Rheological Investigation as Tool to Assess Physicochemical Stability of a Hyaluronic Acid Dermal Filler Cross-Linked with Polyethylene Glycol Diglycidyl Ether and Containing Calcium Hydroxyapatite, Glycine and L-Proline
Abstract
:1. Introduction
2. Results and Discussion
2.1. Frequency Sweep Test
2.2. Rheology as Tool for the Study of Dermal Filler Behaviour at Different Temperatures
2.3. Dermal Fillers’ Viscosity Determination
3. Conclusions
4. Materials and Methods
4.1. Frequency Sweep Test
4.2. Dermal Fillers’ Behaviour at Different Temperatures
4.3. Dermal Fillers’ Viscosity Determination
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.-Y.; Zhong, Y.-Y.; Zheng, J.; Liu, Y.; Li, T.; Hu, E.; Zhu, X.-F.; Ding, R.-Q.; Wu, Y.; Zhang, Y.; et al. Fmoc-amino acid-based hydrogel vehicle for delivery of amygdalin to perform neuroprotection. Smart Mater. Med. 2021, 2, 56–64. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Chen, H.; Chen, K.; Tao, W.; Ouyang, X.K.; Mei, L.; Zeng, X. Polyphenol-based hydrogels: Pyramid evolution from crosslinked structures to biomedical applications and the reverse design. Bioact. Mater. 2022, 17, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Brummer, R.; Griebenow, M.; Hetzel, F.; Schlesiger, V.; Uhlmann, R. Rheological swing test to predict the temperature stability of cosmetic emulsions. In Proceedings of the 21st IFSCC International Congress, Berlin, Germany, 