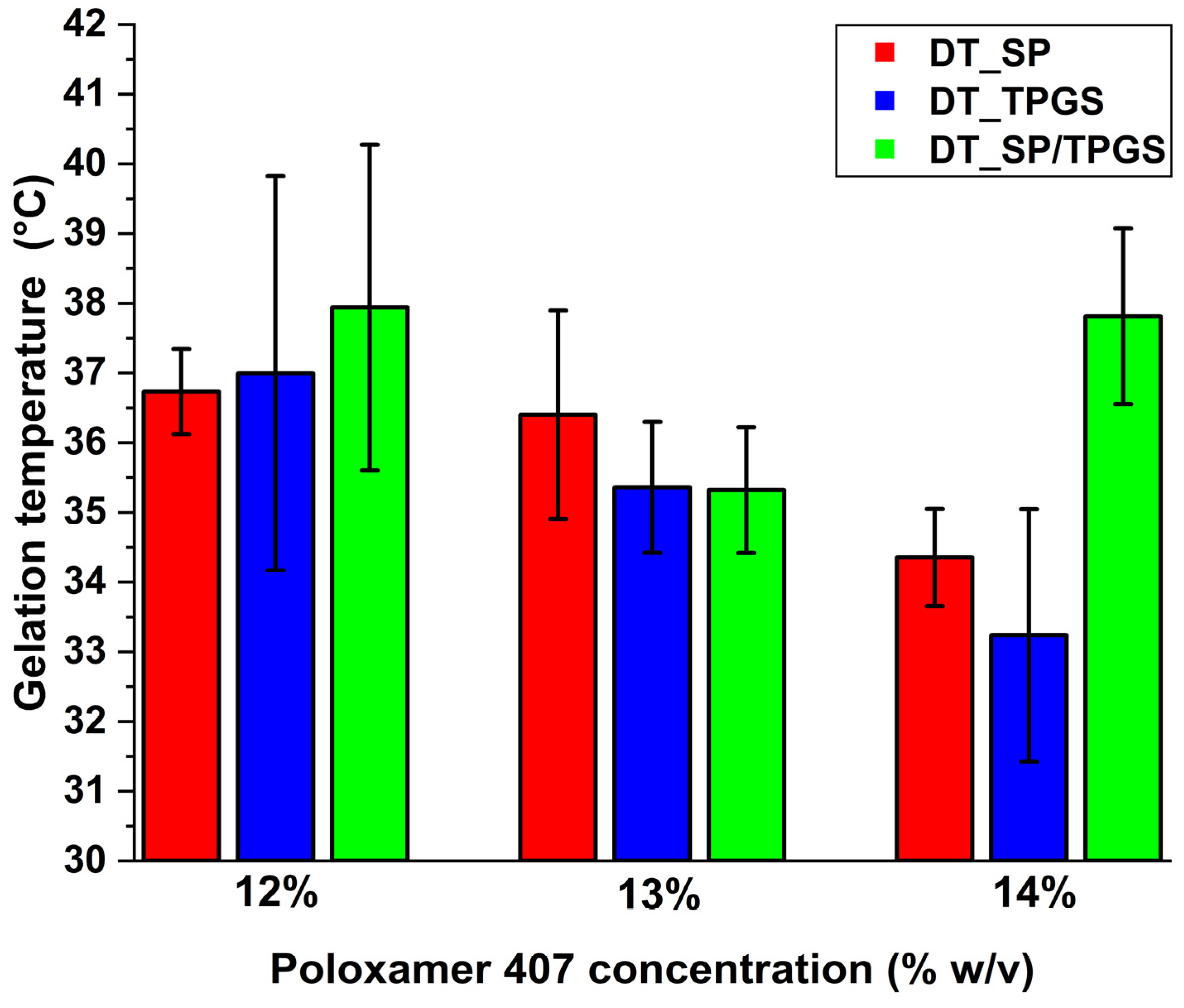
2.4. Finding the Optimal In Situ Gel-Forming Polymer Concentration via the Determination of Gelation Time and Temperature
2.5. Characterization of the In Situ Gel Formulations
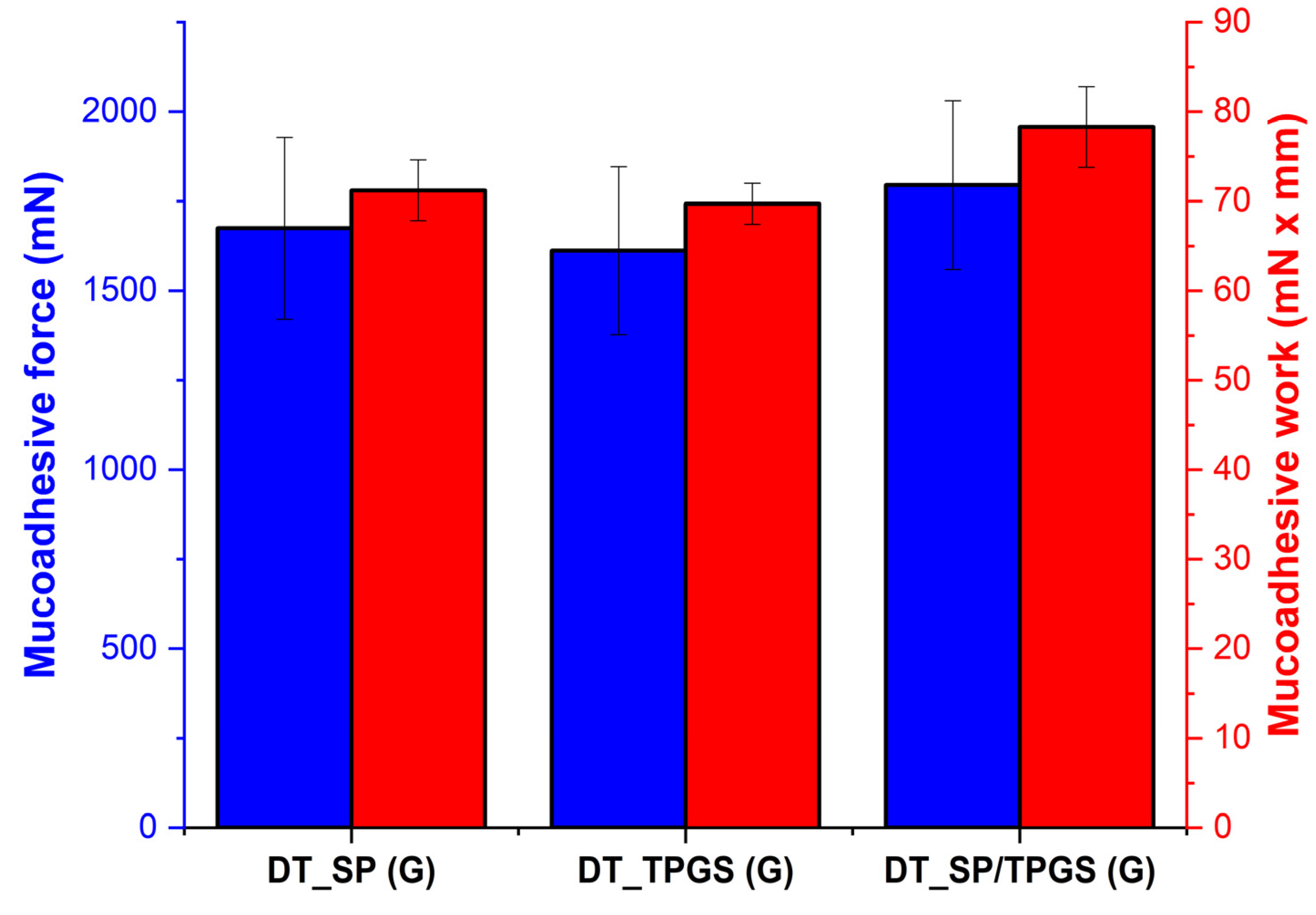
2.6. In Vitro Mucoadhesion Study
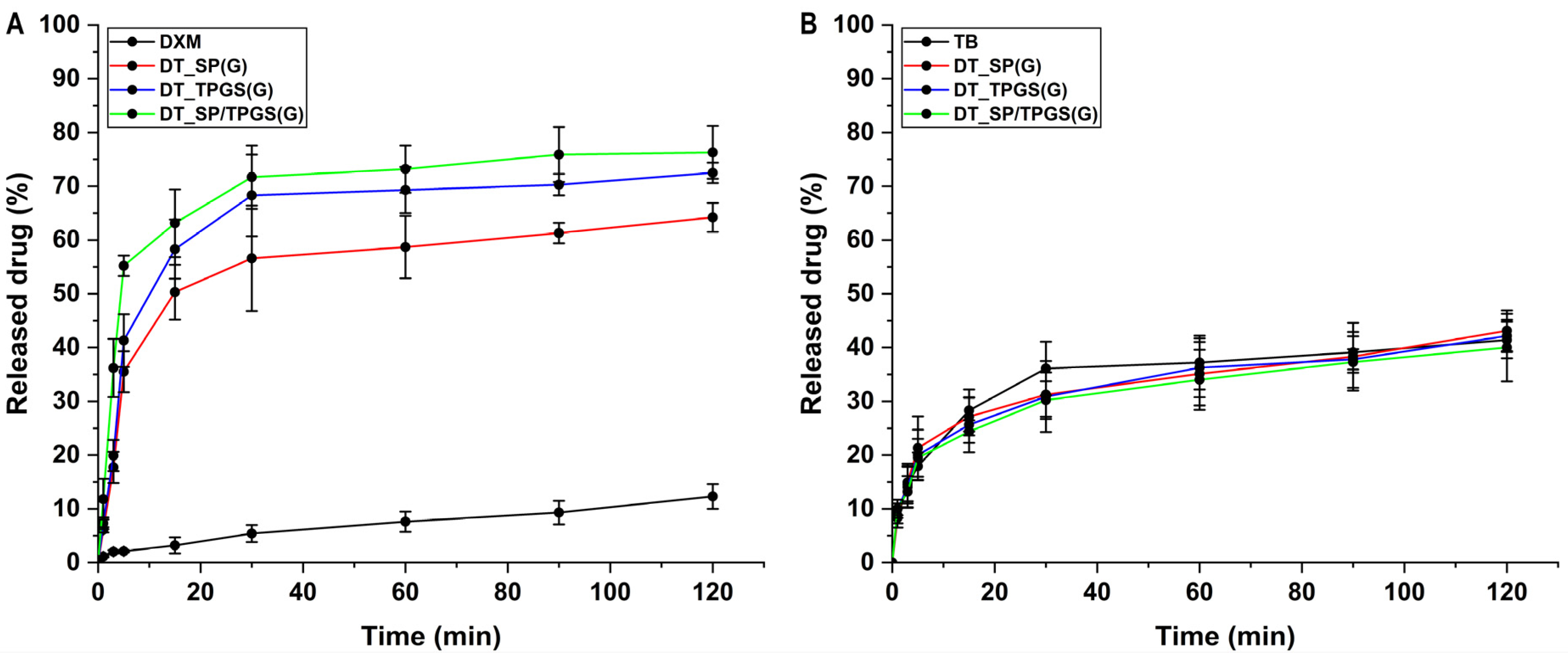
2.7. In Vitro Drug Release Study
2.8. In Vitro Nasal Passive Diffusion Study
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Quantitative Analysis of Dexamethasone via Liquid Chromatography
4.3. Quantitative Analysis of Tobramycin via Liquid Chromatography
4.4. Phase Solubility Study of Dexamethasone
4.5. Formulation of Dexamethasone-Loaded Polymeric Micelles
4.6. Characterization of Polymeric Micelles
4.6.1. Determination of Micelle Size, Size Distribution, and Zeta Potential
4.6.2. Determination of Encapsulation Efficiency
4.6.3. Determination of Thermodynamic Solubility
4.7. Incorporation of Polymeric Micelles and Tobramycin into In Situ Gelling Systems
4.8. Characterization of the In Situ Gelling Formulations
4.8.1. Determination of Gelling Time and Temperature of the In Situ Gelling Formulations
4.8.2. Determination of pH, Clarity, and Drug Content of the In Situ Gelling Formulations
4.9. In Vitro Nasal Applicability Investigations
4.9.1. In Vitro Mucoadhesion Study
4.9.2. In Vitro Drug Release Study
4.9.3. In Vitro Nasal Passive Diffusion Study
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Pandey, A.N.; Jain, S.K. Nasal-nanotechnology: Revolution for efficient therapeutics delivery. Drug Deliv. 2016, 23, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Bahmanpour, A.H.; Ghaffari, M.; Ashraf, S.; Mozafari, M. Nanotechnology for pulmonary and nasal drug delivery. In Nanoengineered Biomaterials for Advanced Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 561–579. [Google Scholar]
- de Barros, C.; Portugal, I.; Batain, F.; Portella, D.; Severino, P.; Cardoso, J.; Arcuri, P.; Chaud, M.; Alves, T. Formulation, design and strategies for efficient nanotechnology-based nasal delivery systems. RPS Pharm. Pharmacol. Rep. 2022, 1, rqac003. [Google Scholar] [CrossRef]
