Hydrogel-Based Adsorbent Material for the Effective Removal of Heavy Metals from Wastewater: A Comprehensive Review
Abstract
:1. Introduction
1.1. Problem Statement
1.2. Heavy Metals and Their Hazardous Effect
| Heavy Metals | Leading Source | Path of Entry | Toxic Effects on Human Health | Environmental Hazards | MCL (mg/L) |
|---|---|---|---|---|---|
| Lead (Pb) | Mining, automobile emissions, smoking, pesticide, paint, burning of coal | Ingestion and inhalation | Damages the central nervous system, fetal brain, kidney, reproductive system, liver, basic cellular processes, and causes diseases, namely, anemia, nephrite syndrome, hepatitis, etc. | Soil and water pollution | 0.015 |
| Cadmium (Cd) | Pesticide fertilizer, electroplating, Cd-Ni batteries, welding | Ingestion and inhalation | Irritation of respiratory system, damages liver, kidney, and lungs | Soil and water pollution | 0.005 |
| Nickel (Ni) | Electrochemical industries | Inhalation | Causes lung, kidney, and gastronomical pain, renal edema, pulmonary fibrosis, and skin dermatitis | Soil and water pollution | 0.1 |
| Zinc (Zn) | Plumbing, refineries, metal plating, brass manufacture | Ingestion, inhalation, and through skin | Vomiting, pain in the stomach, skin irritation, nausea, and anemia | Soil and water pollution | 0.8 |
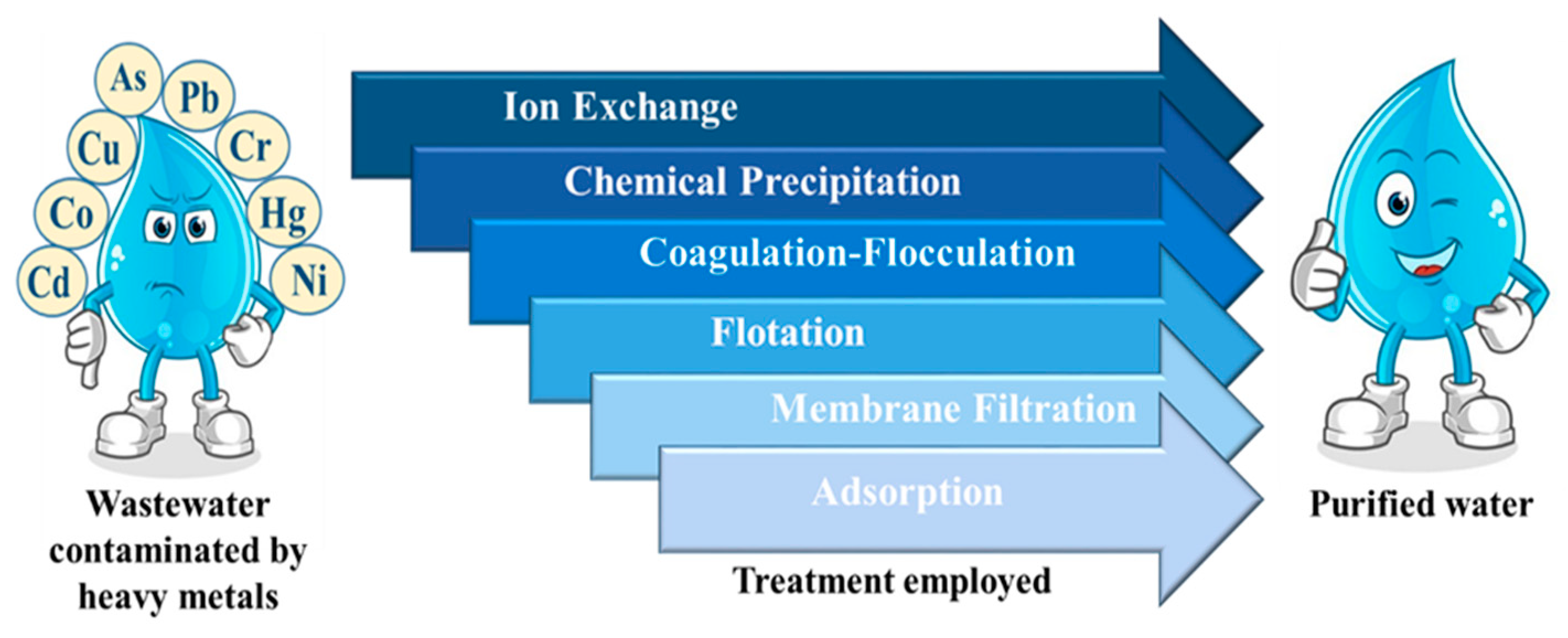
2. Methods Used for Recycling Wastewater
| Methods | Advantages | Disadvantages | References |
|---|---|---|---|
| Ion exchange | Does not produce a large amount of sludge, easy regeneration of resins | High operational cost, selective towards certain metal ions | [25] |
| Chemical precipitation | Low capital cost, simple process | Produces a large amount of sludge, ineffective in treating low concentration of heavy metal ions | [26] |
| Coagulation-flocculation | Easy to employ, inexpensive, low energy consumption | Complete removal of heavy metals is difficult, generation of a large quantity of sludge | [27] |
| Flotation | Economically efficient | Low elimination efficiency, | [28,29] |
| Membrane filtration | Small space requirement, high efficiency, high separation selectivity | complex process, high operational expense due to membrane fouling | [30] |
| Adsorption | Technologically feasible, effective, low-cost adsorbent, no waste generation, easy operation conditions | Low selectivity | [3,13] |
3. Hydrogels for Removal of Heavy Metals
- Cost-effective
- High adsorption capacity to absorb heavy metals from wastewater
- High adsorption rate (determined by porosity and particle size)
- Biodegradable
- Easy to modify
- The low content of the unreacted residual monomer
- High stability and durability during swelling and storage
- Non-toxic, colorless, and odorless
- pH neutrality after swelling in aqueous media
- Deswelling capabilities and re-watering (able to release back the stored water)
4. Properties of Hydrogel
4.1. Swelling or Deswelling of Hydrogels
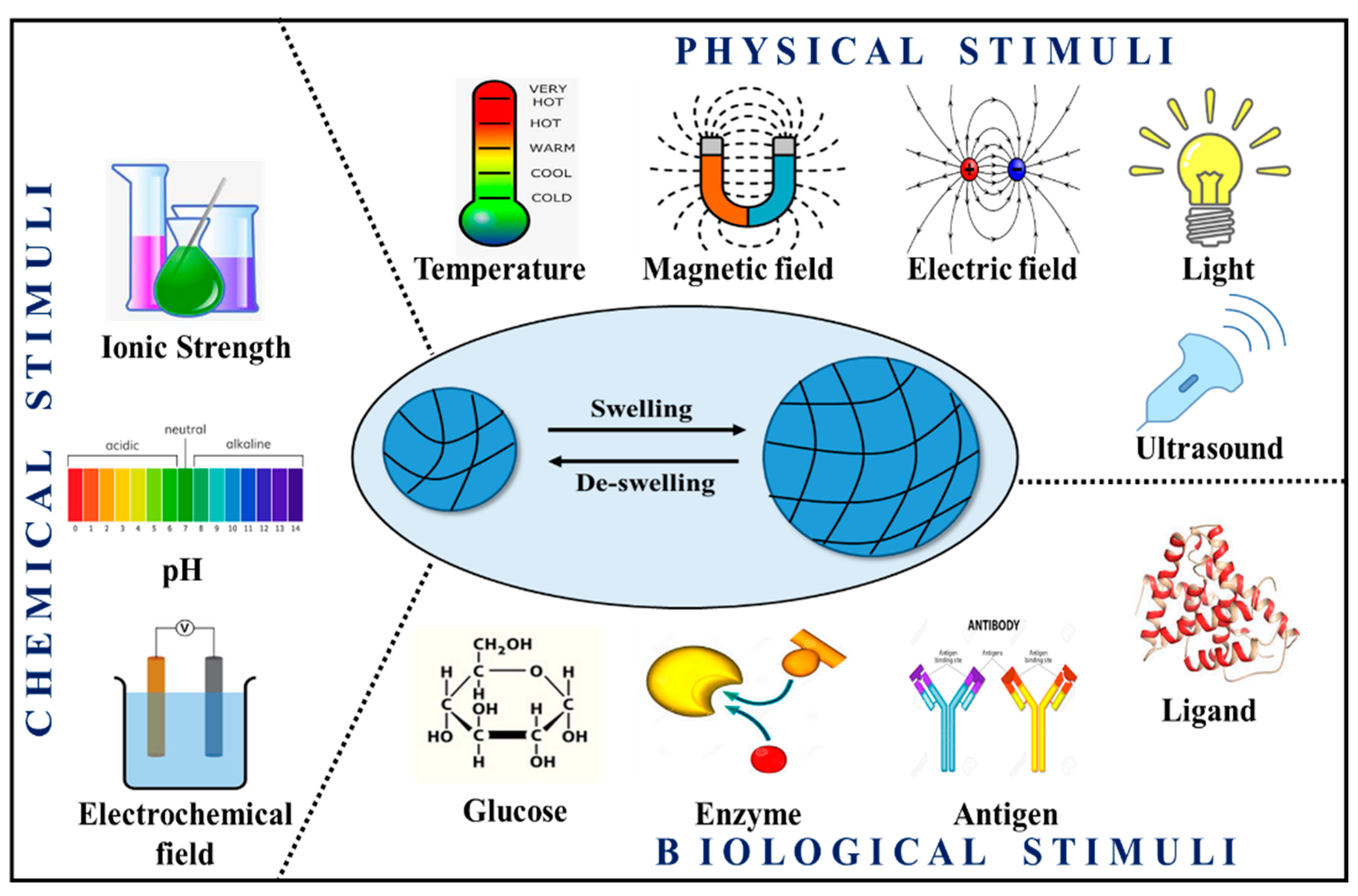
4.2. Stimuli-Responsive Hydrogels
4.3. Biodegradable
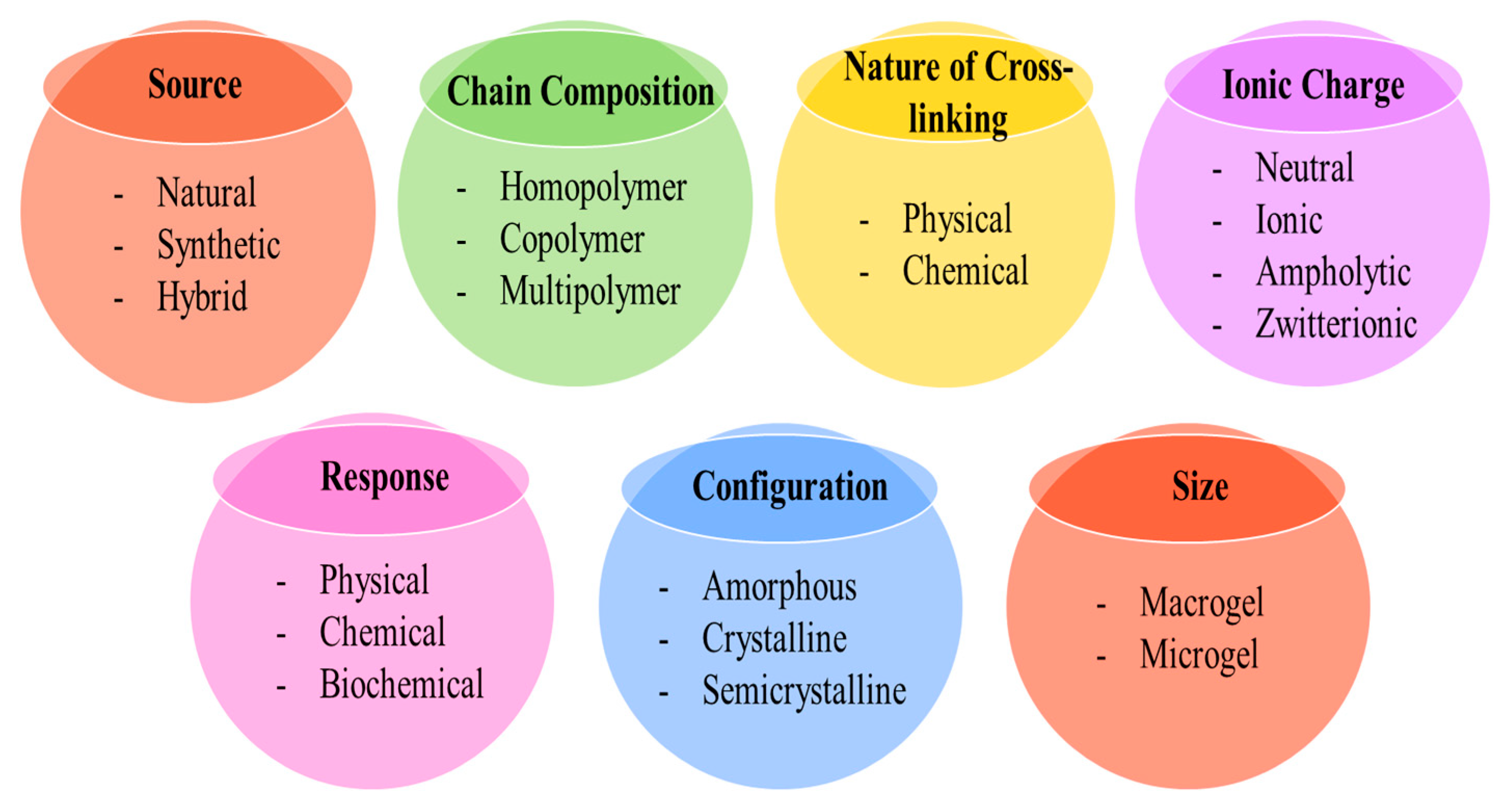
5. Classification of Hydrogel
5.1. Based on Source
- (a)
- Natural hydrogels are synthesized by using natural sources including chitosan, agar-agar, cellulose, lignin, gelatin, alginate, dextran, collagen, and many other materials [54].
