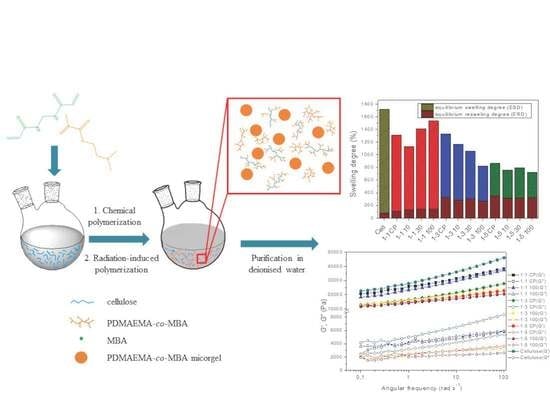
Swelling and Viscoelastic Properties of Cellulose-Based Hydrogels Prepared by Free Radical Polymerization of Dimethylaminoethyl Methacrylate in Cellulose Solution
Abstract
1. Introduction
2. Results and Discussion
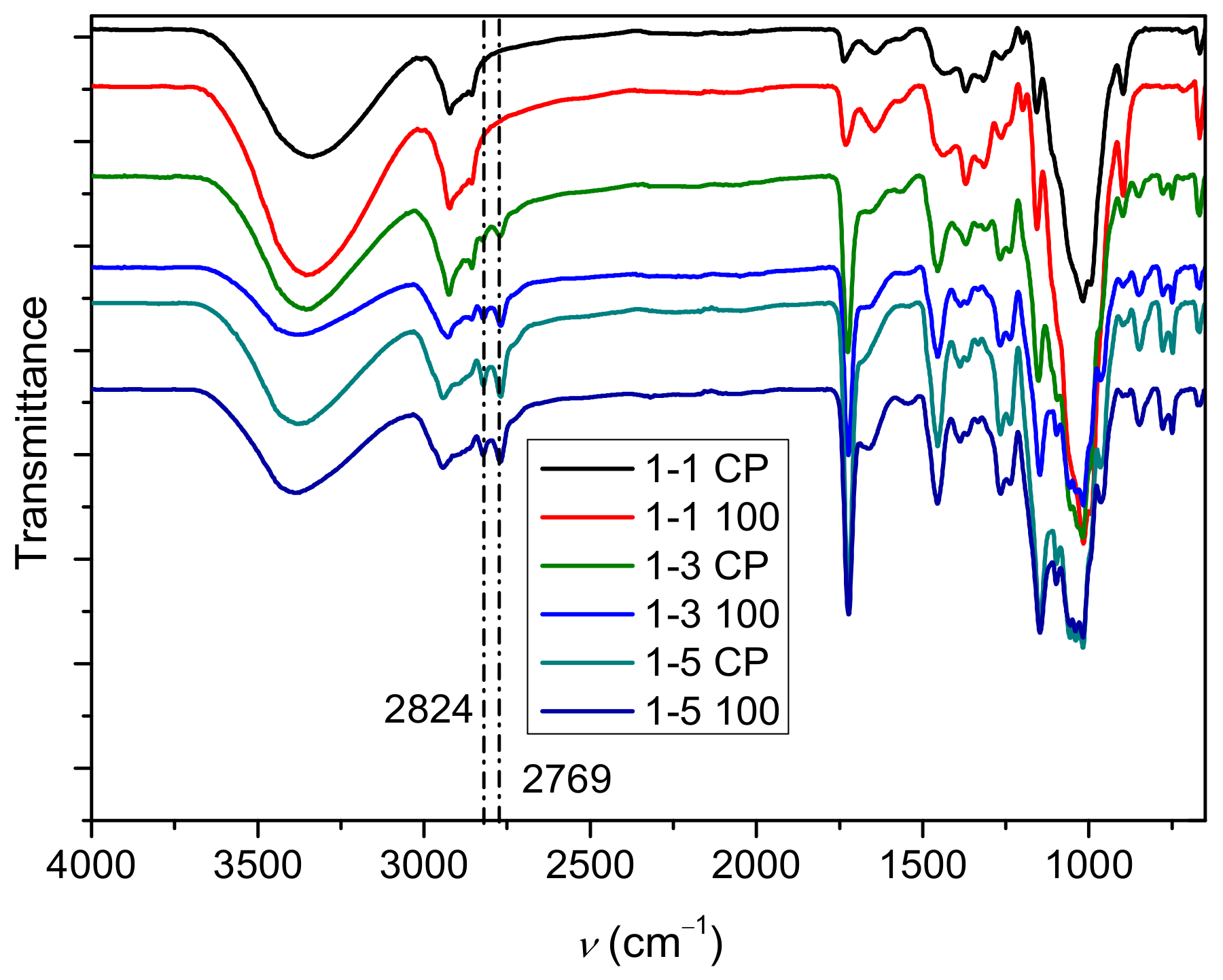
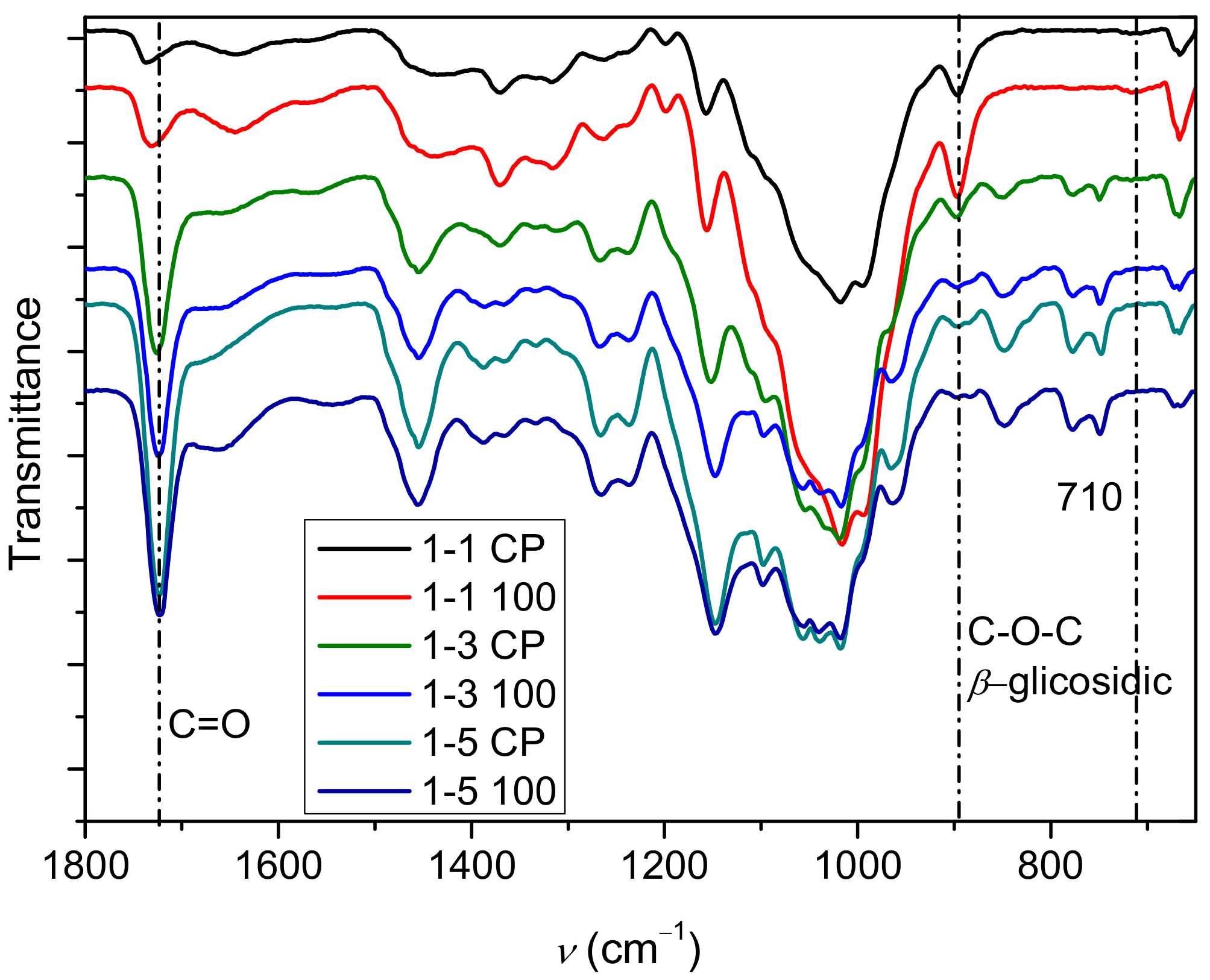
2.1. Polymerization Reaction
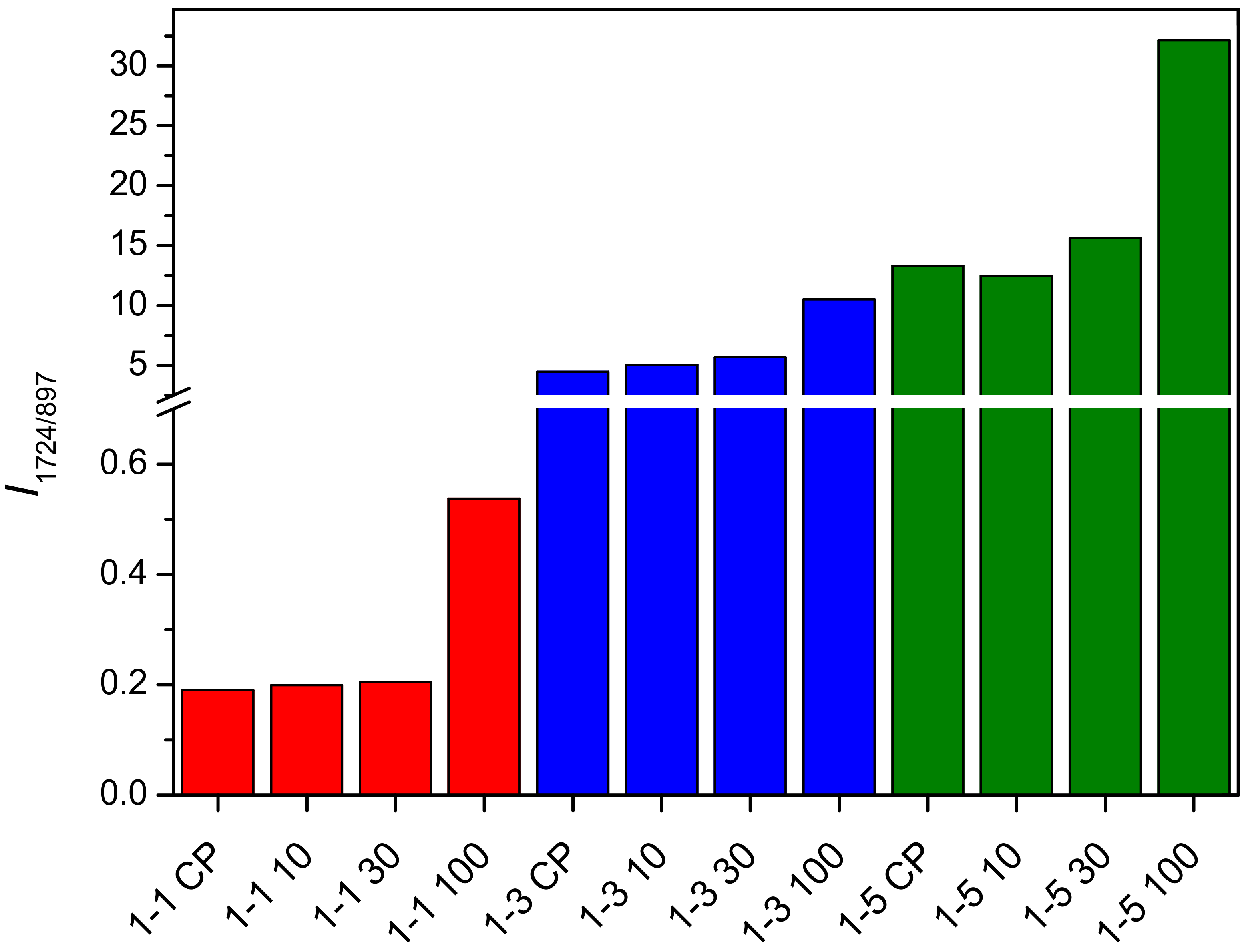
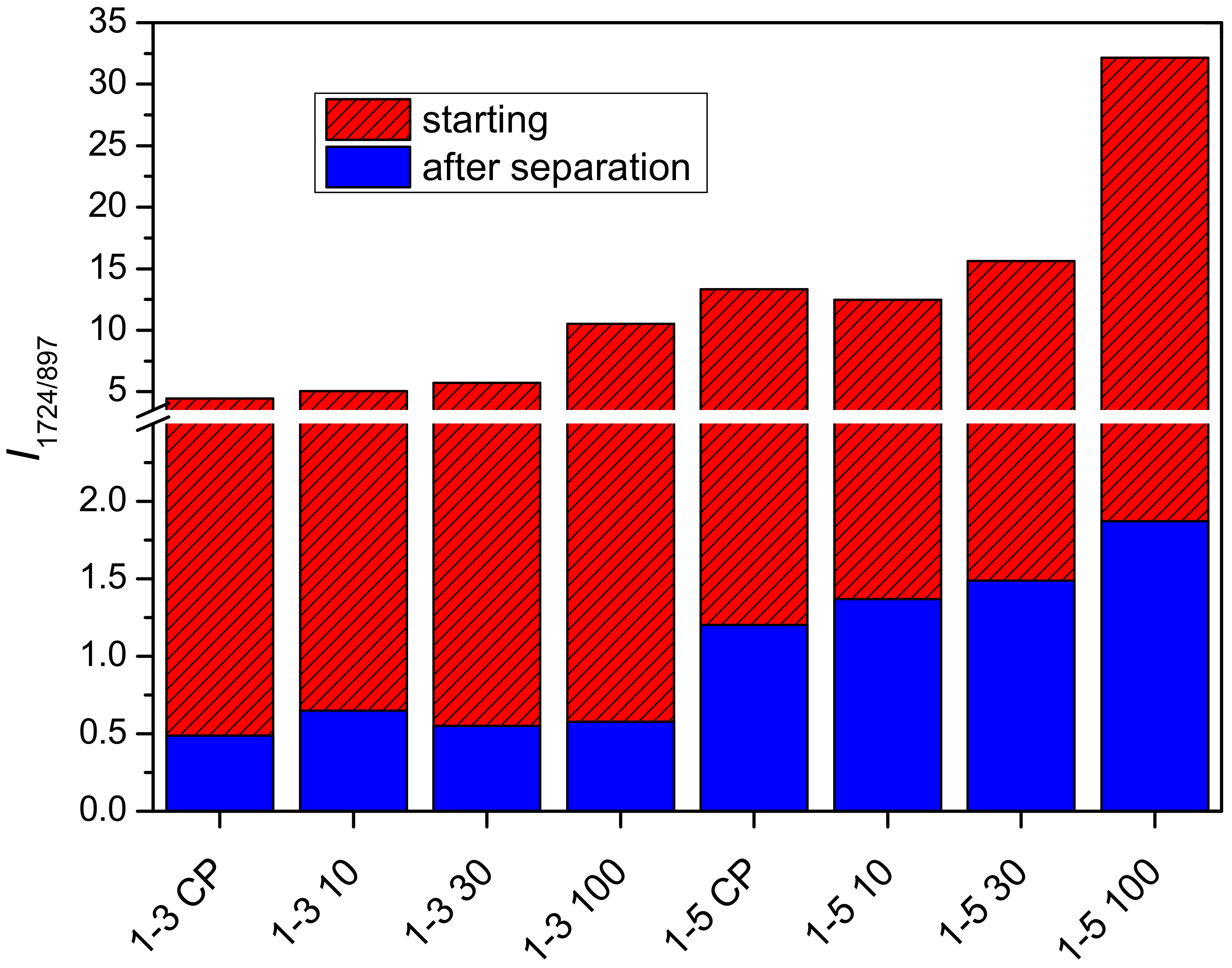
2.2. Determination of PDMAEMA Content in Cel-DMAEMA Hydrogels
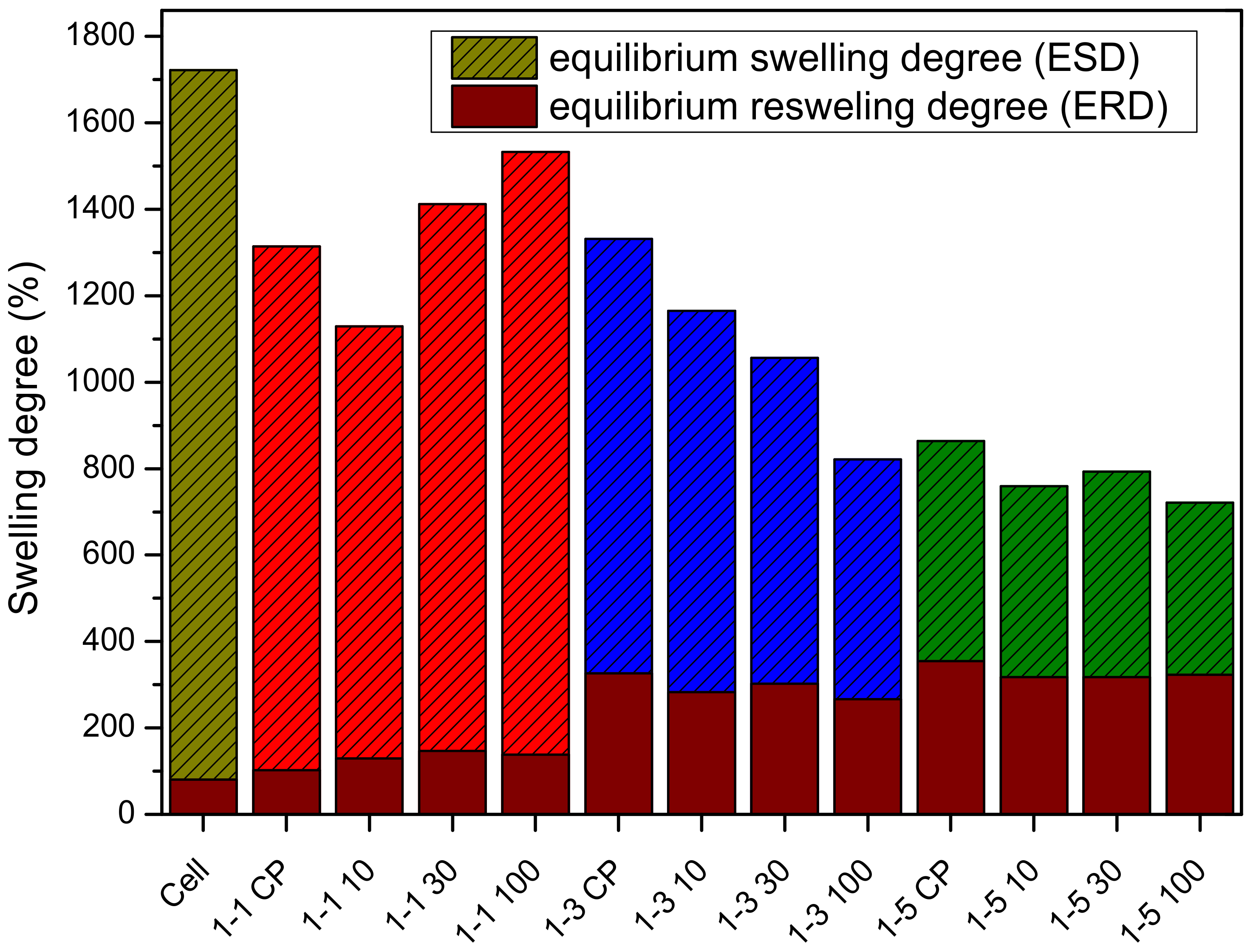
2.3. Influence of Composition and Irradiation on Swelling Behavior of Hydrogels
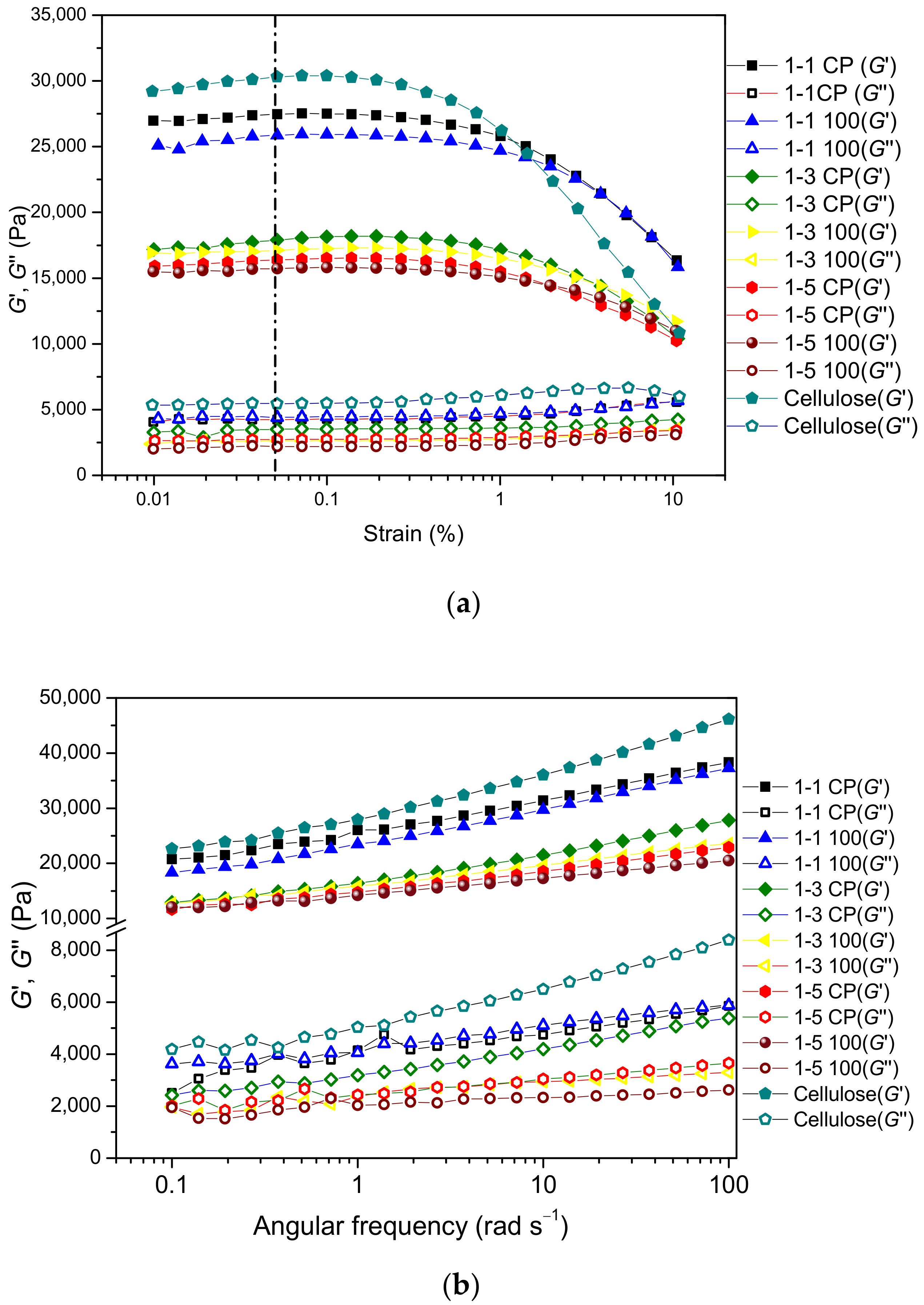
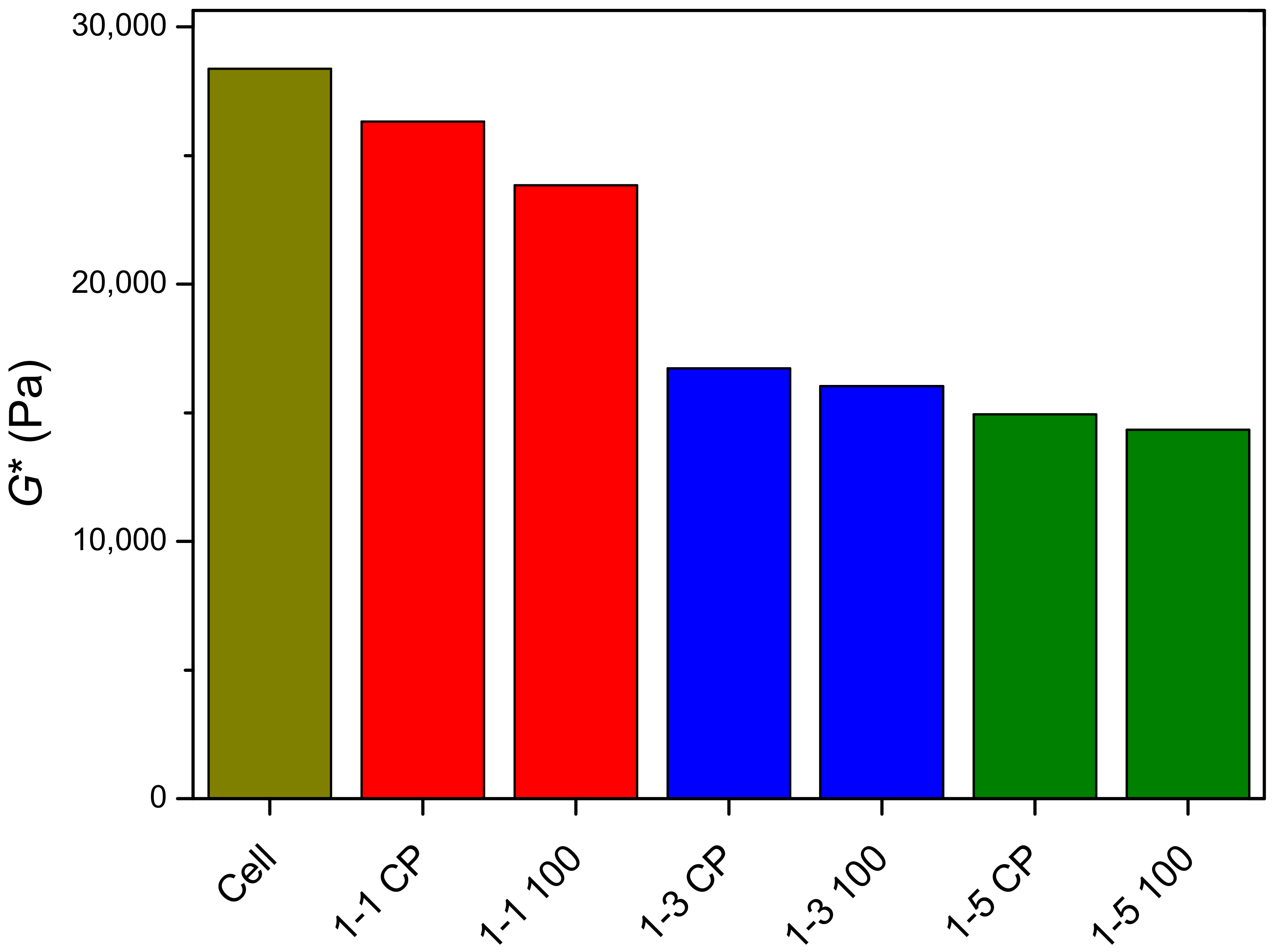
2.4. Rheological Properties of Prepared Hydrogels
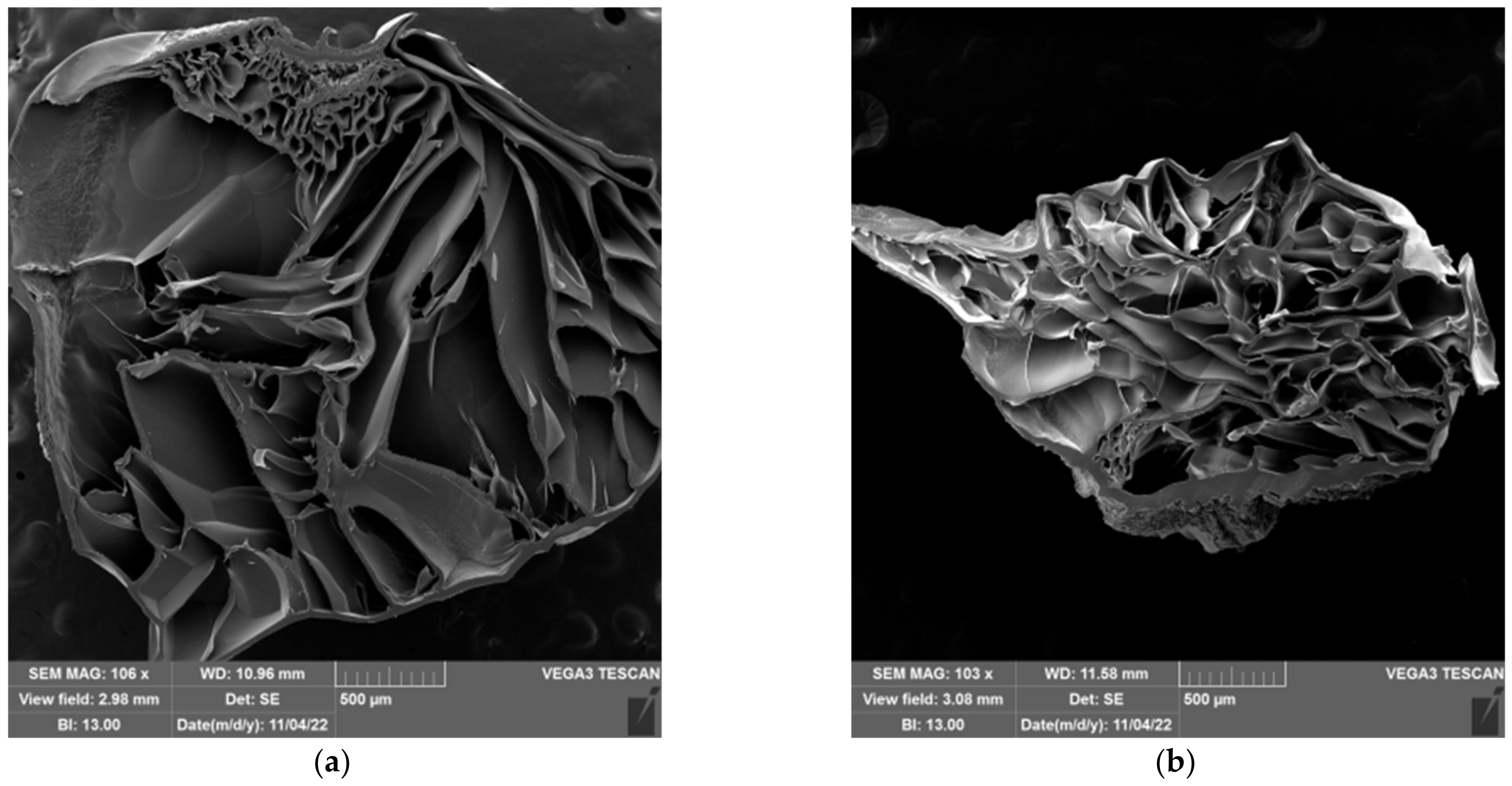
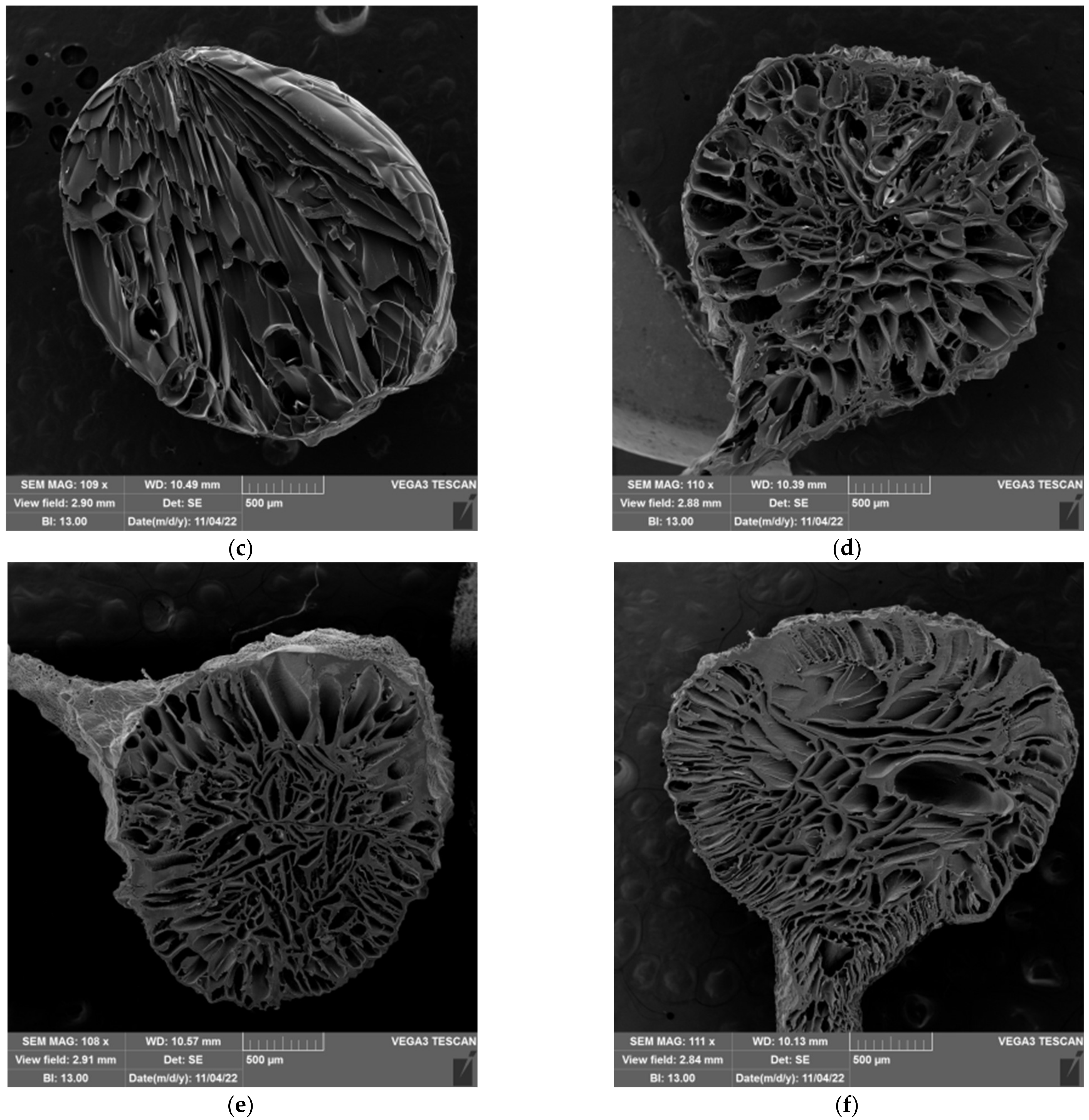
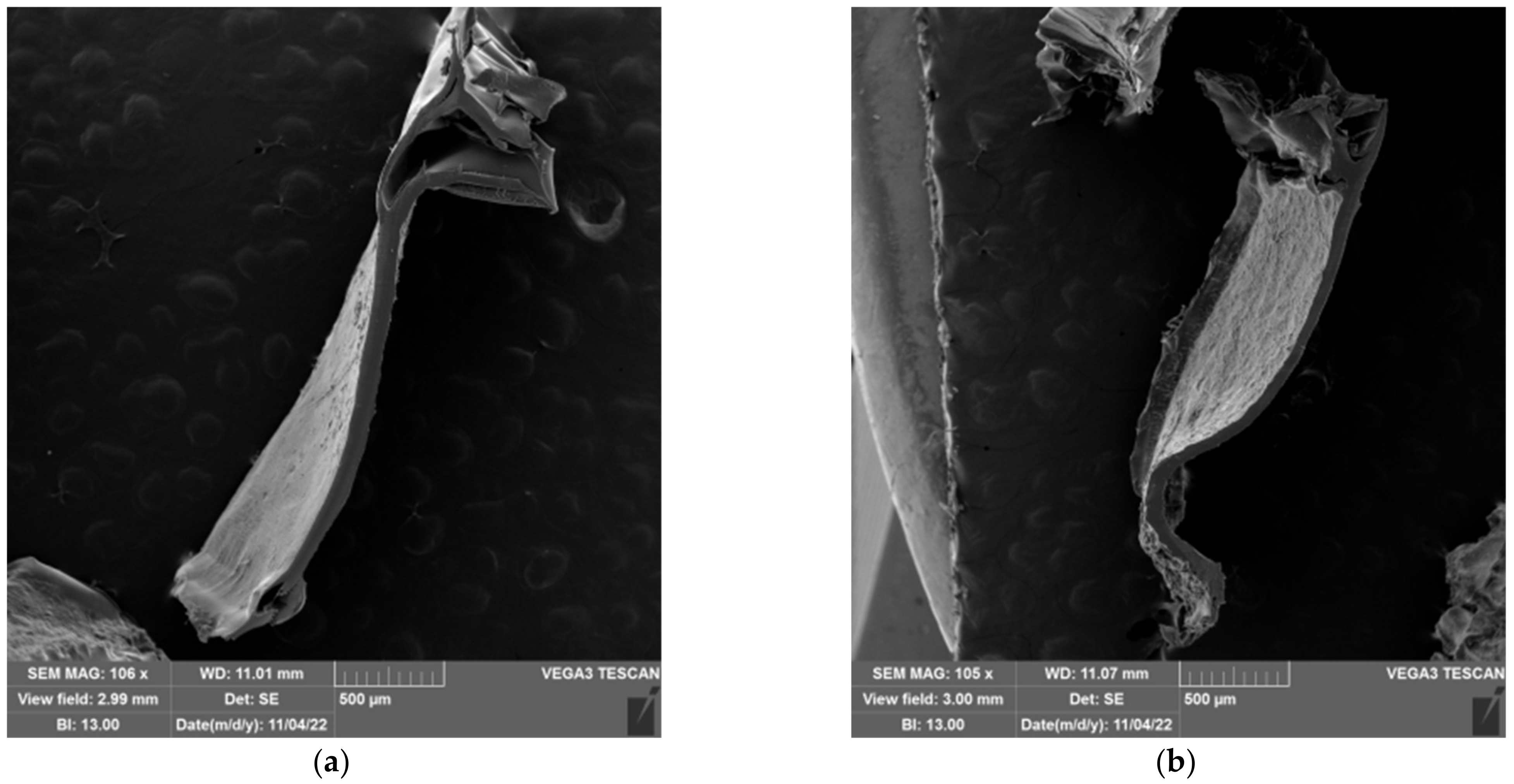
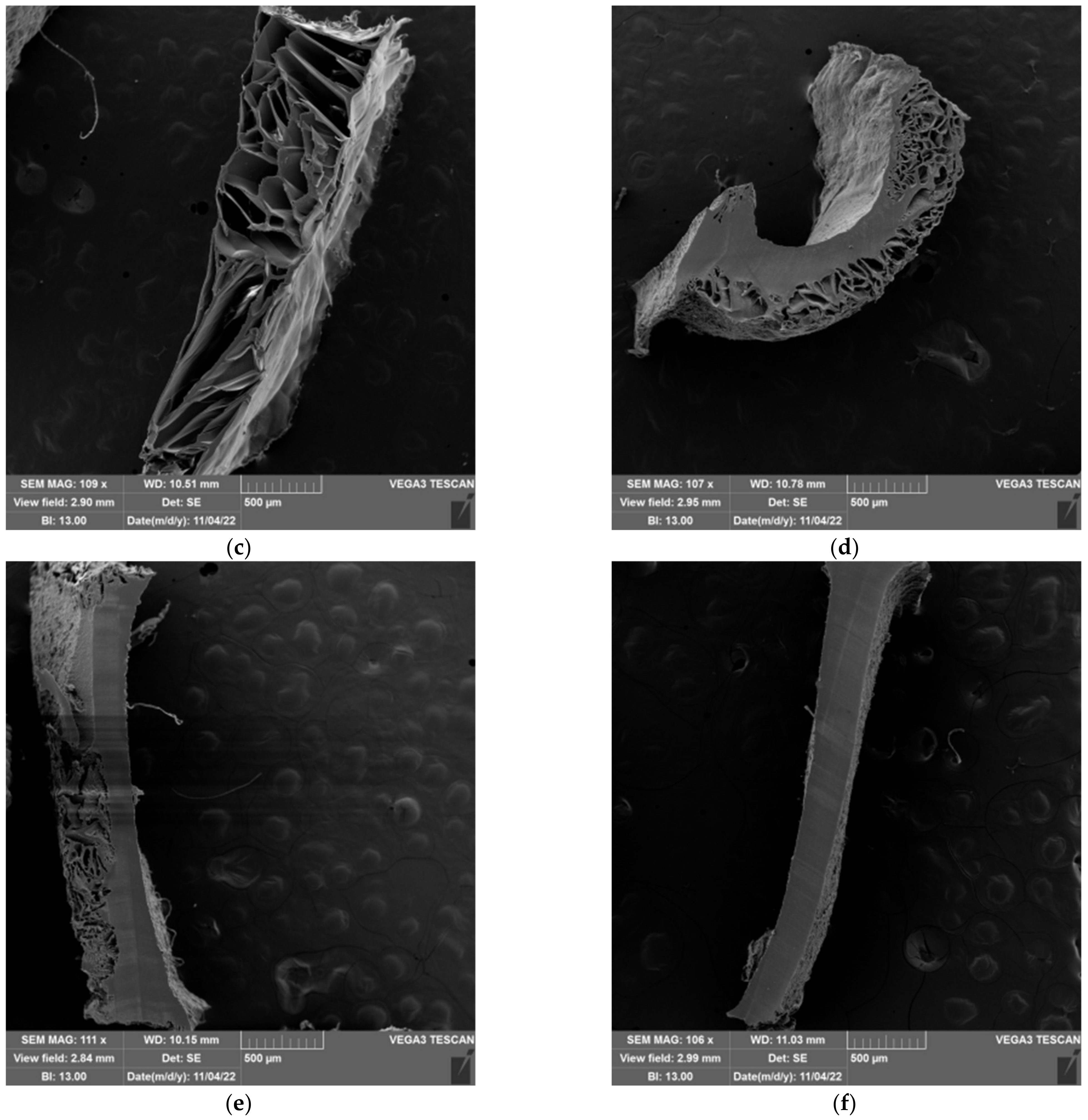
2.5. Morphology of Prepared Hydrogels
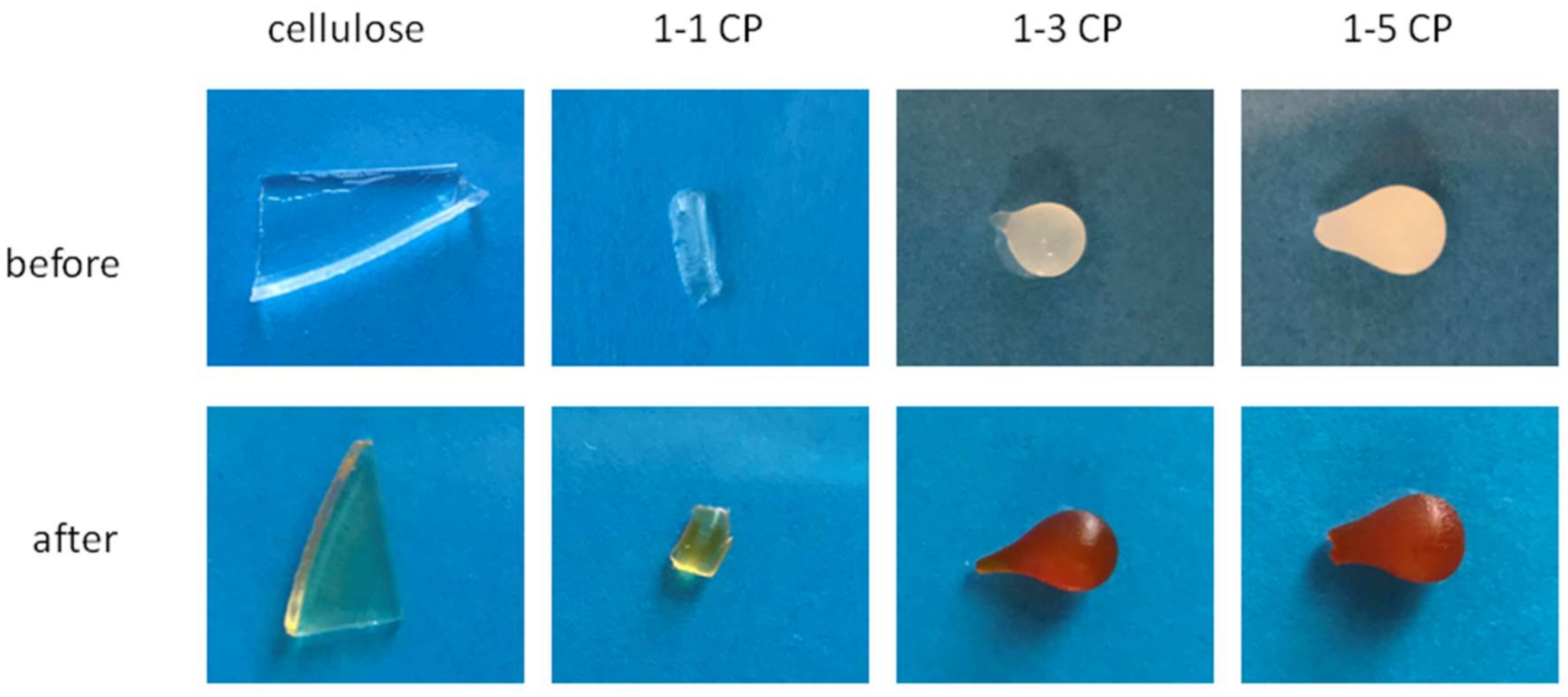
2.6. Hydrogels with Silver Particles
2.7. Adsorption of Fe3+ Ions
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Polymerization Reaction
4.3. Microgelation Examination
4.4. Drying of the Spheres
4.5. Preparation of Hydrogels with Silver Particles
4.6. Adsorption of Fe3+ Ions
4.7. Polymer Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, L.; Liu, R.; Tan, J.; Wang, D.; Jin, X.; Kang, H.; Wu, M.; Huang, Y. Self-Assembly and Dual-Stimuli Sensitivities of Hydroxypropylcellulose-Graft-Poly(N,N-Dimethyl Aminoethyl Methacrylate) Copolymers in Aqueous Solution. Langmuir 2010, 26, 8697–8703. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, L.; Oliveira, B.; Pereira, V.J.; Barreto Crespo, M.T.; Crespo, J.G.; Quemener, D.; Semsarilar, M. Nanocomposite Membranes from Nano-Particles Prepared by Polymerization Induced Self-Assembly and Their Biocidal Activity. Sep. Purif. Technol. 2020, 251, 117375. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Kuznetsov, V.A.; Lavlinskaya, M.S. Synthesis of Graft Copolymers of Carboxymethyl Cellulose and N,N-Dimethylaminoethyl Methacrylate and Their Study as Paclitaxel Carriers. Polym. Bull. 2021, 78, 2975–2992. [Google Scholar] [CrossRef]
- Rawlinson, L.A.B.; Ryan, S.M.; Mantovani, G.; Syrett, J.A.; Haddleton, D.M.; Brayden, D.J. Antibacterial Effects of Poly(2-(Dimethylamino Ethyl)Methacrylate) against Selected Gram-Positive and Gram-Negative Bacteria. Biomacromolecules 2010, 11, 443–453. [Google Scholar] [CrossRef]
- Sun, H.; Gao, Z.; Yang, L.; Gao, L.; Lv, X. Synthesis and Characterization of Novel Four-Arm Star PDMAEMA-Stabilized Colloidal Silver Nanoparticles. Colloid Polym. Sci. 2010, 288, 1713–1722. [Google Scholar] [CrossRef]
