Designing of pH-Sensitive Hydrogels for Colon Targeted Drug Delivery; Characterization and In Vitro Evaluation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of CS/β-CDcPAa Hydrogels
2.2. FTIR Analysis
2.3. Sol–Gel Analysis
2.4. Porosity Study
2.5. Thermogravimetric Analysis (TGA)
2.6. Powder X-ray Diffraction (PXRD) Analysis
2.7. Scanning Electron Microscopy (SEM)
2.8. Dynamic Swelling Studies
2.9. Polymer Volume Fraction
2.10. Drug Loading Analysis
2.11. Dissolution Studies
2.12. Kinetic Modeling
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of CS/β-CDcPAa Hydrogels
4.3. Fourier Transform Infrared Spectroscopy (FTIR)
4.4. Sol–Gel Analysis
4.5. Porosity Study
4.6. Thermogravimetric Analysis (TGA)
4.7. Powder X-ray Diffraction (PXRD) Analysis
4.8. Scanning Electron Microscopy (SEM)
4.9. Dynamic Swelling Studies
4.10. Polymer Volume Fraction
4.11. Drug Loading Analysis
4.12. Dissolution Studies
4.13. Kinetic Modeling
4.14. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113. [Google Scholar] [PubMed]
- Sardo, H.S.; Saremnejad, F.; Bagheri, S.; Akhgari, A.; Garekani, H.A.; Sadeghi, F. A review on 5-aminosalicylic acid colon-targeted oral drug delivery systems. Int. J. Pharm. 2019, 558, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D. Cidofovir. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Klotz, U. Clinical pharmacokinetics of sulphasalazine, its metabolites and other prodrugs of 5-aminosalicylic acid. Clin. Pharmacokinet. 1985, 10, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Mladenovska, K.; Raicki, R.; Janevik, E.; Ristoski, T.; Pavlova, M.; Kavrakovski, Z.; Dodov, M.; Goracinova, K. Colon-specific delivery of 5-aminosalicylic acid from chitosan-Ca-alginate microparticles. Int. J. Pharm. 2007, 342, 124–136. [Google Scholar] [CrossRef]
- Ahmad, A.; Ansari, M.M.; Mishra, R.K.; Kumar, A.; Vyawahare, A.; Verma, R.K.; Raza, S.S.; Khan, R. Enteric-coated gelatin nanoparticles mediated oral delivery of 5-aminosalicylic acid alleviates severity of DSS-induced ulcerative colitis. Mater. Sci. Eng. C 2021, 119, 111582. [Google Scholar] [CrossRef]
