Structural Characterization and Peroxidation Stability of Palm Oil-Based Oleogel Made with Different Concentrations of Carnauba Wax and Processed with Ultrasonication
Abstract
:1. Introduction
2. Results and Discussion
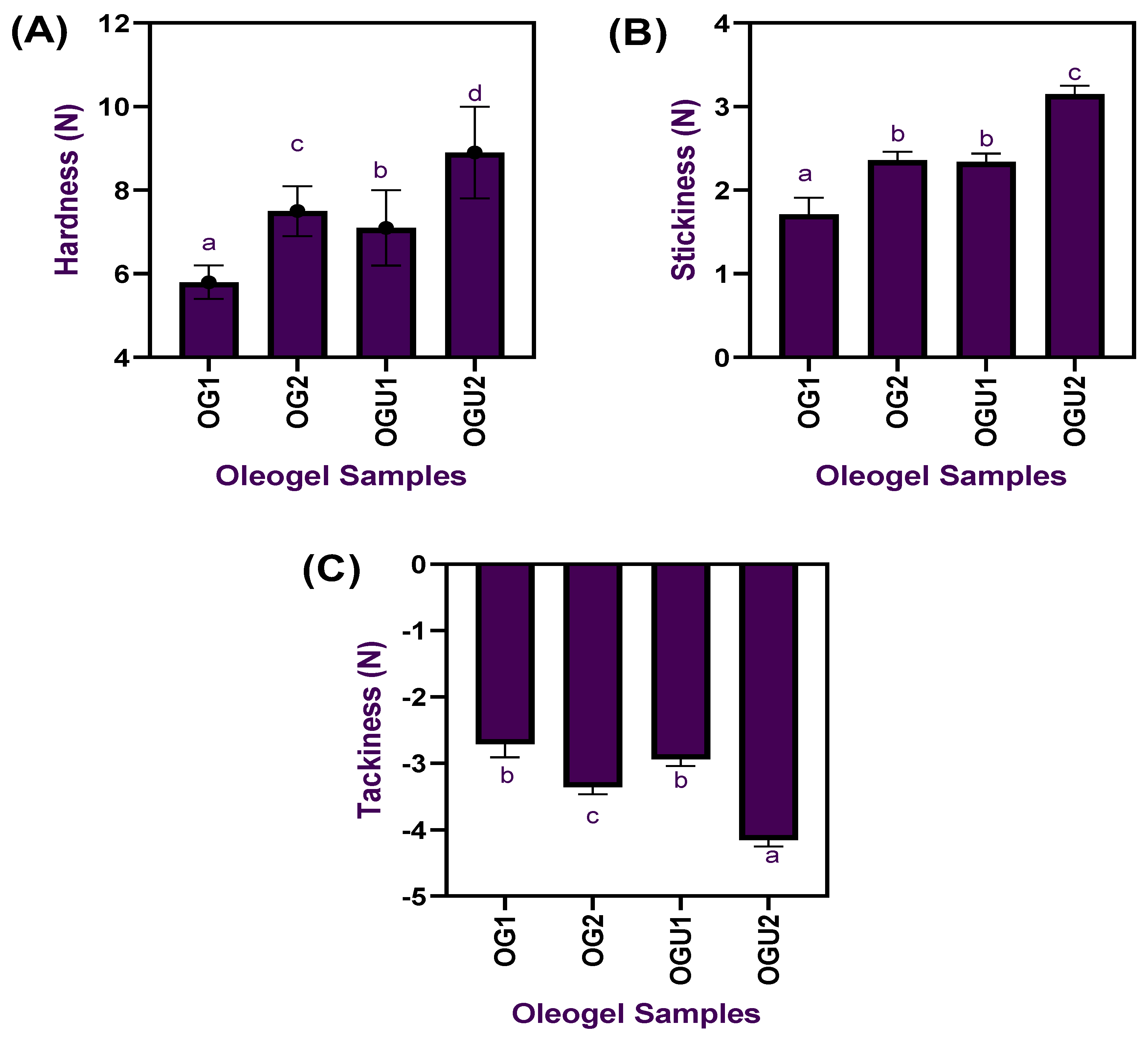
2.1. Color and Textural Profiles
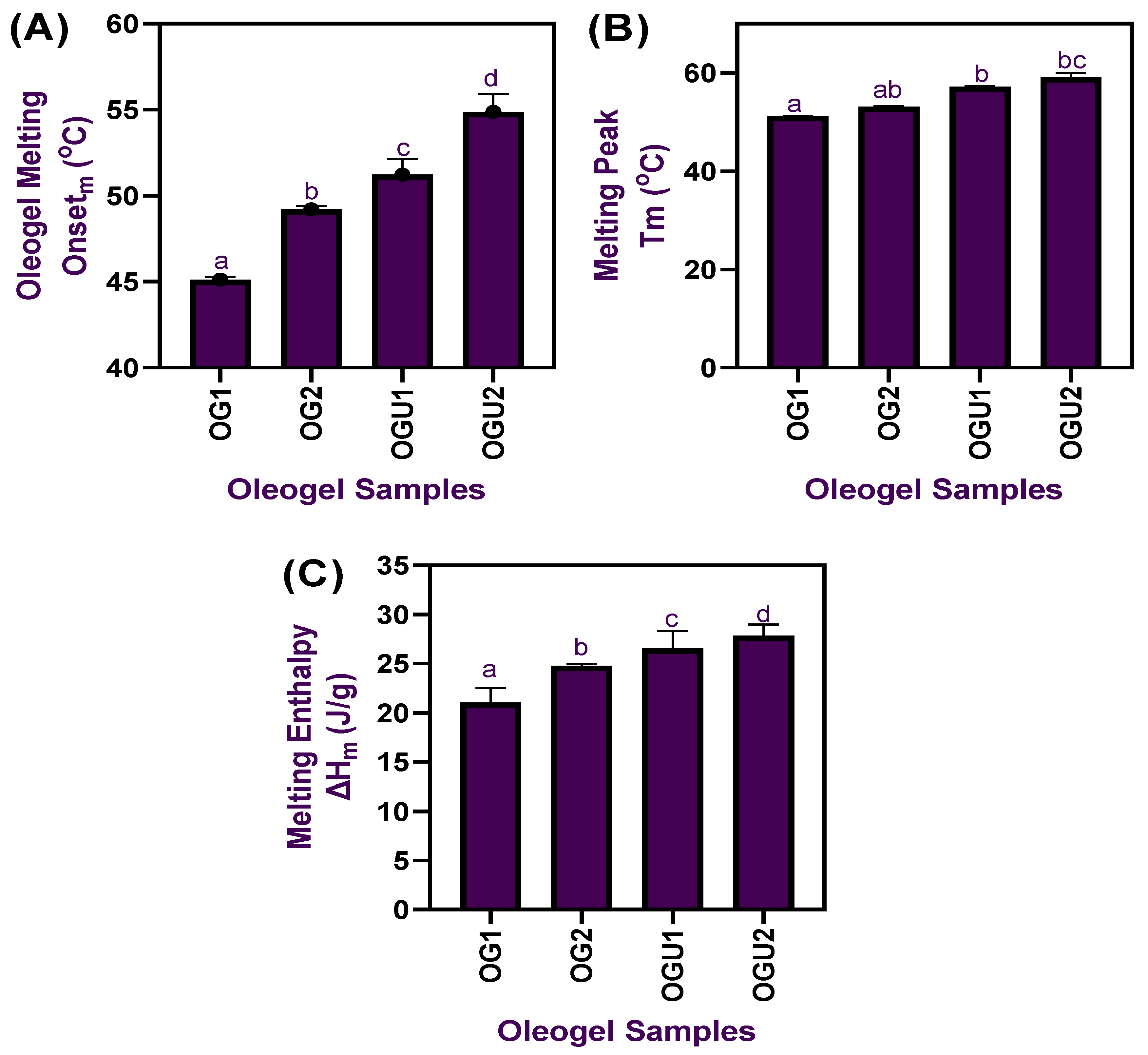
2.2. Thermal Properties
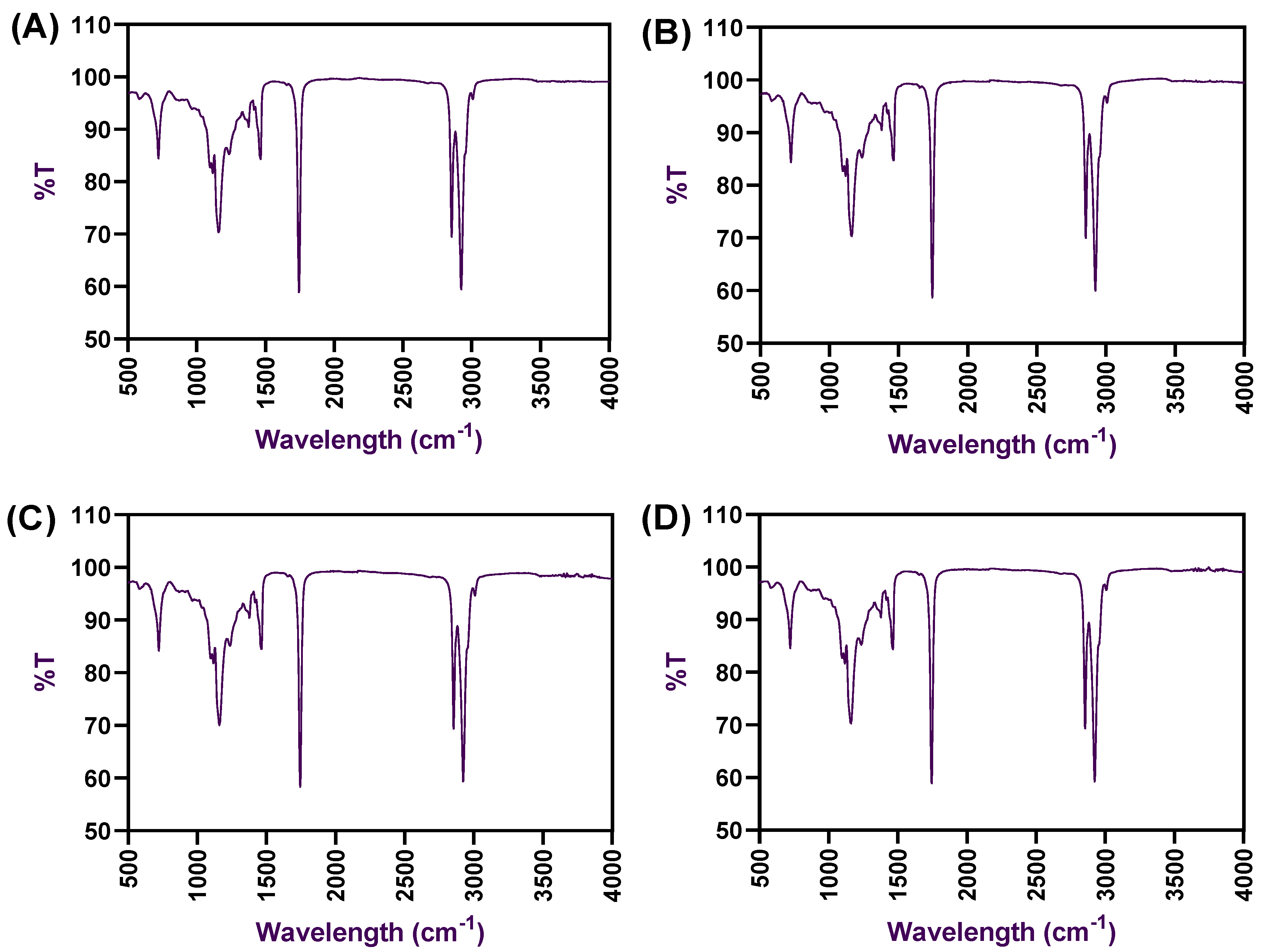
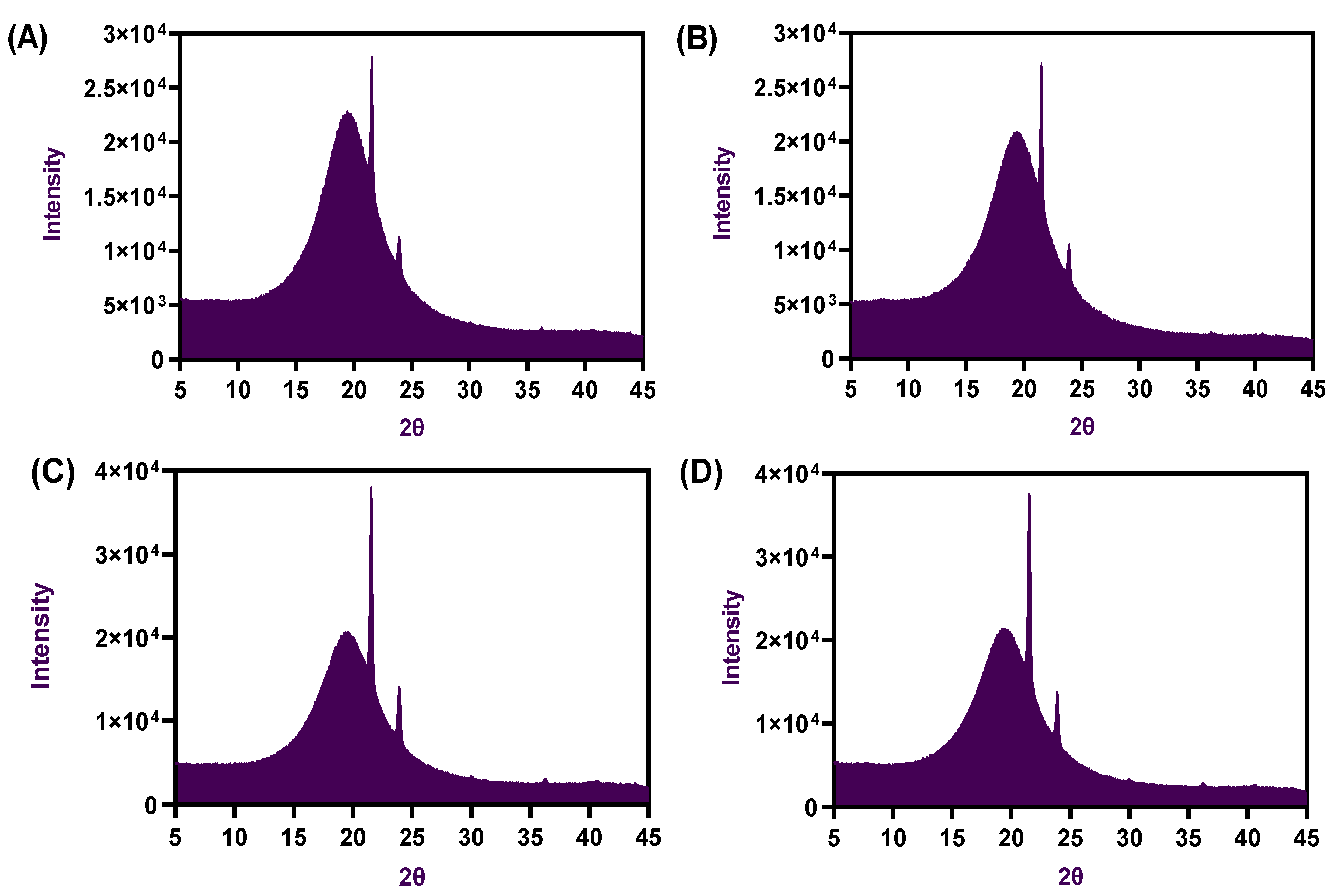
2.3. FTIR Spectra and X-ray Diffractions
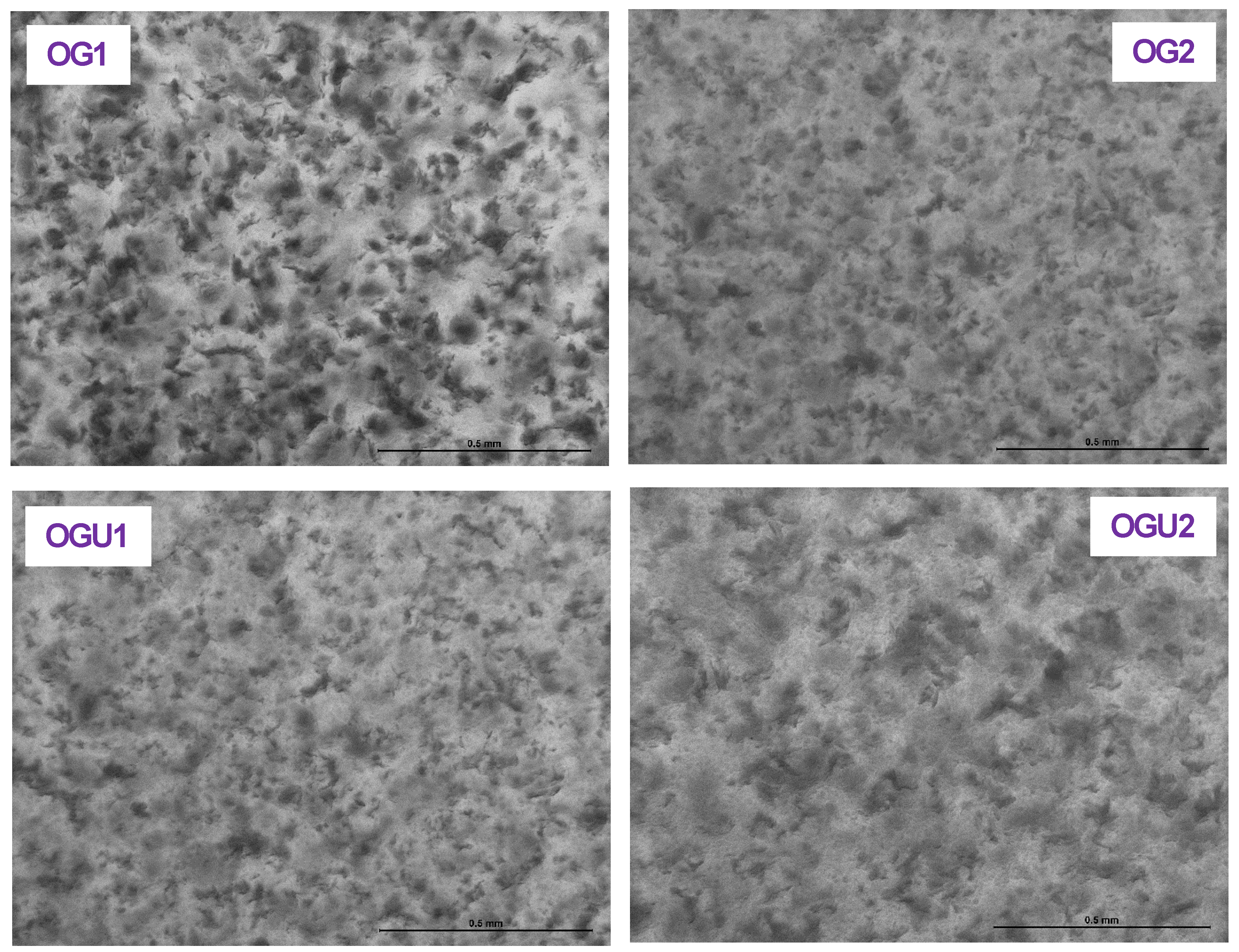
2.4. Microscopical Observations
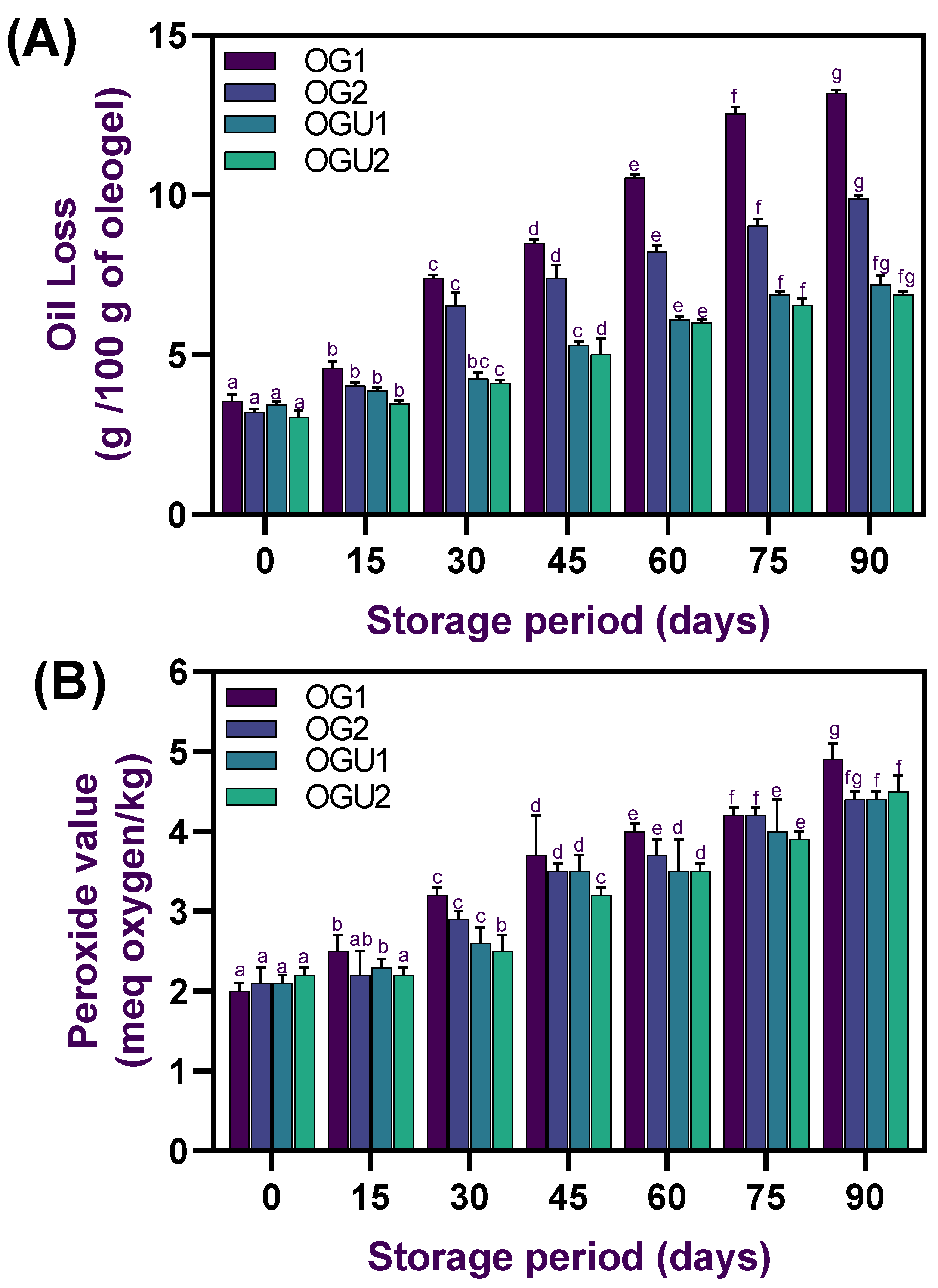
2.5. Oil Loss and Oxidative Stability
3. Conclusions
4. Materials and Methods
4.1. Raw material, Chemicals, and Reagents
4.2. Oleogel Preparation
4.3. Analysis
4.3.1. Color Characteristics
4.3.2. Texture Profile
4.3.3. Thermal Properties
4.3.4. Morphological Observations
4.3.5. XRD Analysis
4.3.6. FTIR Spectroscopy
4.3.7. Oil loss and Oxidative Stability of Oleogels
Oil Loss
Peroxide Value (PV)
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marangoni, G.A.; van Duynhoven, M.P.J.; Acevedo, C.N.; Nicholson, A.R.; Patel, R.A. Advances in our understanding of the structure and functionality of edible fats and fat mimetics. Soft Matters 2019, 16, 289–306. [Google Scholar] [CrossRef]
