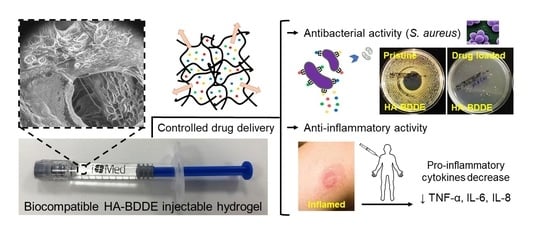
Drug Delivery from Hyaluronic Acid–BDDE Injectable Hydrogels for Antibacterial and Anti-Inflammatory Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of HA-BDDE Hydrogels
2.2. Rheological Properties
| Hydrogels | Reaction Time (h) | BDDE Concentration | MoD (%) | Viscosity (Pa·s) | G′ (Pa) | G′′ (Pa) | tan δ | G* |
|---|---|---|---|---|---|---|---|---|
| HA-BDDE-1 (●) | 2 | 0.5 C | 13.50 | 71.9 ± 5.0 | 103.9 ± 4.7 | 27.1 ± 2.5 | 0.261 | 107.4 |
| HA-BDDE-2 (●) | 2 | C | 31.50 | 84.3 ± 5.7 | 385.7 ± 14.9 | 71.6 ± 3.6 | 0.186 | 392.3 |
| HA-BDDE-3 (●) | 2 | 2 C | 65.25 | 91.7 ± 6.1 | 1142.0 ± 58.4 | 204.4 ± 9.3 | 0.179 | 1160.1 |
| HA-BDDE-4 (●) | 3 | 0.5 C | 16.50 | 80.2 ± 5.1 | 123.2 ± 5.5 | 24.7 ± 2.8 | 0.201 | 125.6 |
| HA-BDDE-5 (●) | 3 | C | 36.75 | 124.8 ± 8.2 | 615.8 ± 20.2 | 107.7 ± 7.5 | 0.174 | 625.1 |
| HA-BDDE-6 (●) | 3 | 2 C | 76.50 | 160.9 ± 10.6 | 2911.1 ± 84.1 | 354.6 ± 22.1 | 0.122 | 2932.5 |
2.3. Morphology, In Vitro Swelling and Degradation, and Thermal Characterization
2.4. Biocompatibility Test
2.5. Drug Loading and Release Studies
2.6. Antibacterial Activity
2.7. Anti-Inflammatory Activity
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of HA-BDDE Hydrogels
4.3. Physicochemical Characterization
4.3.1. Proton Nuclear Magnetic Resonance Spectroscopy (1H-NMR)
4.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
4.3.3. Rheological Properties
4.3.4. Low Vacuum Scanning Electron Microscopy (LVSEM)
4.3.5. Equilibrium Swelling Ratio
4.3.6. In Vitro Degradation
4.3.7. Thermal Characterizations
4.4. Biocompatibility Test
4.4.1. pH and Osmolality
4.4.2. In Vitro Cytotoxicity
4.4.3. In Vivo Acute Systemic Toxicity
4.5. Loading and Sustained Release of Drugs
4.6. In Vitro Antibacterial Activity
4.7. In Vitro Anti-Inflammatory Activity
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salwowska, N.M.; Bebenek, K.A.; Żądło, D.A.; Wcisło-Dziadecka, D.L. Physiochemical properties and application of hyaluronic acid: A systematic review. J. Cosmet. Dermatol. 2016, 15, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic acid in the third millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Gatta, A.; Salzillo, R.; Catalano, C.; D’Agostino, A.; Pirozzi, A.V.A.; De Rosa, M.; Schiraldi, C. Hyaluronan-based hydrogels as dermal fillers: The biophysical properties that translate into a “volumetric” effect. PLoS ONE 2019, 14, e0218287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Zhong, W.; Pang, L.; Feng, H.; Dong, H.; Wang, S.; Cong, H.; Shen, Y.; Bing, Y. Recent advantage of hyaluronic acid for anti-cancer application: A review of “3S” transition approach. Carbohydr. Polym. 2020, 238, 116204. [Google Scholar] [CrossRef]
- Chang, W.H.; Liu, P.Y.; Lin, M.H.; Lu, C.J.; Chou, H.Y.; Nian, C.Y.; Jiang, Y.T.; Hsu, Y.H.H. Applications of hyaluronic acid in ophthalmology and contact lenses. Molecules 2021, 26, 2485. [Google Scholar] [CrossRef] [PubMed]
- De Meo, D.; Ceccarelli, G.; Iaiani, G.; Lo Torto, F.; Ribuffo, D.; Persiani, P.; Villani, C. Clinical Application of Antibacterial Hydrogel and Coating in Orthopaedic and Traumatology Surgery. Gels 2021, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Cervino, G.; Meto, A.; Fiorillo, L.; Odorici, A.; Meto, A.; D’amico, C.; Oteri, G.; Cicciù, M. Surface treatment of the dental implant with hyaluronic acid: An overview of recent data. Int. J. Environ. Res. Public Health 2021, 18, 4670. [Google Scholar] [CrossRef]
