Poly(2-Hydroxyethyl methacrylate-co-N,N-dimethylacrylamide)-Coated Quartz Crystal Microbalance Sensor: Membrane Characterization and Proof of Concept
Abstract
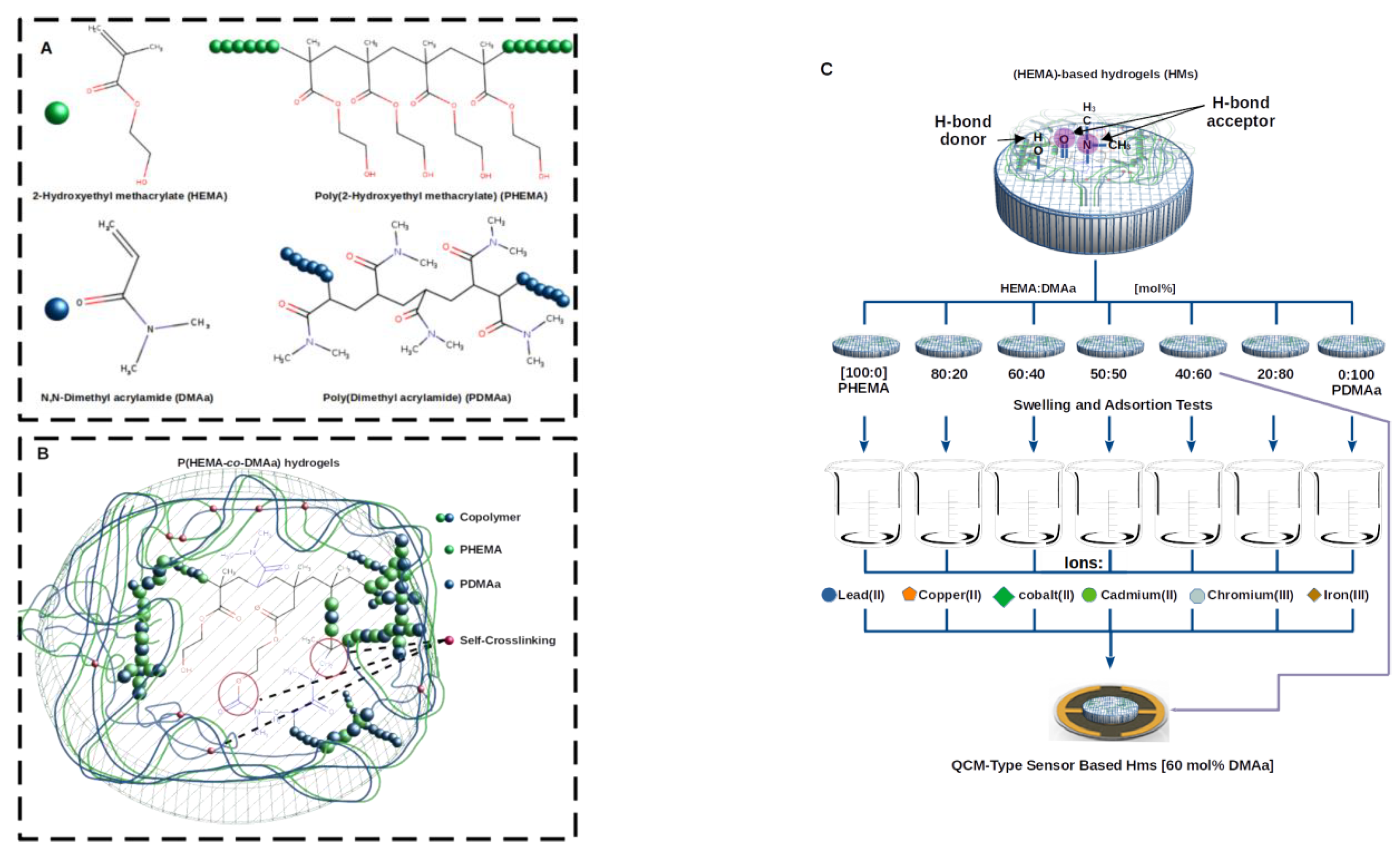
:1. Introduction
2. Results and Discussion
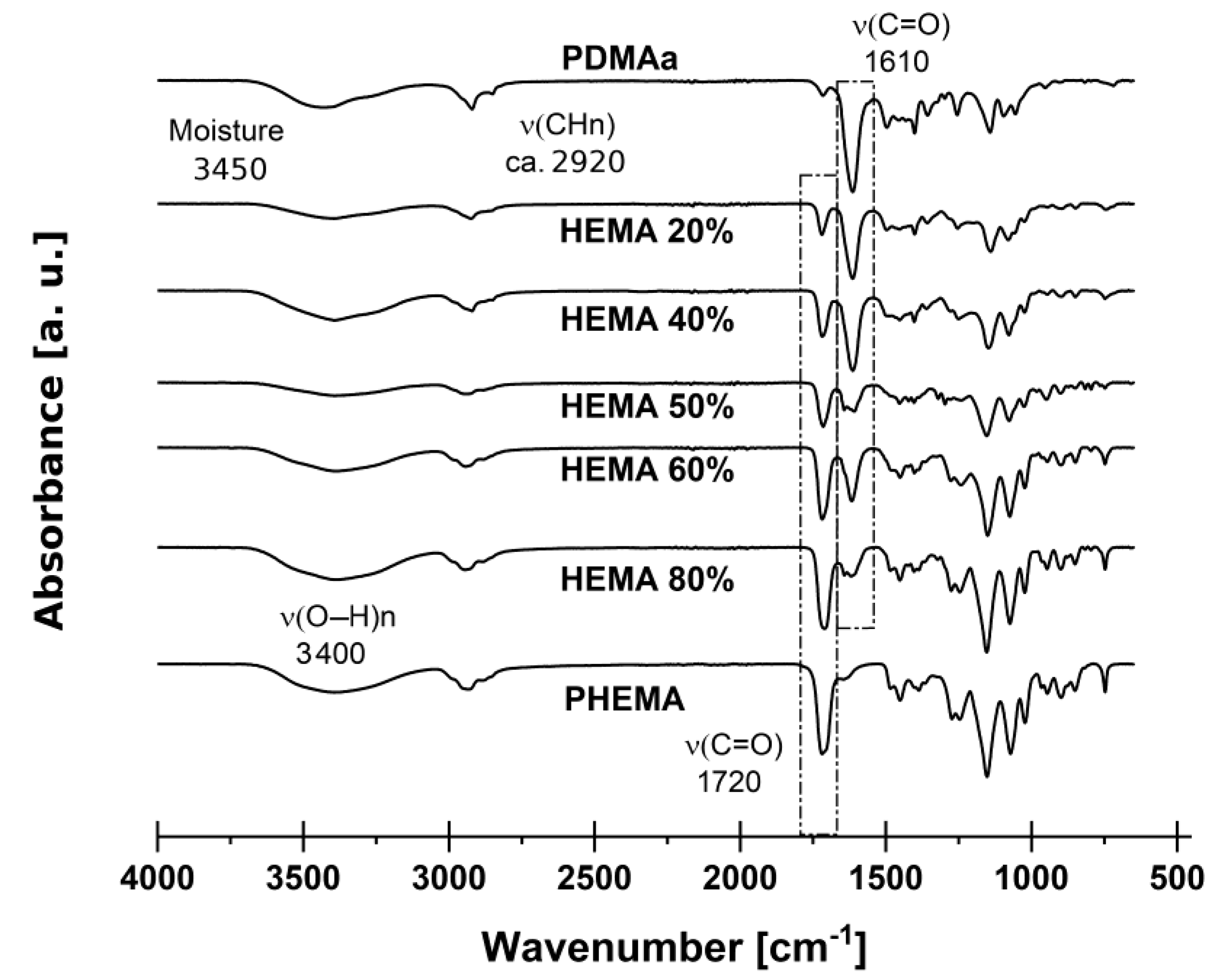
2.1. Synthesis of the Copolymer Membranes and Chemical Characterization