- (b)
- Synthetic hydrogels are prepared by using synthetic polymers namely hydroxy methyl methacrylate (HEMA), acrylic acid (AA), vinyl acetate (VAc), ethylene glycol (EG), ethyleneglycoldimethacrylate (EGDMA), methacrylic acid, N-vinyl 2-pyrrolidone (NVP), and many other materials [54].
- (c)
5.2. Based on the Nature of Chain Composition
- (a)
- (b)
- Co-polymeric hydrogels are composed of two or more types of monomer units with at least one hydrophilic monomer, arranged in a random, block, and irregular structure along the backbone of the polymer network [59]. These hydrogels are prepared by cross-linking or polymerization between both the monomers by using a cross-linker and initiator. An example of such hydrogels is chitosan, k-carrageenan, carboxymethyl cellulose composite hydrogel which is used to remove metal ions.
- (c)
- Multipolymer hydrogels are cross-linked polymer-network prepared by three or more monomer units via cross-linking and polymerization reactions. For example, Kim et al. synthesized chitosan-based multicomponent functional gel comprising multiwall carbon nanotubes, polyaniline, poly (acrylic acid), and poly (4-amino diphenyl amine) [60].
- (d)
- Interpenetrating polymeric hydrogels are comprised of two independent, intertwined polymer networks, having natural and/or synthetic polymer components. In a semi-interpenetrating polymer hydrogel, one polymer has a linear network that diffuses into another cross-linked network. There is no chemical bonding between the polymers [54].
5.3. Based on the Nature of Cross-Linking
- (a)
- Physically cross-linked hydrogels have a transient junction that arises due to physical interaction such as hydrogen bond, ionic interaction, and hydrophobic interaction.
- (b)
- Chemically cross-linked hydrogels have permanent junctions that arise due to covalent bonds [50].
5.4. Based on the Reaction of Hydrogel with the External Stimulus
- (a)
- Traditional hydrogel is not reactive to environmental changes
- (b)
- An environment-sensitive hydrogel can detect changes caused by chemical (pH, concentration), biochemical (antigen, enzyme, ligand), and physical (temperature, pressure, light) factors [50].
5.5. Based on the Configuration
5.6. Based on the Size
5.7. Based on Ionic Charge
- (a)
- Neutral hydrogels are also known as non-ionic hydrogels. These hydrogels contain no charge on side groups or polymer backbone.
- (b)
- Ionic hydrogels are further classified as anionic and cationic. Anionic hydrogels carry negatively charged functional groups like sulfonyl, carboxyl, etc. and at high pH values show an increase in swelling behavior. Cationic hydrogels carry positively charged functional groups like amines, thiol, etc., and at low pH values exhibit an increase in swelling behavior.
