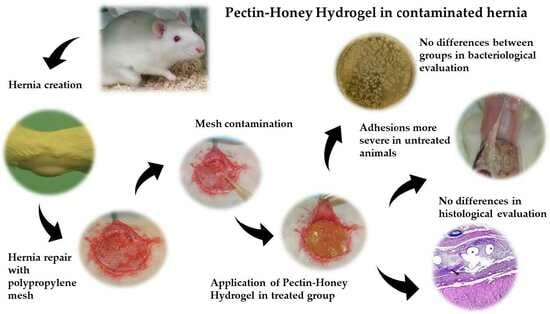
The Effects of Pectin–Honey Hydrogel in a Contaminated Chronic Hernia Model in Rats
Abstract
:1. Introduction
2. Results
2.1. Macroscopical Evaluation
2.2. Bacteriological Evaluation
2.3. Histological Evaluation and Immunohistochemical Analysis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals
5.2. Preparation of PHH
5.3. Bacterial Growth
5.4. First Surgery: Abdominal Defect Creation
5.5. Second Surgery: Abdominal Defect Repair and Contamination
5.6. Macroscopical Evaluation
5.7. Bacteriological Evaluation
5.8. Histological Evaluation and Immunohistochemical Analysis
5.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muysoms, F.E.; Antoniou, S.A.; Bury, K.; Campanelli, G.; Conze, J.; Cuccurullo, D.; De Beaux, A.C.; Deerenberg, E.B.; East, B.; Fortelny, R.H.; et al. European Hernia Society Guidelines on the Closure of Abdominal Wall Incisions. Hernia 2015, 19, 1–24. [Google Scholar] [CrossRef]
- Walming, S.; Angenete, E.; Block, M.; Bock, D.; Gessler, B.; Haglind, E. Retrospective Review of Risk Factors for Surgical Wound Dehiscence and Incisional Hernia. BMC Surg. 2017, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Luijendijk, R.W.; Hop, W.C.; van den Tol, M.P.; de Lange, D.C.; Braaksma, M.M.; IJzermans, J.N.; Boelhouwer, R.U.; de Vries, B.C.; Salu, M.K.; Wereldsma, J.C.; et al. A comparison of suture repair with mesh repair for incisional hernia. N. Engl. J. Med. 2020, 343, 392–398. [Google Scholar] [CrossRef]
- van Ramshorst, G.H.; Eker, H.H.; Hop, W.C.; Jeekel, J.; Lange, J.F. Impact of incisional hernia on health-related quality of life and body image: A prospective cohort study. Am. J. Surg. 2012, 204, 144–150. [Google Scholar] [CrossRef]
- Ortega-Deballon, P.; Renard, Y.; De Launay, J.; Lafon, T.; Roset, Q.; Passot, G. Incidence, Risk Factors, and Burden of Incisional Hernia Repair after Abdominal Surgery in France: A Nationwide Study. Hernia 2023, 27, 861–871. [Google Scholar] [CrossRef]
- Pande, T.; Naidu, C.S. Mesh Infection in Cases of Polypropylene Mesh Hernioplasty. Hernia 2020, 24, 849–856. [Google Scholar] [CrossRef]
- Sanchez, V.M.; Abi-Haidar, Y.E.; Itani, K.M.F. Mesh Infection in Ventral Incisional Hernia Repair: Incidence, Contributing Factors, and Treatment. Surg. Infect. 2011, 12, 205–210. [Google Scholar] [CrossRef]
- Paton, B.L.; Novitsky, Y.W.; Zerey, M.; Sing, R.F.; Kercher, K.W.; Todd Heniford, B. Management of Infections of Polytetrafluoroethylene-Based Mesh. Surg. Infect. 2007, 8, 337–342. [Google Scholar] [CrossRef]
- Brown, C.; Finch, J. Which Mesh for Hernia Repair? Annals 2010, 92, 272–278. [Google Scholar] [CrossRef]
- Owens, C.D.; Stoessel, K. Surgical Site Infections: Epidemiology, Microbiology and Prevention. J. Hosp. Infect. 2008, 70, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am. J. Infect Control. 1999, 27, 97–132; quiz 133–134; discussion 96. [Google Scholar] [CrossRef] [PubMed]
- Kallick, E.; Nistico, L.; Longwell, M.; Byers, B.; Cartieri, F.; Kreft, R.; Edington, H. Resistance of Synthetic and Biologic Surgical Meshes to Methicillin-Resistant Staphylococcus Aureus Biofilm: An In Vitro Investigation. Int. J. Biomater. 2019, 2019, 1063643. [Google Scholar] [CrossRef] [PubMed]