- Mullol, J.; Obando, A.; Pujols, L.; Alobid, I. Corticosteroid treatment in chronic rhinosinusitis: The possibilities and the limits. Immunol. Allergy Clin. 2009, 29, 657–668. [Google Scholar] [CrossRef]
- Suh, J.D.; Kennedy, D.W. Treatment options for chronic rhinosinusitis. Proc. Am. Thorac. Soc. 2011, 8, 132–140. [Google Scholar] [CrossRef]
- Barshak, M.B.; Durand, M.L. The role of infection and antibiotics in chronic rhinosinusitis. Laryngoscope Investig. Otolaryngol. 2017, 2, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Di Cicco, M.; Alcandro, G.; Claut, L.; Cariani, L.; Luca, N.; Defilippi, G.; Constantini, D.; Colombo, C. Efficacy and tolerability of a new nasal spray formulation containing hyaluronate and tobramycin in cystic fibrosis patients with bacterial rhinosinusitis. J. Cyst. Fibros. 2014, 13, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Berkhout, M.C.; van Velzen, A.J.; Touw, D.J.; de Kok, B.M.; Fokkens, W.J.; Hejjerman, H.G.M. Systemic absorption of nasally administered tobramycin and colistin in patients with cystic fibrosis. J. Antimicrob. Chemother. 2014, 69, 3112–3115. [Google Scholar] [CrossRef] [PubMed]
- Chiu, A.G.; Antunes, M.B.; Palmer, J.N.; Cohen, N.A. Evaluation of the in vivo efficacy of topical tobramycin against Pseudomonas sinonasal biofilms. J. Antimicrob. Chemother. 2007, 59, 1130–1134. [Google Scholar] [CrossRef]
- Suzuki, K.; Yoshizaki, Y.; Horii, K.; Murase, N.; Kuzuya, A.; Ohya, Y. Preparation of hyaluronic acid-coated polymeric micelles for nasal vaccine delivery. Biomater. Sci. 2022, 10, 1920–1928. [Google Scholar] [CrossRef]
- Chaudhari, S.P.; Shinde, P.U. Formulation and characterization of tranylcypromine loaded polymeric micellar in-situ nasal gel for treatment of depression. Technology 2020, 5, 149–165. [Google Scholar]
