Preparation and Adsorption Photocatalytic Properties of PVA/TiO2 Colloidal Photonic Crystal Films
Abstract
1. Introduction
2. Results and Discussion
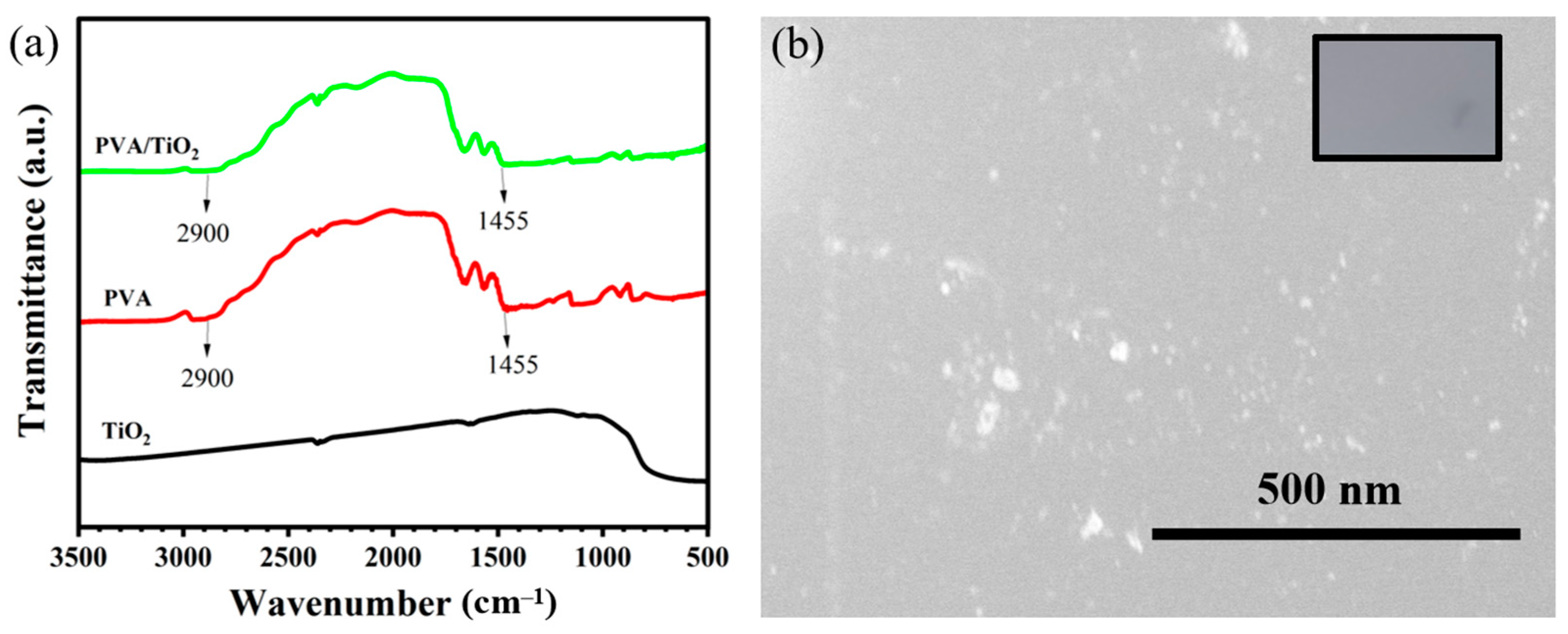
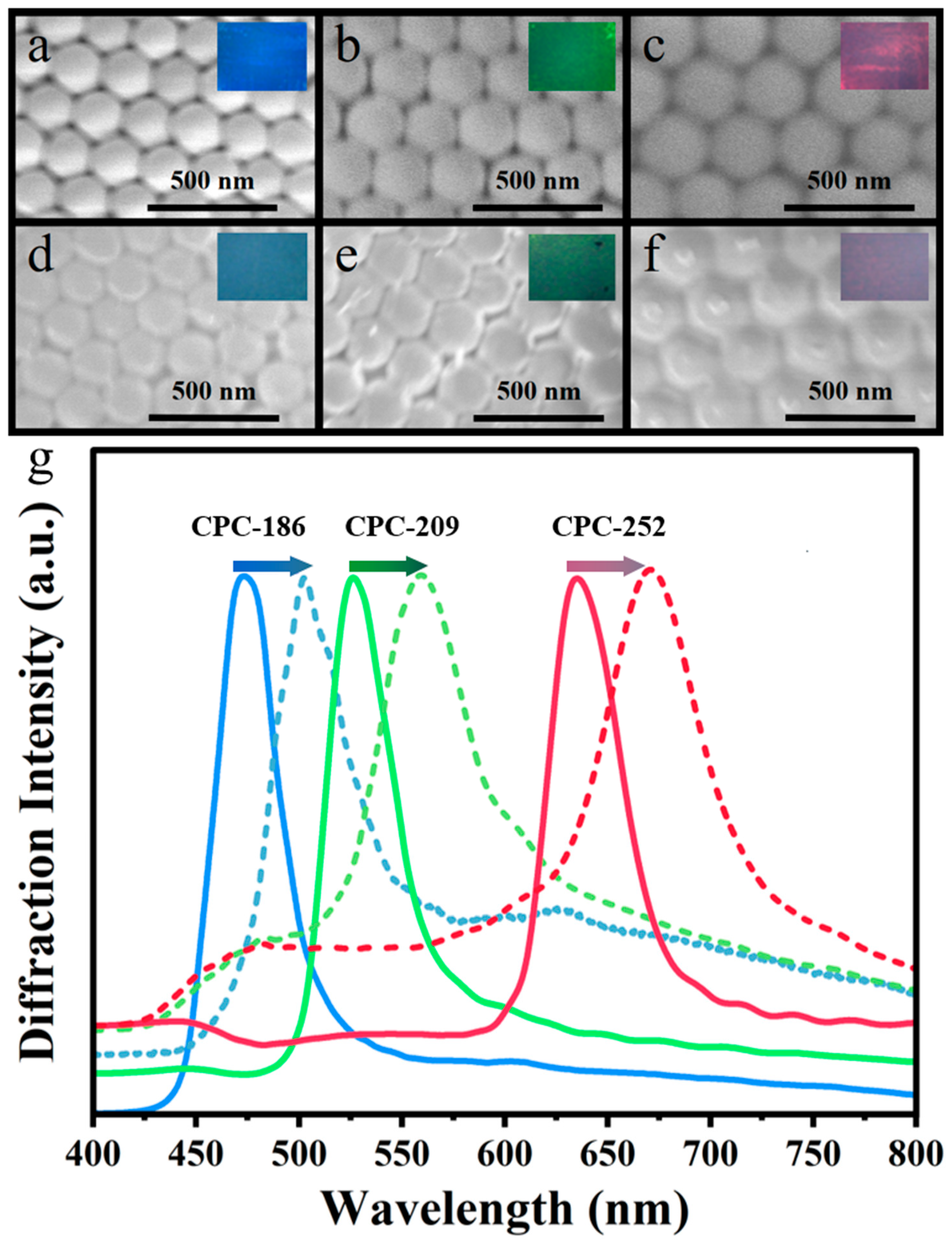
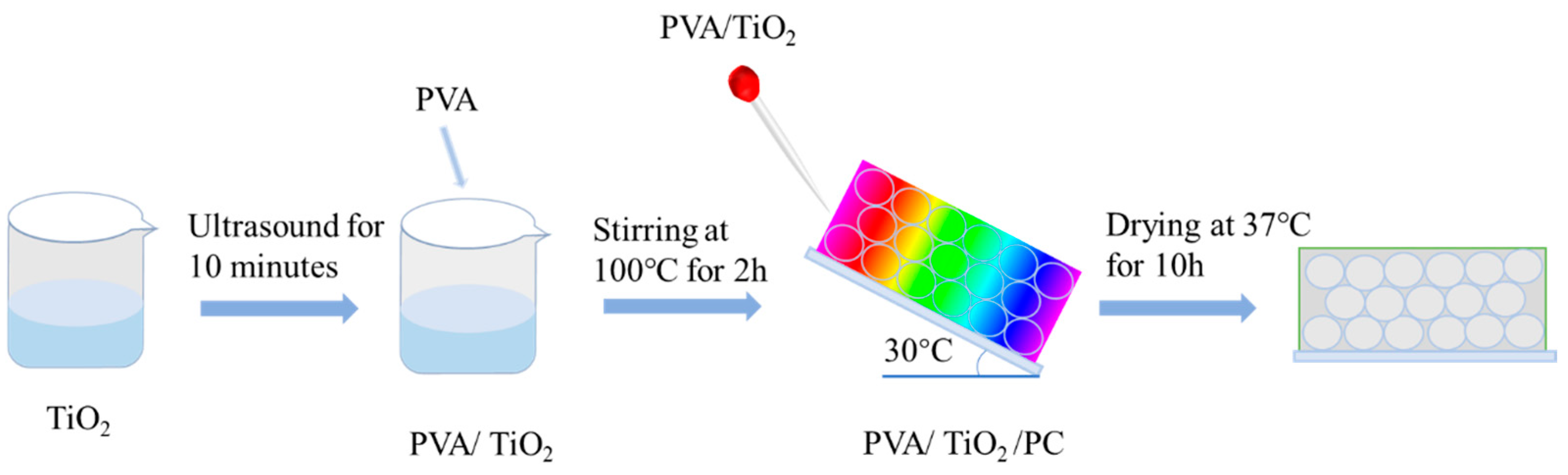
2.1. Preparation and Characterization of PVA/TiO2/CPC Thin Films
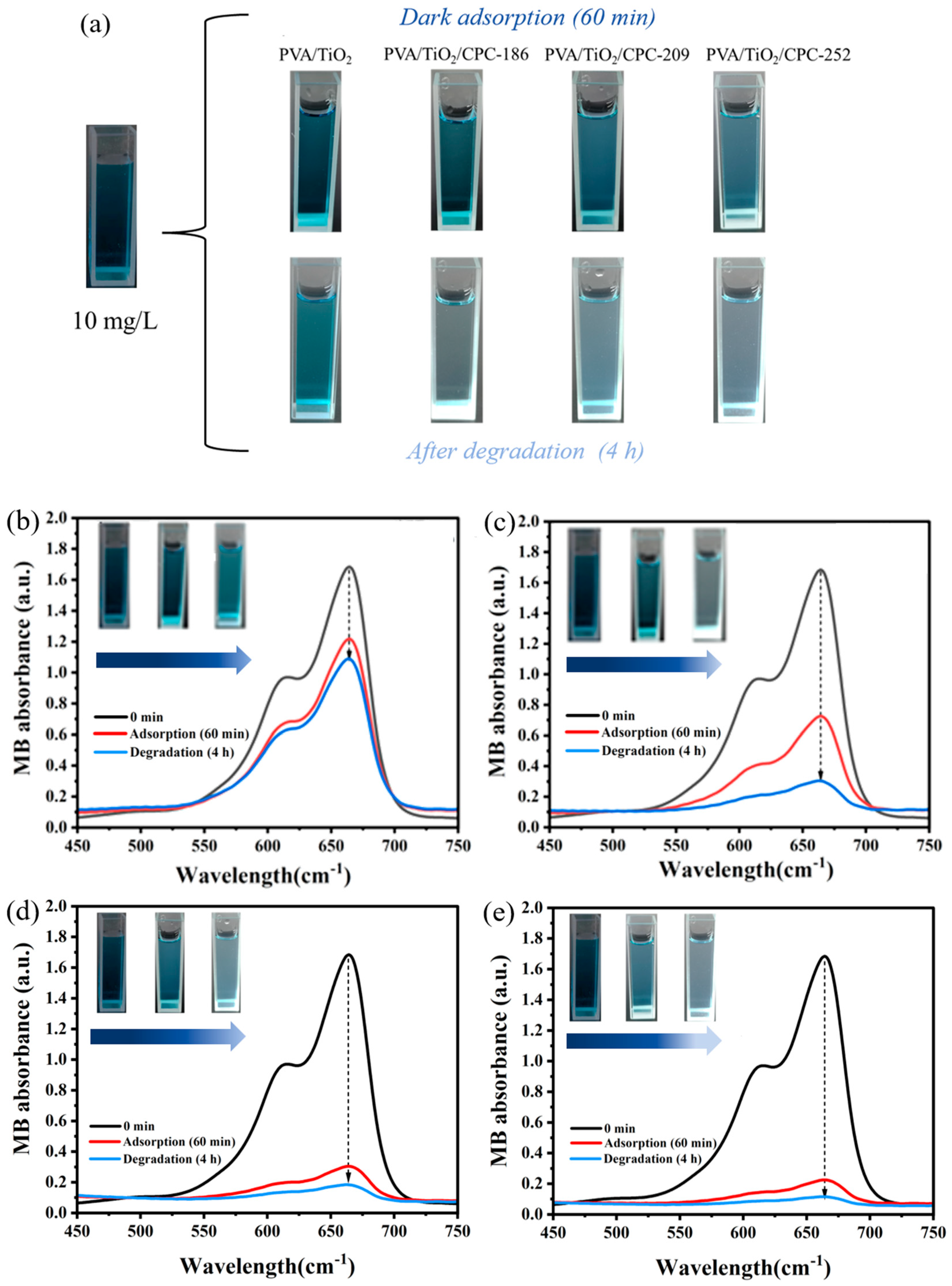
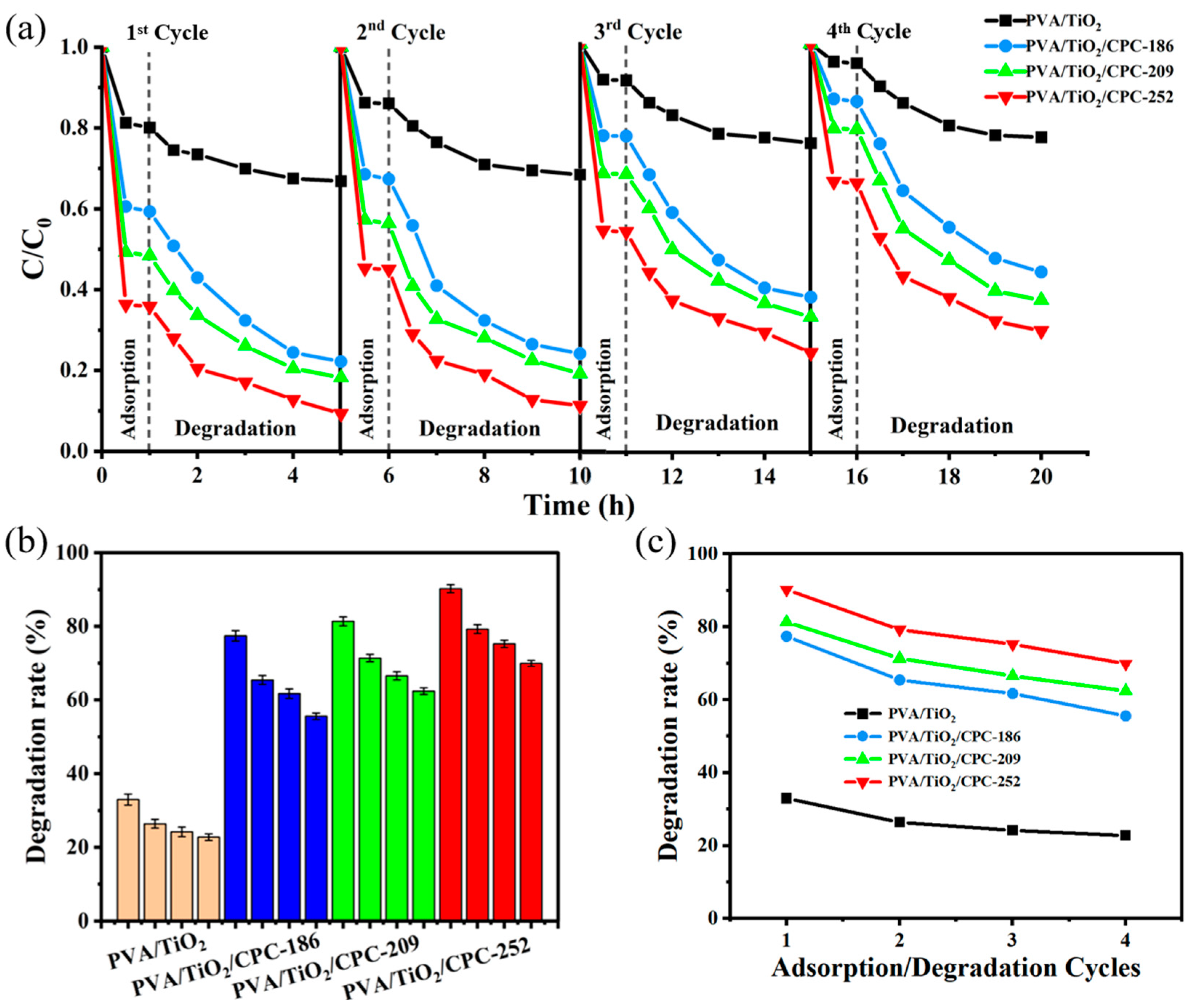
2.2. Dark Adsorption–Photocatalytic Degradation of MB Dye by PVA/TiO2/CPC Film
2.3. Cycling Performance (Repeatability) Study
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of PS Monodisperse Colloids
4.3. Preparation of CPC Template
4.4. Preparation of PVA/TiO2/CPC Adsorption Photocatalytic Films
4.5. PVA/TiO2/CPC Dark Adsorption–Photocatalytic Degradation of MB
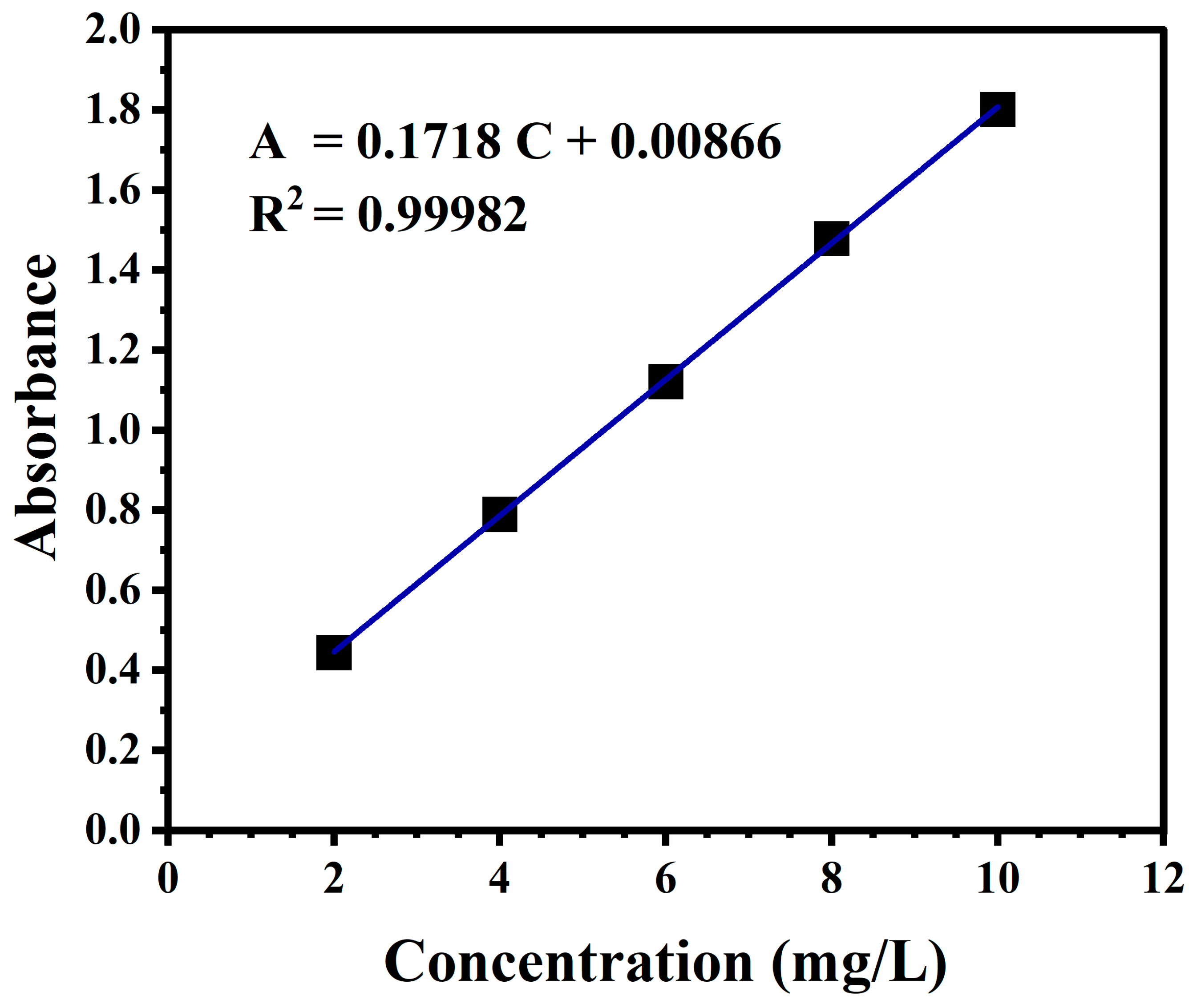
4.6. Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samarasinghe, L.; Muthukumaran, S.; Baskaran, K. Recent advances in visible light-activated photocatalysts for degradation of dyes: A comprehensive review. Chemosphere 2024, 349, 140818. [Google Scholar] [CrossRef] [PubMed]
