Screening of Optimal Konjac Glucomannan–Protein Composite Gel Formulations to Mimic the Texture and Appearance of Tripe
Abstract
1. Introduction
2. Results and Discussion
2.1. Screening of the Most Suitable Recipe for Tripe Simulation
2.1.1. Textural and Color Properties of Beef Reticulum
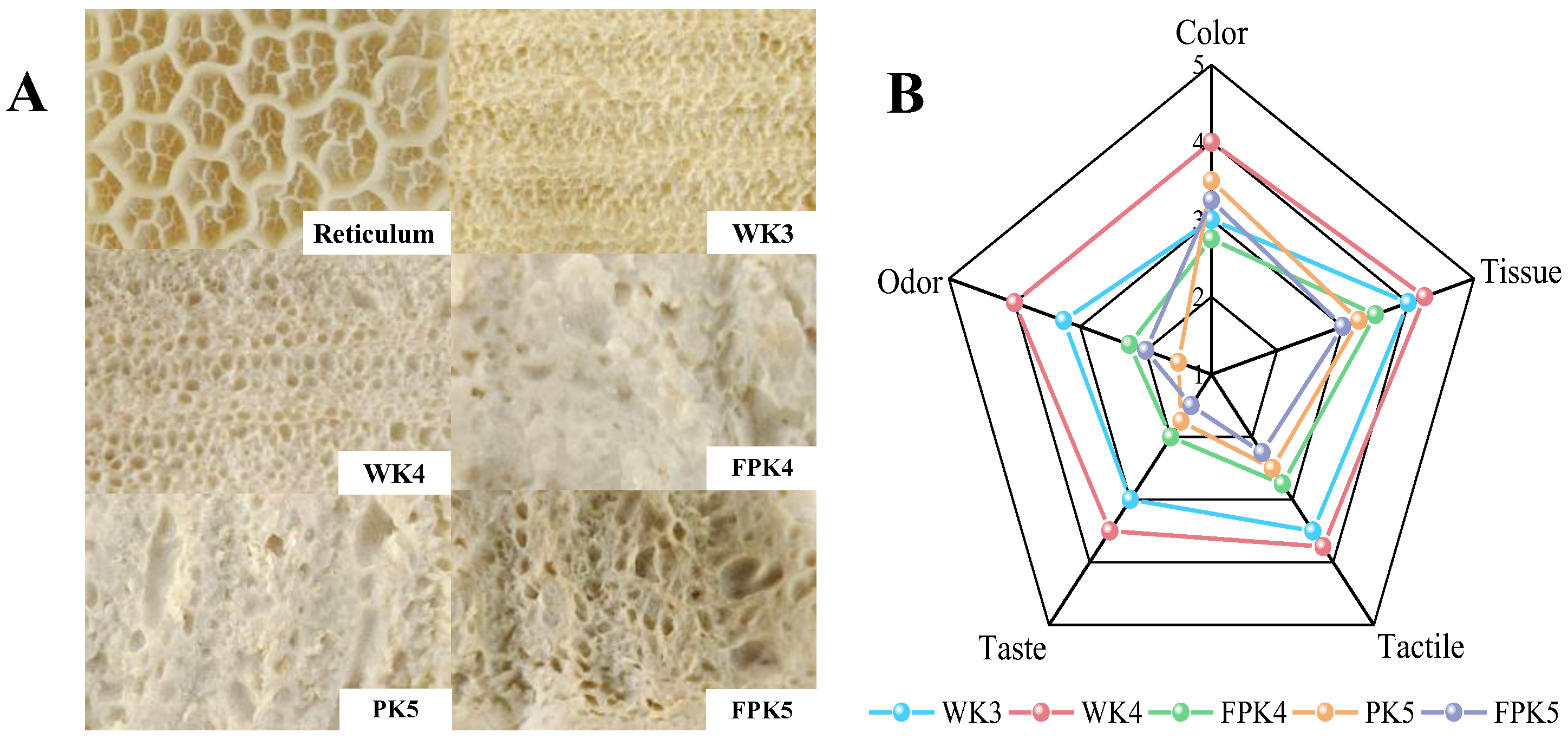
2.1.2. Appearance Properties of Different Protein/KGM Composite Gels
2.1.3. TPA
2.1.4. Shear Force
2.1.5. Color
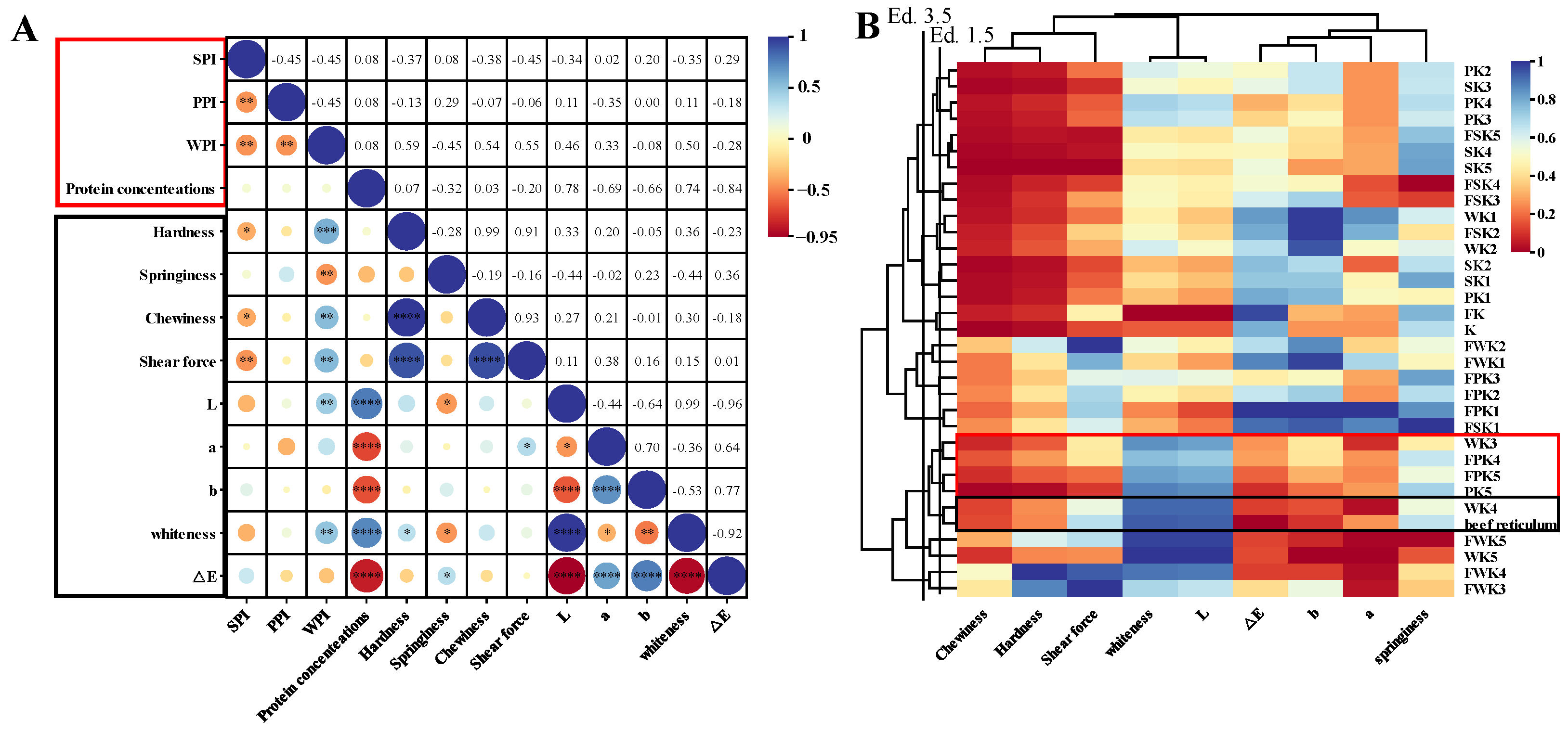
2.1.6. Correlation Analysis
