Opportunities for Ivory Nut Residue Valorization as a Source of Nanocellulose Colloidal Suspensions
Abstract
:1. Introduction
2. Results and Discussion
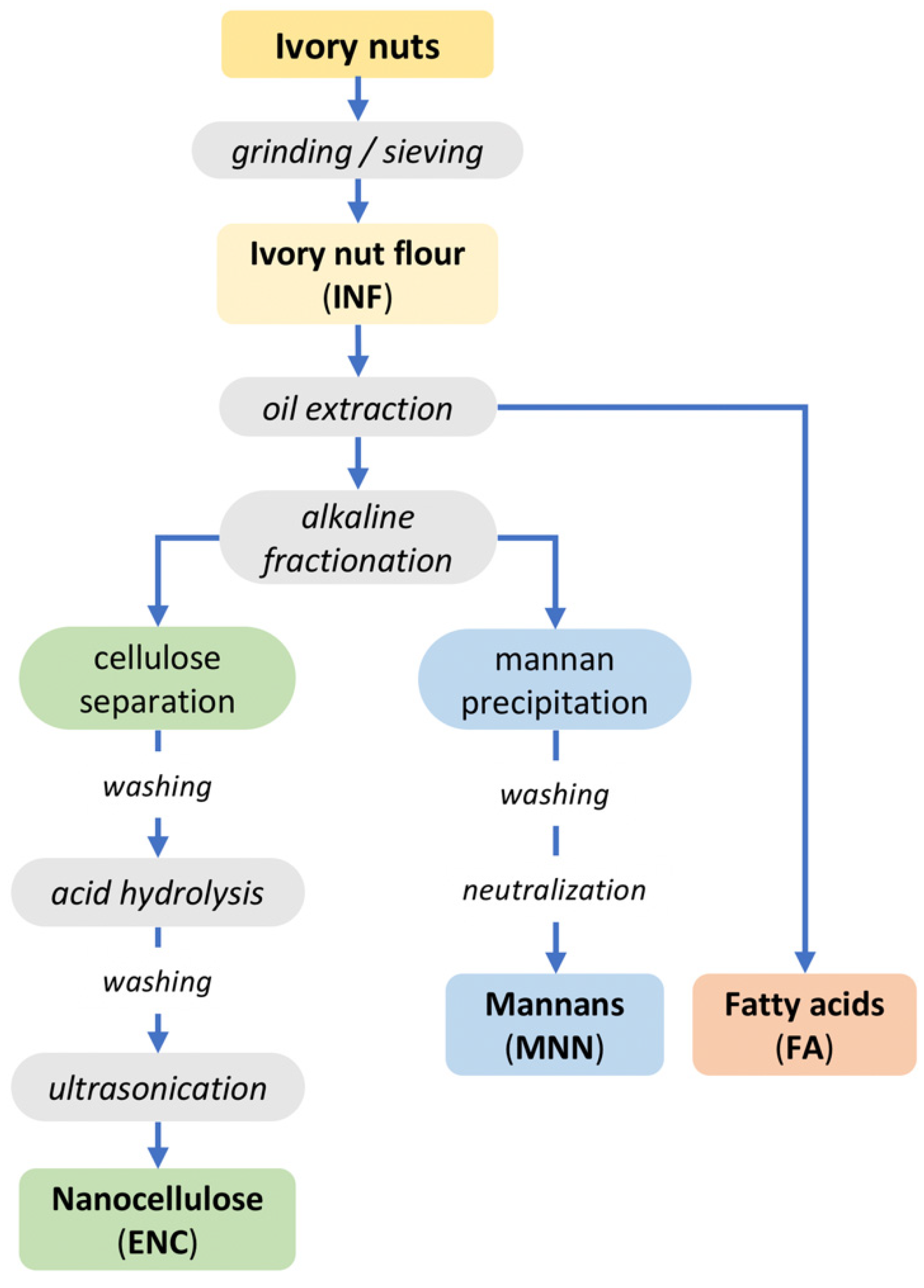
2.1. Fractionation from Ivory Nut Flour
2.2. Characterization of the Fractions
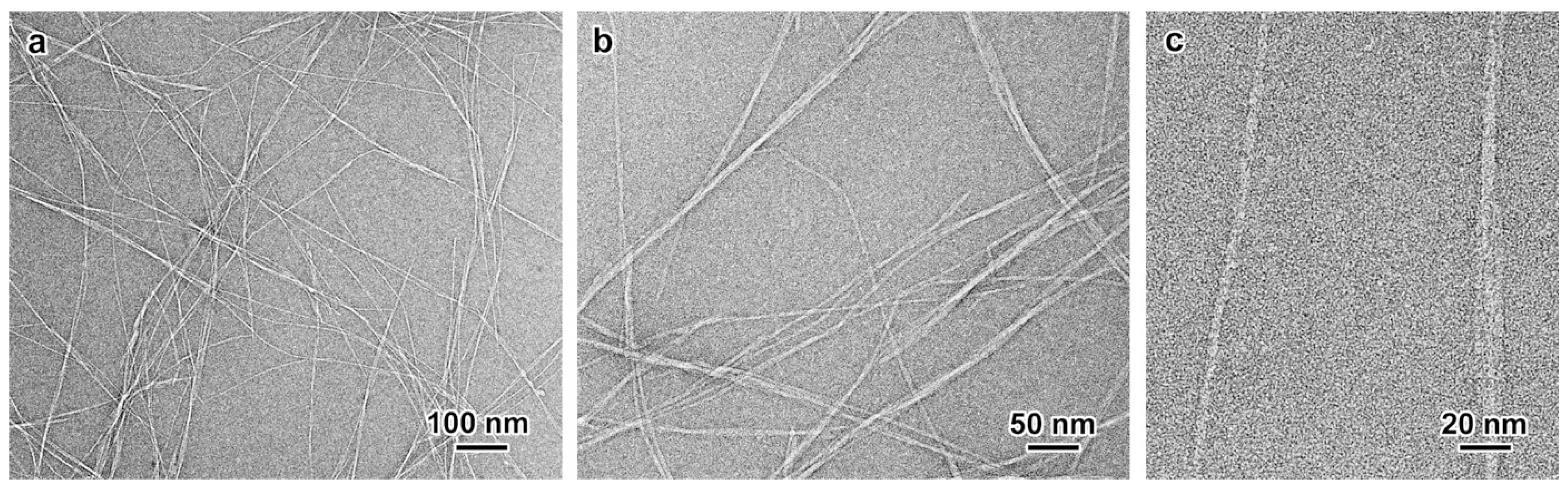
2.2.1. Nanocellulose Morphology
2.2.2. Structural Analyses
2.2.3. Vibrational Characterization of Endospermic Nanocellulose
2.2.4. Sulfation Degree of Endospermic Nanocellulose
2.2.5. Yield
- YieldA obtained was 4.08%
- YieldR obtained was 68.07%
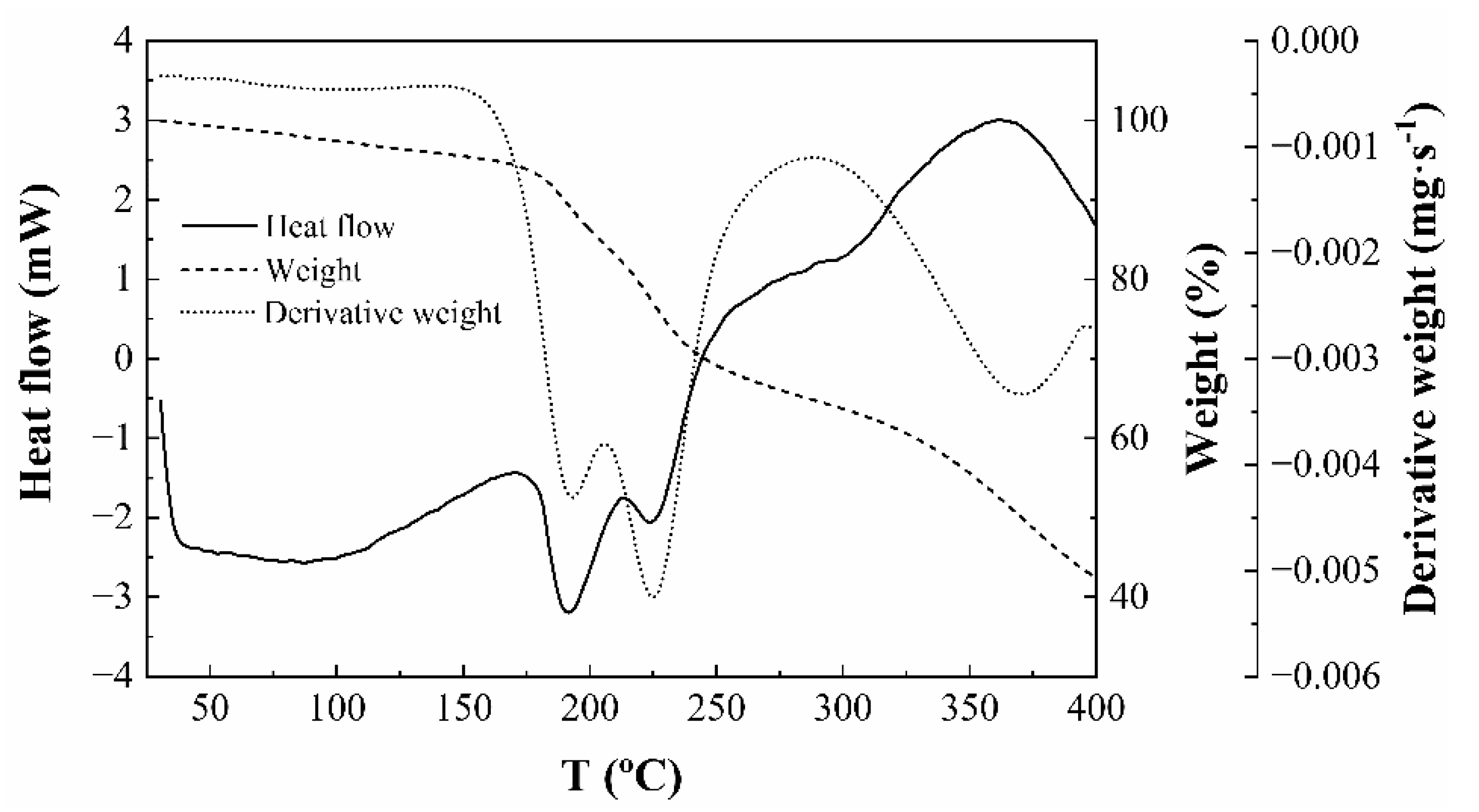
2.2.6. Thermal Analysis of Endospermic Nanocellulose
2.3. Common Methods for Obtaining Nanocellulose and Nanocellulose Suspensions
2.4. Applicability of the Obtained Nanocellulose Colloidal Suspensions
3. Conclusions
4. Material and Methods
4.1. Feedstock
4.2. Characterization
4.2.1. Particle Size Measurement
4.2.2. Transmission Electron Microscopy (TEM)
4.2.3. X-Ray Powder Diffraction (XRD)
4.2.4. Cellulose Nanofibril Sulfation Measurement
4.2.5. Yield
4.2.6. Vibrational Spectroscopy
4.2.7. CP/MAS 13C-NMR Spectroscopy
4.2.8. Thermal Analysis
4.2.9. Density and Cellulose Content in ENC Suspensions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kraikaew, J.; Morakul, S.; Keawsompong, S. Nutritional improvement of copra meal using mannanase and Saccharomyces cerevisiae. 3 Biotech 2020, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Dávila, J.A.; Rosenberg, M.; Castro, E.; Cardona, C.A. A model biorefinery for avocado (Persea americana Mill.) processing. Bioresour. Technol. 2017, 243, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, M.; Durazzo, A.; Romani, A.; Campo, M.; Lombardi-Boccia, G.; Cecchini, F. Bio-based compounds from grape seeds: A biorefinery approach. Molecules 2018, 23, 1888. [Google Scholar] [CrossRef] [Green Version]
- Valencia, R.; Montúfar, R.; Navarrete, H.; Balslev, H. Palmas Ecuatorianas: Biología y uso Sostenible; Herbario QCA de la Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2013; p. 253. [Google Scholar]
- Barfod, A.S.; Bergmann, B.; Pedersen, H.B. The vegetable ivory industry: Surviving and doing well in Ecuador. Economic Botany 1990, 44, 293–300. [Google Scholar] [CrossRef]
