Preparation of Complex Polysaccharide Gels with Zanthoxylum bungeanum Essential Oil and Their Application in Fish Preservation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results of Single Factor
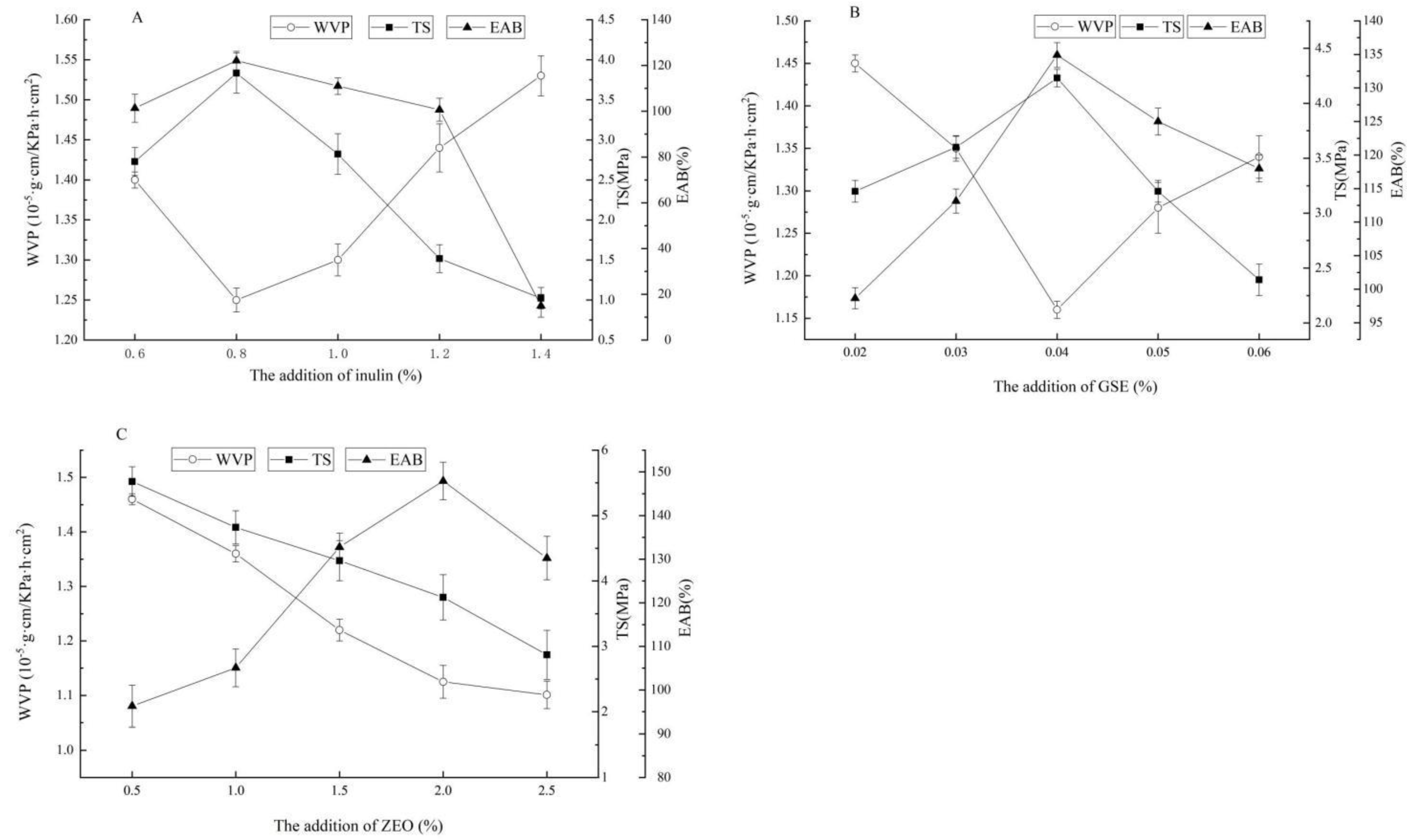
2.1.1. Effect of the Addition of Inulin on Water Vapor Permeability (WVP), Tensile Strength (TS), and Elongation at Break (EAB) of the ZEO-Complex Polysaccharide Gels
2.1.2. Effect of the Addition of GSE on WVP, TS, and EAB of the ZEO-Complex Polysaccharide Gels
2.1.3. Effect of the Addition of ZEO on WVP, TS, and EAB of the ZEO-Complex Polysaccharide Gels
2.2. Results of MATLAB Analysis and Box-Behnken Design
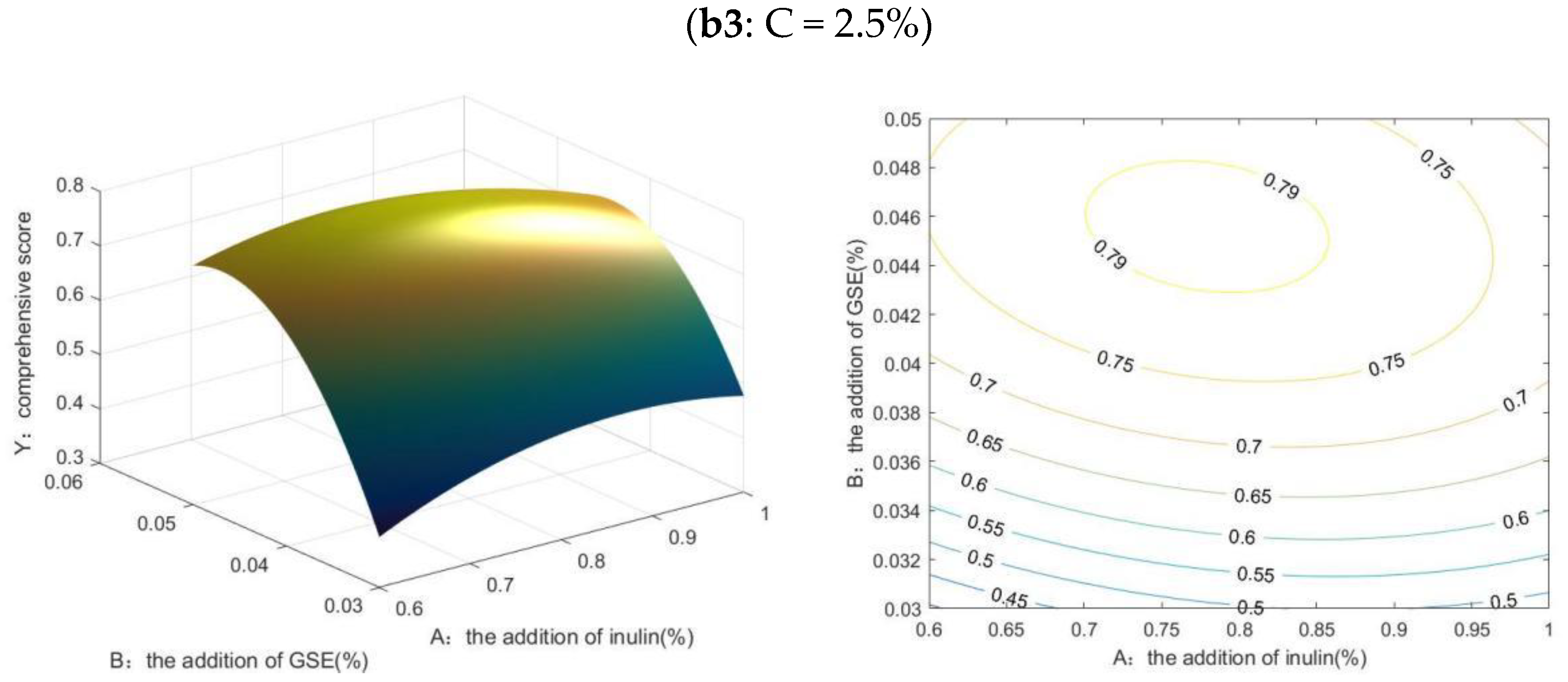
2.2.1. Results of MATLAB Analysis
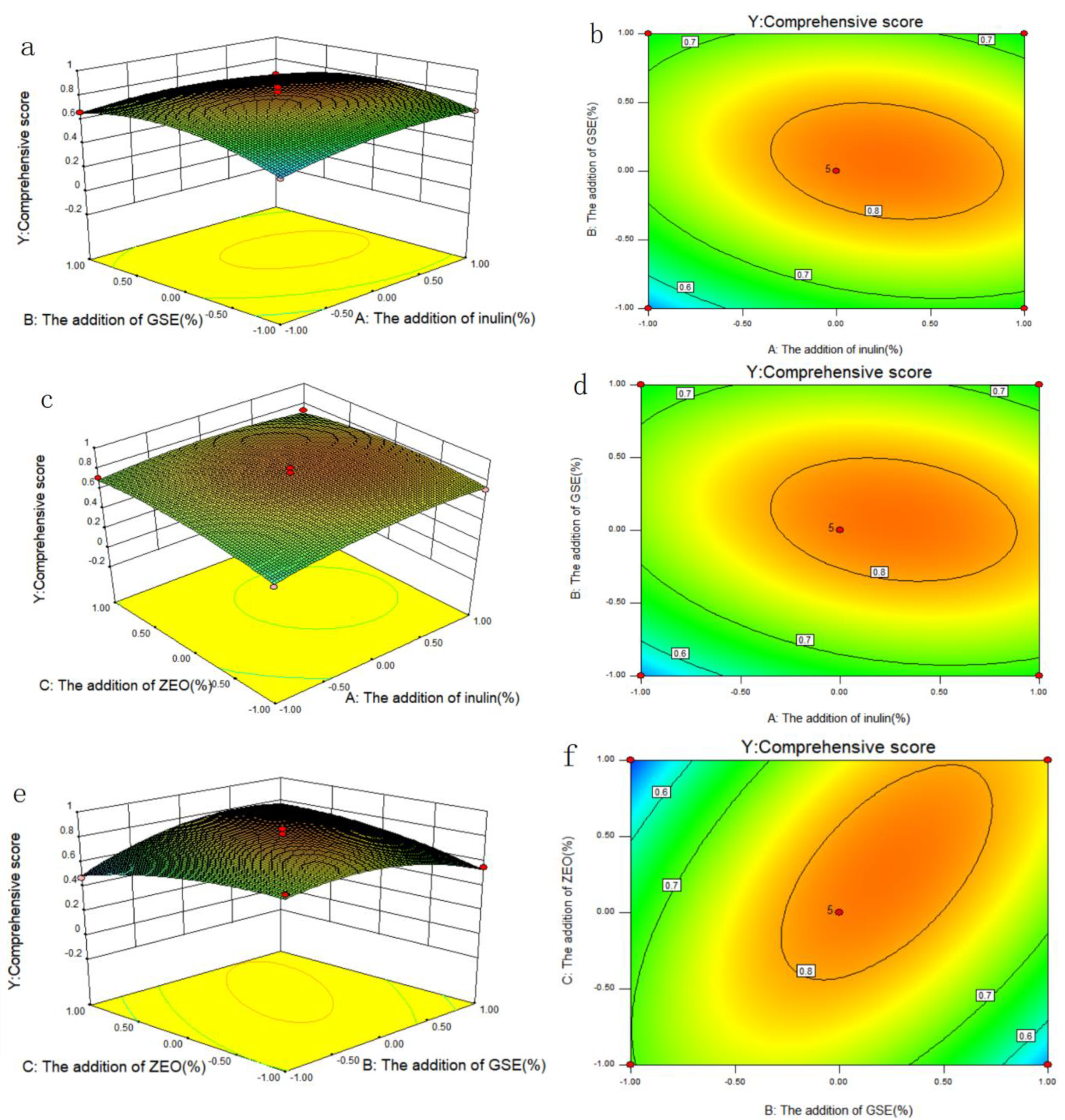
2.2.2. Results of Box-Behnken Design
2.3. Results of Verify Test
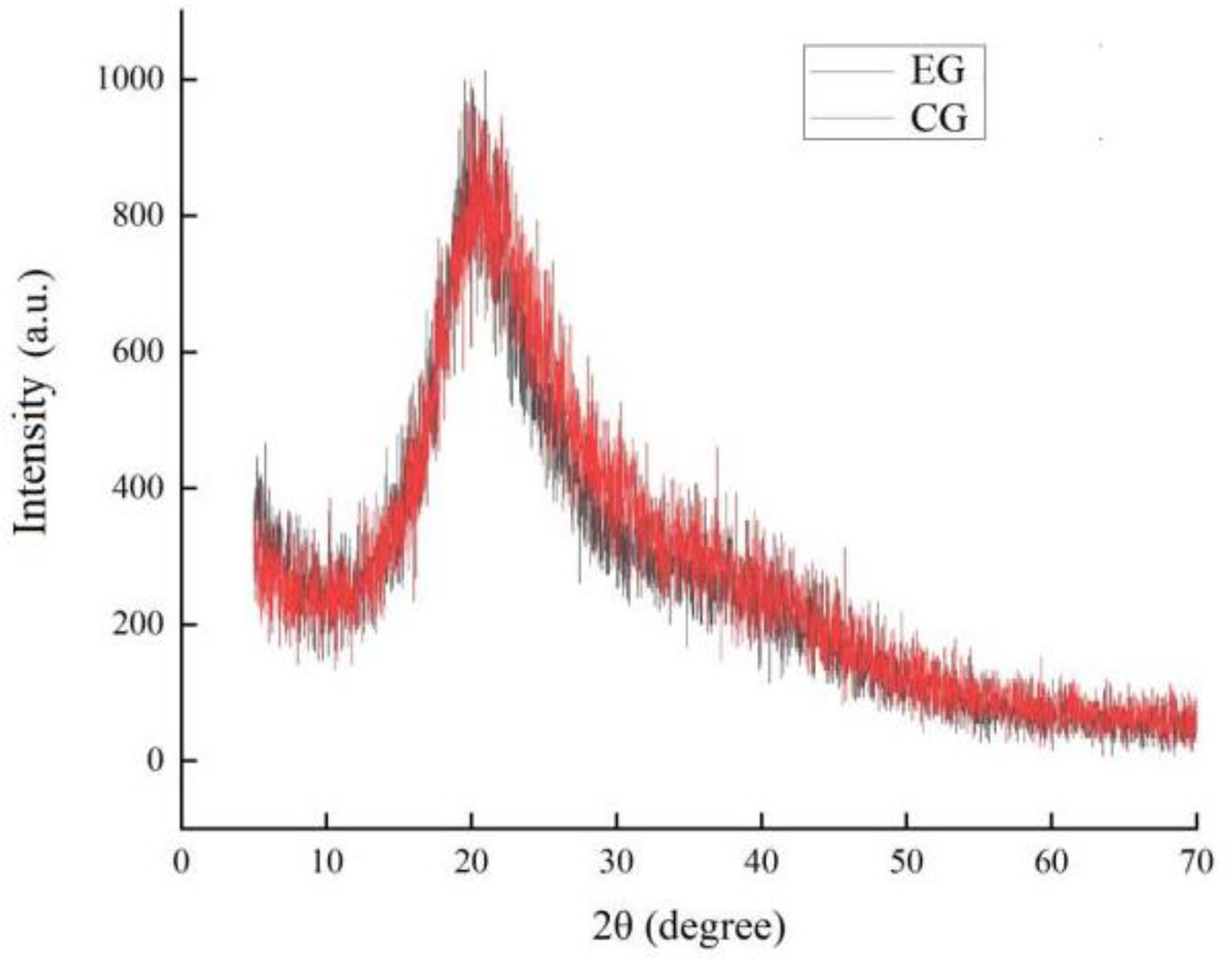
2.4. Results of X-ray Diffraction (XRD)
2.5. Analysis of Scanning Electron Microscopy
2.6. Physical and Chemical Quality Changes in Grass Carp during Storage
2.6.1. Change in Water Holding Capacity
2.6.2. Change in pH Value
2.6.3. Change in TVB-N
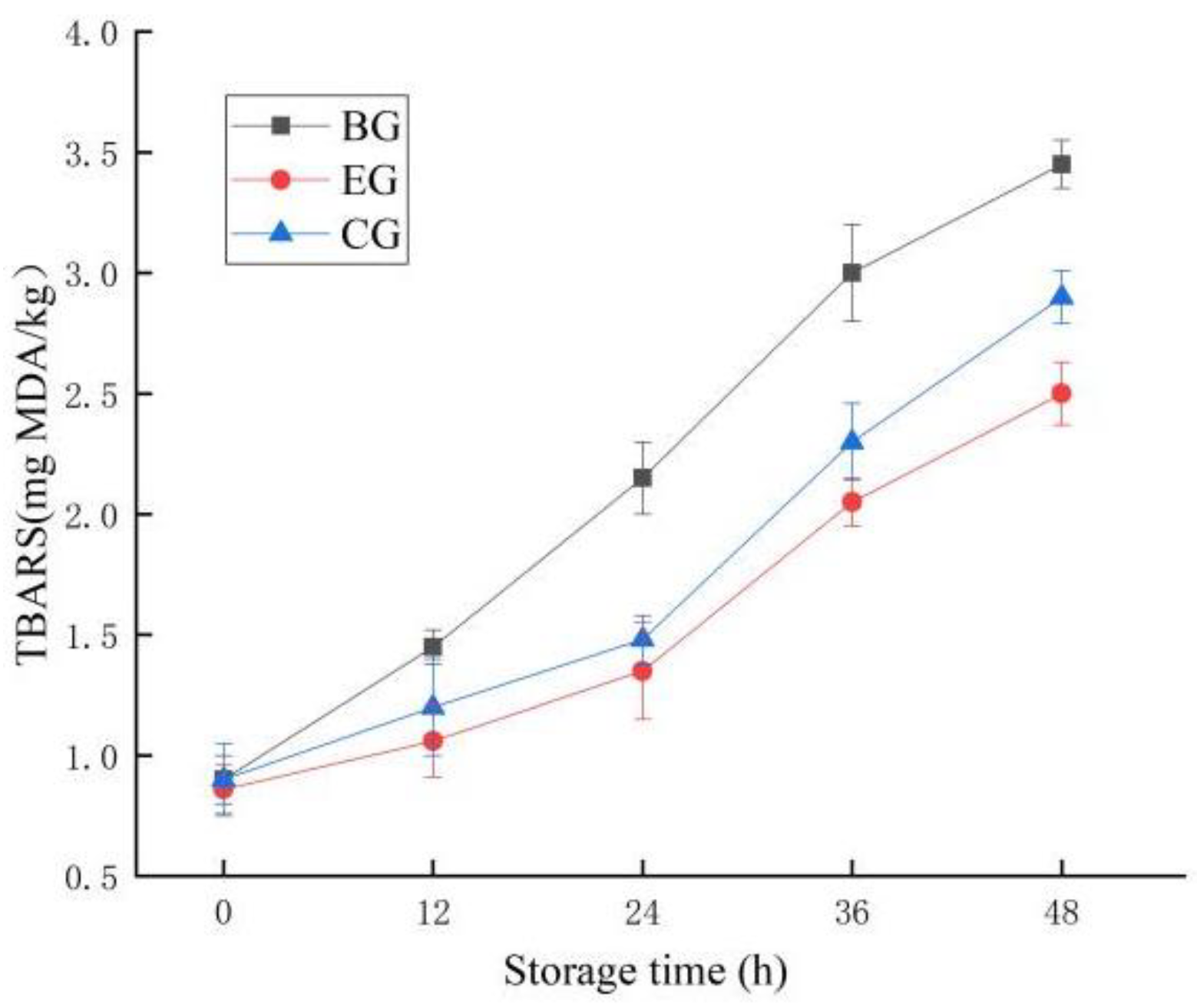
2.6.4. Change in TBARS
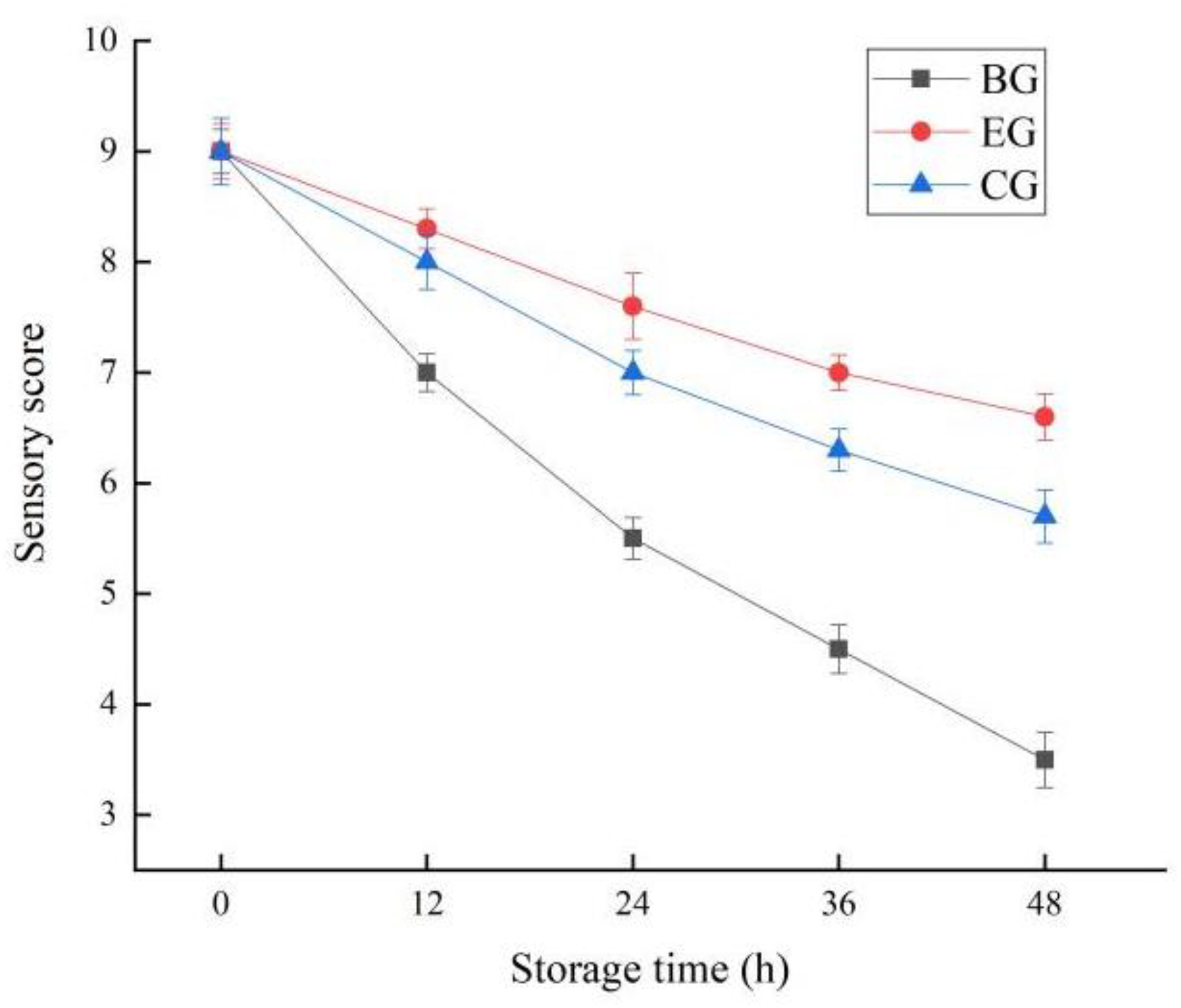
2.6.5. Change in Sensory Score
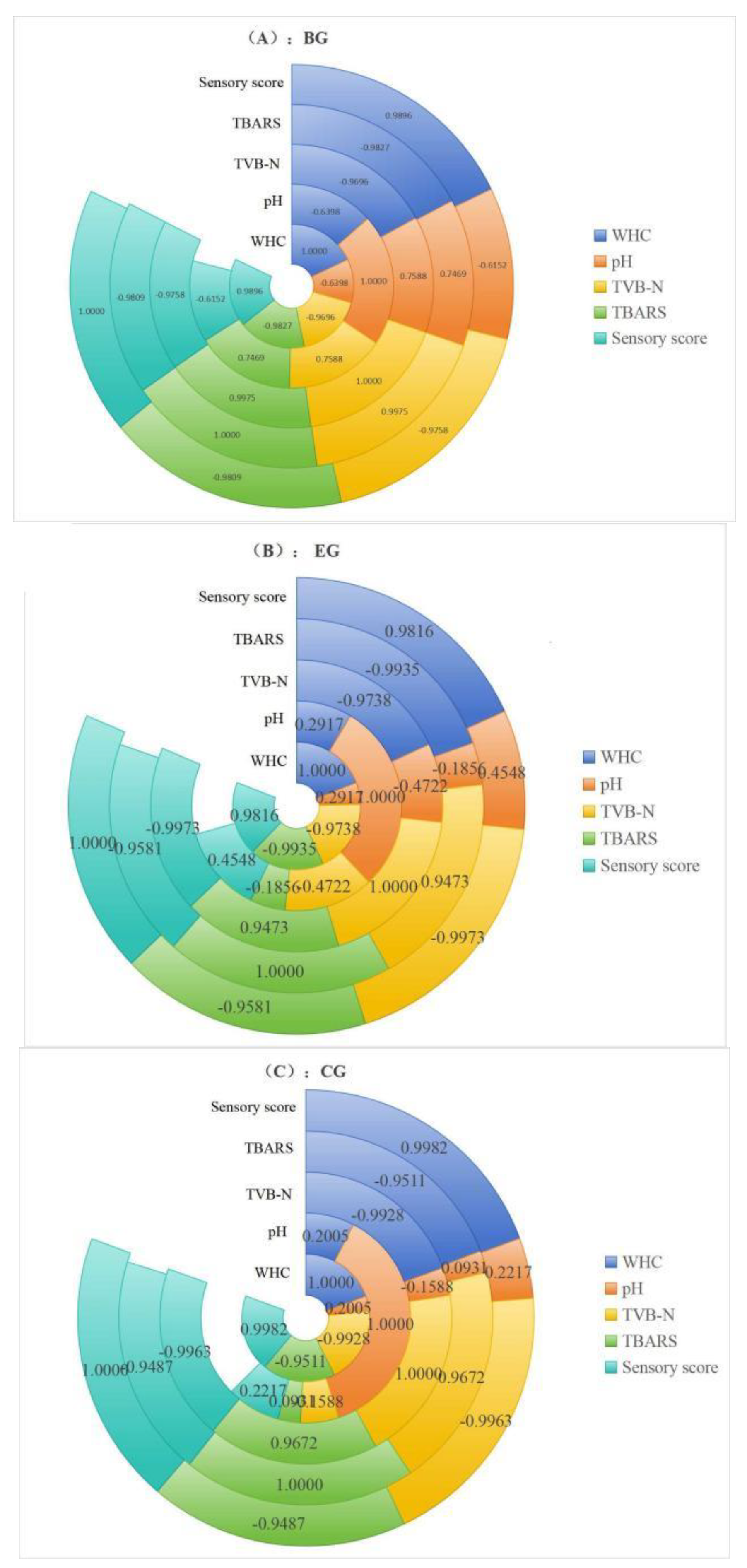
2.6.6. Correlation Analysis of the Change in Each Index
3. Conclusions
4. Materials and Methods
4.1. Material
4.2. Preparation of ZEO-Complex Polysaccharide Gels
4.3. Single Factor Experiment
4.4. Characterization of ZEO-Complex Polysaccharide
4.4.1. Thickness
4.4.2. Water Vapor Permeability (WVP)
4.4.3. Mechanical Properties
4.4.4. X-ray Diffraction (XRD) Analysis of ZEO-Complex Polysaccharide Gels
4.4.5. Scanning Electron Microscope (SEM)
4.4.6. Calculation of Comprehensive Score of ZEO-Complex Polysaccharide Gels
4.5. Four and Three-Dimensional Analysis by MATLAB and Box-Behnken Design
4.6. The Application of the ZEO-Complex Polysaccharide Gels on the Preservation of Grass Carp
4.6.1. Storage Experiment
4.6.2. Change in Water Holding Capacity (WHC)
4.6.3. pH Value
4.6.4. TVB-N Value
4.6.5. Thiobarbituric Acid Reactive Substance (TBARS) Analysis
4.6.6. Sensory Evaluation
