Diosmin-Loaded Nanoemulsion-Based Gel Formulation: Development, Optimization, Wound Healing and Anti-Inflammatory Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Solubility Studies
2.2. Experimental Design
2.2.1. Effect of Independent Variables on Globule Size
2.2.2. Effect of Independent Variables on PDI
2.2.3. Effect of Independent Variables on %EE
2.3. Measurement of Globule Size, PDI, %EE and Zeta Potential
2.4. Determination of Viscosity, Refractive Index (RI) and pH
2.5. Thermodynamic Stability Studies
2.6. Transmission Electron Microscopy (TEM)
2.7. Evaluation of Gel
2.7.1. pH and Drug Content
2.7.2. Spreadability
2.8. In Vitro Release Studies and Kinetics
2.9. Acute Dermal Toxicity
2.10. Evaluation of Wound Healing Activity
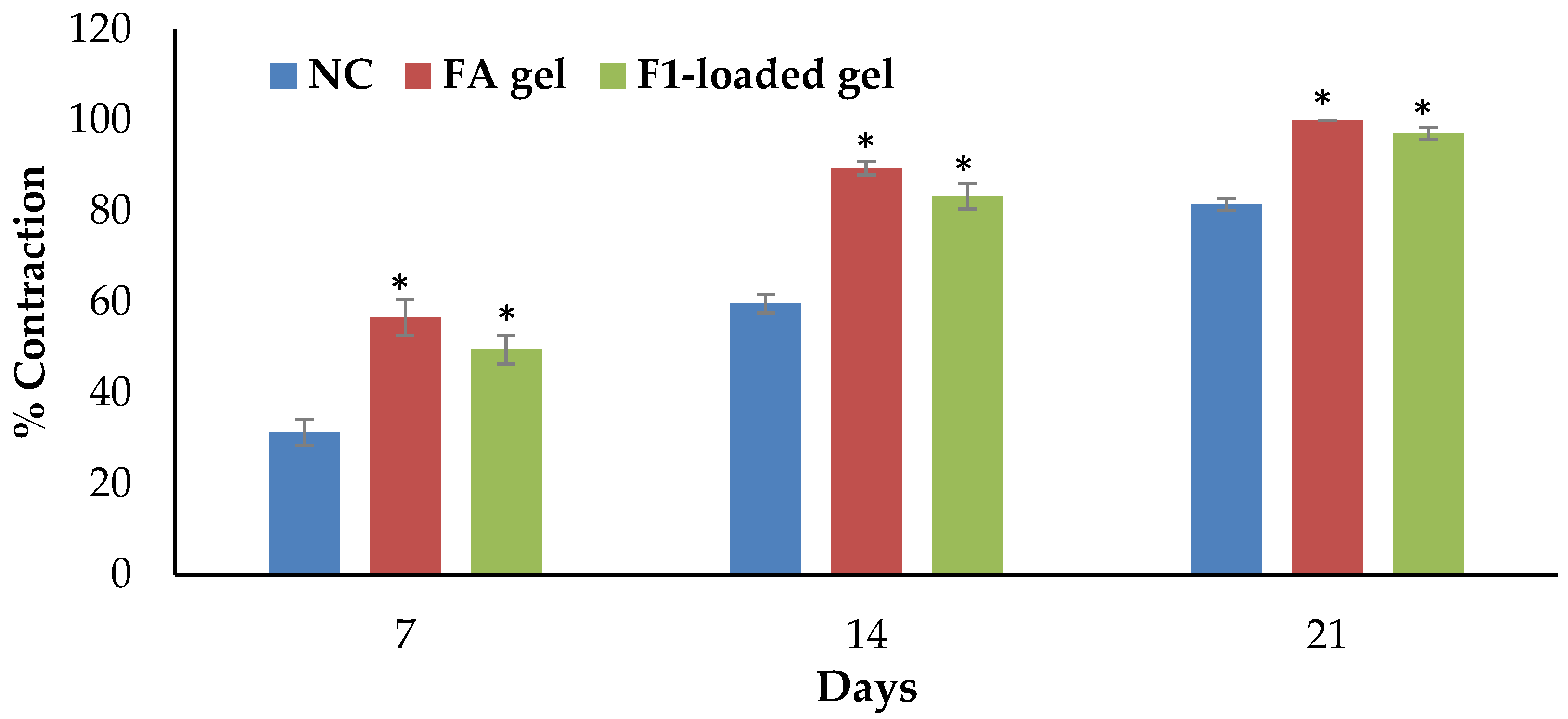
2.10.1. Excision Wound Model
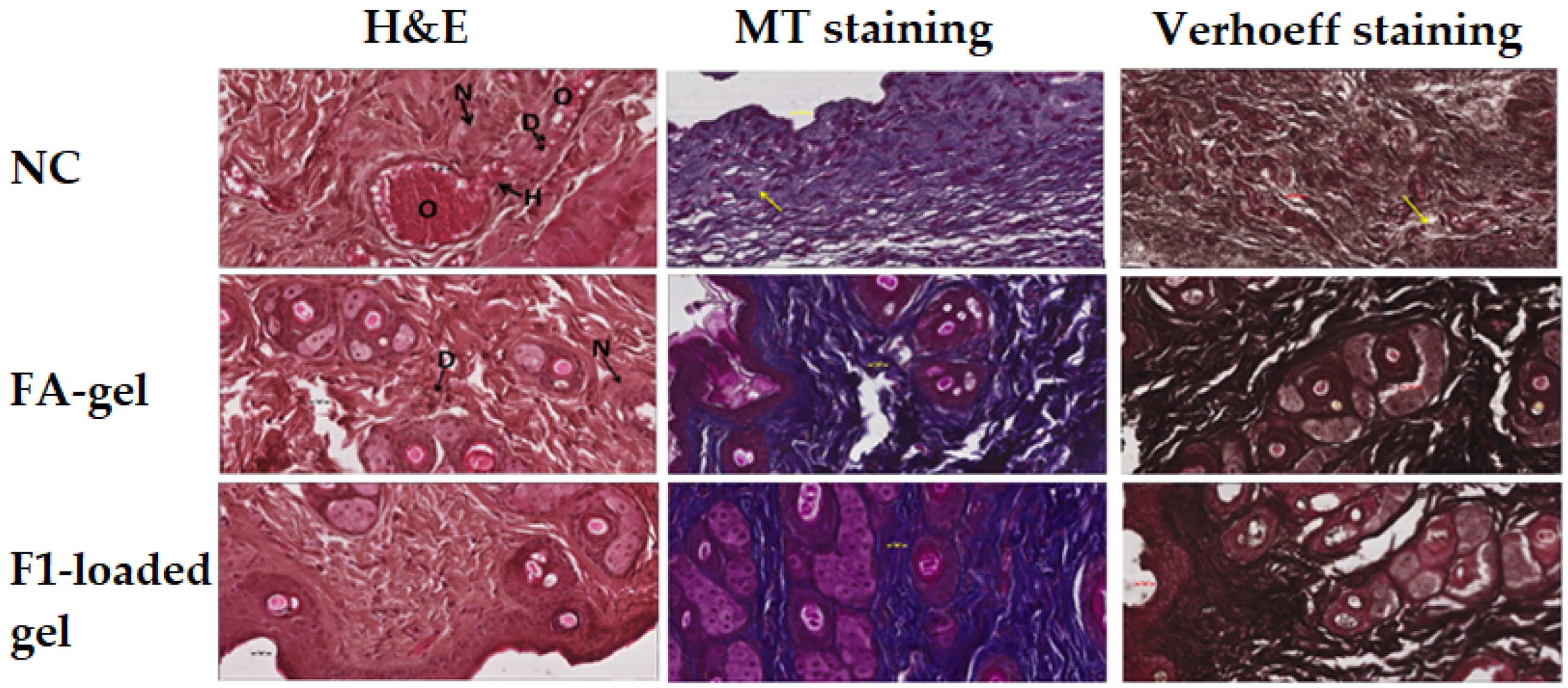
2.10.2. Microscopic Evaluation of the Wound
2.11. Evaluation of Anti-Inflammatory
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Solubility Studies
4.3. Experimental Design
4.4. Selection of Optimized Formulation
4.5. Preparation of DSM-Loaded NEs
4.6. Measurement of Globule Size, PDI, %EE and Zeta Potential
4.7. Determination of Refractive Index (RI) and pH
4.8. Thermodynamic Stability Studies
4.9. Transmission Electron Microscopy (TEM)
4.10. Preparation of DSM-Loaded NEs Gels
4.11. Evaluation of Gel
4.11.1. pH and Drug Content
4.11.2. Spreadability
4.12. In Vitro Diffusion Studies and Release Kinetics
4.13. Experimental Animals
4.14. Acute Dermal Toxicity
4.15. Evaluation of Wound Healing Activity
4.15.1. Excision Wound Model
4.15.2. Microscopic Evaluation of the Wound
4.16. In Vivo Anti-Inflammatory Activity
