Gelation Based on Host–Guest Interactions Induced by Multi-Functionalized Nanosheets
Abstract
:1. Introduction
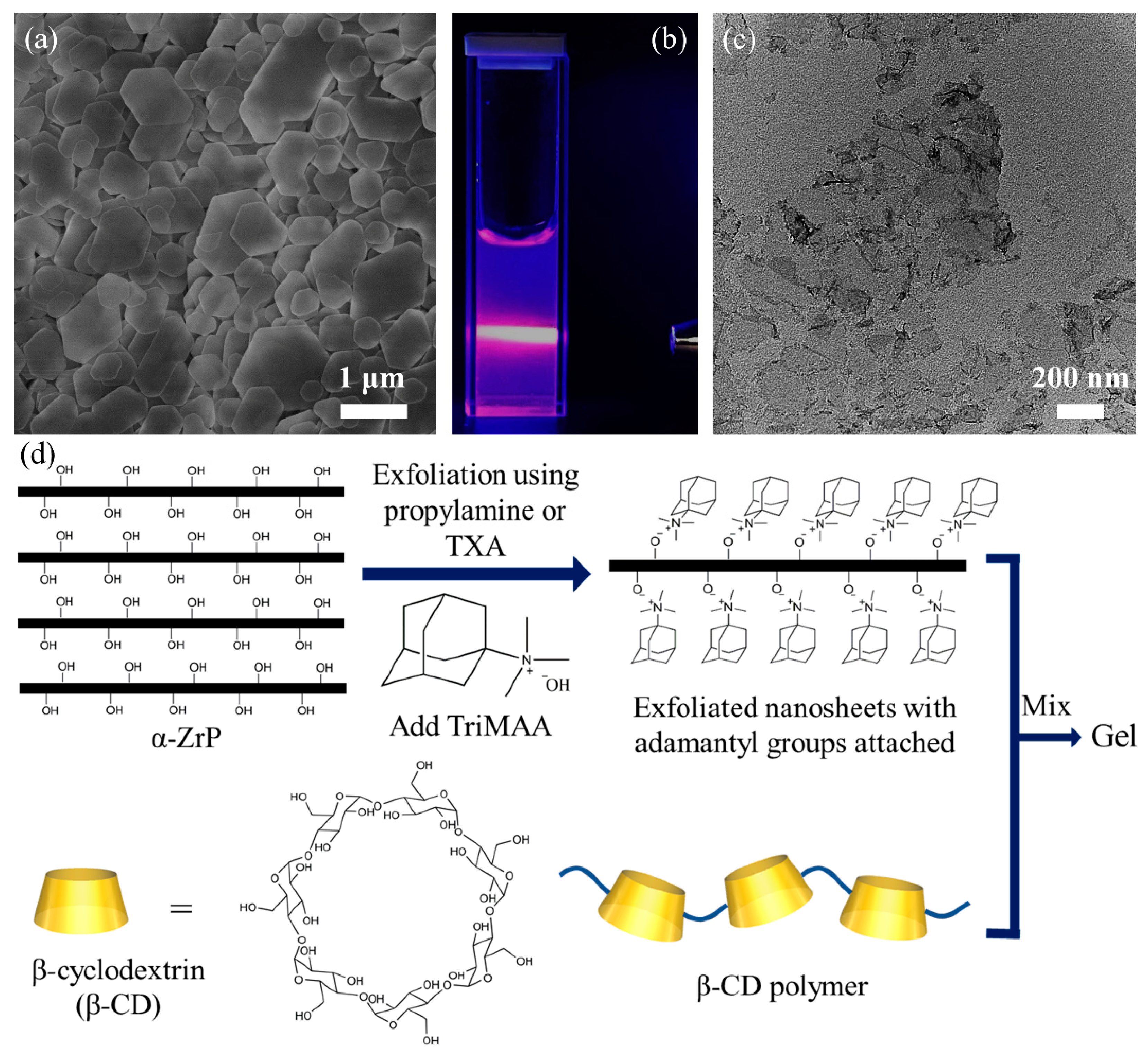
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of α-ZrP
4.3. Preparation of α-ZrP Nanosheets with Adamantyl Groups Attached
4.3.1. Direct Intercalation of TriMAA into α-ZrP
4.3.2. Exfoliated ZrP with Adamantyl Groups Directly Attached
4.3.3. Exfoliated ZrP with Adamantyl Groups Indirectly Attached
4.4. Gelation Based on the Host–Guest Interaction
4.5. Control Samples
4.5.1. Gelation Tests Missing Guest Molecules
4.5.2. Gelation Tests Missing α-ZrP Nanosheets
4.5.3. Gelation Tests Using Host Molecule Monomers
4.5.4. Gelation Tests with Unexfoliated α-ZrP
4.5.5. Reversibility of Gelation
4.6. Structural Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Xue, M.; Yang, Y.; Chi, X.; Zhang, Z.; Huang, F. Pillararenes, a new class of macrocycles for supramolecular chemistry. Acc. Chem. Res. 2012, 45, 1294–1308. [Google Scholar] [CrossRef]
- Hu, Q.-D.; Tang, G.-P.; Chu, P.K. Cyclodextrin-based host–guest supramolecular nanoparticles for delivery: From design to applications. Acc. Chem. Res. 2014, 47, 2017–2025. [Google Scholar] [CrossRef]
- Wang, Y.; Ping, G.; Li, C. Efficient complexation between pillar [5] arenes and neutral guests: From host–guest chemistry to functional materials. Chem. Commun. 2016, 52, 9858–9872. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, Q.; Hollett, G.; Zhao, W.; Kang, Y.; Wu, J. Cyclodextrin-based host–guest supramolecular hydrogel and its application in biomedical fields. Polym. Chem. 2018, 9, 3436–3449. [Google Scholar] [CrossRef]
- Schmidt, B.V.; Barner-Kowollik, C. Dynamic macromolecular material design-The versatility of cyclodextrin-based host–guest chemistry. Angew. Chem. Int. Ed. 2017, 56, 8350–8369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Ma, P.X. Polymeric core-shell assemblies mediated by host–guest interactions: Versatile nanocarriers for drug delivery. Angew. Chem. 2009, 121, 982–986. [Google Scholar] [CrossRef]
- Tilloy, S.; Bertoux, F.; Mortreux, A.; Monflier, E. Chemically modified β-cyclodextrins in biphasic catalysis: A fruitful contribution of the host–guest chemistry to the transition-metal catalyzed reactions. Catal. Today 1999, 48, 245–253. [Google Scholar] [CrossRef]
- Ahn, Y.; Jang, Y.; Selvapalam, N.; Yun, G.; Kim, K. Supramolecular velcro for reversible underwater adhesion. Angew. Chem. Int. Ed. 2013, 52, 3140–3144. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Y.; Ma, X.; Lei, Y. Functional self-healing materials and their potential applications in biomedical engineering. Adv. Compos. Hybrid Mater. 2018, 1, 94–113. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Xie, R.; Zhu, Y.; Liu, X. Ordered 2D layered MoS2/conjugated polymer nanocomposites: Influences of sulfonated β-cyclodextrin on the preparation and properties. Adv. Compos. Hybrid Mater. 2019, 2, 330–338. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Nakahata, M. Supramolecular polymeric materials via cyclodextrin-guest interactions. Acc. Chem. Res. 2014, 47, 2128–2140. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.L.; Zhang, Z.; Li, J. Supramolecular hydrogels based on cyclodextrin-polymer polypseudorotaxanes: Materials design and hydrogel properties. Soft Matter 2011, 7, 11290–11297. [Google Scholar] [CrossRef]
- Zhao, X. Multi-scale multi-mechanism design of tough hydrogels: Building dissipation into stretchy networks. Soft Matter 2014, 10, 672–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clearfield, A.; Stynes, J. The preparation of crystalline zirconium phosphate and some observations on its ion exchange behaviour. J. Inorg. Nucl. Chem. 1964, 26, 117–129. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, R.; Ding, F.; Brittain, A.D.; Liu, J.; Zhang, M.; Xiao, M.; Meng, Y.; Sun, L. Sulfonic acid-functionalized α-zirconium phosphate single-layer nanosheets as a strong solid acid for heterogeneous catalysis applications. ACS Appl. Mater. Interfaces 2014, 6, 7417–7425. [Google Scholar] [CrossRef]
- Troup, J.; Clearfield, A. On the mechanism of ion exchange in zirconium phosphates. 20. refinement of the crystal structure of α-zirconium phosphate. Inorg. Chem. 1977, 16, 3311–3314. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Xiao, M.; Meng, Y.; Sun, L. Designing supported ionic liquids (ILs) within inorganic Nanosheets for CO2 capture applications. ACS Appl. Mater. Interfaces 2016, 8, 5547–5555. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ding, H.; Liu, J.; LaChance, A.M.; Xiao, M.; Meng, Y.; Sun, L. Gold nanoparticles immobilized on single-layer α-zirconium phosphate nanosheets as a highly effective heterogeneous catalyst. Adv. Compos. Hybrid Mater. 2019, 2, 520–529. [Google Scholar] [CrossRef]
- Alberti, G.; Torracca, E. Crystalline insoluble salts of polybasic metals-II. synthesis of crystalline zirconium or titanium phosphate by direct precipitation. J. Inorg. Nucl. Chem. 1968, 30, 317–318. [Google Scholar] [CrossRef]
- Sun, L.; Boo, W.J.; Sue, H.-J.; Clearfield, A. Preparation of α-zirconium phosphate nanoplatelets with wide variations in aspect ratios. New J. Chem. 2007, 31, 39–43. [Google Scholar] [CrossRef]
- Pica, M.; Donnadio, A.; Capitani, D.; Vivani, R.; Troni, E.; Casciola, M. Advances in the chemistry of nanosized zirconium phosphates: A new mild and quick route to the synthesis of nanocrystals. Inorg. Chem. 2011, 50, 11623–11630. [Google Scholar] [CrossRef]
