Smart Hydrogel Based on Derivatives of Natural α-Amino Acids for Efficient Removal of Metal Ions from Wastewater
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sorption Capacity
2.2. Sorption Kinetics
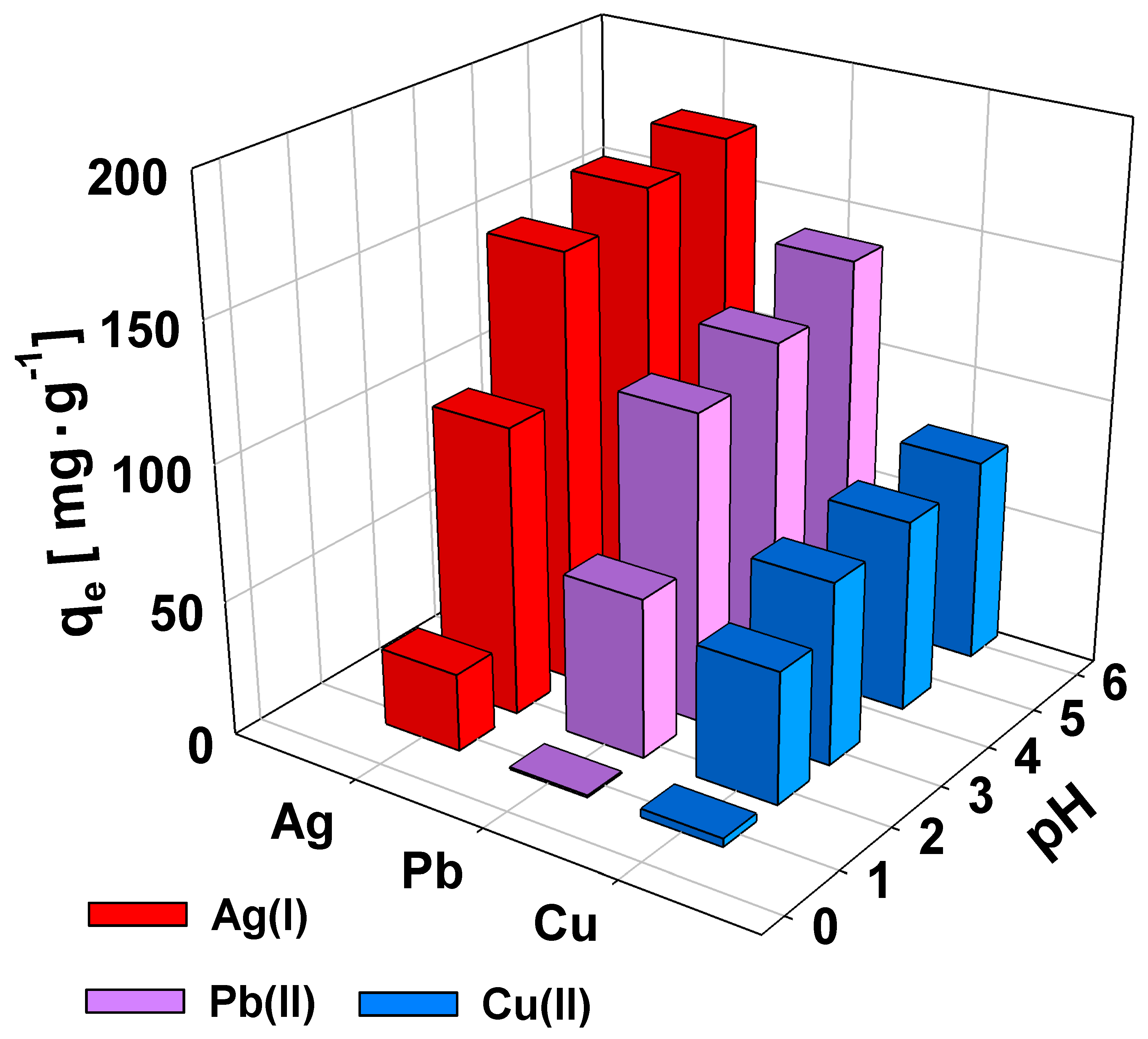
2.3. Effect of pH
2.4. Regeneration of Hydrogel Sorbent
2.5. Swelling Behavior of Hydrogel Sorbent after Metal Absorption
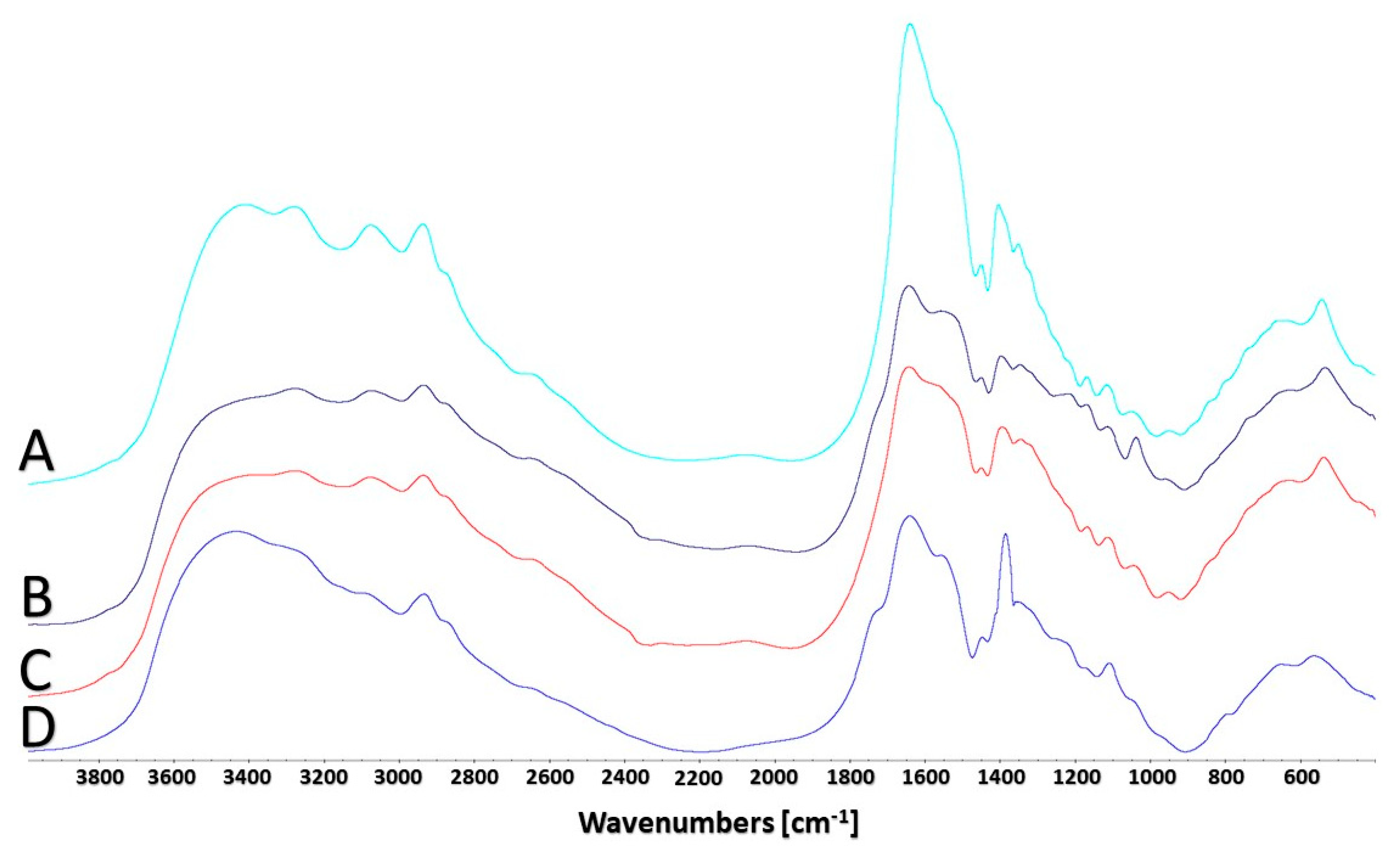
2.6. FTIR Spectroscopic Analysis
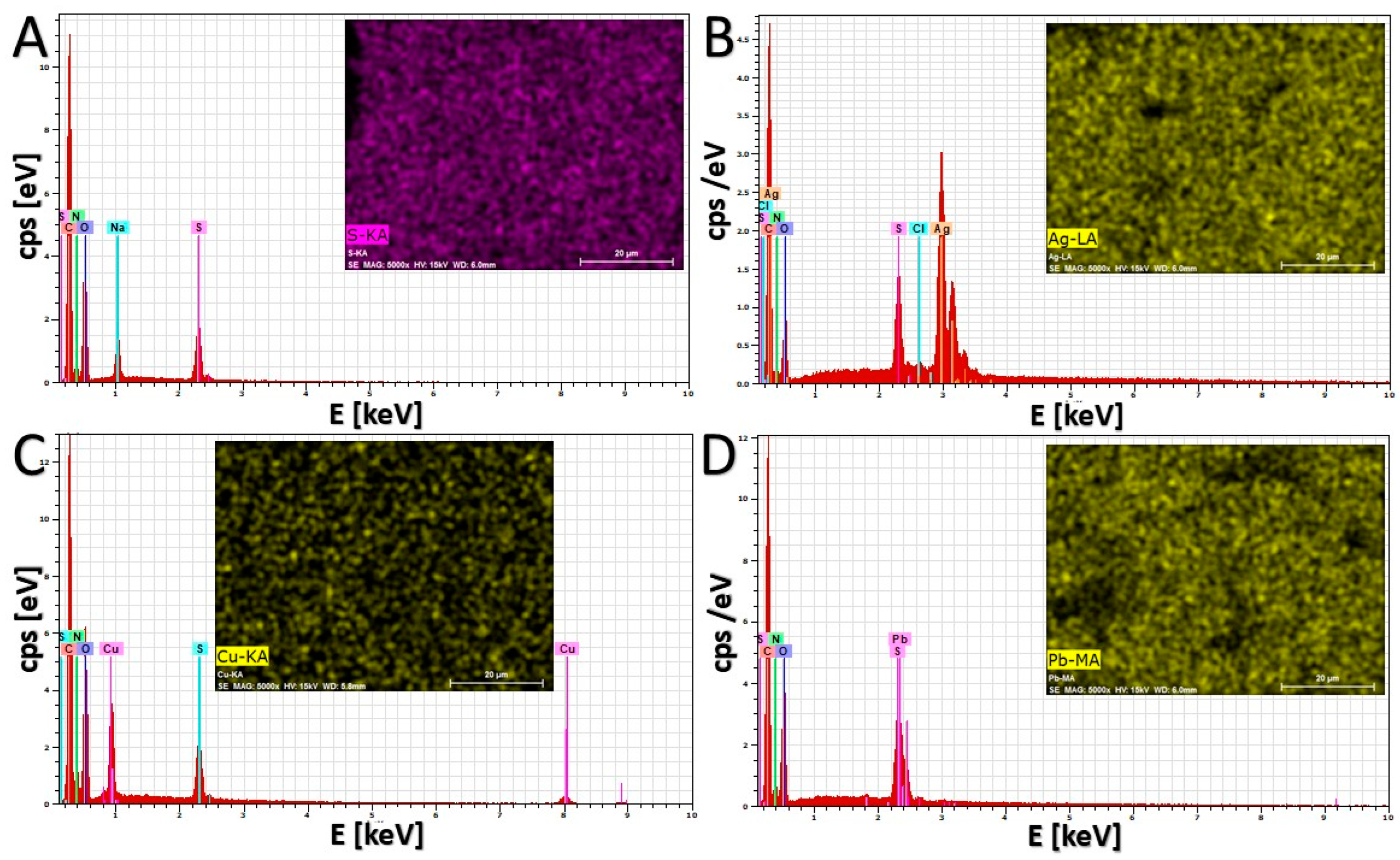
2.7. Characterization of Sorbent Morphology and Element Distribution
3. Conclusions
4. Materials and Methods
4.1. Materials
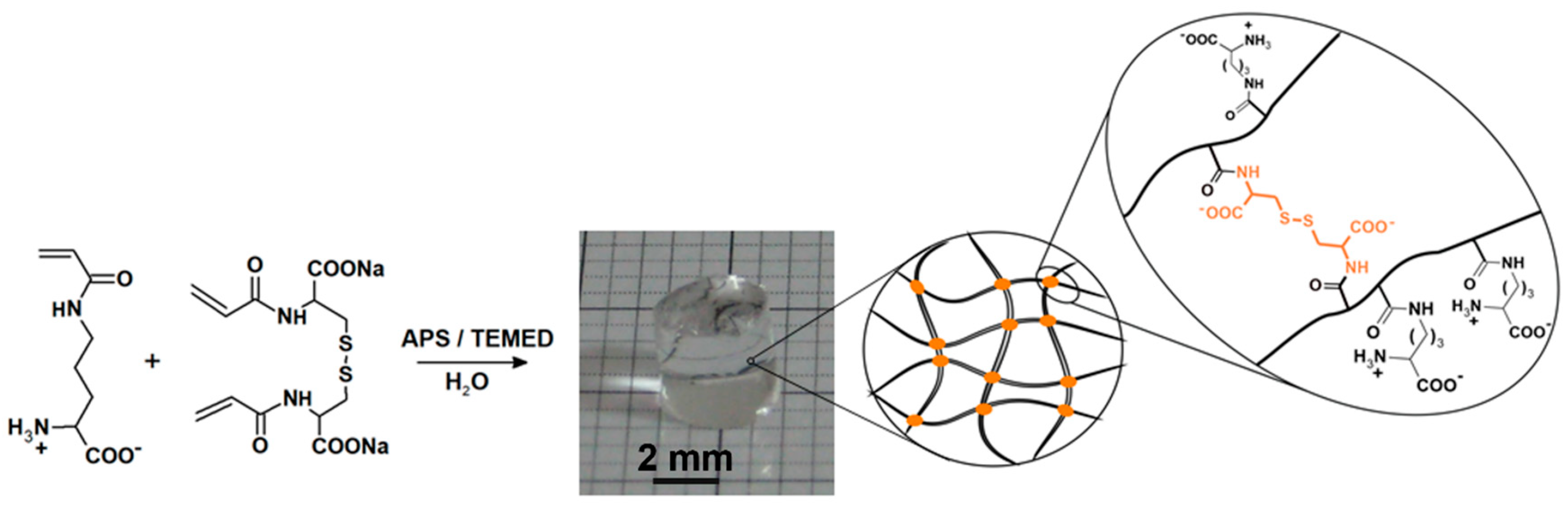
4.2. Synthesis of AcOrn-BISS Hydrogel
4.3. Evaluating the Sorption Properties
4.3.1. Adsorption Isotherm
4.3.2. Adsorption Kinetics
4.3.3. pH Dependence Study
4.3.4. Metal Recovery Experiments
4.4. Measurement of Swelling Ratio of Gel Rods
4.5. Scanning Electron Microscope (SEM) Investigations
4.6. X-ray Photoelectron Spectroscopy (XPS)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Sikdar, P.; Uddin, M.; Dip, T.M.; Islam, S.; Hoque, S.; Dhar, A.K.; Wu, S. Recent advances in the synthesis of smart hydrogels. Mater. Adv. 2021, 2, 4532–4573. [Google Scholar] [CrossRef]