- Perumal, S.; Atchudan, R.; Lee, W. A review of polymeric micelles and their applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantú, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef]
- Luiz, M.T.; Di Filippo, L.D.; Alves, R.C.; Araújo, V.H.S.; Duarte, J.L.; Marchetti, J.M.; Chorilli, M. The use of TPGS in drug delivery systems to overcome biological barriers. Eur. Polym. J. 2021, 142, 110129. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, J.; Adu-Frimpong, M.; Li, X.; Shen, X.; He, Q.; Rong, W.; Ji, H.; Toreniyazov, E.; Xu, X.; et al. Hyperoside-loaded TPGS/mPEG-DDLLA self-assembled polymeric micelles: Preparation, characterization and in vitro/in vivo evaluation. Pharm. Dev. Technol. 2022, 27, 829–841. [Google Scholar] [CrossRef]
- Mi, Y.; Zhao, J.; Feng, S.S. Vitamin E TPGS prodrug micelles for hydrophilic drug delivery with neuroprotective effects. Int. J. Pharm. 2012, 438, 98–106. [Google Scholar] [CrossRef]
- Pignatello, R.; Corsaro, R.; Bonaccorso, A.; Zingale, E.; Carbone, C.; Musumeci, T. Soluplus polymeric nanomicelles improve solubility of BCS-class II drugs. Drug Deliv. Transl. Res. 2022, 12, 1991–2006. [Google Scholar] [CrossRef]
- Jin, I.S.; Jo, M.J.; Park, C.W.; Chung, Y.B.; Kim, J.S.; Shin, D.H. Physicochemical, pharmacokinetic and toxicity evaluation of Soluplus polymeric micelles encapsulating fenbendazole. Pharmaceutics 2020, 12, 1000. [Google Scholar] [CrossRef]
- Sipos, B.; Bella, Z.; Gróf, I.; Veszelka, S.; Deli, A.M.; Szűcs, K.F.; Sztojkov-Ivanov, A.; Ducza, E.; Gáspár, R.; Kecskeméti, G.; et al. Soluplus promotes efficient transport of meloxicam to the central nervous system via nasal administration. Int. J. Pharm. 2023, 632, 122594. [Google Scholar] [CrossRef]
- Sipos, B.; Csóka, I.; Budai-Szűcs, M.; Kozma, G.; Berkesi, D.; Kónya, Z.; Balogh, G.T.; Katona, G. Development of dexamethasone-loaded mixed polymeric micelles for nasal delivery. Eur. J. Pharm. Sci. 2021, 166, 105960. [Google Scholar] [CrossRef]