2.2. Swelling Behavior
2.3. Sorption Study
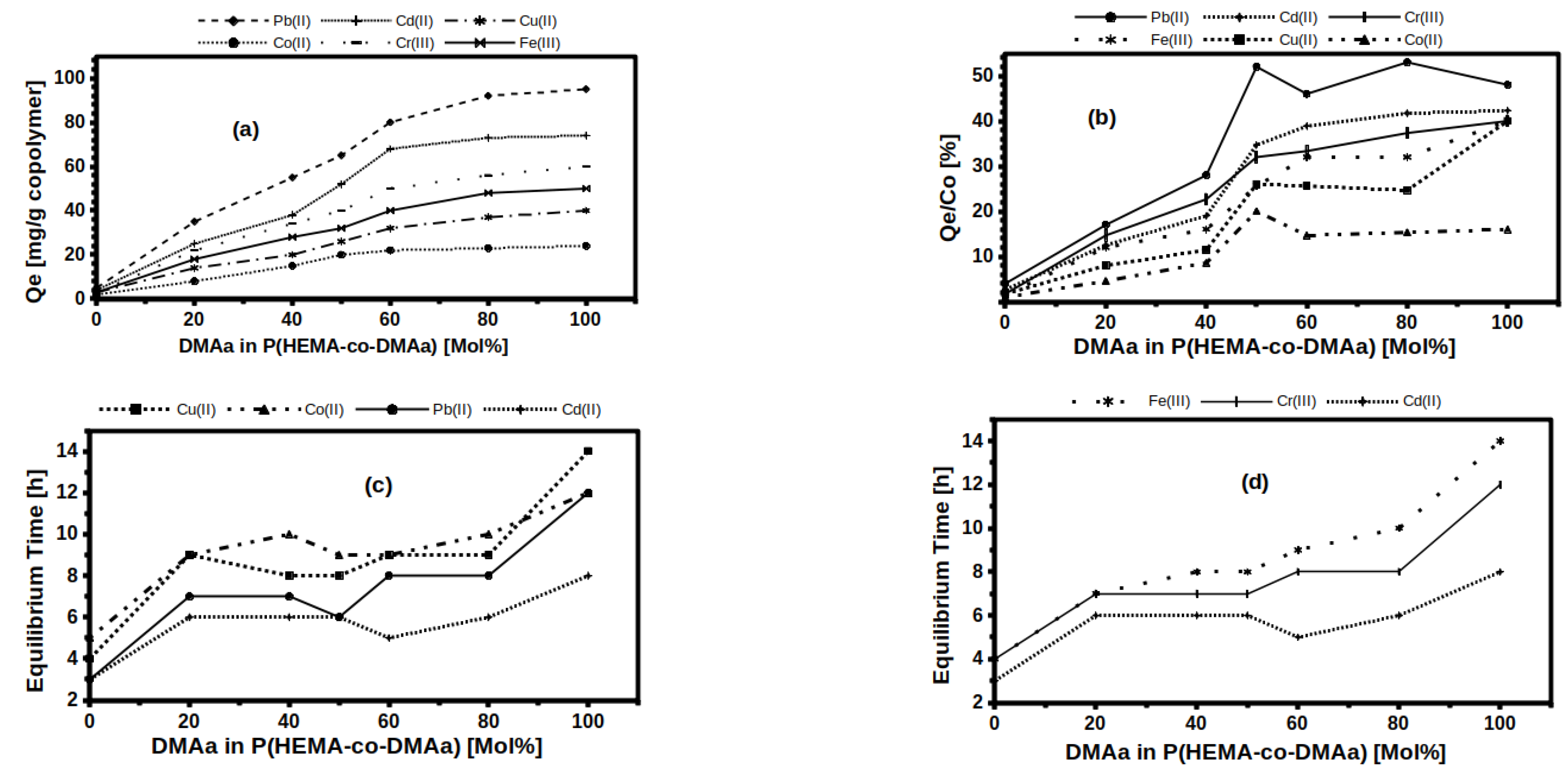
2.4. Sorption Equilibrium
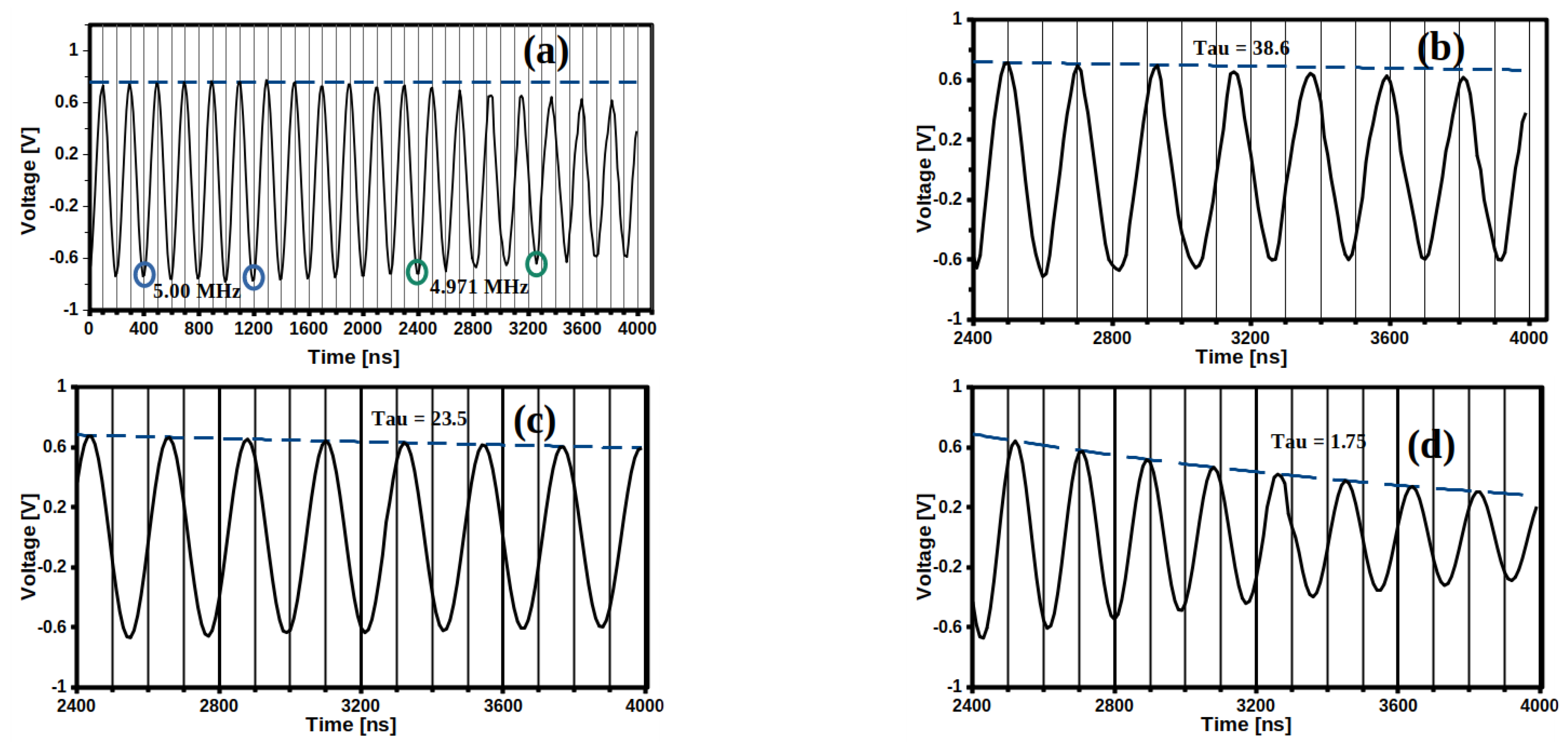
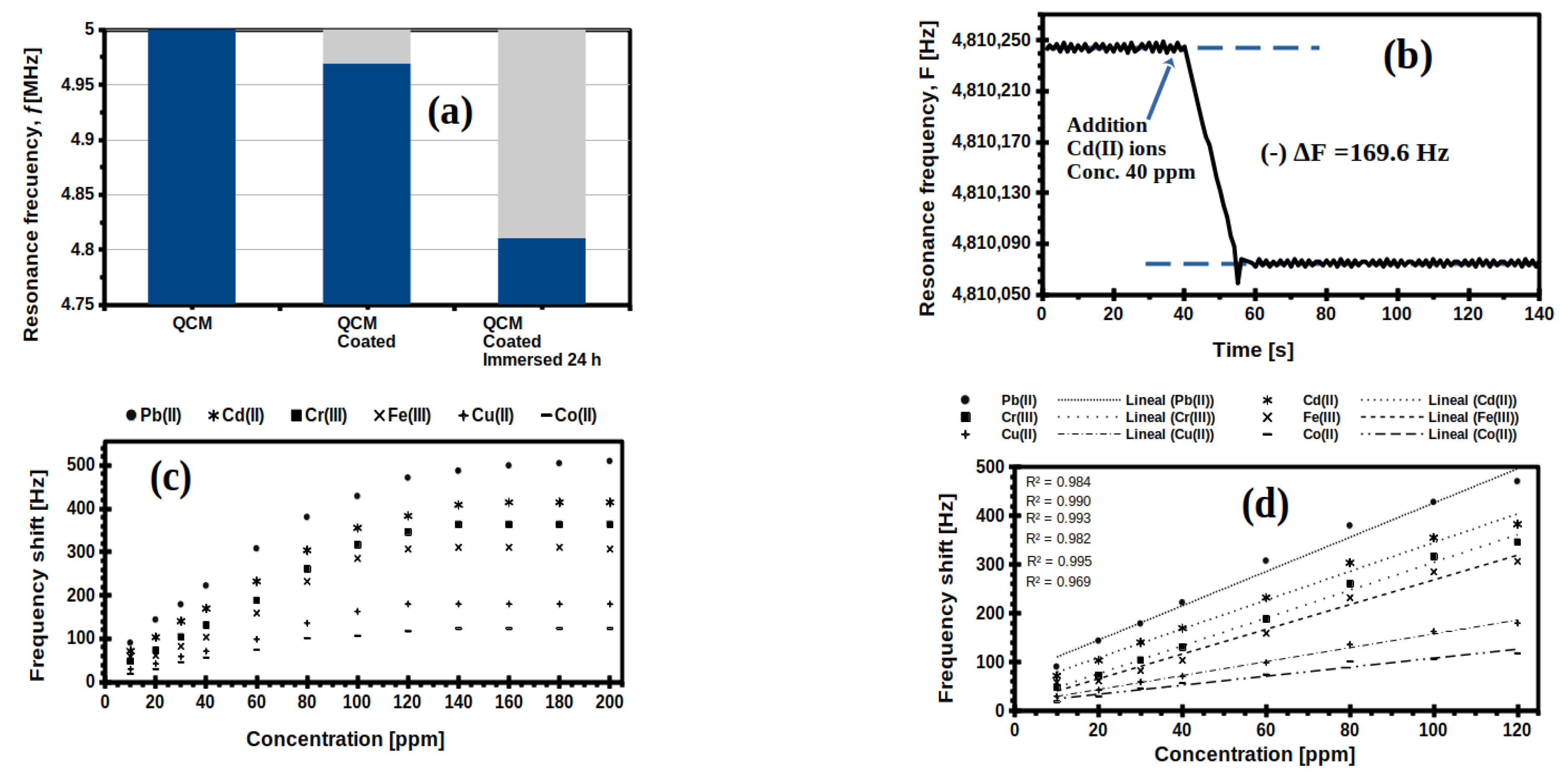
2.5. Evaluation of QCM-Sensors Coated
2.6. Proof of Concept to QCM-Sensors
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Hydrogel Membranes Synthesis
4.3. Characterization and Techniques
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Janata, J. Chemical sensors. Anal. Chem. 1992, 64, 196–219. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.; Wilson, G.S. Electrochemical Biosensors: Recommended Definitions and Classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Turner, A.; Karube, I.; Wilson, G.; Worsfold, P. Biosensors: Fundamentals and Applications; Oxford University Press: Oxford, UK, 1987. [Google Scholar]
- Ruiu, A.; Vonlanthen, M.; Morales-Espinoza, E.G.; Rojas-Montoya, S.M.; González-Méndez, I.; Rivera, E. Pyrene Chemosensors for Nanomolar Detection of Toxic and Cancerogenic Amines. J. Mol. Struct. 2019, 1196, 1–7. [Google Scholar] [CrossRef]
- Gavalas, V.; Wang, J.; Bachas, L. Membranes for the Development of Biosensors. In New Insights into Membrane Science and Technology: Polymeric and Biofunctional Membranes; Bhattacharyya, D., Butterfield, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 8, pp. 379–392. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0927519303800219?via%3Dihub (accessed on 7 August 2021).
