Applying Different Conditions in the OphthalMimic Device Using Polymeric and Hydrogel-Based Hybrid Membranes to Evaluate Gels and Nanostructured Ophthalmic Formulations
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Material
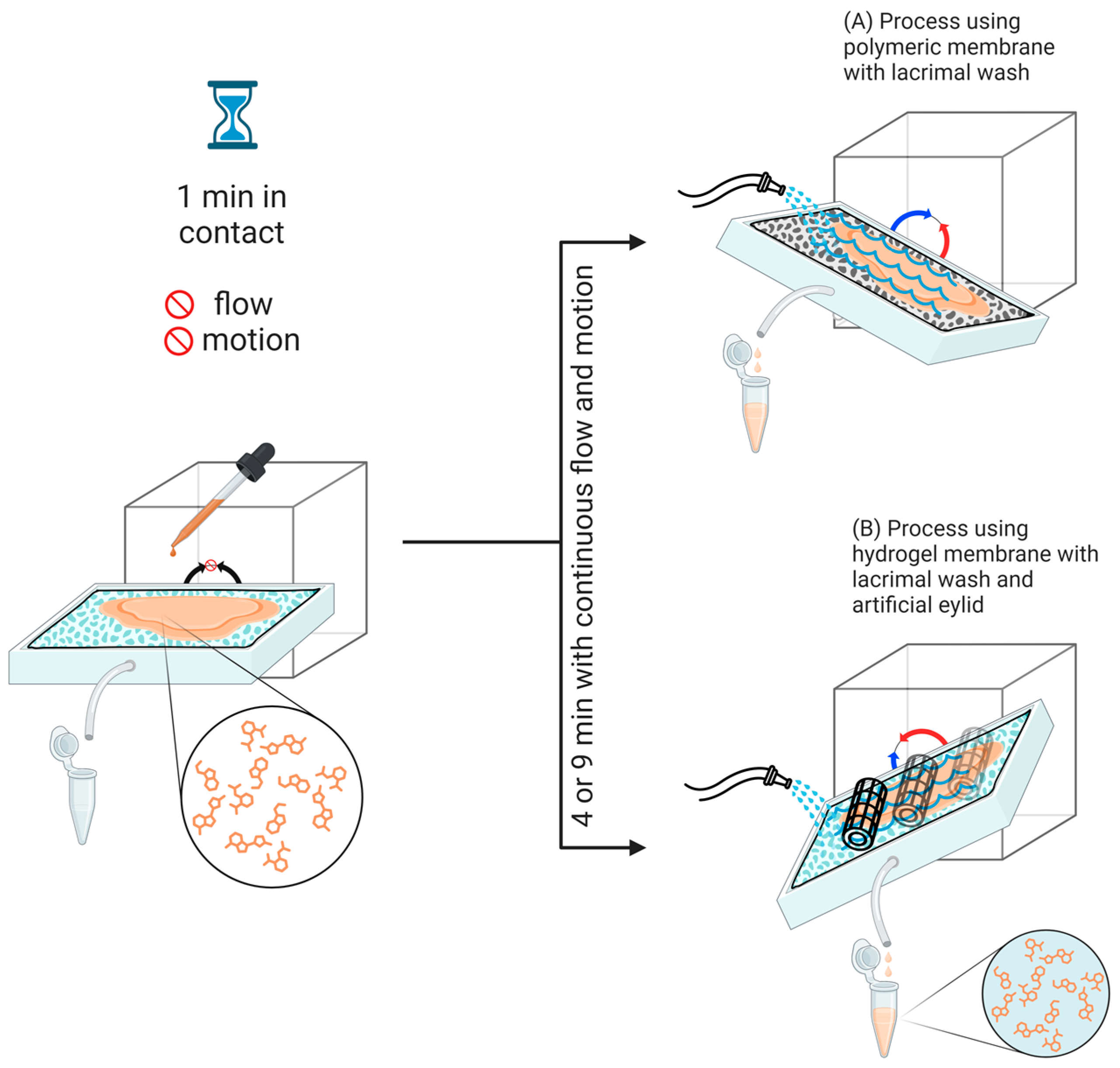
4.2. Preparation of Polymeric and Hydrogel-Based Hybrid Membranes
4.3. Formulation Preparation and Drug Assay
4.4. Varying Test Conditions in OphthalMimic Device
4.5. Statistical Analyses
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bu, H.; Gukasyan, H.; Goulet, L.; Lou, X.; Xiang, C.; Koudriakova, T. Ocular disposition, pharmacokinetics, efficacy and safety of nanoparticle-formulated ophthalmic drugs. Curr. Drug Metab. 2007, 8, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Mofidfar, M.; Abdi, B.; Ahadian, S.; Mostafavi, E.; Desai, T.; Abbasi, F.; Sun, Y.; Manche, E.; Ta, C.; Flowers, C. Drug delivery to the anterior segment of the eye: A review of current and future treatment strategies. Int. J. Pharm. 2021, 607, 120924. [Google Scholar] [CrossRef] [PubMed]
- Gratieri, T.; Gelfuso, G.M.; de Freitas, O.; Rocha, E.M.; Lopez, R.F. Enhancing and sustaining the topical ocular delivery of fluconazole using chitosan solution and poloxamer/chitosan in situ forming gel. Eur. J. Pharm. Biopharm. 2011, 79, 320–327. [Google Scholar] [CrossRef]
- Morrison, P.W.; Khutoryanskiy, V.V. Advances in Ophthalmic Drug Delivery. Ther. Deliv. 2014, 5, 1297–1315. [Google Scholar] [CrossRef]