- (c)
- Ampholytic or amphoteric hydrogels contain acidic as well as basic groups.
- (d)
6. Surface Functional Groups of Hydrogel
6.1. Nitrogen-Containing Functional Groups
6.1.1. Amine Group
6.1.2. Amide Group
6.1.3. Quaternary Ammonium Groups
6.2. Oxygen-Containing Functional Groups
6.2.1. Hydroxyl Group
6.2.2. Carboxyl Group
6.3. Sulfur-Containing Groups
6.3.1. Thiol Group
6.3.2. Sulfonic Acid Group
6.4. Other Functional Groups
6.4.1. Amidoxime Group
6.4.2. Phosphate-Containing Functional Group
6.4.3. Chelating Group
| Hydrogel | Active Functional Group | Heavy Metals Removed | References |
|---|---|---|---|
| Graphene oxide-chitosan-poly(acrylic acid) (GO-CS-AA) hydrogel nanocomposite | R-COOH | Pb2+ | [84] |
| Hydrous ferric oxide-Poly(trans-aconitic acid/2-hydroxyethyl acrylate (HFO-P(TAA/HEA)) hydrogel | R-OH | Cu2+, Cd2+, Pb2+ and Ni2+ | [85] |
| Chitosan-sodium lignosulfonate-acrylic acid (CS-SLS-AA) hydrogel | R-NH2 | Co2+ and Cu2+ | [86] |
| Poly(3-acrylamidopropyl) trimethyl ammonium chloride/ɤ-Fe2O3 | R-N+(CH3)3 | Cr4+ | [87] |
| Sulfathiazole-based novel UV-curved hydrogel | R-SH | Hg2+, Cd2+ and Zn2+ | [88] |
| Magnetic anionic hydrogel (nFeMAH) | R-SO3Na | Cu2+ and Ni2+ | [64] |
| Poly(2-acrylamido-2-methyl-1-propane sulfonic acid) magnetic hydrogel | R-SO3H | Cd2+, Co2+, Fe2+, Pb2+,Cu2+, Cr2+ and Ni2+ | [39] |
| Acrylamide/crotonic acid (AAm/CA) hydrogel | R-COOH, and R-CONH2 | Hg2+ | [89] |
| Glucan/chitosan hydrogel | R-OH and R-NH2 | Co2+, Cu2+, Cd2+, Ni2+ and Pb2+ | [90] |
| Malic acid enhanced chitosan hydrogel beads (mCHBs) | R-COOH and R-NH2 | Cu2+ | [91] |
| Carboxymethyl cellulose/polyacrylamide (CMC/PAM) composite hydrogel | R-OH, R-COOH and R-NH2 | Cd2+, Pb2+ and Cu2+ | [92] |
| Chitosan poly(acrylic acid) supermacroporous hydrogel | R-OH, R-COOH and R-NH2 | Cu2+ and Pb2+ | [93] |
| Lignosulfonate-modified graphene hydrogel | R-C=O, R-OH and R-COOH | Pb2+ | [94] |
| Polyacrylonitrile-chitosan-graphene oxide (PCG) hydrogel composite | R-C(NH2)=N-OH | U6+ | [73] |
7. Synthesis of Hydrogel
7.1. Synthesis via the Chemical Route
7.1.1. Chemical Route of Cross-Linking via Free Radical Polymerization
7.1.2. Chemical Route of Cross-Linking via High Energy Irradiation
7.1.3. Chemical Route of Cross-Linking via Grafting Reactions
7.1.4. Chemical Route of Cross-Linking via Reaction of Functional Groups
7.2. Synthesis via Physical Route
7.2.1. Synthesis via Freeze-Thaw
7.2.2. Synthesis via Self-Assembling
7.2.3. Synthesis via Instantaneous Gelation