- Marangoni, G.A. Fat crystal networks-structure and properties. Microsc. Microanal. 2002, 8, 242–243. [Google Scholar] [CrossRef] [Green Version]
- DiNicolantonio, J.J.; Lucan, C.S.; O’Keefe, H.J. The evidence for saturated fat and for sugar related to coronary heart disease. Prog. Cardiovasc. Dis. 2016, 58, 464–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horlings, G.L.; Marsden, T.K. Towards the real green revolution? Exploring the conceptual dimensions of a new ecological modernization of agriculture that could ‘feed the world’. Glob. Environ. Chang. 2011, 21, 441–452. [Google Scholar] [CrossRef]
- Dias, L.S.F.; Lima, F.M.; Velasco, C.P.; Salles-Costa, R.; Sardinha, C.L.F.; do Carmo, M. Were policies in Brazil effective to reducing trans-fat from industrial origin in foods. Rev. Saude Publica. 2018, 52, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, M.; Allen, B.; Liska, D.; Dourson, M.; Haber, L. Meta regression analysis of the effect of trans fatty acids (TFA) on LDL-Cholesterol. FASEB J. 2015, 29, 923.15. [Google Scholar] [CrossRef]
- Zhang, Z.; Lee, J.W.; Zhou, H.; Wang, Y. Effect of chemical interesterification on the triacylglycerols, solid fat contents and crystallization kinetics of palm oil-based fats. Food Funct. 2019, 10, 7553–7564. [Google Scholar] [CrossRef]