- González-Álvarez, R.J.; Bellido-Milla, D.; Pinto, J.J.; Moreno, C. A handling-free methodology for rapid determination of Cu species in seawater based on direct solid micro-samplers analysis by high-resolution continuum source graphite furnace atomic absorption spectrometry. Talanta 2020, 206, 120249. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Gautam, S.; Kaur, S.; Kajal, N.; Kaur, M.; Gupta, R. Highly functionalized photo-activated metal–organic frameworks for dye degradation: Recent advancements. Mater. Today Commun. 2023, 34, 105180. [Google Scholar] [CrossRef]
- Rodríguez, A.; Binks, B.P. Catalysis in Pickering emulsions. Soft Matter 2020, 16, 10221–10243. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Anusuyadevi, P.; Aymonier, C.; Luque, R.; Marre, S. Nanostructured materials for photocatalysis. Chem. Soc. Rev. 2019, 48, 3868–3902. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Rahimdokht, M.; Pajootan, E. Low cost hydrogels based on gum Tragacanth and TiO2 nanoparticles: Characterization and RBFNN modelling of methylene blue dye removal. Int. J. Biol. Macromol. 2019, 134, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kamali, M.; Zhang, S.; Yu, X.; Appels, L.; Cabooter, D.; Dewil, R. Photo-assisted (waste)water treatment technologies —A scientometric-based critical review. Desalination 2022, 538, 115905. [Google Scholar] [CrossRef]
- Yuan, N.; Shao, K.; Huang, S.; Chen, C. Chitosan, alginate, hyaluronic acid and other novel multifunctional hydrogel dressings for wound healing: A review. Int. J. Biol. Macromol. 2023, 240, 124321. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; James, T.D. Glucose Sensing in Supramolecular Chemistry. Chem. Rev. 2015, 115, 8001–8037. [Google Scholar] [CrossRef]
- Wang, M.; Bai, J.; Shao, K.; Tang, W.; Zhao, X.; Lin, D.; Huang, S.; Chen, C.; Ding, Z.; Ye, J. Poly(vinyl alcohol) Hydrogels: The Old and New Functional Materials. Int. J. Polym. Sci. 2021, 2021, 2225426. [Google Scholar] [CrossRef]