7.2.4. Synthesis via Ionotropic Gelation
7.2.5. Synthesis via Inverse Emulsion Method
8. Characterization Techniques of Hydrogel
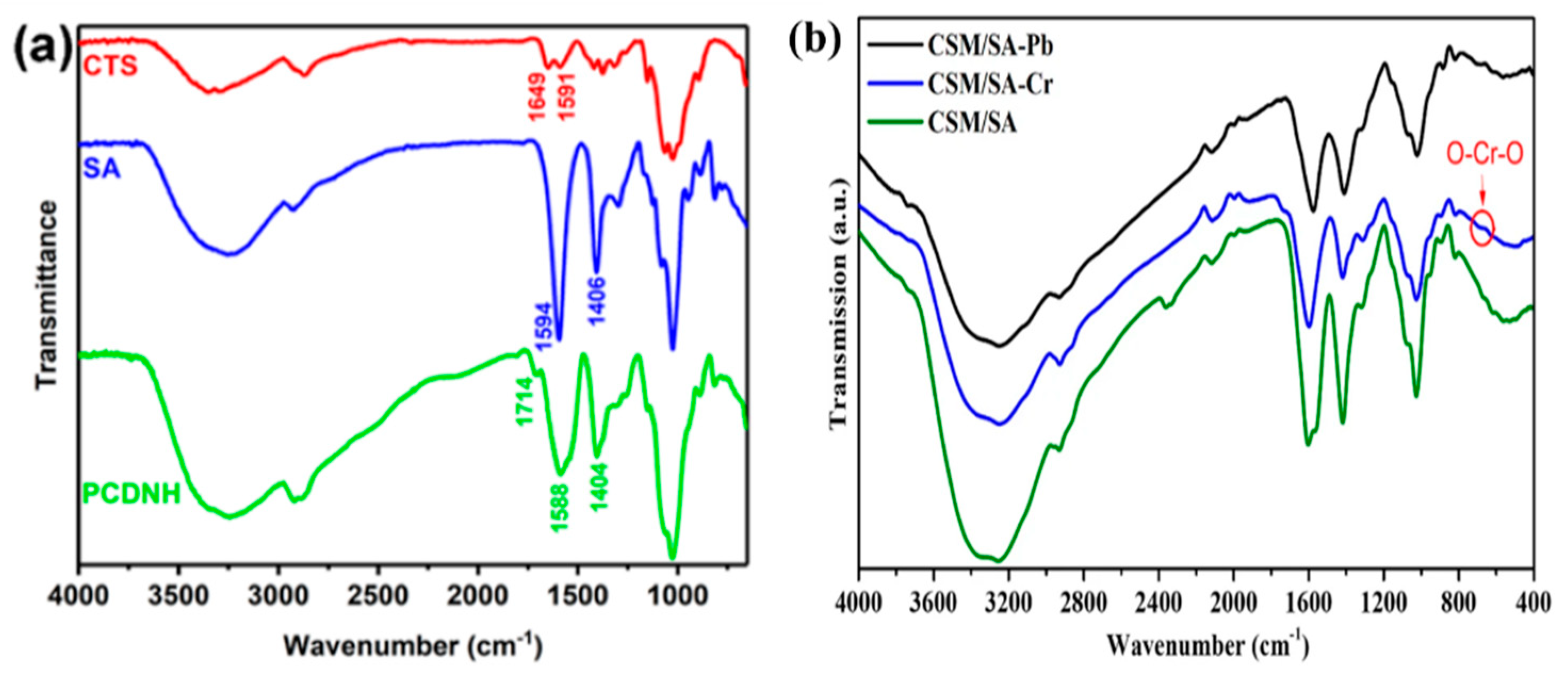
8.1. Functional Group Analysis
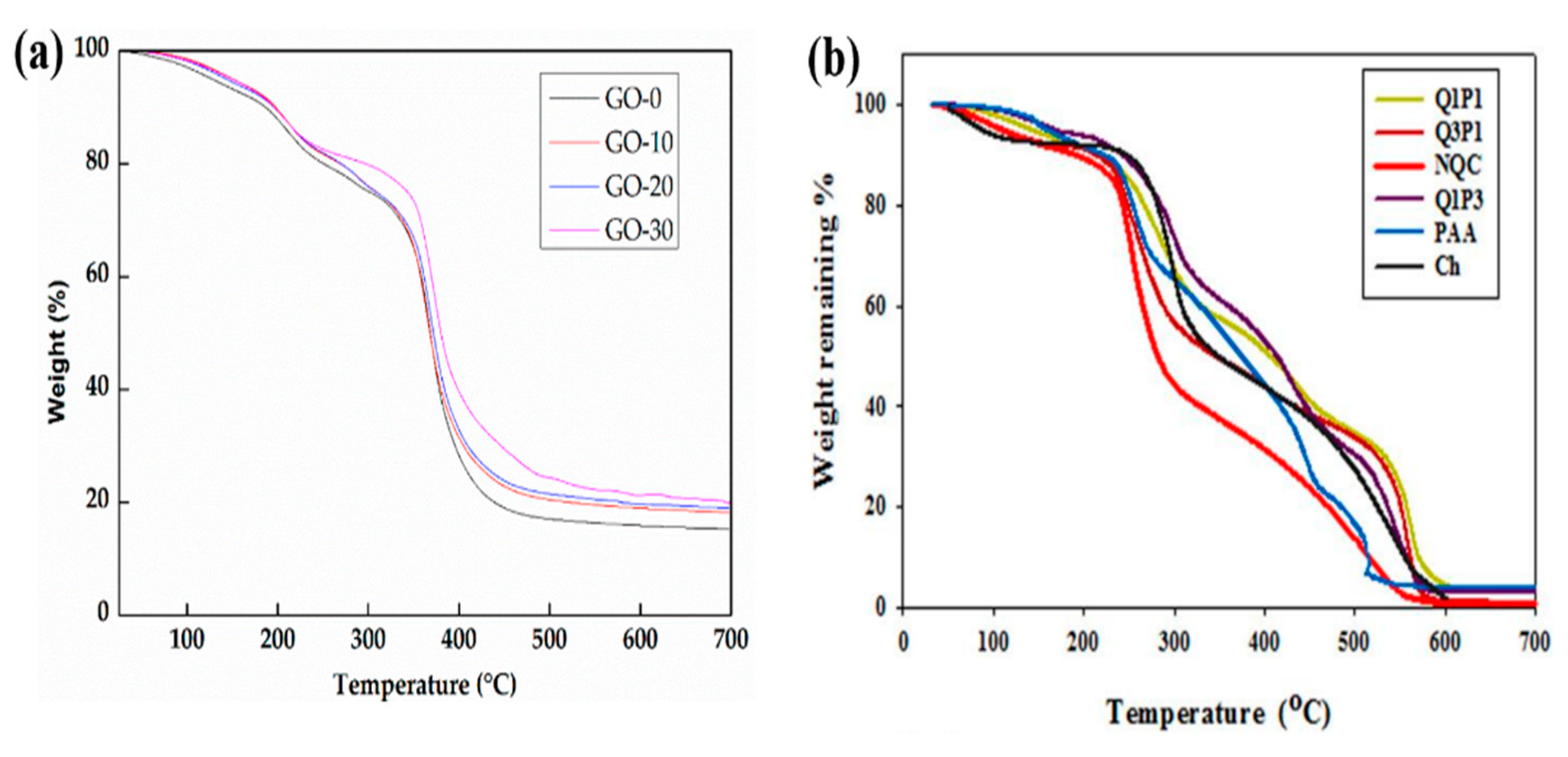
8.2. Thermal Analysis
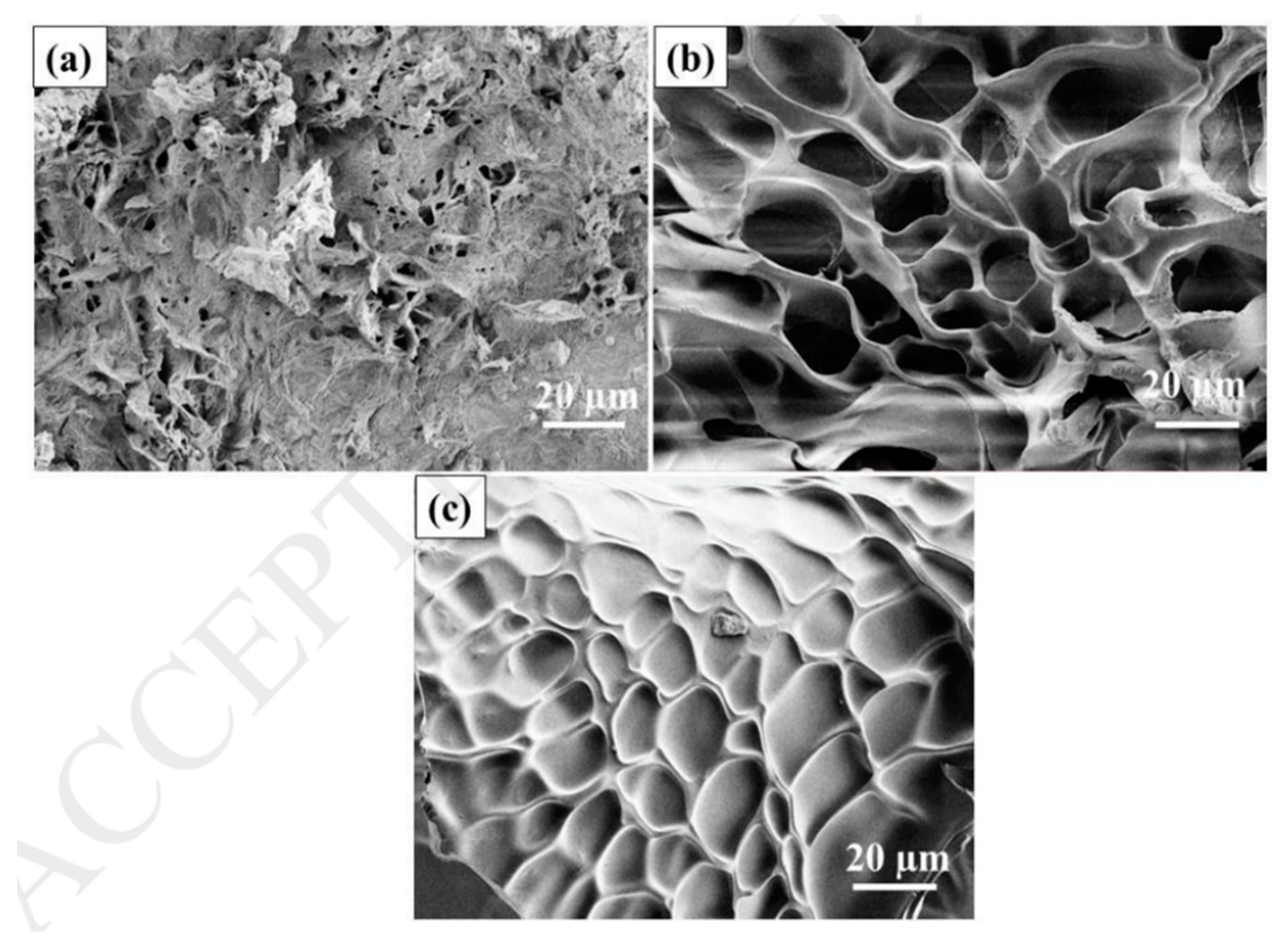
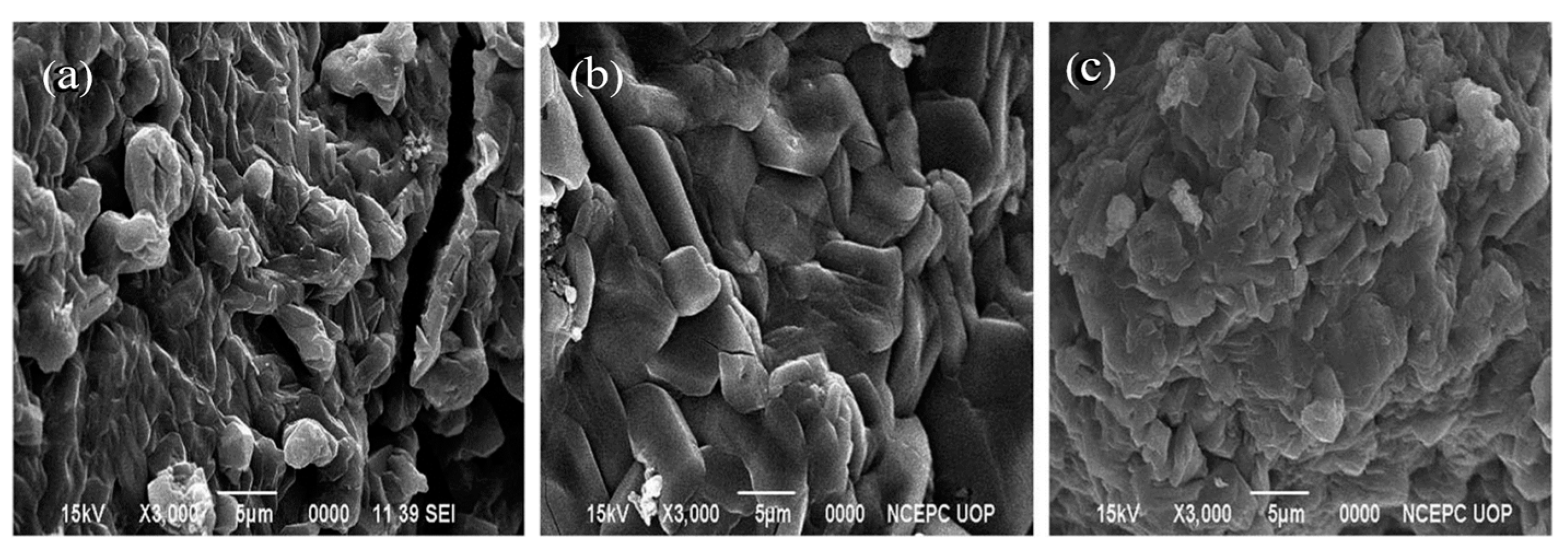
8.3. SEM Analysis
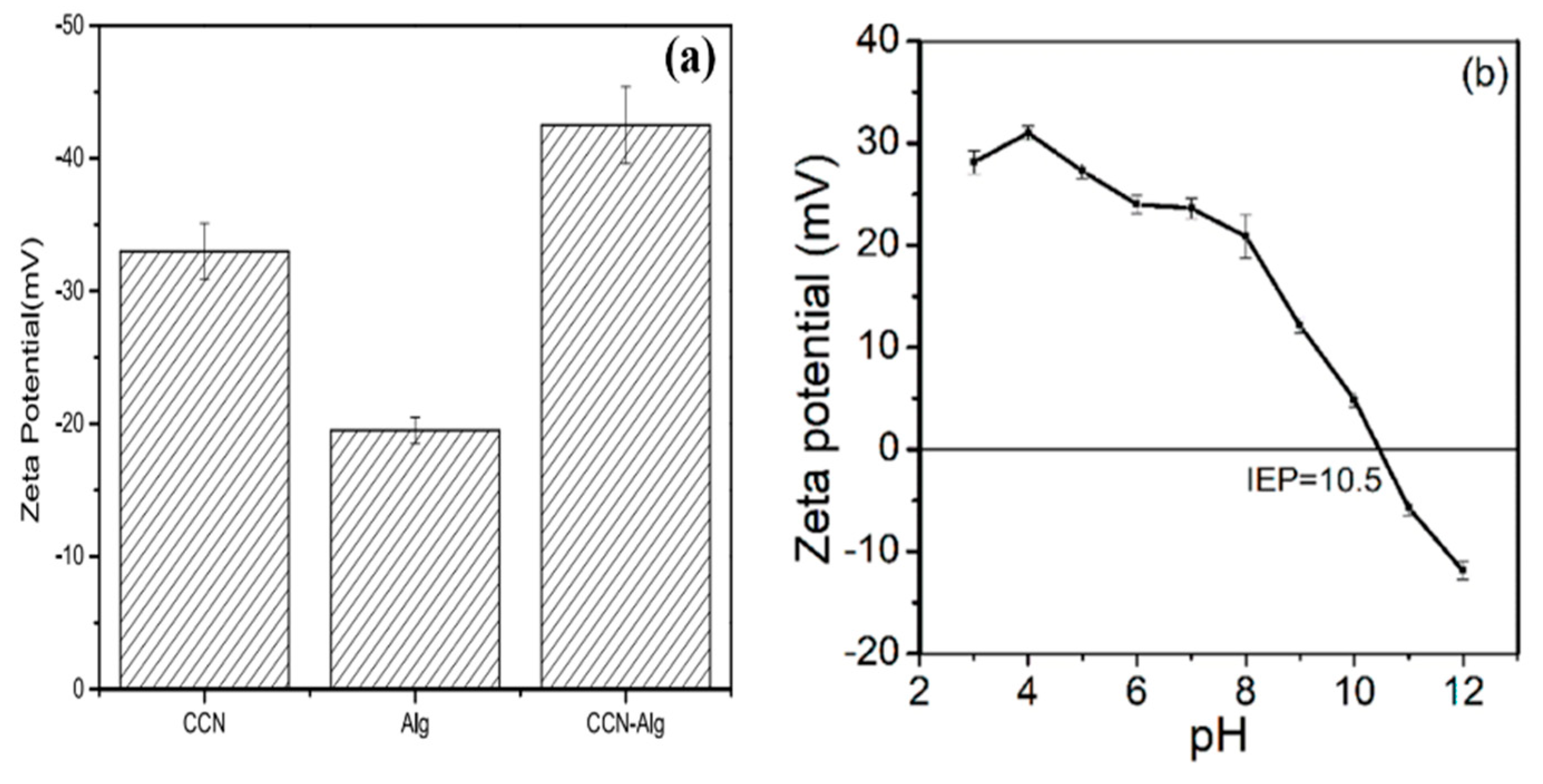
8.4. Zeta Potential Analysis
8.5. EDX Analysis
9. Adsorption Mechanism of Hydrogel
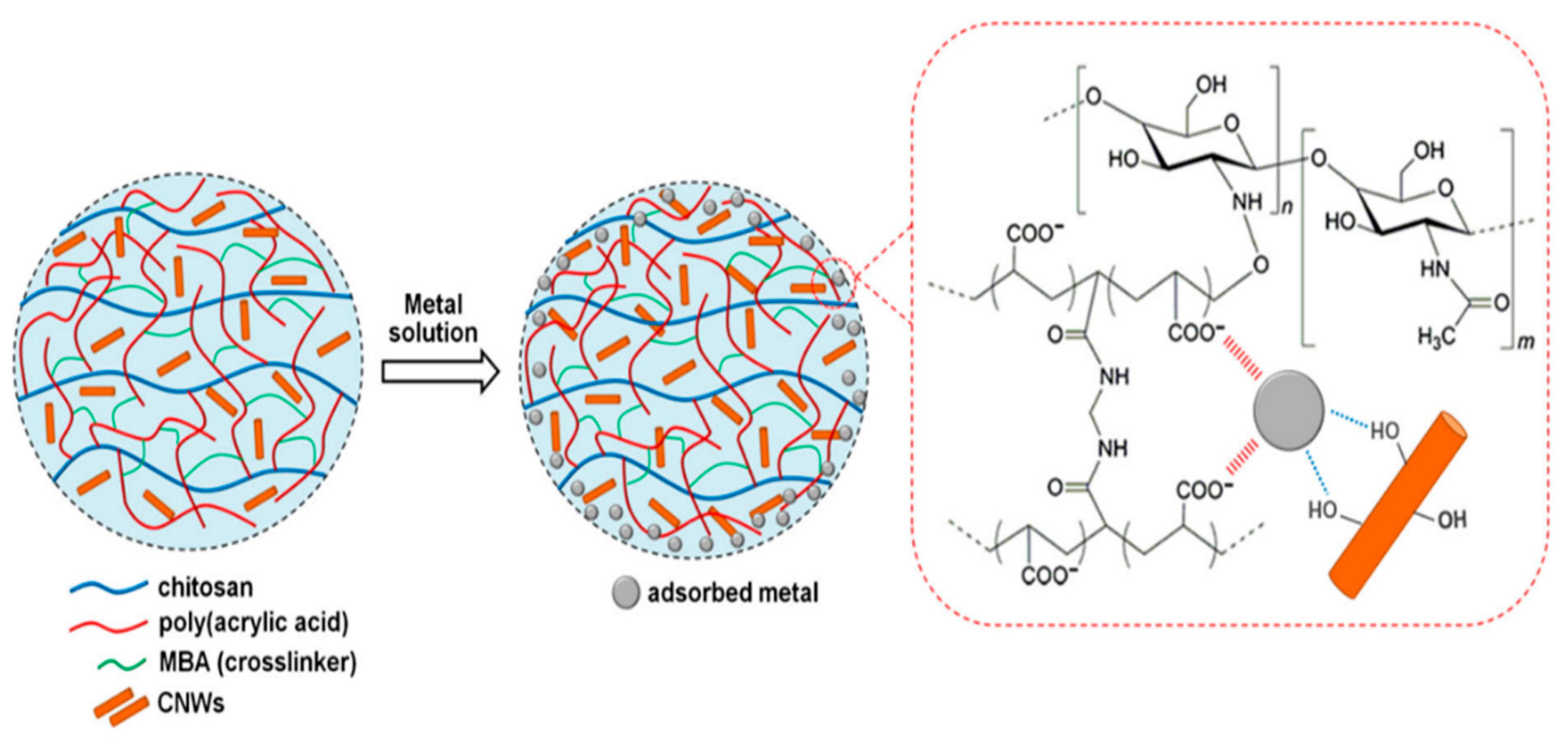
9.1. Electrostatic Interaction
9.2. Ion-Exchange
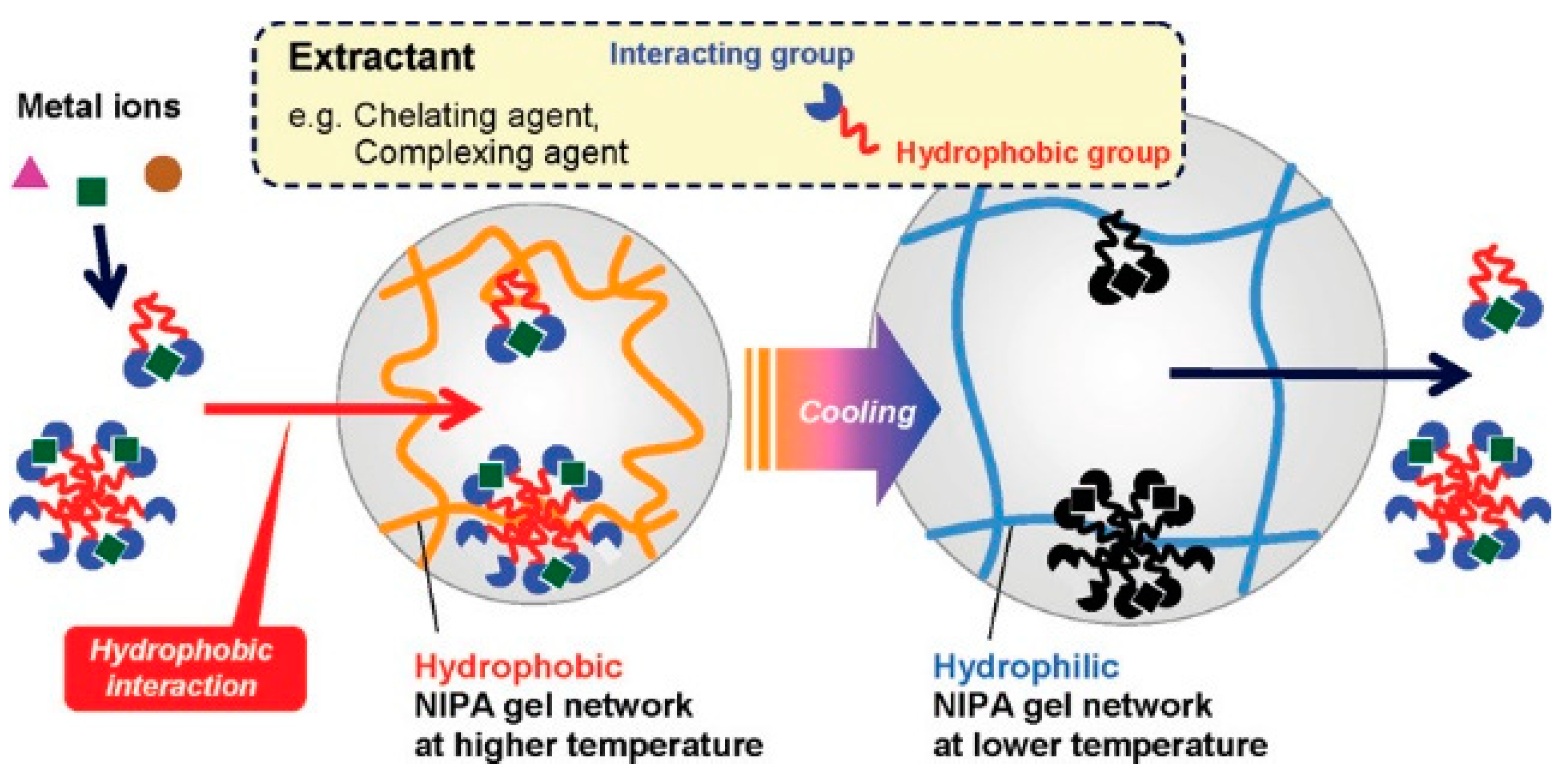
9.3. Hydrophobic Interaction
9.4. Coordination Interaction
| Hydrogel Type | Synthesis Method | Mechanism | Heavy Metals Removed | References |
|---|---|---|---|---|
| Carboxymethyl cellulose-graft-poly(acrylic acid)/monmorillonite hydrogel composite | Graft polymerization | Ion exchange and coordination interaction | Zn2+, Pb2+ | [177] |
| Silk sericin/Lignin hydrogel beads | Graft polymerization | Ion exchange or electrostatic interaction | Cr6+ | [178] |
| Chitosan/multiwall carbon nanotube/poly(acrylic acid)/poly(4-aminodiphenyl amine) functional gel | Free radical polymerization and cross-linking reaction | Complexation interaction | Cr6+ | [60] |
| Sugar cane bagasse cellulose and gelatin-based hydrogel composite | Cross-linking | Coordination and electrostatic interaction | Cu2+ | [179] |
| Carboxy methyl cellulose hydrogel | ɤ-raddiation | Coordination interaction | Cu2+ | [180] |
| Chitin/cellulose composite hydrogel | Freeze-thaw method | Electrostatic and coordination interaction | Hg2+, Cu2+, Pb2+ | [181] |
| Carboxy methyl cellulose hydrogel beads | Inverse suspension method | Coordination interaction | Cu2+, Ni2+, Pb2+ | [182] |
| Hydrogel-biochar composite | Free radical polymerization and cross-linking reactions | Chemisorption | As | [101] |
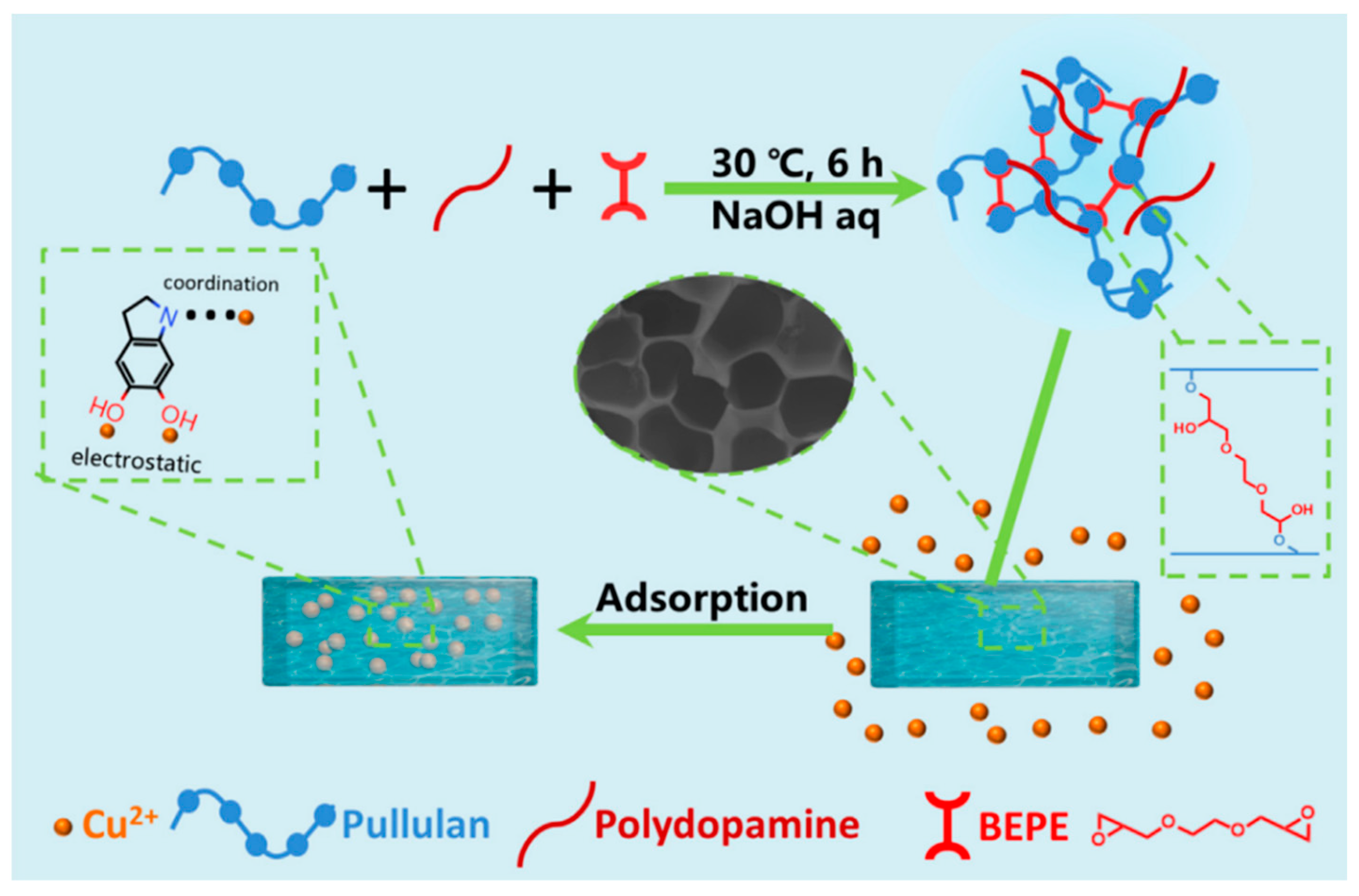
| Pullulan/polydopamine hydrogels | Chemical cross-linking | Electrostatic and coordination interaction | Cu2+ | [158] |
| Jute/poly(acrylic acid) hydrogel | Free radical polymerization | Electrostatic interaction | Cd2+, Pb2+ | [183] |
| Carboxylated chitosan/carboxylated nanocellulose hydrogel beads | Cross-linking | Electrostatic and coordination interaction | Pb2+ | [184] |
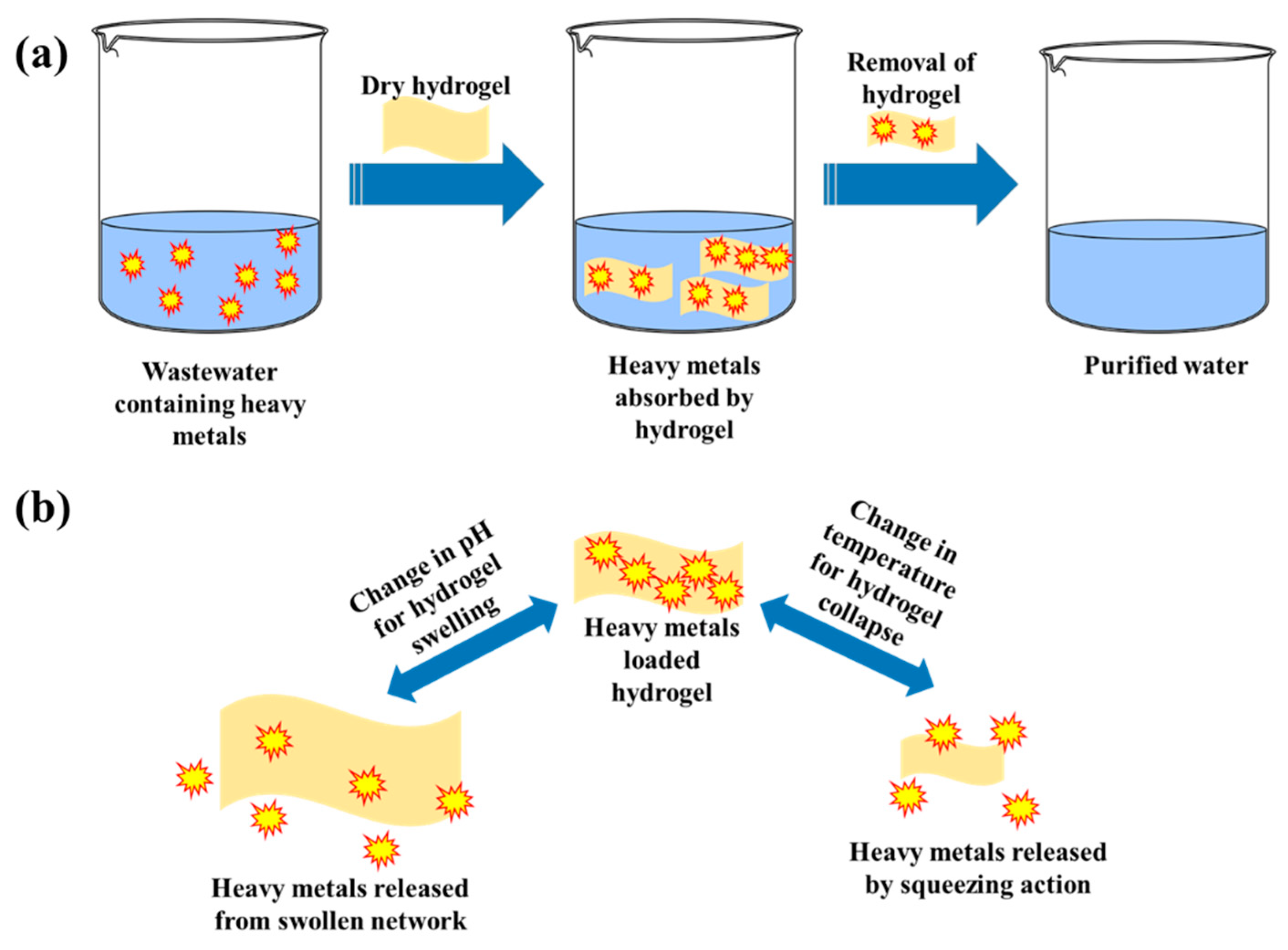
10. Recovery, Regeneration, and Reusability of Hydrogel
11. Conclusions
- Currently, the hydrogel-based adsorbent materials used for heavy metal removal are limited to lab scale. Therefore, further research is required to scale up for a large-scale application.