- Trenkel, M.; Scherließ, R. Nasal powder formulations: In vitro characterisation of the impact of powders on nasal residence time and sensory effects. Pharmaceutics 2021, 13, 385. [Google Scholar] [CrossRef]
- Modaresi, M.A.; Shirani, E. Effect of mucociliary clearance on the particulate airflow inside the nasal sinus and its role in increasing the residence time and absorption of drugs inside the upper respiratory pathway. J. Drug Deliv. Sci. Technol. 2023, 89, 105095. [Google Scholar] [CrossRef]
- Marttin, E.; Schipper, N.G.; Verhoef, J.C.; Merkus, F.W. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv. Drug Deliv. Rev. 1998, 29, 13–38. [Google Scholar] [CrossRef]
- Chen, Y.; Lee, J.H.; Meng, M.; Cui, n.; Dai, C.Y.; Jia, Q.; Lee, E.S.; Jiang, H.B. An Overview on Thermosensitive Oral Gel Based on Poloxamer 407. Materials 2021, 14, 4522. [Google Scholar] [CrossRef]
- Ullah, K.H.; Raza, F.; Munawar, S.M.; Sohail, M.; Zafar, M.I.; Ur-Rehman, T. Poloxamer 407 based gel formulations for transungual delivery of hydrophobic drugs: Selection and optimization of potential additives. Polymers 2021, 13, 3376. [Google Scholar] [CrossRef]
- Bercea, M.; Constantin, M.; Plugariu, I.A.; Daraba, M.O.; Ihcim, D.L. Thermosensitive gels of pullulan and poloxamer 407 as potential injectable biomaterials. J. Mol. Liq. 2022, 362, 119717. [Google Scholar] [CrossRef]
- Xia, Y.; Li, L.; Huang, X.; Wang, Z.; Zhang, H.; Gao, J.; Du, Y.; Chen, W.; Zheng, A. Performance and toxicity of different absorption enhancers used in the preparation of Poloxamer thermosensitive in situ gels for ketamine nasal administration. Drug Dev. Ind. Pharm. 2020, 46, 697–705. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.; Nawale, R.B.; Kulthe, S.S. Polymeric micelles: General considerations and their applications. Indian J. Pharm. Educ. Res. 2011, 45, 128–138. [Google Scholar]
- Pepic, I.; Lovric, J.; Filipovic-Grcic, J. How do polymeric micelles cross epithelial barriers? Eur. J. Pharm. Sci. 2013, 50, 42–55. [Google Scholar] [CrossRef]
