Interaction-Induced Structural Transformations in Polysaccharide and Protein-Polysaccharide Gels as Functional Basis for Novel Soft-Matter: A Case of Carrageenans
Abstract
:1. Introduction
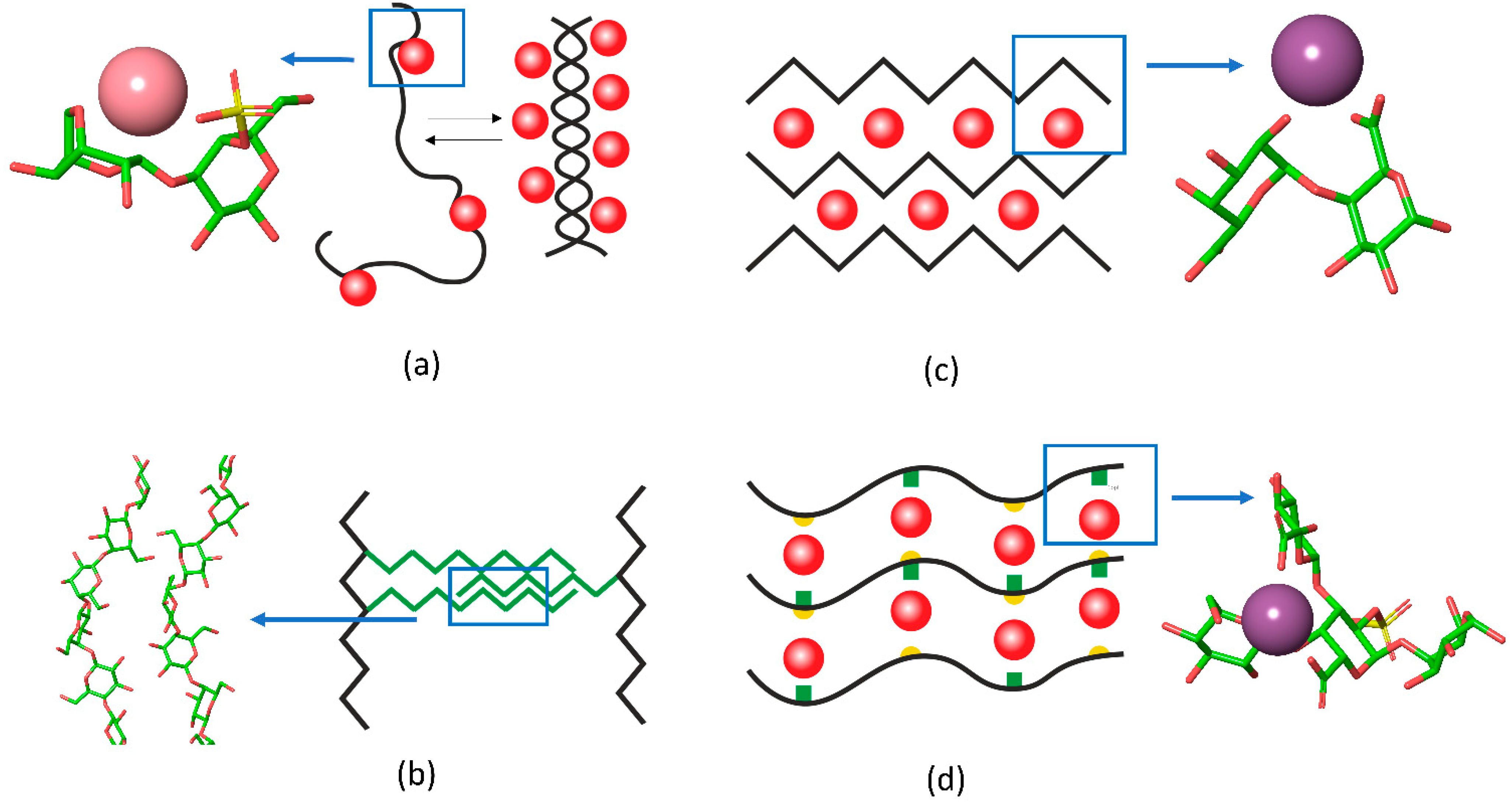
2. Crosslinking in Polysaccharide Gels
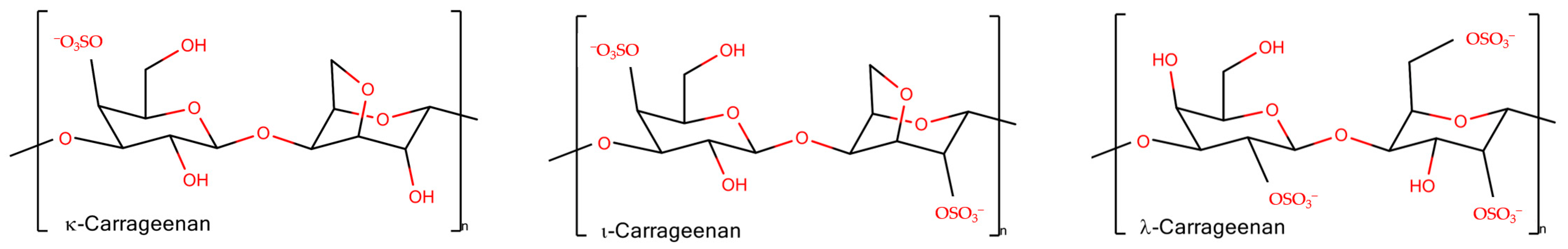
2.1. Chain Ordering in Carrageenans
2.2. 1,4-β-Galactan Crosslinking
2.3. Egg-Box Assembly in Pectins and Alginates
3. Protein–Polysaccharide Gels
3.1. κ-Carrageenan–Gelatin Gels
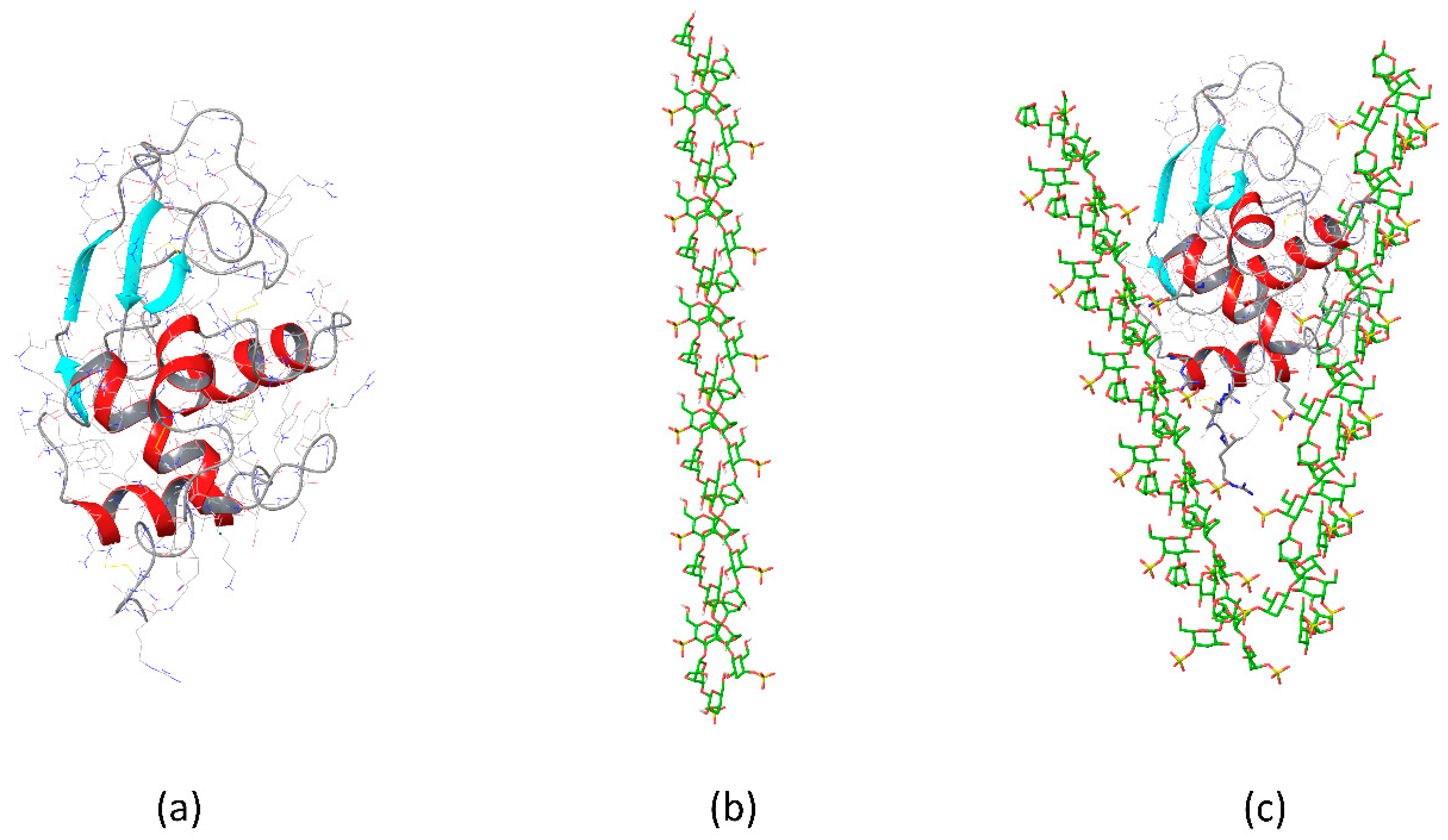
3.2. κ-Carrageenan–Lysozyme Gels
3.3. Gels of κ-Carrageenan-Lysozyme Fibrils
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ali, A.; Ahmed, S. Recent Advances in Edible Polymer Based Hydrogels as a Sustainable Alternative to Conventional Polymers. J. Agric. Food Chem. 2018, 66, 6940–6967. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, A.; Li, X.; Sun, L.; Guo, Y. An overview of classifications, properties of food polysaccharides and their links to applications in improving food textures. Trends Food Sci. Technol. 2020, 102, 1–15. [Google Scholar] [CrossRef]
- Shelke, N.B.; James, R.; Laurencin, C.T.; Kumbar, S.G. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym. Adv. Technol. 2014, 25, 448–460. [Google Scholar] [CrossRef]
- Suntornnond, R.; An, J.; Chua, C.K. Bioprinting of Thermoresponsive Hydrogels for Next Generation Tissue Engineering: A Review. Macromol. Mater. Eng. 2017, 302, 1600266. [Google Scholar] [CrossRef]
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydrogel-Forming Algae Polysaccharides: From Seaweed to Biomedical Applications. Biomacromolecules 2021, 22, 1027–1052. [Google Scholar] [CrossRef] [PubMed]
- Kaliva, M.; Kavasi, R.-M.; Chatzinikolaidou, M.; Vamvakaki, M. 4.01—Polysaccharides and Applications in Regenerative Medicine. In Comprehensive Glycoscience; Barchi, J.J., Ed.; Elsevier: Oxford, UK, 2021; pp. 1–33. [Google Scholar]
- Nakagawa, Y.; Ebara, M. 4.02—Polysaccharides for Drug Delivery: The Development of Polysaccharide-Based Materials and Glycopolymer to Improve Drug Delivery Applications. In Comprehensive Glycoscience; Barchi, J.J., Ed.; Elsevier: Oxford, UK, 2021; pp. 34–50. [Google Scholar]
- Bogdanova, L.R.; Makarova, A.O.; Zueva, O.S.; Zakharova, L.Y.; Zuev, Y.F. Encapsulation of diagnostic dyes in the polysaccharide matrix modified by carbon nanotubes. Russ. Chem. Bull. 2020, 69, 590–595. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Faizullin, D.A.; Mikshina, P.V.; Gorshkova, T.A.; Zuev, Y.F. Spatial structures of rhamnogalacturonan I in gel and colloidal solution identified by 1D and 2D-FTIR spectroscopy. Carbohydr. Polym. 2018, 192, 231–239. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Faizullin, D.A.; Zuev, Y.F. Interplay between secondary structure and ion binding upon thermoreversible gelation of κ-carrageenan. Carbohydr. Polym. 2020, 227, 115342. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Gorshkova, T.A.; Mikshina, P.V.; Zuev, Y.F.; Perez, S. Metrics of rhamnogalacturonan I with β-(1→4)-linked galactan side chains and structural basis for its self-aggregation. Carbohydr. Polym. 2017, 158, 93–101. [Google Scholar] [CrossRef]
- Mikshina, P.V.; Makshakova, O.N.; Petrova, A.A.; Gaifullina, I.Z.; Idiyatullin, B.Z.; Gorshkova, T.A.; Zuev, Y.F. Gelation of rhamnogalacturonan I is based on galactan side chain interaction and does not involve chemical modifications. Carbohydr. Polym. 2017, 171, 143–151. [Google Scholar] [CrossRef]