- Agarwal, P.; Scherer, D.; Günther, B.; Rupenthal, I.D. Semifluorinated alkane based systems for enhanced corneal penetration of poorly soluble drugs. Int. J. Pharm. 2018, 538, 119–129. [Google Scholar] [CrossRef]
- Agarwal, P.; Craig, J.P.; Krösser, S.; Eickhoff, K.; Swift, S.; Rupenthal, I.D. Topical semifluorinated alkane-based azithromycin suspension for the management of ocular infections. Eur. J. Pharm. Biopharm. 2019, 142, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Tan, K.; Akbar, D.; Choulakian, M.Y.; Tran, S.D. A New Era in Ocular Therapeutics: Advanced Drug Delivery Systems for Uveitis and Neuro-Ophthalmologic Conditions. Pharmaceutics 2023, 15, 1952. [Google Scholar] [CrossRef]
- Wróblewska, K.; Jadach, B.; Muszalska-Kolos, I. Progress in drug formulation design and delivery of medicinal substances used in ophthalmology. Int. J. Pharm. 2021, 607, 121012. [Google Scholar] [CrossRef]
- Brown, G.; Brown, M.; Chaudhry, I.; Stein, J. Opportunities to Reduce Potential Bias in Ophthalmic Cost-Utility Analysis. JAMA Ophthalmol. 2021, 139, 389–397. [Google Scholar] [CrossRef]
- Robinson, N.B.; Krieger, K.; Khan, F.M.; Huffman, W.; Chang, M.; Naik, A.; Yongle, R.; Hameed, I.; Krieger, K.; Girardi, L.N.; et al. The current state of animal models in research: A review. Int. J. Surg. 2019, 72, 9–13. [Google Scholar] [CrossRef]