- Bernabeu, E.; Gonzalez, L.; Cagel, M.; Gergic, E.P.; Moretton, M.A.; Chiappetta, D.A. Novel Soluplus-TPGS mixed micelles for encapsulation of paclitaxel with enhanced in vitro cytotoxicity on breast and ovarian cancer cell lines. Colloids Surf. B Biointerfaces 2016, 140, 403–411. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Y.; Wang, C.; Ding, Y.; Chen, M.; Wang, Y.; Peng, J.; Li, L.; Lv, L. Soluplus/TPGS mixed micelles for dioscin delivery in cancer therapy. Drug Dev. Ind. Pharm. 2017, 43, 1197–1204. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, C.; Liiu, W.; Yang, M.; Xu, B.; Chen, Y. Fast in vitro release and in vivo absorption of an anti-schizophrenic drug paliperidone from its Soluplus/TPGS mixed micelles. Pharmaceutics 2022, 14, 889. [Google Scholar] [CrossRef]
- Jiang, S.; Mou, Y.; He, H.; Yang, D.; Qin, L.; Zhang, F.; Zhang, P. Preparation and evaluation of self-assembly Soluplus-sodium cholate-phospholipid ternary mixed micelles of docetaxel. Drug Dev. Ind. Pharm. 2019, 45, 1788–1798. [Google Scholar] [CrossRef]
- Bergh, V.J.V.; Tonnesen, H.H. Interactions and solubilisation of 5, 10, 15, 20-tetrakis (4-hydroxyphenyl) porphyrin with poloxamer 407 and β-cyclodextrin-derivatives in binary and ternary systems. J. Drug Del. Sci. Technol. 2017, 37, 51–60. [Google Scholar] [CrossRef]
- Hirun, N.; Kraisit, P.; Tantishaiyakul, V. Thermosensitive polymer blend composed of poloxamer 407, poloxamer 188 and polycarbophil for the use as mucoadhesive in situ gel. Polymers 2022, 14, 1836. [Google Scholar] [CrossRef]
- Keck, T.; Leiacker, R.; Rettinger, G. Temperature profile in the nasal cavity. Laryngoscope 2000, 110, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Mardikasari, S.A.; Sipos, B.; Csóka, I.; Katona, G. Nasal route for antibiotics delivery: Advances, challenges and future opportunities applying the quality by design concepts. J. Drug. Deliv. Sci. Technol. 2022, 77, 103887. [Google Scholar] [CrossRef]
- Laffleur, F.; Bauer, B. Progress in nasal drug delivery systems. Int. J. Pharm. 2021, 607, 120994. [Google Scholar] [CrossRef]