4.17. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srinivasan, S.; Pari, L. Ameliorative effect of diosmin, a citrus flavonoid against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Chem.-Biol. Interact. 2012, 195, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.T.; Li, H.B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.Y. Citrus Flavonoids as Promising Phytochemicals Targeting Diabetes and Related Complications: A Systematic Review of In Vitro and In Vivo Studies. Nutrients 2020, 12, 2907. [Google Scholar] [CrossRef] [PubMed]
- Gerges, S.H.; Wahdan, S.A.; Elsherbiny, D.A.; El-Demerdash, E. Pharmacology of Diosmin, a Citrus Flavone Glycoside: An Updated Review. Eur. J. Drug Metab. Pharmacokinet. 2022, 47, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kocka, A.B.; Woźniak, M.; Feldo, M.; Kocki, J.; Szewczyk, K. Diosmin–Isolation Techniques, Determination in Plant Material and Pharmaceutical Formulations, and Clinical Use. Nat. Prod. Commun. 2013, 8, 545–550. [Google Scholar]
- Wojnar, W.; Kaczmarczyk-Sedlak, I.; Zych, M. Diosmin ameliorates the effects of oxidative stress in lenses of streptozotocin-induced type 1 diabetic rats. Pharmacol. Rep. 2017, 69, 995–1000. [Google Scholar] [CrossRef]
- Yao, X.; Gu, X.; Jin, S.; Shi, K.; Gao, X.; Wang, Q.; Zhao, J.; Zhang, H.; Lai, X. Anticancer and Anti-inflammatory Effect of Diosmin against Dalton Ascitic Lymphoma Induced Leukemia. J. Oleo Sci. 2021, 70, 665–673. [Google Scholar] [CrossRef]
- Liu, W.Y.; Liou, S.-S.; Hong, T.-Y.; Liu, I.-M. The Benefits of the Citrus Flavonoid Diosmin on Human Retinal Pigment Epithelial Cells under High-Glucose Conditions. Molecules 2017, 22, 2251. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, V.V.; Sathyamurthy, D.; Ramasamy, A.; Das, S.; Anuradha, M.; Pachiappan, S. Evaluation of protective effects of diosmin (a citrus flavonoid) in chemical-induced urolithiasis in experimental rats. Pharm. Biol. 2016, 54, 1513–1521. [Google Scholar] [CrossRef] [Green Version]
- Zhiye, G.; Xiaolong, D.; Yihua, Z.; Chunwan, S.; Feng, R.; Qiulun, l. Diosmin Alleviates Venous Injury and Muscle Damage in a Mouse Model of Iliac Vein Stenosis. Front. Cardiovasc. Med. 2022, 8, 785554. [Google Scholar] [CrossRef]