2.1.7. Cluster Analysis
2.1.8. Sensory Evaluation
2.2. Characterization of Optimal Gel Formulations
2.2.1. Rheological Analyses
2.2.2. FTIR and XRD
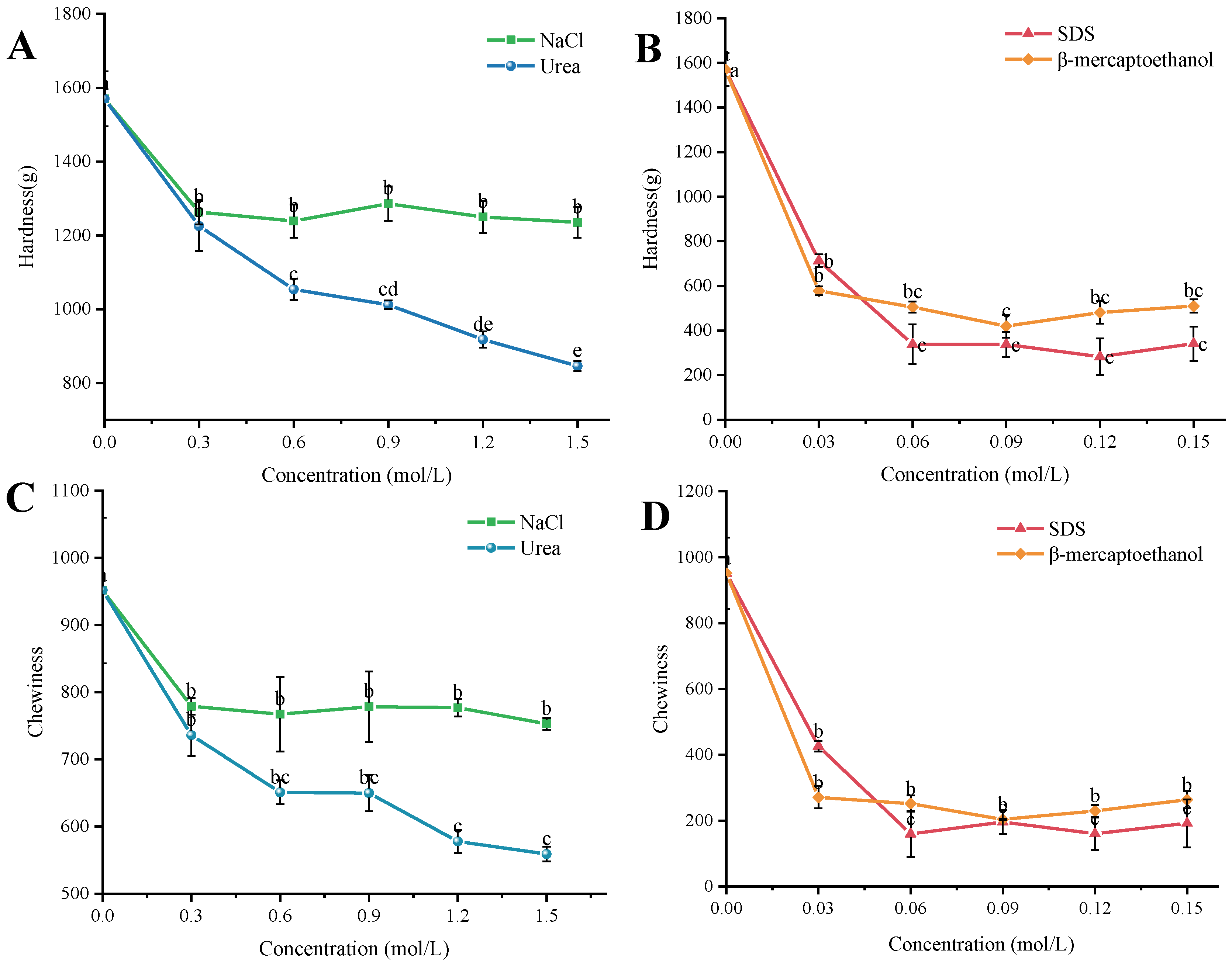
2.2.3. Analysis of the Degree of Contribution of Molecular Interactions
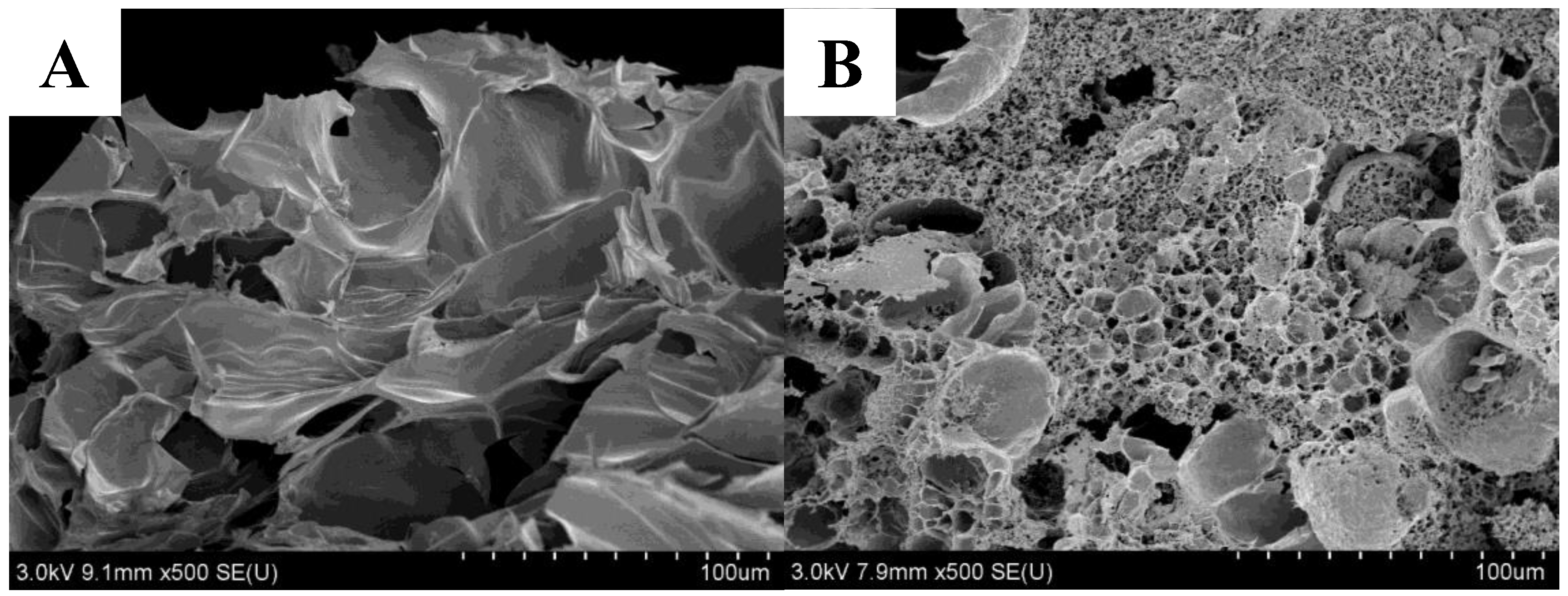
2.2.4. SEM Observations
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Processing of Beef Reticulum
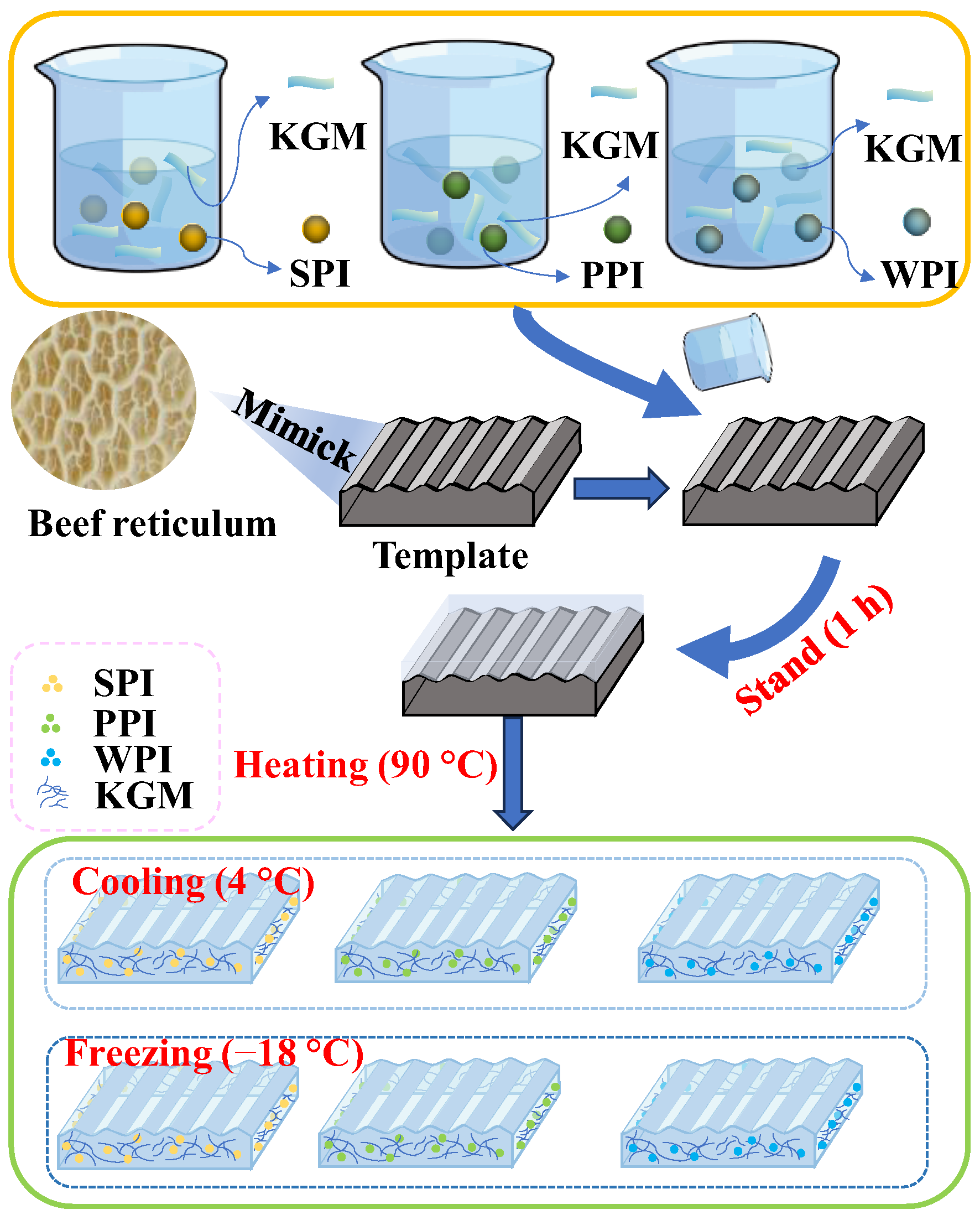
4.3. Preparation of Composite Gels
4.4. Texture Profile Analysis (TPA)
4.5. Shear Force
4.6. Color
4.7. Sensory Evaluation
4.8. Rheological Property
4.9. Fourier Transform Infrared Spectroscopy (FTIR)
4.10. X-ray Diffraction Analysis (XRD)
4.11. Degree of Contribution of Intermolecular Forces
- (1)
- Electrostatic interactions: NaCl was used to break the electrostatic interactions in the gel system, and NaCl was added at concentrations of 0.3 mol/L, 0.6 mol/L, 0.9 mol/L, 1.2 mol/L, and 1.5 mol/L. The electrostatic interactions in the gel system were analyzed by using NaCl.