- Runk, J.V. Productivity and sustainability of a vegetable ivory palm (Phytelephas aequatorialis, Arecaceae) under three management regimes in Northwestern Ecuador. Economic Botany 1998, 52, 168–182. [Google Scholar] [CrossRef]
- Clay, J.W.; Clement, C.R. Selected Species and Strategies to Enhance Income Generation from Amazonian Forests. Misc/93/6. Working Paper; FAO: Rome, Italy, 1993; Volume 93, p. 270. [Google Scholar]
- Koziol, M.J.; Pedersen, H.B. Phytelephas aequatorialis (Arecaceae) in human and animal nutrition. Economic Botany 1993, 47, 401–407. [Google Scholar] [CrossRef]
- Ghysels, A.; Krämer, A.; Venable, R.M.; Teague, W.E.; Lyman, E.; Gawrisch, K.; Pastor, R.W. Permeability of membranes in the liquid ordered and liquid disordered phases. Nat. Commun. 2019, 10, 5616. [Google Scholar] [CrossRef] [Green Version]
- Aspinall, G.O.; Rashbrook, R.B.; Kessler, G. The mannans of ivory nut (Phytelephas macrocarpa). Part II. The partial acid hydrolysis of mannans A and B. J. Chem. Soc. 1958, 1958. [Google Scholar] [CrossRef]
- Chanzy, H.; Dube, M.; Marchessault, R.H.; Revol, J.F. Single crystals and oriented crystallization of ivory nut mannan. Biopolymers 1979, 18, 887–898. [Google Scholar] [CrossRef]
- Chanzy, H.D.; Grosrenaud, A.; Vuong, R.; Mackie, W. The crystalline polymorphism of mannan in plant cell walls and after recrystallisation. Planta 1984, 161, 320–329. [Google Scholar] [CrossRef]
- Meier, H. On the structure of cell walls and cell wall mannans from ivory nuts and from dates. Biochim. Biophys. Acta 1958, 28, 229–240. [Google Scholar] [CrossRef]
- Isogai, A. Wood nanocelluloses: Fundamentals and applications as new bio-based nanomaterials. J. Wood Sci. 2013, 59, 449–459. [Google Scholar] [CrossRef]
- Kumar, V.; Pathak, P.; Bhardwaj, N.K. Waste paper: An underutilized but promising source for nanocellulose mining. Waste Manage. 2020, 102, 281–303. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.; Saba, N.; Asiri, A.M.; Jawaid, M.; Indarti, E.; Wanrosli, W.D. Preparation and characterization of nanocomposite films from oil palm pulp nanocellulose/poly(vinyl alcohol) by casting method. Carbohydr. Polym. 2018, 191, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Dinand, E.; Chanzy, H.; Vignon, R.M. Suspensions of cellulose microfibrils from sugar beet pulp. Food Hydrocoll. 1999, 13, 275–283. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: From Nature to High Performance Tailored Materials; Walter de Gruyter GmbH & Co. KG: Göttingen, Germany, 2012; p. 475. [Google Scholar]