- Rees, D.A. Structure, conformation, and mechanism in the formation of polysaccharide gels and networks. Adv. Carbohydr. Chem. Biochem. 1969, 24, 267–332. [Google Scholar] [CrossRef] [PubMed]
- Pettinelli, N.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Feijoo-Bandín, S.; Lago, F. Carrageenan-based physically crosslinked injectable hydrogel for wound healing and tissue repairing applications. Int. J. Pharm. 2020, 589, 119828. [Google Scholar] [CrossRef] [PubMed]
- Delbarre-Ladrat, C.; Sinquin, C.; Lebellenger, L.; Zykwinska, A.; Colliec-Jouault, S. Exopolysaccharides produced by marine bacteria and their applications as glycosaminoglycan-like molecules. Front. Chem. 2014, 2, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Piculell, L. Gelling Carrageenans. In Food Polysaccharides and Their Applications; Stephen, A.M., Phillips, G.O., Williams, P.A., Eds.; Taylor & Francis: Abingdon, UK, 2006; Volume 67, pp. 239–287. [Google Scholar]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Noferini, D.; Faraone, A.; Rossi, M.; Mamontov, E.; Fratini, E.; Baglioni, P. Disentangling Polymer Network and Hydration Water Dynamics in Polyhydroxyethyl Methacrylate Physical and Chemical Hydrogels. J. Phys. Chem. C 2019, 123, 19183–19194. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Design principles of food gels. Nat. Food 2020, 1, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; Huang, W.; Chen, L. Soluble Pea Protein Aggregates Form Strong Gels in the Presence of κ-Carrageenan. ACS Food Sci. Technol. 2021, 1, 1605–1614. [Google Scholar] [CrossRef]
- Banerjee, S.; Bhattacharya, S. Food Gels: Gelling Process and New Applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 334–346. [Google Scholar] [CrossRef]
- Verbeken, D.; Neirinck, N.; Van Der Meeren, P.; Dewettinck, K. Influence of kappa-carrageenan on the thermal gelation of salt-soluble meat proteins. Meat Sci. 2005, 70, 161–166. [Google Scholar] [CrossRef]
- Wang, X.J.; Feng, T.T.; Xia, S.Q. Saltiness perception related to salt release of surimi emulsified sausages: Modulation in texture and microstructure by polysaccharides. Int. J. Food Sci. Technol. 2021, 56, 3893–3902. [Google Scholar] [CrossRef]
- Sedmeyer, F.; Kulozik, U. Impact of process conditions on the rheological detectable structure of UHT treated milk protein-carrageenan systems. J. Food Eng. 2006, 77, 943–950. [Google Scholar] [CrossRef]
- Shchipunov, Y.A.; Chesnokov, A.V. Carrageenan gels in skim milk: Formation and rheological properties. Colloid J. 2003, 65, 105–113. [Google Scholar] [CrossRef]
- Biglarian, N.; Rafe, A.; Shahidi, S.A. Effect of basil seed gum and kappa-carrageenan on the rheological, textural, and structural properties of whipped cream. J. Sci. Food Agric. 2021, 101, 5851–5860. [Google Scholar] [CrossRef]
- Blaszak, B.; Gozdecka, G.; Shyichuk, A. Carrageenan as a functional additive in the production of cheese and cheese-like products. Acta Sci. Polonorum-Technol. Aliment. 2018, 17, 107–116. [Google Scholar] [CrossRef]
- Shen, Y.R.; Kuo, M.I. Effects of different carrageenan types on the rheological and water holding properties of tofu. Lwt-Food Sci. Technol. 2017, 78, 122–128. [Google Scholar] [CrossRef]
- Schmitt, C.; Sanchez, C.; Desobry-Banon, S.; Hardy, J. Structure and technofunctional properties of protein-polysaccharide complexes: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 689–753. [Google Scholar] [CrossRef]
- Jong, H.G.; Kruyt, H.R. Coacervation (partial miscibility in colloid systems). Proc. K. Ned. Akad. Wet. 1929, 32, 849–856. [Google Scholar]
