Removal of Acetic Acid from Bacterial Culture Media by Adsorption onto a Two-Component Composite Polymer Gel
Abstract
:1. Introduction
2. Results and Discussion
2.1. Concept of Two-Component Copolymer Gel for Acetic Acid Adsorption
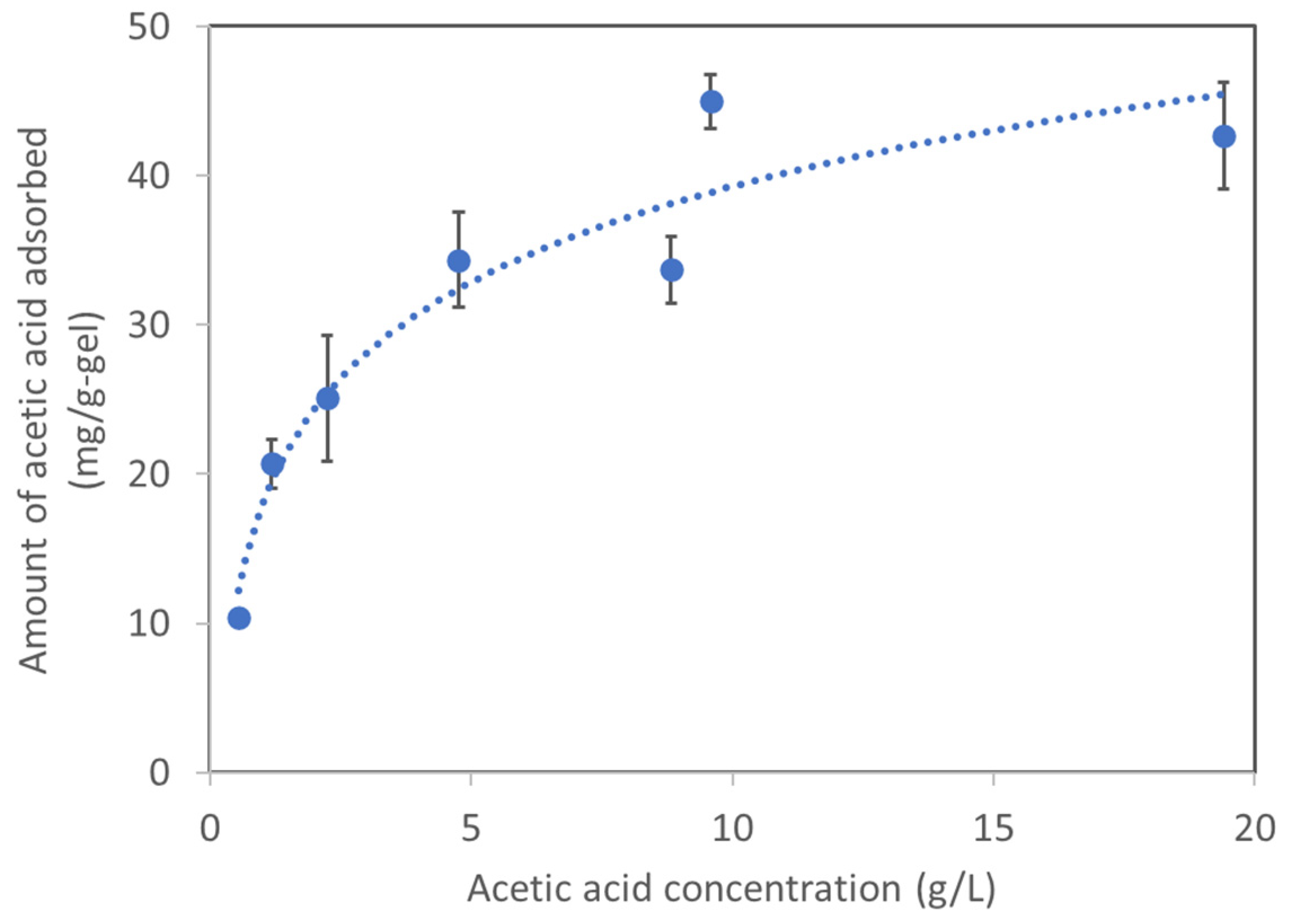
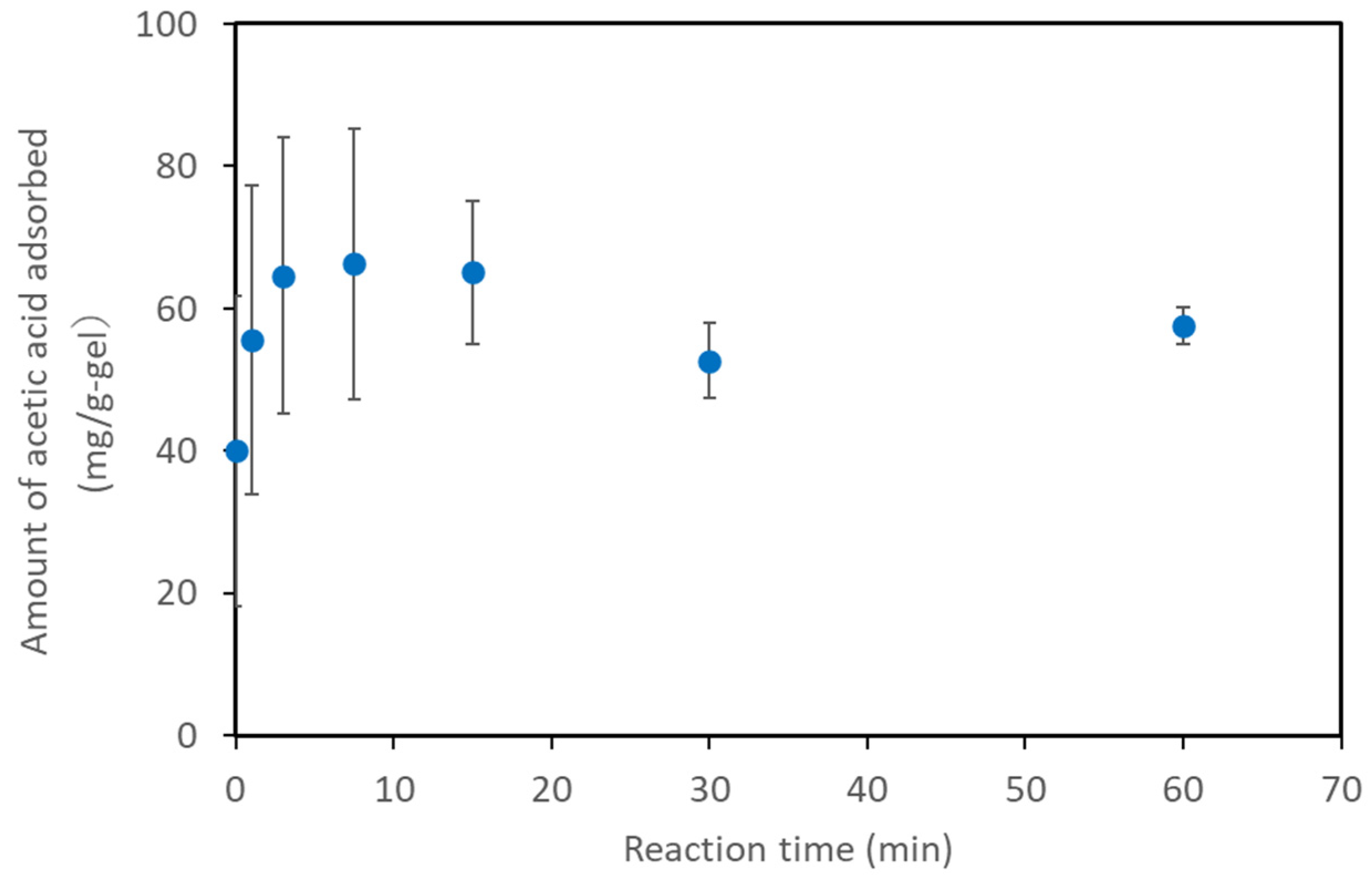
2.2. Basic Characteristic of Acetic Acid Adsorption on AQ11
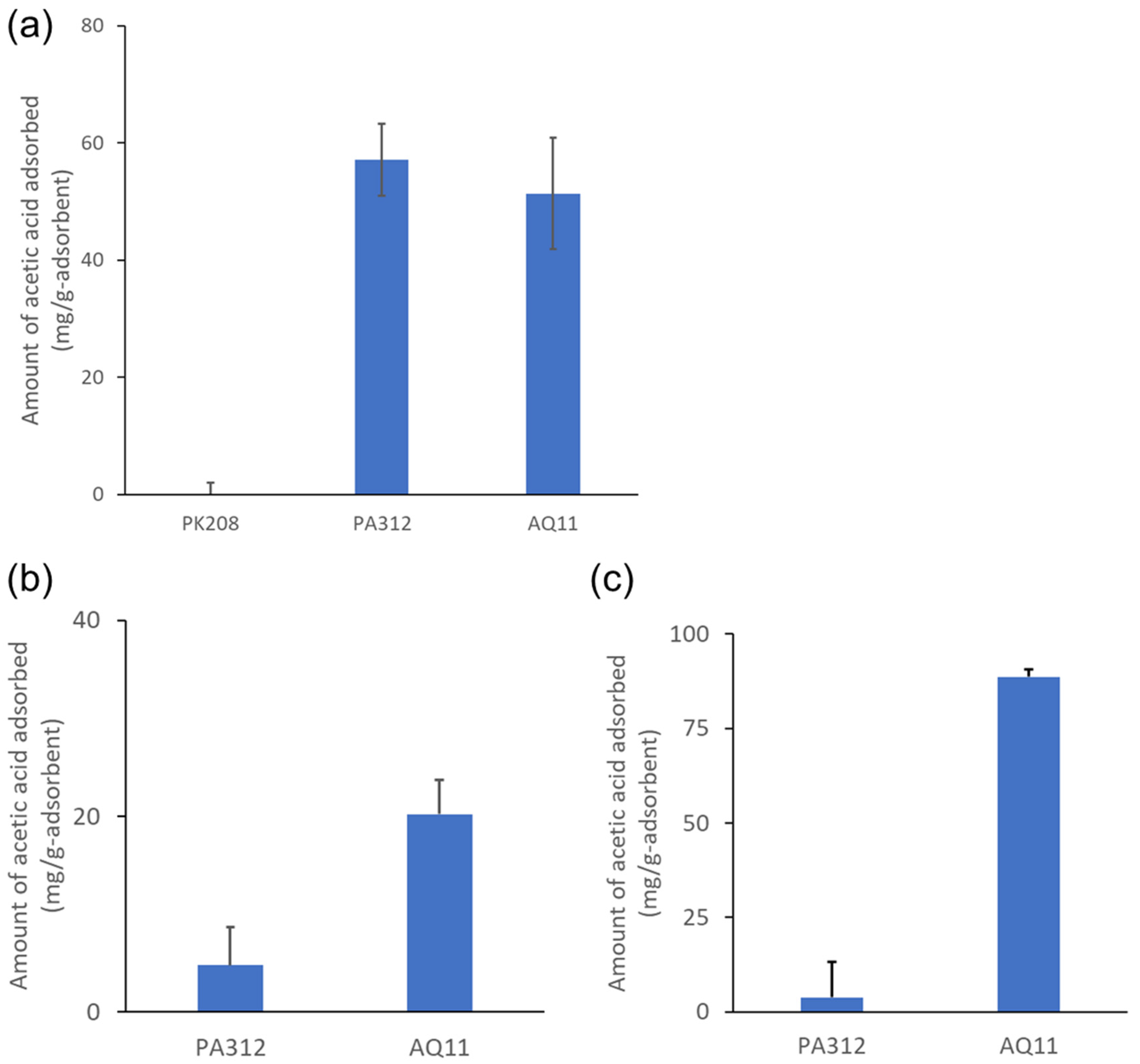
2.3. Comparison of AQ11 with Other Adsorbents
2.4. Adsorption Capability of AQ11 in a Culture Medium and at Various pH Values
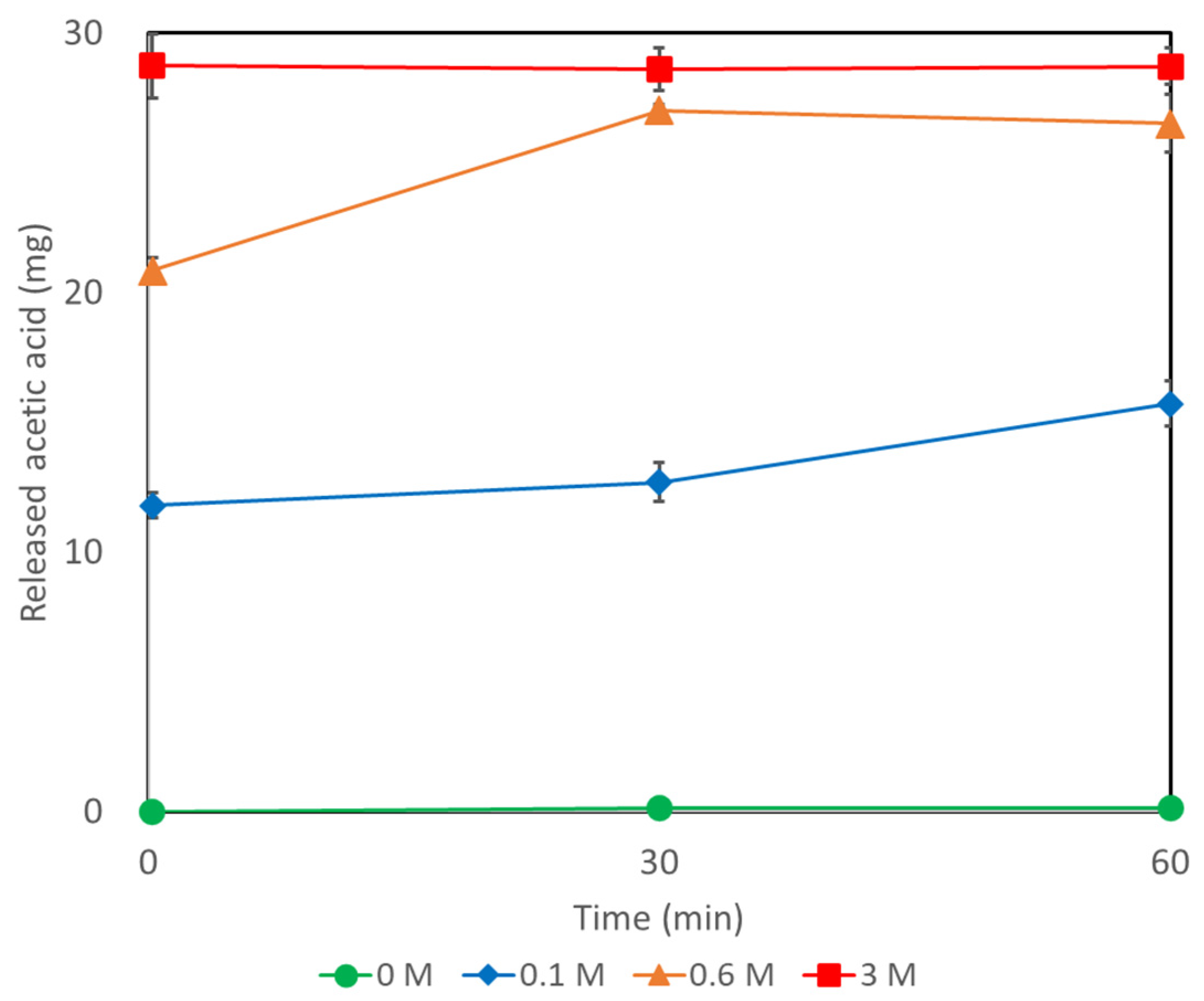
2.5. Recovery of Acetic Acid from AQ11
2.6. Recycling of AQ11-Treated Culture Broth for Fermentation
2.7. Adsorption of Short-Chain Organic Acids by AQ11
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Hydrogel Adsorbent
4.3. Analytical Methods
4.4. Adsorption Assay
4.5. Desorption Assay
4.6. Fermentation Experiment