- Barbalho, G.N.; Falcão, M.A.; Alves Amaral, V.; Contarato, J.L.A.; Barbalho, A.M.; Kaori Diógenes, G.; Mariana Gomes Silva, M.; Carvalho de Barros do Vale Rochelle, B.; Gelfuso, G.M.; Cunha-Filho, M.; et al. OphthalMimic: A new alternative apparatus without animal tissue for the evaluation of topical ophthalmic drug products. Methods 2024, 228, 1–11. [Google Scholar] [CrossRef]
- Davanço, M.G.; Campos, D.R.; Carvalho, P.O. In vitro-In vivo correlation in the development of oral drug formulation: A screenshot of the last two decades. Int. J. Pharm. 2020, 580, 119210. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2016, 66, 159–182. [Google Scholar] [CrossRef]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef]
- Sun, X.; Yu, Z.; Cai, Z.; Yu, L.; Lv, Y. Voriconazole Composited Polyvinyl Alcohol/Hydroxypropyl-β-Cyclodextrin Nanofibers for Ophthalmic Delivery. PLoS ONE 2016, 11, e0167961. [Google Scholar] [CrossRef] [PubMed]
- Åhlén, M.; Tummala, G.K.; Mihranyan, A. Nanoparticle-loaded hydrogels as a pathway for enzyme-triggered drug release in ophthalmic applications. Int. J. Pharm. 2018, 536, 73–81. [Google Scholar] [CrossRef]
- Tummala, G.K.; Lopes, V.R.; Mihranyan, A.; Ferraz, N. Biocompatibility of Nanocellulose-Reinforced PVA Hydrogel with Human Corneal Epithelial Cells for Ophthalmic Applications. J. Funct. Biomater. 2019, 10, 35. [Google Scholar] [CrossRef]