- The present research is confined to removing a single type of heavy metal. More research should be undertaken targeting multiple heavy metals.
- The research should focus on the ability of the hydrogel to regenerate (for example, the adsorption efficiency of hydrogel drops after five sorption cycles).
- To broaden the spectrum of hydrogels application for separation of rare earth metals.
- To develop high mechanical strength tailored hydrogel (for example hydrogel membranes) that are easier to separate from the liquid phase for wastewater treatment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kabir, S.; Cueto, R.; Balamurugan, S.; Romeo, L.D.; Kuttruff, J.T.; Marx, B.D.; Negulescu, I.I. Removal of acid dyes from textile wastewaters using fish scales by absorption process. Clean Technol. 2019, 1, 311–324. [Google Scholar] [CrossRef] [Green Version]
- Hasanpour, M.; Hatami, M. Photocatalytic performance of aerogels for organic dyes removal from wastewaters: Review study. J. Mol. Liq. 2020, 309, 113094. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv. Colloid Interface Sci. 2020, 284, 102247. [Google Scholar] [CrossRef] [PubMed]
- Siti, N.; Mohd, H.; Md, L.K.; Shamsul, I. Adsorption process of heavy metals by low-cost adsorbent: A review. World Appl. Sci. J. 2013, 28, 1518–1530. [Google Scholar]
- Saha, J.; Dikshit, A.; Bandyopadhyay, M.; Saha, K. A review of arsenic poisoning and its effects on human health. Crit. Rev. Environ. Sci. Technol. 1999, 29, 281–313. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, X.; Zhang, M.; Zhuo, X.; Niu, J. Preparation and characterization of modified cellulose for adsorption of Cd (II), Hg (II), and acid fuchsin from aqueous solutions. Ind. Eng. Chem. Res. 2013, 52, 876–884. [Google Scholar] [CrossRef]
- Masindi, V.; Muedi, K.L. Environmental contamination by heavy metals. Heavy Met. 2018, 10, 115–132. [Google Scholar]
- Soleimani, M.; Amini, N.; Sadeghian, B.; Wang, D.; Fang, L. Heavy metals and their source identification in particulate matter (PM2.5) in Isfahan City, Iran. J. Environ. Sci. 2018, 72, 166–175. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Abdel-Raouf, M.; Abdul-Raheim, A. Removal of Heavy Metals from Industrial Waste Water by Biomass-Based Materials: A Review. J. Pollut. Eff. Control. 2017, 5, 1000180. [Google Scholar]
- Saxena, G.; Purchase, D.; Mulla, S.I.; Saratale, G.D.; Bharagava, R.N. Phytoremediation of heavy metal-contaminated sites: Eco-environmental concerns, field studies, sustainability issues, and future prospects. Rev. Environ. Contam. Toxicol. 2019, 249, 71–131. [Google Scholar]
- Ali, R.M.; Hamad, H.A.; Hussein, M.M.; Malash, G.F. Potential of using green adsorbent of heavy metal removal from aqueous solutions: Adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol. Eng. 2016, 91, 317–332. [Google Scholar] [CrossRef]
- Mthombeni, N.H.; Mbakop1and, S.; Onyango, M.S. Adsorptive removal of manganese from industrial and mining wastewater. In Proceedings of the Sustainable Research and Innovation Conference, Nairobi, Kenya, 4 May 2016; College of Engineering and Technology Jomo Kenyatta University of Agriculture and Technology: Nairobi, Kenya, 2016; pp. 36–45. [Google Scholar]
- Yadav, A. Bioremediation of wastewater using various sorbents and vegetable enzymes. Res. Biotechnol. 2015, 6, 1–15. [Google Scholar]
- Ahmad, M.; Ahmed, S.; Swami, B.L.; Ikram, S. Adsorption of heavy metal ions: Role of chitosan and cellulose for water treatment. Langmuir 2015, 79, 109–155. [Google Scholar]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of heavy metals from industrial wastewaters: A review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Rudi, N.N.; Muhamad, M.S.; Te Chuan, L.; Alipal, J.; Omar, S.; Hamidon, N.; Hamid, N.H.A.; Sunar, N.M.; Ali, R.; Harun, H. Evolution of adsorption process for manganese removal in water via agricultural waste adsorbents. Heliyon 2020, 6, e05049. [Google Scholar] [CrossRef]
- Shafiq, M.; Alazba, A.; Amin, M. Removal of heavy metals from wastewater using date palm as a biosorbent: A comparative review. Sains Malays. 2018, 47, 35–49. [Google Scholar]
- Sun, C.; Chen, T.; Huang, Q.; Wang, J.; Lu, S.; Yan, J. Enhanced adsorption for Pb (II) and Cd (II) of magnetic rice husk biochar by KMnO4 modification. Environ. Sci. Pollut. Res. 2019, 26, 8902–8913. [Google Scholar] [CrossRef]
- Nuhanović, M.; Grebo, M.; Draganović, S.; Memić, M.; Smječanin, N. Uranium (VI) biosorption by sugar beet pulp: Equilibrium, kinetic and thermodynamic studies. J. Radioanal. Nucl. Chem. 2019, 322, 2065–2078. [Google Scholar] [CrossRef]
- George, R.; Bahadur, N.; Singh, N.; Singh, R.; Verma, A.; Shukla, A. Environmentally benign TiO2 nanomaterials for removal of heavy metal ions with interfering ions present in tap water. Mater. Today Proc. 2016, 3, 162–166. [Google Scholar] [CrossRef]
- Manjuladevi, M.; Sri, O. Heavy metals removal from industrial wastewater by nano adsorbent prepared from cucumis melopeel activated carbon. J. Nanomed. Res. 2017, 5, 1–4. [Google Scholar]
- Es-Sahbany, H.; Berradi, M.; Nkhili, S.; Hsissou, R.; Allaoui, M.; Loutfi, M.; Bassir, D.; Belfaquir, M.; El Youbi, M. Removal of heavy metals (nickel) contained in wastewater-models by the adsorption technique on natural clay. Mater. Today Proc. 2019, 13, 866–875. [Google Scholar] [CrossRef]
- Weerasundara, L.; Gabriele, B.; Figoli, A.; Ok, Y.-S.; Bundschuh, J. Hydrogels: Novel materials for contaminant removal in water—A review. Crit. Rev. Environ. Sci. Technol. 2020, 51, 1–45. [Google Scholar] [CrossRef]
- Pan, Z.; An, L. Removal of heavy metal from wastewater using ion exchange membranes. In Applications of Ion Exchange Materials in the Environment; Springer: Berlin, Germany, 2019; pp. 25–46. [Google Scholar]
- Liu, L.; Luo, X.-B.; Ding, L.; Luo, S.-L. Application of nanotechnology in the removal of heavy metal from water. In Nanomaterials for the Removal of Pollutants and Resource Reutilization; Elsevier: Oxford, UK, 2019; pp. 83–147. [Google Scholar]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent advancement of coagulation–flocculation and its application in wastewater treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Medina, B.; Torem, M.; De Mesquita, L. On the kinetics of precipitate flotation of Cr III using sodium dodecylsulfate and ethanol. Miner. Eng. 2005, 18, 225–231. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Mukherjee, R.; Bhunia, P.; De, S. Impact of graphene oxide on removal of heavy metals using mixed matrix membrane. Chem. Eng. J. 2016, 292, 284–297. [Google Scholar] [CrossRef]
- Van Bemmelen, J. Das hydrogel und das krystallinische hydrat des kupferoxyds. Z. Anorg. Chem. 1894, 5, 466–483. [Google Scholar] [CrossRef] [Green Version]
- Thakur, S.; Thakur, V.K.; Arotiba, O.A. History, classification, properties and application of hydrogels: An overview. In Hydrogels; Springer: Singapore, 2018; pp. 29–50. [Google Scholar]
- Wichterle, O.; Lim, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Shalla, A.H.; Yaseen, Z.; Bhat, M.A.; Rangreez, T.A.; Maswal, M. Recent review for removal of metal ions by hydrogels. Sep. Sci. Technol. 2019, 54, 89–100. [Google Scholar] [CrossRef]
- Dai, L.; Cheng, T.; Xi, X.; Nie, S.; Ke, H.; Liu, Y.; Tong, S.; Chen, Z. A versatile TOCN/CGG self-assembling hydrogel for integrated wastewater treatment. Cellulose 2020, 27, 915–925. [Google Scholar] [CrossRef]
- Jang, S.H.; Jeong, Y.G.; Min, B.G.; Lyoo, W.S.; Lee, S.C. Preparation and lead ion removal property of hydroxyapatite/polyacrylamide composite hydrogels. J. Hazard. Mater. 2008, 159, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Ozay, O.; Ekici, S.; Baran, Y.; Kubilay, S.; Aktas, N.; Sahiner, N. Utilization of magnetic hydrogels in the separation of toxic metal ions from aqueous environments. Desalination 2010, 260, 57–64. [Google Scholar] [CrossRef]
- Barakat, M.; Sahiner, N. Cationic hydrogels for toxic arsenate removal from aqueous environment. J. Environ. Manag. 2008, 88, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Ozay, O.; Ekici, S.; Baran, Y.; Aktas, N.; Sahiner, N. Removal of toxic metal ions with magnetic hydrogels. Water Res. 2009, 43, 4403–4411. [Google Scholar] [CrossRef]
- Hernández, R.; Mijangos, C. In situ Synthesis of Magnetic Iron Oxide Nanoparticles in Thermally Responsive Alginate-Poly (N-isopropylacrylamide) Semi-Interpenetrating Polymer Networks. Macromol. Rapid Commun. 2009, 30, 176–181. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Rowley, J.A.; Eiselt, P.; Moy, E.M.; Bouhadir, K.H.; Mooney, D.J. Controlling mechanical and swelling properties of alginate hydrogels independently by cross-linker type and cross-linking density. Macromolecules 2000, 33, 4291–4294. [Google Scholar] [CrossRef]
- Sahiner, N. Hydrogels of versatile size and architecture for effective environmental applications. Turk. J. Chem. 2008, 32, 113–123. [Google Scholar]
- Singh, A.; Sharma, P.K.; Garg, V.K.; Garg, G. Hydrogels: A review. Int. J. Pharm. Sci. Rev. Res. 2010, 4, 97–105. [Google Scholar]
- Mahinroosta, M.; Farsangi, Z.J.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Ozay, O.; Aktas, N.; Sahiner, N. Hydrogels as a potential chromatographic system: Absorption, speciation, and separation of chromium species from aqueous media. Sep. Sci. Technol. 2011, 46, 1450–1461. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.; Mujtaba, M.; Alghamdi, N.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Cheng, W.; Nie, W.; Shao, Z. Synthesis and characterization of a temperature-sensitive hydrogel based on sodium alginate and N-isopropylacrylamide. Polym. Adv. Technol. 2015, 26, 1340–1345. [Google Scholar] [CrossRef]
- De, S.K.; Aluru, N.R. A chemo-electro-mechanical mathematical model for simulation of pH sensitive hydrogels. Mech. Mater. 2004, 36, 395–410. [Google Scholar] [CrossRef]
- Mu, R.; Liu, B.; Chen, X.; Wang, N.; Yang, J. Hydrogel adsorbent in industrial wastewater treatment and ecological environment protection. Environ. Technol. Innov. 2020, 20, 101107. [Google Scholar] [CrossRef]
- Kuddushi, M.; Rajput, S.; Shah, A.; Mata, J.; Aswal, V.K.; El Seoud, O.; Kumar, A.; Malek, N.I. Stimuli responsive, self-sustainable, and self-healable functionalized hydrogel with dual gelation, load-bearing, and dye-absorbing properties. ACS Appl. Mater. Interfaces 2019, 11, 19572–19583. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Li, Q.; Yu, C.; Chu, W. Natural polymer-based stimuli-responsive hydrogels. Curr. Med. Chem. 2020, 27, 2631–2657. [Google Scholar] [CrossRef]
- Rittikulsittichai, S.; Kolhatkar, A.G.; Sarangi, S.; Vorontsova, M.A.; Vekilov, P.G.; Brazdeikis, A.; Lee, T.R. Multi-responsive hybrid particles: Thermo-, pH-, photo-, and magneto-responsive magnetic hydrogel cores with gold nanorod optical triggers. Nanoscale 2016, 8, 11851–11861. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Jing, G.; Wang, L.; Yu, H.; Amer, W.A.; Zhang, L. Recent progress on study of hybrid hydrogels for water treatment. Colloids Surf. A Physicochem. Eng. Asp. 2013, 416, 86–94. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, A. Adsorption of Pb (II) from aqueous solution by chitosan-g-poly (acrylic acid)/attapulgite/sodium humate composite hydrogels. J. Chem. Eng. Data 2010, 55, 2379–2384. [Google Scholar] [CrossRef]