11–14 September 2000. [Google Scholar]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Guaratini, T.; Gianeti, M.D.; Campos, P.M. Stability of cosmetic formulations containing esters of vitamins E and A: Chemical and physical aspects. Int. J. Pharm. 2006, 327, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Lukic, M.; Jaksic, I.; Krstonosic, V.; Dokic, L.; Savic, S. Effect of Small Change in Oil Phase Composition on Rheological and Textural Properties of w/o Emulsion. J. Texture Stud. 2012, 44, 34–44. [Google Scholar] [CrossRef]
- Franzol, A.; Banin, T.M.; Brazil, T.R.; Rezende, M.C. Assessment of kinetic stability of cosmetic emulsions formulated with different emulsifiers using rheological and sensory analyses. J. Sol-Gel Sci. Technol. 2021, 99, 469–481. [Google Scholar] [CrossRef]
- Tezel, A.; Fredrickson, G.H. The science of hyaluronic acid dermal fillers. J. Cosmet. Laser. Ther. 2008, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Falcone, S.J.; Doerfler, A.M.; Berg, R.A. Novel synthetic dermal fillers based on sodium carboxymethylcellulose: Comparison with crosslinked hyaluronic acid-based dermal fillers. Dermatol. Surg. 2007, 33, S136–S143. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Garcia, E.S.; Zimmerman, S.C. Intramolecularly Cross-Linked Polymers: From Structure to Function with Applications as Artificial Antibodies and Artificial Enxymes. Acc. Chem. Res. 