- Stavarache, C.E.; Ghebaur, A.; Dinescu, S.; Samoilă, I.; Vasile, E.; Vlasceanu, G.M.; Iovu, H.; Gârea, S.A. 5-Aminosalicylic Acid Loaded Chitosan-Carrageenan Hydrogel Beads with Potential Application for the Treatment of Inflammatory Bowel Disease. Polymers 2021, 13, 2463. [Google Scholar] [CrossRef]
- Bautzová, T.; Rabišková, M.; Béduneau, A.; Pellequer, Y.; Lamprecht, A. Bioadhesive pellets increase local 5-aminosalicylic acid concentration in experimental colitis. Eur. J. Pharm. Biopharm. 2012, 81, 379–385. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Suhail, M.; Rosenholm, J.M.; Minhas, M.U.; Badshah, S.F.; Naeem, A.; Khan, K.U.; Fahad, M. Nanogels as drug-delivery systems: A comprehensive overview. Ther. Deliv. 2019, 10, 697–717. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V. Design, fabrication and drug release potential of dual stimuli-responsive composite hydrogel nanoparticle interfaces. Colloids Surf. B Biointerfaces 2021, 204, 111819. [Google Scholar] [CrossRef]
- Dinu, M.V.; Cocarta, A.I.; Dragan, E.S. Synthesis, characterization and drug release properties of 3D chitosan/clinoptilolite biocomposite cryogels. Carbohydr. Polym. 2016, 153, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Luo, Y. Chitosan-based hydrogel beads: Preparations, modifications and applications in food and agriculture sectors–A review. Int. J. Biol. Macromol. 2020, 152, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, R.M.; Ahmad, M.; Mahmood, A.; Akram, M.R.; Abrar, A. Development of β-cyclodextrin-based hydrogel microparticles for solubility enhancement of rosuvastatin: An in vitro and in vivo evaluation. Drug Des. Dev. Ther. 2017, 11, 3083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, K.U.; Minhas, M.U.; Badshah, S.F.; Sohail, M.; Sarfraz, R.M. β-cyclodextrin modification by cross-linking polymerization as highly porous nanomatrices for olanzapine solubility improvement; synthesis, characterization and bio-compatibility evaluation. J. Drug Deliv. Sci. Technol. 2022, 67, 102952. [Google Scholar] [CrossRef]