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merli, G.; Becci, A.; Amato, A.; Beolchini, F. Acetic acid bioproduction: The technological innovation change. Sci. Total Environ. 2021, 798, 149292. [Google Scholar] [CrossRef] [PubMed]
- Morales-Vera, R.; Crawford, J.; Dou, C.; Bura, R.; Gustafson, R. Techno-Economic Analysis of Producing Glacial Acetic Acid from Poplar Biomass via Bioconversion. Molecules 2020, 25, 4328. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.R.; Barlow, C.Y. Life cycle assessments of biodegradable, commercial biopolymers—A critical review. Resour. Conserv. Recycl. 2013, 78, 54–66. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Modestra, J.A.; Amulya, K.; Butti, S.K.; Velvizhi, G. A Circular Bioeconomy with Biobased Products from CO2 Sequestration. Trends Biotechnol. 2016, 34, 506–519. [Google Scholar] [CrossRef]
- Ma, X.; Liang, H.; Panda, S.; Fung, V.K.Y.; Zhou, J.F.J.; Zhou, K. C2 feedstock-based biomanufacturing of value-added chemicals. Curr. Opin. Biotechnol. 2021, 73, 240–245. [Google Scholar] [CrossRef]
- Budsberg, E.; Morales-Vera, R.; Crawford, J.T.; Bura, R.; Gustafson, R. Production routes to bio-acetic acid: Life cycle assessment. Biotechnol. Biofuels 2020, 13, 154. [Google Scholar] [CrossRef]
- Blank, L.M.; Narancic, T.; Mampel, J.; Tiso, T.; O’Connor, K. Biotechnological upcycling of plastic waste and other non-conventional feedstocks in a circular economy. Curr. Opin. Biotechnol. 2020, 62, 212–219. [Google Scholar] [CrossRef]
- Wang, J.; Lin, M.; Xu, M.; Yang, S.T. Anaerobic Fermentation for Production of Carboxylic Acids as Bulk Chemicals from Renewable Biomass. Adv. Biochem. Eng. Biotechnol. 2016, 156, 323–361. [Google Scholar] [CrossRef]
- Schuchmann, K.; Muller, V. Autotrophy at the thermodynamic limit of life: A model for energy conservation in acetogenic bacteria. Nat. Rev. Microbiol. 2014, 12, 809–821. [Google Scholar] [CrossRef]
- Schuchmann, K.; Muller, V. Energetics and Application of Heterotrophy in Acetogenic Bacteria. Appl. Environ. Microbiol. 2016, 82, 4056–4069. [Google Scholar] [CrossRef] [Green Version]