- Thakur, D.; Singh, A.; Prabhakar, P.; Meghwal, M.; Upadhyay, A. Optimization and characterization of soybean oil-carnauba wax oleogel. LWT 2022, 157, 113108. [Google Scholar] [CrossRef]
- Li, J.; Guo, R.; Wang, M.; Bi, Y.; Zhang, H.; Xu, X. Development and characterization of compound oleogels based on monoglycerides and edible waxes. ACS Food Sci. Technol. 2022, 2, 302–314. [Google Scholar] [CrossRef]
- Noonim, P.; Rajasekaran, B.; Venkatachalam, K. Effect of palm oil-carnauba wax oleogel that processed with ultrasonication on the physicochemical properties of salted duck egg white fortified instant noodles. Gels 2022, 8, 487. [Google Scholar] [CrossRef] [PubMed]
- Winkler-Moser, J.K.; Anderson, J.; Byars, J.A.; Singh, M.; Hwang, H.-S. Evaluation of Beeswax, Candelilla Wax, Rice Bran Wax, and Sunflower Wax as Alternative Stabilizers for Peanut Butter. J. Am. Oil Chem. Soc. 2019, 96, 1235–1248. [Google Scholar] [CrossRef]
- Feichtinger, A.; Scholten, E. Preparation of protein oleogels: Effect on structure and functionality. Foods 2020, 9, 1745. [Google Scholar] [CrossRef] [PubMed]
- Freitas, S.A.C.; Sousa, M.H.P.; Soares, J.D.; Silva, G.Y.J.; Benjamin, R.S.; Guedes, F.I.M. Carnauba wax uses in food–A review. Food Chem. 2019, 291, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.X.; Wang, M.J.; Guo, J.; Wan, L.J.; Yin, W.S.; Yang, Q.X. Hierarchical high internal phase emulsion and transparent oleogels stabilized by quillaja saponin coated nanodroplets for color performance. Food Funct. 2017, 8, 823. [Google Scholar] [CrossRef] [PubMed]