- Habibi, Y.; Mahrouz, M.; Vignon, M.R. Microfibrillated cellulose from the peel of prickly pear fruits. Food Chem. 2009, 115, 423–429. [Google Scholar] [CrossRef]
- Ifuku, S.; Adachi, M.; Morimoto, M.; Saimoto, H. Fabrication of cellulose nanofibers from parenchyma cells of pears and apples. Sen’i Gakkaishi 2011, 67, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Cosgrove, D.J.; Jarvis, M.C. Comparative structure and biomechanics of plant primary and secondary cell walls. Front. Plant Sci. 2012, 3, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef] [PubMed]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Hu, L.; Zheng, G.; Yao, J.; Liu, N.; Weil, B.; Eskilsson, M.; Karabulut, E.; Ruan, Z.; Fan, S.; Bloking, J.T.; et al. Transparent and conductive paper from nanocellulose fibers. Energy Environ. Sci. 2013, 6, 513–518. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, P.; Choi, S.-Y.; Shim, B.; Lee, J.; Cuddihy, M.; Kotov, N.A. Molecularly engineered nanocomposites: Layer-by-layer assembly of cellulose nanocrystals. Biomacromolecules 2005, 6, 2914–2918. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Wang, C.; Bai, J.; Tian, P.; Xie, R.; Duan, Z.; Lv, Q.; Tao, Y. The application status of nanoscale cellulose-based hydrogels in tissue engineering and regenerative biomedicine. Front. Bioeng. Biotechnol. 2021, 18, 732513. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of cellulose nanocrystals produced by acid-hydrolysis from sugarcane bagasse as agro-waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Zuluaga, R.; Putaux, J.L.; Cruz, J.; Vélez, J.; Mondragon, I.; Gañán, P. Cellulose microfibrils from banana rachis: Effect of alkaline treatments on structural and morphological features. Carbohydr. Polym. 2009, 76, 51–59. [Google Scholar] [CrossRef]