2020, 53, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Zerbinati, N.; Sommatis, S.; Maccario, C.; Capillo, M.C.; Grimaldi, G.; Alonci, G.; Protasoni, M.; Rauso, R.; Mocchi, R. Toward Physicochemical and Rheological Characterization of Different Injectable Hyaluronic Acid Dermal Fillers Cross-Linked with Polyethylene Glycol Diglycidyl Ether. Polymers 2021, 13, 948. [Google Scholar] [CrossRef]
- Scarano, A.; Rapone, B.; Amuso, D. Hyaluronic Acid Fillers Enriched with Glycine and Proline in Eyebrow Augmentation Procedure. Aesthet. Plast. Surg. 2021, 46, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Lorenc, Z.P.; Bass, L.M.; Fitzgerald, R.; Goldberg, D.J.; Graivier, M.H. Physiochemical Characteristics of Calcium Hydroxylapatite (CaHA). Aesthet. Surg. J. 2018, 38, S8–S12. [Google Scholar] [CrossRef] [PubMed]
- Zerbinati, N.; Lotti, T.; Monticelli, D.; Rauso, R.; Gonzàlez-Isaza, P.; D’Este, E.; Calligaro, A.; Sommatis, S.; Maccario, C.; Mocchi, R.; et al. In vitro evaluation of the biosafety of hyaluronic acid PEG cross-linked with micromolecules of calcium hydroxyapatite in low concentration. Open Access Maced. J. Med. Sci. 2018, 6, 15–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavard Molliard, S.; Bon Bétemps, J.; Hadjab, B.; Topchian, D.; Micheels, P.; Salomon, D. Key rheological properties of hyaluronic acid fillers: From tissue integration to product degradation. Plast. Aesthet. Res. 2018, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Lorenc, Z.P.; Öhrlund, A.; Edsman, K. Factors Affecting the Rheological Measurement of Hyaluronic Acid Gel Fillers. J. Drugs Dermatol. 