- Yu, J.; Ding, H.; Lampron, J.; Martin, B.R.; Clearfield, A.; Sun, L. Complexing agent directed growth of α-zirconium phosphate-based hexagonal prisms. Inorg. Chem. 2020, 59, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, J.; Huang, R.; Zhang, M.; Xiao, M.; Meng, Y.; Sun, L. Covalently immobilized ionic liquids on single layer nanosheets for heterogeneous catalysis applications. Dalton Trans. 2017, 46, 13126–13134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Noshadi, I.; Ding, H.; Liu, J.; Parnas, R.; Clearfield, A.; Xiao, M.; Meng, Y.; Sun, L. Solid acid catalyst based on single-layer α-zirconium phosphate nanosheets for biodiesel production via esterification. Catalysts 2018, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Ding, F.; Ding, H.; Liu, J.; Xiao, M.; Meng, Y.; Sun, L. Sulfonated poly(fluorenyl ether ketone)/sulfonated α-zirconium phosphate nanocomposite membranes for proton exchange membrane fuel cells. Adv. Compos. Hybrid Mater. 2020, 3, 498–507. [Google Scholar] [CrossRef]
- Clearfield, A. Role of ion exchange in solid-state chemistry. Chem. Rev. 1988, 88, 125–148. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, J.; Liu, J.; Guo, Z.; Umar, A.; Sun, L. Na+ and K+-exchanged zirconium phosphate (ZrP) as high-temperature CO2 Adsorbents. Sci. Adv. Mater. 2013, 5, 469–474. [Google Scholar] [CrossRef]
- Sun, L.; Boo, W.J.; Browning, R.L.; Sue, H.-J.; Clearfield, A. Effect of crystallinity on the intercalation of monoamine in α-zirconium phosphate layer structure. Chem. Mater. 2005, 17, 5606–5609. [Google Scholar] [CrossRef]
- Sun, L.; O’Reilly, J.Y.; Kong, D.; Su, J.Y.; Boo, W.J.; Sue, H.J.; Clearfield, A. The effect of guest molecular architecture and host crystallinity upon the mechanism of the intercalation reaction. J. Colloid Interface Sci. 2009, 333, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Boo, W.J.; Sun, L.; Liu, J.; Clearfield, A.; Sue, H.-J. Effective intercalation and exfoliation of nanoplatelets in epoxy via creation of porous pathways. J. Phys. Chem. C 2007, 111, 10377–10381. [Google Scholar] [CrossRef]
- Hu, H.; Martin, J.C.; Xiao, M.; Southworth, C.S.; Meng, Y.; Sun, L. Immobilization of ionic liquids in layered compounds via mechanochemical intercalation. J. Phys. Chem. C 2011, 115, 5509–5514. [Google Scholar] [CrossRef]
- Lu, N.; Lin, K.-Y.; Kung, C.-C.; Jhuo, J.-W.; Zhou, Y.; Liu, J.; Sun, L. Intercalated polyfluorinated Pd complexes in [small alpha]-zirconium phosphate for Sonogashira and Heck reactions. RSC Adv. 2014, 4, 27329–27336. [Google Scholar] [CrossRef]
- Kaschak, D.M.; Johnson, S.A.; Hooks, D.E.; Kim, H.-N.; Ward, M.D.; Mallouk, T.E. Chemistry on the edge: A microscopic analysis of the intercalation, exfoliation, edge functionalization, and monolayer surface tiling reactions of α-zirconium phosphate. J. Am. Chem. Soc. 1998, 120, 10887–10894. [Google Scholar] [CrossRef]