- De Kruif, C.G.; Weinbreck, F.; De Vries, R. Complex coacervation of proteins and anionic polysaccharides. Curr. Opin. Colloid Interface Sci. 2004, 9, 340–349. [Google Scholar] [CrossRef]
- Schmitt, C.; Turgeon, S.L. Protein/polysaccharide complexes and coacervates in food systems. Adv. Colloid Interface Sci. 2011, 167, 63–70. [Google Scholar] [CrossRef]
- Running, C.A.; Falshaw, R.; Janaswamy, S. Trivalent Iron Induced Gelation in Lambda-Carrageenan. Carbohydr. Polym. 2012, 87, 2735–2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilliou, L. Structure-Elastic Properties Relationships in Gelling Carrageenans. Polymers 2021, 13, 4120. [Google Scholar] [CrossRef] [PubMed]
- Geonzon, L.C.; Descallar, F.B.A.; Du, L.; Bacabac, R.G.; Matsukawa, S. Gelation mechanism and network structure in gels of carrageenans and their mixtures viewed at different length scales—A review. Food Hydrocoll. 2020, 108, 106039. [Google Scholar] [CrossRef]
- Smidsrød, O.; Grasdalen, H. Some physical properties of carrageenan in solution and gel state. Carbohydr. Polym. 1982, 2, 270–272. [Google Scholar] [CrossRef]
- Schefer, L.; Usov, I.; Mezzenga, R. Anomalous Stiffening and Ion-Induced Coil–Helix Transition of Carrageenans under Monovalent Salt Conditions. Biomacromolecules 2015, 16, 985–991. [Google Scholar] [CrossRef]
- Anderson, N.S.; Campbell, J.W.; Harding, M.M.; Rees, D.A.; Samuel, J.W. X-ray diffraction studies of polysaccharide sulphates: Double helix models for k- and l-carrageenans. J. Mol. Biol. 1969, 45, 85–99. [Google Scholar] [CrossRef]
- van de Velde, F.; Rollema, H.S.; Grinberg, N.V.; Burova, T.V.; Grinberg, V.Y.; Tromp, R.H. Coil-helix transition of iota-carrageenan as a function of chain regularity. BioPolymers 2002, 65, 299–312. [Google Scholar] [CrossRef]
- Arnott, S.; Scott, W.E.; Rees, D.A.; McNab, C.G. Iota-carrageenan: Molecular structure and packing of polysaccharide double helices in oriented fibres of divalent cation salts. J. Mol. Biol. 1974, 90, 253–267. [Google Scholar] [CrossRef]
- Gormally, J.; Pereira, M.C.; Wyn-Jones, E.; Morris, E.R. Ultrasonic relaxation of agarose and carrageenan gels. The role of solvent. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1982, 78, 1661–1673. [Google Scholar] [CrossRef]
- Janaswamy, S.; Chandrasekaran, R. Effect of calcium ions on the organization of iota-carrageenan helices: An X-ray investigation. Carbohydr. Res. 2002, 337, 523–535. [Google Scholar] [CrossRef]
- Millane, R.P.; Chandrasekaran, R.; Arnott, S.; Dea, I.C.M. The molecular structure of kappa-carrageenan and comparison with iota-carrageenan. Carbohydr. Res. 1988, 182, 1–17. [Google Scholar] [CrossRef]
- Hirota, N.; Nagai, K. Helical structures and water vapor sorption properties of carrageenan membranes derived from red algae. Carbohydr. Polym. Technol. Appl. 2022, 3, 100200. [Google Scholar] [CrossRef]
- Le Questel, J.Y.; Cros, S.; Mackie, W.; Pérez, S. Computer modelling of sulfated carbohydrates: Applications to carrageenans. Int. J. Biol. Macromol. 1995, 17, 161–175. [Google Scholar] [CrossRef]
- Schefer, L.; Adamcik, J.; Mezzenga, R. Unravelling secondary structure changes on individual anionic polysaccharide chains by atomic force microscopy. Angew Chem. Int. Ed. Engl. 2014, 53, 5376–5379. [Google Scholar] [CrossRef]
- Diener, M.; Adamcik, J.; Sánchez-Ferrer, A.; Jaedig, F.; Schefer, L.; Mezzenga, R. Primary, Secondary, Tertiary and Quaternary Structure Levels in Linear Polysaccharides: From Random Coil, to Single Helix to Supramolecular Assembly. Biomacromolecules 2019, 20, 1731–1739. [Google Scholar] [CrossRef]