- Branco, A.C.; Oliveira, A.S.; Monteiro, I.; Nolasco, P.; Silva, D.C.; Figueiredo-Pina, C.G.; Colaço, R.; Serro, A.P. PVA-Based Hydrogels Loaded with Diclofenac for Cartilage Replacement. Gels 2022, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, W.; Xu, Q.; Zheng, Y. Progress in Antibacterial Hydrogel Dressing. Gels 2022, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Li, J. Active visualized solvent sensor based on copolymer hydrogel photonic crystals containing white LEDs. Sens. Actuators B Chem. 2019, 286, 394–400. [Google Scholar] [CrossRef]
- Sobhanimatin, M.B.; Pourmahdian, S.; Tehranchi, M.M. Colorimetric monitoring of humidity by opal photonic hydrogel. Polym. Test. 2021, 98, 106999. [Google Scholar] [CrossRef]
- Chen, S.L.; Wang, A.J.; Dai, C.; Benziger, J.B.; Liu, X.C. The effect of photonic band gap on the photo-catalytic activity of nc-TiO2/SnO2 photonic crystal composite membranes. Chem. Eng. J. 2014, 249, 48–53. [Google Scholar] [CrossRef]
- Richel, A.; Johnson, N.P.; Mccomb, D.W. Observation of Bragg reflection in photonic crystals synthesized from air spheres in a Titania matrix. Appl. Phys. Lett. 2000, 76, 1816–1818. [Google Scholar] [CrossRef]
- Chu, Z.; Xue, C.; Shao, K.; Xiang, L.; Zhao, X.; Chen, C.; Pan, J.; Lin, D. Photonic Crystal-Embedded Molecularly Imprinted Contact Lenses for Controlled Drug Release. ACS Appl. Bio Mater. 2022, 5, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chu, Z.; Huang, Y.; Chen, G.; Zhao, X.; Zhu, Z.; Chen, C.; Lin, D. Copper Ion Imprinted Hydrogel Photonic Crystal Sensor Film. ACS Appl. Polym. Mater. 2022, 4, 4568–4575. [Google Scholar] [CrossRef]
- Kangwansupamonkon, W.; Klaikaew, N.; Kiatkamjornwong, S. Green synthesis of titanium dioxide/acrylamide-based hydrogel composite, self degradation and environmental applications. Eur. Polym. J. 2018, 107, 118–131. [Google Scholar] [CrossRef]