- Iizawa, T.; Taketa, H.; Maruta, M.; Ishido, T.; Gotoh, T.; Sakohara, S. Synthesis of porous poly (N-isopropylacrylamide) gel beads by sedimentation polymerization and their morphology. J. Appl. Polym. Sci. 2007, 104, 842–850. [Google Scholar] [CrossRef]
- Garg, S.; Garg, A.; Vishwavidyalaya, R. Hydrogel: Classification, properties, preparation and technical features. Asian J. Biomater. Res 2016, 2, 163–170. [Google Scholar]
- Yang, L.; Chu, J.S.; Fix, J.A. Colon-specific drug delivery: New approaches and in vitro/in vivo evaluation. Int. J. Pharm. 2002, 235, 1–15. [Google Scholar] [CrossRef]
- Kim, M.K.; Sundaram, K.S.; Iyengar, G.A.; Lee, K.-P. A novel chitosan functional gel included with multiwall carbon nanotube and substituted polyaniline as adsorbent for efficient removal of chromium ion. Chem. Eng. J. 2015, 267, 51–64. [Google Scholar] [CrossRef]
- Singhal, R.; Gupta, K. A review: Tailor-made hydrogel structures (classifications and synthesis parameters). Polym. Plast. Technol. Eng. 2016, 55, 54–70. [Google Scholar] [CrossRef]
- Kabiri, K.; Omidian, H.; Zohuriaan-Mehr, M.; Doroudiani, S. Superabsorbent hydrogel composites and nanocomposites: A review. Polym. Compos. 2011, 32, 277–289. [Google Scholar] [CrossRef]
- Badsha, M.A.; Khan, M.; Wu, B.; Kumar, A.; Lo, I.M. Role of surface functional groups of hydrogels in metal adsorption: From performance to mechanism. J. Hazard. Mater. 2021, 408, 124463. [Google Scholar] [CrossRef]
- Badsha, M.A.; Lo, I.M. An innovative pH-independent magnetically separable hydrogel for the removal of Cu (II) and Ni (II) ions from electroplating wastewater. J. Hazard. Mater. 2020, 381, 121000. [Google Scholar] [CrossRef]
- He, X.; Cheng, L.; Wang, Y.; Zhao, J.; Zhang, W.; Lu, C. Aerogels from quaternary ammonium-functionalized cellulose nanofibers for rapid removal of Cr (VI) from water. Carbohydr. Polym. 2014, 111, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Cyganowski, P.; Dzimitrowicz, A.; Jamroz, P.; Jermakowicz-Bartkowiak, D.; Pohl, P. Polymerization-driven immobilization of dc-apgd synthesized gold nanoparticles into a quaternary ammonium-based hydrogel resulting in a polymeric nanocomposite with heat-transfer applications. Polymers 2018, 10, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, G.; Ke, W.; Chen, X.; Kong, Y.; Zheng, H.; Yin, Y.; Cai, W. Preparation and properties of quaternary ammonium chitosan-g-poly (acrylic acid-co-acrylamide) superabsorbent hydrogels. React. Funct. Polym. 2017, 111, 14–21. [Google Scholar] [CrossRef]
- Odio, O.F.; Lartundo-Rojas, L.; Palacios, E.G.; Martínez, R.; Reguera, E. Synthesis of a novel poly-thiolated magnetic nano-platform for heavy metal adsorption. Role of thiol and carboxyl functions. Appl. Surf. Sci. 2016, 386, 160–177. [Google Scholar] [CrossRef]
- Saad, D.M.; Cukrowska, E.M.; Tutu, H. Selective removal of mercury from aqueous solutions using thiolated cross-linked polyethylenimine. Appl. Water Sci. 2013, 3, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Mohammadnia, E.; Hadavifar, M.; Veisi, H. Kinetics and thermodynamics of mercury adsorption onto thiolated graphene oxide nanoparticles. Polyhedron 2019, 173, 114139. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Jiang, S.-J.; Tseng, W.-L. Facile synthesis and characterization of thiol-functionalized graphene oxide as effective adsorbent for Hg (II). J. Environ. Chem. Eng. 2016, 4, 2052–2065. [Google Scholar] [CrossRef]
- El-Hag Ali, A. Removal of heavy metals from model wastewater by using carboxymehyl cellulose/2-acrylamido-2-methyl propane sulfonic acid hydrogels. J. Appl. Polym. Sci. 2012, 123, 763–769. [Google Scholar] [CrossRef]
- Lu, W.; Dai, Z.; Li, L.; Liu, J.; Wang, S.; Yang, H.; Cao, C.; Liu, L.; Chen, T.; Zhu, B. Preparation of composite hydrogel (PCG) and its adsorption performance for uranium (VI). J. Mol. Liq. 2020, 303, 112604. [Google Scholar] [CrossRef]
- Bai, J.; Chu, J.; Yin, X.; Wang, J.; Tian, W.; Huang, Q.; Jia, Z.; Wu, X.; Guo, H.; Qin, Z. Synthesis of amidoximated polyacrylonitrile nanoparticle/graphene composite hydrogel for selective uranium sorption from saline lake brine. Chem. Eng. J. 2020, 391, 123553. [Google Scholar] [CrossRef]
- Hamza, M.F.; Roux, J.-C.; Guibal, E. Uranium and europium sorption on amidoxime-functionalized magnetic chitosan micro-particles. Chem. Eng. J. 2018, 344, 124–137. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, Q.; Xu, W.; Wang, G.; Xu, Y.; Liang, E. Graft copolymerization of crosslinked polyvinyl alcohol with acrylonitrile and its amidoxime modification as a heavy metal ion adsorbent. J. Polym. Environ. 2020, 28, 116–122. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, M.; Chen, D. Electrosorption of uranium (VI) by highly porous phosphate-functionalized graphene hydrogel. Appl. Surf. Sci. 2019, 484, 83–96. [Google Scholar] [CrossRef]
- Flora, G.; Mittal, M.; Flora, S.J. Medical countermeasures—Chelation therapy. In Handbook of Arsenic Toxicology; Elsevier: Oxford, UK, 2015; pp. 589–626. [Google Scholar]
- Chang, Y.-C.; Chen, D.-H. Preparation and adsorption properties of monodisperse chitosan-bound Fe3O4 magnetic nanoparticles for removal of Cu (II) ions. J. Colloid Interface Sci. 2005, 283, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-H.; Chen, D.-H. Rapid removal of heavy metal cations and anions from aqueous solutions by an amino-functionalized magnetic nano-adsorbent. J. Hazard. Mater. 2009, 163, 174–179. [Google Scholar] [CrossRef]
- Repo, E.; Warchoł, J.K.; Bhatnagar, A.; Mudhoo, A.; Sillanpää, M. Aminopolycarboxylic acid functionalized adsorbents for heavy metals removal from water. Water Res. 2013, 47, 4812–4832. [Google Scholar] [CrossRef]
- Atzei, D.; Ferri, T.; Sadun, C.; Sangiorgio, P.; Caminiti, R. Structural characterization of complexes between iminodiacetate blocked on Styrene− Divinylbenzene matrix (Chelex 100 Resin) and Fe (III), Cr (III), and Zn (II) in Solid Phase by Energy-Dispersive X-ray Diffraction. J. Am. Chem. Soc. 2001, 123, 2552–2558. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, H.; Shao, T.; Zhao, X.; Peng, H.; Gong, Y.; Wan, H. Enhanced copper adsorption by DTPA-chitosan/alginate composite beads: Mechanism and application in simulated electroplating wastewater. Chem. Eng. J. 2018, 339, 322–333. [Google Scholar] [CrossRef]
- Medina, R.P.; Nadres, E.T.; Ballesteros, F.C.; Rodrigues, D.F. Incorporation of graphene oxide into a chitosan–poly (acrylic acid) porous polymer nanocomposite for enhanced lead adsorption. Environ. Sci. Nano 2016, 3, 638–646. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z. Heavy metals removal using hydrogel-supported nanosized hydrous ferric oxide: Synthesis, characterization, and mechanism. Sci. Total Environ. 2017, 580, 776–786. [Google Scholar] [CrossRef]
- Tian, R.; Liu, Q.; Zhang, W.; Zhang, Y. Preparation of lignin-based hydrogel and its adsorption on Cu2+ ions and Co2+ ions in wastewaters. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2545–2553. [Google Scholar] [CrossRef]
- Wu, B.; Yan, D.Y.; Khan, M.; Zhang, Z.; Lo, I.M. Application of magnetic hydrogel for anionic pollutants removal from wastewater with adsorbent regeneration and reuse. J. Hazard. Toxic Radioact. Waste 2017, 21, 04016008. [Google Scholar] [CrossRef]
- Yetimoğlu, E.K.; Kahraman, M.V.; Bayramoğlu, G.; Ercan, Ö.; Apohan, N.K. Sulfathiazole-based novel UV-cured hydrogel sorbents for mercury removal from aqueous solutions. Radiat. Phys. Chem. 2009, 78, 92–97. [Google Scholar] [CrossRef]
- Saraydın, D.; Yıldırım, E.Ş.; Karadağ, E.; Güven, O. Radiation-Synthesized Acrylamide/Crotonic Acid Hydrogels for Selective Mercury (II) Ion Adsorption. Adv. Polym. Technol. 2018, 37, 822–829. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Wang, G.; Hao, C.; Li, X.; Li, T. Adsorption performance of a polysaccharide composite hydrogel based on crosslinked glucan/chitosan for heavy metal ions. Compos. Part B Eng. 2019, 169, 45–54. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, S.; Qiao, J.; Kołodyńska, D.; Ju, Y.; Zhang, M.; Cai, M.; Deng, D.; Dionysiou, D.D. Malic acid-enhanced chitosan hydrogel beads (mCHBs) for the removal of Cr (VI) and Cu (II) from aqueous solution. Chem. Eng. J. 2018, 353, 225–236. [Google Scholar] [CrossRef]
- Godiya, C.B.; Cheng, X.; Li, D.; Chen, Z.; Lu, X. Carboxymethyl cellulose/polyacrylamide composite hydrogel for cascaded treatment/reuse of heavy metal ions in wastewater. J. Hazard. Mater. 2019, 364, 28–38. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, Y.; Wang, F.; Wang, A. Monolithic supermacroporous hydrogel prepared from high internal phase emulsions (HIPEs) for fast removal of Cu2+ and Pb2+. Chem. Eng. J. 2016, 284, 422–430. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Yuan, T.; Sun, R. A lignosulfonate-modified graphene hydrogel with ultrahigh adsorption capacity for Pb (II) removal. J. Mater. Chem. A 2016, 4, 11888–11896. [Google Scholar] [CrossRef]
- Milani, P.; França, D.; Balieiro, A.G.; Faez, R. Polymers and its applications in agriculture. Polímeros 2017, 27, 256–266. [Google Scholar] [CrossRef]
- Kirillova, A.; Ionov, L. Shape-changing polymers for biomedical applications. J. Mater. Chem. B 2019, 7, 1597–1624. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A.; Masood, F.; Yasin, T.; Hameed, A. Progress in polymeric nanocomposite membranes for wastewater treatment: Preparation, properties and applications. J. Ind. Eng. Chem. 2019, 79, 29–40. [Google Scholar] [CrossRef]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.; Mahanti, N.K. Application of biodegradable polymers in food packaging industry: A comprehensive review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Thakur, S.; Sharma, B.; Verma, A.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent approaches in guar gum hydrogel synthesis for water purification. Int. J. Polym. Anal. Charact. 2018, 23, 621–632. [Google Scholar] [CrossRef] [Green Version]
- Malik, D.; Jain, C.; Yadav, A.K. Removal of heavy metals from emerging cellulosic low-cost adsorbents: A review. Appl. Water Sci. 2017, 7, 2113–2136. [Google Scholar] [CrossRef] [Green Version]
- Sanyang, M.; Ghani, W.A.W.A.K.; Idris, A.; Ahmad, M.B. Hydrogel biochar composite for arsenic removal from wastewater. Desalination Water Treat. 2016, 57, 3674–3688. [Google Scholar] [CrossRef]
- Yan, E.; Cao, M.; Ren, X.; Jiang, J.; An, Q.; Zhang, Z.; Gao, J.; Yang, X.; Zhang, D. Synthesis of Fe3O4 nanoparticles functionalized polyvinyl alcohol/chitosan magnetic composite hydrogel as an efficient adsorbent for chromium (VI) removal. J. Phys. Chem. Solids 2018, 121, 102–109. [Google Scholar] [CrossRef]
- Ali, A.E.-H.; Shawky, H.; Abd El Rehim, H.; Hegazy, E. Synthesis and characterization of PVP/AAc copolymer hydrogel and its applications in the removal of heavy metals from aqueous solution. Eur. Polym. J. 2003, 39, 2337–2344. [Google Scholar]
- Pettinelli, N.; Rodríguez-Llamazares, S.; Abella, V.; Barral, L.; Bouza, R.; Farrag, Y.; Lago, F. Entrapment of chitosan, pectin or κ-carrageenan within methacrylate based hydrogels: Effect on swelling and mechanical properties. Mater. Sci. Eng. C 2019, 96, 583–590. [Google Scholar] [CrossRef]
- Chern, J.M.; Lee, W.F.; Hsieh, M.Y. Preparation and swelling characterization of poly (n-isopropylacrylamide)-based porous hydrogels. J. Appl. Polym. Sci. 2004, 92, 3651–3658. [Google Scholar] [CrossRef]