- Schefer, L.; Adamcik, J.; Diener, M.; Mezzenga, R. Supramolecular chiral self-assembly and supercoiling behavior of carrageenans at varying salt conditions. Nanoscale 2015, 7, 16182–16188. [Google Scholar] [CrossRef]
- Morris, E.R.; Rees, D.A.; Thom, D.; Boyd, J. Chiroptical and stoichiometric evidence of a specific, primary dimerisation process in alginate gelation. Carbohydr. Res. 1978, 66, 145–154. [Google Scholar] [CrossRef]
- Braccini, I.; Grasso, R.P.; Pérez, S. Conformational and configurational features of acidic polysaccharides and their interactions with calcium ions: A molecular modeling investigation. Carbohydr. Res. 1999, 317, 119–130. [Google Scholar] [CrossRef]
- Braccini, I.; Pérez, S. Molecular Basis of Ca2+-Induced Gelation in Alginates and Pectins: The Egg-Box Model Revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Makshakova, O.; Zykwinska, A.; Cuenot, S.; Colliec-Jouault, S.; Perez, S. Three-dimensional structures, dynamics and calcium-mediated interactions of the exopolysaccharide, Infernan, produced by the deep-sea hydrothermal bacterium Alteromonas infernus. Carbohydr. Polym. 2022, 276, 118732. [Google Scholar] [CrossRef] [PubMed]
- Tako, M. The Principle of Polysaccharide Gels. Adv. Biosci. Biotechnol. 2015, 06, 15. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarska, A.; Pieczywek, P.M.; Cybulska, J.; Zdunek, A. Structure and functionality of Rhamnogalacturonan I in the cell wall and in solution: A review. Carbohydr. Polym. 2021, 278, 118909. [Google Scholar] [CrossRef] [PubMed]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Sacco, P.; Furlani, F.; De Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for Developing Physical Gels of Chitosan and of Chitosan Derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef] [Green Version]
- Kayitmazer, A.B.; Seyrek, E.; Dubin, P.L.; Staggemeier, B.A. Influence of Chain Stiffness on the Interaction of Polyelectrolytes with Oppositely Charged Micelles and Proteins. J. Phys. Chem. B 2003, 107, 8158–8165. [Google Scholar] [CrossRef] [Green Version]
- Perez, S.; Fadda, E.; Makshakova, O. 1.15—Computational Modeling in Glycoscience. In Comprehensive Glycoscience; Barchi, J.J., Ed.; Elsevier: Oxford, UK, 2021; pp. 374–404. [Google Scholar]
- Anggara, K.; Zhu, Y.; Delbianco, M.; Rauschenbach, S.; Abb, S.; Seeberger, P.H.; Kern, K. Exploring the Molecular Conformation Space by Soft Molecule–Surface Collision. J. Am. Chem. Soc. 2020, 142, 21420–21427. [Google Scholar] [CrossRef]
- Le, X.T.; Rioux, L.E.; Turgeon, S.L. Formation and functional properties of protein-polysaccharide electrostatic hydrogels in comparison to protein or polysaccharide hydrogels. Adv. Colloid Interface Sci. 2017, 239, 127–135. [Google Scholar] [CrossRef]
- Stenner, R.; Matubayasi, N.; Shimizu, S. Gelation of carrageenan: Effects of sugars and polyols. Food Hydrocoll. 2016, 54, 284–292. [Google Scholar] [CrossRef]
- Tao, H.; Guo, L.; Qin, Z.; Yu, B.; Wang, Y.; Li, J.; Wang, Z.; Shao, X.; Dou, G.; Cui, B. Textural characteristics of mixed gels improved by structural recombination and the formation of hydrogen bonds between curdlan and carrageenan. Food Hydrocoll. 2022, 129, 107678. [Google Scholar] [CrossRef]
- Antonov, Y.A.; Zhuravleva, I.L. The Interaction of Lysozyme with Carrageenans. Appl. Biochem. Microbiol. 2019, 55, 209–217. [Google Scholar] [CrossRef]
- Olsson, C.; Frigard, T.; Andersson, R.; Hermansson, A.M. Effects of amylopectin structure and molecular weight on microstructural and rheological properties of mixed beta-lactoglobulin gels. Biomacromolecules 2003, 4, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Derkach, S.R.; Kuchina, Y.A.; Kolotova, D.S.; Voron’ko, N.G. Polyelectrolyte Polysaccharide-Gelatin Complexes: Rheology and Structure. Polymers 2020, 12, 266. [Google Scholar] [CrossRef] [Green Version]
