Bicontinuous Nanophasic Conetworks of Polystyrene with Poly(dimethylsiloxane) and Divinylbenzene: From Macrocrosslinked to Hypercrosslinked Double-Hydrophobic Conetworks and Their Organogels with Solvent-Selective Swelling
Abstract
1. Introduction
2. Results and Discussion
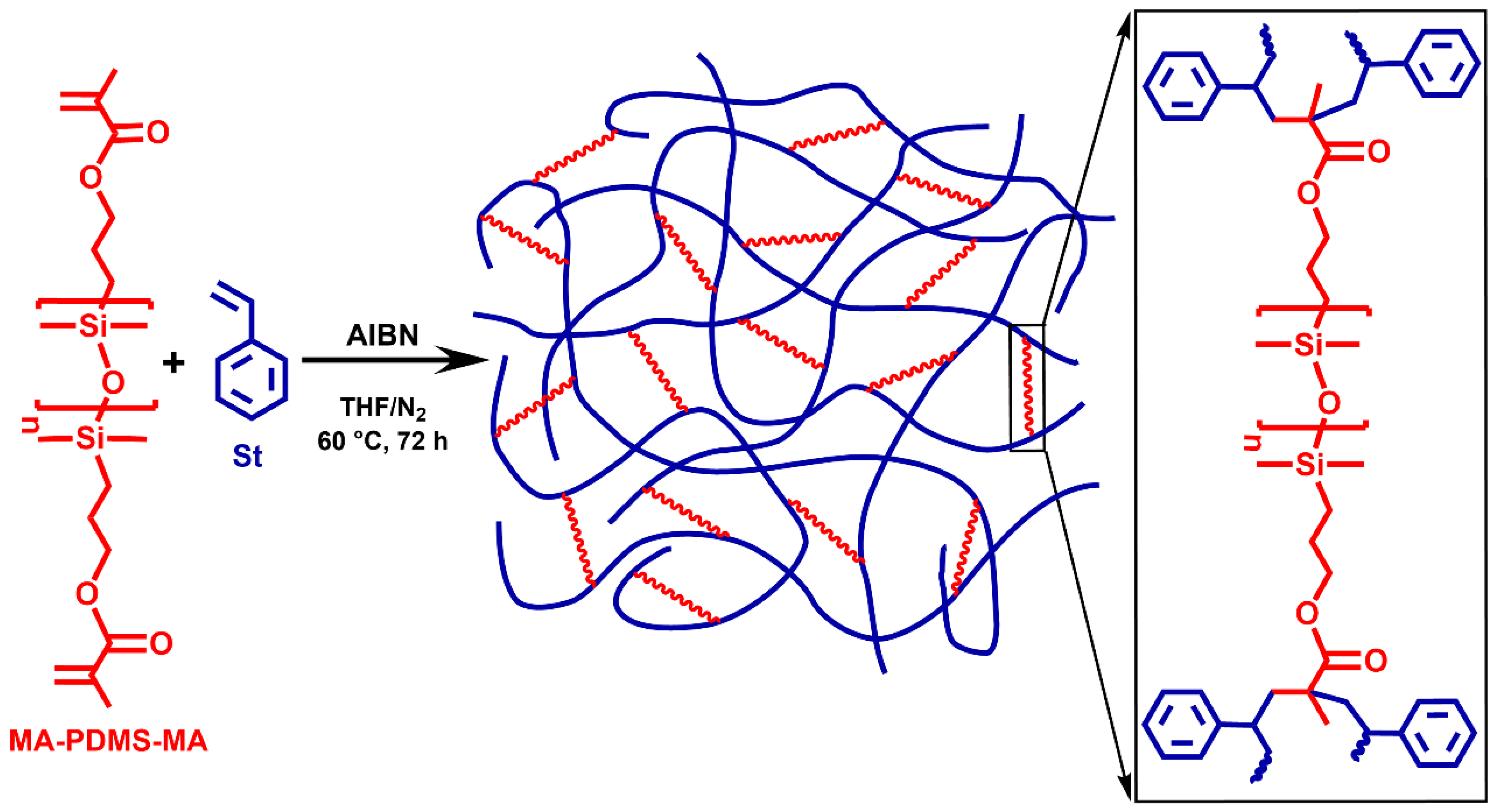
2.1. Synthesis of PSt-l-PDMS and PSt-l-PDMS/DVB Polymer Conetworks
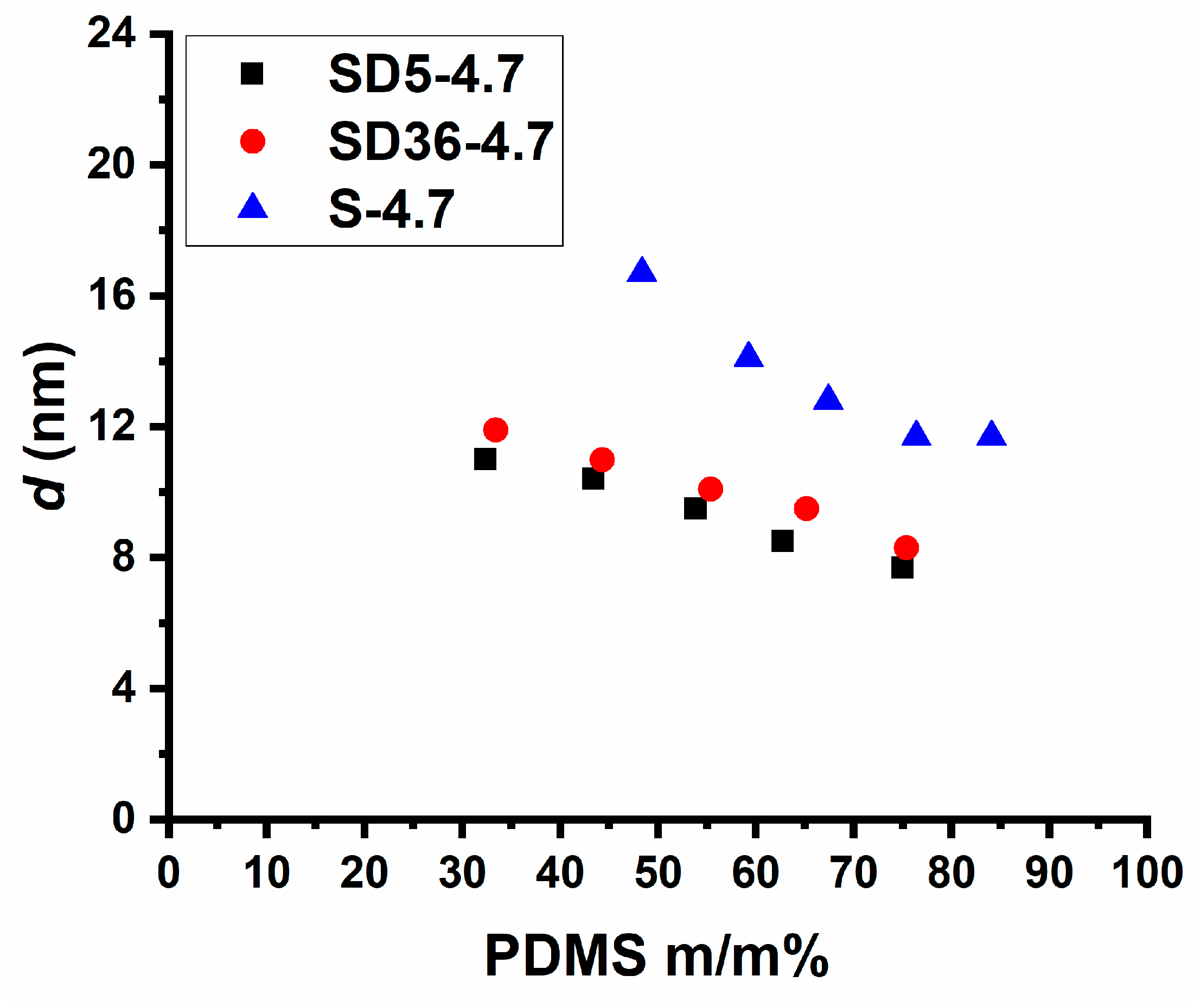
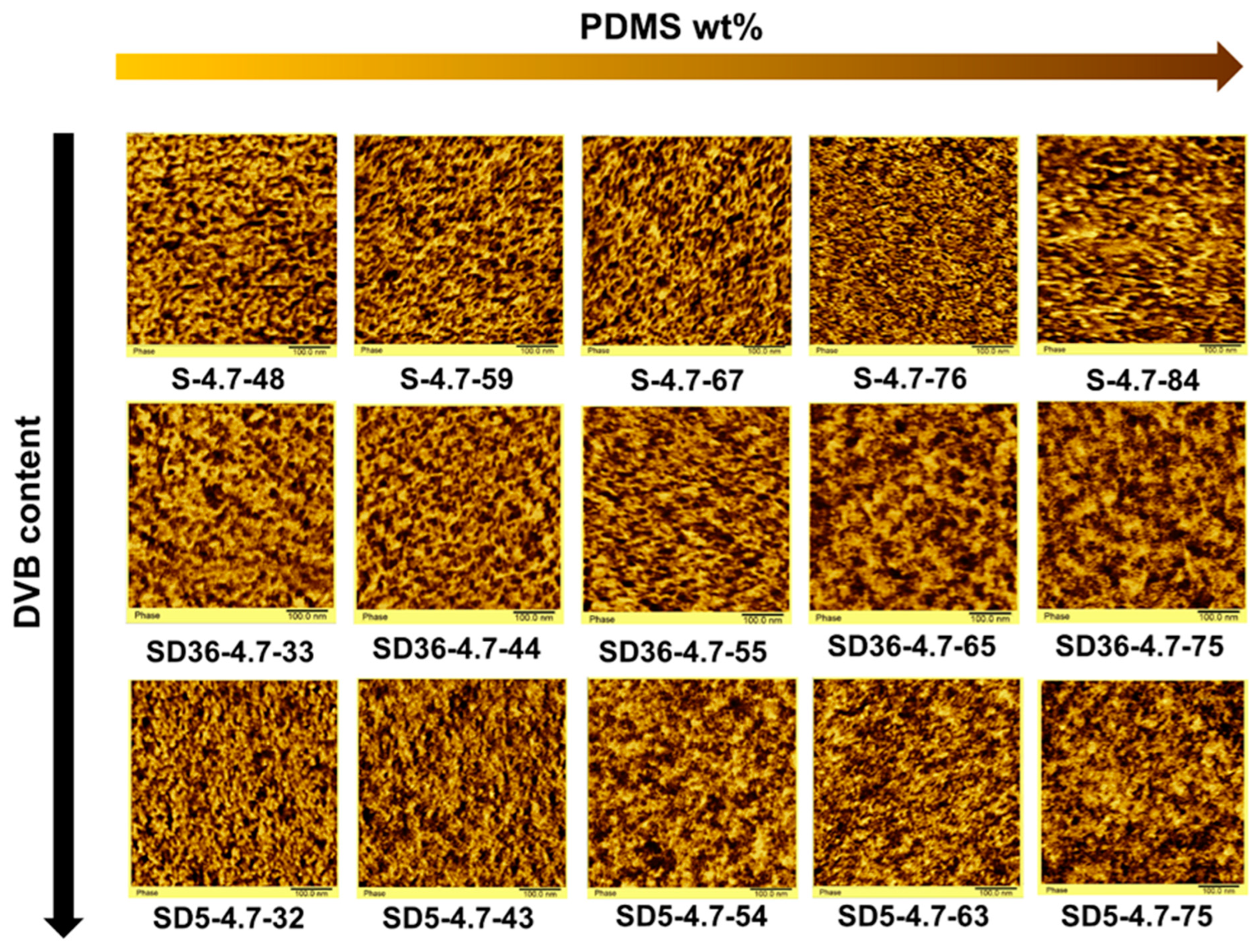
2.2. Structural Characterization of the PSt-l-PDMS and PSt-l-PDMS/DVB Conetworks
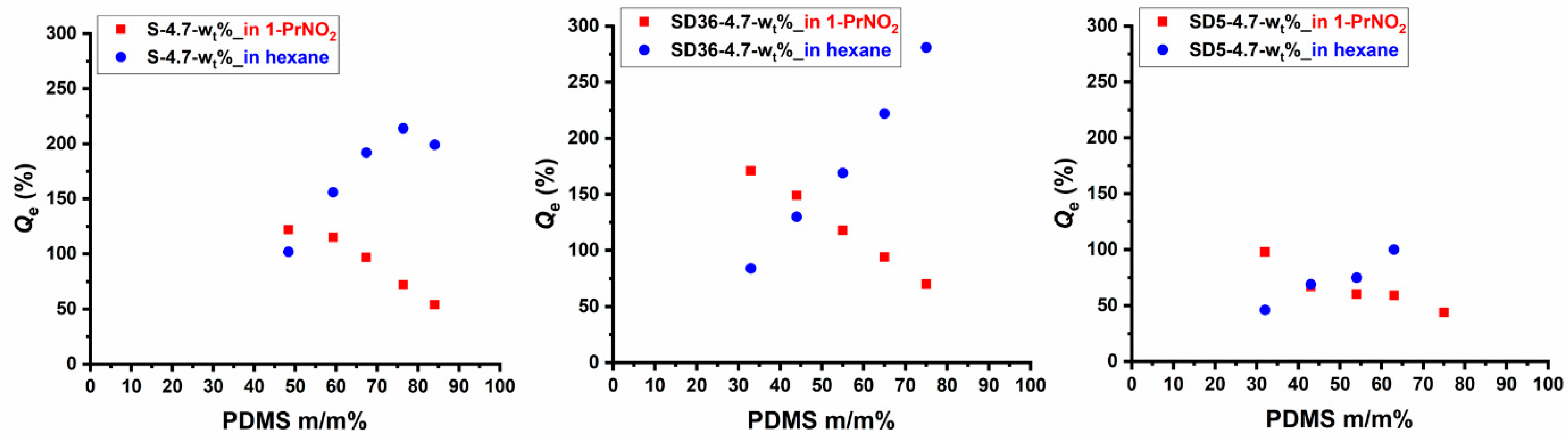
2.3. Solvent-Selective Swelling of the PSt-l-PDMS and PSt-l-PDMS/DVB Conetwork Gels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of PSt-l-PDMS and PSt-l-PDMS/DVB Conetworks
4.3. Characterization
4.3.1. 1H Nuclear Magnetic Resonance (1H NMR) Spectroscopy
4.3.2. Elemental Analysis
4.3.3. Differential Scanning Calorimetry (DSC)
4.3.4. Small Angle X-Ray Scattering (SAXS)
4.3.5. Atomic Force Microscopy (AFM)
4.3.6. Swelling Measurements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erdodi, G.; Kennedy, J.P. Amphiphilic conetworks: Definition, synthesis, applications. Prog. Polym. Sci. 2006, 31, 1–18. [Google Scholar] [CrossRef]
- Amphiphilic Polymer Co-Networks: Synthesis, Properties, Modelling and Applications; Patrickios, C.S., Ed.; Royal Society of Chemistry: Cambridge, UK, 2020. [Google Scholar] [CrossRef]
- Kalulu, M.; Oderinde, O.; Wei, Y.Y.; Zhang, C.; Hussain, I.; Han, X.L.; Jiang, Y. Robust solvent-free fabrication and characterization of (polydimethylsiloxane-co-2-hydroxyethylmethacrylate)/poly(ethylene glycol) methacrylate (PDMS-HEMA)/PEGMA hydrogels. Polym. Adv. Technol. 2019, 30, 1922–1932. [Google Scholar] [CrossRef]
- Karunakaran, R.; Kennedy, J.P. Synthesis, Characterization, and Crosslinking of Methacrylate-Telechelic PDMAAm-b-PDMS-b-PDMAAm Copolymers. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 4284–4290. [Google Scholar] [CrossRef]
- Lin, C.H.; Yeh, Y.H.; Lin, W.C.; Yang, M.C. Novel silicone hydrogel based on PDMS and PEGMA for contactlens application. Colloids Surf. B Biointerfaces 2014, 123, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, X.; Sun, F. In vitro and in vivo evaluation of ketotifen fumarate-loaded silicone hydrogel contact lenses for ocular drug delivery. Drug Deliv. 2011, 18, 150–158. [Google Scholar] [CrossRef]
- Yang, M.C.; Tran-Nguyen, P.L. Evaluation of silicone hydrogel contact lenses based on poly(dimethylsiloxane) dialkanol and hydrophilic polymers. Colloids Surf. B Biointerfaces 2021, 206, 111957. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, X.; Liu, D.; Wang, H.; He, C. Polyvinylpyrrolidone–polydimethylsiloxane amphiphilic co-networks: Synthesis, characterization, and perm-selective behavior. J. Appl. Polym. Sci. 2016, 133, 42985. [Google Scholar] [CrossRef]
- Bruns, N.; Tiller, J.C. Nanophasic Amphiphilic Conetworks with a Fluorophilic Phase. Macromolecules 2006, 39, 4386–4394. [Google Scholar] [CrossRef]
- Bruns, N.; Scherble, J.; Hartmann, L.; Thomann, R.; Iván, B.; Mülhaupt, R.; Tiller, J.C. Nanophase Separated Amphiphilic Conetwork Coatings and Membranes. Macromolecules 2005, 38, 2431–2438. [Google Scholar] [CrossRef]
- Tobis, J.; Thomann, Y.; Tiller, J.C. Synthesis and characterization of chiral and thermo responsive amphiphilic conetworks. Polymer 2010, 51, 35–45. [Google Scholar] [CrossRef]
- Tobis, J.; Boch, L.; Thomann, Y.; Tiller, J.C. Amphiphilic polymer conetworks as chiral separation membranes. J. Membr. Sci. 2011, 372, 219–227. [Google Scholar] [CrossRef]
- Shi, L.; Xie, P.; Li, Z.; Wu, Y.; Deng, J. Chiral pH-Responsive Amphiphilic Polymer Co-networks: Preparation, Chiral Recognition, and Release Abilities. Macromol. Chem. Phys. 2013, 214, 1375–1383. [Google Scholar] [CrossRef]
- Hossain, I.; Kim, D.; Al Munsur, A.Z.; Roh, J.M.; Park, H.B.; Kim, T.H. PEG/PPG−PDMS-Based Cross-Linked Copolymer Membranes Prepared by ROMP and In Situ Membrane Casting for CO2 Separation: An Approach to Endow Rubbery Materials with Properties of Rigid Polymers. ACS Appl. Mater. Interfaces 2020, 12, 27286–27299. [Google Scholar] [CrossRef]