- Patil, M.; Mathad, S.; Patil, A.; Arshad, M.; Alorfi, H.; Puttegowda, M.; Asiri, A.; Khan, A.; Azum, N. Synthesis and Characterization of Microwave-Assisted Copolymer Membranes of Poly(vinyl alcohol)-g-starch-methacrylate and Their Evaluation for Gas Transport Properties. Polymers 2022, 14, 350. [Google Scholar] [CrossRef]
- Takei, T.; Yoshihara, R.; Danjo, S.; Fukuhara, Y.; Evans, C.; Tomimatsu, R.; Ozuno, Y.; Yoshida, M. Hydrophobically-modified gelatin hydrogel as a carrier for charged hydrophilic drugs and hydrophobic drugs. Int. J. Biol. Macromol. 2020, 149, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Burgalassi, S.; Zucchetti, E.; Ling, L.; Chetoni, P.; Tampucci, S.; Monti, D. Hydrogels as Corneal Stroma Substitutes for In Vitro Evaluation of Drug Ocular Permeation. Pharmaceutics 2022, 14, 850. [Google Scholar] [CrossRef]
- Barbalho, G.N.; Falcão, M.A.; Amaral, V.A.; Contarato, J.L.; Gelfuso, G.M.; Cunha-Filho, M.; Gratieri, T. Hydrogel-based hybrid membrane enhances in vitro ophthalmic drug evaluation in the OphthalMimic device. Methods 2024, 230, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Mantelli, F.; Argüeso, P. Functions of ocular surface mucins in health and disease. Curr. Opin. Allergy Clin. Immunol. 2008, 5, 477–483. [Google Scholar] [CrossRef]