- (2)
- Hydrogen-bonding interactions: Urea was used to destroy the hydrogen-bonding interactions in the gel system, and the urea was added at concentrations of 0.3 mol/L, 0.6 mol/L, 0.9 mol/L, 1.2 mol/L, and 1.5 mol/L.
- (3)
- Hydrophobic interactions: Sodium dodecyl sulfate (SDS) was used to destroy the hydrophobic interactions in the gel system, and the concentrations of SDS were 0.03 mol/L, 0.06 mol/L, 0.09 mol/L, 0.12 mol/L, and 0.15 mol/L. The hydrophobic interactions in the gel system were also destroyed by the use of SDS.
- (4)
- Disulfide bonding interactions: β-mercaptoethanol was used to destroy the disulfide bonding interactions in the gel system, and β-mercaptoethanol was added at concentrations of 0.03 mol/L, 0.06 mol/L, 0.09 mol/L, 0.12 mol/L, and 0.15 mol/L.
4.12. Scanning Electron Microscope (SEM) Observation
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Wang, C.C.; Zhou, S.Y.; Li, J.; Song, Z.; Hou, F.M. Research Progress on Processing Technology of Tripe. Adv. Mater. Res. 2012, 554–556, 994–999. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Y.; Zhao, Y.; Fang, Y. Agarose/Konjac Glucomannan Double Network Hydrogels to Mimic the Texture of Beef Tripe. Food Hydrocoll. 2023, 135, 108173. [Google Scholar] [CrossRef]
- Lee, H.J.; Yong, H.I.; Kim, M.; Choi, Y.-S.; Jo, C. Status of Meat Alternatives and Their Potential Role in the Future Meat Market—A Review. Asian Australas. J. Anim. Sci. 2020, 33, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ahmad, M.; Manzoor, K.; Purwar, R.; Ikram, S. A Review on Latest Innovations in Natural Gums Based Hydrogels: Preparations & Applications. Int. J. Biol. Macromol. 2019, 136, 870–890. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, X.; Bu, X.; An, Y.; Bi, H.; Zhao, Z. The Application of Cartilage Tissue Engineering with Cell-Laden Hydrogel in Plastic Surgery: A Systematic Review. Tissue Eng. Regen. Med. 2022, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Semmling, B.; Nagel, S.; Sternberg, K.; Weitschies, W.; Seidlitz, A. Impact of Different Tissue-Simulating Hydrogel Compartments on in Vitro Release and Distribution from Drug-Eluting Stents. Eur. J. Pharm. Biopharm. 2014, 87, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, B.; Sun, S.; Wu, P. Skin-like Mechanoresponsive Self-Healing Ionic Elastomer from Supramolecular Zwitterionic Network. Nat. Commun. 2021, 12, 4082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, L.; Li, H.; Zhao, D.; Cao, J.; Song, Y.; Liu, X. Mimic Pork Rinds from Plant-Based Gel: The Influence of Sweet Potato Starch and Konjac Glucomannan. Molecules 2022, 27, 3103. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Lou, Z.; Zhang, L.; Guo, H.; Wang, Z.; Guo, C.; Fukuda, K.; Ma, S.; Wang, G.; Someya, T.; et al. Ultrathin Hydrogel Films toward Breathable Skin-Integrated Electronics. Adv. Mater. 2023, 35, 2206793. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Lok Toh, S.; Hong Goh, J.C. PLGA Nanofiber-Coated Silk Microfibrous Scaffold for Connective Tissue Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 95B, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Ray, R.C. Konjac Glucomannan, a Promising Polysaccharide of Amorphophallus Konjac K. Koch in Health Care. Int. J. Biol. Macromol. 2016, 92, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Ma, J.; Lei, P.; Wang, L.; Qu, J.; Zhao, J.; Liu, F.; Yan, X.; Wu, W.; Jin, L.; et al. Konjac Glucomannan: An Emerging Specialty Medical Food to Aid in the Treatment of Type 2 Diabetes Mellitus. Foods 2023, 12, 363. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, A.; Dar, A.H.; Pandey, V.K.; Shams, R.; Khan, S.; Panesar, P.S.; Kennedy, J.F.; Fayaz, U.; Khan, S.A. Recent Insights into Polysaccharide-Based Hydrogels and Their Potential Applications in Food Sector: A Review. Int. J. Biol. Macromol. 2022, 213, 987–1006. [Google Scholar] [CrossRef] [PubMed]