- Engelsman, A.F.; Van Der Mei, H.C.; Ploeg, R.J.; Busscher, H.J. The Phenomenon of Infection with Abdominal Wall Reconstruction. Biomaterials 2007, 28, 2314–2327. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.B.; Farooque, Y. Risks and Prevention of Surgical Site Infection After Hernia Mesh Repair and the Predictive Utility of ACS-NSQIP. J. Gastrointest. Surg. 2022, 26, 950–964. [Google Scholar] [CrossRef]
- Catena, F.; Ansaloni, L.; Gazzotti, F.; Gagliardi, S.; Di Saverio, S.; D’Alessandro, L.; Pinna, A.D. Use of porcine dermal collagen graft (Permacol) for hernia repair in contaminated fields. Hernia 2006, 11, 57–60. [Google Scholar] [CrossRef]
- Costa, A.; Naranjo, J.; Turner, N.; Swinehart, I.; Kolich, B.; Shaffiey, S.; Londono, R.; Keane, T.; Reing, J.; Johnson, S.; et al. Mechanical strength vs. degradation of a biologically-derived surgical mesh over time in a rodent full thickness abdominal wall defect. Biomaterials 2016, 108, 81–90. [Google Scholar] [CrossRef]
- Butler, C.E.; Prieto, V.G. Reduction of Adhesions with Composite AlloDerm/Polypropylene Mesh Implants for Abdominal Wall Reconstruction. Plast. Reconstr. Surg. 2004, 114, 464–473. [Google Scholar] [CrossRef]
- Fernandez-Moure, J.S.; Van Eps, J.L.; Scherba, J.C.; Haddix, S.; Livingston, M.; Bryan, N.S.; Cantu, C.; Valson, C.; Taraballi, F.; Kaplan, L.J.; et al. Polyester Mesh Functionalization with Nitric Oxide-Releasing Silica Nanoparticles Reduces Early Methicillin-Resistant Staphylococcus Aureus Contamination. Surg. Infect. 2021, 22, 910–922. [Google Scholar] [CrossRef]
- Mirel, S.; Pusta, A.; Moldovan, M.; Moldovan, S. Antimicrobial Meshes for Hernia Repair: Current Progress and Perspectives. JCM 2022, 11, 883. [Google Scholar] [CrossRef]
- Giusto, G.; Vercelli, C.; Comino, F.; Caramello, V.; Tursi, M.; Gandini, M. A New, Easy-to-Make Pectin-Honey Hydrogel Enhances Wound Healing in Rats. BMC Complement Altern. Med. 2017, 17, 266. [Google Scholar] [CrossRef]
- Giusto, G.; Beretta, G.; Vercelli, C.; Valle, E.; Iussich, S.; Borghi, R.; Odetti, P.; Monacelli, F.; Tramuta, C.; Grego, E.; et al. Pectin-honey hydrogel: Characterization, antimicrobial activity and biocompatibility. Biomed. Mater. Eng. 2018, 29, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Tramuta, C.; Nebbia, P.; Robino, P.; Giusto, G.; Gandini, M.; Chiadò-Cutin, S.; Grego, E. Antibacterial activities of Manuka and Honeydew honey-based membranes against bacteria that cause wound infections in animals. Antibakterielle Aktivität von Membranen aus Manuka- und Honigtauhonig gegen Wundkeime bei Tieren. Schweiz. Arch. Tierheilkd. 2017, 159, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Giusto, G.; Vercelli, C.; Iussich, S.; Audisio, A.; Morello, E.; Odore, R.; Gandini, M. A Pectin-Honey Hydrogel Prevents Postoperative Intraperitoneal Adhesions in a Rat Model. BMC Vet. Res. 2016, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Vercelli, C.; Re, G.; Iussich, S.; Odore, R.; Morello, E.M.; Gandini, M.; Giusto, G. In Vivo Evaluation of a Pectin-Honey Hydrogel Coating on Polypropylene Mesh in a Rat Model of Acute Hernia. Gels 2021, 7, 132. [Google Scholar] [CrossRef]