2017, 16, 876–882. [Google Scholar] [PubMed]
- Zerbinati, N.; Sommatis, S.; Maccario, C.; Capillo, M.C.; Grimaldi, G.; Alonci, G.; Rauso, R.; Guida, S.; Mocchi, R. Comparative Physicochemical Analysis among 1,4-Butanediol Diglycidyl Ether Cross-Linked Hyaluronic Acid Dermal Fillers. Gels 2021, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Gołezbiowski, M.; Cerkowniak, M.; Urbanek, A.; Dawgul, M.; Kamysz, M.; Sosnowska, D.; Stepnowski, P. Antimicrobial activity of untypical lipid compounds in the cuticular and internal lipids of four fly species. J. Appl. Microbiology 2013, 116, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Duranti, F.; Salti, G.; Bovani, B.; Calandra, M.; Rosati, M.L. Injectable hyaluronic acid gel for soft tissue augmentation. A clinical and histological study. Dermatol. Surg. 1998, 24, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
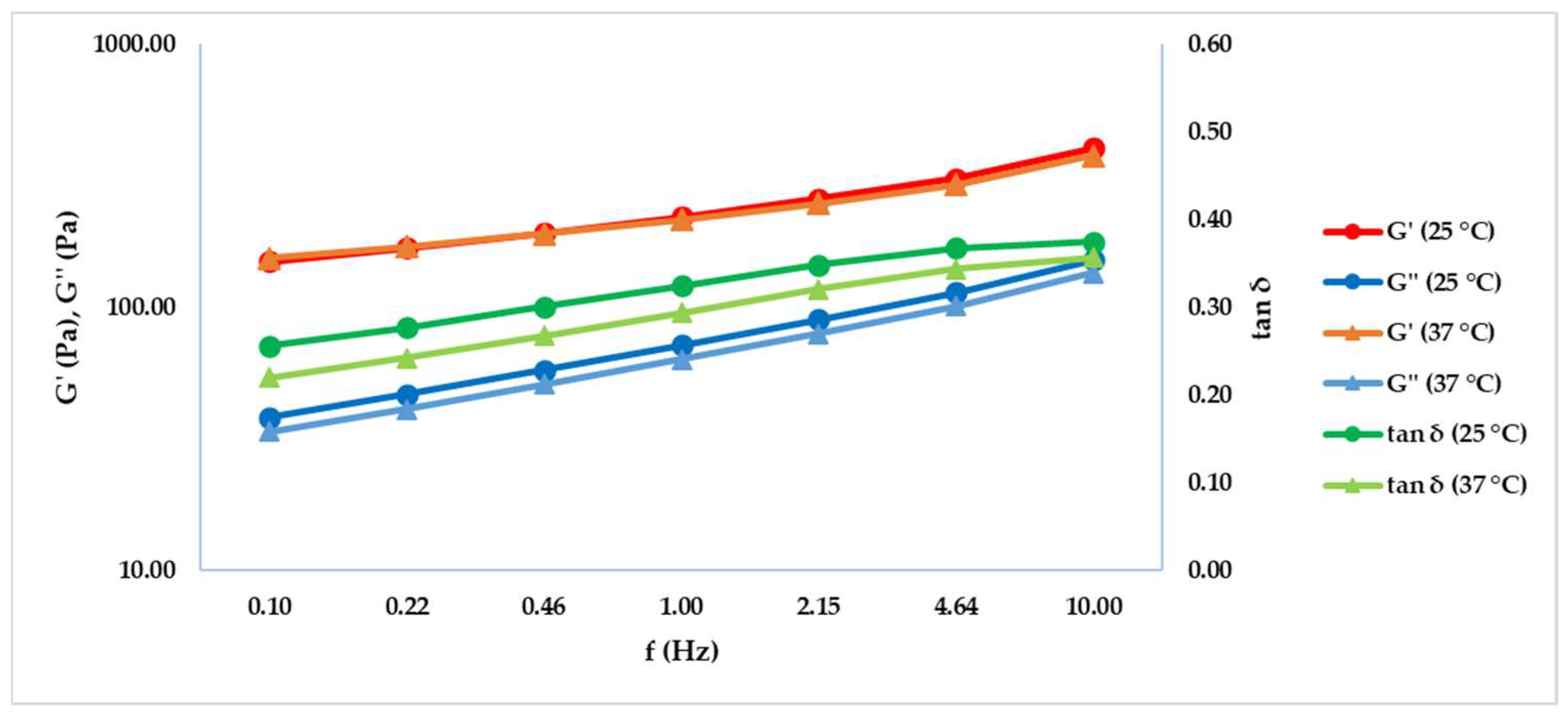
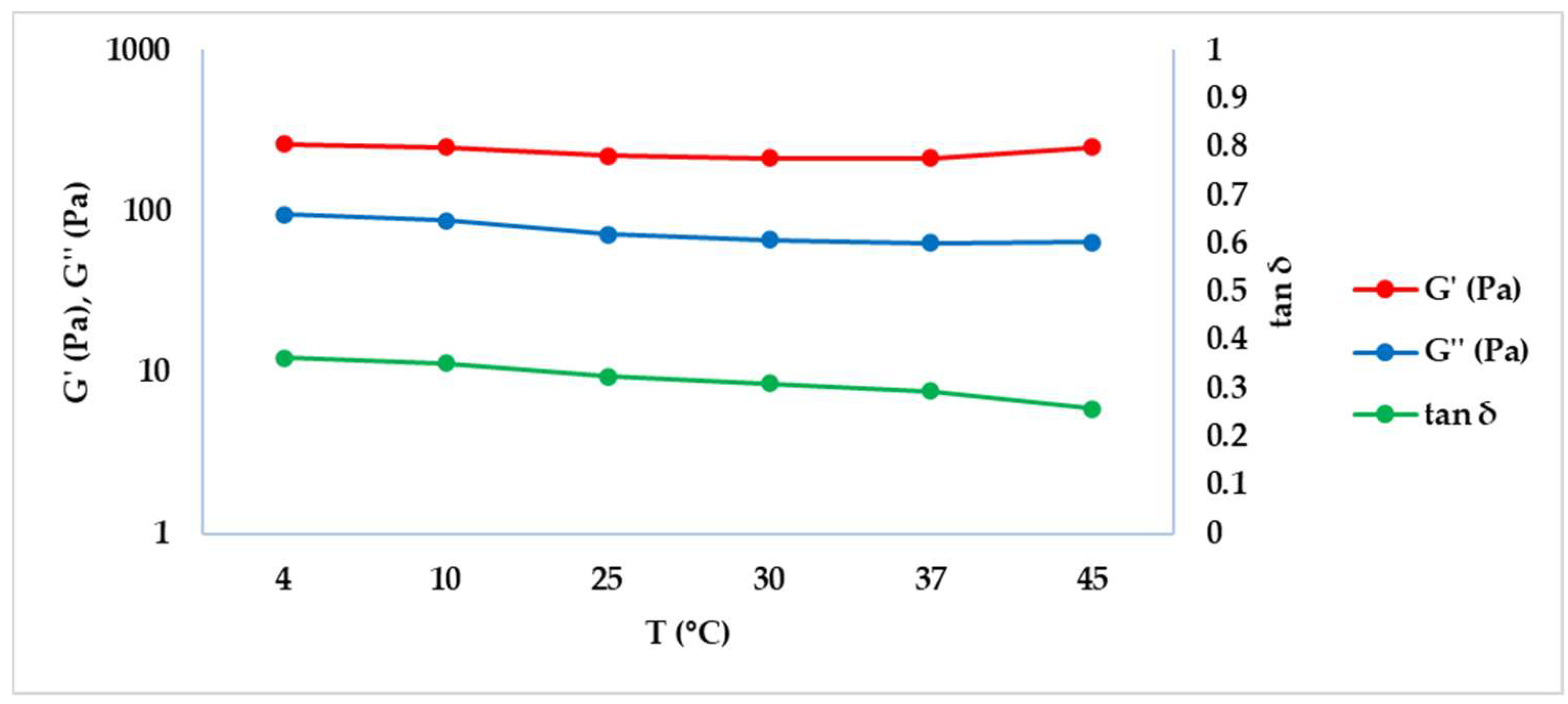
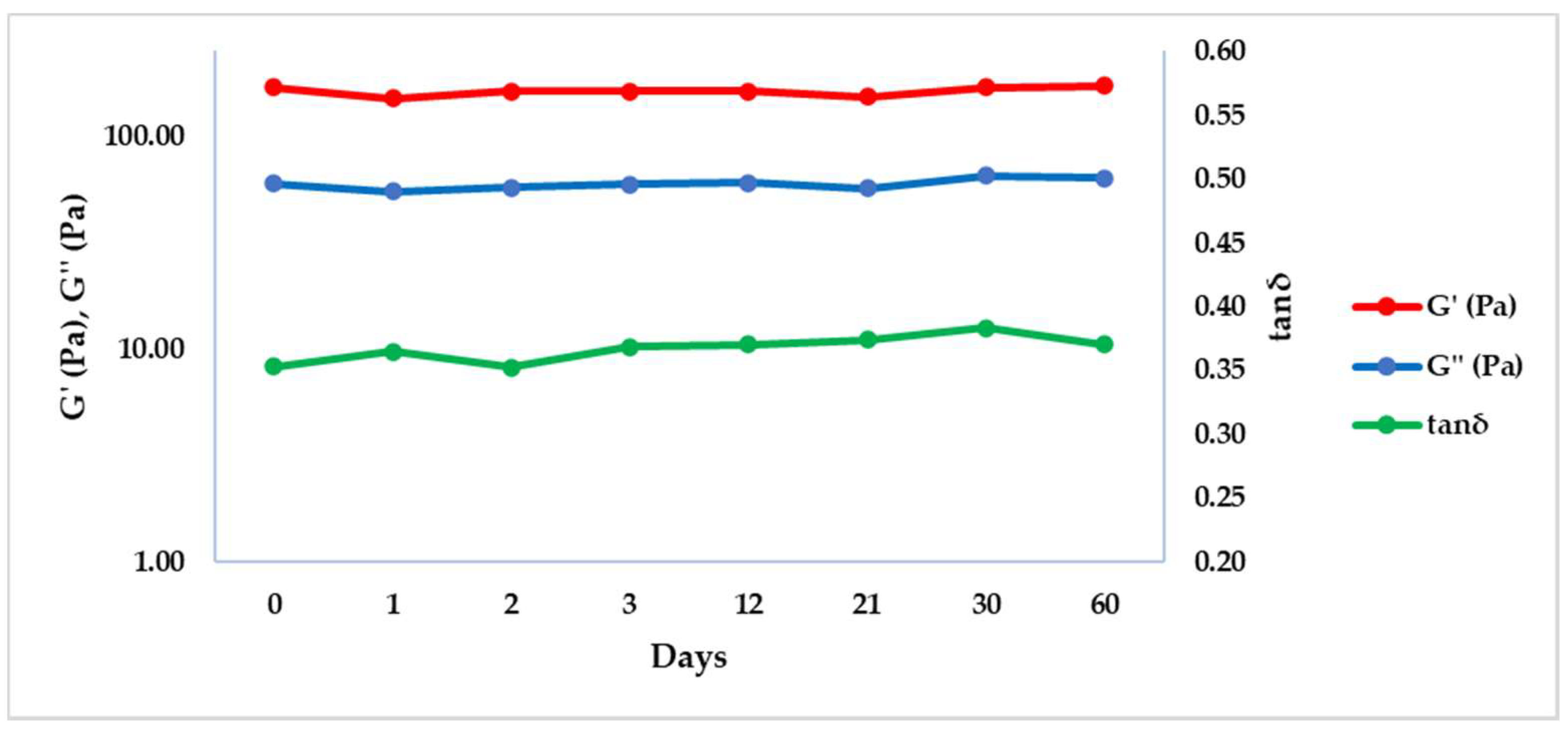
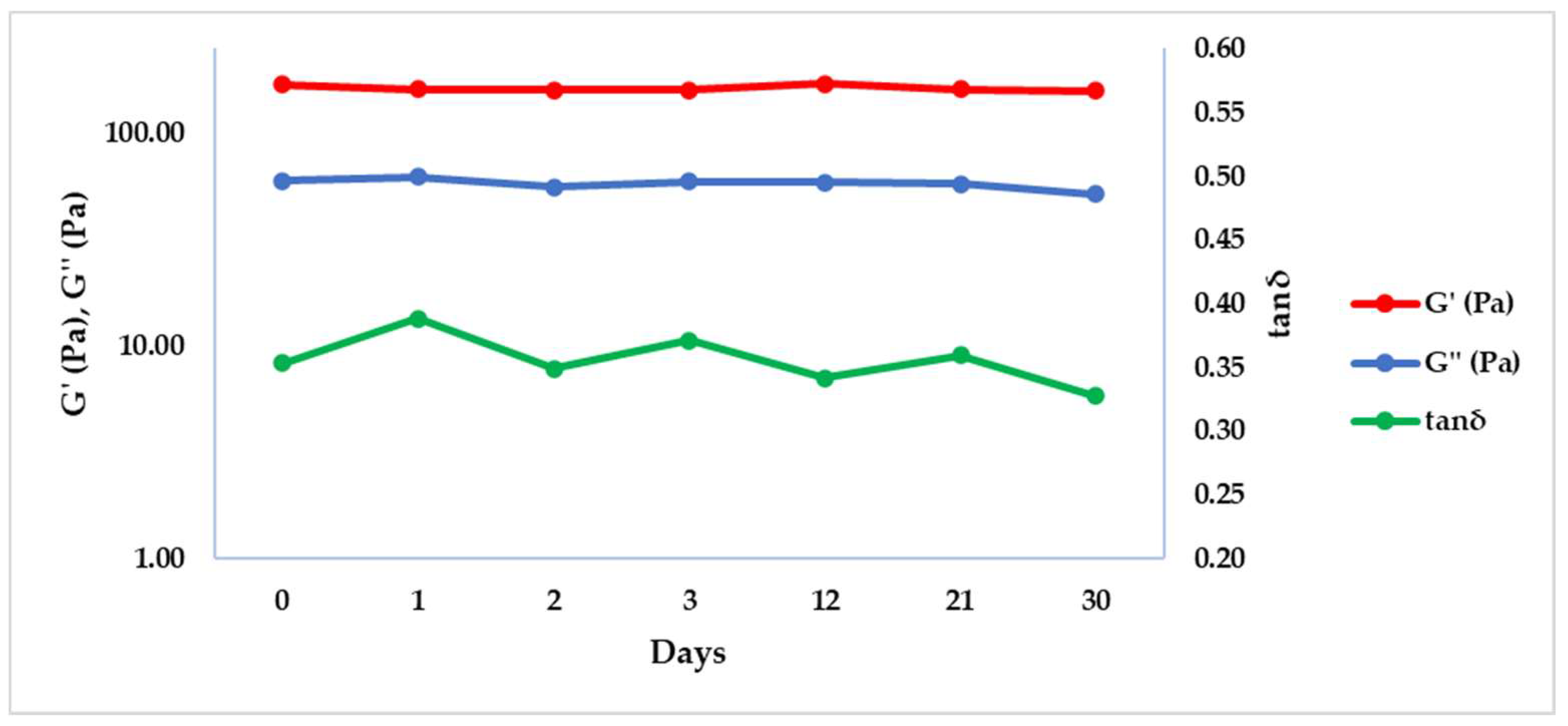

| T (°C) | f (Hz) | G’ (Pa) ± SD | G’’ (Pa) ± SD | tan δ ± SD |
|---|---|---|---|---|
| 25 | 0.10 | 149.40 ± 8.61 | 38.30 ± 4.03 | 0.26 ± 0.02 |
| 25 | 0.22 | 168.33 ± 10.13 | 46.71 ± 4.62 | 0.28 ± 0.02 |