- Shvedchenko, D.O.; Nekrasova, T.N.; Nazarova, O.V.; Buffat, P.A.; Suvorova, E.I. Mechanism of Formation of Silver Nanoparticles in MAG–DMAEMA Copolymer Aqueous Solutions. J. Nanoparticle Res. 2015, 17, 275. [Google Scholar] [CrossRef]
- Zhu, T.; Mao, J.; Cheng, Y.; Liu, H.; Lv, L.; Ge, M.; Li, S.; Huang, J.; Chen, Z.; Li, H.; et al. Recent Progress of Polysaccharide-Based Hydrogel Interfaces for Wound Healing and Tissue Engineering. Adv. Mater. Interfaces 2019, 6, 1900761. [Google Scholar] [CrossRef]
- Xia, Z.; Patchan, M.; Maranchi, J.; Elisseeff, J.; Trexler, M. Determination of Crosslinking Density of Hydrogels Prepared from Microcrystalline Cellulose. J. Appl. Polym. Sci. 2013, 127, 4537–4541. [Google Scholar] [CrossRef]
- Stumpf, T.R.; Yang, X.; Zhang, J.; Cao, X. In Situ and Ex Situ Modifications of Bacterial Cellulose for Applications in Tissue Engineering. Mater. Sci. Eng. C 2018, 82, 372–383. [Google Scholar] [CrossRef]
- Ciecholewska-Juśko, D.; Żywicka, A.; Junka, A.; Drozd, R.; Sobolewski, P.; Migdał, P.; Kowalska, U.; Toporkiewicz, M.; Fijałkowski, K. Superabsorbent Crosslinked Bacterial Cellulose Biomaterials for Chronic Wound Dressings. Carbohydr. Polym. 2021, 253, 117247. [Google Scholar] [CrossRef]
- Wang, H.; Yu, X.; Tang, X.; Sun, Y.; Zeng, X.; Lin, L. A Self-Healing Water-Dissolvable and Stretchable Cellulose-Hydrogel for Strain Sensor. Cellulose 2022, 29, 341–354. [Google Scholar] [CrossRef]
- Salama, A.; Shukry, N.; El-Sakhawy, M. Carboxymethyl Cellulose-g-Poly(2-(Dimethylamino) Ethyl Methacrylate) Hydrogel as Adsorbent for Dye Removal. Int. J. Biol. Macromol. 2015, 73, 72–75. [Google Scholar] [CrossRef]
- Li, W.; Zuo, P.; Xu, D.; Xu, Y.; Wang, K.; Bai, Y.; Ma, H. Tunable Adsorption Properties of Bentonite/Carboxymethyl Cellulose-g-Poly(2-(Dimethylamino) Ethyl Methacrylate) Composites toward Anionic Dyes. Chem. Eng. Res. Des. 2017, 124, 260–270. [Google Scholar] [CrossRef]
- Yuan, H.; Chi, H.; Yuan, W. Ethyl Cellulose Amphiphilic Graft Copolymers with LCST-UCST Transition: Opposite Self-Assembly Behavior, Hydrophilic-Hydrophobic Surface and Tunable Crystalline Morphologies. Carbohydr. Polym. 2016, 147, 261–271. [Google Scholar] [CrossRef]
- Takács, E.; Mirzadeh, H.; Wojnárovits, L.; Borsa, J.; Mirzataheri, M.; Benke, N. Comparison of Simultaneous and Pre-Irradiation Grafting of N-Vinylpyrrolidone to Cotton-Cellulose. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 265, 217–220. [Google Scholar] [CrossRef]
- Estrada-Villegas, G.M.; Bucio, E. Comparative Study of Grafting a Polyampholyte in a Fluoropolymer Membrane by Gamma Radiation in One or Two-Steps. Radiat. Phys. Chem. 2013, 92, 61–65. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Peng, J.; Wu, H.; Li, J.; Zhai, M. Adsorption of Cr(VI) Using Cellulose Microsphere-Based Adsorbent Prepared by Radiation-Induced Grafting. Radiat. Phys. Chem. 2012, 81, 967–970. [Google Scholar] [CrossRef]