- Ahmad, S.; Minhas, M.U.; Ahmad, M.; Sohail, M.; Abdullah, O.; Badshah, S.F. Preparation and evaluation of skin wound healing chitosan-based hydrogel membranes. AAPS PharmSciTech 2018, 19, 3199–3209. [Google Scholar] [CrossRef]
- Khan, M.Z.; Makreski, P.; Murtaza, G. Preparation, optimization, in vitro evaluation and ex vivo permeation studies of finasteride loaded gel formulations prepared by using response surface methodology. Curr. Drug Deliv. 2018, 15, 1312–1322. [Google Scholar] [CrossRef]
- Gatiganti, D.L.; Srimathkandala, M.H.; Ananthula, M.B.; Bakshi, V. Formulation and evaluation of oral natural polysaccharide hydrogel microbeads of Irbesartan. Anal. Chem. Lett. 2016, 6, 334–344. [Google Scholar] [CrossRef]
- Mura, C.; Nácher, A.; Merino, V.; Merino-Sanjuan, M.; Manconi, M.; Loy, G.; Fadda, A.M.; Díez-Sales, O. Design, characterization and in vitro evaluation of 5-aminosalicylic acid loaded N-succinyl-chitosan microparticles for colon specific delivery. Colloids Surf. B Biointerfaces 2012, 94, 199–205. [Google Scholar] [CrossRef]
- Patil, J.S.; Yadava, S.; Mokale, V.J.; Naik, J.B. Preparation and characterization of single pulse sustained release ketorolac nanoparticles to reduce their side-effects at gastrointestinal tract. In Proceedings of the International Conference on Advances in Engineering and Technology, Kollam, India, October 2014; pp. 59–62. [Google Scholar]
- Khan, S.; Ranjha, N.M. Effect of degree of cross-linking on swelling and on drug release of low viscous chitosan/poly (vinyl alcohol) hydrogels. Polym. Bull. 2014, 71, 2133–2158. [Google Scholar] [CrossRef]
- Khanum, H.; Ullah, K.; Murtaza, G.; Khan, S.A. Fabrication and in vitro characterization of HPMC-g-poly (AMPS) hydrogels loaded with loxoprofen sodium. Int. J. Biol. Macromol. 2018, 120, 1624–1631. [Google Scholar] [CrossRef]