- Ogutcu, M.; Arifoglu, N.; Yilmaz, E. Preparation and characterization of virgin olive oil-beeswax oleogel emulsion products. J. Am. Oil Chem. Soc. 2015, 92, 459–471. [Google Scholar] [CrossRef]
- Pucas, A.; Tanislav, A.E.; Muresan, E.A.; Farcas, C.A.; Muresan, V. Walnut oil oleogels as milk fat replacing system for commercially available chocolate butter. Gels 2022, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, S.; Masoodi, F.A.; Naqash, F.; Rashid, R. Oleogels: Promising alternatives to solid fats for food applications. Food Hydrocol. Health 2022, 2, 100058. [Google Scholar] [CrossRef]
- Meng, Z.; Qi, K.; Guo, Y.; Wang, Y.; Liu, Y. Effects of thickening agents on the formation and properties of edible oleogels based on hydroxypropyl methyl cellulose. Food Chem. 2018, 246, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Campanella, O.; Maleky, F. The effects of whey protein and oleogel interactions on mechanical properties of oleocolloids and hydro-oleocolloids matrices. Food Hydrocoll. 2022, 124, 107285. [Google Scholar] [CrossRef]
- Sharifi, M.; Goli, S.A.H.; Fayaz, G. Exploitation of high-intensity ultrasound to modify the structure of olive oil organogel containing propolis wax. Int. J. Food Sci. Technol. 2019, 54, 509–515. [Google Scholar] [CrossRef]