- Grasselli, M.; Betz, N. Electron-Beam Induced RAFT-Graft Polymerization of Poly(Acrylic Acid) onto PVDF. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 236, 201–207. [Google Scholar] [CrossRef]
- Nasef, M.M.; Güven, O. Radiation-Grafted Copolymers for Separation and Purification Purposes: Status, Challenges and Future Directions. Prog. Polym. Sci. 2012, 37, 1597–1656. [Google Scholar] [CrossRef]
- Gupta, B.; Grover, N.; Mohanty, S.; Jain, K.G.; Singh, H. Radiation Grafting of Acrylic Acid/N-Vinyl Pyrrolidone Binary Mixture onto Poly(Ethylene Terephthalate) Fabric and Growth of Human Mesenchymal Stem Cell. J. Appl. Polym. Sci. 2010, 115, 116–126. [Google Scholar] [CrossRef]
- Abou Taleb, M.F.; Mahmoud, G.A.; Elsigeny, S.M.; Hegazy, E.S.A. Adsorption and Desorption of Phosphate and Nitrate Ions Using Quaternary (Polypropylene-g-N,N-Dimethylamino Ethylmethacrylate) Graft Copolymer. J. Hazard. Mater. 2008, 159, 372–379. [Google Scholar] [CrossRef]
- Desmet, G.; Takács, E.; Wojnárovits, L.; Borsa, J. Cellulose Functionalization via High-Energy Irradiation-Initiated Grafting of Glycidyl Methacrylate and Cyclodextrin Immobilization. Radiat. Phys. Chem. 2011, 80, 1358–1362. [Google Scholar] [CrossRef]
- Ishak, W.H.W.; Jia, O.Y.; Ahmad, I. PH-Responsive Gamma-Irradiated Poly(Acrylic Acid)-Cellulose-Nanocrystal-Reinforced Hydrogels. Polymers 2020, 12, 1932. [Google Scholar] [CrossRef]
- Abeer, M.M.; Amin, M.C.I.M.; Lazim, A.M.; Pandey, M.; Martin, C. Synthesis of a Novel Acrylated Abietic Acid-g-Bacterial Cellulose Hydrogel by Gamma Irradiation. Carbohydr. Polym. 2014, 110, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L. Synthesis of Carboxymethylcellulose/Acrylic Acid Hydrogels with Superabsorbent Properties by Radiation-Initiated Crosslinking. Radiat. Phys. Chem. 2016, 124, 135–139. [Google Scholar] [CrossRef]
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L. Synthesis of Cellulose-Based Superabsorbent Hydrogels by High-Energy Irradiation in the Presence of Crosslinking Agent. Radiat. Phys. Chem. 2014, 118, 114–119. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, J.; Xie, Y.; Gao, S.; Ling, Z.; Lai, C.; Wang, J.; Wang, C.; Chu, F.; Dumont, M.J.; et al. Mimicking Skin Cellulose Hydrogels for Sensor Applications. Chem. Eng. J. 2022, 427, 130921. [Google Scholar] [CrossRef]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering Nanocellulose Hydrogels for Biomedical Applications. Adv. Colloid Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Sakurai, A.; Sakakibara, M.; Saga, H. Preparation and Properties of Transparent Cellulose Hydrogels. J. Appl. Polym. Sci. 2003, 90, 3020–3025. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, M.; Qin, C.; Qian, X.; Zhang, L.; Zhou, J.; Lu, A. Transparent, Conductive Cellulose Hydrogel for Flexible Sensor and Triboelectric Nanogenerator at Subzero Temperature. Carbohydr. Polym. 2021, 265, 118078. [Google Scholar] [CrossRef]