- Kim, D.; Hossain, I.; Kim, Y.; Choi, O.; Kim, T.H. PEG/PPG-PDMS Adamantane-Based Crosslinked Terpolymer Using the ROMP Technique to Prepare a Highly Permeable and CO2-Selective Polymer Membrane. Polymers 2020, 12, 1674. [Google Scholar] [CrossRef]
- Mugemana, C.; Martin, A.; Grysan, P.; Dieden, R.; Ruch, D.; Dubois, P. Scratch-Healing Surface-Attached Coatings from Metallo-Supramolecular Polymer Conetworks. Macromol. Chem. Phys. 2021, 222, 2000331. [Google Scholar] [CrossRef]
- Mugemana, C.; Grysan, P.; Dieden, R.; Ruch, D.; Bruns, N.; Dubois, P. Self-Healing Metallo-Supramolecular Amphiphilic Polymer Conetworks. Macromol. Chem. Phys. 2020, 221, 1900432. [Google Scholar] [CrossRef]
- Hanko, M.; Bruns, N.; Tiller, J.C.; Heinze, J. Optical biochemical sensor for determining hydroperoxides in nonpolar organic liquids as archetype for sensors consisting of amphiphilic conetworks as immobilisation matrices. Anal. Bioanal. Chem. 2006, 386, 1273–1283. [Google Scholar] [CrossRef]
- Meskath, S.; Urban, G.; Heinze, J. Nanophase separated amphiphilic polymer co-networks as efficient matrices for optical sensors: Rapid and sensitive detection of NO2. Sens. Actuators B Chem. 2013, 186, 367–373. [Google Scholar] [CrossRef]
- Meskath, S.; Urban, G.; Heinze, J. A new optochemical chlorine gas sensor based on the application of amphiphilic co-networks as matrices. Sens. Actuators B Chem. 2011, 151, 327–332. [Google Scholar] [CrossRef]
- Ulrich, S.; Osypova, A.; Panzarasa, G.; Rossi, R.M.; Bruns, N.; Boesel, L.F. Pyranine-Modified Amphiphilic Polymer Conetworks as Fluorescent Ratiometric pH Sensors. Macromol. Rapid Commun. 2019, 40, 190036. [Google Scholar] [CrossRef]
- Guo, F.; Schulte, L.; Vigild, M.E.; Ndoni, S. Load–release of small and macromolecules from elastomers with reversible gyroid mesoporosity. Soft Matter 2012, 8, 11499–11507. [Google Scholar] [CrossRef]
- Schulte, L.; Grydgaard, A.; Jakobsen, M.R.; Szewczykowski, P.P.; Guo, F.; Vigild, M.E.; Berg, R.H.; Ndoni, S. Nanoporous materials from stable and metastable structures of 1,2-PB-b-PDMS block copolymers. Polymer 2011, 52, 422–429. [Google Scholar] [CrossRef]
- Ndoni, S.; Li, L.; Schulte, L.; Szewczykowski, P.P.; Hansen, T.W.; Guo, F.; Berg, R.H.; Vigild, M.E. Controlled Photooxidation of Nanoporous Polymers. Macromolecules 2009, 42, 3877–3880. [Google Scholar] [CrossRef]
- Li, L.; Schulte, L.; Clausen, L.D.; Hansen, K.M.; Jonsson, G.E.; Ndoni, S. Gyroid Nanoporous Membranes with Tunable Permeability. ACS Nano 2011, 5, 7754–7766. [Google Scholar] [CrossRef]
- Guo, F.; Andreasen, J.W.; Vigild, M.E.; Ndoni, S. Influence of 1,2-PB Matrix Cross-Linking on Structure and Properties of Selectively Etched 1,2-PB-b-PDMS Block Copolymers. Macromolecules 2007, 40, 3669–3675. [Google Scholar] [CrossRef]
- Szewczykowski, P.P.; Andersen, K.; Schulte, L.; Mortensen, K.; Vigild, M.E.; Ndoni, S. Elastomers with Reversible Nanoporosity. Macromolecules 2009, 42, 5636–5641. [Google Scholar] [CrossRef]
- Cavicchi, K.A.; Zalusky, A.S.; Hillmyer, M.A.; Lodge, T.P. An Ordered Nanoporous Monolith from an Elastomeric Crosslinked Block Copolymer Precursor. Macromol. Rapid Commun. 2004, 25, 704–709. [Google Scholar] [CrossRef]
- Hansen, M.S.; Vigild, M.E.; Berg, R.H.; Ndoni, S. Nanoporous Crosslinked Polyisoprene from Polyisoprene-Polydimethylsiloxane Block Copolymer. Polym. Bull. 2004, 51, 403–409. [Google Scholar] [CrossRef]
- Bruns, N.; Tiller, J.C. Amphiphilic Network as Nanoreactor for Enzymes in Organic Solvents. Nano Lett. 2005, 5, 45–48. [Google Scholar] [CrossRef]
- Tobis, J.; Tiller, J.C. Impact of the configuration of a chiral, activating carrier on the enantioselectivity of entrapped lipase from Candida rugosa in cyclohexane. Biotechnol. Lett. 2014, 36, 1661–1667. [Google Scholar] [CrossRef]
- Bruns, N.; Hanko, M.; Dech, S.; Ladisch, R.; Tobis, J.; Tiller, J.C. Amphiphilic Polymer Conetworks as Matrices for Phase Transfer Reactions. Macromol. Symp. 2010, 291–292, 293–301. [Google Scholar] [CrossRef]