- Lassila, P.; Valoppi, F.; Tommiska, O.; Hyvonen, J.; Holmstrom, A.; Hietala, S.; Salmi, A.; Haeggstrom, E. Practical scale modification of oleogels by ultrasonic standing waves. Ultrasonic. Sonochem. 2022, 85, 105970. [Google Scholar] [CrossRef] [PubMed]
- Ogutcu, M.; Yilmaz, E. Comparison of the pomegranate seed oil organogels of carnauba wax and monoglyceride. J. App. Polym. Sci. 2015, 132, 41343. [Google Scholar] [CrossRef]
- Aranda-Ledesma, E.N.; Bautista-Hernandez, I.; Rojas, R.; Aguilar-Zarate, P.; Medina-Herrera, P.N.; Castro-Lopez, C.; Martinez-Avila, G.C.G. Candelilla wax: Prospective suitable applications within the food field. LWT 2022, 159, 113170. [Google Scholar] [CrossRef]
- Co, E.D.; Marangoni, A.G. Organogels: An alternative edible oil-structuring method. J. Am. Oil Chem. Soc. 2012, 89, 749–780. [Google Scholar] [CrossRef]
- Gordon, S.H.; Cao, X.; Willett, L.J.M. Infrared spectroscopy method reveals hydrogen bonding and intermolecular interactions between components in polymer blends. J. App. Polym. Sci. 2015, 97, 813–821. [Google Scholar] [CrossRef]
- den Adel, R.; Heussen, P.C.M.; Bot, A. Effect of water on self-assembled tubules in β-sitosterol + γ-oryzanol-based oleogels. J. Phys. Conf. Ser. 2010, 247, 012025. [Google Scholar] [CrossRef]