- Sheiko, S.S.; Dobrynin, A.V. Architectural Code for Rubber Elasticity: From Supersoft to Superfirm Materials. Macromolecules 2019, 52, 7531–7546. [Google Scholar] [CrossRef]
- Sheiko, S.S.; Vashahi, F.; Morgan, B.J.; Maw, M.; Dashtimoghadam, E.; Fahimipour, F.; Jacobs, M.; Keith, A.N.; Vatankhah-Varnosfaderani, M.; Dobrynin, A.V.; et al. Mechanically Diverse Gels with Equal Solvent Content. ACS Cent. Sci. 2022, 8, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Teper, P.; Sotirova, A.; Mitova, V.; Oleszko-Torbus, N.; Utrata-Wesołek, A.; Koseva, N.; Kowalczuk, A.; Mendrek, B. Antimicrobial Activity of Hybrid Nanomaterials Based on Star and Linear Polymers of N,N’-Dimethylaminoethyl Methacrylate with In Situ Produced Silver Nanoparticles. Materials 2020, 13, 3037. [Google Scholar] [CrossRef]
- Chekini, M.; Krivoshapkina, E.; Shkodenko, L.; Koshel, E.; Shestovskaya, M.; Dukhinova, M.; Kheiri, S.; Khuu, N.; Kumacheva, E. Nanocolloidal Hydrogel with Sensing and Antibacterial Activities Governed by Iron Ion Sequestration. Chem. Mater. 2020, 32, 10066–10075. [Google Scholar] [CrossRef]
- O’Brien, N.; McKee, A.; Sherrington, D.C.; Slark, A.T.; Titterton, A. Facile, Versatile and Cost Effective Route to Branched Vinyl Polymers. Polymer 2000, 41, 6027–6031. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, D.; Lyu, J.; Sigen, A.; Xu, Q.; Newland, B.; Matyjaszewski, K.; Tai, H.; Wang, W. Complex Polymer Architectures through Free-Radical Polymerization of Multivinyl Monomers. Nat. Rev. Chem. 2020, 4, 194–212. [Google Scholar] [CrossRef]
- Cerid, H.; Okay, O. Minimization of Spatial Inhomogeneity in Polystyrene Gels Formed by Free-Radical Mechanism. Eur. Polym. J. 2004, 40, 579–587. [Google Scholar] [CrossRef]
- Besenius, P.; Slavin, S.; Vilela, F.; Sherrington, D.C. Synthesis and Characterization of Water-Soluble Densely Branched Glycopolymers. React. Funct. Polym. 2008, 68, 1524–1533. [Google Scholar] [CrossRef]
- Zhao, L.; Li, L.; Wang, Y.; Wu, J.; Meng, G.; Liu, Z.; Guo, X. Preparation and Characterization of Thermo- and PH Dual-Responsive 3D Cellulose-Based Aerogel for Oil/Water Separation. Appl. Phys. A 2018, 124, 9. [Google Scholar] [CrossRef]
- Shi, K.; Yang, X.; Xu, J.; Sha, D.; Wang, B.; Liu, X.; Liu, Z.; Ji, X. Preparation of Polyvinyl Alcohol Formaldehyde-g-Poly(2-(Dimethylamino)Ethyl Methacrylate) Macroporous Hydrogels and Their Dual Thermo/PH-Responsive Behavior and Antibacterial Performance. React. Funct. Polym. 2021, 164, 104916. [Google Scholar] [CrossRef]