- Mat Zain, N.F. Preparation and characterization of cellulose and nanocellulose from pomelo (Citrus grandis) albedo. J. Nutr. Food Sci. 2014, 05, 1000334. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, J.; Vuong, R.; Chanzy, H. Electron diffraction study on the two crystalline phases occurring in native cellulose from an algal cell wall. Macromolecules 2002, 24, 4168–4175. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2013, 21, 885–896. [Google Scholar] [CrossRef]
- Lai-Kee-Him, J.; Chanzy, H.; Müller, M.; Putaux, J.-L.; Imai, T.; Bulone, V. In vitro versus in vivo cellulose microfibrils from plant primary wall synthases: Structural differences. J. Biol. Chem. 2002, 277, 36931–36939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, M.; Heux, L.; Sugiyama, J. Polymorphism of cellulose I family: Reinvestigation of cellulose IVI. Biomacromolecules 2004, 5, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Montanari, S.; Roumani, M.; Heux, L.; Vignon, M.R. Topochemistry of carboxylated cellulose nanocrystals resulting from TEMPO-mediated oxidation. Macromolecules 2005, 38, 1665–1671. [Google Scholar] [CrossRef]
- Foston, M. Advances in solid-state NMR of cellulose. Curr. Opin. Biotechnol. 2014, 27, 176–184. [Google Scholar] [CrossRef]
- Marchessault, R.H.; Taylor, M.G.; Winter, W.T. 13C CP/MAS NMR spectra of poly-β-D(1→4) mannose: Mannan. Can. J. Chem. 1990, 68, 1192–1195. [Google Scholar] [CrossRef] [Green Version]
- Heux, L.; Hägglund, P.; Putaux, J.L.; Chanzy, H. Structural aspects in semicrystalline samples of the mannan II family. Biomacromolecules 2004, 6, 324–332. [Google Scholar] [CrossRef]
- Li, Q.Q. Nanocellulose: Preparation, Characterization, Supramolecular Modeling, and Its life Cycle Assessment; Virginia Polytechnic Institute & State University,: Blacksburg, VA, USA, 2012. [Google Scholar]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Yang, P.; Kobayashi, H.; Hara, K.; Fukuoka, A. Phase change of nickel phosphide catalysts in the conversion of cellulose into sorbitol. ChemSusChem 2012, 5, 920–926. [Google Scholar] [CrossRef]
- Jahan, M.S.; Saeed, A.; He, Z.; Ni, Y. Jute as raw material for the preparation of microcrystalline cellulose. Cellulose 2010, 18, 451–459. [Google Scholar] [CrossRef]
