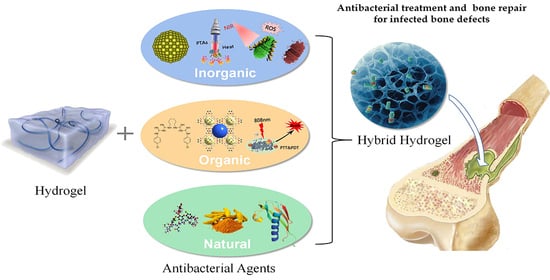
Recent Research on Hybrid Hydrogels for Infection Treatment and Bone Repair
Abstract
:1. Introduction
2. Hybrid Hydrogels with Inorganic Antibacterial Agents for Infected Bone Repair
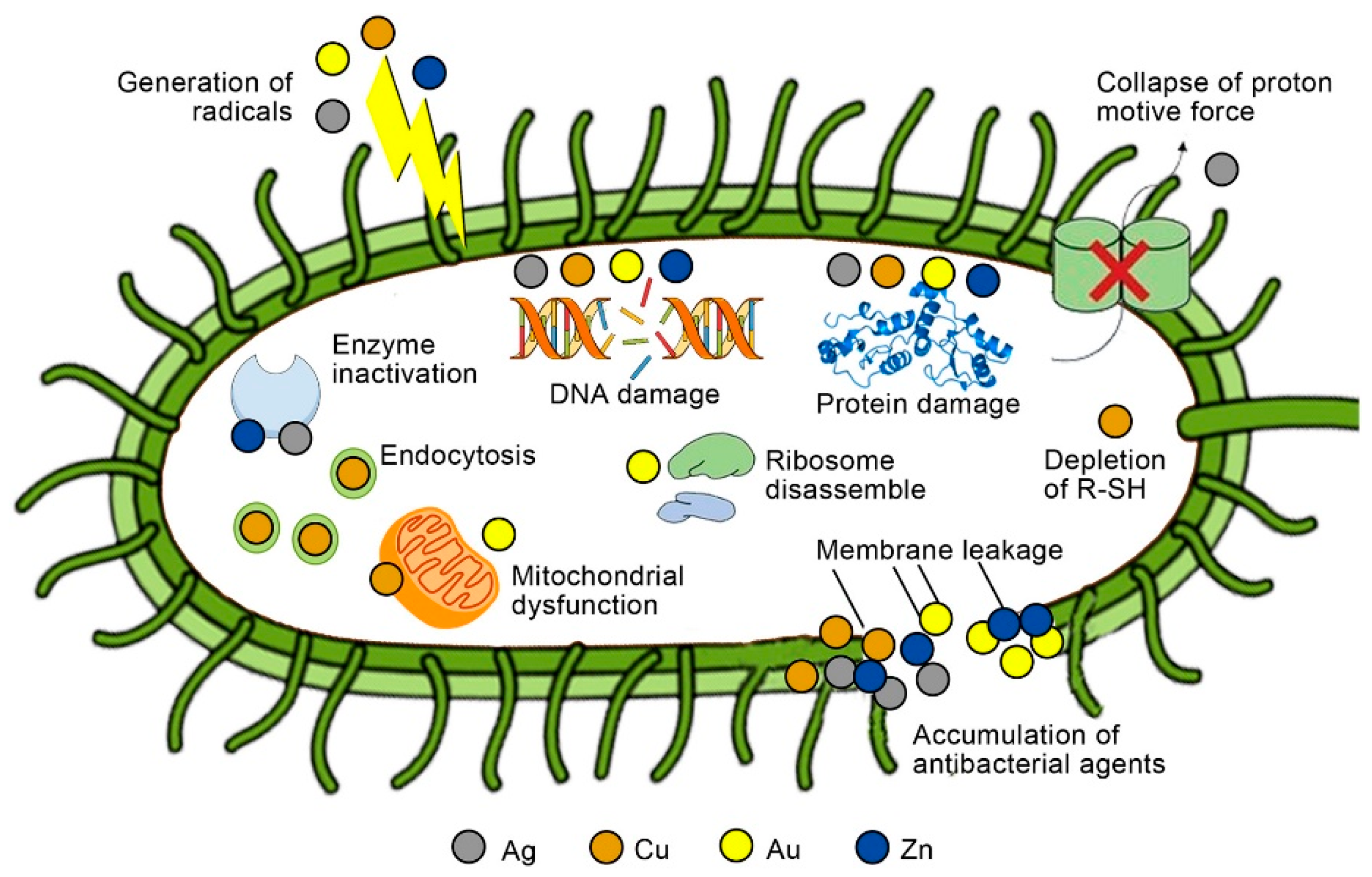
2.1. Hydrogels with Metal Nanomaterials
2.2. Light-Mediated Inorganic Antibacterial Hydrogels
3. Hybrid Hydrogels with Organic Antibacterial Agents for Infected Bone Repair
3.1. Hybrid Hydrogels with Organic Antibacterial Agents
3.2. Hybrid Hydrogels with Metal-Organic Frameworks
3.3. Light-Mediated Organic Antibacterial Hydrogels
4. Hybrid Hydrogels with Natural Antibacterial Agents for Bone Defect Repair
4.1. Hybrid Hydrogels with Microorganism Origin Natural Antibacterial Agents
4.2. Hybrid Hydrogels with Plant Origin Natural Antibacterial Agents
4.3. Hybrid Hydrogels with Animal Origin Natural Antibacterial Agents
5. Hydrogels with the Inherent Antibacterial Ability for Bone Defect Repair
6. Summary and Challenges
| Category | Representative Agent | Antibacterial Mechanism | Effect on Bone Repair | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|
| Hydrogels with metal nanomaterials | AgNPs | Attach onto the cell wall and membrane, damage intracellular biomolecules and structures | Promote the expression and mineralization of osteogenic proteins, alter microRNA expression associated with bone formation | Broad-spectrum antimicrobial properties, stimulate bone growth | Long-term use produces multidrug-resistant bacteria and is difficult to biodegrade | [51,195] |
| Light-mediated inorganic antibacterial nanoparticle hybrid hydrogels | rGO | Mechanical breakage of the cell membrane results in intracellular substance leakage | Promote cell proliferation and differentiation | Do not elicit bacterial resistance | Low photothermal conversion efficiency, non-biodegradable nature | [196,197] |
| Hydrogels with organic antibacterial agent | Quaternary ammonium salts | Binding to the cell membrane, bacteria lysis | Promote more osteogenic differentiation | Can be used as a modification factor | Short-term functionality, environmental toxicity, rapid antimicrobial resistance, and skin penetration | [96,97,198] |
| Hydrogel with MOFs | ZIF-8 | Synergistic action, such as Zn2+ and ligand release, ROS production, photothermal effect | Activate the ERK pathway primarily, activates MAPK signaling eventually, and promotes the osteogenesis of rBMSCs | Can be used as carriers and have electrostatic interaction with negatively charged bacterial cells | Excess metal ions may be harmful to host tissues | [112,199] |
| Light-mediated organic antibacterial agent hybrid hydrogels | ICG | Combination of PTT and PDT to kill bacteria through ROS generation and thermal ablation | Increase ALP activity and enhanced mineralization of osteoblasts | Water-soluble, very low cytotoxicity | Rapid clearance from the body, instability in aqueous solutions, an photobleaching | [200,201,202,203,204,205] |
| Hydrogels with microorganisms origin natural antibacterial agents | Doxycycline | Interfere with prokaryotic protein synthesis at the ribosome level | Promote by low concentration, but inhibit by high concentration | Broad-spectrum antibacterial drug | Antibiotic-resistant bacteria, toxic to mammalian cells | [140,141,206] |
| Hydrogels with plant origin natural antibacterial agents | Cur | Target the bacterial DNA, protein, cell membrane, cell wall, and other biological components | Enhance osteoblast proliferation, and induce osteogenesis-related gene expression | Wide sources and good biodegradability | Poor solubility and bioavailability | [149,150,151,155] |
| Hydrogels with animal origin natural antibacterial agents | LL37 | Induce membrane rupture | Enhance proliferation, migration, and osteogenic differentiation of MSCs and block bone resorption | Broad-spectrum activity against | Insufficient antimicrobial activities or unstable antimicrobial activities | [176,207,208] |
| Hydrogels with inherent self-antibacterial ability | CS | Disrupt cytomembrane structure, cellular energy metabolism, and protein synthesis | Up-regulate genes associated with calcium binding and mineralization | Environmentally friendly agent and cytocompatibility | Limited bacterial activity against Gram-negative bacteria | [209,210] |
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lima, A.L.L.; Oliveira, P.R.; Carvalho, V.C.; Cimerman, S.; Savio, E.; Diretrizes Panamer Tratamiento, O. Recommendations for the treatment of osteomyelitis. Braz. J. Infect. Dis. 2014, 18, 526–534. [Google Scholar] [CrossRef] [Green Version]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef]
- Agarwal, R.; García, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Keating, J.F.; Simpson, A.H.; Robinson, C.M. The management of fractures with bone loss. J. Bone Jt. Surg. Br. Vol. 2005, 87, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.S.; Bemenderfer, T.B.; Wessel, A.R.; Kacena, M.A. A review of mouse critical size defect models in weight bearing bones. Bone 2013, 55, 241–247. [Google Scholar] [CrossRef] [Green Version]
- O’Keefe, R.J.; Mao, J. Bone tissue engineering and regeneration: From discovery to the clinic—An overview. Tissue Eng. Part B Rev. 2011, 17, 389–392. [Google Scholar] [CrossRef] [Green Version]
- Mountziaris, P.M.; Mikos, A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng. Part B Rev. 2008, 14, 179–186. [Google Scholar] [CrossRef]
- Waters, R.V.; Gamradt, S.C.; Asnis, P.; Vickery, B.H.; Avnur, Z.; Hill, E.; Bostrom, M. Systemic corticosteroids inhibit bone healing in a rabbit ulnar osteotomy model. Acta Orthop. Scand. 2000, 71, 316–321. [Google Scholar] [CrossRef]
- Toh, C.L.; Jupiter, J.B. The infected nonunion of the tibia. Clin. Orthop. Relat. Res. 1995, 315, 176–191. Available online: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=ppvovftb&NEWS=N&AN=00003086-199506000-00019 (accessed on 19 April 2022). [CrossRef]
- Patzakis, M.J.; Wilkins, J. Factors influencing infection rate in open fracture wounds. Clin. Orthop. Relat. Res. 1989, 243, 36–40. Available online: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=ppvovfta&NEWS=N&AN=00003086-198906000-00006 (accessed on 19 April 2022). [CrossRef]