- Taghian Dinani, S.; De Jong, S.; Vardhanabhuti, B.; Van Der Goot, A.J. Improving the Quality of Gluten-Free Plant-Based Meat Analogs Based on Soy Protein Isolate with Insoluble Soy Fibers and Low Acyl Gellan Gum. Eur. Food Res. Technol. 2024, 250, 389–408. [Google Scholar] [CrossRef]
- Tan, M.; Nawaz, M.A.; Buckow, R. Functional and Food Application of Plant Proteins—A Review. Food Rev. Int. 2023, 39, 2428–2456. [Google Scholar] [CrossRef]
- Zhang, Z.; Kobata, K.; Pham, H.; Kos, D.; Tan, Y.; Lu, J.; McClements, D.J. Production of Plant-Based Seafood: Scallop Analogs Formed by Enzymatic Gelation of Pea Protein-Pectin Mixtures. Foods 2022, 11, 851. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Lou, X.; Zheng, H.; Gu, Q.; Yang, H. Improving the Texture and Rheological Qualities of a Plant-Based Fishball Analogue by Using Konjac Glucomannan to Enhance Crosslinks with Soy Protein. Innov. Food Sci. Emerg. 2022, 75, 102910. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, W.; Naik, R.R.; Spicer, P.T.; Selomulya, C. Tuning the Pea Protein Gel Network to Mimic the Heterogenous Microstructure of Animal Protein. Food Hydrocoll. 2023, 140, 108611. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, M.; Chen, H. Effect of Whey Protein on the 3D Printing Performance of Konjac Hybrid Gel. LWT 2021, 140, 110716. [Google Scholar] [CrossRef]
- Genevro, G.M.; De Moraes, M.A.; Beppu, M.M. Freezing Influence on Physical Properties of Glucomannan Hydrogels. Int. J. Biol. Macromol. 2019, 128, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Shang, L.; Liang, H.; Li, B.; Li, J. Preparation of Konjac Glucomannan/Xanthan Gum/Sodium Alginate Composite Gel by Freezing Combining Moisture Regulation. Food Hydrocoll. 2022, 127, 107499. [Google Scholar] [CrossRef]
- Solo-de-Zaldívar, B.; Herranz, B.; Borderías, A.J.; Tovar, C.A. Effect of Freezing and Frozen Storage on Restructured FISH Prototypes Made with Glucomannan and FISH Mince. Food Hydrocoll. 2014, 41, 233–240. [Google Scholar] [CrossRef]
- Du, H.; Shi, S.; Liu, W.; Teng, H.; Piao, M. Processing and Modification of Hydrogel and Its Application in Emerging Contaminant Adsorption and in Catalyst Immobilization: A Review. Environ. Sci. Pollut. Res. Int. 2020, 27, 12967–12994. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zongo, A.W.-S.; Geng, F.; Li, J.; Li, B. Effect of Ethanol on Preparation of Konjac Emulgel-Based Fat Analogue by Freeze-Thaw Treatment. Foods 2022, 11, 3173. [Google Scholar] [CrossRef] [PubMed]
- Takeno, H.; Nishino, M.; Hsieh, W.-C. Effects of Freeze–Thawing Treatment on Mechanical and Structural Properties of Konjac Glucomannan Gels Containing Additives. Food Hydrocoll. 2023, 144, 109045. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, J.; Wang, G.; Zhang, A.; Wang, X.; Zhao, X. Effect of Freeze-Thaw Treatment on the Structure and Texture of Soy Protein-Dextran Conjugate Gels Crosslinked by Transglutaminase. LWT 2022, 153, 112443. [Google Scholar] [CrossRef]
- Liu, X.; Ji, L.; Zhang, T.; Xue, Y.; Xue, C. Effects of Pre-Emulsification by Three Food-Grade Emulsifiers on the Properties of Emulsified Surimi Sausage. J. Food Eng. 2019, 247, 30–37. [Google Scholar] [CrossRef]
- Brodkorb, A.; Croguennec, T.; Bouhallab, S.; Kehoe, J.J. Heat-Induced Denaturation, Aggregation and Gelation of Whey Proteins. In Advanced Dairy Chemistry; McSweeney, P.L.H., O’Mahony, J.A., Eds.; Springer: New York, NY, USA, 2016; pp. 155–178. ISBN 978-1-4939-2799-9. [Google Scholar]
- Youssef, M.K.; Barbut, S. Effects of Two Types of Soy Protein Isolates, Native and Preheated Whey Protein Isolates on Emulsified Meat Batters Prepared at Different Protein Levels. Meat Sci. 2011, 87, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Dong, Y.; Niu, F.; Wang, C.; Liu, Y.; Yang, Y. Study on the Gel Properties and Secondary Structure of Soybean Protein Isolate/Egg White Composite Gels. Eur. Food Res. Technol. 2015, 240, 367–378. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, L.; Su, Y.; Chang, C.; Gu, L.; Yang, Y.; Li, J. Effect of Soybean Protein Isolate and Egg White Mixture on Gelation of Chicken Myofibrillar Proteins under Salt/-Free Conditions. LWT 2021, 149, 111871. [Google Scholar] [CrossRef]