- Sun, L.; Boo, W.J.; Sun, D.; Clearfield, A.; Sue, H.-J. Preparation of exfoliated epoxy/α-zirconium phosphate nanocomposites containing high aspect ratio nanoplatelets. Chem. Mater. 2007, 19, 1749–1754. [Google Scholar] [CrossRef]
- Ding, H.; Khan, S.T.; Aguirre, K.N.; Camarda, R.S.; Gafney, J.B.; Clearfield, A.; Sun, L. Exfoliation of α-zirconium phosphate using tetraalkylammonium hydroxides. Inorg. Chem. 2020, 59, 7822–7829. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Khan, S.T.; Zeng, S.; Sun, L. Exfoliation of nanosized α-zirconium phosphate in methanol. Inorg. Chem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Boo, W.J.; Sun, L.; Liu, J.; Clearfield, A.; Sue, H.-J.; Mullins, M.J.; Pham, H. Morphology and mechanical behavior of exfoliated epoxy/α-zirconium phosphate nanocomposites. Compos. Sci. Technol. 2007, 67, 262–269. [Google Scholar] [CrossRef]
- Boo, W.J.; Sun, L.; Liu, J.; Moghbelli, E.; Clearfield, A.; Sue, H.-J.; Pham, H.; Verghese, N. Effect of nanoplatelet dispersion on mechanical behavior of polymer nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 1459–1469. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, L.; Zeng, S.; Liu, J.; Xiao, M.; Wang, S.; Meng, Y.; Sun, L. Synthesis of polylactide nanocomposites using an α-zirconium phosphate nanosheet-supported zinc catalyst via in situ polymerization. ACS Appl. Polym. Mater. 2019, 1, 1382–1389. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L. Facile one-pot synthesis of silver nanoparticles supported on α-zirconium phosphate single-layer nanosheets. ES Mater. Manuf. 2019, 5, 24–28. [Google Scholar] [CrossRef]
- Sun, L.; Boo, W.J.; Clearfield, A.; Sue, H.-J.; Pham, H.Q. Barrier properties of model epoxy nanocomposites. J. Membr. Sci. 2008, 318, 129–136. [Google Scholar] [CrossRef]
- Ding, F.; Hu, H.; Ding, H.; Liu, J.; Chen, Y.; Xiao, M.; Meng, Y.; Sun, L. Sulfonated poly(fluorene ether ketone) (SPFEK)/α-zirconium phosphate (ZrP) nanocomposite membranes for fuel cell applications. Adv. Compos. Hybrid Mater. 2020, 3, 546–550. [Google Scholar] [CrossRef]
- Boo, W.J.; Sun, L.; Warren, G.L.; Moghbelli, E.; Pham, H.; Clearfield, A.; Sue, H.-J. Effect of nanoplatelet aspect ratio on mechanical properties of epoxy nanocomposites. Polymer 2007, 48, 1075–1082. [Google Scholar] [CrossRef]
- Sun, L.; Boo, W.-J.; Liu, J.; Clearfield, A.; Sue, H.-J.; Verghese, N.E.; Pham, H.Q.; Bicerano, J. Effect of nanoplatelets on the rheological behavior of epoxy monomers. Macromol. Mater. Eng. 2009, 294, 103–113. [Google Scholar] [CrossRef]
- Moghbelli, E.; Sun, L.; Jiang, H.; Boo, W.J.; Sue, H.-J. Scratch behavior of epoxy nanocomposites containing alpha-zirconium phosphate and core-shell rubber particles. Polym. Eng. Sci. 2009, 49, 483–490. [Google Scholar] [CrossRef]