- Patzakis, M.J.; Zalavras, C.G. Chronic posttraumatic osteomyelitis and infected nonunion of the tibia: Current management concepts. J. Am. Acad. Orthop. Surg. 2005, 13, 417–427. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Liu, Y.; Yan, L.; Zaat, S.A.J.; Wismeijer, D.; Pathak, J.L.; Wu, G. A dual functional bone-defect-filling material with sequential antibacterial and osteoinductive properties for infected bone defect repair. J. Biomed. Mater. Res. Part A 2019, 107, 2360–2370. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef]
- Arron, J.R.; Choi, Y. Bone versus immune system. Nature 2000, 408, 535–536. [Google Scholar] [CrossRef]
- Arboleya, L.; Castañeda, S. Osteoimmunology: The study of the relationship between the immune system and bone tissue. Reumatol. Clin. 2013, 9, 303–315. [Google Scholar] [CrossRef]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef]
- Walsh, M.C.; Kim, N.; Kadono, Y.; Rho, J.; Lee, S.Y.; Lorenzo, J.; Choi, Y. Osteoimmunology: Interplay between the immune system and bone metabolism. Annu. Rev. Immunol. 2006, 24, 33–63. [Google Scholar] [CrossRef] [Green Version]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.-h.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, R.; Kundu, B.; Nandi, S.K.; Basu, D. Systematic approach to treat chronic osteomyelitis through localized drug delivery system: Bench to bed side. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3986–3993. [Google Scholar] [CrossRef]
- Cheng, T.; Qu, H.; Zhang, G.; Zhang, X. Osteogenic and antibacterial properties of vancomycin-laden mesoporous bioglass/PLGA composite scaffolds for bone regeneration in infected bone defects. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1935–1947. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Liu, Y.; Guo, J.; Wu, H.; Wang, J.; Wu, G. Biomaterials with Antibacterial and Osteoinductive Properties to Repair Infected Bone Defects. Int. J. Mol. Sci. 2016, 17, 334. [Google Scholar] [CrossRef]
- Thomas, M.V.; Puleo, D.A. Infection, Inflammation, and Bone Regeneration: A Paradoxical Relationship. J. Dent. Res. 2011, 90, 1052–1061. [Google Scholar] [CrossRef]
- Soundrapandian, C.; Sa, B.; Datta, S. Organic–Inorganic Composites for Bone Drug Delivery. AAPS Pharm. Sci. Tech. 2009, 10, 1158–1171. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.N.; Cammisa, F.P., Jr.; Sandhu, H.S.; Diwan, A.D.; Girardi, F.P.; Lane, J.M. The biology of bone grafting. J. Am. Acad. Orthop. Surg. 2005, 13, 77–86. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, G.; Moghaddam, A. Allograft bone matrix versus synthetic bone graft substitutes. Injury 2011, 42 (Suppl. 2), S16–S21. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, Y.; Dong, Y.; Wu, Y.; Cheng, L.; Wang, Y.; Xing, M.; Yuan, Q. A Novel Nanosilver/Nanosilica Hydrogel for Bone Regeneration in Infected Bone Defects. ACS Appl. Mater. Interfaces 2016, 8, 13242–13250. [Google Scholar] [CrossRef]
- Wan, X.; Zhao, Y.; Li, Z.; Li, L. Emerging polymeric electrospun fibers: From structural diversity to application in flexible bioelectronics and tissue engineering. Exploration 2022, 2, 20210029. [Google Scholar] [CrossRef]
- Altay, G.; Tosi, S.; García-Díaz, M.; Martínez, E. Imaging the Cell Morphological Response to 3D Topography and Curvature in Engineered Intestinal Tissues. Front. Bioeng. Biotechnol. 2020, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial Hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermonden, T.; Klumperman, B. The past, present and future of hydrogels. Eur. Polym. J. 2015, 72, 341–343. [Google Scholar] [CrossRef]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.-Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Vermonden, T.; Hennink, W.E. Hydrogels for Therapeutic Delivery: Current Developments and Future Directions. Biomacromolecules 2017, 18, 316–330. [Google Scholar] [CrossRef]
- Liu, M.; Guo, R.; Ma, Y. Construction of a specific and efficient antibacterial agent against Pseudomonas aeruginosa based on polyethyleneimine cross-linked fucose. J. Mater. Sci. 2021, 56, 6083–6094. [Google Scholar] [CrossRef]
- Chung, Y.C.; Wang, H.L.; Chen, Y.M.; Li, S.L. Effect of abiotic factors on the antibacterial activity of chitosan against waterborne pathogens. Bioresour. Technol. 2003, 88, 179–184. [Google Scholar] [CrossRef]
- Mombelli, A.; Samaranayake, L.P. Topical and systemic antibiotics in the management of periodontal diseases. Int. Dent. J. 2004, 54, 3–14. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Mou, J.; Liu, Z.; Liu, J.; Lu, J.; Zhu, W.; Pei, D. Hydrogel containing minocycline and zinc oxide-loaded serum albumin nanopartical for periodontitis application: Preparation, characterization and evaluation. Drug Deliv. 2019, 26, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Gil, J.; Natesan, S.; Li, J.; Valdes, J.; Harding, A.; Solis, M.; Davis, S.C.; Christy, R.J. A PEGylated fibrin hydrogel-based antimicrobial wound dressing controls infection without impeding wound healing. Int. Wound J. 2017, 14, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Shultz, R.B. Hydrogel-based local drug delivery strategies for spinal cord repair. Neural Regen. Res. 2021, 16, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Dubey, A.K. Various Biomaterials and Techniques for Improving Antibacterial Response. ACS Appl. Bio Mater. 2018, 1, 3–20. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Shi, Y.; Song, H.; Yu, C. Antibiotic-Free Antibacterial Strategies Enabled by Nanomaterials: Progress and Perspectives. Adv. Mater. 2019, 32, e1904106. [Google Scholar] [CrossRef] [PubMed]
- Seil, J.T.; Webster, T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomed. 2012, 7, 2767–2781. [Google Scholar] [CrossRef] [Green Version]
- Babu, K.S.; Anandkumar, M.; Tsai, T.Y.; Kao, T.H.; Inbaraj, B.S.; Chen, B.H. Cytotoxicity and antibacterial activity of gold-supported cerium oxide nanoparticles. Int. J. Nanomed. 2014, 9, 5515–5531. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Kong, Y.; Kundu, S.; Cirillo, J.D.; Liang, H. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guérin. J. Nanobiotechnol. 2012, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Kruk, T.; Szczepanowicz, K.; Stefańska, J.; Socha, R.P.; Warszyński, P. Synthesis and antimicrobial activity of monodisperse copper nanoparticles. Colloids Surf. B Biointerfaces 2015, 128, 17–22. [Google Scholar] [CrossRef]