- Khalid, I.; Ahmad, M.; Minhas, M.U.; Barkat, K. Synthesis and evaluation of chondroitin sulfate based hydrogels of loxoprofen with adjustable properties as controlled release carriers. Carbohydr. Polym. 2018, 181, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Fei, L.; Cui, F.; Tang, C.; Yin, C. Superporous hydrogels containing poly (acrylic acid-co-acrylamide)/O-carboxymethyl chitosan interpenetrating polymer networks. Biomaterials 2007, 28, 1258–1266. [Google Scholar] [CrossRef]
- Sarika, P.R.; James, N.R.; Kumar, P.R.A.; Raj, D.K. Preparation, characterization and biological evaluation of curcumin loaded alginate aldehyde-gelatin nanogels. Mater. Sci. Eng. C 2016, 68, 251–257. [Google Scholar] [CrossRef]
- Barkat, K.; Ahmad, M.; Usman Minhas, M.; Khalid, I.; Nasir, B. Development and characterization of pH-responsive polyethylene glycol-co-poly (methacrylic acid) polymeric network system for colon target delivery of oxaliplatin: Its acute oral toxicity study. Adv. Polym. Technol. 2018, 37, 1806–1822. [Google Scholar] [CrossRef]
- Minhas, M.U.; Ahmad, M.; Khan, S.; Ali, L.; Sohail, M. Synthesis and characterization of β-cyclodextrin hydrogels: Crosslinked polymeric network for targeted delivery of 5-fluorouracil. Drug Deliv. 2016, 9, 10. [Google Scholar]
- Wei, W.; Hu, X.; Qi, X.; Yu, H.; Liu, Y.; Li, J.; Zhang, J.; Dong, W. A novel thermo-responsive hydrogel based on salecan and poly (N-isopropylacrylamide): Synthesis and characterization. Colloids Surf. B Biointerfaces 2015, 125, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Feng, L.; Wei, W.; Xie, A.; Wang, S.; Zhang, J.; Dong, W. Synthesis and characterization of a novel semi-IPN hydrogel based on Salecan and poly (N, N-dimethylacrylamide-co-2-hydroxyethyl methacrylate). Carbohydr. Polym. 2014, 105, 135–144. [Google Scholar] [CrossRef]
- Ray, M.; Pal, K.; Anis, A.; Banthia, A. Development and characterization of chitosan-based polymeric hydrogel membranes. Des. Monomers Polym. 2010, 13, 193–206. [Google Scholar] [CrossRef] [Green Version]
- Barkat, K.; Ahmad, M.; Minhas, M.U.; Khalid, I. Oxaliplatin-loaded crosslinked polymeric network of chondroitin sulfate-co-poly (methacrylic acid) for colorectal cancer: Its toxicological evaluation. J. Appl. Polym. Sci. 2017, 134, 45312. [Google Scholar] [CrossRef]