- Van Eps, J.; Fernandez-Moure, J.; Cabrera, F.; Wang, X.; Karim, A.; Corradetti, B.; Chan, P.; Dunkin, B.; Tasciotti, E.; Weiner, B.; et al. Decreased hernia recurrence using autologous platelet-rich plasma (PRP) with Strattice™ mesh in a rodent ventral hernia model. Surg. Endosc. 2016, 30, 3239–3249. [Google Scholar] [CrossRef]
- He, L.; Wang, X.; Fan, G.; Zhao, Y. Hernia mesh infection treatment following the repair of abdominal wall hernias: A single-center experience. Front Surg. 2022, 9, 993855. [Google Scholar] [CrossRef]
- Primus, F.E.; Harris, H.W. A Critical Review of Biologic Mesh Use in Ventral Hernia Repairs under Contaminated Conditions. Hernia 2013, 17, 21–30. [Google Scholar] [CrossRef]
- Lee, L.; Mata, J.; Landry, T.; Khwaja, K.A.; Vassiliou, M.C.; Fried, G.M.; Feldman, L.S. A Systematic Review of Synthetic and Biologic Materials for Abdominal Wall Reinforcement in Contaminated Fields. Surg. Endosc. 2014, 28, 2531–2546. [Google Scholar] [CrossRef]
- Bondre, I.L.; Holihan, J.L.; Askenasy, E.P.; Greenberg, J.A.; Keith, J.N.; Martindale, R.G.; Roth, J.S.; Liang, M.K. Suture, synthetic, or biologic in contaminated ventral hernia repair. J. Surg. Res. 2016, 200, 488–494. [Google Scholar] [CrossRef]
- Birolini, C.; Massazo Utiyama, E.; Junqueira Rodrigues, A.; Birolini, D. Elective Colonic Operation and Prosthetic Repair of Incisional Hernia: Does Contamination Contraindicate Abdominal Wall Prosthesis Use?11No Competing Interests Declared. J. Am. Coll. Surg. 2000, 191, 366–372. [Google Scholar] [CrossRef]
- Warren, J.; Desai, S.S.; Boswell, N.D.; Hancock, B.H.; Abbad, H.; Ewing, J.A.; Carbonell, A.M.; Cobb, W.S. Safety and Efficacy of Synthetic Mesh for Ventral Hernia Repair in a Contaminated Field. J. Am. Coll. Surg. 2020, 230, 405–413. [Google Scholar] [CrossRef]
- Felemovicius, I.; Bonsack, M.E.; Hagerman, G.; Delaney, J.P. Prevention of Adhesions to Polypropylene Mesh1. J. Am. Coll. Surg. 2004, 198, 543–548. [Google Scholar] [CrossRef]
- Baptista, M.L.; Bonsack, M.E.; Delaney, J.P. Seprafilm Reduces Adhesions to Polypropylene Mesh. Surgery 2000, 128, 86–92. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, L.; Ceci, F.; Mattia, S.; Fabbi, M.; Negro, P.; Gossetti, F. Adhesion Prevention in Ventral Hernia Repair: An Experimental Study Comparing Three Lightweight Porous Meshes Recommended for Intraperitoneal Use. Hernia 2017, 21, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Van Steensel, S.; Gielen, M.; Vercoulen, T.; Melenhorst, J.; Winkens, B.; Bouvy, N.D. Comparison of Coated Meshes for Intraperitoneal Placement in Animal Studies: A Systematic Review and Meta-Analysis. Hernia 2020, 24, 1253–1261. [Google Scholar] [CrossRef]