- Moon, I.; Kang, H.; Yeo, A.; Noh, H.; Kim, H.; Song, J.; Ji, Y.; Lee, H. Comparison of Ocular Surface Mucin Expression After Topical Ophthalmic Drug Administration in Dry Eye-Induced Mouse Model. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2018, 34, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Seiler, T.; Trahms, L.; Wollensak, J. The distinction of corneal water in free and bound fractions. Graefes Arch. Clin. Exp. Ophthalmol. 2005, 221, 244. [Google Scholar] [CrossRef]
- Singh, M.; Bharadwaj, S.; Lee, K.E.; Kang, S.G. Therapeutic nanoemulsions in ophthalmic drug administration: Concept in formulations and characterization techniques for ocular drug delivery. J. Control Release 2020, 328, 895–916. [Google Scholar] [CrossRef] [PubMed]
- Perioli, L.; Ambrogi, V.; Venezia, L.; Pagano, C.; Ricci, M.; Róssi, C. Chitosan and a modified chitosan as agents to improve performances of mucoadhesive vaginal gels. Colloids Surf. B Biointerfaces 2008, 66, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Popa, L.; Ghica, M.; Popescu, R.; Irimia, T.; Dinu-Pîrvu, C. Development and Optimization of Chitosan-Hydroxypropyl Methylcellulose In Situ Gelling Systems for Ophthalmic Delivery of Bupivacaine Hydrochloride. Processes 2021, 9, 1694. [Google Scholar] [CrossRef]
- Dos Santos, G.A.; Ferreira-Nunes, R.; Dalmolin, L.F.; Dos Santos Ré, A.C.; Anjos, J.L.V.; Mendanha, S.A.; Aires, C.P.; Lopez, R.F.V.; Cunha-Filho, M.; Gelfuso, G.M.; et al. Besifloxacin liposomes with positively charged additives for an improved topical ocular delivery. Sci. Rep. 2020, 10, 19285. [Google Scholar] [CrossRef] [PubMed]