- Milosavljević, N.B.; Kljajević, L.M.; Popović, I.G.; Filipović, J.M.; Kalagasidis Krušić, M.T. Chitosan, itaconic acid and poly (vinyl alcohol) hybrid polymer networks of high degree of swelling and good mechanical strength. Polym. Int. 2010, 59, 686–694. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M.; Dong, W.; Zhang, J. Redox/pH dual stimuli-responsive degradable Salecan-g-SS-poly (IA-co-HEMA) hydrogel for release of doxorubicin. Carbohydr. Polym. 2017, 155, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-T.; Huang, C.-P.; Lee, Y.-D. Synthesis and characterizations of amphiphilic poly (l-lactide)-grafted chondroitin sulfate copolymer and its application as drug carrier. Biomol. Eng. 2007, 24, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Ubaidulla, U.; Nayak, A.K. Okra (Hibiscus esculentus) gum-alginate blend mucoadhesive beads for controlled glibenclamide release. Int. J. Biol. Macromol. 2015, 72, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Anwar, N. Highly porous pH-responsive carboxymethyl chitosan-grafted-poly (acrylic acid) based smart hydrogels for 5-fluorouracil controlled delivery and colon targeting. Int. J. Polym. Sci. 2019, 2019, 6579239. [Google Scholar] [CrossRef] [Green Version]
- Malik, N.S.; Ahmad, M.; Minhas, M.U.; Tulain, R.; Barkat, K.; Khalid, I.; Khalid, Q. Chitosan/xanthan gum based hydrogels as potential carrier for an antiviral drug: Fabrication, characterization, and safety evaluation. Front. Chem. 2020, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.F.; Ranjha, N.M.; Hanif, M. Effect of ethylene glycol dimethacrylate on swelling and on metformin hydrochloride release behavior of chemically crosslinked pH–sensitive acrylic acid–polyvinyl alcohol hydrogel. DARU J. Pharm. Sci. 2015, 23, 41. [Google Scholar] [CrossRef] [Green Version]
- Mandal, B.; Ray, S.K. Swelling, diffusion, network parameters and adsorption properties of IPN hydrogel of chitosan and acrylic copolymer. Mater. Sci. Eng. C 2014, 44, 132–143. [Google Scholar] [CrossRef]