- Spotti, M.J.; Santiago, L.G.; Rubiolo, A.C.; Carrara, C.R. Mechanical and Microstructural Properties of Milk Whey Protein/Espina Corona Gum Mixed Gels. LWT 2012, 48, 69–74. [Google Scholar] [CrossRef]
- Andrés-Bello, A.; Iborra-Bernad, C.; García-Segovia, P.; Martínez-Monzó, J. Effect of Konjac Glucomannan (KGM) and Carboxymethylcellulose (CMC) on Some Physico-Chemical and Mechanical Properties of Restructured Gilthead Sea Bream (Sparus Aurata) Products. Food Bioproc. Tech. 2013, 6, 133–145. [Google Scholar] [CrossRef]
- Kono, S.; Kon, M.; Araki, T.; Sagara, Y. Effects of Relationships among Freezing Rate, Ice Crystal Size and Color on Surface Color of Frozen Salmon Fillet. J. Food Eng. 2017, 214, 158–165. [Google Scholar] [CrossRef]
- Li, Y.; Cai, M.; Liu, H.; Liu, X. Properties of Whey Protein Isolation/Konjac Glucomannan Composite Gels: Effects of Deacetylation Degrees. Int. J. Biol. Macromol. 2023, 238, 124138. [Google Scholar] [CrossRef] [PubMed]
- Abbastabar, B.; Azizi, M.H.; Adnani, A.; Abbasi, S. Determining and Modeling Rheological Characteristics of Quince Seed Gum. Food Hydrocoll. 2015, 43, 259–264. [Google Scholar] [CrossRef]
- Keskin Uslu, E.; Yılmaz, E. Preparation and Characterization of Glycerol Monostearate and Polyglycerol Stearate Oleogels with Selected Amphiphiles. Food Struct. 2021, 28, 100192. [Google Scholar] [CrossRef]
- Chemeda, Y.C.; Deneele, D.; Christidis, G.E.; Ouvrard, G. Influence of Hydrated Lime on the Surface Properties and Interaction of Kaolinite Particles. Appl. Clay Sci. 2015, 107, 1–13. [Google Scholar] [CrossRef]
- Tunick, M.H. Small-Strain Dynamic Rheology of Food Protein Networks. J. Agric. Food Chem. 2011, 59, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Alavi, F.; Emam-Djomeh, Z.; Yarmand, M.S.; Salami, M.; Momen, S.; Moosavi-Movahedi, A.A. Cold Gelation of Curcumin Loaded Whey Protein Aggregates Mixed with K-Carrageenan: Impact of Gel Microstructure on the Gastrointestinal Fate of Curcumin. Food Hydrocoll. 2018, 85, 267–280. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Qin, Z.; Fang, W.; Guo, Z.; Zou, X. 3D Printing Properties of Heat-Induced Sodium Alginate–Whey Protein Isolate Edible Gel. Gels 2024, 10, 425. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Kennedy, J.F.; Li, B.; Xie, B.J. Condensed State Structure and Biocompatibility of the Konjac Glucomannan/Chitosan Blend Films. Carbohyd. Polym. 2006, 64, 532–538. [Google Scholar] [CrossRef]
- Wang, W.; Shen, M.; Liu, S.; Jiang, L.; Song, Q.; Xie, J. Gel Properties and Interactions of Mesona Blumes Polysaccharide-Soy Protein Isolates Mixed Gel: The Effect of Salt Addition. Carbohydr. Polym. 2018, 192, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, J.; Zhang, Y.; He, R.; Du, Y.; Zhong, G. Hydration Properties and Mesoscopic Structures of Different Depolymerized Konjac Glucomannan: Experiments and Molecular Dynamics Simulations. Food Hydrocoll. 2024, 151, 109853. [Google Scholar] [CrossRef]
- Ran, X.; Yang, H. Promoted Strain-Hardening and Crystallinity of a Soy Protein-Konjac Glucomannan Complex Gel by Konjac Glucomannan. Food Hydrocoll. 2022, 133, 107959. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- ISO 8586:2012; International Organization for Standardization ISO 8586:2012. ISO: Geneva, Switzerland, 2012. Available online: https://www.iso.org/standard/45352.html (accessed on 10 August 2024).
- Williams, J.; Ringsdorf, A. Human Odour Thresholds Are Tuned to Atmospheric Chemical Lifetimes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190274. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Do Trong, N.; Rizzolo, A.; Herremans, E.; Vanoli, M.; Cortellino, G.; Erkinbaev, C.; Tsuta, M.; Spinelli, L.; Contini, D.; Torricelli, A.; et al. Reprint of “Optical Properties–Microstructure–Texture Relationships of Dried Apple Slices: Spatially Resolved Diffuse Reflectance Spectroscopy as a Novel Technique for Analysis and Process Control”. Innov. Food Sci. Emerg. 2014, 24, 145–153. [Google Scholar] [CrossRef]