- Zhao, C.; Si, Y.; Pan, B.; Taha, A.Y.; Pan, T.; Sun, G. Design and Fabrication of a Highly Sensitive and Naked-Eye Distinguishable Colorimetric Biosensor for Chloramphenicol Detection by Using ELISA on Nanofibrous Membranes. Talanta 2020, 217, 121054. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Malmagro, J.; García-Molina, G.; De Lacey, A. Electrochemical Biosensors Based on Membrane-Bound Enzymes in Biomimetic Configurations. Sensors 2020, 20, 3393. [Google Scholar] [CrossRef]
- Hernandez-Martinez, A. Materials Science and Lab-on-a-Chip for Environmental and Industrial Analysis. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, Boddula, R., Asiri, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 225–236. [Google Scholar] [CrossRef]
- Ahmad, O.S.; Bedwell, T.S.; Esen, C.; Cruz, A.G.; Piletsky, S.A. Molecularly Imprinted Polymers in Electrochemical and Optical Sensors. Trends Biotechnol. 2018, 37, 294–309. [Google Scholar] [CrossRef]
- Wang, J.; Liang, R.; Qin, W. Molecularly Imprinted Polymer-Based Potentiometric Sensors. TrAC Trends Anal. Chem. 2020, 130, 115980. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel Scaffolds for Tissue Engineering: Progress and Challenges. Glob. Cardiol. Sci. Pr. 2013, 2013, 316–342. [Google Scholar] [CrossRef] [Green Version]
- Spicer, C.D. Hydrogel Scaffolds for Tissue Engineering: The Importance of Polymer Choice. Polym. Chem. 2019, 11, 184–219. [Google Scholar] [CrossRef]
- Liang, K.; Bae, K.H.; Kurisawa, M. Recent Advances in the Design of Injectable Hydrogels for Stem Cell-Based Therapy. J. Mater. Chem. B 2019, 7, 3775–3791. [Google Scholar] [CrossRef]
- Kousalová, J.; Etrych, T. Polymeric Nanogels as Drug Delivery Systems. Physiol. Res. 2018, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Bjørge, I.M.; Costa, M.; Silva, A.; Vidal, J.P.O.; Nobrega, J.; Mano, J.F. Tuneable Spheroidal Hydrogel Particles for Cell and Drug Encapsulation. Soft Matter 2018, 14, 5622–5627. [Google Scholar] [CrossRef]
- Wakuda, Y.; Nishimoto, S.; Suye, S.-I.; Fujita, S. Native Collagen Hydrogel Nanofibres with Anisotropic Structure Using Core-Shell Electrospinning. Sci. Rep. 2018, 8, 6248. [Google Scholar] [CrossRef]
- Yazdi, M.K.; Vatanpour, V.; Taghizadeh, A.; Taghizadeh, M.; Ganjali, M.R.; Munir, M.T.; Habibzadeh, S.; Saeb, M.R.; Ghaedi, M. Hydrogel membranes: A review. Mater. Sci. Eng. C 2020, 114, 111023. [Google Scholar] [CrossRef]
- Qi, M.; Zhao, K.; Bao, Q.; Pan, P.; Zhao, Y.; Yang, Z.; Wang, H.; Wei, J. Adsorption and Electrochemical Detection of Bovine Serum Albumin Imprinted Calcium Alginate Hydrogel Membrane. Polymers 2019, 11, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, J.P.H.; López-Cabarcos, E.; López-Ruiz, B. The Application of Methacrylate-Based Polymers to Enzyme Biosensors. Biomol. Eng. 2006, 23, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Rosiak, J.M.; Yoshii, F. Hydrogels and Their Medical Applications. In Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms; Elsevier: Amsterdam, The Netherlands, 1999; Volume 151, pp. 56–64. [Google Scholar] [CrossRef]
- Ozcelik, B. Degradable Hydrogel Systems for Biomedical Applications. In Biosynthetic Polymers for Medical Applications; Poole-Warren, L., Martens, P., Green, R., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 173–188. [Google Scholar] [CrossRef]