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sundqvist-Andberg, H.; Åkerman, M. Sustainability governance and contested plastic food packaging—An integrative review. J. Clean. Prod. 2021, 306, e127111. [Google Scholar] [CrossRef]
- Ureña, M.; Fournier, P.; Phùng, T.T.-T.; Lagorce, A.; Karbowiak, T. Potential of polysaccharides for food packaging applications. Part 2/2: An experimental review of the effect of aging conditions on the functional properties of polysaccharide films. Food Hydrocoll. 2023, 144, e108954. [Google Scholar] [CrossRef]
- Yang, Y.C.; Lin, H.S.; Chen, H.X.; Wang, P.K.; Zheng, B.D.; Huang, Y.Y.; Zhang, N.; Zhang, X.Q.; Ye, J.; Xiao, M.T. Plant polysaccharide-derived edible film packaging for instant food: Rapid dissolution in hot water coupled with exceptional mechanical and barrier characteristics. Int. J. Biol. Macromol. 2024, 270, e132066. [Google Scholar] [CrossRef]
- Long, J.Y.; Zhang, W.Y.; Zhao, M.Z.; Ruan, C.Q. The reduce of water vapor permeability of polysaccharide-based films in food packaging: A comprehensive review. Carbohyd. Polym. 2023, 321, e121267. [Google Scholar] [CrossRef]
- Xue, S.; Gong, Z.T.; Lin, J.J.; Zheng, S.C. Optimization of extraction process of crude polysaccharide from chicory root of Cichorium intybus L. var. Foliosum Hegi, and analysis of antioxidant activity in vitro and relative molecular weight. Sci. Technol. Food Ind. 2021, 42, 138–145. (In Chinese) [Google Scholar]
- Wan, X.H.; Guo, H.; Liang, Y.Y.; Zhou, C.Z.; Liu, Z.H.; Li, K.W.; Niu, F.J.; Zhai, X.; Wang, L.Z. The physiological functions and pharmaceutical applications of inulin: A review. Carbohyd. Polym. 2020, 246, e116589. [Google Scholar]
- Shoaib, M.; Shehzad, A.; Omar, A.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohyd. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef]
- Shi, S.; Wu, X.D.; Wang, Y.; Li, W.X.; Zhang, H.; Lou, X.J.; Xia, X.F.; Liang, W.W. Sodium-alginate-based indicator film containing a hydrophobic nanosilica layer for monitoring fish freshness. Int. J. Biol. Macromol. 2024, 265, e130714. [Google Scholar] [CrossRef]