| 25 | 0.46 | 191.70 ± 12.16 | 57.71 ± 5.51 | 0.30 ± 0.02 |
| 25 | 1.00 | 220.90 ± 14.62 | 71.85 ± 6.56 | 0.33 ± 0.01 |
| 25 | 2.15 | 258.30 ± 17.71 | 90.09 ± 7.87 | 0.35 ± 0.01 |
| 25 | 4.64 | 309.83 ± 21.47 | 113.90 ± 9.45 | 0.37 ± 0.01 |
| 25 | 10.00 | 403.40 ± 24.50 | 151.1 ± 13.75 | 0.37 ± 0.02 |
| T (°C) | f (Hz) | G’ (Pa) ± SD | G’’ (Pa) ± SD | tan δ ± SD |
|---|---|---|---|---|
| 37 | 0.10 | 153.70 ± 8.00 | 33.78 ± 2.23 | 0.22 ± 0.02 |
| 37 | 0.22 | 170.03 ± 8.17 | 41.13 ± 2.75 | 0.24 ± 0.02 |
| 37 | 0.46 | 190.23 ± 8.57 | 50.90 ± 3.25 | 0.27 ± 0.02 |
| 37 | 1.00 | 215.57 ± 9.35 | 63.42 ± 3.95 | 0.29 ± 0.02 |
| 37 | 2.15 | 248.17 ± 10.57 | 79.64 ± 4.89 | 0.32 ± 0.01 |
| 37 | 4.64 | 293.67 ± 12.30 | 101.07 ± 5.95 | 0.34 ± 0.01 |
| 37 | 10.00 | 378.73 ± 13.48 | 135.23 ± 8.57 | 0.36 ± 0.01 |
| T (°C) | G’ (Pa) ± SD | G’’ (Pa) ± SD | tan δ ± SD |
|---|---|---|---|
| 4 | 261.83 ± 17.96 | 95.43 ± 8.35 | 0.36 ± 0.01 |
| 10 | 249.70 ± 18.38 | 88.08 ± 7.95 | 0.35 ± 0.01 |
| 25 | 220.90 ± 14.62 | 71.85 ± 6.56 | 0.32 ± 0.01 |
| 30 | 214.97 ± 12.16 | 66.89 ± 5.66 | 0.31 ± 0.01 |
| 37 | 215.57 ± 9.35 | 63.42 ± 3.95 | 0.29 ± 0.02 |
| 45 | 251.03 ± 9.46 | 64.67 ± 2.77 | 0.26 ± 0.02 |
| T (°C) | Time (Day) | G’ (Pa) ± SD | G’’ (Pa) ± SD | tan δ ± SD |
|---|---|---|---|---|
| 50 | 0 | 169.55 ± 0.21 | 59.90 ± 0.72 | 0.35 ± 0.00 |
| 50 | 1 | 150.40 ± 5.94 | 54.98 ± 6.14 | 0.37 ± 0.03 |