- Seida, Y.; Tokuyama, H. Hydrogel Adsorbents for the Removal of Hazardous Pollutants—Requirements and Available Functions as Adsorbent. Gels 2022, 8, 220. [Google Scholar] [CrossRef]
- Luo, Z.; He, J.; Polle, A.; Rennenberg, H. Heavy metal accumulation and signal transduction in herbaceous and woody plants: Paving the way for enhancing phytoremediation efficiency. Biotechnol. Adv. 2016, 34, 1131–1148. [Google Scholar] [CrossRef]
- Lemos, V.A.; Teixeira, L.S.G.; Bezerra, M.A.; Costa, A.C.S.; Castro, J.T.; Cardodo, L.A.M.; de Jesus, D.S.; Santos, E.S.; Baliza, P.X.; Santos, L.N. New materials for solid-phase extraction of trace elements. Appl. Spectrosc. Rev. 2008, 43, 303–334. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Shangbin, S.; Berkland, C.; Liang, J.-T. Chelator-mimetic multi-functionalized hydrogel: Highly efficient and reusable sorbent for Cd, Pb, and As removal from waste water. Chem. Eng. J. 2017, 307, 496–502. [Google Scholar] [CrossRef]
- Pyrzynska, K. Application of Carbon Sorbents for the Concentration and Separation of Metal Ions. Anal. Sci. 2007, 23, 631–637. [Google Scholar] [CrossRef]
- Wang, F.; Lu, X.; Li, X. Selective removals of heavy metals (Pb2+, Cu2+, and Cd2+) from wastewater by gelation with alginate for effective metal recovery. J. Hazard. Mater. 2016, 308, 75–83. [Google Scholar] [CrossRef]