- Bruns, N.; Bannwarth, W.; Tiller, J.C. Amphiphilic Conetworks as Activating Carriers for the Enhancement of Enzymatic Activity in Supercritical CO2. Biotechnol. Bioeng. 2008, 101, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Tiller, J.C.; Sprich, C.; Hartmann, L. Amphiphilic conetworks as regenerative controlled releasing antimicrobial coatings. J. Control. Release 2005, 103, 355–367. [Google Scholar] [CrossRef]
- Hanko, M.; Bruns, N.; Rentmeister, S.; Tiller, J.C.; Heinze, J. Nanophase-Separated Amphiphilic Conetworks as Versatile Matrixes for Optical Chemical and Biochemical Sensors. Anal. Chem. 2006, 78, 6376–6383. [Google Scholar] [CrossRef]
- Szabó, L.S.; Iván, B.; Scherble, J.; Mülhaupt, R. New Amphiphilic Conetworks from Methacrylate-Telechelic Polydimethylsiloxane Bismacromonomers. Polym. Mater. Sci. Eng. 2004, 91, 486–487. [Google Scholar]
- Rother, M.; Barmettler, J.; Reichmuth, A.; Araujo, J.V.; Rytka, C.; Glaied, O.; Pieles, U.; Bruns, N. Self-Sealing and Puncture Resistant Breathable Membranes for Water-Evaporation Applications. Adv. Mater. 2015, 27, 6620–6624. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qiu, M.; Ma, B.; He, C. “Near Perfect” Amphiphilic Conetwork Based on End-Group Cross-Linking of Polydimethylsiloxane Triblock Copolymer via Atom Transfer Radical Polymerization. ACS Appl. Mater. Interfaces 2014, 6, 15283–15290. [Google Scholar] [CrossRef]
- Karunakaran, R.; Kennedy, J.P. Novel Amphiphilic Conetworks by Synthesis and Crosslinking of Allyl-Telechelic Block Copolymers. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 4254–4257. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Peng, X.; He, C. A clean synthesis approach to biocompatible amphiphilic conetworks via reversible addition–fragmentation chain transfer polymerization and thiol–ene chemistry. RSC Adv. 2016, 6, 17228–17238. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Z.; Wang, H.; Feng, X.; He, C. Novel Anti-Biofouling Soft Contact Lens: L-Cysteine Conjugated Amphiphilic Conetworks via RAFT and Thiol–Ene Click Chemistry. Macromol. Biosci. 2017, 17, 1600444. [Google Scholar] [CrossRef]
- De Bruycker, K.; Mertens, C.; Du Prez, F.E. Thiolactone Chemistry for the Synthesis of Functional Silicone-Based Amphiphilic Co-Networks. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 322–333. [Google Scholar] [CrossRef]
- Sundararajan, S.; Samui, A.B.; Kulkarni, P.S. Crosslinked polymer networks of poly(ethylene glycol) (PEG) and hydroxyl terminated poly(dimethyl siloxane) (HTPDMS) as polymeric phase change material for thermal energy storage. Sol. Energy 2019, 181, 187–194. [Google Scholar] [CrossRef]
- Lin, G.; Zhang, X.; Kumar, S.R.; Mark, J.E. Improved Hydrophilicity from Poly(ethylene glycol) in Amphiphilic Conetworks with Poly(dimethylsiloxane). Silicon 2009, 1, 173–181. [Google Scholar] [CrossRef]
- Erdodi, G.; Kennedy, J.P. Ideal Tetrafunctional Amphiphilic PEG/PDMS Conetworks by a Dual-Purpose Extender/Crosslinker. I. Synthesis. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4953–4964. [Google Scholar] [CrossRef]
- Erdodi, G.; Kennedy, J.P. Ideal Tetrafunctional Amphiphilic PEG/PDMS Conetworks by a Dual-Purpose Extender/Crosslinker. II. Characterization and Properties of Water-Swollen Membranes. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4965–4971. [Google Scholar] [CrossRef]
- Kurian, P.; Kasibhatla, B.; Daum, J.; Burns, C.A.; Moosa, M.; Rosenthal, K.S.; Kennedy, J.P. Synthesis, permeability and biocompatibility of tricomponent membranes containing polyethylene glycol, polydimethylsiloxane and polypentamethylcyclopentasiloxane domains. Biomaterials 2003, 24, 3493–3503. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Lackey, M.A.; Tew, G.N.; Crosby, A.J. Mechanical Properties of End-Linked PEG/PDMS Hydrogels. Macromolecules 2012, 45, 6104–6110. [Google Scholar] [CrossRef]
- Cui, J.; Lackey, M.A.; Madkour, A.E.; Saffer, E.M.; Griffin, D.M.; Bhatia, S.R.; Crosby, A.J.; Tew, G.N. Synthetically Simple, Highly Resilient Hydrogels. Biomacromolecules 2012, 13, 584–588. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, Y.; Tang, Z.; Zhang, C.; Wu, J.; Wu, B. Surface Reconstruction of Silicone-Based Amphiphilic Polymers for Mitigating Marine Biofouling. Polymers 2024, 16, 1570. [Google Scholar] [CrossRef]