- Chung, S.; Kim, H.J.; Choi, H.K.; Park, J.H.; Hwang, J.T. Comparative study of the effects of diosmin and diosmetin on fat accumulation, dyslipidemia, and glucose intolerance in mice fed a high-fat high-sucrose diet. Food Sci. Nutr. 2020, 8, 5976–5984. [Google Scholar] [CrossRef]
- Shabani, S.; Mirshekar, M.A. Diosmin is neuroprotective in a rat model of scopolamine-induced cognitive impairment. Biomed. Pharmacother. 2018, 108, 1376–1383. [Google Scholar] [CrossRef]
- Crespo, M.E.; Gálvez, J.; Cruz, T.; Ocete, M.A.; Zarzuelo, A. Anti-inflammatory activity of diosmin and esperidin in rat colitis induced by TNBS. Planta Med. 1999, 65, 651–653. [Google Scholar] [CrossRef]
- Naso, L.; Martínez, V.R.; Lezama, L.; Salado, C.; Valcarcel, M.; Ferrer, E.G.; Williams, P.A.M. Antioxidant, anticancer activities and mechanistic studies of the flavone glycoside diosmin and its oxidovanadium(IV) complex. Interactions with bovine serum albumin. Bioorg. Med. Chem. 2016, 24, 4108–4119. [Google Scholar] [CrossRef]
- Damon, M.; Flandre, O.; Michel, F.; Perdrix, L.; Labrid, C.; Crastes de Paulet, A. Effect of chronic treatment with a purified flavonoid fraction on inflammatory granuloma in the rat. Study of prostaglandin E2 and F2 alpha and thromboxane B2 release and histological changes. Arzneimittel-Forschung 1987, 37, 1149–1153. [Google Scholar]
- Jiang, H.; Ying, X.; Chen, H. Effect of oral diosmin tablets on the pain and wound healing after modified anal fissure resection. Chin. J. Biochem. Pharm. 2017, 6, 243–245, 248. [Google Scholar]
- Feldo, M.; Wójciak, M.; Ziemlewska, A.; Dresler, S.; Sowa, I. Modulatory Effect of Diosmin and Diosmetin on Metalloproteinase Activity and Inflammatory Mediators in Human Skin Fibroblasts Treated with Lipopolysaccharide. Molecules 2022, 27, 4264. [Google Scholar] [CrossRef]