- Rashid, A.; Qayum, A.; Bacha, S.A.S.; Liang, Q.F.; Liu, Y.X.; Kang, L.X.; Chi, Z.Z.; Chi, R.H.; Han, X.; Ekumah, J.N.; et al. Novel pullulan-sodium alginate film incorporated with anthocyanin-loaded casein-carboxy methyl cellulose nanocomplex for real-time fish and shrimp freshness monitoring. Food Hydrocoll. 2024, 156, e110356. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohyd. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef]
- Trinetta, V.; Morgan, M.T.; Coupland, J.N.; Yucel, U. Essential oils against pathogen and spoilage microorganisms of fruit juices: Use of versatile antimicrobial delivery systems. J. Food Sci. 2017, 82, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Bi, F.; Zhang, X.; Bai, R.; Liu, Y.P.; Liu, J.; Liu, J. Preparation and characterization of antioxidant and antimicrobial packaging films based on chitosan and proanthocyanidins. Int. J. Biol. Macromol. 2019, 134, 11–19. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Tian, W.K.; Qin, X.L.; Wang, H.; Tan, L.L.; Liu, X. Chitosan/oxidized konjac glucomannan films incorporated with zanthoxylum bungeanum essential oil: A novel approach for extending the shelf life of meat. Int. J. Biol. Macromol. 2024, 262, e129683. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sui, J.; Yu, B.; Yuan, C.; Guo, L.; Abd El-Aty, A.M.; Cui, B. Physicochemical properties and antibacterial activity of corn starch-based films incorporated with Zanthoxylum bungeanum essential oil. Carbohyd. Polym. 2021, 254, e117314. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.X.; Zhang, D.; Zhao, L.; Shi, B.L.; Xiao, J.B.; Liu, X.H.; Marcillinus, Z.; Hu, Y.Q.; Auriol, N.; Shi, J.Y.; et al. Antagonistic interaction of phenols and alkaloids in Sichuan pepper (Zanthoxylum bungeanum) pericarp. Ind. Crops Prod. 2020, 152, 112551. (In Chinese) [Google Scholar] [CrossRef]
- Li, Q.; Chen, Z.H.; Zeng, L.X.; Bi, Y.G.; Kong, F.S.; Wang, Z.; Tan, S.F. Characterization, in-vitro digestion, antioxidant, anti-hyperlipidemic and antibacterial activities of Zanthoxylum bungeanum Maxim essential oil nano-emulsion. Food Biosci. 2023, 56, e103082. [Google Scholar] [CrossRef]
- Nowshehri, A.J.; Bhat, A.Z.; Shah, Y.M. Blessings in disguise: Bio-functional benefits of grape seed extracts. Food Res. Int. 2015, 77, 333–348. [Google Scholar] [CrossRef]