- Strasser, P.; Walliser, C.; Ajvazi, E.; Bauer, F.; Brüggemann, O.; Lammermann, S.; Major, Z.; Minarcikova, A.; Majercikova, M.; Micusík, M.; et al. Metal-Free Curing of 3D Printable Silicone Elastomers via Thermally Triggered 2-Oxazoline Cross-Linkers. Macromolecules 2025, 58, 2709–2718. [Google Scholar] [CrossRef]
- Mugemana, C.; Mertz, G.; Grysan, P.; Dieden, R.; Ruch, D. Adhesive Films from Dopamine-Functionalized Polydimethylsiloxane Polymer Conetworks. Macromol. Chem. Phys. 2023, 224, 2200456. [Google Scholar] [CrossRef]
- Clarke, B.R.; Tew, G.N. Bottlebrush Amphiphilic Polymer Co-Networks. Macromolecules 2022, 55, 5131–5139. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.S.; Yakunin, S.; Avaro, J.; Kang, X.; Bodnarchuk, M.I.; Liebi, M.; Sun, X.; Rossi, R.M.; Kovalenko, M.V.; Boesel, L.F. Amphiphilic Polymer Co-Network: A Versatile Matrix for Tailoring the Photonic Energy Transfer in Wearable Energy Harvesting Devices. Adv. Energy Mater. 2022, 12, 2200441. [Google Scholar] [CrossRef]
- Huang, C.S.; Jakubowski, K.; Ulrich, S.; Yakunin, S.; Clerc, M.; Toncelli, C.; Rossi, R.M.; Kovalenko, M.V.; Boesel, L.F. Nano-domains assisted energy transfer in amphiphilic polymer conetworks for wearable luminescent solar concentrators. Nano Energy 2020, 76, 105039. [Google Scholar] [CrossRef]
- Guzman, G.; Nugay, T.; Nugay, I.; Nugay, N.; Kennedy, J.; Cakmak, M. High strength bimodal amphiphilic conetworks for immunoisolation membranes: Synthesis, characterization, and properties. Macromolecules 2015, 48, 6251–6262. [Google Scholar] [CrossRef]
- Velasquez, S.T.R.; Belluati, A.; Tervoort, E.; Mattich, I.; Hertel, B.; Russell, S.; Gouveia, M.G.; Grysan, P.; Mugemana, C.; Studart, A.R.; et al. Microfluidically Produced Microcapsules with Amphiphilic Polymer Conetwork Shells. Adv. Mater. Technol. 2024, 9, 2400109. [Google Scholar] [CrossRef]
- Velasquez, S.T.; Jang, D.; Jenkins, P.; Liu, P.; Yang, L.; Korley, L.T.; Bruns, N. Peptide-Reinforced Amphiphilic Polymer Conetworks. Adv. Funct. Mater. 2022, 32, 2207317. [Google Scholar] [CrossRef]
- Wilhelm, S.A.; Maricanov, M.; Brandt, V.; Katzenberg, F.; Tiller, J.C. Amphiphilic polymer conetworks with ideal and non-ideal swelling behavior demonstrated by small angle X-ray scattering. Polymer 2022, 242, 124582. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Jia, L.; Li, J.; Liu, S. Stereo-Complex and Click-Chemical Bicrosslinked Amphiphilic Network Gels with Temperature/pH Response. Gels 2023, 9, 647. [Google Scholar] [CrossRef]
- Tironi, C.N.; Graf, R.; Lieberwirth, I.; Klapper, M.; Müllen, K. Synthesis and Selective Loading of Polyhydroxyethyl Methacrylate-l-Polysulfone Amphiphilic Polymer Conetworks. ACS Macro Lett. 2015, 4, 1302–1306. [Google Scholar] [CrossRef]
- Moon, J.D.; Sujanani, R.; Geng, Z.; Freeman, B.D.; Segalman, R.A.; Hawker, C.J. Versatile synthetic platform for polymer membrane libraries using functional networks. Macromolecules 2021, 54, 866–873. [Google Scholar] [CrossRef]
- Némethy, Á.; Solti, K.; Kiss, L.; Gyarmati, B.; Deli, M.A.; Csányi, E.; Szilágyi, A. pH-and temperature-responsive poly(aspartic acid)-l-poly(N-isopropylacrylamide) conetwork hydrogel. Eur. Polym. J. 2013, 49, 2392–2403. [Google Scholar] [CrossRef]
- Ida, S.; Morimura, M.; Kitanaka, H.; Hirokawa, Y.; Kanaoka, S. Swelling and mechanical properties of thermoresponsive/hydrophilic conetworks with crosslinked domain structures prepared from various triblock precursors. Polym. Chem. 2019, 10, 6122–6130. [Google Scholar] [CrossRef]
- Getya, D.; Lucas, A.; Gitsov, I. Composite Hydrogels Based on Poly(Ethylene Glycol) and Cellulose Macromonomers as Fortified Materials for Environmental Cleanup and Clean Water Safeguarding. Int. J. Mol. Sci. 2023, 24, 7558. [Google Scholar] [CrossRef] [PubMed]
- Anuradha; Das, A.; Pal, S.; Jewrajka, S.K. Physical, electrochemical, and solvent permeation properties of amphiphilic conetwork membranes formed through interlinking of poly(vinylidene fluoride)-graft-Poly[(2-dimethylamino) ethyl methacrylate] with telechelic poly(ethylene glycol) and small molecular weight cross-linkers. Langmuir 2022, 38, 15340–15352. [Google Scholar] [CrossRef]