- Herman, T.F.; Bordoni, B. Wound Classification. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kujath, P.; Michelsen, A. Wounds-from physiology to wound dressing. Dtsch. Ärzteblatt Int. 2008, 105, 239–248. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Aboudzadeh, M.A. Emulsion-Based Encapsulation of Antioxidants; Springer Nature: Cham, Switzerland, 2021. [Google Scholar]
- Aboudzadeh, M.A.; Mehravar, E.; Fernandez, M.; Lezama, L.; Tomovska, R. Low-Energy Encapsulation of α-Tocopherol Using Fully Food Grade Oil-in-Water Microemulsions. ACS Omega 2018, 3, 10999–11008. [Google Scholar] [CrossRef]
- Nikolic, I.; Gledovic, A.; Tamburic, S.; Major, T.; Savic, S. Nanoemulsions as Carriers for Natural Antioxidants: Formulation Development and Optimisation. In Emulsion-Based Encapsulation of Antioxidants; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Ansari, M.N.; Soliman, G.A.; Rehman, N.U.; Anwer, M.K. Crisaborole Loaded Nanoemulsion Based Chitosan Gel: Formulation, Physicochemical Characterization and Wound Healing Studies. Gels 2022, 8, 318. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.; Anwer, M.K.; Fatima, F.; Alali, A.S.; Kalam, M.A.; Zafar, A.; Alshehri, S.; Ghoneim, M.M. Development of Apremilast Nanoemulsion-Loaded Chitosan Gels: In Vitro Evaluations and Anti-Inflammatory and Wound Healing Studies on a Rat Model. Gels 2022, 8, 253. [Google Scholar] [CrossRef]
- Khan, R.; Mirza, M.A.; Aqil, M.; Hassan, N.; Zakir, F.; Ansari, M.J.; Iqbal, Z. A Pharmaco-Technical Investigation of Thymoquinone and Peat-Sourced Fulvic Acid Nanoemulgel: A Combination Therapy. Gels 2022, 8, 733. [Google Scholar] [CrossRef] [PubMed]
- Anwer, M.K.; Jamil, S.; Ansari, M.J.; Al-Shdefat, R.; Ali, B.E.; Ganaie, M.A.; Abdel-Kader, M.S.; Shakeel, F. Water soluble binary and ternary complexes of diosmin with β-cyclodextrin: Spectroscopic characterization, release studies and anti-oxidant activity. J. Mol. Liq. 2014, 199, 35–41. [Google Scholar] [CrossRef]