- Bosso, A.; Guaita, M.; Petrozziello, M. Influence of solvents on the composition of condensed tannins in grape pomace seed extracts. Food Chem. 2016, 207, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, G.; Singh, J. Study of coating effectiveness of grape fruit seed extract incorporated Chitosan/cornstarch based active packaging film on grapes. Food Chem. Adv. 2024, 4, e100651. [Google Scholar] [CrossRef]
- Zheng, M.X.; Zhu, Y.J.; Zhuang, Y.H.; Tan, K.B.; Chen, J.F. Effects of grape seed extract on the properties of pullulan polysaccharide/xanthan gum active films for apple preservation. Int. J. Biol. Macromol. 2023, 241, e124617. [Google Scholar] [CrossRef]
- Zhang, U.Q.; Li, J.; Huang, X.J.; Yang, C.X.; Wu, C.; Yang, Z.L.; Li, D.Q. Performance-enhanced regenerated cellulose film by adding grape seed extract. Int. J. Biol. Macromol. 2023, 232, e123290. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, Y.; Zhang, J.Y.; Zhang, J.Y. Review of the Effect of Inulin on the Thermal Properties and Digestibilityof Starch. Modern Food Sci. Technol. 2025, 41, 1–10. (In Chinese) [Google Scholar]
- Jiang, Y.X.; Fan, F.Y.; Yang, X.L.; Sun, Y.X. Effect of grape seed extract/tea polyphenols on performance of sodium alginate nanocomposite films. Food Ferment. Ind. 2024, 50, 108–115. (In Chinese) [Google Scholar]
- Jiang, G.Y.; Hou, X.Y.; Zeng, X.D.; Zhang, C.; Wu, H.J.; Shen, G.H.; Li, S.S.; Luo, Q.Y.; Li, M.L.; Liu, X.Y.; et al. Preparation and characterization of indicator films from carboxymethyl-cellulose/starch and purple sweet potato (Ipomoea batatas (L.) lam) anthocyanins for monitoring fish freshness. Int. J. Biol. Macromol. 2020, 143, 359–372. [Google Scholar] [CrossRef]
- Zhao, M.N.; Nuerjiang, M.; Bai, X.; Feng, J.; Kong, B.H.; Sun, F.D.; Li, Y.; Xia, X.F. Monitoring dynamic changes in chicken freshness at 4 °C and 25 °C using pH-sensitive intelligent films based on sodium alginate and purple sweet potato peel extracts. Int. J. Biol. Macromol. 2022, 216, 361–373. [Google Scholar] [CrossRef]
- Jalalvand, A.; Roushani, M.; Goicoechea, H.; Rutledge, D.N.; Gu, H.W. MATLAB in electrochemistry: Areview. Talanta 2019, 194, 205–225. [Google Scholar] [CrossRef] [PubMed]