- Nutan, B.; Chandel, A.K.S.; Jewrajka, S.K. Liquid prepolymer-based in situ formation of degradable poly(ethylene glycol)-linked-poly(caprolactone)-linked-poly(2-dimethylaminoethyl) methacrylate amphiphilic conetwork gels showing polarity driven gelation and bioadhesion. ACS Appl. Bio Mater. 2018, 1, 1606–1619. [Google Scholar] [CrossRef] [PubMed]
- Löser, L.; Bunk, C.; Scholz, R.; Lang, M.; Böhme, F.; Saalwachter, K. Structural Characterization of Amphiphilic Conetworks in Selective and Nonselective Solvents Using 1H NMR and SAXS. Macromolecules 2024, 57, 940–954. [Google Scholar] [CrossRef]
- Fribiczer, N.; Hagmann, K.; Bunk, C.; Böhme, F.; von Klitzing, R.; Seiffert, S. Impact of Swelling on Macroscopic and Nanoscopic Mechanical Properties of Amphiphilic Polymer Co-Networks in Non-Selective and Selective Solvents. Macromol. Chem. Phys. 2024, 225, 2300389. [Google Scholar] [CrossRef]
- Hagmann, K.; Bunk, C.; Böhme, F.; von Klitzing, R. Amphiphilic Polymer Conetwork Gel Films Based on Tetra-Poly(ethylene Glycol) and Tetra-Poly(ε-Caprolactone). Polymers 2022, 14, 2555. [Google Scholar] [CrossRef]
- Nakagawa, S.; Li, X.; Shibayama, M.; Kamata, H.; Sakai, T.; Gilbert, E.P. Insight into the microscopic structure of module-assembled thermoresponsive conetwork hydrogels. Macromolecules 2018, 51, 6645–6652. [Google Scholar] [CrossRef]
- Ju, J.; Hayward, R.C. Interconnected Nanoporous Polysulfone by the Self-Assembly of Randomly Linked Copolymer Networks and Linear Multiblocks. ACS Appl. Mater. Interfaces 2024, 16, 34079–34088. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Chandel, A.K.S.; Anuradha; Vadadoriya, N.; Mamtani, V.; Jewrajka, S.K. Structurally heterogeneous amphiphilic conetworks of poly(vinyl imidazole) derivatives with potent antimicrobial properties and cytocompatibility. ACS Appl. Mater. Interfaces 2023, 15, 46333–46346. [Google Scholar] [CrossRef] [PubMed]
- Malo de Molina, P.; Kafouris, D.; Patrickios, C.S.; Noirez, L.; Gradzielski, M. Amphiphilic Polymer Conetworks Studied by SANS: Effect of the Type of Solubilizate and Molecular Architecture on the Swollen Gel Structure. Macromolecules 2023, 56, 8323–8332. [Google Scholar] [CrossRef]
- Ida, S.; Suzuki, S.; Toda, S.; Takeshita, H.; Oyama, M.; Nakajima, K.; Kanaoka, S. Structure-Property Correlation of Hydrogels Obtained via Radical Polymerization Using Central Cores of Multiarm Star Polymers as Crosslinkers. Polym. Chem. 2025; advance article. [Google Scholar] [CrossRef]
- Tamer, Y.B. A New Design of Poly(N-Isopropylacrylamide) Hydrogels Using Biodegradable Poly(Beta-Aminoester) Crosslinkers as Fertilizer Reservoirs for Agricultural Applications. Gels 2023, 9, 127. [Google Scholar] [CrossRef]
- Scherble, J.; Thomann, R.; Iván, B.; Mülhaupt, R. Formation of CdS nanoclusters in phase-separated poly(2-hydroxyethyl methacrylate)-l-polyisobutylene amphiphilic conetworks. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 1429–1436. [Google Scholar] [CrossRef]
- Iván, B.; Almdal, K.; Mortensen, K.; Johannsen, I.; Kops, J. Synthesis, Characterization, and Structural Investigations of Poly(ethyl acrylate)-l -polyisobutylene Bicomponent Conetwork. Macromolecules 2001, 34, 1579–1585. [Google Scholar] [CrossRef]
- Domján, A.; Erdődi, G.; Wilhelm, M.; Neidhofer, M.; Landfester, K.; Iván, B.; Spiess, H.W. Structural Studies of Nanophase-Separated Poly(2-hydroxyethyl methacrylate)-l-polyisobutylene Amphiphilic Conetworks by Solid-State NMR and Small-Angle X-ray Scattering. Macromolecules 2003, 36, 9107–9114. [Google Scholar] [CrossRef]
- Iván, B.; Haraszti, M.; Erdődi, G.; Scherble, J.; Thomann, R.; Mülhaupt, R. New Nanophase Separated Intelligent Amphiphilic Conetworks and Gels. Macromol. Symp. 2005, 227, 265–273. [Google Scholar] [CrossRef]
- Fodor, C.; Kali, G.; Thomann, R.; Thomann, Y.; Iván, B.; Mülhaupt, R. Nanophasic morphologies as a function of the composition and molecular weight of the macromolecular cross-linker in poly(N-vinylimidazole)-l-poly(tetrahydrofuran) amphiphilic conetworks: Bicontinuous domain structure in broad composition ranges. RSC Adv. 2017, 7, 6827–6834. [Google Scholar] [CrossRef]