- Ozay, O.; Ekici, S.; Aktas, N.; Sahiner, N. P (4-vinyl pyridine) hydrogel use for the removal of UO22+ and Th4+ from aqueous environments. J. Environ. Manag. 2011, 92, 3121–3129. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Peng, B.; Ji, Y.; Chen, J.; Li, D. Chitosan (chitin)/cellulose composite biosorbents prepared using ionic liquid for heavy metal ions adsorption. AIChE J. 2009, 55, 2062–2069. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Liu, C.; Jiang, Y.; Yu, G.; Mu, X.; Wang, X. Magnetic cellulose–chitosan hydrogels prepared from ionic liquids as reusable adsorbent for removal of heavy metal ions. Chem. Commun. 2012, 48, 7350–7352. [Google Scholar] [CrossRef]
- Yan, H.; Row, K.H. Characteristic and synthetic approach of molecularly imprinted polymer. Int. J. Mol. Sci. 2006, 7, 155–178. [Google Scholar] [CrossRef] [Green Version]
- Haraguchi, K.; Li, H.-J.; Matsuda, K.; Takehisa, T.; Elliott, E. Mechanism of forming organic/inorganic network structures during in-situ free-radical polymerization in PNIPA− clay nanocomposite hydrogels. Macromolecules 2005, 38, 3482–3490. [Google Scholar] [CrossRef]
- Fu, J.; Chen, L.; Li, J.; Zhang, Z. Current status and challenges of ion imprinting. J. Mater. Chem. A 2015, 3, 13598–13627. [Google Scholar] [CrossRef]
- Pakdel, P.M.; Peighambardoust, S.J. A review on acrylic based hydrogels and their applications in wastewater treatment. J. Environ. Manag. 2018, 217, 123–143. [Google Scholar] [CrossRef]
- Shah, L.A.; Khan, M.; Javed, R.; Sayed, M.; Khan, M.S.; Khan, A.; Ullah, M. Superabsorbent polymer hydrogels with good thermal and mechanical properties for removal of selected heavy metal ions. J. Clean. Prod. 2018, 201, 78–87. [Google Scholar] [CrossRef]
- Kaşgöz, H. New sorbent hydrogels for removal of acidic dyes and metal ions from aqueous solutions. Polym. Bull. 2006, 56, 517–528. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, G.; Chu, L.; Liu, Y.; Liu, C.; Luo, S.; Wei, Y. Efficient removal of heavy metal ions with an EDTA functionalized chitosan/polyacrylamide double network hydrogel. ACS Sustain. Chem. Eng. 2017, 5, 843–851. [Google Scholar] [CrossRef]
- Evren, M.; Acar, I.; Güçlü, K.; Güçlü, G. Removal of Cu2+ and Pb2+ ions by N-vinyl 2-pyrrolidone/itaconic acid hydrogels from aqueous solutions. Can. J. Chem. Eng. 2014, 92, 52–59. [Google Scholar] [CrossRef]
- Zhou, G.; Luo, J.; Liu, C.; Chu, L.; Ma, J.; Tang, Y.; Zeng, Z.; Luo, S. A highly efficient polyampholyte hydrogel sorbent based fixed-bed process for heavy metal removal in actual industrial effluent. Water Res. 2016, 89, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Hu, X.; Zhang, X.; Huang, L.; Liu, Z.; Sun, G. Highly efficient removal of trace metal ions by using poly (acrylic acid) hydrogel adsorbent. Mater. Des. 2019, 181, 107934. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Wang, J.-L. Removal of cesium from radioactive wastewater using magnetic chitosan beads cross-linked with glutaraldehyde. Nucl. Sci. Tech. 2016, 27, 43. [Google Scholar] [CrossRef]
- Mishra, A.; Nath, A.; Pande, P.P.; Shankar, R. Treatment of gray wastewater and heavy metal removal from aqueous medium using hydrogels based on novel crosslinkers. J. Appl. Polym. Sci. 2021, 138, 50242. [Google Scholar] [CrossRef]
- Dil, N.N.; Sadeghi, M. Free radical synthesis of nanosilver/gelatin-poly (acrylic acid) nanocomposite hydrogels employed for antibacterial activity and removal of Cu (II) metal ions. J. Hazard. Mater. 2018, 351, 38–53. [Google Scholar] [CrossRef]
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L. Synthesis of cellulose-based superabsorbent hydrogels by high-energy irradiation in the presence of crosslinking agent. Radiat. Phys. Chem. 2016, 118, 114–119. [Google Scholar] [CrossRef]
- Khan, M.; Lo, I.M. A holistic review of hydrogel applications in the adsorptive removal of aqueous pollutants: Recent progress, challenges, and perspectives. Water Res. 2016, 106, 259–271. [Google Scholar] [CrossRef]
- Delbecq, F.; Kono, F.; Kawai, T. Preparation of PVP–PVA–exfoliated graphite cross-linked composite hydrogels for the incorporation of small tin nanoparticles. Eur. Polym. J. 2013, 49, 2654–2659. [Google Scholar] [CrossRef]
- Yang, J.; Dong, X.; Gao, Y.; Zhang, W. One-step synthesis of methacrylated POSS cross-linked poly (N-isopropylacrylamide) hydrogels by γ-irradiation. Mater. Lett. 2015, 157, 81–84. [Google Scholar] [CrossRef]
- Abd El-Mohdy, H. Water sorption behavior of CMC/PAM hydrogels prepared by γ-irradiation and release of potassium nitrate as agrochemical. React. Funct. Polym. 2007, 67, 1094–1102. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Rivera, C.; Wu, C.-J.; Chan, B.K.; Schmidt, G. Photocrosslinked nanocomposite hydrogels from PEG and silica nanospheres: Structural, mechanical and cell adhesion characteristics. Mater. Sci. Eng. C 2013, 33, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels—A review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Maziad, N.; Mohsen, M.; Gomaa, E.; Mohammed, R. Radiation copolymerization of hydrogels based in polyacrylic acid/polyvinyl alcohol applied in water treatment processes. J. Mater. Sci. Eng. A 2015, 5, 381–390. [Google Scholar]
- Tran, T.H.; Okabe, H.; Hidaka, Y.; Hara, K. Removal of metal ions from aqueous solutions using carboxymethyl cellulose/sodium styrene sulfonate gels prepared by radiation grafting. Carbohydr. Polym. 2017, 157, 335–343. [Google Scholar] [CrossRef]
- Aly, A.A.; El-Bisi, M.K. Grafting of polysaccharides: Recent advances. In Biopolym. Grafting; Elsevier: Amsterdam, The Netherlands, 2018; pp. 469–519. [Google Scholar] [CrossRef]
- Qi, X.; Lin, L.; Shen, L.; Li, Z.; Qin, T.; Qian, Y.; Wu, X.; Wei, X.; Gong, Q.; Shen, J. Efficient decontamination of lead ions from wastewater by salecan polysaccharide-based hydrogels. ACS Sustain. Chem. Eng. 2019, 7, 11014–11023. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, W.; Li, G. The microstructure and stability of collagen hydrogel cross-linked by glutaraldehyde. Polym. Degrad. Stab. 2016, 130, 264–270. [Google Scholar] [CrossRef]
- Gennen, S.; Grignard, B.; Thomassin, J.-M.; Gilbert, B.; Vertruyen, B.; Jerome, C.; Detrembleur, C. Polyhydroxyurethane hydrogels: Synthesis and characterizations. Eur. Polym. J. 2016, 84, 849–862. [Google Scholar] [CrossRef]
- Kassem, I.; Kassab, Z.; Khouloud, M.; Sehaqui, H.; Bouhfid, R.; Jacquemin, J.; El Achaby, M. Phosphoric acid-mediated green preparation of regenerated cellulose spheres and their use for all-cellulose cross-linked superabsorbent hydrogels. Int. J. Biol. Macromol. 2020, 162, 136–149. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, G.; Zeng, X.; Li, J.; Li, G.; Huang, W.; Sun, R.; Wong, C. High-strength, tough, fatigue resistant, and self-healing hydrogel based on dual physically cross-linked network. ACS Appl. Mater. Interfaces 2016, 8, 24030–24037. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of synthesis of hydrogels…A review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhang, F.; Wu, J. Physically crosslinked hydrogels from polysaccharides prepared by freeze–thaw technique. React. Funct. Polym. 2013, 73, 923–928. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Wang, M.-J. Removal of heavy metal ions by poly (vinyl alcohol) and carboxymethyl cellulose composite hydrogels prepared by a freeze–thaw method. ACS Sustain. Chem. Eng. 2016, 4, 2830–2837. [Google Scholar] [CrossRef]
- Guan, Y.; Bian, J.; Peng, F.; Zhang, X.-M.; Sun, R.-C. High strength of hemicelluloses based hydrogels by freeze/thaw technique. Carbohydr. Polym. 2014, 101, 272–280. [Google Scholar] [CrossRef]
- Qi, X.; Hu, X.; Wei, W.; Yu, H.; Li, J.; Zhang, J.; Dong, W. Investigation of Salecan/poly(vinyl alcohol) hydrogels prepared by freeze/thaw method. Carbohydr. Polym. 2015, 118, 60–69. [Google Scholar] [CrossRef]
- Yang, Z.; Liang, G.; Xu, B. Enzymatic control of the self-assembly of small molecules: A new way to generate supramolecular hydrogels. Soft Matter 2007, 3, 515–520. [Google Scholar] [CrossRef]
- Cui, H.; Webber, M.J.; Stupp, S.I. Self-assembly of peptide amphiphiles: From molecules to nanostructures to biomaterials. Pept. Sci. Orig. Res. Biomol. 2010, 94, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Rajbhandary, A.; Nilsson, B.L. Self-Assembling Hydrogels. In GELS HANDBOOK: Fundamentals, Properties and Applications Volume 1: Fundamentals of Hydrogels; World Scientific: Singapore, 2016; pp. 219–250. [Google Scholar]
- Feng, Y.; Gong, J.-L.; Zeng, G.-M.; Niu, Q.-Y.; Zhang, H.-Y.; Niu, C.-G.; Deng, J.-H.; Yan, M. Adsorption of Cd (II) and Zn (II) from aqueous solutions using magnetic hydroxyapatite nanoparticles as adsorbents. Chem. Eng. J. 2010, 162, 487–494. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, S.; Zhang, L.; Zhan, H.; Levit, M.V. Use of carboxylated cellulose nanofibrils-filled magnetic chitosan hydrogel beads as adsorbents for Pb (II). Carbohydr. Polym. 2014, 101, 75–82. [Google Scholar] [CrossRef]
- Pedroso-Santana, S.; Fleitas-Salazar, N. Ionotropic gelation method in the synthesis of nanoparticles/microparticles for biomedical purposes. Polym. Int. 2020, 69, 443–447. [Google Scholar] [CrossRef]
- Guerrero, R.; Acibar, C.; Alarde, C.M.; Maslog, J.; Pacilan, C.J. Evaluation of Pb (II) Removal from Water Using Sodium Alginate/Hydroxypropyl Cellulose Beads. E3S Web Conf. 2020, 148, 02002. [Google Scholar] [CrossRef] [Green Version]
- Omidian, H.; Zohuriaan-Mehr, M.; Bouhendi, H. Polymerization of sodium acrylate in inverse-suspension stabilized by sorbitan fatty esters. Eur. Polym. J. 2003, 39, 1013–1018. [Google Scholar] [CrossRef]
- Klinpituksa, P.; Kosaiyakanon, P. Superabsorbent polymer based on sodium carboxymethyl cellulose grafted polyacrylic acid by inverse suspension polymerization. Int. J. Polym. Sci. 2017, 2017, 3476921. [Google Scholar] [CrossRef]
- Tang, S.; Yang, J.; Lin, L.; Peng, K.; Chen, Y.; Jin, S.; Yao, W. Construction of physically crosslinked chitosan/sodium alginate/calcium ion double-network hydrogel and its application to heavy metal ions removal. Chem. Eng. J. 2020, 393, 124728. [Google Scholar] [CrossRef]
- Ablouh, E.-H.; Hanani, Z.; Eladlani, N.; Rhazi, M.; Taourirte, M. Chitosan microspheres/sodium alginate hybrid beads: An efficient green adsorbent for heavy metals removal from aqueous solutions. Sustain. Environ. Res. 2019, 29, 5. [Google Scholar] [CrossRef]
- Kong, W.; Chang, M.; Zhang, C.; Liu, X.; He, B.; Ren, J. Preparation of Xylan-G-/P (AA-co-AM)/GO nanocomposite hydrogel and its adsorption for heavy metal ions. Polymers 2019, 11, 621. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, R.R.; Elella, M.H.A.; Sabaa, M.W. Cytotoxicity and metal ions removal using antibacterial biodegradable hydrogels based on N-quaternized chitosan/poly (acrylic acid). Int. J. Biol. Macromol. 2017, 98, 302–313. [Google Scholar] [CrossRef]
- Javed, R.; Shah, L.A.; Sayed, M.; Khan, M.S. Uptake of heavy metal ions from aqueous media by hydrogels and their conversion to nanoparticles for generation of a catalyst system: Two-fold application study. RSC Adv. 2018, 8, 14787–14797. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.-H.; Omer, A.M.; Ouyang, X.K.; Yu, D. Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb (II) from aqueous solution. Int. J. Biol. Macromol. 2018, 108, 149–157. [Google Scholar] [CrossRef]
- Bandara, P.C.; Perez, J.V.D.; Nadres, E.T.; Nannapaneni, R.G.; Krakowiak, K.J.; Rodrigues, D.F. Graphene oxide nanocomposite hydrogel beads for removal of selenium in contaminated water. ACS Appl. Polym. Mater. 2019, 1, 2668–2679. [Google Scholar] [CrossRef]