- Khan, S.A.; Azam, W.; Ashames, A.; Fahelelbom, K.M.; Ullah, K.; Mannan, A.; Murtaza, G. β-Cyclodextrin-based (IA-co-AMPS) Semi-IPNs as smart biomaterials for oral delivery of hydrophilic drugs: Synthesis, characterization, In-Vitro and In-Vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 60, 101970. [Google Scholar] [CrossRef]
- Nasir, N.; Ahmad, M.; Minhas, M.U.; Barkat, K.; Khalid, M.F. pH-responsive smart gels of block copolymer [pluronic F127-co-poly (acrylic acid)] for controlled delivery of Ivabradine hydrochloride: Its toxicological evaluation. J. Polym. Res. 2019, 26, 212. [Google Scholar] [CrossRef]
- Badshah, S.F.; Akhtar, N.; Minhas, M.U.; Khan, K.U.; Khan, S.; Abdullah, O.; Naeem, A. Porous and highly responsive cross-linked β-cyclodextrin based nanomatrices for improvement in drug dissolution and absorption. Life Sci. 2021, 267, 118931. [Google Scholar] [CrossRef]
- Serra, L.; Doménech, J.; Peppas, N.A. Drug transport mechanisms and release kinetics from molecularly designed poly (acrylic acid-g-ethylene glycol) hydrogels. Biomaterials 2006, 27, 5440–5451. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A.; Pervaiz, F.; Shoukat, H.; Shabbir, K.; Noreen, S.; Anwar, M. Fabrication and evaluation of pH sensitive chemically cross-linked interpenetrating network [Gelatin/Polyvinylpyrrolidone-co-poly(acrylic acid)] for targeted release of 5-fluorouracil. Polym. Bull. 2020, 79, 1–20. [Google Scholar] [CrossRef]
- Abdullah, O.; Usman Minhas, M.; Ahmad, M.; Ahmad, S.; Ahmad, A. Synthesis of hydrogels for combinatorial delivery of 5-fluorouracil and leucovorin calcium in colon cancer: Optimization, in vitro characterization and its toxicological evaluation. Polym. Bull. 2019, 76, 3017–3037. [Google Scholar] [CrossRef]
- Ullah, K.; Khan, S.A.; Murtaza, G.; Sohail, M.; Manan, A.; Afzal, A. Gelatin-based hydrogels as potential biomaterials for colonic delivery of oxaliplatin. Int. J. Pharm. 2019, 556, 236–245. [Google Scholar] [CrossRef]