- Stumphauser, T.; Kasza, G.; Domján, A.; Wacha, A.; Varga, Z.; Thomann, Y.; Thomann, R.; Pásztói, B.; Trötschler, T.M.; Kerscher, B.; et al. Nanoconfined Crosslinked Poly(ionic liquid)s with Unprecedented Selective Swelling Properties Obtained by Alkylation in Nanophase-Separated Poly(1-vinylimidazole)-l-poly(tetrahydrofuran) Conetworks. Polymers 2020, 12, 2292. [Google Scholar] [CrossRef]
- Pásztor, S.; Becsei, B.; Szarka, G.; Thomann, Y.; Thomann, R.; Mühlhaupt, R.; Iván, B. The Scissors Effect in Action: The Fox-Flory Relationship between the Glass Transition Temperature of Crosslinked Poly(Methyl Methacrylate) and Mc in Nanophase Separated Poly(Methyl Methacrylate)-l-Polyisobutylene Conetworks. Materials 2020, 13, 4822. [Google Scholar] [CrossRef] [PubMed]
- Majewska-Smolarek, K. Synergistic Self-Healing Enhancement in Multifunctional Silicone Elastomers and Their Application in Smart Materials. Polymers 2024, 16, 487. [Google Scholar] [CrossRef]
- Xu, X.; Xiao, T.; Wen, J.; Li, J.; Chen, Y.; Lu, A.; Tyan, H.; Tang, C. 3D printing of High-Strength Silicone-Based Polyurethane-Polyurea enabled by growth of covalent Cross-Linked network. Chem. Eng. J. 2024, 488, 150810. [Google Scholar] [CrossRef]
- Kim, S.U.; Kim, J.Y. Comparative Performance Analysis of Inverse Phase Active Vibration Cancellation Using Macro Fiber Composite (MFC) and Vibration Absorption of Silicone Gel for Vibration Reduction. Polymers 2023, 15, 4672. [Google Scholar] [CrossRef]
- Porpora, F.; Dei, L.; Duncan, T.T.; Olivadese, F.; London, S.; Berrie, B.H.; Weiss, R.G.; Carretti, E. Non-Aqueous Poly(dimethylsiloxane) Organogel Sponges for Controlled Solvent Release: Synthesis, Characterization, and Application in the Cleaning of Artworks. Gels 2023, 9, 985. [Google Scholar] [CrossRef]
- O’Shea, M.S.; George, G.A. Bulk copolymerization of methacryloyloxypropyl functionalized siloxane macromonomers with styrene: 1. Network formation. Polymer 1994, 35, 4181–4189. [Google Scholar] [CrossRef]
- Hamurcu, E.E.; Hazer, B.; Baysal, B.M. Polystyrene-b-polydimethyl siloxane (PDMS) multicomponent polymer networks: Styrene polymerization with macromonomeric initiators (macroinimers) having PDMS units. Polymer 1997, 38, 2981–2987. [Google Scholar] [CrossRef]
- Uddin, M.H.; Alshali, S.; Alqurashi, E.; Alyoubi, S.; Walters, N.; Khan, I.M. Recyclable Thermoplastic Elastomer from Furan Functionalized Hairy Nanoparticles with Polystyrene Core and Polydimethylsiloxane Hairs. Polymers 2024, 16, 3117. [Google Scholar] [CrossRef] [PubMed]
- Tenhu, H.; Vaahtera, K. Phase Separation in Polystyrene Crosslinked with Samples of Poly(dimethylsiloxane) of Various Chain Length. Eur. Polym. J. 1991, 27, 717–722. [Google Scholar] [CrossRef]
- Tenhu, H.; Heino, E.L. Polystyrene Crosslinked with Oligomeric and Polymeric Poly(dimethylsiloxane) Derivatives. Thermal and Dynamic Mechanical Studies. J. Appl. Polym. Sci. 1992, 44, 55–64. [Google Scholar] [CrossRef]
- Zhao, C.; Xie, H.; Huang, H.; Cai, Y.; Chen, Z.; Cheng, J.; Xiang, D.; Li, D.; Li, Z.; Wu, Y. Superhydrophobic/superoleophilic polystyrene-based porous material with superelasticity for highly efficient and continuous oil/water separation in harsh environments. J. Hazard. Mater. 2024, 472, 134566. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, I.F.; Sun, H.J.; Fu, Q.; Lotz, B.; Cavicchi, K.A.; Cheng, S.Z. Phase structural formation and oscillation in polystyrene-block-polydimethylsiloxane thin films. Soft Matter 2012, 8, 7937–7944. [Google Scholar] [CrossRef]
- Agarwal, S.; Lu, M.; Sarkar, J. Fabrication of micropatterned thin films through controlled phase separation of polystyrene/polydimethylsiloxane blends by spin coating. Front. Soft Matter 2023, 3, 1306346. [Google Scholar] [CrossRef]
- Getya, D.; Gitsov, I. Stronger Together. Poly(Styrene) Gels Reinforced by Soft Gellan Gum. Gels 2022, 8, 607. [Google Scholar] [CrossRef]
- Getya, D.; Gitsov, I. Reactive Cellu-mers—A Novel Approach to Improved Cellulose/Polymer Composites. Polymers 2022, 14, 1670. [Google Scholar] [CrossRef]
- McLeod, K.R.; Tew, G.N. Microphase-separated thiol–ene conetworks from telechelic macromonomers with asymmetric molecular weights. Macromolecules 2017, 50, 8042–8047. [Google Scholar] [CrossRef]