- Gharakhloo, M.; Jagleniec, D.; Romanski, J.; Karbarz, M. A novel self-healing hydrogel based on derivatives of natural α-amino acids with potential applications as a strain sensor. J. Mater. Chem. B 2022, 10, 4463. [Google Scholar] [CrossRef] [PubMed]
- Ninni, L.; Ermatchkov, V.; Hasse, H.; Maurer, G. Swelling behavior of chemically cross-linked poly(N-IPAAm-allylglycine) hydrogels: Effects of NaCl and pH. Fluid Phase Equilibria 2014, 361, 257–265. [Google Scholar] [CrossRef]
- Bauri, K.; Nandi, M.; De, P. Amino acid-derived stimuli-responsive polymers and their applications. Polym. Chem. 2018, 9, 1257–1287. [Google Scholar] [CrossRef]
- Karbarz, M.; Romanski, J.; Michniewicz, K.; Jurczak, J.; Stojek, Z. Influence of polymer network-metal ion complexation on the swelling behaviour of new gels with incorporated α-amino acid groups. Soft Matter 2010, 6, 1336–1342. [Google Scholar] [CrossRef]
- Mackiewicz, M.; Kaniewska, K.; Romanski, J.; Augustin, E.; Stojek, Z.; Karbarz, M. Stable and degradable microgels linked with cystine for storing and environmentally triggered release of drugs. J. Mater. Chem. B 2015, 3, 7262–7270. [Google Scholar] [CrossRef]
- de Vargas Brião, G.; Hashim, M.A.; Chu, K.H. The Sips isotherm equation: Often used and sometimes misused. Sep. Sci. Technol. 2023, 58, 884–892. [Google Scholar] [CrossRef]