- Potthast, A.; Rosenau, T.; Sixta, H.; Kosma, P. Degradation of Cellulosic Materials by Heating in DMAc/LiCl. Tetrahedron Lett. 2002, 43, 7757–7759. [Google Scholar] [CrossRef]
- Potthast, A.; Rosenau, T.; Sartori, J.; Sixta, H.; Kosma, P. Hydrolytic Processes and Condensation Reactions in the Cellulose Solvent System N,N-Dimethylacetamide/Lithium Chloride. Part 2: Degradation of Cellulose. Polymer 2002, 44, 7–17. [Google Scholar] [CrossRef]
- Tang, J.; Lee, M.F.X.; Zhang, W.; Zhao, B.; Berry, R.M.; Tam, K.C. Dual Responsive Pickering Emulsion Stabilized by Poly[2-(Dimethylamino) Ethyl Methacrylate] Grafted Cellulose Nanocrystals. Biomacromolecules 2014, 15, 3052–3060. [Google Scholar] [CrossRef]
- Li, T.; Shen, J.; Zhang, Z.; Wang, S.; Wei, D. A Poly(2-(Dimethylamino)Ethyl Methacrylate-Co-Methacrylic Acid) Complex Induced Route to Fabricate a Super-Hydrophilic Hydrogel and Its Controllable Oil/Water Separation. RSC Adv. 2016, 6, 40656–40663. [Google Scholar] [CrossRef]
- Geyik, G.; Işıklan, N. PH/Temperature-Responsive Poly(Dimethylaminoethyl Methacrylate) Grafted κ-Carrageenan Copolymer: Synthesis and Physicochemical Properties. J. Appl. Polym. Sci. 2020, 137, 49596. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, G.; Chu, L.; Liu, Y.; Liu, C.; Luo, S.; Wei, Y. Efficient Removal of Heavy Metal Ions with An EDTA Functionalized Chitosan/Polyacrylamide Double Network Hydrogel. ACS Sustain. Chem. Eng. 2017, 5, 843–851. [Google Scholar] [CrossRef]
- Barletta, F.; Liguori, A.; Leys, C.; Colombo, V.; Gherardi, M.; Nikiforov, A. Novel Method for NH-Rich Coatings Engineering by Means of Aerosol Assisted Atmospheric Pressure Plasma Deposition. Mater. Lett. 2018, 214, 76–79. [Google Scholar] [CrossRef]
- Rahmatpour, A.; Zamani, M. MBA-Cross-Linked Poly(N-Vinyl-2-Pyrrolidone)/Ferric Chloride Macromolecular Coordination Complex as a Novel and Recyclable Lewis Acid Catalyst: Synthesis, Characterization, and Performance toward for Regioselective Ring-Opening Alcoholysis of Epoxides. React. Funct. Polym. 2021, 168, 105032. [Google Scholar] [CrossRef]
- Gao, H.; Sun, Y.; Wang, M.; Wang, Z.; Han, G.; Jin, L.; Lin, P.; Xia, Y.; Zhang, K. Mechanically Robust and Reprocessable Acrylate Vitrimers with Hydrogen-Bond-Integrated Networks for Photo-3D Printing. ACS Appl. Mater. Interfaces 2021, 13, 1581–1591. [Google Scholar] [CrossRef]
- Sankar Panda, S.; Kumar Bisaria, S.; Singh, M.R. The Spectroscopic and Microscopic Evaluation of Cellulose Used in Conservation of Archival Materials. Microchem. J. 2021, 160, 105707. [Google Scholar] [CrossRef]
- Guo, X.; Liu, L.; Wu, J.; Fan, J.; Wu, Y. Qualitatively and Quantitatively Characterizing Water Adsorption of a Cellulose Nanofiber Film Using Micro-FTIR Spectroscopy. RSC Adv. 2018, 8, 4214–4220. [Google Scholar] [CrossRef]