- Burger, J.W.A.; Halm, J.A.; Wijsmuller, A.R.; Raa, S.T.; Jeekel, J. Evaluation of New Prosthetic Meshes for Ventral Hernia Repair. Surg. Endosc. 2006, 20, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Pandey, H.; Thakur, D.S.; Somashekar, U.; Kothari, R.; Agarwal, P.; Sharma, D. Use of Polypropylene Mesh in Contaminated and Dirty Strangulated Hernias: Short-Term Results. Hernia 2018, 22, 1045–1050. [Google Scholar] [CrossRef]
- Anastasio, A.T.; Van Eps, J.L.; Fernandez-Moure, J.S. Surgical Technique for Development of a Clinically-Representative Ventral Hernia Repair Infection Rat Model. MethodsX 2020, 7, 100887. [Google Scholar] [CrossRef]
- Sotocinal, S.G.; Sorge, R.E.; Zaloum, A.; Tuttle, A.H.; Martin, L.J.; Wieskopf, J.S.; Mapplebeck, J.C.; Wei, P.; Zhan, S.; Zhang, S.; et al. The Rat Grimace Scale: A Partially Automated Method for Quantifying Pain in the Laboratory Rat via Facial Expressions. Mol. Pain. 2011, 7, 1744–8069. [Google Scholar] [CrossRef]
- Pereira-Lucena, C.G.; Neto, R.A.; De Rezende, D.T.; Lopes-Filho, G.D.J.; Matos, D.; Linhares, M.M. Early and late postoperative inflammatory and collagen deposition responses in three different meshes: An experimental study in rats. Hernia 2013, 18, 563–570. [Google Scholar] [CrossRef] [PubMed]










| T (n = 9) | C (n = 8) | p Value | |
|---|---|---|---|
| CFU/mL 24 h | 2 | 100 | 0.672 |
| 2 | 59 | ||
| 20 | 0 | ||
| 15 | 12 | ||
| 0 | 0 | ||
| 0 | 0 | ||
| 25 | 0 | ||
| 1 | 0 | ||
| 0 | |||
| CFU/mL 48 h | 2 | 406 | 0.869 |
| 0 | 62 | ||
| 25 | 0 | ||
| 27 | 12 | ||
| 0 | 1 | ||
| 0 | 0 | ||
| 28 | 2 | ||
| 1 | 0 | ||
| 1 |
| T (n = 9) | C (n = 8) | p Value | |
|---|---|---|---|
| Median (range) | |||
| Cells layers at margins of the granulomas | 3 (2–3) | 2,5 (2–3) | 0.59 |
| Inflammatory reaction in the host tissue | 3 (2–4) | 2 (2–3) | 0.23 |
| Inflammatory response on the mesh surface | 3 (2–4) | 3 (2–4) | 0.63 |
| Tissue maturation | 3 (2–4) | 2 (1–3) | 0.28 |
| T (n = 9) | C (n = 8) | p Value | |
|---|---|---|---|
| Median (range) | |||
| COX % cell | 2 (1–3) | 2 (1–3) | 0.59 |
| COX intensity | 2 (1–3) | 2 (1–3) | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerullo, A.; Giusto, G.; Maniscalco, L.; Nebbia, P.; von Degerfeld, M.M.; Serpieri, M.; Vercelli, C.; Gandini, M. The Effects of Pectin–Honey Hydrogel in a Contaminated Chronic Hernia Model in Rats. Gels 2023, 9, 811. https://doi.org/10.3390/gels9100811
Cerullo A, Giusto G, Maniscalco L, Nebbia P, von Degerfeld MM, Serpieri M, Vercelli C, Gandini M. The Effects of Pectin–Honey Hydrogel in a Contaminated Chronic Hernia Model in Rats. Gels. 2023; 9(10):811. https://doi.org/10.3390/gels9100811
Chicago/Turabian StyleCerullo, Anna, Gessica Giusto, Lorella Maniscalco, Patrizia Nebbia, Mitzy Mauthe von Degerfeld, Matteo Serpieri, Cristina Vercelli, and Marco Gandini. 2023. "The Effects of Pectin–Honey Hydrogel in a Contaminated Chronic Hernia Model in Rats" Gels 9, no. 10: 811. https://doi.org/10.3390/gels9100811
APA StyleCerullo, A., Giusto, G., Maniscalco, L., Nebbia, P., von Degerfeld, M. M., Serpieri, M., Vercelli, C., & Gandini, M. (2023). The Effects of Pectin–Honey Hydrogel in a Contaminated Chronic Hernia Model in Rats. Gels, 9(10), 811. https://doi.org/10.3390/gels9100811