- Sánchez-Bodón, J.; Del Olmo, J.A.; Alonso, J.M.; Moreno-Benítez, I.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Bioactive Coatings on Titanium: A Review on Hydroxylation, Self-Assembled Monolayers (SAMs) and Surface Modification Strategies. Polymers 2022, 14, 165. [Google Scholar] [CrossRef]
- Buwalda, S.J. Bio-based composite hydrogels for biomedical applications. Multifunct. Mater. 2020, 3, 022001. [Google Scholar] [CrossRef]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.V.; Huynh, D.P.; Park, J.H.; Lee, D.S. Injectable polymeric hydrogels for the delivery of therapeutic agents: A review. Eur. Polym. J. 2015, 72, 602–619. [Google Scholar] [CrossRef]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef]
- Pérez, L.A.; Hernández, R.; Alonso, J.M.; Pérez-González, R.; Sáez-Martínez, V. Hyaluronic acid hydrogels crosslinked in physiological conditions: Synthesis and biomedical applications. Biomedicines 2021, 9, 1113. [Google Scholar] [CrossRef] [PubMed]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef]
- Faivre, J.; Pigweh, A.I.; Iehl, J.; Maffert, P.; Goekjian, P.; Bourdon, F. Crosslinking hyaluronic acid soft-tissue fillers: Current status and perspectives from an industrial point of view. Expert Rev. Med. Devices 2021, 18, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Mundada, P.; Kohler, R.; Boudabbous, S.; Toutous Trellu, L.; Platon, A.; Becker, M. Injectable facial fillers: Imaging features, complications, and diagnostic pitfalls at MRI and PET CT. Insights Imaging 2017, 8, 557–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glogau, R.G.; Kane, M.A.C. Effect of injection techniques on the rate of local adverse events in patients implanted with nonanimal hyaluronic acid gel dermal fillers. Dermatol. Surg. 2008, 34, 105–109. [Google Scholar] [CrossRef]
- Christensen, L. Normal and pathologic tissue reactions to soft tissue gel fillers. Dermatol. Surg. 2007, 33, 168–175. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Fernández-Figueras, M.T.; Puig, L. Inflammatory, immune-mediated adverse reactions related to soft tissue dermal fillers. Semin. Arthritis Rheum. 2013, 43, 241–258. [Google Scholar] [CrossRef] [PubMed]
- El-Khalawany, M.; Fawzy, S.; Saied, A.; Al Said, M.; Amer, A.; Eassa, B. Dermal filler complications: A clinicopathologic study with a spectrum of histologic reaction patterns. Ann. Diagn. Pathol. 2015, 19, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Virych, P.; Nadtoka, O.; Doroschuk, V.; Lelyushok, S.; Chumachenko, V.; Bezugla, T.; Kutsevol, N. Cefuroxime-Loaded Hydrogels for Prevention and Treatment of Bacterial Contamination of Open Wounds. Int. J. Polym. Sci. 2021, 2021, 4935642. [Google Scholar] [CrossRef]
- Grossman, T.H. Tetracycline antibiotics and resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, S.P.; Rao, R.; Nanda, S. Amoxicillin: A broad spectrum antibiotic. Int. J. Pharm. Pharm. Sci. 2011, 3, 30–37. [Google Scholar]
- Di Bella, S.; Luzzati, R.; Principe, L.; Zerbato, V.; Meroni, E.; Giuffrè, M.; Crocè, L.S.; Merlo, M.; Perotto, M.; Dolso, E.; et al. Aspirin and Infection: A Narrative Review. Biomedicines 2022, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Darby, I.A.; Weller, C.D. Aspirin treatment for chronic wounds: Potential beneficial and inhibitory effects. Wound Repair Regen. 2017, 25, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial Hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef] [Green Version]