- Ercengiz Yildirim, M.; Demiral, H. Recovery of acetic acid from waste streams by extractive distillation. Water Sci. Technol. 2003, 47, 183–188. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, Y.H. Energy saving in acetic acid process using an azeotropic distillation column with a side stripper. Chem. Eng. Commun. 2018, 205, 1311–1322. [Google Scholar] [CrossRef]
- Wang, G.; Wang, D.I. Elucidation of Growth Inhibition and Acetic Acid Production by Clostridium thermoaceticum. Appl. Environ. Microbiol. 1984, 47, 294–298. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Yang, S.T. Extractive fermentation for enhanced propionic acid production from lactose by Propionibacterium acidipropionici. Biotechnol. Prog. 1998, 14, 457–465. [Google Scholar] [CrossRef]
- Safi, S.R.; Gotoh, T.; Iizawa, T.; Nakai, S. Development and regeneration of composite of cationic gel and iron hydroxide for adsorbing arsenic from ground water. Chemosphere 2019, 217, 808–815. [Google Scholar] [CrossRef]
- Safi, S.R.; Senmoto, K.; Gotoh, T.; Iizawa, T.; Nakai, S. The effect of γ-FeOOH on enhancing arsenic adsorption from groundwater with DMAPAAQ + FeOOH gel composite. Sci. Rep. 2019, 9, 11909. [Google Scholar] [CrossRef] [PubMed]
- Safi, S.R.; Gotoh, T. Removal of Manganese Using Polymer Gel Composites. Mater. Proc. 2020, 4, 68. [Google Scholar] [CrossRef]
- Safi, S.R.; Gotoh, T. Simultaneous Removal of Arsenic and Manganese from Synthetic Aqueous Solutions Using Polymer Gel Composites. Nanomaterials 2021, 11, 1032. [Google Scholar] [CrossRef]
- Song, Y.; Gotoh, T.; Nakai, S. Synthesis of Oxidant Functionalised Cationic Polymer Hydrogel for Enhanced Removal of Arsenic (III). Gels 2021, 7, 197. [Google Scholar] [CrossRef]
- Ion Exchange Resins. Available online: https://www.diaion.com/en/products/ion_exchange_resins/strongly_basic_anion/index.html (accessed on 10 January 2022).