- Yahya, M.B.; Lee, H.V.; Abd Hamid, S.B. Preparation of nanocellulose via transition metal salt-catalyzed hydrolysis pathway. BioResources 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Maiti, S.; Jayaramudu, J.; Das, K.; Reddy, S.M.; Sadiku, R.; Ray, S.S.; Liu, D. Preparation and characterization of nano-cellulose with new shape from different precursor. Carbohydr. Polym. 2013, 98, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Ureña-Benavides, E.E.; Ao, G.; Davis, V.A.; Kitchens, C.L. Rheology and phase behavior of lyotropic cellulose nanocrystal suspensions. Macromolecules 2011, 44, 8990–8998. [Google Scholar] [CrossRef]
- Fraschini, C.; Chauve, G.; Bouchard, J. TEMPO-mediated surface oxidation of cellulose nanocrystals (CNCs). Cellulose 2017, 24, 2775–2790. [Google Scholar] [CrossRef]
- Johnston, L.J.; Jakubek, Z.J.; Beck, S.; Araki, J.; Cranston, E.D.; Danumah, C.; Fox, D.; Li, H.; Wang, J.; Mester, Z.; et al. Determination of sulfur and sulfate half-ester content in cellulose nanocrystals: An interlaboratory comparison. Metrologia 2018, 55, 872–882. [Google Scholar] [CrossRef]
- Savage, N.; Diallo, M.S. Nanomaterials and water purification: Opportunities and challenges. J. Nanopart. Res. 2005, 7, 331–342. [Google Scholar] [CrossRef]
- Cheng, M.; Qin, Z.; Chen, Y.; Liu, J.; Ren, Z. Facile one-step extraction and oxidative carboxylation of cellulose nanocrystals through hydrothermal reaction by using mixed inorganic acids. Cellulose 2017, 24, 3243–3254. [Google Scholar] [CrossRef]
- Ketabchi, M.R.; Khalid, M.; Ratnam, C.T.; Manickam, S.; Walvekar, R.; Hoque, M.E. Sonosynthesis of Cellulose Nanoparticles (CNP) from Kenaf Fiber: Effects of Processing Parameters. Fibers Polym. 2016, 17, 1352–1358. [Google Scholar] [CrossRef]
- Gan, P.G.; Sam, S.T.; Abdullah, M.F.b.; Omar, M.F. Thermal properties of nanocellulose-reinforced composites: A review. J. Appl. Polym. Sci. 2019, 137, 48544. [Google Scholar] [CrossRef] [Green Version]
- Morais, J.P.S.; Rosa, M.d.F.; de Souza Filho, M.d.s.M.; Nascimento, L.D.; do Nascimento, D.M.; Cassales, A.R. Extraction and characterization of nanocellulose structures from raw cotton linter. Carbohydr. Polym. 2013, 91, 229–235. [Google Scholar] [CrossRef]
- Espinosa, E.; Rol, F.; Bras, J.; Rodríguez, A. Production of lignocellulose nanofibers from wheat straw by different fibrillation methods. Comparison of its viability in cardboard recycling process. J. Clean. Prod. 2019, 239, 118083. [Google Scholar] [CrossRef]