- del Olmo, J.A.; Alonso, J.M.; Martínez, V.S.; Ruiz-Rubio, L.; González, R.P.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Biocompatible hyaluronic acid-divinyl sulfone injectable hydrogels for sustained drug release with enhanced antibacterial properties against Staphylococcus aureus. Mater. Sci. Eng. C 2021, 125, 112102. [Google Scholar] [CrossRef] [PubMed]
- Andrade del Olmo, J.; Pérez-Álvarez, L.; Sáez-Martínez, V.; Benito-Cid, S.; Ruiz-Rubio, L.; Pérez-González, R.; Vilas-Vilela, J.L.; Alonso, J.M. Wound healing and antibacterial chitosan-genipin hydrogels with controlled drug delivery for synergistic anti-inflammatory activity. Int. J. Biol. Macromol. 2022, 203, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Pérez-álvarez, L.; Ruiz-Rubio, L.; González, R.P.; Sáez-Martínez, V.; Pérez, J.R.; Vilas-Vilela, J.L. Synthesis and characterization of covalently crosslinked pH-responsive hyaluronic acid nanogels: Effect of synthesis parameters. Polymers 2019, 11, 742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faivre, J.; Gallet, M.; Tremblais, E.; Trévidic, P.; Bourdon, F. Advanced Concepts in Rheology for the Evaluation of Hyaluronic Acid-Based Soft Tissue Fillers. Dermatol. Surg. 2021, 47, e159–e167. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Williams, R.; Gagliardi, S.; Vicini, S.; Alloisio, M.; Castellano, M. A micro-rheological and rheological study of biopolymers solutions: Hyaluronic acid. Carbohydr. Polym. 2019, 203, 349–355. [Google Scholar] [CrossRef]
- Ouyang, L.; Highley, C.B.; Rodell, C.B.; Sun, W.; Burdick, J.A. 3D Printing of Shear-Thinning Hyaluronic Acid Hydrogels with Secondary Cross-Linking. ACS Biomater. Sci. Eng. 2016, 2, 1743–1751. [Google Scholar] [CrossRef]
- Santoro, S.; Russo, L.; Argenzio, V.; Borzacchiello, A. Rheological properties of cross-linked hyaluronic acid dermal fillers. J. Appl. Biomater. Biomech. 2011, 9, 127–136. [Google Scholar] [CrossRef]
- Lee, W.; Hwang, S.G.; Oh, W.; Kim, C.Y.; Lee, J.L.; Yang, E.J. Practical Guidelines for Hyaluronic Acid Soft-Tissue Filler Use in Facial Rejuvenation. Dermatol. Surg. 2020, 46, 41–49. [Google Scholar] [CrossRef]
- Alonso, J.M.; Andrade del Olmo, J.; Perez Gonzalez, R.; Saez-Martinez, V. Injectable Hydrogels: From Laboratory to Industrialization. Polymers 2021, 13, 650. [Google Scholar] [CrossRef]
- Jeong, C.H.; Kim, D.H.; Yune, J.H.; Kwon, H.C.; Shin, D.M.; Sohn, H.; Lee, K.H.; Choi, B.; Kim, E.S.; Kang, J.H.; et al. In vitro toxicity assessment of crosslinking agents used in hyaluronic acid dermal filler. Toxicol. Vitr. 2021, 70, 105034. [Google Scholar] [CrossRef]
- De Boulle, K.; Glogau, R.; Kono, T.; Nathan, M.; Tezel, A.; Roca-Martinez, J.X.; Paliwal, S.; Stroumpoulis, D. A review of the metabolism of 1,4-butanediol diglycidyl ether-crosslinked hyaluronic acid dermal fillers. Dermatol. Surg. 2013, 39, 1758–1766. [Google Scholar] [CrossRef] [Green Version]
- International Organization for Standarization Ophthalmic Implants. Ophthalmic Viscosurgical Devices. (ISO 15798:2013); ISO: Geneva, Switzerland, 2013. [Google Scholar]