- Wei, S.; Lizu, M.; Zhang, X.; Sampathi, J.; Sun, L.; Milner, M.F. Electrospun poly(vinyl alcohol)/α-zirconium phosphate nanocomposite fibers. High Perform. Polym. 2013, 25, 25–32. [Google Scholar] [CrossRef]
- Kim, S.; Healy, K.E. Synthesis and characterization of injectable poly (N-isopropylacrylamide-co-acrylic acid) hydrogels with proteolytically degradable cross-links. Biomacromolecules 2003, 4, 1214–1223. [Google Scholar] [CrossRef]
- Cho, J.; Heuzey, M.-C.; Bégin, A.; Carreau, P.J. Physical gelation of chitosan in the presence of β-glycerophosphate: The effect of temperature. Biomacromolecules 2005, 6, 3267–3275. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Ding, H.; Shen, Z.; Qian, L.; Ouyang, J.; Zeng, S.; Seery, T.A.P.; Li, J.; Wu, G.; Chavez, S.E.; et al. Super stretchable and compressible hydrogels inspired by hook-and-loop fasteners. Langmuir 2021, 37, 7760–7770. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mynar, J.L.; Yoshida, M.; Lee, E.; Lee, M.; Okuro, K.; Kinbara, K.; Aida, T. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature 2010, 463, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, T.; Takashima, Y.; Nakahata, M.; Otsubo, M.; Yamaguchi, H.; Harada, A. Preorganized hydrogel: Self-healing properties of supramolecular hydrogels formed by polymerization of host–guest-monomers that contain cyclodextrins and hydrophobic guest groups. Adv. Mater. 2013, 25, 2849–2853. [Google Scholar] [CrossRef] [PubMed]
- Cafferty, B.; Avirah, R.; Schuster, G.; Hud, N. Ultra-sensitive pH control of supramolecular polymers and hydrogels: pKa matching of biomimetic monomers. Chem. Sci. 2014, 5, 4681–4686. [Google Scholar] [CrossRef]
- John, G.; Jung, J.H.; Masuda, M.; Shimizu, T. Unsaturation effect on gelation behavior of aryl glycolipids. Langmuir 2004, 20, 2060–2065. [Google Scholar] [CrossRef]
- Nouri, V.; Moura, M.P.D.S.; Payre, B.; De Almeida, O.; Déjugnat, C.; Franceschi, S.; Perez, E. How an organogelator can gelate water: Gelation transfer from oil to water induced by a nanoemulsion. Soft Matter 2020, 16, 2371–2378. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.; Khan, S.T.; Liu, J.; Sun, L. Gelation Based on Host–Guest Interactions Induced by Multi-Functionalized Nanosheets. Gels 2021, 7, 106. https://doi.org/10.3390/gels7030106
Ding H, Khan ST, Liu J, Sun L. Gelation Based on Host–Guest Interactions Induced by Multi-Functionalized Nanosheets. Gels. 2021; 7(3):106. https://doi.org/10.3390/gels7030106
Chicago/Turabian StyleDing, Hao, Sana T. Khan, Jingjing Liu, and Luyi Sun. 2021. "Gelation Based on Host–Guest Interactions Induced by Multi-Functionalized Nanosheets" Gels 7, no. 3: 106. https://doi.org/10.3390/gels7030106