- Freag, M.S.; Elnaggar, Y.S.; Abdallah, O.Y. Development of novel polymer-stabilized diosmin nanosuspensions: In vitro appraisal and ex vivo permeation. Int. J. Pharm. 2013, 454, 462–471. [Google Scholar] [CrossRef]
- Mohapatra, S.; Mirza, M.A.; Hilles, A.R.; Zakir, F.; Gomes, A.C.; Ansari, M.J.; Iqbal, Z.; Mahmood, S. Biomedical Application, Patent Repository, Clinical Trial and Regulatory Updates on Hydrogel: An Extensive Review. Gels 2021, 7, 207. [Google Scholar] [CrossRef]
- Cui, M.; Liu, Y.; Hu, Q.; Guan, F.; Ma, Y. Study on Preparation and in vitro Release Property of Diosmin Gel. China Pharm. 2019, 12, 2778–2782. [Google Scholar]
- Anwer, M.K.; Ahmed, M.M.; Alshetaili, A.; Almutairy, B.K.; Alalaiwe, A.; Fatima, F.; Ansari, M.N.; Iqbal, M. Preparation of spray dried amorphous solid dispersion of diosmin in soluplus with improved hepato-renoprotective activity: In vitro anti-oxidant and in-vivo safety studies. J. Drug Deliv. Sci. Technol. 2020, 60, 102101. [Google Scholar] [CrossRef]
- Freag, M.S.; Elnaggar, Y.S.; Abdallah, O.Y. Lyophilized phytosomal nanocarriers as platforms for enhanced diosmin delivery: Optimization and ex vivo permeation. Int. J. Nanomed. 2013, 8, 2385–2397. [Google Scholar] [CrossRef] [Green Version]
- Zingale, E.; Rizzo, S.; Bonaccorso, A.; Consoli, V.; Vanella, L.; Musumeci, T.; Spadaro, A.; Pignatello, R. Optimization of Lipid Nanoparticles by Response Surface Methodology to Improve the Ocular Delivery of Diosmin: Characterization and In-Vitro Anti-Inflammatory Assessment. Pharmaceutics 2022, 14, 1961. [Google Scholar] [CrossRef]
- Rahman, M.; Almalki, W.; Afzal, O.; Kazmi, I.; Altamimi, A.S.; Alghamdi, S.; Al-Abbasi, F.; Altowayan, W.; Alrobaian, M.; Alharbi, K.; et al. Diosmin-loaded solid nanoparticles as nano-antioxidant therapy for management of hepatocellular carcinoma: QbD-based optimization, in vitro and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 61, 102213. [Google Scholar] [CrossRef]