- Dhahir, R.K.; Al-Nima, A.M.; Al-Bazzaz, F.Y. Nanoemulsions as Ophthalmic Drug Delivery Systems. Turk. J. Pharm. Sci. 2021, 18, 652–664. [Google Scholar] [CrossRef]
- Gallarate, M.; Chirio, D.; Bussano, R.; Peira, E.; Battaglia, L.; Baratta, F.; Trotta, M. Development of O/W nanoemulsions for ophthalmic administration of timolol. Int. J. Pharm. 2013, 440, 126–134. [Google Scholar] [CrossRef]
- Cardoso, C.O.; Ferreira-Nunes, R.; Cunha-Filho, M.; Gratieri, T.; Gelfuso, G.M. In situ gelling microemulsion for topical ocular delivery of moxifloxacin and betamethasone. J. Mol. Liq. 2022, 360, 119559. [Google Scholar] [CrossRef]
- Gratieri, T.; Gelfuso, G.M.; Rocha, E.M.; Sarmento, V.H.; de Freitas, O.; Lopez, R.F.V. A poloxamer/chitosan in situ forming a gel with prolonged retention time for ocular delivery. Eur. J. Pharm. Biopharm. 2010, 75, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Rozier, A.; Mazuel, C.; Grove, J.; Plazonnet, B. Gelrite: A novel, ion-activated, in-situ gelling polymer for ophthalmic vehicles. Effect on bioavailability of timol. Int. J. Pharm. 1989, 57, 163–168. [Google Scholar] [CrossRef]

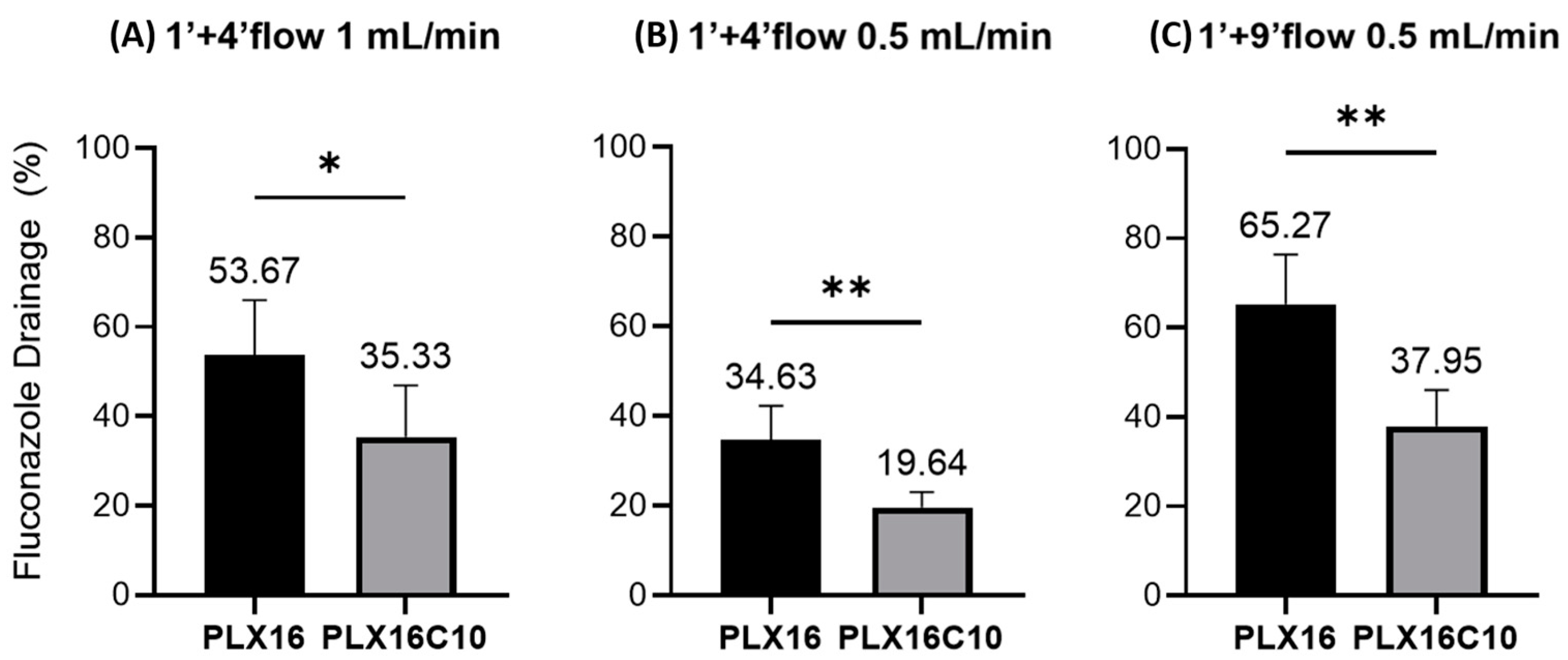
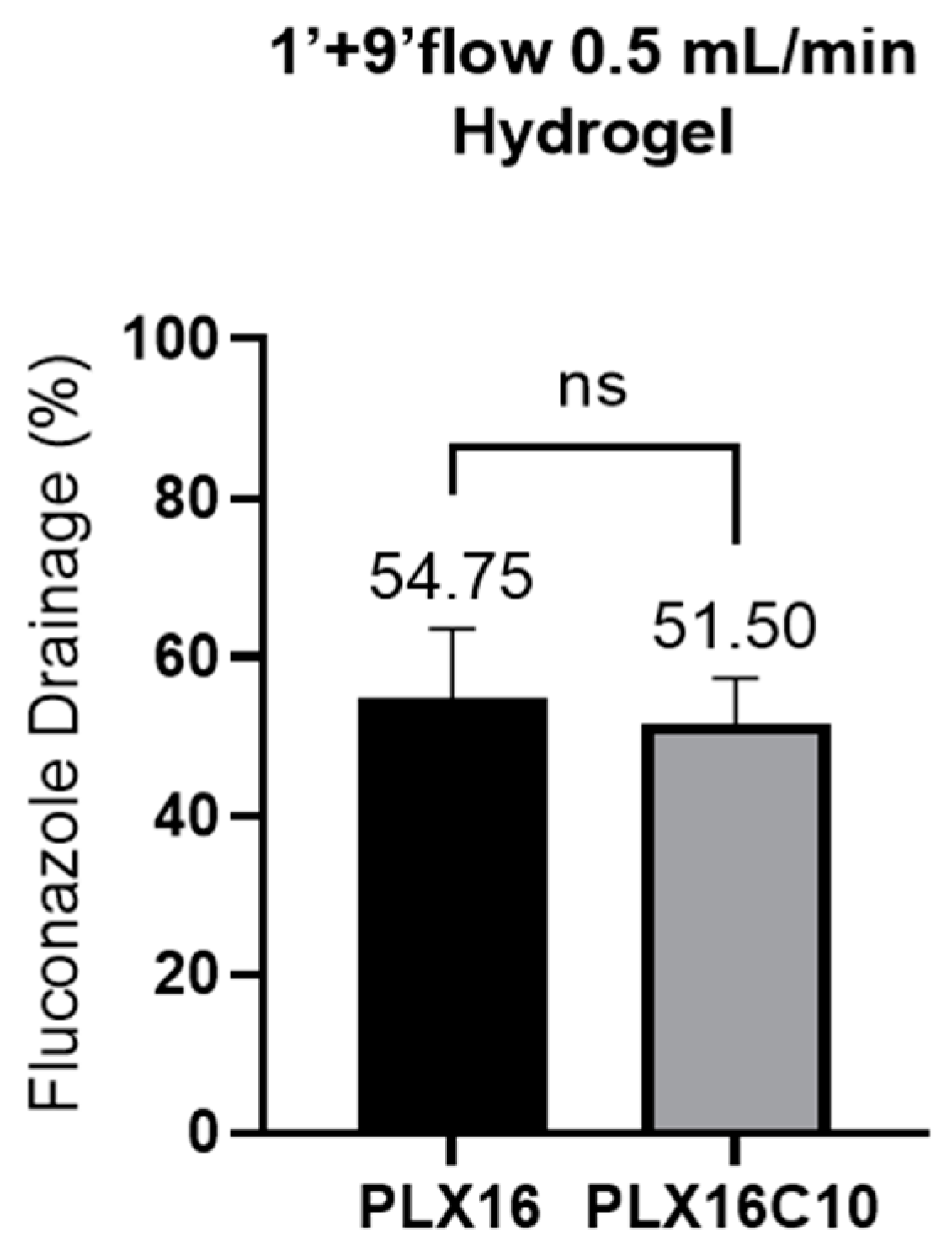
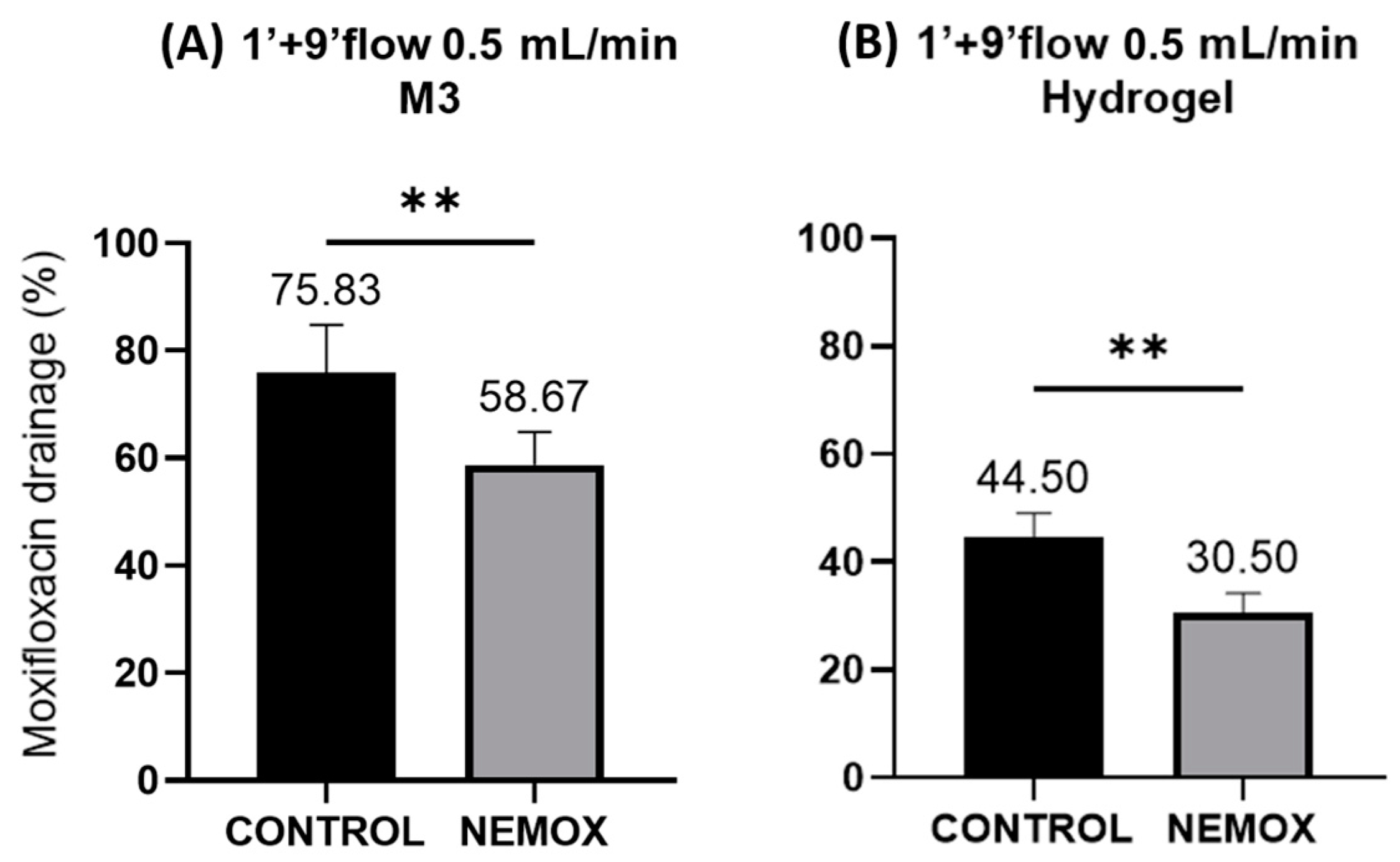
| Test Time | Flow Rate (mL/min) | Membrane | Formulation Tested | |