- Mohan, T.; Spirk, S.; Kargl, R.; Doliška, A.; Vesel, A.; Salzmann, I.; Resel, R.; Ribitsch, V.; Stana-Kleinschek, K. Exploring the Rearrangement of Amorphous Cellulose Model Thin Films upon Heat Treatment. Soft Matter 2012, 8, 9807–9815. [Google Scholar] [CrossRef]
- Jiang, P.; Li, G.; Lv, L.; Ji, H.; Li, Z.; Chen, S.; Chu, S. Effect of DMAEMA Content and Polymerization Mode on Morphologies and Properties of PH and Temperature Double-Sensitive Cellulose-Based Hydrogels. J. Macromol. Sci. 2020, 57, 207–216. [Google Scholar] [CrossRef]
- Capanema, N.S.V.; Mansur, A.A.P.; de Jesus, A.C.; Carvalho, S.M.; de Oliveira, L.C.; Mansur, H.S. Superabsorbent Crosslinked Carboxymethyl Cellulose-PEG Hydrogels for Potential Wound Dressing Applications. Int. J. Biol. Macromol. 2018, 106, 1218–1234. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Huang, J.; Zhong, Y.; Li, K.; Zhang, L.; Cai, J. High-Strength and High-Toughness Double-Cross-Linked Cellulose Hydrogels: A New Strategy Using Sequential Chemical and Physical Cross-Linking. Adv. Funct. Mater. 2016, 26, 6279–6287. [Google Scholar] [CrossRef]
- Guo, Y.Z.; Nakajima, T.; Mredha, M.T.I.; Guo, H.L.; Cui, K.; Zheng, Y.; Cui, W.; Kurokawa, T.; Gong, J.P. Facile Preparation of Cellulose Hydrogel with Achilles Tendon-like Super Strength through Aligning Hierarchical Fibrous Structure. Chem. Eng. J. 2022, 428, 132040. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Peppas, N.A. The Swollen Polymer Network Hypothesis: Quantitative Models of Hydrogel Swelling, Stiffness, and Solute Transport. Prog. Polym. Sci. 2020, 105, 101243. [Google Scholar] [CrossRef]
- An, H.; Bo, Y.; Chen, D.; Wang, Y.; Wang, H.; He, Y.; Qin, J. Cellulose-Based Self-Healing Hydrogel through Boronic Ester Bonds with Excellent Biocompatibility and Conductivity. RSC Adv. 2020, 10, 11300–11310. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, X.; Yang, B.; Zhang, L.; Lu, A. Highly Self-Healable and Injectable Cellulose Hydrogels via Rapid Hydrazone Linkage for Drug Delivery and 3D Cell Culture. Carbohydr. Polym. 2021, 273, 118547. [Google Scholar] [CrossRef]
- Le Goff, K.J.; Gaillard, C.; Helbert, W.; Garnier, C.; Aubry, T. Rheological Study of Reinforcement of Agarose Hydrogels by Cellulose Nanowhiskers. Carbohydr. Polym. 2015, 116, 117–123. [Google Scholar] [CrossRef]
- Bertasa, M.; Dodero, A.; Alloisio, M.; Vicini, S.; Riedo, C.; Sansonetti, A.; Scalarone, D.; Castellano, M. Agar Gel Strength: A Correlation Study between Chemical Composition and Rheological Properties. Eur. Polym. J. 2020, 123, 109442. [Google Scholar] [CrossRef]
- Jacobs, M.; Vashahi, F.; Maw, M.; Sheiko, S.S.; Dobrynin, A.V. Brush Gels: Where Theory, Simulations, and Experiments Meet. Macromolecules 2022, 55, 7922–7931. [Google Scholar] [CrossRef]