- Hyk, W. Statistical Analysis in Laboratory. Available online: www.e-stat.pl (accessed on 1 January 2024).
- Patel, H. Batch and continuous fixed bed adsorption of heavy metals removal using activated charcoal from neem (Azadirachta indica) leaf powder. Sci. Rep. 2020, 10, 16895. [Google Scholar] [CrossRef]
- El-Shafey, E.I.; Al-Hashmi, A.H.R. Sorption of lead and silver from aqueous solution on phosphoric acid dehydrated carbon. J. Environ. Chem. Eng. 2013, 1, 934–944. [Google Scholar] [CrossRef]
- Li, Y.-H.; Ding, J.; Luan, Z.; Di, Z.; Zhu, Y.; Xu, C.; Wu, D.; Wei, B. Competitive adsorption of Pb2+, Cu2+ and Cd2+ ions from aqueous solutions by multiwalled carbon nanotubes. Carbon 2003, 41, 2787–2792. [Google Scholar] [CrossRef]
- Kang, K.C.; Ju, J.H.; Kim, S.S.; Baik, M.H.; Rhee, S.W. Sorption of aqueous Pb2+ ion on synthetic manganese oxides-intercalated with exchangeable cations. J. Ind. Eng. Chem. 2011, 17, 565–569. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Al-Bogami, A.S.; Guibal, E. 2-Mercaptobenzimidazole derivative of chitosan for silver sorption–Contribution of magnetite incorporation and sonication effects on enhanced metal recovery. Chem. Eng. J. 2021, 403, 126265. [Google Scholar] [CrossRef]