- Ahmadi, P.; Tabibiazar, M.; Roufegarinejad, L.; Babazadeh, A. Development of behenic acid-ethyl cellulose oleogel stabilized pickering emulsions as low-calorie fat replacer. Int. J. Biol. Macromol. 2020, 150, 974–981. [Google Scholar] [CrossRef]
- Sullivan, M.C.; Barbut, S.; Marangoni, G.A. Edible oleogels for the oral delivery of lipid soluble molecules: Composition and structural design considerations. Trends Food Sci. Technol. 2016, 57, 59–73. [Google Scholar] [CrossRef] [Green Version]
- Okuro, P.K.; Malfatti-Gasperini, A.A.; Vicente, A.A.; Cunha, R.L. Lecithin and phytosterols-based mixtures as hybrid structuring agents in different organic phases. Food Res. Int. 2018, 111, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, M.; Begg, F.; Bhattacharyya, K.D.; Bandyopadhya, N.; Ghose, M. Nutritional evaluations of oleogel made from micronutrient rich edible oils. J. Oleo Sci. 2017, 66, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, G.; Saleh, M.S.A.; Yang, H.; Wang, N.; Wang, P.; Yue, X.; Xiao, Z. Functional characteristics of oleogel prepared from sunflower oil with β-sitosterol and stearic acid. J. Am. Oil Chem. Soc. 2017, 94, 1153–1164. [Google Scholar] [CrossRef]