- Shen, J.; Karges, J.; Xiong, K.; Chen, Y.; Ji, L.; Chao, H. Cancer cell membrane camouflaged iridium complexes functionalized black-titanium nanoparticles for hierarchical-targeted synergistic NIR-II photothermal and sonodynamic therapy. Biomaterials 2021, 275, 120979. [Google Scholar] [CrossRef]
- Saidin, S.; Jumat, M.A.; Mohd Amin, N.A.A.; Saleh Al-Hammadi, A.S. Organic and inorganic antibacterial approaches in combating bacterial infection for biomedical application. Mater. Sci Eng. C Mater. Biol. Appl. 2021, 118, 111382. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Jang, J. Antibacterial Properties of Novel Poly(methyl methacrylate) Nanofiber Containing Silver Nanoparticles. Langmuir 2008, 24, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, M.; Li, Z.; Casciano, D.; Khodakovskaya, M.V.; Chen, T.; Karmakar, A.; Dervishi, E.; Xu, Y.; Mustafa, T.; Watanabe, F.; et al. Nanostructural materials increase mineralization in bone cells and affect gene expression through miRNA regulation. J. Cell. Mol. Med. 2010, 15, 2297–2306. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; He, J.; Wang, Z.; Bai, Z.; Qu, P.; Song, Z.; Wang, W. Fabrication of silver nanoparticles/gelatin hydrogel system for bone regeneration and fracture treatment. Drug Deliv. 2021, 28, 319–324. [Google Scholar] [CrossRef]
- Yang, X.; Wei, Q.; Shao, H.; Jiang, X. Multivalent Aminosaccharide-Based Gold Nanoparticles as Narrow-Spectrum Antibiotics in Vivo. ACS Appl. Mater. Interfaces 2019, 11, 7725–7730. [Google Scholar] [CrossRef]
- Liang, H.; Jin, C.; Ma, L.; Feng, X.; Deng, X.; Wu, S.; Liu, X.; Yang, C. Accelerated Bone Regeneration by Gold-Nanoparticle-Loaded Mesoporous Silica through Stimulating Immunomodulation. ACS Appl. Mater. Interfaces 2019, 11, 41758–41769. [Google Scholar] [CrossRef]
- Yi, C.; Liu, D.; Fong, C.C.; Zhang, J.; Yang, M. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano 2010, 4, 6439–6448. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.; Mao, H.; Zhang, Y.; Zheng, L.; Yu, P.; Guo, Z.; Li, L.; Jiang, Q. PEGylated gold nanoparticles promote osteogenic differentiation in in vitro and in vivo systems. Mater. Des. 2021, 197, 109231. [Google Scholar] [CrossRef]
- Lee, D.; Heo, D.N.; Nah, H.R.; Lee, S.J.; Ko, W.K.; Lee, J.S.; Moon, H.J.; Bang, J.B.; Hwang, Y.S.; Reis, R.L.; et al. Injectable hydrogel composite containing modified gold nanoparticles: Implication in bone tissue regeneration. Int. J. Nanomed. 2018, 13, 7019–7031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, J.; Zhang, P.; Hu, F.; Du, Y.; Yuan, H.; Zhu, J.; Wang, Z.; Zhou, J.; Li, C. Near-infrared light-sensitive liposomes for the enhanced photothermal tumor treatment by the combination with chemotherapy. Pharm. Res. 2014, 31, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.S.; El Zowalaty, M.E.; Shameli, K.; Zainuddin, N.; Salama, M.; Ibrahim, N.A. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int. J. Nanomed. 2013, 8, 4467–4479. [Google Scholar] [CrossRef] [Green Version]
- Dai, Q.; Li, Q.; Gao, H.; Yao, L.; Lin, Z.; Li, D.; Zhu, S.; Liu, C.; Yang, Z.; Wang, G.; et al. 3D printing of Cu-doped bioactive glass composite scaffolds promotes bone regeneration through activating the HIF-1α and TNF-α pathway of hUVECs. Biomater. Sci. 2021, 9, 5519–5532. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- Lin, J.; He, Z.; Liu, F.; Feng, J.; Huang, C.; Sun, X.; Deng, H. Hybrid Hydrogels for Synergistic Periodontal Antibacterial Treatment with Sustained Drug Release and NIR-Responsive Photothermal Effect. Int. J. Nanomed. 2020, 15, 5377–5387. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Liang, Y.; Li, Z.; Zhu, S.; Zheng, Y.; Yeung, K.W.K.; et al. Zinc-doped Prussian blue enhances photothermal clearance of Staphylococcus aureus and promotes tissue repair in infected wounds. Nat. Commun. 2019, 10, 4490. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J. Control. Release Off. J. Control. Release Soc. 2020, 328, 251–262. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, Y.; Meng, S.; Yang, B.; Pang, L.; Wang, C.; Liu, T. Mechanism and In Vivo Evaluation: Photodynamic Antibacterial Chemotherapy of Lysine-Porphyrin Conjugate. Front. Microbiol. 2016, 7, 242. [Google Scholar] [CrossRef]
- Geng, B.; Li, P.; Fang, F.; Shi, W.; Glowacki, J.; Pan, D.; Shen, L. Antibacterial and osteogenic carbon quantum dots for regeneration of bone defects infected with multidrug-resistant bacteria. Carbon 2021, 184, 375–385. [Google Scholar] [CrossRef]
- Bianco, A.; Cheng, H.-M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family—A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Prasadh, S.; Suresh, S.; Wong, R. Osteogenic Potential of Graphene in Bone Tissue Engineering Scaffolds. Materials 2018, 11, 1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Guo, W.; Li, L.; Yu, F.; Li, J.; Liu, L.; Fang, B.; Xia, L. Photothermally triggered biomimetic drug delivery of Teriparatide via reduced graphene oxide loaded chitosan hydrogel for osteoporotic bone regeneration. Chem. Eng. J. 2020, 413, 127413. [Google Scholar] [CrossRef]
- Li, Y.; He, J.; Zhou, J.; Li, Z.; Liu, L.; Hu, S.; Guo, B.; Wang, W. A conductive photothermal non-swelling nanocomposite hydrogel patch accelerating bone defect repair. Biomater. Sci. 2022, 10, 1326–1341. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Wang, Q.; Zhang, D.; Zhang, Y.; Mu, X.; Kaplan, D.L.; Buehler, M.J. Integration of stiff graphene and tough silk for the design and fabrication of versatile electronic materials. Adv. Funct. Mater. 2018, 28, 1705291. [Google Scholar] [CrossRef]
- Yang, B.; Yin, J.; Chen, Y.; Pan, S.; Yao, H.; Gao, Y.; Shi, J. 2D-Black-Phosphorus-Reinforced 3D-Printed Scaffolds:A Stepwise Countermeasure for Osteosarcoma. Adv. Mater. 2018, 30, 1705611. [Google Scholar] [CrossRef]