- Anwer, M.K. Development of diosmin loaded eudragit s100 polymeric nanoparticles: An investigation of antioxidant effect. IJBPAS 2014, 3, 2015–2026. [Google Scholar]
- Kamble, R.N.; Mehta, P.P.; Kumar, A. Efavirenz Self-Nano-Emulsifying Drug Delivery System: In Vitro and In Vivo Evaluation. AAPS PharmSciTech 2016, 17, 1240–1247. [Google Scholar] [CrossRef] [Green Version]
- Gokhale, J.P.; Mahajan, H.S.; Surana, S.J. Quercetin loaded nanoemulsion-based gel for rheumatoid arthritis: In vivo and in vitro studies. Biomed. Pharmacother. 2019, 112, 108622. [Google Scholar] [CrossRef]
- Mujtaba, M.A.; Alotaibi, N.M.; Alshehri, S.M.; Yusuf, M.; Anwer, M.K.; Rahman, M.A.; Parveen, A. Novel Therapeutic Approach in PEGylated Chitosan Nanoparticles of Apigenin for the Treatment of Cancer via Oral Nanomedicine. Polymers 2022, 14, 4344. [Google Scholar] [CrossRef]
- Youssef, A.A.A.; Cai, C.; Dudhipala, N.; Majumdar, S. Design of Topical Ocular Ciprofloxacin Nanoemulsion for the Management of Bacterial Keratitis. Pharmaceuticals 2021, 14, 210. [Google Scholar] [CrossRef]
- Fachel, F.N.S.; Medeiros-Neves, B.; Dal Prá, M.; Schuh, R.S.; Veras, K.S.; Bassani, V.L.; Koester, L.S.; Henriques, A.T.; Braganhol, E.; Teixeira, H.F. Box-Behnken design optimization of mucoadhesive chitosan-coated nanoemulsions for rosmarinic acid nasal delivery-In vitro studies. Carbohydr. Polym. 2018, 199, 572–582. [Google Scholar] [CrossRef]
- Alshahrani, S.M.; Alshetaili, A.S.; Alalaiwe, A.; Alsulays, B.B.; Anwer, M.K.; Al-Shdefat, R.; Imam, F.; Shakeel, F. Anticancer Efficacy of Self-Nanoemulsifying Drug Delivery System of Sunitinib Malate. AAPS PharmSciTech 2018, 19, 123–133. [Google Scholar] [CrossRef]
- Anwer, M.K.; Iqbal, M.; Aldawsari, M.F.; Alalaiwe, A.S.; Ahmed, M.M.; Muharram, M.M.; Ezzeldin, E.; Mahmoud, M.A.; Imam, F.; Ali, R. Improved antimicrobial activity and oral bioavailability of delafloxacin by self-nanoemulsifying drug delivery system (SNEDDS). J. Drug Deliv. Sci. Technol. 2021, 64, 102572. [Google Scholar] [CrossRef]