- Zeng, Q.; Qi, X.; Zhang, M.; Tong, X.; Jiang, N.; Pan, W.; Xiong, W.; Li, Y.; Xu, J.; Shen, J. Efficient decontamination of heavy metals from aqueous solution using pullulan/polydopamine hydrogels. Int. J. Biol. Macromol. 2020, 145, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.; Chakma, S. Advances in the preparation of hydrogel for wastewater treatment: A concise review. J. Environ. Chem. Eng. 2019, 7, 103295. [Google Scholar] [CrossRef]
- Maity, S.; Naskar, N.; Lahiri, S.; Ganguly, J. Polysaccharide-derived hydrogel water filter for the rapid and selective removal of arsenic. Environ. Sci. Water Res. Technol. 2019, 5, 1318–1327. [Google Scholar] [CrossRef]
- Basri, S.N.; Zainuddin, N.; Hashim, K.; Yusof, N.A. Preparation and characterization of irradiated carboxymethyl sago starch-acid hydrogel and its application as metal scavenger in aqueous solution. Carbohydr. Polym. 2016, 138, 34–40. [Google Scholar] [CrossRef]
- Shen, X.; Shamshina, J.L.; Berton, P.; Gurau, G.; Rogers, R.D. Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green Chem. 2016, 18, 53–75. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Cheng, R.; Deng, J.; Wu, Y. Chiral, pH responsive hydrogels constructed by N-Acryloyl-alanine and PEGDA/α-CD inclusion complex: Preparation and chiral release ability. Polym. Adv. Technol. 2016, 27, 169–177. [Google Scholar] [CrossRef]
- Li, S. Removal of crystal violet from aqueous solution by sorption into semi-interpenetrated networks hydrogels constituted of poly (acrylic acid-acrylamide-methacrylate) and amylose. Bioresour. Technol. 2010, 101, 2197–2202. [Google Scholar] [CrossRef]
- Pereira, R.C.; Anizelli, P.R.; Di Mauro, E.; Valezi, D.F.; da Costa, A.C.S.; Zaia, C.T.B.; Zaia, D.A. The effect of pH and ionic strength on the adsorption of glyphosate onto ferrihydrite. Geochem. Trans. 2019, 20, 3. [Google Scholar] [CrossRef]
- Eskandari, S.; Dong, A.; De Castro, L.T.; Rahman, F.B.A.; Lipp, J.; Blom, D.A.; Regalbuto, J.R. Pushing the limits of electrostatic adsorption: Charge enhanced dry impregnation of SBA-15. Catal. Today 2019, 338, 60–71. [Google Scholar] [CrossRef]
- Akter, M.; Bhattacharjee, M.; Dhar, A.K.; Rahman, F.B.A.; Haque, S.; Rashid, T.U.; Kabir, S. Cellulose-based hydrogels for wastewater treatment: A concise review. Gels 2021, 7, 30. [Google Scholar] [CrossRef]
- Jørgensen, S.E. Adsorption and ion exchange. In Developments in Environmental Modelling; Elsevier: Amsterdam, The Netherlands, 1989; Volume 14, pp. 65–81. [Google Scholar]
- Wawrzkiewicz, M.; Hubicki, Z. Anion exchange resins as effective sorbents for removal of acid, reactive, and direct dyes from textile wastewaters. In Ion Exchange-Studies and Applications; IntechOpen: London, UK, 2015; pp. 37–72. [Google Scholar]
- Kumar, P. Fundamentals and Techniques of Biophysics and Molecular Biology; Pathfinder Publication Unit of PAPL: New Delhi, India, 2016. [Google Scholar]
- Saber-Samandari, S.; Saber-Samandari, S.; Gazi, M. Cellulose-graft-polyacrylamide/hydroxyapatite composite hydrogel with possible application in removal of Cu (II) ions. React. Funct. Polym. 2013, 73, 1523–1530. [Google Scholar] [CrossRef]
- Xiong, Y.; Xu, L.; Jin, C.; Sun, Q. Cellulose hydrogel functionalized titanate microspheres with self-cleaning for efficient purification of heavy metals in oily wastewater. Cellulose 2020, 27, 7751–7763. [Google Scholar] [CrossRef]
- Van Oss, C.; Good, R.; Chaudhury, M. The role of van der Waals forces and hydrogen bonds in “hydrophobic interactions” between biopolymers and low energy surfaces. J. Colloid Interface Sci. 1986, 111, 378–390. [Google Scholar] [CrossRef]
- Tokuyama, H.; Iwama, T. Temperature-swing solid-phase extraction of heavy metals on a poly (N-isopropylacrylamide) hydrogel. Langmuir 2007, 23, 13104–13108. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yu, F.; Chen, H.; Zheng, J.; Ma, J.; Chen, J. Alginate/graphene double-network nanocomposite hydrogel beads with low-swelling, enhanced mechanical properties, and enhanced adsorption capacity. J. Mater. Chem. A 2016, 4, 10885–10892. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, F.H.; de C Magalhães, C.E.; Medina, A.L.; Fajardo, A.R. Hydrogel composites containing nanocellulose as adsorbents for aqueous removal of heavy metals: Design, optimization, and application. Cellulose 2019, 26, 9119–9133. [Google Scholar] [CrossRef]
- Astrini, N.; Anah, L.; Haryadi, H.R. Adsorption of heavy metal ion from aqueous solution by using cellulose based hydrogel composite. Macromol. Symp. 2015, 353, 191–197. [Google Scholar] [CrossRef]
- Kwak, H.W.; Shin, M.; Yun, H.; Lee, K.H. Preparation of silk sericin/lignin blend beads for the removal of hexavalent chromium ions. Int. J. Mol. Sci. 2016, 17, 1466. [Google Scholar] [CrossRef] [Green Version]
- Maity, J.; Ray, S.K. Removal of Cu (II) ion from water using sugar cane bagasse cellulose and gelatin based composite hydrogels. Int. J. Biol. Macromol. 2017, 97, 238–248. [Google Scholar] [CrossRef]
- Hara, K.; Iida, M.; Yano, K.; Nishida, T. Metal ion absorption of carboxymethylcellulose gel formed by γ-ray irradiation: For the environmental purification. Colloids Surf. B Biointerfaces 2004, 38, 227–230. [Google Scholar]
- Tang, H.; Chang, C.; Zhang, L. Efficient adsorption of Hg2+ ions on chitin/cellulose composite membranes prepared via environmentally friendly pathway. Chem. Eng. J. 2011, 173, 689–697. [Google Scholar] [CrossRef]
- Yang, S.; Fu, S.; Liu, H.; Zhou, Y.; Li, X. Hydrogel beads based on carboxymethyl cellulose for removal heavy metal ions. J. Appl. Polym. Sci. 2011, 119, 1204–1210. [Google Scholar] [CrossRef]
- Zhou, G.; Luo, J.; Liu, C.; Chu, L.; Crittenden, J. Efficient heavy metal removal from industrial melting effluent using fixed-bed process based on porous hydrogel adsorbents. Water Res. 2018, 131, 246–254. [Google Scholar] [CrossRef]
- Xu, X.; Ouyang, X.-K.; Yang, L.-Y. Adsorption of Pb (II) from aqueous solutions using crosslinked carboxylated chitosan/carboxylated nanocellulose hydrogel beads. J. Mol. Liq. 2021, 322, 114523. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Shangbin, S.; Berkland, C.; Liang, J.-T. Chelator-mimetic multi-functionalized hydrogel: Highly efficient and reusable sorbent for Cd, Pb, and As removal from waste water. Chem. Eng. J. 2017, 307, 496–502. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Tehrani, Z.M.; Salimi, H.; Banazadeh, A.; Abedini, N. Hydrogel nanocomposite based on chitosan-g-acrylic acid and modified nanosilica with high adsorption capacity for heavy metal ion removal. Iran. Polym. J. 2015, 24, 725–734. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, X.; Yang, L.; Chang, Z.; Luo, S. Progress toward Hydrogels in Removing Heavy Metals from Water: Problems and Solutions—A Review. ACS ES&T Water 2021, 1, 1098–1116. [Google Scholar]
- Tang, S.C.; Yan, D.Y.; Lo, I.M. Sustainable wastewater treatment using microsized magnetic hydrogel with magnetic separation technology. Ind. Eng. Chem. Res. 2014, 53, 15718–15724. [Google Scholar] [CrossRef]
- Tang, S.C.; Yin, K.; Lo, I.M. Column study of Cr (VI) removal by cationic hydrogel for in-situ remediation of contaminated groundwater and soil. J. Contam. Hydrol. 2011, 125, 39–46. [Google Scholar] [CrossRef]





| Hydrogel | Monomer | Cross-Linker | Initiator/Accelerator | References |
|---|---|---|---|---|
| Poly(2-acrylamido-2-methyl-1-propansulfonic acid-co- vinylimidazole) hydrogel | 2-acrylamido-2-methyl-1-propansulfonicacid (AMPS), N- vinyl imidazole | N,N′ methylenebisacrylamide (MBA) | 2,2′-azobis(2-methyl propionamide) (MPA) dihydrochloride | [37] |
| Cationic hydrogel | (3-acrylamidopropyl) trimethylammonium chloride (APTMCI) | N,N′ methylenebisacrylamide (MBA) | Ammoniumpersulfate (APS)/N,N,N′,N′-tetramethylenediamine (TEMED) | [38] |
| Hydrogel biochar composite | Acrylamide (AAm) | N,N′ methylenebisacrylamide (MBA) | Ammonium persulfate (APS) | [101] |
| Fe2O3 nanoparticles functionalized polyvinyl alcohol/chitosan magnetic composite hydrogel | Polyvinyl alcohol (PVA) | Glutaraldehyde vapor | Glacial acetic acid | [102] |
| Methacrylate-based hydrogel | Polyethylene glycol methyl ether methacrylate (PEGMEM), 2-dimethylamino ethyl methacrylate | N,N′ methylenebisacrylamide (MBA) | Ammonium persulfate (APS) | [104] |
| (p-4-VP-co-HEMA) composite hydrogel | 4-vinyl pyridine (4-VP), 2- hydroxyethylmetacrylate (HEMA) | N,N′ methylenebisacrylamide (MBA) | Ammonium persulfate (APS), N,N,N′,N′-tetramethylenediamine (TEMED) | [106] |
| Chitosan-cellulose hydrogel | Chitosan | Cellulose | - | [107] |
| Superabsorbent polymer hydrogels | Acrylic acid (AA), acrylamide (AAm) | N,N′ methylenebisacrylamide (MBA) | Ammoniumpersulfate (APS) | [113] |
| Poly(N-hydroxymethylacrylamide) hydrogel | N-hydroxymethylacrylamide | Polyethylene glycol (400) diacrylate | Ammonium persulfate (APS)/N,N,N′,N′-tetramethylenediamine (TEMED) | [114] |
| EDTA Functionalized Chitosan/Polyacrylamide double network hydrogel | Chitosan, acrylamide | N,N′ methylenebisacrylamide (MBA) | Potassium persulfate (KPS) | [115] |
| N-vinyl-2-pyrrolidone/Itaconic acid hydrogel | Itaconic acid (IA), N-vinyl-2-pyrrolidone | N,N′ methylenebisacrylamide (MBA) | Ammoniumpersulfate (APS/N,N,N′,N′- tetramethylenediamine (TEMED) | [116] |
| Polyampholyte hydrogel | Methyl methacrylate (MMA), acrylic acid (AA) | N,Nʹ methylenebisacrylamide (MBA) | Ammonium persulfate (APS)/N,N,N′,N′- tetramethylenediamine (TEMED) | [117] |
| Poly(acrylic acid) hydrogel adsorbent | Acrylic acid (AA) | Calcium hydroxide (Ca(OH)2) nano-spherulites (CNS) | Ammonium persulfate (APS)/N,N,N′,N′-tetramethylenediamine (TEMED) | [118] |
| Magnetic chitosan hydrogel beads | Chitosan | Glutaraldehyde | - | [119] |
| Hydrogel-based on novel cross-linker | Chitosan, acrylic acid, glucose | Allyl pentaerythritol(AP)15/allyl mannitol (AP)14/allyl sorbitol | Potassium persulfate (KPS) | [120] |
| Characterization Techniques | Characteristics |
|---|---|
| Fourier Transform Infrared Spectroscopy (FTIR) | Functional group |
| Field Emission-Scanning Electron Microscopy (FE-SEM) | Surface morphology |
| Thermo Gravimetric Analysis (TGA) | Thermal stability |
| Zeta Sizer | Surface charge |
| Energy Dispersive X-ray (EDX) | Elemental composition |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darban, Z.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Sridewi, N. Hydrogel-Based Adsorbent Material for the Effective Removal of Heavy Metals from Wastewater: A Comprehensive Review. Gels 2022, 8, 263. https://doi.org/10.3390/gels8050263
Darban Z, Shahabuddin S, Gaur R, Ahmad I, Sridewi N. Hydrogel-Based Adsorbent Material for the Effective Removal of Heavy Metals from Wastewater: A Comprehensive Review. Gels. 2022; 8(5):263. https://doi.org/10.3390/gels8050263
Chicago/Turabian StyleDarban, Zenab, Syed Shahabuddin, Rama Gaur, Irfan Ahmad, and Nanthini Sridewi. 2022. "Hydrogel-Based Adsorbent Material for the Effective Removal of Heavy Metals from Wastewater: A Comprehensive Review" Gels 8, no. 5: 263. https://doi.org/10.3390/gels8050263
APA StyleDarban, Z., Shahabuddin, S., Gaur, R., Ahmad, I., & Sridewi, N. (2022). Hydrogel-Based Adsorbent Material for the Effective Removal of Heavy Metals from Wastewater: A Comprehensive Review. Gels, 8(5), 263. https://doi.org/10.3390/gels8050263