| 50 | 2 | 162.55 ± 14.35 | 57.46 ± 8.87 | 0.35 ± 0.02 |
| 50 | 3 | 161.95 ± 22.56 | 59.49 ± 5.06 | 0.37 ± 0.02 |
| 50 | 12 | 162.25 ± 8.56 | 60.20 ± 7.91 | 0.37 ± 0.03 |
| 50 | 21 | 152.90 ± 21.07 | 56.92 ± 4.21 | 0.37 ± 0.02 |
| 50 | 30 | 170.00 ± 27.15 | 65.16 ± 10.41 | 0.38 ± 0.00 |
| 50 | 60 | 171.50 ± 11.03 | 63.39 ± 1.03 | 0.37 ± 0.02 |
| T (°C) | Time (Day) | G’ (Pa) ± SD | G’’ (Pa) ± SD | tan δ ± SD |
|---|---|---|---|---|
| −20 | 0 | 169.55 ± 0.21 | 59.90 ± 0.72 | 0.35 ± 0.00 |
| −20 | 1 | 160.90 ± 15.70 | 62.45 ± 4.89 | 0.39 ± 0.01 |
| −20 | 2 | 159.47 ± 3.61 | 55.65 ± 4.17 | 0.35 ± 0.03 |
| −20 | 3 | 160.50 ± 11.60 | 59.52 ± 2.57 | 0.37 ± 0.01 |
| −20 | 12 | 171.75 ± 13.93 | 58.58 ± 2.61 | 0.34 ± 0.01 |
| −20 | 21 | 160.80 ± 1.70 | 57.79 ± 0.21 | 0.36 ± 0.00 |
| −20 | 30 | 150.40 ± 8.49 | 50.11 ± 1.18 | 0.33 ± 0.03 |
| −20 | 60 | 169.00 ± 3.39 | 60.41 ± 0.18 | 0.36 ± 0.01 |
| Condition of the Product | Temperature (°C) | Shear Rate (s−1) | η (Pa × s) ± SD |
|---|---|---|---|
| Rest | 25 | 10−2 | 1811.33 ± 25.74 |
| Extrusion | 25 | 102 | 13.43 ± 1.81 |
| Application | 37 | 102 | 8.39 ± 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerbinati, N.; Capillo, M.C.; Sommatis, S.; Maccario, C.; Alonci, G.; Rauso, R.; Galadari, H.; Guida, S.; Mocchi, R. Rheological Investigation as Tool to Assess Physicochemical Stability of a Hyaluronic Acid Dermal Filler Cross-Linked with Polyethylene Glycol Diglycidyl Ether and Containing Calcium Hydroxyapatite, Glycine and L-Proline. Gels 2022, 8, 264. https://doi.org/10.3390/gels8050264