- Turgeon, S.L.; Beaulieu, M. Improvement and modification of whey protein gel texture using polysaccharides. Food Hydrocoll. 2001, 15, 583–591. [Google Scholar] [CrossRef]
- Morris, V.J. Multicomponent gels. In Gums and Stabilisers for the Food Industry; Phillips, G., Wedlock, D., Williams, P., Eds.; Elsevier: London, UK, 1986; p. 87. [Google Scholar]
- Tolstoguzov, V. Some physico-chemical aspects of protein processing in foods. Multicomponent gels. Food Hydrocoll. 1995, 9, 317–332. [Google Scholar] [CrossRef]
- Donato, L.; Garnier, C.; Novales, B.; Durand, S.; Doublier, J.L. Heat-induced gelation of bovine serum albumin/low-methoxyl pectin systems and the effect of calcium ions. Biomacromolecules 2005, 6, 374–385. [Google Scholar] [CrossRef]
- Tromp, R.H.; Rennie, A.R.; Jones, R.A.L. Kinetics of the Simultaneous Phase Separation and Gelation in Solutions of Dextran and Gelatin. Macromolecules 1995, 28, 4129–4138. [Google Scholar] [CrossRef]
- Çakır, E.; Foegeding, E.A. Combining protein micro-phase separation and protein–polysaccharide segregative phase separation to produce gel structures. Food Hydrocoll. 2011, 25, 1538–1546. [Google Scholar] [CrossRef]
- Burla, F.; Mulla, Y.; Vos, B.E.; Aufderhorst-Roberts, A.; Koenderink, G.H. From mechanical resilience to active material properties in biopolymer networks. Nat. Rev. Phys. 2019, 1, 249–263. [Google Scholar] [CrossRef]
- Turgeon, S.L.; Beaulieu, M. Effect of thermal treatments on large and small deformation properties of whey protein/pectin mixed gels. In Gums and Stabilisers for the Food Industry 12; The Royal Society of Chemistry: London, UK, 2004; pp. 211–226. [Google Scholar]
- Eleya, M.M.O.; Turgeon, S.L. The effects of pH on the rheology of beta-lactoglobulin/kappa-carrageenan mixed gels. Food Hydrocoll. 2000, 14, 245–251. [Google Scholar] [CrossRef]
- Croguennoc, P.; Nicolai, T.; Durand, D.; Clark, A. Phase separation and association of globular protein aggregates in the presence of polysaccharides: 2. Heated mixtures of native beta-Lactoglobulin and k-Carrageenan. Langmuir 2001, 17, 4380–4385. [Google Scholar] [CrossRef]
- Ako, K.; Durand, D.; Nicolai, T. Phase separation driven by aggregation can be reversed by elasticity in gelling mixtures of polysaccharides and proteins. Soft Matter. 2011, 7, 2507–2516. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Nicolai, T.; Chassenieux, C.; Benyahia, L. The effect of protein aggregate morphology on phase separation in mixtures with polysaccharides. J. Phys. Cond. Matter. 2014, 26, 464102. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, Y.T.; Lu, N.Y.; Liu, D.S.; Regenstein, J.M.; Zhou, P. Effects of hydrocolloids on the rheological and microstructural properties of semisolid whey protein-rich systems. Food Biosci. 2019, 30, 100424. [Google Scholar] [CrossRef]
- Selig, M.J.; Dar, B.N.; Kierulf, A.; Ravanfar, R.; Rizvi, S.S.H.; Abbaspourrad, A. Modulation of whey protein-kappa carrageenan hydrogel properties via enzymatic protein modification. Food Funct. 2018, 9, 2313–2319. [Google Scholar] [CrossRef]
- Nunes, M.; Raymundo, A.; Sousa, I. Gelled vegetable desserts containing pea protein, kappa-carrageenan and starch. Eur. Food Res. Technol. 2006, 222, 622–628. [Google Scholar] [CrossRef] [Green Version]