|---|---|---|---|---|
| 1 min contact no flow + 4 min with flow | 1.0 | Polymeric | PLX16 | PLX16C10 |
| 1 min contact no flow + 4 min with flow | 0.5 | Polymeric | PLX16 | PLX16C10 |
| 1 min contact no flow + 9 min with flow | 0.5 | Polymeric | PLX16 CONTROL | PLX16C10 NEMOX |
| 1 min contact no flow + 9 min with flow | 0.5 | Hydrogel | PLX16 CONTROL | PLX16C10 NEMOX |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contarato, J.L.A.; Barbalho, G.N.; Cunha-Filho, M.; Gelfuso, G.M.; Gratieri, T. Applying Different Conditions in the OphthalMimic Device Using Polymeric and Hydrogel-Based Hybrid Membranes to Evaluate Gels and Nanostructured Ophthalmic Formulations. Gels 2024, 10, 538. https://doi.org/10.3390/gels10080538
Contarato JLA, Barbalho GN, Cunha-Filho M, Gelfuso GM, Gratieri T. Applying Different Conditions in the OphthalMimic Device Using Polymeric and Hydrogel-Based Hybrid Membranes to Evaluate Gels and Nanostructured Ophthalmic Formulations. Gels. 2024; 10(8):538. https://doi.org/10.3390/gels10080538
Chicago/Turabian StyleContarato, Jonad L. A., Geisa N. Barbalho, Marcilio Cunha-Filho, Guilherme M. Gelfuso, and Tais Gratieri. 2024. "Applying Different Conditions in the OphthalMimic Device Using Polymeric and Hydrogel-Based Hybrid Membranes to Evaluate Gels and Nanostructured Ophthalmic Formulations" Gels 10, no. 8: 538. https://doi.org/10.3390/gels10080538