- Horsholt, S.; Nick, H.M.; Jorgensen, I.B. Oil production optimization of black-oil Models by integration of Matlab and Eclipse E300. IFAC-PapersOnLine 2018, 51, 88–93. [Google Scholar] [CrossRef]
- Xue, S.; Huang, Y.T.; Li, D.D. Optimization of extraction process of pumpkin seed oil with high linoleic acid content based on box-behnken-matlab analysis. J. Chin. Cereals Oils Assoc. 2022, 37, 102–109. [Google Scholar]
- Xue, S.; Xiao, X.; Chen, S.Y.; Liu, B.H.; Lan, G.J. Dual response surface method combined with matlab tooptimize extraction process of grape seed polyphenols and the evaluation on its hydroxylradical scavenging rate. Sci. Technol. Food Ind. 2020, 41, 160–167. (In Chinese) [Google Scholar]
- Xue, S.; Xiao, X.; Liu, B.H.; Chen, S.Y.; Lan, G.J. Extraction technique of pineapple peel residue polyphenols with high antioxidant activity by dual response surface method combined with matlab analysis. Storage Process 2020, 20, 63–71. (In Chinese) [Google Scholar]
- Bilgiç-Keleş, S.; Şahin-Yeşilçubuk, N.; Barla-Demirkoz, A.; Karakaş, M. Response surface optimization and modelling for supercritical carbon dioxide extraction of Echium vulgare seed oil. J. Supercrit. Fluids 2019, 143, 365–369. [Google Scholar] [CrossRef]
- Sreedharan, A.; Ong, S.T. Combination of Plackett Burman and response surface methodology experimental design to optimize Malachite Green dye emoval from aqueous environment. Chem. Data Collect. 2020, 25, e100317. [Google Scholar] [CrossRef]
- Almasi, H.; Azizi, S.; Amjadi, S. Development and characterization of pectin films activated by nanoemulsion and Pickering emulsion stabilized marjoram (Origanum majorana L.) essential oil. Food Hydrocoll. 2020, 99, e105338. [Google Scholar] [CrossRef]
- Guo, Z.L.; Ge, X.Z.; Li, W.Z.; Yang, L.H.; Han, L.; Yu, Q.L. Active-intelligent film based on pectin from watermelon peel containing beetroot extract to monitor the freshness of packaged chilled beef. Food Hydrocoll. 2021, 119, e106751. [Google Scholar] [CrossRef]
- Omar-Aziz, M.; Khodaiyan, F.; Yarmand, M.S.; Mousavi, M.; Gharaghani, M.; Kennedy, J.; Hosseini, S.S. Combined effects of octenylsuccination and beeswax on pullulan films: Water-resistant and mechanical properties. Carbohyd. Polym. 2021, 255, e117471. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Pobiega, K.; Gniewosz, M. Pullulan–biopolymer with potential for use as food packaging. Int. J. Food Eng. 2019, 15, e20190030. [Google Scholar] [CrossRef]