- Wisotzki, E.I.; Hennes, M.; Schuldt, C.; Engert, F.; Knolle, W.; Decker, U.; Käs, J.A.; Zink, M.; Mayr, S.G. Tailoring the Material Properties of Gelatin Hydrogels by High Energy Electron Irradiation. J. Mater. Chem. B 2014, 2, 4297–4309. [Google Scholar] [CrossRef]
- Krömmelbein, C.; Mütze, M.; Konieczny, R.; Schönherr, N.; Griebel, J.; Gerdes, W.; Mayr, S.G.; Riedel, S. Impact of High-Energy Electron Irradiation on Mechanical, Structural and Chemical Properties of Agarose Hydrogels. Carbohydr. Polym. 2021, 263, 117970. [Google Scholar] [CrossRef]
- Ressler, A.; Kamboj, N.; Ledinski, M.; Rogina, A.; Urlić, I.; Hussainova, I.; Ivanković, H.; Ivanković, M. Macroporous Silicon-Wollastonite Scaffold with Sr/Se/Zn/Mg-Substituted Hydroxyapatite/Chitosan Hydrogel. Open Ceram. 2022, 12, 100306. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; de Marco, I.; Reverchon, E. Chitosan Scaffolds Formation by a Supercritical Freeze Extraction Process. J. Supercrit. Fluids 2014, 90, 27–34. [Google Scholar] [CrossRef]
- Soyekwo, F.; Zhang, Q.G.; Deng, C.; Gong, Y.; Zhu, A.M.; Liu, Q.L. Highly Permeable Cellulose Acetate Nanofibrous Composite Membranes by Freeze-Extraction. J. Membr. Sci. 2014, 454, 339–345. [Google Scholar] [CrossRef]
- Rogina, A.; Pribolšan, L.; Hanžek, A.; Gómez-Estrada, L.; Gallego Ferrer, G.; Marijanović, I.; Ivanković, M.; Ivanković, H. Macroporous Poly(Lactic Acid) Construct Supporting the Osteoinductive Porous Chitosan-Based Hydrogel for Bone Tissue Engineering. Polymer 2016, 98, 172–181. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, L.; Feng, J.; Dong, H.; Zhang, C.; Ma, F.; Wang, Q. Efficient Uranium Adsorption by Amidoximized Porous Polyacrylonitrile with Hierarchical Pore Structure Prepared by Freeze-Extraction. J. Mol. Liq. 2021, 328, 115304. [Google Scholar] [CrossRef]
- Ye, D.; Zhong, Z.; Xu, H.; Chang, C.; Yang, Z.; Wang, Y.; Ye, Q.; Zhang, L. Construction of Cellulose/Nanosilver Sponge Materials and Their Antibacterial Activities for Infected Wounds Healing. Cellulose 2016, 23, 749–763. [Google Scholar] [CrossRef]
- Nuñez, Y.A.R.; Castro, R.I.; Arenas, F.A.; López-Cabaña, Z.E.; Carreño, G.; Carrasco-Sánchez, V.; Marican, A.; Villaseñor, J.; Vargas, E.; Santos, L.S.; et al. Preparation of Hydrogel/Silver Nanohybrids Mediated by Tunable-Size Silver Nanoparticles for Potential Antibacterial Applications. Polymers 2019, 11, 716. [Google Scholar] [CrossRef]
- Murali Mohan, Y.; Vimala, K.; Thomas, V.; Varaprasad, K.; Sreedhar, B.; Bajpai, S.K.; Mohana Raju, K. Controlling of Silver Nanoparticles Structure by Hydrogel Networks. J. Colloid Interface Sci. 2010, 342, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Blažic, R.; Kučić Grgić, D.; Kraljić Roković, M.; Vidović, E. Cellulose-g-Poly(2-(Dimethylamino)Ethylmethacrylate) Hydrogels: Synthesis, Characterization, Antibacterial Testing and Polymer Electrolyte Application. Gels 2022, 8, 636. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xu, D.; Yuan, H.; Luo, Q.; Tang, S.; Liu, L.; Wu, Y. A Novel Fluorescent Nanocellulosic Hydrogel Based on Carbon Dots for Efficient Adsorption and Sensitive Sensing in Heavy Metals. J. Mater. Chem. A 2019, 7, 27081–27088. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Wang, G.; Hao, C.; Li, X.; Li, T. Adsorption Performance of a Polysaccharide Composite Hydrogel Based on Crosslinked Glucan/Chitosan for Heavy Metal Ions. Compos. Part B Eng. 2019, 169, 45–54. [Google Scholar] [CrossRef]
- Blažic, R.; Lenac, K.; Vidović, E. Priprava celuloznih hidrogelova modificiranih 2-Dimetilaminoetil-metakrilatom i srebrovim nanočesticama. Kem. u Ind. 2020, 69, 269–279. [Google Scholar] [CrossRef]
- Fu, L.; Li, H. Toward Quantitative Prediction of the Mechanical Properties of Tandem Modular Elastomeric Protein-Based Hydrogels. Macromolecules 2020, 53, 4704–4710. [Google Scholar] [CrossRef]
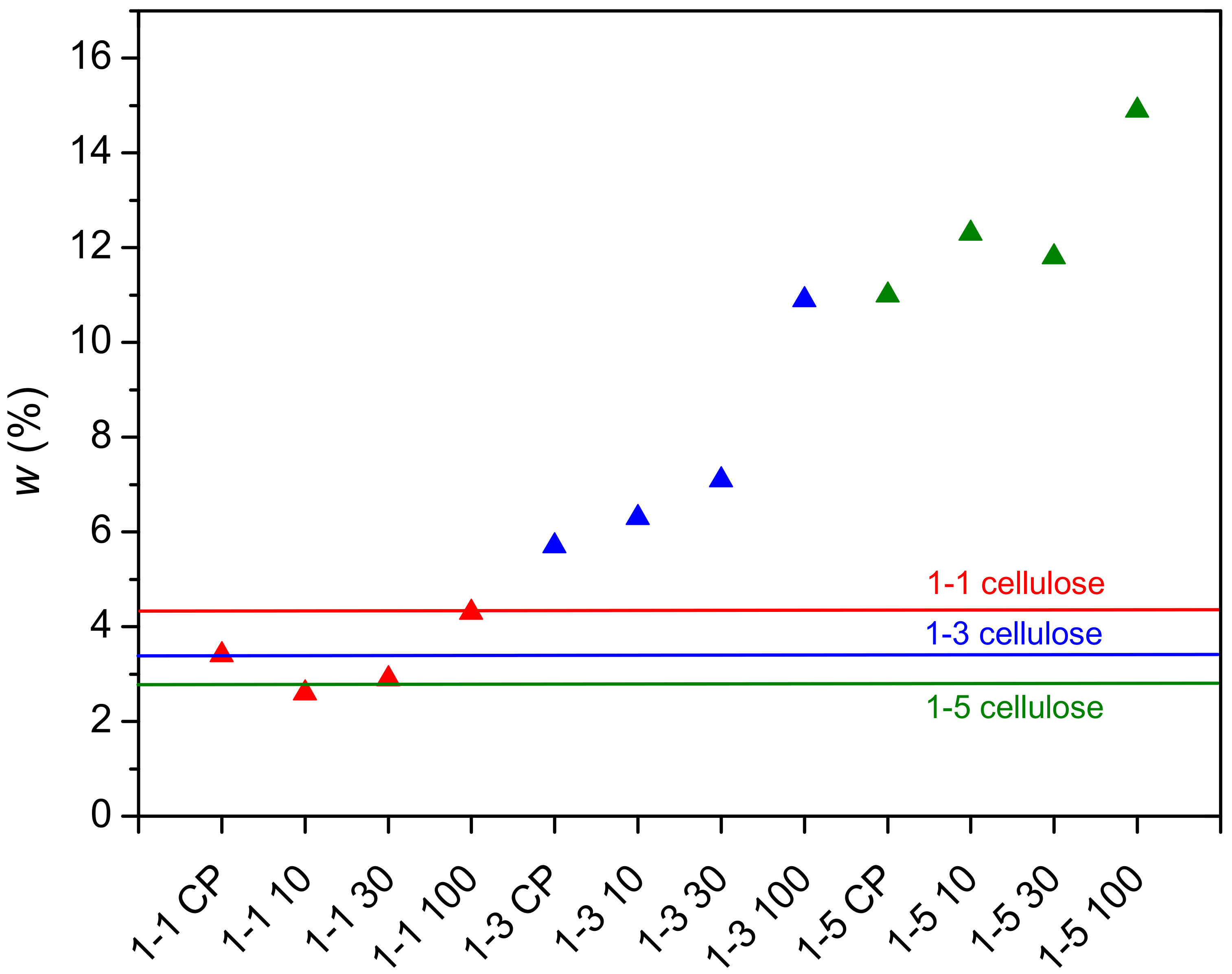





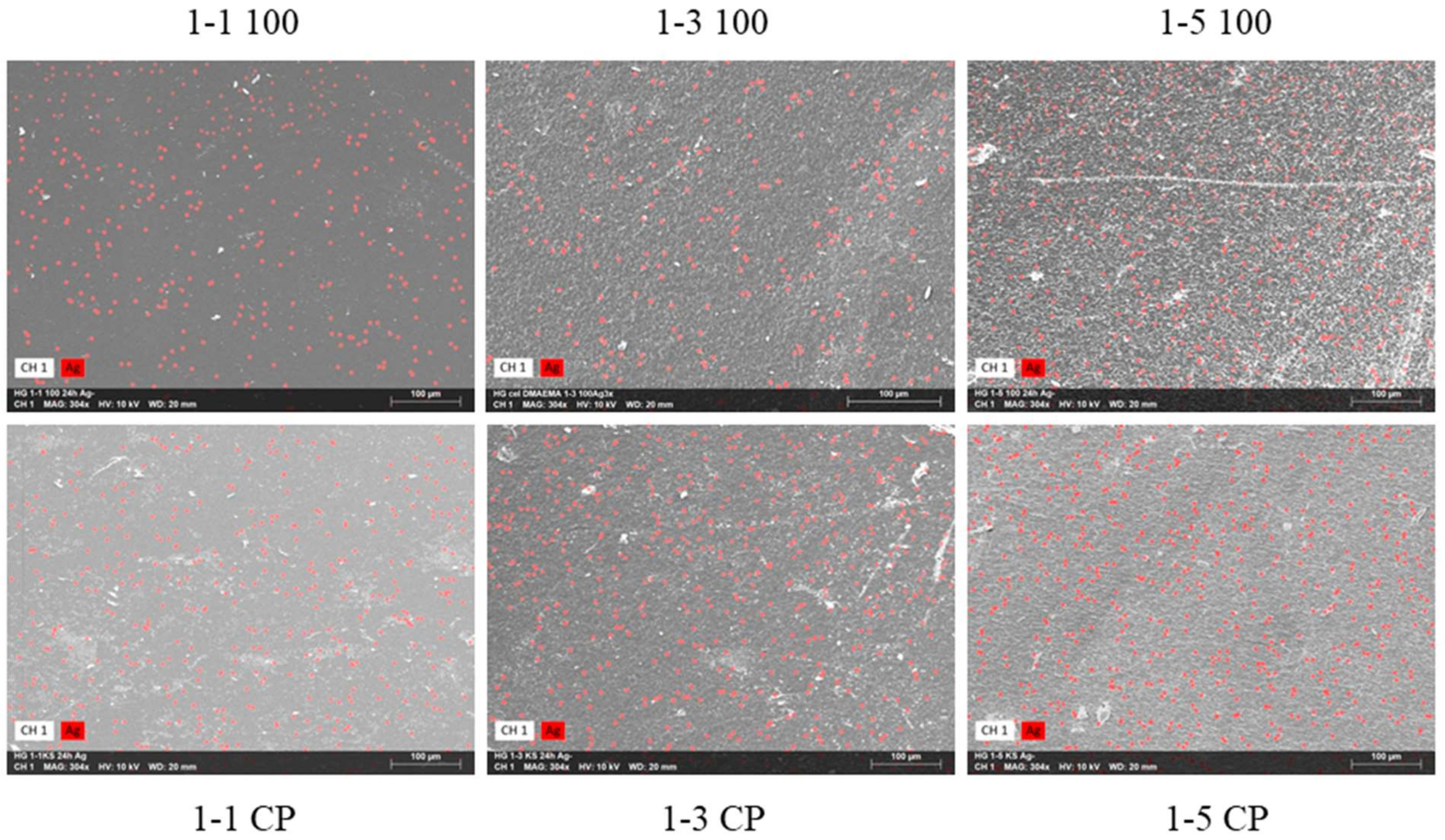
| Sample | w (N) (wt%) | ±σ (wt%) |
|---|---|---|
| 1-1 CP | n. d. * | n. d. * |
| 1-1 100 | 1.8 | 0.1 |
| 1-3 CP | 3.1 | 0.2 |
| 1-3 100 | 5.9 | 0.4 |
| 1-5 CP | 4.8 | 0.2 |
| 1-5 100 | 7.9 | 0.3 |
| Sample | Mc (g mol−1) |
|---|---|
| Cellulose | 5373 |
| 1-1 CP | 5239 |
| 1-1 100 | 7165 |
| 1-3 CP | 9411 |
| 1-3 100 | 9431 |
| 1-5 CP | 10231 |
| 1-5 100 | 7945 |
| Sample | w (Ag) (wt%) | ±σ (wt%) |
|---|---|---|
| Cellulose | 0.9 | 0.2 |
| 1-1 CP | 1.2 | 0.2 |
| 1-1 100 | 1.8 | 0.2 |
| 1-3 CP | 4.0 | 0.3 |
| 1-3 100 | 3.9 | 0.5 |
| 1-5 CP | 3.1 | 0.3 |
| 1-5 100 | 5.5 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blažic, R.; Marušić, K.; Vidović, E. Swelling and Viscoelastic Properties of Cellulose-Based Hydrogels Prepared by Free Radical Polymerization of Dimethylaminoethyl Methacrylate in Cellulose Solution. Gels 2023, 9, 94. https://doi.org/10.3390/gels9020094
Blažic R, Marušić K, Vidović E. Swelling and Viscoelastic Properties of Cellulose-Based Hydrogels Prepared by Free Radical Polymerization of Dimethylaminoethyl Methacrylate in Cellulose Solution. Gels. 2023; 9(2):94. https://doi.org/10.3390/gels9020094
Chicago/Turabian StyleBlažic, Roko, Katarina Marušić, and Elvira Vidović. 2023. "Swelling and Viscoelastic Properties of Cellulose-Based Hydrogels Prepared by Free Radical Polymerization of Dimethylaminoethyl Methacrylate in Cellulose Solution" Gels 9, no. 2: 94. https://doi.org/10.3390/gels9020094
APA StyleBlažic, R., Marušić, K., & Vidović, E. (2023). Swelling and Viscoelastic Properties of Cellulose-Based Hydrogels Prepared by Free Radical Polymerization of Dimethylaminoethyl Methacrylate in Cellulose Solution. Gels, 9(2), 94. https://doi.org/10.3390/gels9020094