- Hyk, W.; Kitka, K. Water purification using sponge like behaviour of poly(N-isopropylacrylamide) ferrogels. Studies on silver removal from water samples. J. Environ. Chem. Eng. 2018, 6, 6108–6117. [Google Scholar] [CrossRef]
- Kiss, T.; Sovago, I.; Gergely, A. Critical survey of stability constants of complexes of glycine. Pure Appl. Chem. 1991, 63, 597–638. [Google Scholar] [CrossRef]
- Pettit, L.D. Critical survey of formation constants of complexes of histidine, phenylalanine, tyrosine, L-DOPA and tryptophan. Pure Appl. Chem. 1984, 56, 247–292. [Google Scholar] [CrossRef]
- Furia, E.; Sindona, G. Complexation of l-Cystine with Metal Cations. J. Chem. Eng. Data 2010, 55, 2985–2989. [Google Scholar] [CrossRef]
- Petrov, A.I. Interaction of disulfides with metal ions and spectroscopic identification of the products. Coord. Chem. Rev. 2024, 505, 215678. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced Analysis of Copper X-ray Photoelectron (XPS) Spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Wagner, C.D.; Naumkin, A.V.; Kraut-Vass, A.; Allison, J.W.; Powell, C.J.; Rumble, J.R., Jr. NIST Standard Reference Database 20, Version 3.4 (Web Version). 2023. Available online: https://srdata.nist.gov/xps/ (accessed on 10 August 2024).