- Wulandari, W.T.; Rochliadi, A.; Arcana, I.M. Nanocellulose prepared by acid hydrolysis of isolated cellulose from sugarcane bagasse. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107. [Google Scholar] [CrossRef]
- Bansal, M.; Kumar, D.; Chauhan, G.S.; Kaushik, A. Preparation, characterization and trifluralin degradation of laccase-modified cellulose nanofibers. Mater. Sci. Energy Technol. 2018, 1, 29–37. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Dufresne, A. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: A review. Nanoscale 2012, 4. [Google Scholar] [CrossRef]
- Theivasanthi, T.; Anne Christma, F.L.; Toyin, A.J.; Gopinath, S.C.B.; Ravichandran, R. Synthesis and characterization of cotton fiber-based nanocellulose. Int. J. Biol. Macromol. 2018, 109, 832–836. [Google Scholar] [CrossRef] [Green Version]
- Thakur, M.; Sharma, A.; Ahlawat, V.; Bhattacharya, M.; Goswami, S. Process optimization for the production of cellulose nanocrystals from rice straw derived α-cellulose. Mater. Sci. Energy Technol. 2020, 3, 328–334. [Google Scholar] [CrossRef]
- Thakur, V.; Guleria, A.; Kumar, S.; Sharma, S.; Singh, K. Recent advances in nanocellulose processing, functionalization and applications: A review. Mater. Adv. 2021, 2, 1872–1895. [Google Scholar] [CrossRef]
- Carvajal Barriga, E.J.; Fitzgerald, W.; Dimitriadis, E.K.; Margolis, L.; Fields, R.D. Sulfated endospermic nanocellulose crystals prevent the transmission of SARS-CoV-2 and HIV-1. Res. Sq. Preprint. 2022. [Google Scholar] [CrossRef]
- Ossa-Paredes, R.; Bastidas, B.; Carvajal-Barriga, E.J. Remediation of contaminated water with chromium VI by sorption in surface-activated-nanocellulose spheroids. Pollution 2022, 8, 489–500. [Google Scholar]
- Carvajal Barriga, E.J.; Bastidas Mayorga, B.D.; Portero Barahona, P. Proceso de Obtención de Nanocelulosa Parenquimatica a Partir de Semillas de Nuez de Marfil de Hidrogeles Obtenidos del Proceso. No. IEPI-2016-61010. 10 June 2016. [Google Scholar]
- Dorris, A.; Gray, D.G. Gelation of cellulose nanocrystal suspensions in glycerol. Cellulose 2012, 19, 687–694. [Google Scholar] [CrossRef]