Zerbinati N, Capillo MC, Sommatis S, Maccario C, Alonci G, Rauso R, Galadari H, Guida S, Mocchi R. Rheological Investigation as Tool to Assess Physicochemical Stability of a Hyaluronic Acid Dermal Filler Cross-Linked with Polyethylene Glycol Diglycidyl Ether and Containing Calcium Hydroxyapatite, Glycine and L-Proline. Gels. 2022; 8(5):264. https://doi.org/10.3390/gels8050264
Chicago/Turabian StyleZerbinati, Nicola, Maria Chiara Capillo, Sabrina Sommatis, Cristina Maccario, Giuseppe Alonci, Raffaele Rauso, Hassan Galadari, Stefania Guida, and Roberto Mocchi. 2022. "Rheological Investigation as Tool to Assess Physicochemical Stability of a Hyaluronic Acid Dermal Filler Cross-Linked with Polyethylene Glycol Diglycidyl Ether and Containing Calcium Hydroxyapatite, Glycine and L-Proline" Gels 8, no. 5: 264. https://doi.org/10.3390/gels8050264
APA StyleZerbinati, N., Capillo, M. C., Sommatis, S., Maccario, C., Alonci, G., Rauso, R., Galadari, H., Guida, S., & Mocchi, R. (2022). Rheological Investigation as Tool to Assess Physicochemical Stability of a Hyaluronic Acid Dermal Filler Cross-Linked with Polyethylene Glycol Diglycidyl Ether and Containing Calcium Hydroxyapatite, Glycine and L-Proline. Gels, 8(5), 264. https://doi.org/10.3390/gels8050264