- Thakur, S.; Pandey, S.; Arotiba, O. Development of a sodium alginate-based organic/inorganic superabsorbent composite hydrogel for adsorption of methylene blue. Carbohydr. Polym. 2016, 153, 34–46. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, J.; Wang, X.; Xu, H.; Yuan, S.; Zhan, Q.; Zhang, M. Preparation of inverse opal titanium dioxide for photocatalytic performance research. Opt. Mater. 2019, 96, 109287. [Google Scholar] [CrossRef]
- Likodimos, V. Photonic crystal-assisted visible light activated TiO2 photocatalysis. Appl. Catal. B Environ. 2018, 230, 269–303. [Google Scholar] [CrossRef]
- Melvin Ng, H.; Leo, C. Translucent and adsorptive PVA thin film containing microfibrillated cellulose intercalated with TiO2 nanoparticles for dye removal. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123590. [Google Scholar]
- Zhang, Y.; Zheng, W.; Qi, A.; Qu, H.; Chen, L. Optical amplification based on slow light effects in the photonic crystal waveguide. Microw. Opt. Technol. Lett. 2011, 53, 2997–3001. [Google Scholar] [CrossRef]
- Grant, A.; Lonergan, A.; O’Dwyer, C. Thickness control of dispersion in opal photonic crystals. Opt. Mater. 2023, 142, 114053. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, J.; Dai, B.; Kou, J.; Lu, C.; Xu, Z. Photothermal effect enhanced photocatalysis realized by photonic crystal and microreactor. Appl. Surf. Sci. 2020, 534, 147640. [Google Scholar] [CrossRef]
- Ginter, J.; Piwoński, I. Evaluation of the synergistic effect between slow photons and electron trapping on the photocatalytic properties of TiO2 photonic crystals. Mater. Res. Bull. 2018, 107, 100–110. [Google Scholar] [CrossRef]
- Lai, C.; Li, J. Self-assembly of colloidal Poly(St-MMA-AA) core/shell photonic crystals with tunable structural colors of the full visible spectrum. Opt. Mater. 2019, 88, 128–133. [Google Scholar] [CrossRef]
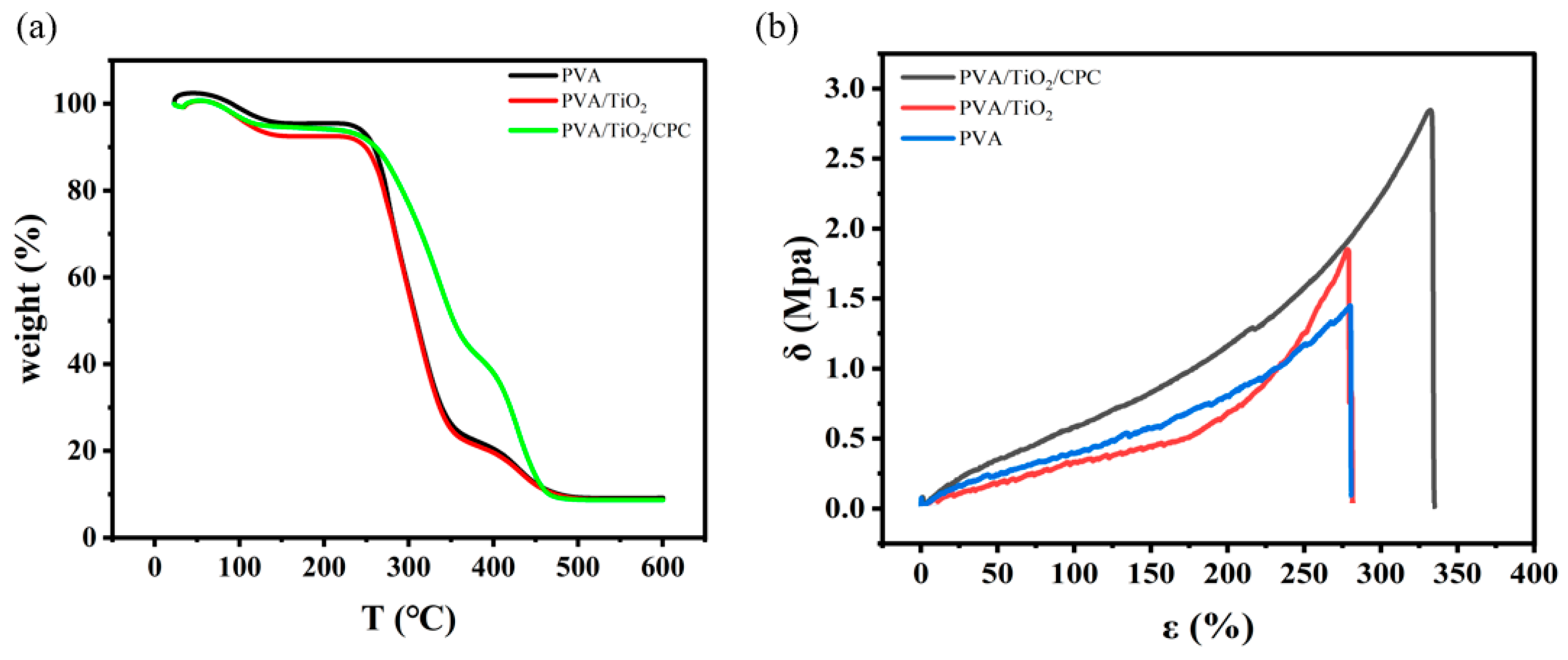

| Sample | Elongation (%) | Tensile Strength (Mpa) | Young’s Modulus (Mpa) |
|---|---|---|---|
| PVA | 280.31 | 1.43 | 0.44 ± 0.001 |
| PVA/TiO2 | 277.20 | 1.83 | 0.49 ± 0.003 |