- Pulp—Total Acidic Group Content: Conductometric Titration Method. SCAN-CM 65:02; Scandinavian Pulp, Paper and Board Testing Committee: Stockholm, Sweden, 2002; p. 4. [Google Scholar]
- Timell, T.E. Vegetable ivory as a source of a mannan polysaccharide. Can. J. Chem. 1957, 35, 333–338. [Google Scholar] [CrossRef]
- Kooiman, P.; Kreger, D.R. Some observations on X-ray diffraction and monosaccharide composition in mannose-containing polysaccharides from seeds. K. Ned. Akad. Van Wet. Proc. Series C 1960, 63, 634. [Google Scholar]




| Treatment | H2SO4 (M) | T (°C) | Time (h) | Consistency | Color | Turbidity | Number of Phases | Dh (nm) | PDI |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 30 | 1 | watery | translucent | no | 2 | 828 | 1.16 |
| 2 | 4 | 30 | 4 | watery | translucent | no | 2 | 839 | 1.34 |
| 3 | 8 | 30 | 1 | gel | hazy | low | 1 | 584 | 0.24 |
| 4 | 8 | 30 | 4 | gel | translucent | very low | 1 | 398 | 0.13 |
| 5 | 4 | 60 | 1 | gel | white | high | 1 | 483 | 0.18 |
| 6 | 4 | 60 | 4 | gel | white | high | 1 | 312 | 0.25 |
| 7 | 8 | 60 | 1 | gel | red | high | 1 | 475 | 0.09 |
| 8 | 8 | 60 | 4 | watery | dark | no | 1 | n/a | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvajal-Barriga, E.J.; Putaux, J.-L.; Martín-Ramos, P.; Simbaña, J.; Portero-Barahona, P.; Martín-Gil, J. Opportunities for Ivory Nut Residue Valorization as a Source of Nanocellulose Colloidal Suspensions. Gels 2023, 9, 32. https://doi.org/10.3390/gels9010032
Carvajal-Barriga EJ, Putaux J-L, Martín-Ramos P, Simbaña J, Portero-Barahona P, Martín-Gil J. Opportunities for Ivory Nut Residue Valorization as a Source of Nanocellulose Colloidal Suspensions. Gels. 2023; 9(1):32. https://doi.org/10.3390/gels9010032
Chicago/Turabian StyleCarvajal-Barriga, Enrique Javier, Jean-Luc Putaux, Pablo Martín-Ramos, Jennifer Simbaña, Patricia Portero-Barahona, and Jesús Martín-Gil. 2023. "Opportunities for Ivory Nut Residue Valorization as a Source of Nanocellulose Colloidal Suspensions" Gels 9, no. 1: 32. https://doi.org/10.3390/gels9010032
APA StyleCarvajal-Barriga, E. J., Putaux, J.-L., Martín-Ramos, P., Simbaña, J., Portero-Barahona, P., & Martín-Gil, J. (2023). Opportunities for Ivory Nut Residue Valorization as a Source of Nanocellulose Colloidal Suspensions. Gels, 9(1), 32. https://doi.org/10.3390/gels9010032