- Dadsetan, M.; Giuliani, M.; Wanivenhaus, F.; Brett Runge, M.; Charlesworth, J.E.; Yaszemski, M.J. Incorporation of phosphate group modulates bone cell attachment and differentiation on oligo(polyethylene glycol) fumarate hydrogel. Acta Biomater. 2012, 8, 1430–1439. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.; Shi, X.; Li, Q.; Hao, L.; Liu, L.; Liu, X.; Chen, Y.; Wang, Y. Engineering natural matrices with black phosphorus nanosheets to generate multi-functional therapeutic nanocomposite hydrogels. Biomater. Sci. 2019, 7, 4046–4059. [Google Scholar] [CrossRef]
- Tan, L.; Li, J.; Liu, X.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Yuan, X.; Zheng, Y.; Yeung, K.W.K.; et al. Rapid Biofilm Eradication on Bone Implants Using Red Phosphorus and Near-Infrared Light. Adv. Mater. 2018, 30, 1801808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, X.; Yang, Y.; Chi, R.; Shi, J.; Hang, R.; Huang, X.; Yao, X.; Chu, P.K.; Zhang, X. Dual light-induced in situ antibacterial activities of biocompatibleTiO(2)/MoS(2)/PDA/RGD nanorod arrays on titanium. Biomater. Sci. 2020, 8, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Pu, K. Organic Semiconducting Agents for Deep-Tissue Molecular Imaging: Second Near-Infrared Fluorescence, Self-Luminescence, and Photoacoustics. Adv. Mater. 2018, 30, e1801778. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Pu, K. Molecular Fluorescence and Photoacoustic Imaging in the Second Near-Infrared Optical Window Using Organic Contrast Agents. Adv. Biosyst. 2018, 2, 1700262. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Antaris, A.L.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 10. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Y.; Shi, J.; Yao, X.; Chen, W.; Wei, X.; Zhang, X.; Chu, P.K. Near-infrared light II—Assisted rapid biofilm elimination platform for bone implants at mild temperature. Biomaterials 2021, 269, 120634. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, Z.; Yang, Y.; Shi, J.; Lv, J.; Fang, Y.; Shen, Z.; Lv, Z.; Li, P.; Yao, X.; et al. A multifunctional antibacterial coating on bone implants for osteosarcoma therapy and enhanced osteointegration. Chem. Eng. J. 2022, 428, 131155. [Google Scholar] [CrossRef]
- Hu, B.; Berkey, C.; Feliciano, T.; Chen, X.; Li, Z.; Chen, C.; Amini, S.; Nai, M.H.; Lei, Q.L.; Ni, R.; et al. Thermal-Disrupting Interface Mitigates Intercellular Cohesion Loss for Accurate Topical Antibacterial Therapy. Adv. Mater. 2020, 32, e1907030. [Google Scholar] [CrossRef]
- Xu, X.; An, H.; Zhang, D.; Tao, H.; Dou, Y.; Li, X.; Huang, J.; Zhang, J. A self-illuminating nanoparticle for inflammation imaging and cancer therapy. Sci. Adv. 2019, 5, eaat2953. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Li, S.; Luo, J.; Wang, X. Latest Advances on Bacterial Cellulose-Based Antibacterial Materials as Wound Dressings. Front. Bioeng. Biotechnol. 2020, 8, 593768. [Google Scholar] [CrossRef]
- Akca, A.E.; Akca, G.; Topçu, F.T.; Macit, E.; Pikdöken, L.; Özgen, I.Ş. The Comparative Evaluation of the Antimicrobial Effect of Propolis with Chlorhexidine against Oral Pathogens: An In Vitro Study. BioMed Res. Int. 2016, 2016, 3627463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, T.; Guo, Q.; Shen, X. Polyethyleneimine and quaternized ammonium polyethyleneimine: The versatile materials for combating bacteria and biofilms. J. Biomater. Sci. Polym. Ed. 2019, 30, 1243–1259. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Forghani, F.; Kong, X.; Liu, D.; Ye, X.; Chen, S.; Ding, T. Antibacterial applications of metal–organic frameworks and their composites. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1397–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hismiogullari, S.E.; Hismiogullari, A.A.; Sahin, F.; Oner, E.T.; Yenice, S.; Karasartova, D. Investigation of Antibacterial and Cytotoxic Effects of Organic Acids Including Ascorbic Acid, Lactic Acid and Acetic Acids on Mammalian Cells. J. Anim. Vet. Adv. 2008, 7, 681–684. Available online: https://medwelljournals.com/abstract/?doi=javaa.2008.681.684 (accessed on 19 April 2022).
- Weber, D.J.; Rutala, W.A.; Sickbert-Bennett, E.E. Outbreaks associated with contaminated antiseptics and disinfectants. Antimicrob. Agents Chemother. 2007, 51, 4217–4224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenoir, S.; Pagnoulle, C.; Galleni, M.; Compère, P.; Jérôme, R.; Detrembleur, C. Polyolefin Matrixes with Permanent Antibacterial Activity: Preparation, Antibacterial Activity, and Action Mode of the Active Species. Biomacromolecules 2006, 7, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liuyang, R.; Dong, C.; Lei, Y.; Zhang, A.; Lin, Y. Polymeric quaternary ammonium salt activity against Fusarium oxysporum f. sp. cubense race 4: Synthesis, structure-activity relationship and mode of action. React. Funct. Polym. 2017, 114, 13–22. [Google Scholar] [CrossRef]
- Lin, M.C.; Chen, C.C.; Wu, I.T.; Ding, S.J. Enhanced antibacterial activity of calcium silicate-based hybrid cements for bone repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110727. [Google Scholar] [CrossRef]
- McClure, J.-A.; Zaal DeLongchamp, J.; Conly, J.M.; Zhang, K. Novel Multiplex PCR Assay for Detection of Chlorhexidine-Quaternary Ammonium, Mupirocin, and Methicillin Resistance Genes, with Simultaneous Discrimination of Staphylococcus aureus from Coagulase-Negative Staphylococci. J. Clin. Microbiol. 2017, 55, 1857–1864. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Wang, D. Novel technologies for the prevention and treatment of dental caries: A patent survey. Expert Opin. Ther. Pat. 2010, 20, 681–694. [Google Scholar] [CrossRef] [Green Version]
- Barbour, M.E.; Gandhi, N.; el-Turki, A.; O’Sullivan, D.J.; Jagger, D.C. Differential adhesion of Streptococcus gordonii to anatase and rutile titanium dioxide surfaces with and without functionalization with chlorhexidine. J. Biomed. Mater. Res. Part A 2009, 90, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Ye, Q.; Xie, J.; Yang, J.; Jiang, W.; Yuan, H.; Li, J. An injectable gellan gum-based hydrogel that inhibits Staphylococcus aureus for infected bone defect repair. J. Mater. Chem. B 2022, 10, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Alavijeh, R.K.; Beheshti, S.; Akhbari, K.; Morsali, A. Investigation of reasons for metal-organic framework’s antibacterial activities. Polyhedron 2018, 156, 257–278. [Google Scholar] [CrossRef]