| PVA/TiO2/CPC | 330.65 | 2.84 | 0.72 ± 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, Z.; Wang, M.; Li, J.; Chu, Z.; Tang, W.; Chen, C. Preparation and Adsorption Photocatalytic Properties of PVA/TiO2 Colloidal Photonic Crystal Films. Gels 2024, 10, 520. https://doi.org/10.3390/gels10080520
Qian Z, Wang M, Li J, Chu Z, Tang W, Chen C. Preparation and Adsorption Photocatalytic Properties of PVA/TiO2 Colloidal Photonic Crystal Films. Gels. 2024; 10(8):520. https://doi.org/10.3390/gels10080520
Chicago/Turabian StyleQian, Zhangyi, Menghan Wang, Junling Li, Zhaoran Chu, Wenwei Tang, and Cheng Chen. 2024. "Preparation and Adsorption Photocatalytic Properties of PVA/TiO2 Colloidal Photonic Crystal Films" Gels 10, no. 8: 520. https://doi.org/10.3390/gels10080520
APA StyleQian, Z., Wang, M., Li, J., Chu, Z., Tang, W., & Chen, C. (2024). Preparation and Adsorption Photocatalytic Properties of PVA/TiO2 Colloidal Photonic Crystal Films. Gels, 10(8), 520. https://doi.org/10.3390/gels10080520