- Sohail, M.; Ahmad, M.; Minhas, M.U.; Ali, L.; Munir, A.; Khalid, I. Synthesis and characterization of graft PVA composites for controlled delivery of valsartan. Lat. Am. J. Pharm 2014, 33, 1237–1244. [Google Scholar]
- Khalid, I.; Ahmad, M.; Usman Minhas, M.; Barkat, K.; Sohail, M. Cross-Linked Sodium Alginate-g-Poly (Acrylic Acid) Structure: A Potential Hydrogel Network for Controlled Delivery of Loxoprofen Sodium. Adv. Polym. Technol. 2018, 37, 985–995. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Aminabhavi, T.M. Novel interpenetrating network chitosan-poly (ethylene oxide-g-acrylamide) hydrogel microspheres for the controlled release of capecitabine. Int. J. Pharm. 2006, 324, 103–115. [Google Scholar] [CrossRef]
- Khalid, Q.; Ahmad, M.; Usman Minhas, M. Hydroxypropyl-β-cyclodextrin hybrid nanogels as nano-drug delivery carriers to enhance the solubility of dexibuprofen: Characterization, in vitro release, and acute oral toxicity studies. Adv. Polym. Technol. 2018, 37, 2171–2185. [Google Scholar] [CrossRef]
- Shoaib, M.H.; Tazeen, J.; Merchant, H.A.; Yousuf, R.I. Evaluation of drug release kinetics from ibuprofen matrix tablets using HPMC. Pak. J. Pharm. Sci. 2006, 19, 119–124. [Google Scholar]
- Maziad, N.A.; El-Hamouly, S.; Zied, E.; EL Kelani, T.A.; Nasef, N.R. Radiation preparation of smart hydrogel has antimicrobial properties for controlled release of ciprofloxacin in drug delivery systems. Asian J. Pharm. Clin. Res. 2015, 14, 15. [Google Scholar]


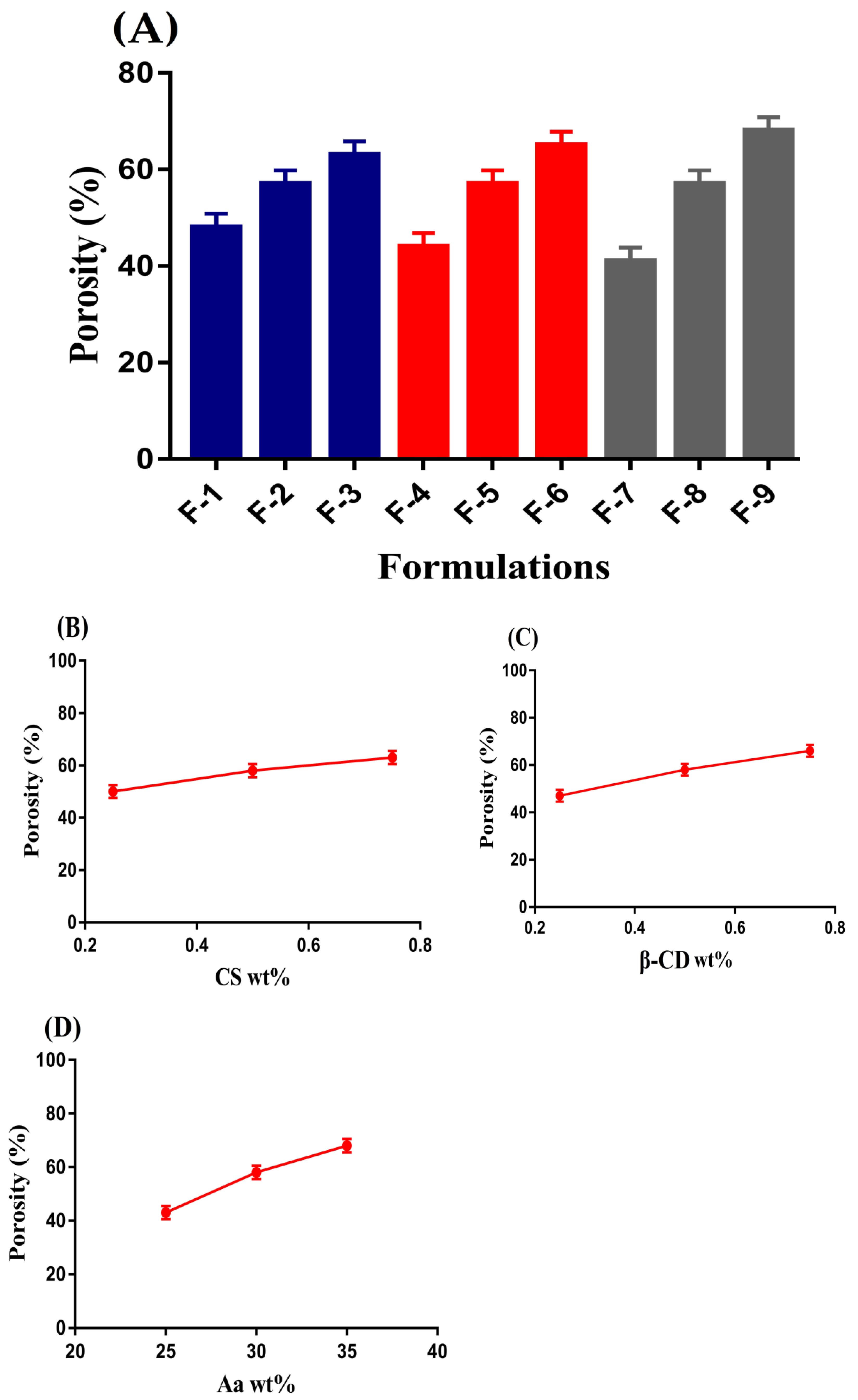
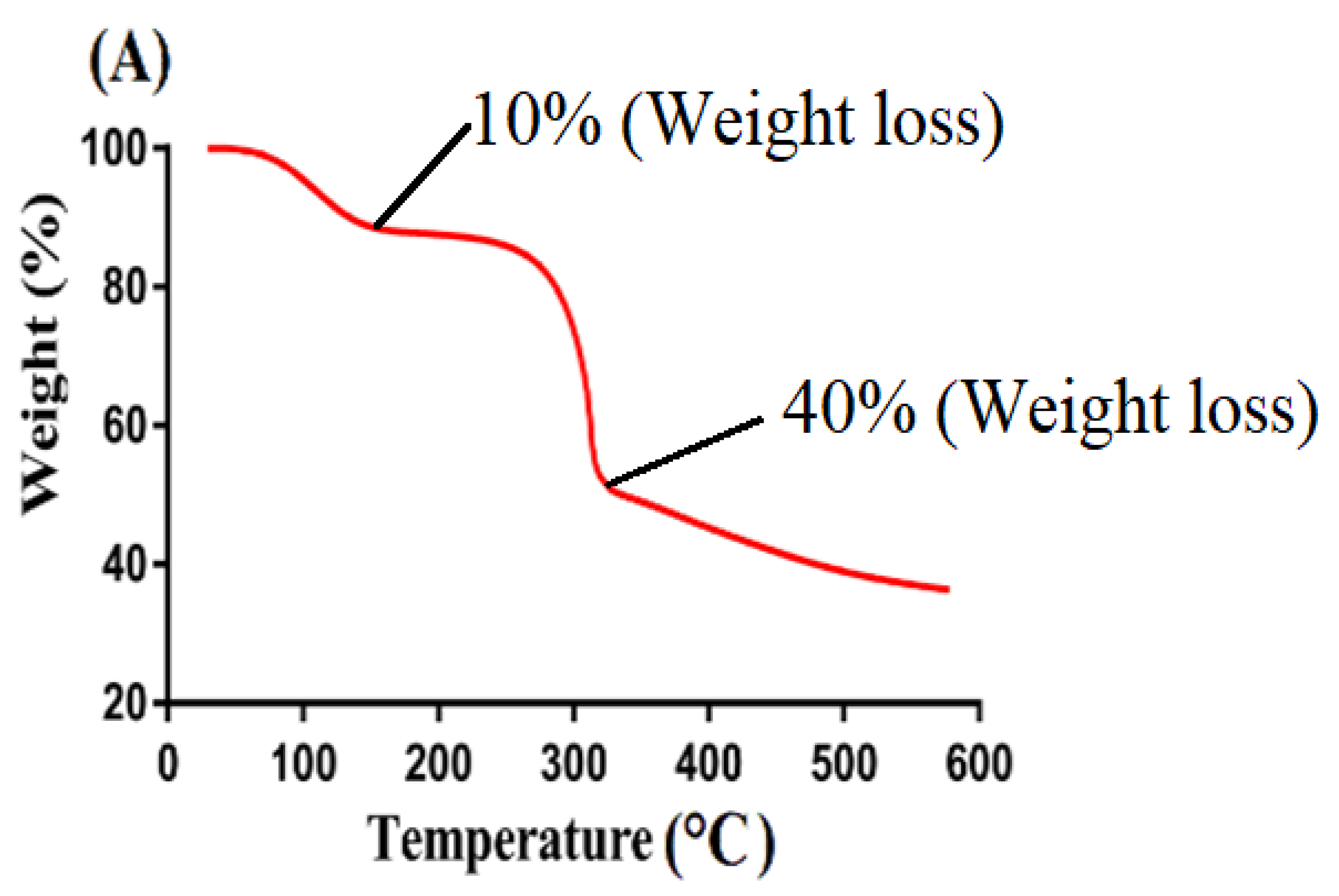


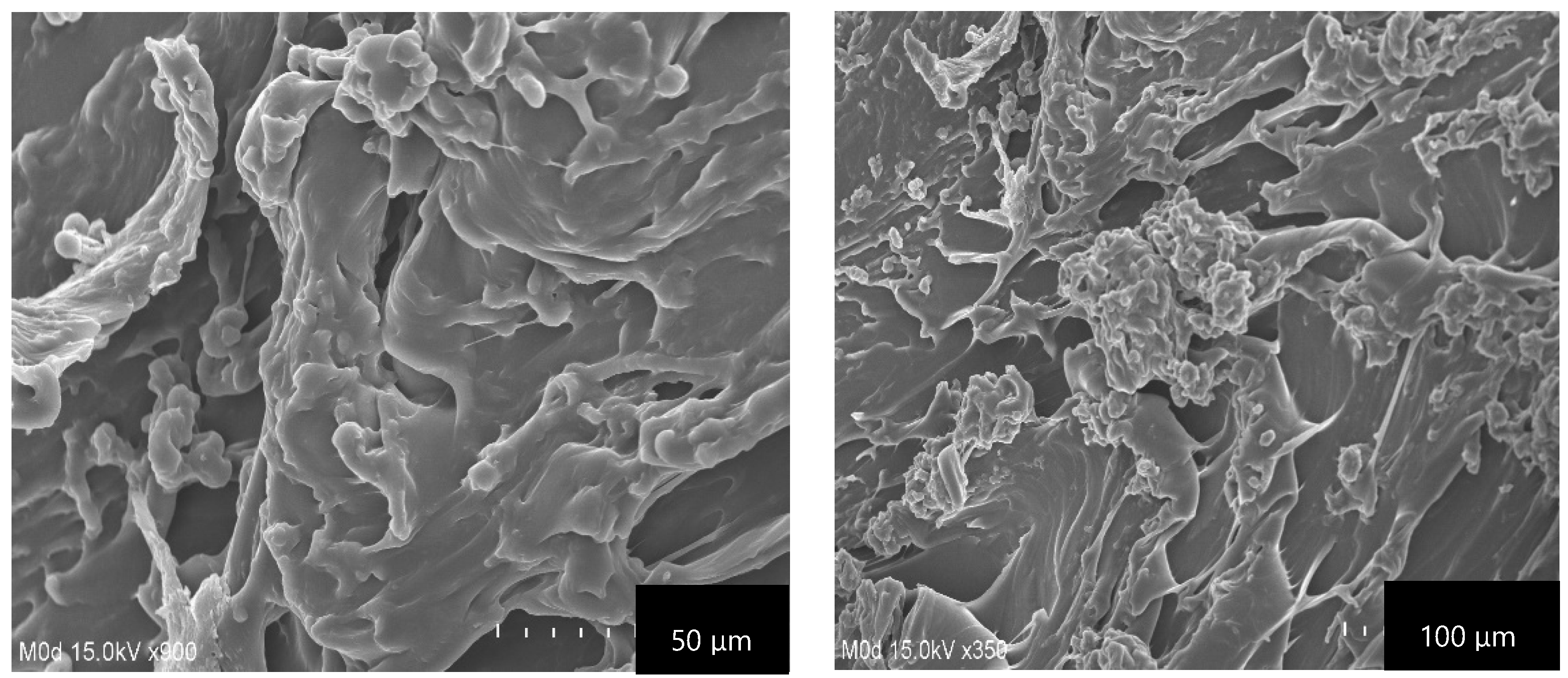
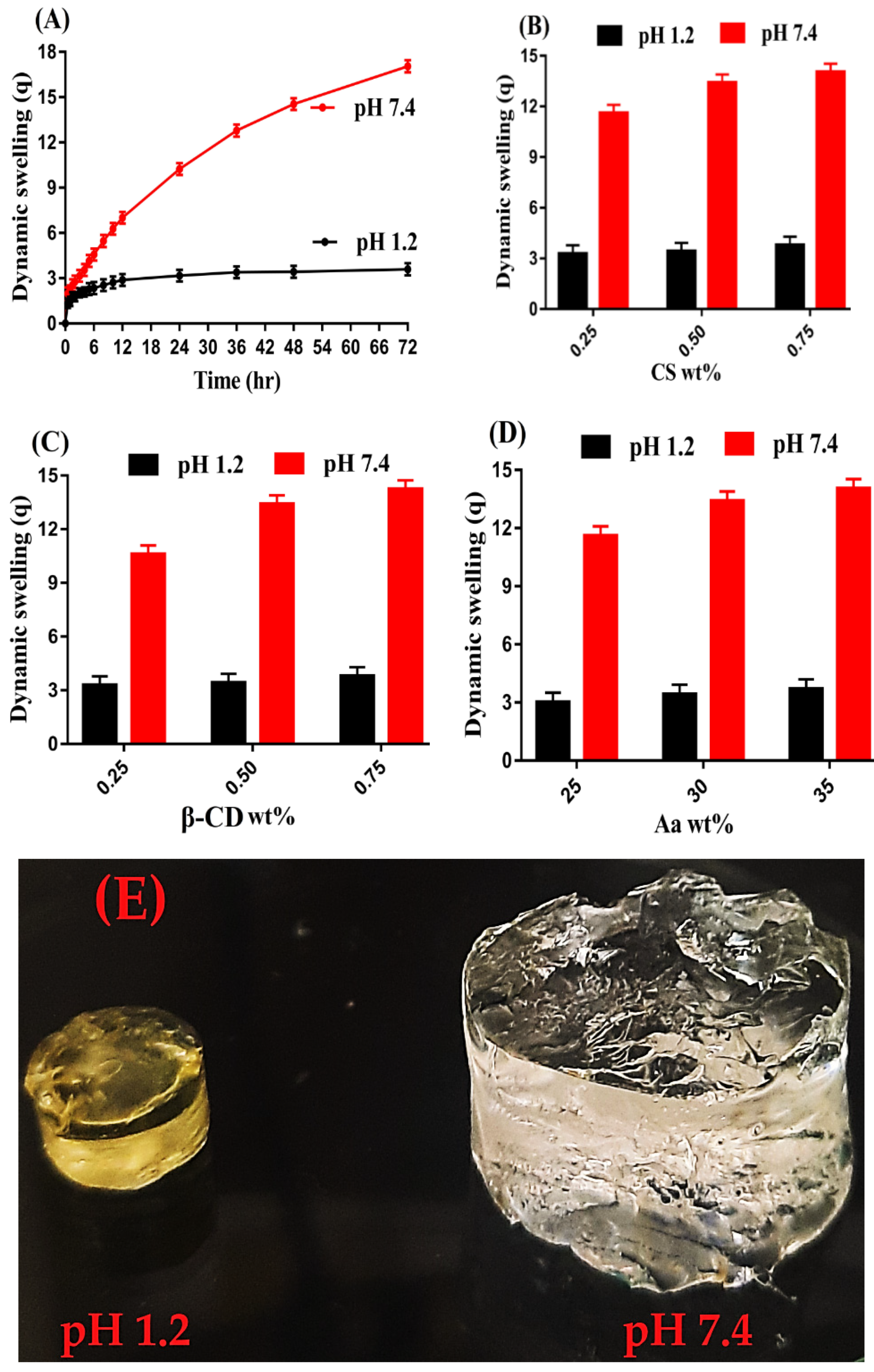


| F. Code | Polymer (CS) g/100 g | Polymer (β-CD) g/100 g | Monomer (Aa) g/100 g | Polymer Volume Fraction | |
|---|---|---|---|---|---|
| pH 1.2 | pH 7.4 | ||||
| F-1 | 0.25 | 0.50 | 30 | 0.300 | 0.083 |
| F-2 | 0.50 | 0.50 | 30 | 0.282 | 0.077 |
| F-3 | 0.75 | 0.50 | 30 | 0.271 | 0.073 |
| F-4 | 0.50 | 0.25 | 30 | 0.304 | 0.086 |
| F-5 | 0.50 | 0.50 | 30 | 0.282 | 0.077 |
| F-6 | 0.50 | 0.75 | 30 | 0.267 | 0.070 |
| F-7 | 0.50 | 0.50 | 25 | 0.308 | 0.093 |
| F-8 | 0.50 | 0.50 | 30 | 0.282 | 0.077 |