- Zane, A.; Zuo, R.; Villamena, F.A.; Rockenbauer, A.; Digeorge Foushee, A.M.; Flores, K.; Dutta, P.K.; Nagy, A. Biocompatibility and antibacterial activity of nitrogen-doped titanium dioxide nanoparticles for use in dental resin formulations. Int. J. Nanomed. 2016, 11, 6459–6470. [Google Scholar] [CrossRef] [Green Version]
- Tsai, D.-S.; Yang, T.-S.; Huang, Y.-S.; Peng, P.-W.; Ou, K.-L. Disinfection effects of undoped and silver-doped ceria powders of nanometer crystallite size. Int. J. Nanomed. 2016, 11, 2531–2542. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Yang, Y. Metal–Organic Frameworks for Biomedical Applications. Small 2020, 16, e1906846. [Google Scholar] [CrossRef]
- Restrepo, J.; Serroukh, Z.; Santiago-Morales, J.; Aguado, S.; Gómez-Sal, P.; Mosquera, M.E.G.; Rosal, R. An Antibacterial Zn–MOF with Hydrazinebenzoate Linkers. Eur. J. Inorg. Chem. 2016, 2017, 574–580. [Google Scholar] [CrossRef]
- Tamames-Tabar, C.; Imbuluzqueta, E.; Guillou, N.; Serre, C.; Miller, S.R.; Elkaim, E.; Horcajada, P.; Blanco-Prieto, M.J. A Zn azelate MOF: Combining antibacterial effect. Crystengcomm 2015, 17, 456–462. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, S.; Liu, Y.; Gao, X.; Hu, S.; Zhang, X.; Huang, C.; Wan, Q.; Wang, J.; Pei, X. Micro or nano: Evaluation of biosafety and biopotency of magnesium metal organic framework-74 with different particle sizes. Nano Res. 2020, 13, 511–526. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nyström, A.M.; Zou, X. One-pot Synthesis of Metal-Organic Frameworks with Encapsulated Target Molecules and Their Applications for Controlled Drug Delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.T.; Shiu, B.C.; Lin, J.H.; Lou, C.W. Two methods for constructing ZIF-8 nanomaterials with good bio compatibility and robust antibacterial applied to biomedical. J. Biomater. Appl. 2022, 36, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xue, Y.; Zhu, Z.; Chen, J.; Liu, Y.; Cheng, X.; Zhang, X.; Wang, J.; Pei, X.; Wan, Q. Nanoscale Zeolitic Imidazolate Framework-8 Activator of Canonical MAPK Signaling for Bone Repair. ACS Appl. Mater. Interfaces 2021, 13, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Z.; Pei, X.; Zhang, X.; Cheng, X.; Hu, S.; Gao, X.; Wang, J.; Chen, J.; Wan, Q. ZIF-8-Modified Multifunctional Bone-Adhesive Hydrogels Promoting Angiogenesis and Osteogenesis for Bone Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 36978–36995. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sonshine, D.A.; Shervani, S.; Hurt, R.H. Controlled Release of Biologically Active Silver from Nanosilver Surfaces. ACS Nano 2010, 4, 6903–6913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.; Chen, Y.; Guo, B.; Wang, Y.; Liu, W.; Sun, J.; Wang, X. Magnesium-organic framework-based stimuli-responsive systems that optimize the bone microenvironment for enhanced bone regeneration. Chem. Eng. J. 2020, 396, 125241. [Google Scholar] [CrossRef]
- Soomro, N.A.; Wu, Q.; Amur, S.A.; Liang, H.; Ur Rahman, A.; Yuan, Q.; Wei, Y. Natural drug physcion encapsulated zeolitic imidazolate framework, and their application as antimicrobial agent. Colloids Surf. B Biointerfaces 2019, 182, 110364. [Google Scholar] [CrossRef]
- Huang, G.; Li, Y.; Qin, Z.; Liang, Q.; Xu, C.; Lin, B. Hybridization of carboxymethyl chitosan with MOFs to construct recyclable, long-acting and intelligent antibacterial agent carrier. Carbohydr. Polym. 2020, 233, 115848. [Google Scholar] [CrossRef]
- Li, J.; Rao, J.; Pu, K. Recent progress on semiconducting polymer nanoparticles for molecular imaging and cancer phototherapy. Biomaterials 2018, 155, 217–235. [Google Scholar] [CrossRef]
- Li, J.; Pu, K. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem. Soc. Rev. 2019, 48, 38–71. [Google Scholar] [CrossRef]
- Lu, R.; Zhu, J.; Yu, C.; Nie, Z.; Gao, Y. Cu(3)BiS(3) Nanocrystals as Efficient Nanoplatforms for CT Imaging Guided Photothermal Therapy of Arterial Inflammation. Front. Bioeng. Biotechnol. 2020, 8, 981. [Google Scholar] [CrossRef]
- Kuang, L.J.; Huang, J.H.; Liu, Y.T.; Li, X.L.; Yuan, Y.; Liu, C.S. Injectable Hydrogel with NIR Light-Responsive, Dual-Mode PTH Release for Osteoregeneration in Osteoporosis. Adv. Funct. Mater. 2021, 31, 2105383. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wu, J.; Jia, Z.; Tang, P.; Sheng, J.; Xie, C.; Liu, C.; Gan, D.; Hu, D.; Zheng, W.; et al. An Injectable, Bifunctional Hydrogel with Photothermal Effects for Tumor Therapy and Bone Regeneration. Macromol. Biosci. 2019, 19, e1900047. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.Y.; Zou, Q.X.; Zou, W.W.; Xie, Z.Z.; Li, Z.H.; Zhao, X.J.; Du, C. Bifunctional scaffolds of hydroxyapatite/poly(dopamine)/carboxymethyl chitosan with osteogenesis and anti-osteosarcoma effect. Biomater. Sci. 2021, 9, 3319–3333. [Google Scholar] [CrossRef]
- Lü, B.; Chen, Y.; Li, P.; Wang, B.; Müllen, K.; Yin, M. Stable radical anions generated from a porous perylenediimide metal-organic framework for boosting near-infrared photothermal conversion. Nat. Commun. 2019, 10, 767. [Google Scholar] [CrossRef]
- Eirich, J.; Orth, R.; Sieber, S.A. Unraveling the Protein Targets of Vancomycin in Living S. aureus and E. faecalis Cells. J. Am. Chem. Soc. 2011, 133, 12144–12153. [Google Scholar] [CrossRef]
- King, A.M.; Reid-Yu, S.A.; Wang, W.; King, D.T.; De Pascale, G.; Strynadka, N.C.; Walsh, T.R.; Coombes, B.K.; Wright, G.D. Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature 2014, 510, 503–506. [Google Scholar] [CrossRef] [Green Version]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Clardy, J.; Fischbach, M.A.; Walsh, C.T. New antibiotics from bacterial natural products. Nat. Biotechnol. 2006, 24, 1541–1550. [Google Scholar] [CrossRef]
- Genilloud, O. Actinomycetes: Still a source of novel antibiotics. Nat. Prod. Rep. 2017, 34, 1203–1232. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Aoki, T.; Pellegrini, A. Strategies for new antimicrobial proteins and peptides: Lysozyme and aprotinin as model molecules. Curr. Pharm. Des. 2002, 8, 671–693. [Google Scholar] [CrossRef] [PubMed]
- Habermann, E. Bee and wasp venoms. Science 1972, 177, 314–322. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Lichtenstein, A.K.; Ganz, T. Defensins: Antimicrobial and cytotoxic peptides of mammalian cells. Annu. Rev. Immunol. 1993, 11, 105–128. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [Green Version]