- England, R.J.A.; Homer, J.J.; Knight, L.C.; Ell, S.R. Nasal pH measurement: A reliable and repeatable parameter. Clin. Otolaryng. All. Sci. 1999, 24, 67–68. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal applications of poloxamer 407-based hydrogels: An overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.; Bruschi, M.L.; de Freitas, O.; Gremiao, M.P.D.; Lara, E.H.G.; Andrews, G.P. Rheological, mechanical and mucoadhesive properties of thermoresponsive, bioadhesive binary mixtures composed of poloxamer 407 and carbopol 974P designed as platforms for implantable drug delivery systems for use in the oral cavity. Int. J. Pharm. 2009, 372, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Akkari, A.C.S.; Papini, J.Z.B.; Garcia, G.K.; Franco, M.K.K.D.; Cavalcanti, L.P.; Gasperini, A.; Alkschbirs, M.I.; Yokaichyia, F.; de Paula, E.; Tófoli, G.R.; et al. Poloxamer 407/188 binary thermosensitive hydrogels as delivery systems for infiltrative local anesthesica: Physcio-chemical characterization and pharmacological evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Ricci, E.J.; Lunard, L.O.; Nanclares, D.M.A.; Marchetti, J.M. Sustained release of lidocaine from Poloxamer 407 gels. Int. J. Pharm. 2005, 288, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Katona, G.; Sipos, B.; Budai-Szűcs, M.; Balogh, G.T.; Veszelka, S.; Gróf, I.; Deli, A.M.; Volk, B.; Szabó-Révész, P.; Csóka, I. Development of in situ gelling meloxicam-human serum albumin nanoparticle formulation for nose-to-brain application. Pharmaceutics 2021, 13, 646. [Google Scholar] [CrossRef] [PubMed]


| DT_SP | DT_TPGS | DT_SP/TPGS | |
|---|---|---|---|
| DH (nm) | 72.41 ± 6.32 | 21.43 ± 7.26 | 92.30 ± 3.4 |
| PdI | 0.119 ± 0.004 | 0.169 ± 0.011 | 0.249 ± 0.027 |
| ζ (mV) | −18.4 ± 4.1 | −13.4 ± 2.3 | −21.2 ± 2.8 |
| DXM | DT_SP | DT_TPGS | DT_SP/TPGS | |
|---|---|---|---|---|
| EEDXM (%) | - | 89.13 ± 2.70 | 83.05 ± 3.3 | 93.77 ± 4.1 |