- Tian, D.; Park, S.; Jo, S.; Ryu, C.Y.; Ryu, D.Y.; Bae, C. Simultaneous Postfunctionalization and Cross-Linking of Epoxidized Polystyrene-b-polybutadiene-b-polystyrene for Anion Exchange Membrane. ACS Appl. Energy Mater. 2024, 7, 6209–6219. [Google Scholar] [CrossRef]
- Admoni, S.; Cohen, O.; Matyjaszewski, K.; Silverstein, M.S. Hierarchical Porosity in Emulsion-Templated Triblock Copolymer-like Structures: Mid-block Degradation and End-block Hypercrosslinking. Polymer 2025, 323, 128158. [Google Scholar] [CrossRef]
- Gorbovskaia, A.V.; Kvachenok, I.K.; Stavrianidi, A.N.; Chernobrovkina, A.V.; Uzhel, A.S.; Shpigun, O.A. Polyelectrolyte-grafted mixed-mode stationary phases based on poly(styrene–divinylbenzene). Microchem. J. 2024, 199, 110075. [Google Scholar] [CrossRef]
- Ardelean, R.; Popa, A.; Visa, A.A.; Dragan, E.S.; Davidescu, C.M. Synthesis, characterization and applications of poly(styrene-co-divinylbenzene) functionalized with aminophosphinic acid pendant groups as high-performance adsorbents for acetylsalicylic acid. Polym. Bull. 2024, 81, 8783–8809. [Google Scholar] [CrossRef]
- Martiz, A.; Károly, Z.; Bereczki, L.; Trif, L.; Farkas, A.; Menyhárd, A.; Kótai, L. Carbonization of Zr-Loaded Thiourea-Functionalized Styrene-Divinylbenzene Copolymers: An Easy Way to Synthesize Nano-ZrO2@C and Nano-(ZrC, ZrO2)@C Composites. J. Compos. Sci. 2023, 7, 306. [Google Scholar] [CrossRef]
- Saifutdinov, B.R.; Buryak, A.K. Thermodynamic Characteristics and Selectivity of the Liquid-Phase Adsorption of Aromatic Compounds on Hypercrosslinked Polystyrene Networks with Ultimate-High Crosslinking Densities by Data of Liquid Chromatography. Int. J. Mol. Sci. 2024, 25, 1551. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, S.; Yan, K.; Zhang, R.; Liu, S.; Ye, Q.; Zhou, F. Preparation of hydrophilic hyper-cross-linked polystyrene nanospheres with antibacterial for improved water lubrication performance. Langmuir 2024, 40, 8992–9000. [Google Scholar] [CrossRef] [PubMed]
- Lyubimov, S.E.; Zvinchuk, A.A.; Korlyukov, A.A.; Davankov, V.A.; Parenago, O.P. Palladium nanoparticles in hypercrosslinked polystyrene: Synthesis and application in the hydrogenation of arenes. Petrol. Chem. 2021, 61, 76–80. [Google Scholar] [CrossRef]
- Coote, M.L.; Johnston, L.P.; Davis, T.P. Copolymerization propagation kinetics of styrene and methyl methacrylate-revisited. 2. Kinetic analysis. Macromolecules 1997, 30, 8191–8204. [Google Scholar] [CrossRef]
- Moad, G.; Solomon, D.H. The Chemsitry of Free Radical Polymerization; Elsevier: Oxford, UK, 1995; p. 282. [Google Scholar]
- Clarson, S.J.; Dodgso, K.; Semlyen, J.A. Studies of cyclic and linear poly(dimethylsiloxanes): 19. Glass transition temperatures and crystallization behaviour. Polymer 1985, 26, 930–934. [Google Scholar] [CrossRef]
- Erdődi, G.; Iván, B. Novel Amphiphilic Conetworks Composed of Telechelic Poly(ethylene oxide) and Three-Arm Star Polyisobutylene. Chem. Mater. 2004, 16, 959–962. [Google Scholar] [CrossRef]
- Fodor, C.; Kali, G.; Iván, B. Poly(N-vinylimidazole)-l-Poly(tetrahydrofuran) Amphiphilic Conetworks and Gels: Synthesis, Characterization, Thermal and Swelling Behavior. Macromolecules 2011, 44, 4496–4502. [Google Scholar] [CrossRef]
- Fodor, C.; Domján, A.; Iván, B. Unprecedented scissors effect of macromolecular cross-linkers on the glass transition temperature of poly (N-vinylimidazole), crystallinity suppression of poly (tetrahydrofuran) and molecular mobility by solid state NMR in poly(N-vinylimidazole)-l-poly(tetrahydrofuran) conetworks. Polym. Chem. 2013, 4, 3714–3724. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Masaro, L.; Zhu, X.X. Physical models of diffusion for polymer solutions, gels and solids. Prog. Polym. Sci. 1999, 24, 731–775. [Google Scholar] [CrossRef]
- Ahmed, L.; Atif, R.; Eldeen, T.S.; Yahya, I.; Omara, A.; Eltayeb, M. Study the Using of Nanoparticles as Drug Delivery System Based on Mathematical Models for Controlled Release. Int. J. Latest Technol. Eng. Manag. Appl. Sci. 2019, 8, 52–56. Available online: www.ijltemas.in/DigitalLibrary/Vol.8Issue5/52-56.pdf (accessed on 20 April 2025).