- Shafiq, S.; Shakeel, F.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K.; Ali, M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur. J. Pharm. Biopharm. 2007, 66, 227–243. [Google Scholar] [CrossRef]
- Zafar, A.; Imam, S.S.; Alruwaili, N.K.; Yasir, M.; Alsaidan, O.A.; Alshehri, S.; Ghoneim, M.M.; Khalid, M.; Alquraini, A.; Alharthi, S.S. Formulation and Evaluation of Topical Nano-Lipid-Based Delivery of Butenafine: In Vitro Characterization and Antifungal Activity. Gels 2022, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Pathan, I.B.; Dwivedi, R.; Ambekar, W. Formulation and evaluation of ketoprofen loaded chitosan nanogel for pain management: Ex-vivo and In-vivo study. Ars Pharm. 2019, 60, 101–108. [Google Scholar] [CrossRef]
- Ghose, A.; Nabi, B.; Rehman, S.; Md, S.; Alhakamy, N.A.; Ahmad, O.A.A.; Baboota, S.; Ali, J. Development and Evaluation of Polymeric Nanosponge Hydrogel for Terbinafine Hydrochloride: Statistical Optimization, In Vitro and In Vivo Studies. Polymers 2020, 12, 2903. [Google Scholar] [CrossRef] [PubMed]
- Anwer, M.K.; Jamil, S.; Ansari, M.J.; Al-Shdefat, R.; Abdel-Kader, M.S. A new improved Stability-Indicating RP-HPLC method for Determination of Diosmin and hesperidin in combination. Int. Res. J. Biol. Sci. 2014, 3, 41–46. [Google Scholar]
- Almutairy, B.K.; Alshetaili, A.; Alali, A.S.; Ahmed, M.M.; Anwer, M.K.; Aboudzadeh, M.A. Design of Olmesartan Medoxomil-Loaded Nanosponges for Hypertension and Lung Cancer Treatments. Polymers 2021, 13, 2272. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Pedersen, J.N.; Marie, R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 2020, 11, 2337. [Google Scholar] [CrossRef]