- Diamond, G.; Beckloff, N.; Weinberg, A.; Kisich, K.O. The roles of antimicrobial peptides in innate host defense. Curr. Pharm. Des. 2009, 15, 2377–2392. [Google Scholar] [CrossRef] [Green Version]
- Rudramurthy, G.R.; Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Nanoparticles: Alternatives Against Drug-Resistant Pathogenic Microbes. Molecules 2016, 21, 836. [Google Scholar] [CrossRef]
- Wei, S.; Jian, C.; Xu, F.; Bao, T.; Lan, S.; Wu, G.; Qi, B.; Bai, Z.; Yu, A. Vancomycin-impregnated electrospun polycaprolactone (PCL) membrane for the treatment of infected bone defects: An animal study. J. Biomater. Appl. 2018, 32, 1187–1196. [Google Scholar] [CrossRef]
- Giavaresi, G.; Bertazzoni Minelli, E.; Sartori, M.; Benini, A.; Della Bora, T.; Sambri, V.; Gaibani, P.; Borsari, V.; Salamanna, F.; Martini, L.; et al. Microbiological and pharmacological tests on new antibiotic-loaded PMMA-based composites for the treatment of osteomyelitis. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2012, 30, 348–355. [Google Scholar] [CrossRef]
- Feng, K.; Sun, H.; Bradley, M.A.; Dupler, E.J.; Giannobile, W.V.; Ma, P.X. Novel antibacterial nanofibrous PLLA scaffolds. J. Control. Release Off. J. Control. Release Soc. 2010, 146, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-B. Low dose of doxycyline promotes early differentiation of preosteoblasts by partially regulating the expression of estrogen receptors. J. Surg. Res. 2012, 178, 737–742. [Google Scholar] [CrossRef]
- Park, J.-B. Effects of Doxycycline, Minocycline, and Tetracycline on Cell Proliferation, Differentiation, and Protein Expression in Osteoprecursor Cells. J. Craniofacial Surg. 2011, 22, 1839–1842. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.W.; Oh, S.H.; Lee, I.S.; Byun, J.H.; Lee, J.H. In Situ Gelling Hydrogel with Anti-Bacterial Activity and Bone Healing Property for Treatment of Osteomyelitis. Tissue Eng. Regen. Med. 2019, 16, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-M.; Chen, W.-C.; Ko, C.-L.; Chang, H.-T.; Chen, Y.-S.; Haung, S.-M.; Chang, K.-C.; Chen, J.-C. In Vitro Evaluation of Calcium Phosphate Bone Cement Composite Hydrogel Beads of Cross-Linked Gelatin-Alginate with Gentamicin-Impregnated Porous Scaffold. Pharmaceuticals 2021, 14, 1000. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, Y.; Ren, L.; Huang, W.; Wang, D.A. A protein/antibiotic releasing poly(lactic-co-glycolic acid)/lecithin scaffold for bone repair applications. Int. J. Pharm. 2009, 373, 85–92. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.A.; Nair, S.P. Interaction of staphylococci with bone. Int. J. Med. Microbiol. 2010, 300, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Kalghatgi, S.; Spina Catherine, S.; Costello James, C.; Liesa, M.; Morones-Ramirez, J.R.; Slomovic, S.; Molina, A.; Shirihai Orian, S.; Collins James, J. Bactericidal Antibiotics Induce Mitochondrial Dysfunction and Oxidative Damage in Mammalian Cells. Sci. Transl. Med. 2013, 5, 192ra185. [Google Scholar] [CrossRef] [Green Version]
- Reddy, R.C.; Vatsala, P.G.; Keshamouni, V.G.; Padmanaban, G.; Rangarajan, P.N. Curcumin for malaria therapy. Biochem. Biophys. Res. Commun. 2005, 326, 472–474. [Google Scholar] [CrossRef]
- Kant, V.; Gopal, A.; Pathak, N.N.; Kumar, P.; Tandan, S.K.; Kumar, D. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int. Immunopharmacol. 2014, 20, 322–330. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, K.; Zhu, Y.; Wang, D.; Shao, Y.; Zhang, J. Curcumin inhibits hypoxia-induced proliferation and invasion of MG-63 osteosarcoma cells via downregulating Notch1. Mol. Med. Rep. 2017, 15, 1747–1752. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Wang, H.; Yang, F.; Chen, H.; He, W.; Wang, J. Curcumin Promotes Osteosarcoma Cell Death by Activating miR-125a/ERRα Signal Pathway. J. Cell. Biochem. 2016, 118, 74–81. [Google Scholar] [CrossRef]
- Hussain, Y.; Alam, W.; Ullah, H.; Dacrema, M.; Daglia, M.; Khan, H.; Arciola, C.R. Antimicrobial Potential of Curcumin: Therapeutic Potential and Challenges to Clinical Applications. Antibiotics 2022, 11, 322. [Google Scholar] [CrossRef]
- Morão, L.G.; Polaquini, C.R.; Kopacz, M.; Torrezan, G.S.; Ayusso, G.M.; Dilarri, G.; Cavalca, L.B.; Zielińska, A.; Scheffers, D.J.; Regasini, L.O.; et al. A simplified curcumin targets the membrane of Bacillus subtilis. MicrobiologyOpen 2019, 8, e00683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, S.; Modi, N.H.; Panda, D.; Roy, N. Probing the binding site of curcumin in Escherichia coli and Bacillus subtilis FtsZ--a structural insight to unveil antibacterial activity of curcumin. Eur. J. Med. Chem. 2010, 45, 4209–4214. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huang, L. Antibacterial Mechanism of Curcumin: A Review. Chem. Biodivers. 2020, 17, e2000171. [Google Scholar] [CrossRef] [PubMed]
- Leite, D.P.; Paolillo, F.R.; Parmesano, T.N.; Fontana, C.R.; Bagnato, V.S. Effects of photodynamic therapy with blue light and curcumin as mouth rinse for oral disinfection: A randomized controlled trial. Photomed. Laser Surg. 2014, 32, 627–632. [Google Scholar] [CrossRef] [Green Version]
- Koon, H.K.; Leung, A.W.N.; Yue, K.; Mak, N.K. Photodynamic Effect of Curcumin on NPC/CNE2 Cells. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 205–216. [Google Scholar] [CrossRef]
- Han, S.; Yang, Y. Antimicrobial activity of wool fabric treated with curcumin. Dyes Pigment. 2005, 64, 157–161. [Google Scholar] [CrossRef]
- Yu, Q.; Meng, Z.; Liu, Y.; Li, Z.; Sun, X.; Zhao, Z. Photocuring Hyaluronic Acid/Silk Fibroin Hydrogel Containing Curcumin Loaded CHITOSAN Nanoparticles for the Treatment of MG-63 Cells and ME3T3-E1 Cells. Polymers 2021, 13, 2302. [Google Scholar] [CrossRef]
- Virk, R.S.; Rehman, M.A.U.; Munawar, M.A.; Schubert, D.W.; Goldmann, W.H.; Dusza, J.; Boccaccini, A.R. Curcumin-Containing Orthopedic Implant Coatings Deposited on Poly-Ether-Ether-Ketone/Bioactive Glass/Hexagonal Boron Nitride Layers by Electrophoretic Deposition. Coatings 2019, 9, 572. [Google Scholar] [CrossRef] [Green Version]