| SDXM,25°C (µg/mL) | 124.13 ± 4.51 | 1432.21 ± 28.79 | 896.25 ± 32.88 | 1658.02 ± 22.49 |
| DT_SP (G) (13%) | DT_TPGS (G) (14%) | DT_SP/TPGS (G) (13%) | |
|---|---|---|---|
| pHsol | 6.58 ± 0.23 | 6.67 ± 0.13 | 6.71 ± 0.15 |
| pHgel | 6.83 ± 0.11 | 6.91 ± 0.20 | 7.02 ± 0.09 |
| Clarity | Transparent | Transparent | Transparent |
| Drug content (DXM) (%) | 92.13 ± 4.12 | 91.39 ± 3.55 | 97.35 ± 1.06 |
| Drug content (TB) (%) | 94.24 ± 2.31 | 92.11 ± 0.87 | 95.45 ± 2.15 |
| DT_SP (13%) | DT_TPGS (14%) | DT_SP/TPGS (13%) | ||
|---|---|---|---|---|
| DXM | ||||
| J (µg/cm2 × h) | 24.5 ± 2.90 | 137.76 ± 17.72 | 171.3 ± 13.21 | 184.6 ± 25.09 |
| Kp (cm/h) | 0.0245 | 0.1378 | 0.1713 | 0.1846 |
| Papp (×10−4 cm/s) | 1.23 | 6.89 | 8.57 | 9.23 |
| TB | ||||
| J (µg/cm2 × h) | 141.4 ± 9.6 | 341.3 ± 29.9 | 381.9 ± 28.9 | 395.8 ± 21.9 |
| Kp (cm/h) | 0.0236 | 0.0569 | 0.0634 | 0.0659 |
| Papp (×10−4 cm/s) | 1.18 | 2.84 | 3.18 | 3.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sipos, B.; Földes, F.; Budai-Szűcs, M.; Katona, G.; Csóka, I. Comparative Study of TPGS and Soluplus Polymeric Micelles Embedded in Poloxamer 407 In Situ Gels for Intranasal Administration. Gels 2024, 10, 521. https://doi.org/10.3390/gels10080521
Sipos B, Földes F, Budai-Szűcs M, Katona G, Csóka I. Comparative Study of TPGS and Soluplus Polymeric Micelles Embedded in Poloxamer 407 In Situ Gels for Intranasal Administration. Gels. 2024; 10(8):521. https://doi.org/10.3390/gels10080521
Chicago/Turabian StyleSipos, Bence, Frézia Földes, Mária Budai-Szűcs, Gábor Katona, and Ildikó Csóka. 2024. "Comparative Study of TPGS and Soluplus Polymeric Micelles Embedded in Poloxamer 407 In Situ Gels for Intranasal Administration" Gels 10, no. 8: 521. https://doi.org/10.3390/gels10080521
APA StyleSipos, B., Földes, F., Budai-Szűcs, M., Katona, G., & Csóka, I. (2024). Comparative Study of TPGS and Soluplus Polymeric Micelles Embedded in Poloxamer 407 In Situ Gels for Intranasal Administration. Gels, 10(8), 521. https://doi.org/10.3390/gels10080521