- Nunes, M.C.; Raymundo, A.; Sousa, I. Rheological behaviour and microstructure of pea protein/kappa-carrageenan/starch gels with different setting conditions. Food Hydrocoll. 2006, 20, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Bi, C.H.; Zhu, Y.D.; Li, L.T.; Zhang, Y.L.; Hua, Z.; Zhu, J.Y.; Liu, Y.; Liu, Y.D.; Huang, Z.G. Rheological properties and microstructure of soy protein isolate/kappa-carrageenan gels under high-speed shear treatment. J. Food Eng. 2018, 236, 44–50. [Google Scholar] [CrossRef]
- Nieto, T.V.N.; Wang, Y.X.; Ozimek, L.; Chen, L.Y. Improved thermal gelation of oat protein with the formation of controlled phase-separated networks using dextrin and carrageenan polysaccharides. Food Res. Int. 2016, 82, 95–103. [Google Scholar] [CrossRef]
- Tang, M.X.; Lei, Y.C.; Wang, Y.; Li, D.; Wang, L.J. Rheological and structural properties of sodium caseinate as influenced by locust bean gum and kappa-carrageenan. Food Hydrocoll. 2021, 112, 106251. [Google Scholar] [CrossRef]
- Ma, F.; Chen, C.G.; Zheng, L.; Zhou, C.L.; Cai, K.Z.; Han, Z. Effect of high pressure processing on the gel properties of salt-soluble meat protein containing CaCl2 and kappa-carrageenan. Meat Sci. 2013, 95, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Babaei, J.; Khodaiyan, F.; Mohammadian, M.; Sheikhi, M. In vitro digestibility and functional attributes of the whey protein heat-induced hydrogels reinforced by various polysaccharides and CaCl2. J. Food Measur. Charact. 2022, 16, 19–28. [Google Scholar] [CrossRef]
- Duran, N.M.; Spelzini, D.; Boeris, V. Characterization of acid—Induced gels of quinoa proteins and carrageenan. LWT-Food Sci. Technol. 2019, 108, 39–47. [Google Scholar] [CrossRef]
- Duran, N.M.; Galante, M.; Spelzini, D.; Boeris, V. The effect of carrageenan on the acid-induced aggregation and gelation conditions of quinoa proteins. Food Res. Int. 2018, 107, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.N.; Du, Y.N.; Jiang, X.Y.; Han, J.R.; Pan, J.F.; Wu, H.T. Intermolecular interaction in the hybrid gel of scallop (Patinopecten yessoensis) male gonad hydrolysates and kappa-carrageenan. J. Food Sci. 2021, 86, 792–802. [Google Scholar] [CrossRef]
- Palanisamy, M.; Töpfl, S.; Aganovic, K.; Berger, R.G. Influence of iota carrageenan addition on the properties of soya protein meat analogues. LWT 2018, 87, 546–552. [Google Scholar] [CrossRef]
- Jiang, X.-Y.; Yan, J.-N.; Du, Y.-N.; Xu, S.-Q.; Han, J.-R.; Wu, H.-T. Hybrid gelation of scallop (Patinopecten yessoensis) male gonad hydrolysates combined with different concentrations of iota-carrageenan. J. Food Measur. Charact. 2022, 16, 1974–1982. [Google Scholar] [CrossRef]
- Lam, R.S.; Nickerson, M.T. Effect of the biopolymer mixing ratio on the formation of electrostatically coupled whey protein-κ- and ι-carrageenan networks in the presence and absence of oil droplets. J. Agric. Food Chem. 2014, 62, 8730–8739. [Google Scholar] [CrossRef]
- Míšková, Z.; Salek, R.N.; Křenková, B.; Kůrová, V.; Němečková, I.; Pachlová, V.; Buňka, F. The effect of κ- and ι-carrageenan concentrations on the viscoelastic and sensory properties of cream desserts during storage. LWT 2021, 145, 111539. [Google Scholar] [CrossRef]
- Bocker, L.; Ruhs, P.A.; Boni, L.; Fischer, P.; Kuster, S. Fiber-Enforced Hydrogels: Hagfish Slime Stabilized with Biopolymers including kappa-Carrageenan. ACS Biomater. Sci. Eng. 2016, 2, 90–95. [Google Scholar] [CrossRef]
- Huang, M.; Mao, Y.Z.; Li, H.L.; Yang, H.S. Kappa-carrageenan enhances the gelation and structural changes of egg yolk via electrostatic interactions with yolk protein. Food Chem. 2021, 360, 129972. [Google Scholar] [CrossRef] [PubMed]