- Lopez-Garzon, C.S.; Straathof, A.J. Recovery of carboxylic acids produced by fermentation. Biotechnol. Adv. 2014, 32, 873–904. [Google Scholar] [CrossRef]
- Hassan, M.A.; Shirai, Y.; Umeki, H.; Yamazumi, H.; Jin, S.; Yamamoto, S.; Karim, M.I.A.; Nakanishi, K.; Hashimoto, K. Acetic Acid Separation from Anaerobically Treated Palm Oil Mill Effluent by Ion Exchange Resins for the Production of Polyhydroxyalkanoate by Alcaligenes eutrophus. Biosci. Biotechnol. Biochem. 2014, 61, 1465–1468. [Google Scholar] [CrossRef] [Green Version]
- da Silva, A.H.; Miranda, E.A. Adsorption/Desorption of Organic Acids onto Different Adsorbents for Their Recovery from Fermentation Broths. J. Chem. Eng. Data 2013, 58, 1454–1463. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, X.; Liu, C.; Wu, Y.; Zheng, G.; Chen, Y. Advances in downstream processes and applications of biological carboxylic acids derived from organic wastes. Bioresour. Technol. 2021, 346, 126609. [Google Scholar] [CrossRef]
- Arafiles, K.H.; Iwasaka, H.; Eramoto, Y.; Okamura, Y.; Tajima, T.; Matsumura, Y.; Nakashimada, Y.; Aki, T. Value-added lipid production from brown seaweed biomass by two-stage fermentation using acetic acid bacterium and thraustochytrid. Appl. Microbiol. Biotechnol. 2014, 98, 9207–9216. [Google Scholar] [CrossRef]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef]
- Miura, T.; Kita, A.; Okamura, Y.; Aki, T.; Matsumura, Y.; Tajima, T.; Kato, J.; Nakashimada, Y. Evaluation of marine sediments as microbial sources for methane production from brown algae under high salinity. Bioresour. Technol. 2014, 169, 362–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, J.; Takemura, K.; Kato, S.; Fujii, T.; Wada, K.; Iwasaki, Y.; Aoi, Y.; Matsushika, A.; Murakami, K.; Nakashimada, Y. Metabolic engineering of Moorella thermoacetica for thermophilic bioconversion of gaseous substrates to a volatile chemical. AMB Express 2021, 11, 59. [Google Scholar] [CrossRef]
- Tanner, R.S.; Miller, L.M.; Yang, D. Clostridium ljungdahlii sp. nov., an acetogenic species in clostridial rRNA homology group I. Int. J. Syst. Bacteriol. 1993, 43, 232–236. [Google Scholar] [CrossRef] [Green Version]
- Tanner, R.S. Monitoring Sulfate-Reducing Bacteria—Comparison of Enumeration Media. J. Microbiol. Methods 1989, 10, 83–90. [Google Scholar] [CrossRef]
- Hungate, R.E. Chapter IV A Roll Tube Method for Cultivation of Strict Anaerobes. In Methods in Microbiology; Academic Press: Cambridge, MA, USA, 1969; pp. 117–132. [Google Scholar]


| Medium Composite | Fructose Consumption (mM) | Acetic Acid Production (mM) |
|---|---|---|
| Fresh medium | 57.4 ± 0.4 | 108.1 ± 3.2 |
| Recycled medium | 56.5 ± 1.1 | 111.6 ± 3.1 |
| Organic Acid | Tested Concentration (mM) | Amount Adsorbed (mmol/g-gel) |
|---|---|---|
| Formic acid | 160 | 0.88 ± 0.07 |
| Acetic acid | 162 | 0.67 ± 0.08 |
| Propionic acid | 178 | 0.75 ± 0.03 |
| Butyric acid | 193 | 0.80 ± 0.09 |
| Component Name | Component Type | Molecular Weight | mol/m3 | g |
|---|---|---|---|---|
| DMAPAA | monomer | 105.22 | 500 | 1.841 |
| DMAPAA-Q | monomer | 206.71 | 500 | 3.617 |
| MBAA | linker | 154.17 | 50 | 0.2698 |
| TEMED | accelerator | 116.21 | 20 | 0.08135 |
| APS | Initiator | 228.19 | 20 | 0.15973 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, J.; Gotoh, T.; Nakashimada, Y. Removal of Acetic Acid from Bacterial Culture Media by Adsorption onto a Two-Component Composite Polymer Gel. Gels 2022, 8, 154. https://doi.org/10.3390/gels8030154
Kato J, Gotoh T, Nakashimada Y. Removal of Acetic Acid from Bacterial Culture Media by Adsorption onto a Two-Component Composite Polymer Gel. Gels. 2022; 8(3):154. https://doi.org/10.3390/gels8030154
Chicago/Turabian StyleKato, Junya, Takehiko Gotoh, and Yutaka Nakashimada. 2022. "Removal of Acetic Acid from Bacterial Culture Media by Adsorption onto a Two-Component Composite Polymer Gel" Gels 8, no. 3: 154. https://doi.org/10.3390/gels8030154