- Kaderli, S.; Boulocher, C.; Pillet, E.; Watrelot-Virieux, D.; Rougemont, A.L.; Roger, T.; Viguier, E.; Gurny, R.; Scapozza, L.; Jordan, O. A novel biocompatible hyaluronic acid-chitosan hybrid hydrogel for osteoarthrosis therapy. Int. J. Pharm. 2015, 483, 158–168. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standarization. Biological Evaluation of Medical Devices-Tests for In Vitro Cytotoxicity (ISO 10993-5:2009); ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Choi, S.C.; Yoo, M.A.; Lee, S.Y.; Lee, H.J.; Son, D.H.; Jung, J.; Noh, I.; Kim, C.W. Modulation of biomechanical properties of hyaluronic acid hydrogels by crosslinking agents. J. Biomed. Mater. Res.-Part A 2015, 103, 3072–3080. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standarization. Biological Evaluation of Medical Devices-Test for Systemic Toxicity (ISO 10993-11:2017); ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Rizzo, F.; Kehr, N.S. Recent Advances in Injectable Hydrogels for Controlled and Local Drug Delivery. Adv. Healthc. Mater. 2021, 10, 2001341. [Google Scholar] [CrossRef]
- Andrade del Olmo, J.; Sáez-Martínez, V.; Pérez-González, R.; Alonso, J.M. Sustained Drug Release from Biopolymer-Based Hydrogels and Hydrogel Coatings. In Hydrogels—From Tradition to Innovative Platforms With Multiple Applications; Lacramioara Popa, Ghica, M., Dinu-Pirvu, C., Eds.; IntechOpen: London, UK, 2022; pp. 1–20. [Google Scholar]
- Andrade-Del Olmo, J.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Sáez-Martínez, V.; Vilas-Vilela, J.L. Antibacterial Coatings for Improving the Performance of Biomaterials. Coatings 2020, 10, 139. [Google Scholar] [CrossRef] [Green Version]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Askari, E.; Seyfoori, A.; Amereh, M.; Gharaie, S.S.; Ghazali, H.S.; Ghazali, Z.S.; Khunjush, B.; Akbari, M. Stimuli-responsive hydrogels for local post-surgical drug delivery. Gels 2020, 6, 14. [Google Scholar] [CrossRef]
- Carton, F.; Chevalier, Y.; Nicoletti, L.; Tarnowska, M.; Stella, B.; Arpicco, S.; Malatesta, M.; Jordheim, L.P.; Briançon, S.; Lollo, G. Rationally designed hyaluronic acid-based nano-complexes for pentamidine delivery. Int. J. Pharm. 2019, 568, 118526. [Google Scholar] [CrossRef]
- Sueke, H.; Kaye, S.; Neal, T.; Murphy, C.; Hall, A.; Whittaker, D.; Tuft, S.; Parry, C. Minimum inhibitory concentrations of standard and novel antimicrobials for isolates from bacterial keratitis. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2519–2524. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Gao, L.; Xu, T.; Chen, Y.; Yang, X.; Han, M.; He, X.; Li, C.; Zhou, R.; Yang, Y. Amoxicillin Administration Regimen and Resistance Mechanisms of Staphylococcus aureus Established in Tissue Cage Infection Model. Front. Microbiol. 2019, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chan, E.W.L.; Yee, Z.Y.; Raja, I.; Yap, J.K.Y. Synergistic effect of non-steroidal anti-inflammatory drugs (NSAIDs) on antibacterial activity of cefuroxime and chloramphenicol against methicillin-resistant Staphylococcus aureus. J. Glob. Antimicrob. Resist. 2017, 10, 70–74. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef]
- Christensen, L.; Breiting, V.; Bjarnsholt, T.; Eickhardt, S.; Høgdall, E.; Janssen, M.; Pallua, N.; Zaat, S.A.J. Bacterial infection as a likely cause of adverse reactions to polyacrylamide hydrogel fillers in cosmetic surgery. Clin. Infect. Dis. 2013, 56, 1438–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saththianathan, M.; Johani, K.; Taylor, A.; Hu, H.; Vickery, K.; Callan, P.; Deva, A.K. The Role of Bacterial Biofilm in Adverse Soft-Tissue Filler Reactions: A Combined Laboratory and Clinical Study. Plast. Reconstr. Surg. 2017, 139, 613–621. [Google Scholar] [CrossRef] [PubMed]