- Martín-Moreno, A.M.; Reigada, D.; Ramírez, B.G.; Mechoulam, R.; Innamorato, N.; Cuadrado, A.; de Ceballos, M.L. Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: Relevance to Alzheimer’s disease. Mol. Pharmacol. 2011, 79, 964–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Cervantes, R.; Méndez-Díaz, M.; Prospéro-García, Ó.; Morales-Montor, J. Immunoregulatory Role of Cannabinoids during Infectious Disease. Neuroimmunomodulation 2017, 24, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Bab, I.; Ofek, O.; Tam, J.; Rehnelt, J.; Zimmer, A. Endocannabinoids and the Regulation of Bone Metabolism. J. Neuroendocr. 2008, 20, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Schmuhl, E.; Ramer, R.; Salamon, A.; Peters, K.; Hinz, B.J.B.p. Increase of mesenchymal stem cell migration by cannabidiol via activation of p42/44 MAPK. Biochem. Pharmacol. 2014, 87, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zheng, Z.; Hu, L.; Wang, H.; Tang, B.; Lin, L. Development and characterization of cannabidiol-loaded alginate copper hydrogel for repairing open bone defects in vitro. Colloids Surf. B Biointerfaces 2022, 212, 112339. [Google Scholar] [CrossRef] [PubMed]
- Izadpanah, A.; Gallo, R.L. Antimicrobial peptides. J. Am. Acad. Dermatol. 2005, 52, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Hilchie, A.L.; Wuerth, K.; Hancock, R.E. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem. Biol. 2013, 9, 761–768. [Google Scholar] [CrossRef]
- De Smet, K.; Contreras, R. Human antimicrobial peptides: Defensins, cathelicidins and histatins. Biotechnol. Lett. 2005, 27, 1337–1347. [Google Scholar] [CrossRef]
- Lee, J.; Kang, D.; Choi, J.; Huang, W.; Wadman, M.; Barron, A.E.; Seo, J. Effect of side chain hydrophobicity and cationic charge on antimicrobial activity and cytotoxicity of helical peptoids. Bioorganic. Med. Chem. Lett. 2018, 28, 170–173. [Google Scholar] [CrossRef]
- Jantaruk, P.; Roytrakul, S.; Sitthisak, S.; Kunthalert, D. Potential role of an antimicrobial peptide, KLK in inhibiting lipopolysaccharide-induced macrophage inflammation. PLoS ONE 2017, 12, e0183852. [Google Scholar] [CrossRef] [Green Version]
- Kazemzadeh-Narbat, M.; Kindrachuk, J.; Duan, K.; Jenssen, H.; Hancock, R.E.; Wang, R. Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections. Biomaterials 2010, 31, 9519–9526. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Huang, T.; Wang, Y.; Wang, H.; Li, Y.; Yu, K.; Dong, L. Sustained Release of Antimicrobial Peptide from Self-Assembling Hydrogel Enhanced Osteogenesis. J. Biomater. Sci. Polym. Ed. 2018, 29, 1812–1824. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yue, K.; Kazemzadeh-Narbat, M.; Liu, Y.; Khalilpour, A.; Li, B.; Zhang, Y.S.; Annabi, N.; Khademhosseini, A. Mussel-Inspired Multifunctional Hydrogel Coating for Prevention of Infections and Enhanced Osteogenesis. ACS Appl. Mater. Interfaces 2017, 9, 11428–11439. [Google Scholar] [CrossRef] [Green Version]
- Sani, E.S.; Lara, R.P.; Aldawood, Z.; Bassir, S.H.; Nguyen, D.; Kantarci, A.; Intini, G.; Annabi, N. An Antimicrobial Dental Light Curable Bioadhesive Hydrogel for Treatment of Peri-Implant Diseases. Matter 2019, 1, 926–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Quan, J.; Long, W.; Chen, H.; Wang, R.; Guo, J.; Lin, X.; Mai, S. LL-37 inhibits LPS-induced inflammation and stimulates the osteogenic differentiation of BMSCs via P2X7 receptor and MAPK signaling pathway. Exp. Cell Res. 2018, 372, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lu, F.; Zhang, G.; Liu, Z. Overview of signal transduction between LL37 and bone marrow-derived MSCs. Histochem. J. 2022, 1–9. [Google Scholar] [CrossRef]
- Liu, P.; Li, M.; Yu, H.; Fang, H.; Yin, J.; Zhu, D.; Yang, Q.; Ke, Q.; Huang, Y.; Guo, Y.; et al. Biphasic CK2.1-coated β-glycerophosphate chitosan/LL37-modified layered double hydroxide chitosan composite scaffolds enhance coordinated hyaline cartilage and subchondral bone regeneration. Chem. Eng. J. 2021, 418, 129531. [Google Scholar] [CrossRef]
- Raafat, D.; von Bargen, K.; Haas, A.; Sahl, H.G. Insights into the mode of action of chitosan as an antibacterial compound. Appl Env. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef] [Green Version]
- Nair, L.S.; Laurencin, C.T. Polymers as biomaterials for tissue engineering and controlled drug delivery. Adv. Biochem. Eng. Biotechnol. 2006, 102, 47–90. [Google Scholar] [CrossRef]
- Mathews, S.; Gupta, P.K.; Bhonde, R.; Totey, S. Chitosan enhances mineralization during osteoblast differentiation of human bone marrow-derived mesenchymal stem cells, by upregulating the associated genes. Cell Prolif. 2011, 44, 537–549. [Google Scholar] [CrossRef]
- Shi, Z.; Neoh, K.G.; Kang, E.T.; Poh, C.; Wang, W. Bacterial adhesion and osteoblast function on titanium with surface-grafted chitosan and immobilized RGD peptide. J. Biomed. Mater. Res. Part A 2008, 86, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Chen, M.; Tian, J.; Zhang, Y.; Dai, Z.; Li, J.; Zhang, W. Oxygen-Carrying and Antibacterial Fluorinated Nano-hydroxyapatite Incorporated Hydrogels for Enhanced Bone Regeneration. Adv. Healthc. Mater. 2022, e2102540. [Google Scholar] [CrossRef]
- Xu, K.J.; Dai, Q.Y.; Dong, K.Q.; Wei, N.S.; Qin, Z.Y. Double noncovalent network chitosan/hyperbranched polyethylenimine/Fe3+ films with high toughness and good antibacterial activity. RSC Adv. 2022, 12, 5255–5264. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Chen, T.; Riffon, R.; Wang, R.; Wang, Z. Synergy between polyethylenimine and different families of antibiotics against a resistant clinical isolate of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2008, 52, 1635–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, M.M.; Ramalho, P.; Silva, A.P.; Teixeira-Santos, R.; Pina-Vaz, C.; Rodrigues, A.G. Polyethyleneimine and polyethyleneimine-based nanoparticles: Novel bacterial and yeast biofilm inhibitors. J. Med. Microbiol. 2014, 63, 1167–1173. [Google Scholar] [CrossRef]
- Haldar, J.; An, D.; Alvarez de Cienfuegos, L.; Chen, J.; Klibanov, A.M. Polymeric coatings that inactivate both influenza virus and pathogenic bacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 17667–17671. [Google Scholar] [CrossRef] [Green Version]