- Holback, H.; Yeo, Y.; Park, K. Hydrogel Swelling Behavior and Its Biomedical Applications. In Biomedical Hydrogels; Rimmer, S., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 3–24. [Google Scholar] [CrossRef] [Green Version]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the Porosity and Microarchitecture of Hydrogels for Tissue Engineering. Tissue Eng. Part. B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2013, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Peppas, N. Hydrogels in Pharmaceutical Formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Bezemer, J.; Radersma, R.; Grijpma, D.; Dijkstra, P.; Feijen, J.; van Blitterswijk, C. Zero-Order Release of Lysozyme from poly(ethylene Glycol)/Poly(butylene Terephthalate) Matrices. J. Control. Release 2000, 64, 179–192. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Peppas, N.A. Equilibrium Swelling Behavior of pH-Sensitive Hydrogels. Chem. Eng. Sci. 1991, 46, 715–722. [Google Scholar] [CrossRef]
- Brahim, S.; Narinesingh, D.; Guiseppi-Elie, A. Amperometric Determination of Cholesterol in Serum Using a Biosensor of Cholesterol Oxidase Contained within a Polypyrrole–Hydrogel Membrane. Anal. Chim. Acta 2001, 448, 27–36. [Google Scholar] [CrossRef]
- Demir, E.F.; Özçalışkan, E.; Karakaş, H.; Uygun, M.; Uygun, D.A.; Akgöl, S.; Denizli, A. Synthesis and Characterization of Albumin Imprinted Polymeric Hydrogel Membranes for Proteomic Studies. J. Biomater. Sci. Polym. Ed. 2018, 29, 2218–2236. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.F.; Kuru, C.I.; Uygun, M.; Uygun, D.A.; Akgöl, S. Antibody Separation Using Lectin Modified poly(HEMA-EDMA) Hydrogel Membranes. J. Biomater. Sci. Polym. Ed. 2017, 29, 344–359. [Google Scholar] [CrossRef] [PubMed]
- Hacker, M.C.; Krieghoff, J.; Mikos, A.G. Synthetic Polymers. In Principles of Regenerative Medicine, 3rd ed.; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 559–590. [Google Scholar] [CrossRef]
- Montheard, J.-P.; Chatzopoulos, M.; Chappard, D. 2-Hydroxyethyl Methacrylate (HEMA): Chemical Properties and Applications in Biomedical Fields. J. Macromol. Sci. Part. C 1992, 32, 1–34. [Google Scholar] [CrossRef]
- Holly, F.J.; Refojo, M.F. Wettability of Hydrogels I. poly(2-Hydroxyethyl Methacrylate). J. Biomed. Mater. Res. 1975, 9, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Refojo, M.F.; Yasuda, H. Hydrogels from 2-Hydroxyethyl Methacrylate and Propylene Glycol Monoacrylate. J. Appl. Polym. Sci. 1965, 9, 2425–2435. [Google Scholar] [CrossRef]
- Roointan, A.; Farzanfar, J.; Mohammadi-Samani, S.; Behzad-Behbahani, A.; Farjadian, F. Smart pH Responsive Drug Delivery System Based on poly(HEMA-Co-DMAEMA) Nanohydrogel. Int. J. Pharm. 2018, 552, 301–311. [Google Scholar] [CrossRef]
- Satish, C.S.; Shivakumar, H.G. Formulation and Evaluation of Self-Regulated Insulin Delivery System Based on poly(HEMA-co-DMAEMA) Hydrogels. J. Macromol. Sci. Part. A 2007, 44, 379–387. [Google Scholar] [CrossRef]
- Benjamin, C.; Craven, R.; Crone, W.; Lakes, R. Viscoelastic Characterization of pH-Sensitive 2-Hydroxyethyl Methacrylate(2-dimethylamino) Ethyl Methacrylate HEMA-DMAEMA Hydrogels. Polym. Test. 2018, 72, 372–376. [Google Scholar] [CrossRef]
- Erol, O.; Pantula, A.; Liu, W.; Gracias, D.H. Transformer Hydrogels: A Review. Adv. Mater. Technol. 2019, 4. [Google Scholar] [CrossRef] [Green Version]