- Fosca, M.; Rau, J.V.; Uskoković, V. Factors influencing the drug release from calcium phosphate cements. Bioact. Mater. 2022, 7, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Atmani, Z.; Heinze, T.; Gericke, M. Development of tailored polysaccharide gels through selective Diels–Alder crosslinking. Cellulose 2025, 32, 187–209. [Google Scholar] [CrossRef]
- Daglar, S.; Balta, D.K.; Temel, B.A.; Temel, G. From Single-Chain Polymeric Nanoparticles to Interpenetrating Polymer Network Organogels: A One-Pot Fabrication Approach. Gels 2025, 11, 122. [Google Scholar] [CrossRef]
- Bowman, Z.; Baker, J.G.; Hughes, M.J.; Nguyen, J.D.; Garcia, M.; Tamrat, N.; Worch, J.C.; Figg, C.A. Customizing STEM organogels using PET-RAFT polymerization. Polym. Chem. 2024, 15, 3907–3915. [Google Scholar] [CrossRef]
- Kuzina, M.A.; Kartsev, D.D.; Stratonovich, A.V.; Levkin, P.A. Organogels versus hydrogels: Advantages, challenges, and applications. Adv. Funct. Mater. 2023, 33, 2301421. [Google Scholar] [CrossRef]
- Feng, X.; Li, G. Photo-crosslinkable and ultrastable poly (1, 4-butadiene) based organogel with record-high reversible elongation upon cooling and contraction upon heating. Polymer 2022, 262, 125477. [Google Scholar] [CrossRef]
- Apostolides, D.E.; Patrickios, C.S. Dynamic covalent polymer hydrogels and organogels crosslinked through acylhydrazone bonds: Synthesis, characterization and applications. Polym. Int. 2018, 67, 627–649. [Google Scholar] [CrossRef]
- Li, Y.; Plummer, A.; Werner, J.G. Chemically Nanostructured Organogel Monoliths from Cross-Linked Block Copolymers for Selective Infusion Templating. ACS Nano 2024, 18, 19150–19160. [Google Scholar] [CrossRef]
- Daniels, E.L.; Runge, J.R.; Oshinowo, M.; Leese, H.S.; Buchard, A. Cross-linking of sugar-derived polyethers and boronic acids for renewable, self-healing, and single-ion conducting organogel polymer electrolytes. ACS Appl. Energy Mater. 2023, 6, 2924–2935. [Google Scholar] [CrossRef] [PubMed]
- Wacha, A.; Varga, Z.; Bóta, A. CREDO: A new general-purpose laboratory instrument for small-angle X-ray scattering. J. Appl. Crystallogr. 2014, 47, 1749–1754. [Google Scholar] [CrossRef]
- Wacha, A. Optimized pinhole geometry for small-angle scattering. J. Appl. Crystallogr. 2015, 48, 1843–1848. [Google Scholar] [CrossRef]
- Babutan, I.; Atanase, L.I.; Botiz, I. Self-Assembly of Lamellar/Micellar Block Copolymers Induced Through Their Rich Exposure to Various Solvent Vapors: An AFM Study. Materials 2025, 18, 1759. [Google Scholar] [CrossRef]
- Harniman, R.L.; Pearce, S.; Manners, I. Exploring the “living” growth of block copolymer nanofibers from surface-confined seeds by In situ solution-phase atomic force microscopy. J. Am. Chem. Soc. 2022, 144, 951–962. [Google Scholar] [CrossRef]
- Murphy, J.G.; Raybin, J.G.; Ansay, G.E.; Sibener, S.J. Spatiotemporal mapping of hole nucleation and growth during block copolymer terracing with high-speed atomic force microscopy. ACS Nano 2023, 17, 5644–5652. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Huang, Z.; Kentzinger, E.; Rücker, U.; Brückel, T.; Xiao, Y. Nanoparticle-induced morphological transformation in block copolymer-based nanocomposites. Nanoscale 2022, 14, 8766–8775. [Google Scholar] [CrossRef]
- Werner, E.; Güth, U.; Brockhagen, B.; Döpke, C.; Ehrmann, A. Examination of Polymer Blends by AFM Phase Images. Technologies 2023, 11, 56. [Google Scholar] [CrossRef]





| Sample ID | mPDMS,feed (%) | Gel Fraction (%) | mPDMS,EA by Elemental Analysis (%) | mPDMS,extr by Composition of Extractables (%) |
|---|---|---|---|---|
| S-4.7-48 | 30 | 55.9 | n. a. | 48.4 |
| S-4.7-59 | 40 | 60.4 | 61.3 | 59.3 |
| S-4.7-67 | 50 | 60.2 | 67.1 | 67.4 |
| S-4.7-76 | 60 | 63.0 | 77.2 | 76.4 |
| S-4.7-84 | 70 | 53.2 | 84.1 | n. a. |
| SD36-4.7-33 | 30 | 88.1 | n. a. | 33.4 |
| SD36-4.7-44 | 40 | 86.3 | n. a. | 44.3 |
| SD36-4.7-55 | 50 | 86.7 | n. a. | 55.4 |
| SD36-4.7-65 | 60 | 87.1 | n. a. | 65.2 |
| SD36-4.7-75 | 70 | 86.8 | n. a. | 75.4 |
| SD5-4.7-32 | 30 | 92.1 | 33.8 | 32.4 |
| SD5-4.7-43 | 40 | 88.9 | 42.3 | 43.4 |
| SD5-4.7-54 | 50 | 87.8 | 52.6 | 53.9 |
| SD5-4.7-63 | 60 | 85.9 | 61.3 | 62.7 |
| SD5-4.7-75 | 70 | 84.1 | 73.6 | 75.0 |
| Sample ID | d-Spacing (nm) by SAXS | d (nm) by AFM |
|---|---|---|
| S-4.7-48 | 16.7 | 16 |
| S-4.7-59 | 14.1 | 14 |
| S-4.7-67 | 12.8 | 13 |
| S-4.7-76 | 11.7 | 11 |
| S-4.7-84 | 11.7 | 12 |
| SD36-4.7-33 | 11.9 | 15 |
| SD36-4.7-44 | 11.0 | 15 |
| SD36-4.7-55 | 10.1 | 14 |
| SD36-4.7-65 | 9.5 | 13 |
| SD36-4.7-75 | 8.3 | 12 |
| SD5-4.7-32 | 11.0 | 11 |
| SD5-4.7-43 | 10.4 | 10 |
| SD5-4.7-54 | 9.5 | 9 |
| SD5-4.7-63 | 8.5 | 9 |
| SD5-4.7-75 | 7.7 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petróczy, A.; Szanka, I.; Wacha, A.; Varga, Z.; Thomann, Y.; Thomann, R.; Mülhaupt, R.; Bereczki, L.; Hegyesi, N.; Iván, B. Bicontinuous Nanophasic Conetworks of Polystyrene with Poly(dimethylsiloxane) and Divinylbenzene: From Macrocrosslinked to Hypercrosslinked Double-Hydrophobic Conetworks and Their Organogels with Solvent-Selective Swelling. Gels 2025, 11, 318. https://doi.org/10.3390/gels11050318
Petróczy A, Szanka I, Wacha A, Varga Z, Thomann Y, Thomann R, Mülhaupt R, Bereczki L, Hegyesi N, Iván B. Bicontinuous Nanophasic Conetworks of Polystyrene with Poly(dimethylsiloxane) and Divinylbenzene: From Macrocrosslinked to Hypercrosslinked Double-Hydrophobic Conetworks and Their Organogels with Solvent-Selective Swelling. Gels. 2025; 11(5):318. https://doi.org/10.3390/gels11050318
Chicago/Turabian StylePetróczy, Anna, István Szanka, András Wacha, Zoltán Varga, Yi Thomann, Ralf Thomann, Rolf Mülhaupt, Laura Bereczki, Nóra Hegyesi, and Béla Iván. 2025. "Bicontinuous Nanophasic Conetworks of Polystyrene with Poly(dimethylsiloxane) and Divinylbenzene: From Macrocrosslinked to Hypercrosslinked Double-Hydrophobic Conetworks and Their Organogels with Solvent-Selective Swelling" Gels 11, no. 5: 318. https://doi.org/10.3390/gels11050318
APA StylePetróczy, A., Szanka, I., Wacha, A., Varga, Z., Thomann, Y., Thomann, R., Mülhaupt, R., Bereczki, L., Hegyesi, N., & Iván, B. (2025). Bicontinuous Nanophasic Conetworks of Polystyrene with Poly(dimethylsiloxane) and Divinylbenzene: From Macrocrosslinked to Hypercrosslinked Double-Hydrophobic Conetworks and Their Organogels with Solvent-Selective Swelling. Gels, 11(5), 318. https://doi.org/10.3390/gels11050318