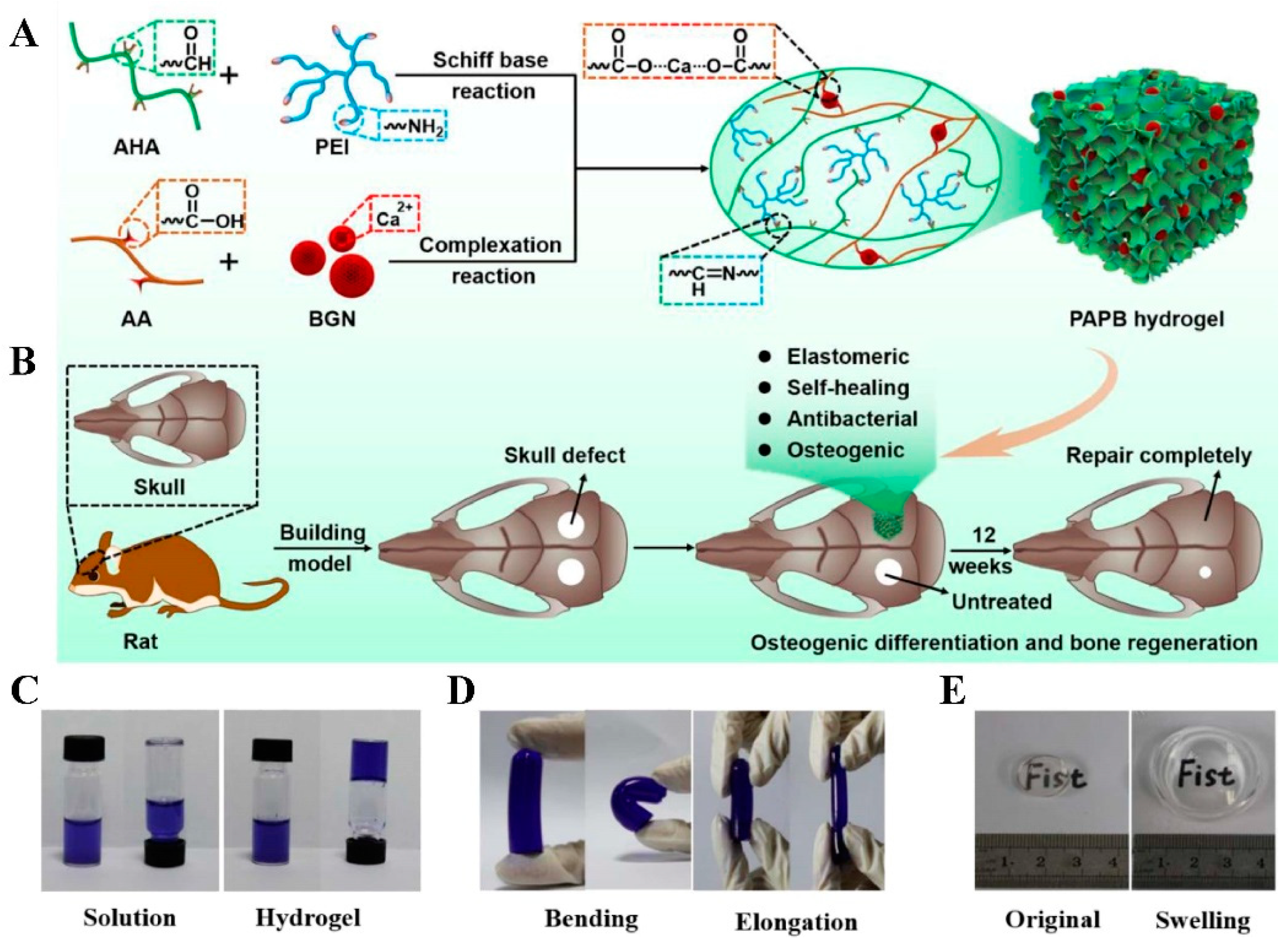
- Li, Y.; Ge, J.; Luo, M.; Niu, W.; Ling, X.; Xu, K.; Lin, C.; Lei, B.; Zhang, X. Elastomeric self-healing antibacterial bioactive nanocomposites scaffolds for treating skull defect. Appl. Mater. Today 2022, 26, 101254. [Google Scholar] [CrossRef]
- Kundu, B.; Soundrapandian, C.; Nandi, S.K.; Mukherjee, P.; Dandapat, N.; Roy, S.; Datta, B.K.; Mandal, T.K.; Basu, D.; Bhattacharya, R.N. Development of New Localized Drug Delivery System Based on Ceftriaxone-Sulbactam Composite Drug Impregnated Porous Hydroxyapatite: A Systematic Approach for In Vitro and In Vivo Animal Trial. Pharm. Res. 2010, 27, 1659–1676. [Google Scholar] [CrossRef]
- Wenke, J.C.; Guelcher, S.A. Dual delivery of an antibiotic and a growth factor addresses both the microbiological and biological challenges of contaminated bone fractures. Expert Opin. Drug Deliv. 2011, 8, 1555–1569. [Google Scholar] [CrossRef]
- Qayoom, I.; Teotia, A.K.; Panjla, A.; Verma, S.; Kumar, A. Local and Sustained Delivery of Rifampicin from a Bioactive Ceramic Carrier Treats Bone Infection in Rat Tibia. ACS Infect. Dis. 2020, 6, 2938–2949. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Mei, L.; Zhu, S.; Yin, W.; Chen, C.; Nie, G.; Gu, Z.; Zhao, Y. Two-dimensional nanomaterials beyond graphene for antibacterial applications: Current progress and future perspectives. Theranostics 2020, 10, 757–781. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Yang, G.; Wang, Y.; Jiang, H.; Fu, Y.; Yue, G.; Ju, R. Phototherapy-based combination strategies for bacterial infection treatment. Theranostics 2020, 10, 12241–12262. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, Z.; Joseph, J.; Zhang, X.; Ferdows, B.E.; Patel, D.N.; Chen, W.; Banfi, G.; Molinaro, R.; Cosco, D.; et al. Biomaterials and nanomedicine for bone regeneration: Progress and future prospects. Exploration 2021, 1, 20210011. [Google Scholar] [CrossRef]
- He, W.; Zheng, Y.; Feng, Q.; Elkhooly, T.A.; Liu, X.; Yang, X.; Wang, Y.; Xie, Y. Silver nanoparticles stimulate osteogenesis of human mesenchymal stem cells through activation of autophagy. Nanomedicine 2020, 15, 337–353. [Google Scholar] [CrossRef]
- Choudhary, P.; Parandhaman, T.; Ramalingam, B.; Duraipandy, N.; Kiran, M.S.; Das, S.K. Fabrication of Nontoxic Reduced Graphene Oxide Protein Nanoframework as Sustained Antimicrobial Coating for Biomedical Application. ACS Appl. Mater. Interfaces 2017, 9, 38255–38269. [Google Scholar] [CrossRef]
- Jodati, H.; Yilmaz, B.; Evis, Z. In vitro and in vivo properties of graphene-incorporated scaffolds for bone defect repair. Ceram. Int. 2021, 47, 29535–29549. [Google Scholar] [CrossRef]
- Lei, Y.F.; Zhou, S.W.; Dong, C.Y.; Zhang, A.Q.; Lin, Y.L. PDMS tri-block copolymers bearing quaternary ammonium salts for epidermal antimicrobial agents: Synthesis, surface adsorption and non-skin penetration. React. Funct. Polym. 2018, 124, 20–28. [Google Scholar] [CrossRef]
- Li, P.; Li, J.; Feng, X.; Li, J.; Hao, Y.; Zhang, J.; Wang, H.; Yin, A.; Zhou, J.; Ma, X.; et al. Metal-organic frameworks with photocatalytic bactericidal activity for integrated air cleaning. Nat. Commun. 2019, 10, 2177. [Google Scholar] [CrossRef]
- Mamoon, A.M.; Gamal-Eldeen, A.M.; Ruppel, M.E.; Smith, R.J.; Tsang, T.; Miller, L.M. In vitro efficiency and mechanistic role of indocyanine green as photodynamic therapy agent for human melanoma. Photodiagn. Photodyn. Ther. 2009, 6, 105–116. [Google Scholar] [CrossRef]
- Genina, E.A.; Bashkatov, A.N.; Simonenko, G.V.; Odoevskaya, O.D.; Tuchin, V.V.; Altshuler, G.B. Low-intensity indocyanine-green laser phototherapy of acne vulgaris: Pilot study. J. Biomed. Opt. 2004, 9, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Kirchherr, A.K.; Briel, A.; Mäder, K. Stabilization of indocyanine green by encapsulation within micellar systems. Mol. Pharm. 2009, 6, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Sadoqi, M.; Shao, J. Degradation kinetics of indocyanine green in aqueous solution. J. Pharm. Sci. 2003, 92, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Ateş, G.B.; Ak, A.; Garipcan, B.; Gülsoy, M. Indocyanine green-mediated photobiomodulation on human osteoblast cells. Lasers Med. Sci. 2018, 33, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Hu, J.; Zhu, H.; Shang, Y.; Chen, D.; Chen, Y.; Liu, H. In situ formation of a near-infrared controlled dual-antibacterial platform. New J. Chem. 2022, 46, 1569–1576. [Google Scholar] [CrossRef]
- Rempe, S.; Hayden, J.M.; Robbins, R.A.; Hoyt, J.C. Tetracyclines and pulmonary inflammation. Endocr. Metab. Immune Disord. Drug Targets 2007, 7, 232–236. [Google Scholar] [CrossRef]
- Lakshmaiah Narayana, J.; Chen, J.Y. Antimicrobial peptides: Possible anti-infective agents. Peptides 2015, 72, 88–94. [Google Scholar] [CrossRef]
- Vandamme, D.; Landuyt, B.; Luyten, W.; Schoofs, L. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell. Immunol. 2012, 280, 22–35. [Google Scholar] [CrossRef]
- Zhou, C.; Ao, H.Y.; Han, X.; Jiang, W.W.; Yang, Z.F.; Ma, L.; Deng, X.Y.; Wan, Y.Z. Engineering a novel antibacterial agent with multifunction: Protocatechuic acid-grafted-quaternized chitosan. Carbohydr. Polym. 2021, 258, 117683. [Google Scholar] [CrossRef]
- Ao, H.; Yang, S.; Nie, B.; Fan, Q.; Zhang, Q.; Zong, J.; Guo, S.; Zheng, X.; Tang, T. Improved antibacterial properties of collagen I/hyaluronic acid/quaternized chitosan multilayer modified titanium coatings with both contact-killing and release-killing functions. J. Mater. Chem. B 2019, 7, 1951–1961. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, M.; Liu, C.; Li, M.; Zhang, X.; Peng, L.; Liu, L.; Liao, J.; Yang, J. Recent Research on Hybrid Hydrogels for Infection Treatment and Bone Repair. Gels 2022, 8, 306. https://doi.org/10.3390/gels8050306
Cao M, Liu C, Li M, Zhang X, Peng L, Liu L, Liao J, Yang J. Recent Research on Hybrid Hydrogels for Infection Treatment and Bone Repair. Gels. 2022; 8(5):306. https://doi.org/10.3390/gels8050306
Chicago/Turabian StyleCao, Mengjiao, Chengcheng Liu, Mengxin Li, Xu Zhang, Li Peng, Lijia Liu, Jinfeng Liao, and Jing Yang. 2022. "Recent Research on Hybrid Hydrogels for Infection Treatment and Bone Repair" Gels 8, no. 5: 306. https://doi.org/10.3390/gels8050306