- Yang, D.; Gao, S.; Yang, H. Effects of Sucrose Addition on the Rheology and Structure of Iota-Carrageenan. Food Hydrocoll. 2020, 99, 105317. [Google Scholar] [CrossRef]
- Jiang, W.; Yang, X.; Yin, S.; Li, R.; Zhang, S.; Li, L. Gelation Behaviour of Auricularia Polytricha Polysaccharides-Whey Protein Isolate. J. Food Eng. 2024, 377, 112079. [Google Scholar] [CrossRef]
- Yu, N.; Xu, Y.; Jiang, Q.; Xia, W. Molecular Forces Involved in Heat-Induced Freshwater Surimi Gel: Effects of Various Bond Disrupting Agents on the Gel Properties and Protein Conformation Changes. Food Hydrocoll. 2017, 69, 193–201. [Google Scholar] [CrossRef]



| TPA | Hardness (g) | Springiness | Chewiness | Shear force (N) |
| 1344.37 ± 87.67 | 0.97 ± 0.01 | 1103.36 ± 49.92 | 7.31 ± 0.30 | |
| Color | L* | a* | b* | whiteness |
| 60.01 ± 0.61 | −5.22 ± 0.97 | 2.96 ± 0.25 | 60.47 ± 0.50 |
| Protein Type | Concentrations | Hardness (g) | Springiness | Chewiness |
|---|---|---|---|---|
| SPI | 0% | 480.95 ± 9.84 A,b | 0.87 ± 0.02 A,a | 227.92 ± 7.00 A,c |
| 2% | 552.27 ± 22.40 B,a | 0.89 ± 0.01 A,a | 299.71 ± 16.67 B,a | |
| 4% | 500.03 ± 13.43 C,b | 0.87 ± 0.01 A,a | 272.43 ± 8.80 C,ab | |
| 6% | 491.10 ± 8.04 C,b | 0.87 ± 0.01 A,a | 253.11 ± 10.41 C,bc | |
| 8% | 465.62 ± 19.80 C,b | 0.89 ± 0.01 A,a | 256.66 ± 18.92 C,bc | |
| 10% | 400.40 ± 29.41 C,c | 0.89 ± 0.01 A,a | 224.32 ± 14.62 C,c | |
| PPI | 0% | 480.95 ± 9.84 A,d | 0.87 ± 0.02 A,a | 227.92 ± 7.10 A,d |
| 2% | 590.31 ± 17.65 B,c | 0.85 ± 0.01 B,a | 295.77 ± 12.42 B,c | |
| 4% | 614.89 ± 20.70 B,bc | 0.87 ± 0.01 A,a | 333.52 ± 12.48 B,b | |
| 6% | 650.42 ± 24.34 B,ab | 0.87 ± 0.01 A,a | 346.78 ± 13.11 B,ab | |
| 8% | 696.23 ± 31.94 B,a | 0.87 ± 0.02 AB,a | 372.48 ± 27.34 B,a | |
| 10% | 490.35 ± 9.42 B,d | 0.88 ± 0.01 A,a | 266.15 ± 6.59 B,c | |
| WPI | 0% | 480.95 ± 9.84 A,d | 0.87 ± 0.02 A,a | 227.92 ± 7.10 A,e |
| 2% | 747.65 ± 59.51 A,c | 0.86 ± 0.02 B,a | 376.76 ± 27.48 A,d | |
| 4% | 1021.97 ± 5.15 A,b | 0.86 ± 0.03 A,a | 526.17 ± 15.82 A,c | |
| 6% | 1078.86 ± 5.85 A,b | 0.84 ± 0.03 A,ab | 533.09 ± 32.48 A,c | |
| 8% | 1391.37 ± 2.65 A,a | 0.86 ± 0.01 B,ab | 739.63 ± 0.77 A,a | |
| 10% | 1344.60 ± 15.42 A,a | 0.86 ± 0.02 B,c | 646.07 ± 6.83 A,b |
| Protein Type | Concentrations | Hardness (g) | Springiness | Chewiness |
|---|---|---|---|---|
| SPI | 0% | 742.79 ± 22.98 A,cd | 0.89 ± 0.03 A,ab | 455.89 ± 31.88 A,b |
| 2% | 2055.32 ± 81.31 A,a | 0.91 ± 0.01 A,a | 1258.22 ± 12.01 A,a | |
| 4% | 969.79 ± 26.38 C,b | 0.84 ± 0.02 A,bc | 474.29 ± 13.45 C,b | |
| 6% | 810.71 ± 8.84 C,c | 0.80 ± 0.01 C,c | 360.13 ± 14.24 C,c | |
| 8% | 688.07 ± 8.10 C,d | 0.79 ± 0.06 A,c | 325.00 ± 28.47 C,cd | |
| 10% | 561.79 ± 6.53 C,e | 0.68 ± 0.01 C,d | 286.64 ± 10.68 C,d | |
| PPI | 0% | 742.79 ± 22.98 A,e | 0.89 ± 0.03 A,a | 455.89 ± 31.88 A,e |
| 2% | 1593.31 ± 73.63 B,c | 0.89 ± 0.02 A,a | 1025.44 ± 58.67 C,b | |
| 4% | 2063.17 ± 98.95 B,a | 0.87 ± 0.01 A,a | 1204.70 ± 38.43 B,a | |
| 6% | 1825.85 ± 45.54 B,b | 0.89 ± 0.02 A,a | 1147.82 ± 22.12 B,a | |
| 8% | 1473.95 ± 21.95 B,c | 0.87 ± 0.04 A,a | 881.06 ± 77.45 B,c | |
| 10% | 1062.31 ± 66.74 B,d | 0.86 ± 0.01 A,a | 577.79 ± 11.67 B,d | |
| WPI | 0% | 742.79 ± 22.98 A,e | 0.88 ± 0.03 A,a | 455.89 ± 31.88 A,f |
| 2% | 2058.88 ± 71.29 A,d | 0.85 ± 0.02 B,b | 1139.17 ± 42.79 B,e | |
| 4% | 2863.26 ± 96.15 A,c | 0.86 ± 0.01 A,ab | 1669.18 ± 37.40 A,c | |
| 6% | 3811.11 ± 181.49 A,b | 0.83 ± 0.02 B,b | 1987.82 ± 63.29 A,b | |
| 8% | 4338.07 ± 46.15 A,a | 0.84 ± 0.01 A,b | 2313.76 ± 26.99 A,a | |
| 10% | 2783.61 ± 127.93 A,c | 0.79 ± 0.01 B,c | 1444.36 ± 85.50 A,d |
| Index | Protein Type | 0% | 2% | 4% | 6% | 8% | 10% |
|---|---|---|---|---|---|---|---|
| L* | SPI | 29.92 ± 0.24 A,e | 36.52 ± 1.05 A,c | 40.48 ± 0.02 C,d | 41.20 ± 0.06 C,a | 42.16 ± 0.15 C,a | 39.72 ± 0.43 C,b |