- Rueda, J.C.; Santillan, F.; Komber, H.; Voit, B. Synthesis and Characterization of Stiff, Self-Crosslinked Thermoresponsive DMAA Hydrogels. Polymers 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and Trends for Next-Generation Rechargeable Lithium and Lithium-Ion Batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Chen, S.; Zhao, S.; Zhang, G.; Cui, J.; Yan, Y. The Interaction between N, N-Dimethylacrylamide and Pristine Graphene and Its Role in Fabricating a Strong Nanocompo-Site Hydrogel. J. Mater. Sci. 2020, 55, 7652–7664. [Google Scholar] [CrossRef]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide Degradation and Its Implications in Environmental Systems. NPJ Clean Water 2018, 1, 17. [Google Scholar] [CrossRef]
- Hernandez-Martínez, A.; Lujan-Montelongo, J.; Silva-Cuevas, C.; Mota-Morales, J.D.; Cortez-Valadez, M.; Ruíz-Baltazar, D.J.; Cruz, M.; Herrera-Ordonez, J. Swelling and Methylene Blue Adsorption of poly(N, N-Dimethylacrylamide-Co-2-Hydroxyethyl Methacrylate) Hydrogel. React. Funct. Polym. 2018, 122, 75–84. [Google Scholar] [CrossRef]
- Ramos-Jacques, A.; Lujan-Montelongo, J.; Silva-Cuevas, C.; Cortez-Valadez, M.; Estevez, M.; Hernández-Martínez, A. Lead (II) Removal by poly(N, N-Dimethylacrylamide-Co-2-Hydroxyethyl Methacrylate). Eur. Polym. J. 2018, 101, 262–272. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. J. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Birkholz, M.; Rudert, R. Interatomic Distances in Pyrite-Structure Disulfides-A Case for Ellipsoidal Modeling of Sulfur Ions. Phys. Status Solidi B 2008, 245, 1858–1864. [Google Scholar] [CrossRef]
- Feng, J.; Reponen, A.M.; Romanov, A.S.; Linnolahti, M.; Bochmann, M.; Greenham, N.C.; Penfold, T.; Credgington, D. Influence of Heavy Atom Effect on the Photophysics of Coinage Metal Carbene-Metal-Amide Emitters. Adv. Funct. Mater. 2020, 31. [Google Scholar] [CrossRef]
- Barnes, D.J.; Chapman, R.L.; Stephens, F.S.; Vagg, R.S. Studies on the Metal-Amide Bond. VII. Metal Complexes of the Flexible N4 Ligand N, N′-bis(2′-pyridinecarboxamide)1,2-Ethane. Inorg. Chim. Acta 1981, 51, 155–162. [Google Scholar] [CrossRef]
- Chapman, R.; Stephens, F.S.; Vagg, R.S. Studies on the Metal-Amide Bond. II. The Crystal Structure of the Deprotonated copper(II) Complex of N, N′-Bis-(2′-Pyridinecarboxamide)-1,2-Benzene. Inorg. Chim. Acta 1980, 43, 29–33. [Google Scholar] [CrossRef]
- Stennett, C.R.; Wagner, C.L.; Fettinger, J.C.; Vasko, P.; Power, P.P. Reductions of M{N(SiMe3)2}3 (M = V, Cr, Fe): Terminal and Bridging Low-Valent First-Row Transition Metal Hydrido Complexes and “Metallo-Transamination”. Inorg. Chem. 2021, 60, 11401–11411. [Google Scholar] [CrossRef] [PubMed]
- Voinova, M.V.; Rodahl, M.; Jonson, M.; Kasemo, B. Viscoelastic Acoustic Response of Layered Polymer Films at Fluid-Solid Interfaces: Continuum Mechanics Approach. Phys. Scr. 1999, 59, 391–396. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Martinez, A.R. Poly(2-Hydroxyethyl methacrylate-co-N,N-dimethylacrylamide)-Coated Quartz Crystal Microbalance Sensor: Membrane Characterization and Proof of Concept. Gels 2021, 7, 151. https://doi.org/10.3390/gels7040151
Hernandez-Martinez AR. Poly(2-Hydroxyethyl methacrylate-co-N,N-dimethylacrylamide)-Coated Quartz Crystal Microbalance Sensor: Membrane Characterization and Proof of Concept. Gels. 2021; 7(4):151. https://doi.org/10.3390/gels7040151
Chicago/Turabian StyleHernandez-Martinez, Angel Ramon. 2021. "Poly(2-Hydroxyethyl methacrylate-co-N,N-dimethylacrylamide)-Coated Quartz Crystal Microbalance Sensor: Membrane Characterization and Proof of Concept" Gels 7, no. 4: 151. https://doi.org/10.3390/gels7040151