- Szymanska, I.; Zbikowska, A.; Onacik-Gür, S. Candelilla wax-based oleogels versus palm oil: Evaluation of physical properties of innovative and conventional lipids using optical techniques. J. Sci. Food Agric. 2022, 102, 2309–2320. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, T.; Gong, Y.; Wang, W.; Xue, W.; Yu, D.; Wu, F.; Wang, L. Effect of ultrasound on the structural characteristics and oxidative stability of walnut oil oleogel coated with soy protein isolate-phosphatidylserine. Ultrason. Sonochem. 2022, 83, 105945. [Google Scholar] [CrossRef] [PubMed]
- Bascuas, S.; Hernando, I.; Moraga, G.; Quiles, A. Structure and stability of edible oleogels prepared with different unsaturated oils and hydrocolloids. Int. J. Food Sci. Technol. 2020, 55, 1458–1467. [Google Scholar] [CrossRef]
- Gaudino, N.; Ghazani, S.M.; Clark, S.; Marangoni, A.G.; Acevedo, N.C. Development of lecithin and stearic acid based oleogels and oleogel emulsions for edible semisolid applications. Food Res. Int. 2019, 116, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.I.; Co, E.D.; Marangoni, A.G. Structure and physical properties of plant wax crystal networks and their relationship to oil binding capacity. J. Am. Oil Chem. Soc. 2014, 91, 885–903. [Google Scholar] [CrossRef]
- Codex Alimentarius. GSFA Online Food Additive Details for Candelilla Wax. 2005. Available online: http://www.fao.org/gsfaonline/additives/details.html?id=263 (accessed on 10 October 2022).