- Nychas, G.-J.E.; Skandamis, P.N.; Tassou, C.C.; Koutsoumanis, K.P. Meat spoilage during distribution. Meat Sci. 2008, 78, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.A.; Sumon, T.A.; Mazumder, S.K.; Ali, M.M.; Jang, W.J.; Abualreesh, M.H.; Sharifuzzaman, S.M.; Brown, C.L.; Lee, H.T.; Lee, E.W.; et al. Essential oils and chitosan as alternatives to chemical preservatives for fish and fisheries products: A review. Food Control 2021, 129, e108244. [Google Scholar] [CrossRef]
- Yousuf, B.; Wu, S.M.; Gao, Y. Characteristics of karaya gum based films: Amelioration by inclusion of Schisandra chinensis oil and its oleogel in the film formulation. Food Chem. 2021, 345, e128859. [Google Scholar] [CrossRef]
- da Silva, B.D.; do Rosário, D.K.A.; Weitz, D.A.; Conte-Junior, C.A. Essential oil nanoemulsions: Properties, development, and application in meat and meat products. Trends Food Sci. Technol. 2022, 121, 1–13. [Google Scholar] [CrossRef]
- Li, J.X.; Zhao, Q.C.; Song, G.L.; Su, X.R.; Liu, S.C.; Ma, Y.S. Research Progress on the Application And Migration Characteristics of Plant Essential Oils in Antibacterial Active Packaging: A review. Sci. Technol. Food Ind. 2024. (In Chinese) [Google Scholar] [CrossRef]
- Gasti, T.; Dixit, S.; Hiremani, V.D.; Chougale, R.B.; Masti, S.P.; Vootla, S.K.; Mudigoudra, B.S. Chitosan/pullulan based films incorporated with clove essential oil loaded chitosan-ZnO hybrid nanoparticles for active food packaging. Carbohyd. Polym. 2022, 277, e118866. [Google Scholar] [CrossRef]
- Fasihi, H.; Fazilati, M.; Hashemi, M.; Noshirvani, N. Novel carboxymethyl incorporation method. Carbohyd. Polym. 2017, 167, 79–89. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, X.Z.; Xie, F.; Fan, Y.; Xu, X.L.; Qi, J.; Xiong, G.Y.; Gao, X.L.; Zhang, F. pH-responsive double-layer indicator films based on konjac glucomannan/camellia oil and carrageenan/anthocyanin/curcumin for monitoring meat freshness. Food Hydrocoll. 2021, 118, e106695. [Google Scholar] [CrossRef]