- del Olmo, J.A.; Pérez-Álvarez, L.; Pacha-Olivenza, M.Á.; Ruiz-Rubio, L.; Gartziandia, O.; Vilas-Vilela, J.L.; Alonso, J.M. Antibacterial catechol-based hyaluronic acid, chitosan and poly (N-vinyl pyrrolidone) coatings onto Ti6Al4V surfaces for application as biomedical implant. Int. J. Biol. Macromol. 2021, 183, 1222–1235. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.L.; Ren, K.F.; Chang, H.; Wang, J.L.; Ji, J. Construction of degradable multilayer films for enhanced antibacterial properties. ACS Appl. Mater. Interfaces 2013, 5, 4136–4143. [Google Scholar] [CrossRef]
- Salvo, F.; De Sarro, A.; Caputi, A.P.; Polimeni, G. Amoxicillin and amoxicillin plus clavulanate: A safety review. Expert Opin. Drug Saf. 2009, 8, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guan, A.; Isayeva, I.; Vorvolakos, K.; Das, S.; Li, Z.; Phillips, K.S. Interactions of Staphylococcus aureus with ultrasoft hydrogel biomaterials. Biomaterials 2016, 95, 74–85. [Google Scholar] [CrossRef] [Green Version]
- Torimiro, N.; Moshood, A.A.; Eylolawi, S.A. Analysis of Beta-lactamase production and Antibiotics resistance in Staphylococcus aureus strains. J. Infect. Dis. Immun. 2013, 5, 24–28. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [Green Version]
- Khan, J.; Noboru, N.; Young, A.; Thomas, D. Pro and anti-inflammatory cytokine levels (TNF-α, IL-1β, IL-6 and IL-10) in rat model of neuroma. Pathophysiology 2017, 24, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin acute wound healing: A comprehensive review. Int. J. Inflam. 2019, 2019, 3706315. [Google Scholar] [CrossRef] [PubMed]







| Hydrogels | Reaction Time (h) | BDDE Concentration |
|---|---|---|
| HA-BDDE-1 (●) | 2 | 0.5 C |
| HA-BDDE-2 (●) | 2 | 1 C |
| HA-BDDE-3 (●) | 2 | 2 C |
| HA-BDDE-4 (●) | 3 | 0.5 C |
| HA-BDDE-5 (●) | 3 | 1 C |
| HA-BDDE-6 (●) | 3 | 2 C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade del Olmo, J.; Pérez-Álvarez, L.; Sáez Martínez, V.; Benito Cid, S.; Pérez González, R.; Vilas-Vilela, J.L.; Alonso, J.M. Drug Delivery from Hyaluronic Acid–BDDE Injectable Hydrogels for Antibacterial and Anti-Inflammatory Applications. Gels 2022, 8, 223. https://doi.org/10.3390/gels8040223
Andrade del Olmo J, Pérez-Álvarez L, Sáez Martínez V, Benito Cid S, Pérez González R, Vilas-Vilela JL, Alonso JM. Drug Delivery from Hyaluronic Acid–BDDE Injectable Hydrogels for Antibacterial and Anti-Inflammatory Applications. Gels. 2022; 8(4):223. https://doi.org/10.3390/gels8040223
Chicago/Turabian StyleAndrade del Olmo, Jon, Leyre Pérez-Álvarez, Virginia Sáez Martínez, Sandra Benito Cid, Raúl Pérez González, José Luis Vilas-Vilela, and José María Alonso. 2022. "Drug Delivery from Hyaluronic Acid–BDDE Injectable Hydrogels for Antibacterial and Anti-Inflammatory Applications" Gels 8, no. 4: 223. https://doi.org/10.3390/gels8040223
APA StyleAndrade del Olmo, J., Pérez-Álvarez, L., Sáez Martínez, V., Benito Cid, S., Pérez González, R., Vilas-Vilela, J. L., & Alonso, J. M. (2022). Drug Delivery from Hyaluronic Acid–BDDE Injectable Hydrogels for Antibacterial and Anti-Inflammatory Applications. Gels, 8(4), 223. https://doi.org/10.3390/gels8040223