- Raei, M.; Rafe, A.; Shahidi, F. Rheological and structural characteristics of whey protein-pectin complex coacervates. J. Food Eng. 2018, 228, 25–31. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Safarova, E.R.; Zuev, Y.F. Structural insights in interactions between RNase from Bacillus Intermedius and rhamnogalacturonan I from potato. Carbohydr. Polym. 2021, 251, 117038. [Google Scholar] [CrossRef] [PubMed]
- Le, X.T.; Turgeon, S.L. Rheological and structural study of electrostatic cross-linked xanthan gum hydrogels induced by β-lactoglobulin. Soft Matter. 2013, 9, 3063–3073. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Feroz, A.; Khaki, P.S.S.; Bano, B. Binding of lambda-carrageenan (a food additive) to almond cystatin: An insight involving spectroscopic and thermodynamic approach. Int. J. Biol. Macromol. 2017, 98, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.G.; Tan, L.L.; Chen, Y.F.; Zhang, J.; Li, H.H.; Chen, L.H. Effect of kappa-carrageenan addition on protein structure and gel properties of salted duck egg white. J. Sci. Food Agric. 2021, 101, 1389–1395. [Google Scholar] [CrossRef]
- Chen, J.X.; Deng, T.Y.; Wang, C.; Mi, H.B.; Yi, S.M.; Li, X.P.; Li, J.R. Effect of hydrocolloids on gel properties and protein secondary structure of silver carp surimi. J. Sci. Food Agric. 2020, 100, 2252–2260. [Google Scholar] [CrossRef]
- Zheng, H.N.; Beamer, S.K.; Matak, K.E.; Jaczynski, J. Effect of kappa-carrageenan on gelation and gel characteristics of Antarctic krill (Euphausia superba) protein isolated with isoelectric solubilization/precipitation. Food Chem. 2019, 278, 644–652. [Google Scholar] [CrossRef]
- Burova, T.V.; Grinberg, N.V.; Grinberg, V.Y.; Usov, A.I.; Tolstoguzov, V.B.; de Kruif, C.G. Conformational Changes in ι- and κ-Carrageenans Induced by Complex Formation with Bovine β-Casein. Biomacromolecules 2007, 8, 368–375. [Google Scholar] [CrossRef]
- Micsonai, A.; Wien, F.; Kernya, L.; Lee, Y.-H.; Goto, Y.; Réfrégiers, M.; Kardos, J. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, E3095. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Zhang, B.; Qiao, D.; Yan, X.; Zhao, S.; Jia, C.; Niu, M.; Xu, Y. Addition of κ-carrageenan increases the strength and chewiness of gelatin-based composite gel. Food Hydrocoll. 2022, 128, 107565. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S.; Gordeeva, A.M.; Faizullin, D.A.; Gusev, Y.A.; Zuev, Y.F.; Makshakova, O.N. Molecular structure and properties of κ-carrageenan-gelatin gels. Carbohydr. Polym. 2018, 197, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Kang, X.Z.; Fang, Q.; Yang, W.G.; Cen, S.J.; Lou, Q.M.; Huang, T. Rheological properties and interactions of fish gelatin-kappa-carrageenan polyelectrolyte hydrogels: The effects of salt. J. Texture Stud. 2022, 53, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Makshakova, O.N.; Bogdanova, L.R.; Faizullin, D.A.; Ermakova, E.A.; Zuev, Y.F.; Sedov, I.A. Interaction-induced structural transformation of lysozyme and kappa-carrageenan in binary complexes. Carbohydr. Polym. 2021, 252, 117181. [Google Scholar] [CrossRef] [PubMed]
- Van De Weert, M.; Andersen, M.B.; Frokjaer, S. Complex coacervation of lysozyme and heparin: Complex characterization and protein stability. Pharm. Res. 2004, 21, 2354–2359. [Google Scholar] [CrossRef] [PubMed]
- Antonov, Y.A.; Zhuravleva, I.L. Complexation of lysozyme with lambda carrageenan: Complex characterization and protein stability. Food Hydrocoll. 2019, 87, 519–529. [Google Scholar] [CrossRef]