- Kumar, R.; Ghoshal, G.; Goyal, M. Moth Bean Starch (Vigna aconitifolia): Isolation, characterization, and development of edible/biodegradable films. J. Food Sci. Technol. 2019, 56, 4891–4900. [Google Scholar] [CrossRef]
- Jiang, G.Y.; Hou, X.Y.; Ren, W.; Wu, H.J.; Li, S.S.; Shen, G.H.; Chen, A.Y.; Wang, Z.Y.; Zhang, Z.Q. Preparation of indicator films based on sodium carboxymethyl cellulose/starch and purple sweet potato anthocyanins for monitoring fish freshness. Food Sci. 2020, 41, 250–258. (In Chinese) [Google Scholar]
- Xue, S.; Huang, Q. Preparation of Novel Flaxseed Oil/Beeswax Oleogel Systems and Its Application in the Improvement of Sodium Alginate Films. Gels 2024, 10, 78. [Google Scholar] [CrossRef]
- Xue, S.; He, L. Optimization of adding polysaccharides from chicory root based on fuzzy mathematics to improve physicochemical properties of silver carp surimi balls during storage. J. Food Process. Preserv. 2021, 45, e15307. [Google Scholar] [CrossRef]
- Yi, S.; Li, J.; Zhu, J.; Lin, Y.; Fu, L.; Chen, W.; Li, X. Effect of tea polyphenols on microbiological and biochemical quality of Collichthys fish ball. J. Sci. Food Agric. 2011, 91, 1591–1597. [Google Scholar] [CrossRef] [PubMed]






| Test | The Addition of Inulin (A) | The addition of GSE (B) | The Addition of ZEO (C) | WVP (10−5·g·cm/KPa·h·cm2) | TS (MPa) | EAB (%) | Comprehensive Score (Y) |
|---|---|---|---|---|---|---|---|
| 1 | 1 (1%) | 0 (0.04%) | 1 (2.5%) | 1.39 | 3.00 | 68.75 | 0.7335 |
| 2 | 0 (0.8%) | 0 | 0 (2%) | 1.15 | 4.50 | 79.60 | 0.8642 |
| 3 | 1 | 0 | −1 (1.5%) | 1.43 | 2.73 | 68.62 | 0.7186 |
| 4 | 1 | −1 (0.03%) | 0 | 1.45 | 1.41 | 100.00 | 0.6657 |
| 5 | 0 | 0 | 0 | 1.21 | 2.86 | 39.06 | 0.7862 |
| 6 | 0 | 1 (0.05%) | −1 | 1.59 | 2.34 | 45.79 | 0.5526 |
| 7 | −1 (0.6%) | 1 | 0 | 1.47 | 2.63 | 66.24 | 0.6623 |
| 8 | 1 | 1 | 0 | 1.52 | 2.16 | 64.37 | 0.6613 |
| 9 | −1 | 0 | −1 | 1.54 | 1.35 | 59.78 | 0.5931 |
| 10 | 0 | −1 | −1 | 1.41 | 2.15 | 93.50 | 0.7254 |
| 11 | −1 | 0 | 1 | 1.43 | 2.73 | 68.62 | 0.7186 |
| 12 | 0 | 0 | 0 | 1.16 | 4.12 | 77.25 | 0.8221 |
| 13 | −1 | −1 | 0 | 1.55 | 1.43 | 58.06 | 0.5273 |
| 14 | 0 | 1 | 1 | 1.38 | 3.15 | 68.23 | 0.7421 |
| 15 | 0 | −1 | 1 | 1.60 | 1.54 | 73.52 | 0.4741 |