| PPI | 29.92 ± 0.24 A,f | 34.13 ± 0.15 B,e | 44.05 ± 0.07 A,d | 48.45 ± 0.08 B,c | 52.05 ± 0.04 B,b | 57.37 ± 0.01 B,a | |
| WPI | 29.92 ± 0.24 A,f | 36.82 ± 0.04 A,e | 43.38 ± 0.10 B,d | 55.48 ± 0.29 A,c | 59.56 ± 0.07 A,b | 62.78 ± 0.02 A,a | |
| a* | SPI | −0.96 ± 0.45 A,c | 1.96 ± 0.26 B,a | 0.18 ± 0.01 B,b | −0.93 ± 0.10 B,c | −3.45 ± 0.01 B,d | −5.20 ± 0.02 C,e |
| PPI | −0.96 ± 0.45 A,b | 2.06 ± 0.21 B,a | −2.23 ± 0.07 C,c | −3.89 ± 0.10 C,d | −4.91 ± 0.06 C,e | −4.52 ± 0.01 B,e | |
| WPI | −0.96 ± 0.45 A,d | 4.14 ± 0.03 A,a | 2.14 ± 0.05 A,b | −0.37 ± 0.15 A,c | −0.73 ± 0.08 A,cd | −0.88 ± 0.04 A,d | |
| b* | SPI | 6.70 ± 0.35 A,d | 17.78 ± 0.59 B,a | 16.50 ± 0.23 B,b | 15.65 ± 0.18 A,b | 9.91 ± 0.58 A,c | 6.97 ± 0.28 A,d |
| PPI | 6.70 ± 0.35 A,e | 18.33 ± 0.19 B,a | 15.54 ± 0.06 C,b | 11.89 ± 0.12 B,c | 10.03 ± 0.08 A,d | 5.38 ± 0.10 B,f | |
| WPI | 6.70 ± 0.35 A,c | 23.32 ± 0.06 A,a | 22.43 ± 0.20 A,a | 10.64 ± 0.96 C,b | 4.15 ± 0.12 B,d | 0.74 ± 0.26 C,e |
| Index | Protein Type | 0% | 2% | 4% | 6% | 8% | 10% |
|---|---|---|---|---|---|---|---|
| L* | SPI | 23.09 ± 0.35 A,f | 31.82 ± 0.16 B,e | 36.38 ± 0.13 C,d | 39.82 ± 0.07 C,b | 40.50 ± 0.12 C,a | 38.83 ± 0.38 C,c |
| PPI | 23.09 ± 0.35 A,f | 28.71 ± 0.14 C,e | 39.50 ± 0.59 B,d | 45.20 ± 0.05 B,c | 50.19 ± 0.23 B,b | 55.03 ± 0.15 B,a | |
| WPI | 23.09 ± 0.35 A,f | 34.35 ± 0.09 A,e | 41.50 ± 0.09 A,d | 52.23 ± 0.04 A,c | 58.53 ± 0.12 A,b | 62.09 ± 0.07 A,a | |
| a* | SPI | −0.63 ± 0.32 A,b | 4.29 ± 0.14 B,a | 3.78 ± 0.20 A,a | −0.26 ± 0.09 A,b | −3.07 ± 0.12 B,c | −4.09 ± 0.43 C,d |
| PPI | −0.63 ± 0.32 A,b | 5.04 ± 0.20 A,a | −1.97 ± 0.57 C,c | −4.39 ± 0.06 C,d | −3.84 ± 0.05 C,d | −3.79 ± 0.15 B,d | |
| WPI | −0.63 ± 0.32 A,c | 3.24 ± 0.27 C,a | 1.36 ± 0.10 B,b | −0.62 ± 0.05 B,c | −0.74 ± 0.02 A,c | −0.83 ± 0.04 A,c | |
| b* | SPI | 7.96 ± 0.63 A,f | 22.35 ± 0.43 C,b | 23.47 ± 0.18 A,a | 16.94 ± 0.37 A,c | 12.07 ± 0.08 A,d | 10.03 ± 0.76 A,e |
| PPI | 7.96 ± 0.63 A,e | 23.65 ± 0.12 A,a | 17.16 ± 1.72 C,b | 12.23 ± 0.03 C,c | 10.34 ± 0.18 B,d | 7.86 ± 0.27 B,e | |
| WPI | 7.96 ± 0.63 A,d | 23.16 ± 0.21 B,a | 20.40 ± 0.17 B,b | 13.47 ± 0.10 B,c | 3.49 ± 0.18 C,e | 2.66 ± 0.04 C,f |
| Processing Conditions | KGM (%) | SPI (%) | PPI (%) | WPI (%) | Ca(OH)2 (wt%) | Water | |
|---|---|---|---|---|---|---|---|
| Heating-Cooling | Heating-Freezing | ||||||
| K | FK | 5 | 4 | 94.8 | |||
| SK1 | FSK1 | 5 | 2 | 4 | 92.8 | ||
| SK2 | FSK2 | 5 | 4 | 4 | 90.8 | ||
| SK3 | FSK3 | 5 | 6 | 4 | 88.8 | ||
| SK4 | FSK4 | 5 | 8 | 4 | 86.8 | ||
| SK5 | FSK5 | 5 | 10 | 4 | 84.8 | ||
| PK1 | FPK1 | 5 | 2 | 4 | 92.8 | ||
| PK2 | FPK2 | 5 | 4 | 4 | 90.8 | ||
| PK3 | FPK3 | 5 | 6 | 4 | 88.8 | ||
| PK4 | FPK4 | 5 | 8 | 4 | 86.8 | ||
| PK5 | FPK5 | 5 | 10 | 4 | 84.8 | ||
| WK1 | FWK1 | 5 | 2 | 4 | 92.8 | ||
| WK2 | FWK2 | 5 | 4 | 4 | 90.8 | ||
| WK3 | FWK3 | 5 | 6 | 4 | 88.8 | ||
| WK4 | FWK4 | 5 | 8 | 4 | 86.8 | ||
| WK5 | FWK5 | 5 | 10 | 4 | 84.8 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Q.; Liu, Y.; Luo, L.; Chen, Y.; Zheng, Y.; Ran, G.; Liu, D. Screening of Optimal Konjac Glucomannan–Protein Composite Gel Formulations to Mimic the Texture and Appearance of Tripe. Gels 2024, 10, 528. https://doi.org/10.3390/gels10080528
Zou Q, Liu Y, Luo L, Chen Y, Zheng Y, Ran G, Liu D. Screening of Optimal Konjac Glucomannan–Protein Composite Gel Formulations to Mimic the Texture and Appearance of Tripe. Gels. 2024; 10(8):528. https://doi.org/10.3390/gels10080528
Chicago/Turabian StyleZou, Qiang, Yudie Liu, Linghui Luo, Yuyou Chen, Yuhan Zheng, Guilian Ran, and Dayu Liu. 2024. "Screening of Optimal Konjac Glucomannan–Protein Composite Gel Formulations to Mimic the Texture and Appearance of Tripe" Gels 10, no. 8: 528. https://doi.org/10.3390/gels10080528
APA StyleZou, Q., Liu, Y., Luo, L., Chen, Y., Zheng, Y., Ran, G., & Liu, D. (2024). Screening of Optimal Konjac Glucomannan–Protein Composite Gel Formulations to Mimic the Texture and Appearance of Tripe. Gels, 10(8), 528. https://doi.org/10.3390/gels10080528