| F-9 | 0.50 | 0.50 | 35 | 0.263 | 0.066 |
| Formulation Code | Sol | Gel | Drug Loaded (mg)/400 mg of Dry Gel | |
|---|---|---|---|---|
| Fraction | Fraction | |||
| % | % | Weight Method | Extraction Method | |
| F-1 | 10.60 | 89.40 | 125.32 ± 0.94 | 123.90 ± 0.98 |
| F-2 | 09.21 | 90.79 | 132.63 ± 1.04 | 131.48 ± 0.94 |
| F-3 | 08.72 | 91.28 | 137.28 ± 0.98 | 136.12 ± 1.04 |
| F-4 | 11.52 | 88.48 | 121.84 ± 1.08 | 119.73 ± 0.91 |
| F-5 | 09.21 | 90.79 | 132.63 ± 1.04 | 131.48 ± 0.94 |
| F-6 | 07.90 | 92.10 | 140.87 ± 0.97 | 138.11 ± 1.05 |
| F-7 | 11.80 | 88.20 | 114.54 ± 1.07 | 113.25 ± 0.97 |
| F-8 | 09.21 | 90.79 | 132.63 ± 1.04 | 131.48 ± 0.94 |
| F-9 | 08.94 | 91.06 | 144.82 ± 1.04 | 143.51 ± 1.14 |
| F. Code | Zero Order | First Order | Higuchi | Korsmeyer-Peppas | |
|---|---|---|---|---|---|
| r2 | r2 | r2 | r2 | n | |
| F-1 | 0.9364 | 0.9912 | 0.9876 | 0.9848 | 0.5470 |
| F-2 | 0.9636 | 0.9974 | 0.9890 | 0.9753 | 0.5228 |
| F-3 | 0.9598 | 0.9864 | 0.9740 | 0.9823 | 0.6054 |
| F-4 | 0.9312 | 0.9853 | 0.9065 | 0.9739 | 0.5993 |
| F-5 | 0.9636 | 0.9974 | 0.9890 | 0.9753 | 0.5228 |
| F-6 | 0.9512 | 0.9932 | 0.9910 | 0.9810 | 0.6183 |
| F-7 | 0.9765 | 0.9880 | 0.9612 | 0.9636 | 0.6310 |
| F-8 | 0.9636 | 0.9974 | 0.9890 | 0.9753 | 0.5228 |
| F-9 | 0.9390 | 0.9781 | 0.9543 | 0.9584 | 0.5864 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhail, M.; Shao, Y.-F.; Vu, Q.L.; Wu, P.-C. Designing of pH-Sensitive Hydrogels for Colon Targeted Drug Delivery; Characterization and In Vitro Evaluation. Gels 2022, 8, 155. https://doi.org/10.3390/gels8030155
Suhail M, Shao Y-F, Vu QL, Wu P-C. Designing of pH-Sensitive Hydrogels for Colon Targeted Drug Delivery; Characterization and In Vitro Evaluation. Gels. 2022; 8(3):155. https://doi.org/10.3390/gels8030155
Chicago/Turabian StyleSuhail, Muhammad, Yu-Fang Shao, Quoc Lam Vu, and Pao-Chu Wu. 2022. "Designing of pH-Sensitive Hydrogels for Colon Targeted Drug Delivery; Characterization and In Vitro Evaluation" Gels 8, no. 3: 155. https://doi.org/10.3390/gels8030155