| 16 | 0 | 0 | 0 | 1.17 | 3.80 | 78.78 | 0.8145 |
| 17 | 0 | 0 | 0 | 1.18 | 3.50 | 81.55 | 0.7941 |
| Source | Sum of squares | Mean square | F Value | p Value | |||
| Model | 0.18 | 0.02 | 15.95 | 0.0007 | ** | ||
| A | 0.009647 | 0.009647 | 7.5 | 0.0289 | |||
| B | 0.006373 | 0.006373 | 4.96 | 0.0613 | |||
| C | 0.0007722 | 0.0007722 | 0.6 | 0.4637 | |||
| AB | 0.004858 | 0.004858 | 3.78 | 0.093 | |||
| AC | 0.003058 | 0.003058 | 2.38 | 0.1669 | |||
| BC | 0.049 | 0.049 | 37.79 | 0.0005 | ** | ||
| A2 | 0.015 | 0.015 | 11.73 | 0.0111 | ** | ||
| B2 | 0.068 | 0.068 | 53.02 | 0.0002 | ** | ||
| C2 | 0.018 | 0.018 | 14.02 | 0.0072 | ** | ||
| Residual | 0.008998 | 0.001285 | |||||
| Lack of Fit | 0.005268 | 0.001756 | 1.88 | 0.2735 | not significant | ||
| Pure Error | 0.00373 | 0.0009325 | |||||
| Cor Total | 0.19 |
| Score | Odor | Color | Tissue | Elasticity |
|---|---|---|---|---|
| 9–10 | Fish smell was rich, no odor | White | Close section, clear texture | Rapid recovery after compression |
| 7–8 | Fish smell, no odor | Yellowish white | Partial close and clear | Slow recovery after pressing |
| 5–6 | Fish smell was light, slight odor | Yellowish | Not tight, but not loose | Recovery after compression is slow |
| 3–4 | Fishy and unpleasant smell | Grayish yellow | Soft section, partial loose | It is difficult to recover after pressing |
| 1–2 | Very fishy and smelly | Gray | Oar-shaped and loose | It will not recover after compression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, S.; Li, C.; Xiong, Z. Preparation of Complex Polysaccharide Gels with Zanthoxylum bungeanum Essential Oil and Their Application in Fish Preservation. Gels 2024, 10, 533. https://doi.org/10.3390/gels10080533
Xue S, Li C, Xiong Z. Preparation of Complex Polysaccharide Gels with Zanthoxylum bungeanum Essential Oil and Their Application in Fish Preservation. Gels. 2024; 10(8):533. https://doi.org/10.3390/gels10080533
Chicago/Turabian StyleXue, Shan, Chao Li, and Zhouyi Xiong. 2024. "Preparation of Complex Polysaccharide Gels with Zanthoxylum bungeanum Essential Oil and Their Application in Fish Preservation" Gels 10, no. 8: 533. https://doi.org/10.3390/gels10080533