- Jiang, S.; Ma, Y.; Wang, Y.; Wang, R.; Zeng, M. Effect of κ-carrageenan on the gelation properties of oyster protein. Food Chem. 2022, 382, 132329. [Google Scholar] [CrossRef]
- Pérez, S.; Bonnardel, F.; Lisacek, F.; Imberty, A.; Ricard Blum, S.; Makshakova, O. GAG-DB, the New Interface of the Three-Dimensional Landscape of Glycosaminoglycans. Biomolecules 2020, 10, 1660. [Google Scholar] [CrossRef]
- Magnus, J.H.; Husby, G.; Kolset, S.O. Presence of glycosaminoglycans in purified AA type amyloid fibrils associated with juvenile rheumatoid arthritis. Ann. Rheum. Dis. 1989, 48, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Cohlberg, J.A.; Li, J.; Uversky, V.N.; Fink, A.L. Heparin and Other Glycosaminoglycans Stimulate the Formation of Amyloid Fibrils from α-Synuclein in Vitro. Biochemistry 2002, 41, 1502–1511. [Google Scholar] [CrossRef]
- Maïza, A.; Chantepie, S.; Vera, C.; Fifre, A.; Huynh, M.B.; Stettler, O.; Ouidja, M.O.; Papy-Garcia, D. The role of heparan sulfates in protein aggregation and their potential impact on neurodegeneration. FEBS Lett. 2018, 592, 3806–3818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannuzzi, C.; Irace, G.; Sirangelo, I. The effect of glycosaminoglycans (GAGs) on amyloid aggregation and toxicity. Molecules 2015, 20, 2510–2528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodard, D.; Bell, D.; Tipton, D.; Durrance, S.; Burnett, L.C.; Li, B.; Xu, S. Gel formation in protein amyloid aggregation: A physical mechanism for cytotoxicity. PLoS ONE 2014, 9, e94789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenyuk, P.; Tiainen, T.; Hietala, S.; Tenhu, H.; Aseyev, V.; Muronetz, V. Artificial chaperones based on thermoresponsive polymers recognize the unfolded state of the protein. Int. J. Biol. Macromol. 2019, 121, 536–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenyuk, P.I.; Kurochkina, L.P.; Gusev, N.B.; Izumrudov, V.A.; Muronetz, V.I. Chaperone-like activity of synthetic polyanions can be higher than the activity of natural chaperones at elevated temperature. Biochem. Biophys. Res. Commun. 2017, 489, 200–205. [Google Scholar] [CrossRef]
- Wang, R.; Yang, X.; Cui, L.; Yin, H.; Xu, S. Gels of Amyloid Fibers. Biomolecules 2019, 9, 210. [Google Scholar] [CrossRef] [Green Version]
- Usuelli, M.; Germerdonk, T.; Cao, Y.; Peydayesh, M.; Bagnani, M.; Handschin, S.; Nyström, G.; Mezzenga, R. Polysaccharide-reinforced amyloid fibril hydrogels and aerogels. Nanoscale 2021, 13, 12534–12545. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makshakova, O.N.; Zuev, Y.F. Interaction-Induced Structural Transformations in Polysaccharide and Protein-Polysaccharide Gels as Functional Basis for Novel Soft-Matter: A Case of Carrageenans. Gels 2022, 8, 287. https://doi.org/10.3390/gels8050287
Makshakova ON, Zuev YF. Interaction-Induced Structural Transformations in Polysaccharide and Protein-Polysaccharide Gels as Functional Basis for Novel Soft-Matter: A Case of Carrageenans. Gels. 2022; 8(5):287. https://doi.org/10.3390/gels8050287
Chicago/Turabian StyleMakshakova, Olga N., and Yuriy F. Zuev. 2022. "Interaction-Induced Structural Transformations in Polysaccharide and Protein-Polysaccharide Gels as Functional Basis for Novel Soft-Matter: A Case of Carrageenans" Gels 8, no. 5: 287. https://doi.org/10.3390/gels8050287
APA StyleMakshakova, O. N., & Zuev, Y. F. (2022). Interaction-Induced Structural Transformations in Polysaccharide and Protein-Polysaccharide Gels as Functional Basis for Novel Soft-Matter: A Case of Carrageenans. Gels, 8(5), 287. https://doi.org/10.3390/gels8050287