- Valoppi, F.; Salmi, A.; Ratilainen, M.; Barba, L.; Puranen, T.; Tommiska, O.; Helander, P.; Heikkila, J.; Haeggstrom, E. Controlling oleogel crystallization using ultrasonic standing waves. Sci. Rep. 2020, 10, 14448. [Google Scholar] [CrossRef] [PubMed]
- Tiga, H.B.; Hwang, H.; Lee, S. Thermal and pasting properties of Quinoa—Wheat flour blends and their effects on production of extruded instant noodles. J. Cereal Sci. 2021, 60, 17–22. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Barbut, S.; Quinton, M.; Marangoni, A.G. Towards the development of a predictive model of the formulation-dependent mechanical behavior of edible oil-based ethyl cellulose oleogels. J. Food Eng. 2014, 143, 114–122. [Google Scholar] [CrossRef]
- Sahu, S.; Ghosh, M.; Bhattacharyya, D.K. Utilization of unsaponifiable matter from rice bran oil fatty acid distillate for preparing an antioxidant-rich oleogel and evaluation of its properties. Grasas Aceites 2020, 71, e336. [Google Scholar] [CrossRef]
- Totosaus, A.; Gonzalez-Gonzalez, R.; Fragoso, M. Influence of the type of cellulosic derivatives on the texture, and oxidative and thermal stability of soybean oil oleogel. Grasas Aceites 2016, 67, e152. [Google Scholar] [CrossRef] [Green Version]
- Doan, C.D.; Patel, A.R.; Tavernier, I.; Clercq, N.; Raemdonck, V.K.; de Walle, V.D.; Delbaere, C.; Dewettinck, K. The feasibility of wax-based oleogel as a potential co-structurant with palm oil in low-saturated fat confectionery fillings. Eur. J. Lipid Sci. Technol. 2016, 118, 1903–1914. [Google Scholar] [CrossRef]
- Pudtikajorn, K.; Benjakul, S. Simple Wet Rendering Method for Extraction of Prime Quality Oil from Skipjack Tuna Eyeballs. Eur. J. Lipid Sci. Technol. 2020, 122, 2000077. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noonim, P.; Rajasekaran, B.; Venkatachalam, K. Structural Characterization and Peroxidation Stability of Palm Oil-Based Oleogel Made with Different Concentrations of Carnauba Wax and Processed with Ultrasonication. Gels 2022, 8, 763. https://doi.org/10.3390/gels8120763
Noonim P, Rajasekaran B, Venkatachalam K. Structural Characterization and Peroxidation Stability of Palm Oil-Based Oleogel Made with Different Concentrations of Carnauba Wax and Processed with Ultrasonication. Gels. 2022; 8(12):763. https://doi.org/10.3390/gels8120763
Chicago/Turabian StyleNoonim, Paramee, Bharathipriya Rajasekaran, and Karthikeyan Venkatachalam. 2022. "Structural Characterization and Peroxidation Stability of Palm Oil-Based Oleogel Made with Different Concentrations of Carnauba Wax and Processed with Ultrasonication" Gels 8, no. 12: 763. https://doi.org/10.3390/gels8120763
APA StyleNoonim, P., Rajasekaran, B., & Venkatachalam, K. (2022). Structural Characterization and Peroxidation Stability of Palm Oil-Based Oleogel Made with Different Concentrations of Carnauba Wax and Processed with Ultrasonication. Gels, 8(12), 763. https://doi.org/10.3390/gels8120763