- Chastain, J.; King, R.C., Jr. (Eds.) Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics, Inc.: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Biesinger, M.C. Accessing the robustness of adventitious carbon for charge referencing (correction) purposes in XPS analysis: Insights from a multi-user facility data review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]






| Metal Ion | Parameter | Langmuir Model | Sips Model |
|---|---|---|---|
| Ag(I) | a | 0.95 ± 0.43 | 3.44 ± 0.41 |
| b | 0.0014 ± 0.0037 | 0.00534 ± 0.00071 | |
| n | – | 1.69107524 | |
| R2 | 0.43 | 0.91 | |
| Cu(II) | a | 0.0684 ± 0.0016 | 0.0684 ± 0.0016 |
| b | 0.01154 ± 0.00080 | 0.01154 ± 0.00080 | |
| n | – | 1.0003975 | |
| R2 | 0.997 | 0.997 | |
| Pb(II) | a | 0.068 ± 0.019 | 0.0688 ± 0.0072 |
| b | 0.0069 ± 0.0013 | 0.00772 ± 0.00032 | |
| n | – | 1.43989721 | |
| R2 | 0.65 | 0.95 |
| Sorbent | Sorption Capacity (mg g−1) | Reference | ||
|---|---|---|---|---|
| Cu(II) | Pb(II) | Ag(I) | ||
| Activated charcoal from Azadirachta indica | 185.8 | 205.6 | – | [18] |
| H3PO4 dehydrated carbon | – | 41.5 | 312.5 | [19] |
| Multiwalled carbon nanotubes | 24.5 | 97 | – | [20] |
| MnO2 intercalated with H+ or K+ | – | 517 | – | [21] |
| Chitosan modified with 2-mercaptobenzimidazole | – | – | 350 | [22] |
| AcOrn-BISS gel | 90 | 155 | 215 | This work |
| Metal Ion | Parameter | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model |
|---|---|---|---|
| Ag(I) | a | −0.117 ± 0.019 | 0.000440 ± 0.000057 |
| b | 5.911 ± 0.038 | 0.002656 ± 0.000076 | |
| R2 | 0.90 | 0.94 | |
| Cu(II) | a | −0.40 ± 0.14 | 0.0042 ± 0.0011 |
| b | 5.50 ± 0.19 | 0.00363 ± 0.00037 | |
| R2 | 0.72 | 0.84 | |
| Pb(II) | a | −0.097 ± 0.015 | 0.00065 ± 0.00012 |
| b | 5.240 ± 0.039 | 0.00522 ± 0.00025 | |
| R2 | 0.94 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamowska, M.; Kaniewska, K.; Muszyńska, M.; Romański, J.; Hyk, W.; Karbarz, M. Smart Hydrogel Based on Derivatives of Natural α-Amino Acids for Efficient Removal of Metal Ions from Wastewater. Gels 2024, 10, 560. https://doi.org/10.3390/gels10090560
Adamowska M, Kaniewska K, Muszyńska M, Romański J, Hyk W, Karbarz M. Smart Hydrogel Based on Derivatives of Natural α-Amino Acids for Efficient Removal of Metal Ions from Wastewater. Gels. 2024; 10(9):560. https://doi.org/10.3390/gels10090560
Chicago/Turabian StyleAdamowska, Monika, Klaudia Kaniewska, Magdalena Muszyńska, Jan Romański, Wojciech Hyk, and Marcin Karbarz. 2024. "Smart Hydrogel Based on Derivatives of Natural α-Amino Acids for Efficient Removal of Metal Ions from Wastewater" Gels 10, no. 9: 560. https://doi.org/10.3390/gels10090560