- Shakeel, F.; Raish, M.; Anwer, M.K.; Al-Shdefat, R. Self-nanoemulsifying drug delivery system of sinapic acid: In vitro and in vivo evaluation. J. Mol. Liq. 2016, 224, 351–358. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, X.; Yu, G.; Suo, M. Optimization of propofol loaded niosomal gel for transdermal delivery. J. Biomater. Sci. Polym. Ed. 2021, 32, 858–873. [Google Scholar] [CrossRef]
- Kalam, M.A.; Parvez, N.; Yadav, S.; Garg, A.; Amin, S.; Sultana, Y.; Ali, A. Release Kinetics of Modified Pharmaceutical Dosage Forms: A Review. Cont. J. Pharm. Sci. 2007, 1, 30–35. [Google Scholar]
- OECD. Guideline for Testing of Chemicals: Draft Updated Test Guideline 434 on Acute Dermal Toxicity; Draft Guideline; OECD: Paris, France, 2015; pp. 1–12. [Google Scholar]
- Ahmad, M.; Ansari, M.N.; Alam, A.; Khan, T.H. Oral Dose of Citrus Peel Extracts Promotes Wound Repair in Diabetic Rats. Pak. J. Biol. Sci. 2013, 16, 1086–1094. [Google Scholar] [CrossRef] [Green Version]
- Saeedan, A.S.; Gabr, G.A.; Soliman, G.A.; Fayed, M.H.; Ansari, M.N. The potential anti-inflammatory and wound healing activities of chitosan in rats. Adv. Bioresearch 2016, 7, 1–7. [Google Scholar]
- Hamad, A.M.; Ahmed, H.G. Association of Connective Tissue Fibers with Estrogen Expression in Breast Lesions among Sudanese Females. Int. Clin. Pathol. J. 2016, 2, 00051. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Layton, C. The hematoxylins and eosin. In Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Suvarna, S.K., Layton, C., Bancroft, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; p. 131. [Google Scholar]
- Ansari, M.J.; Aldayel, I.A.; Alablan, F.M.; Alsulays, B.; Alshetaili, A.; Alshahrani, S.; Alalaiwe, A.; Ansari, M.N.; Rehman, N.U. Formulation, characterization, in vitro and in vivo evaluations of self-nanoemulsifying drug delivery system of luteolin. J. Taibah Univ. Sci. 2020, 14, 1386–1401. [Google Scholar] [CrossRef]

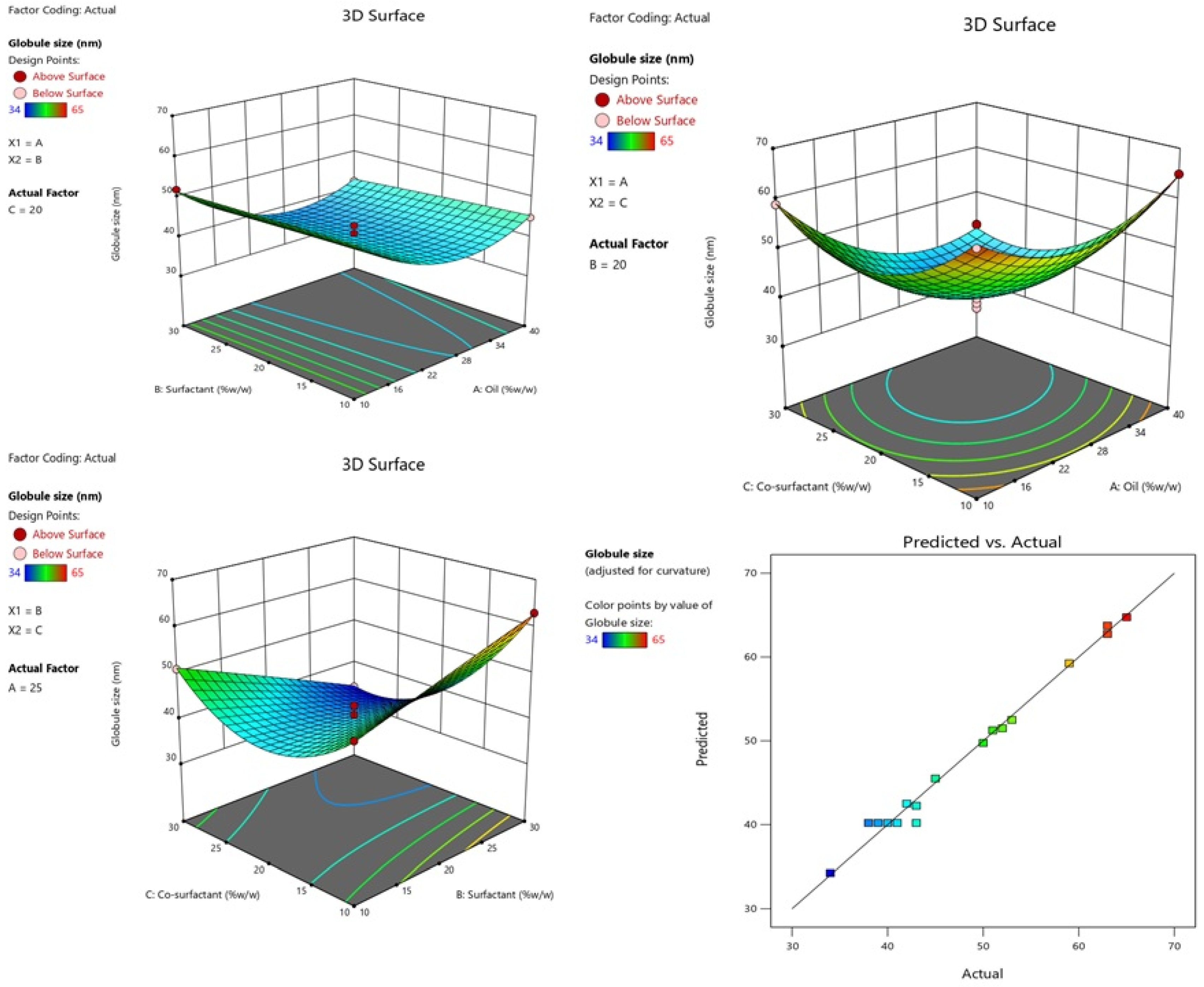

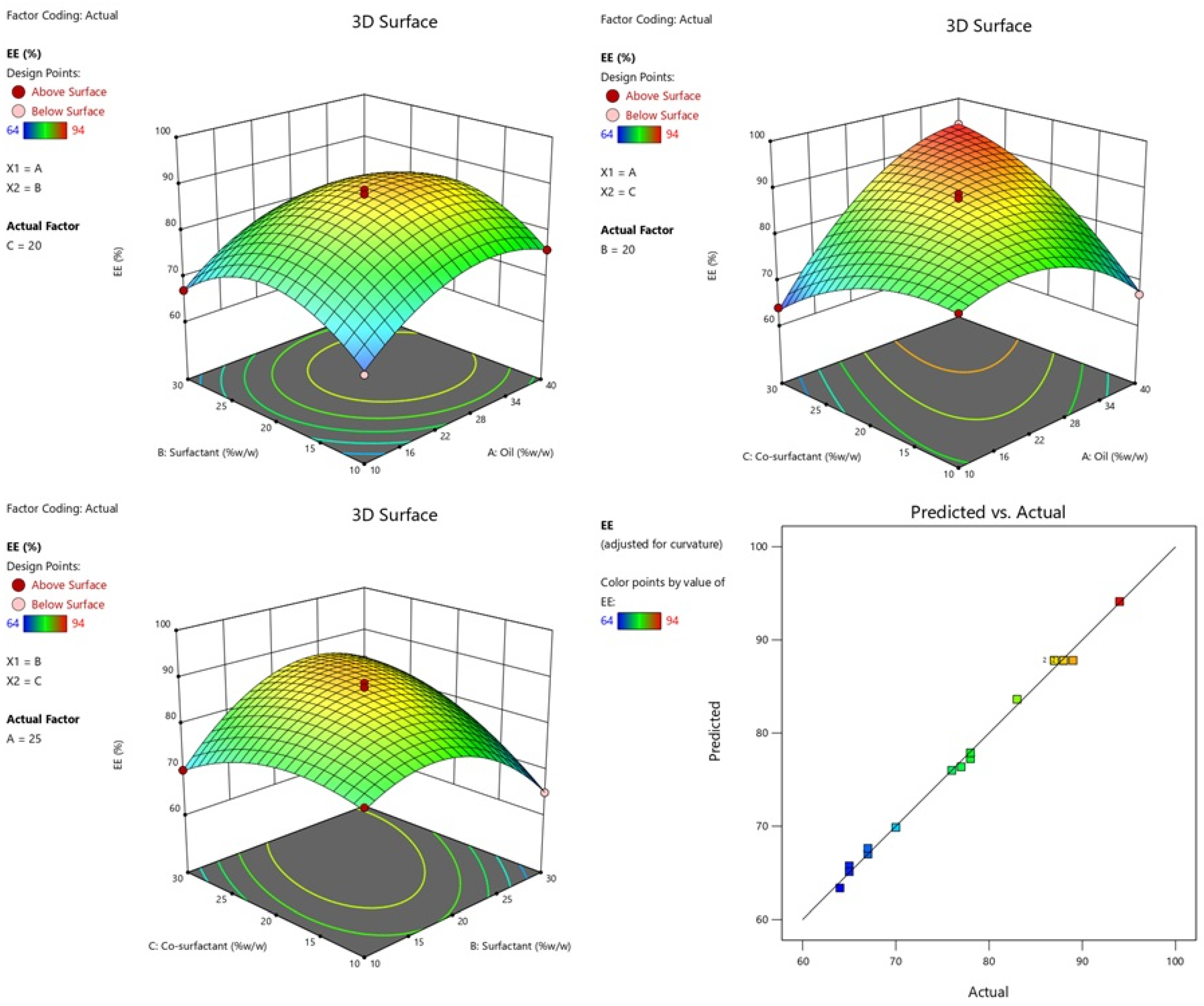

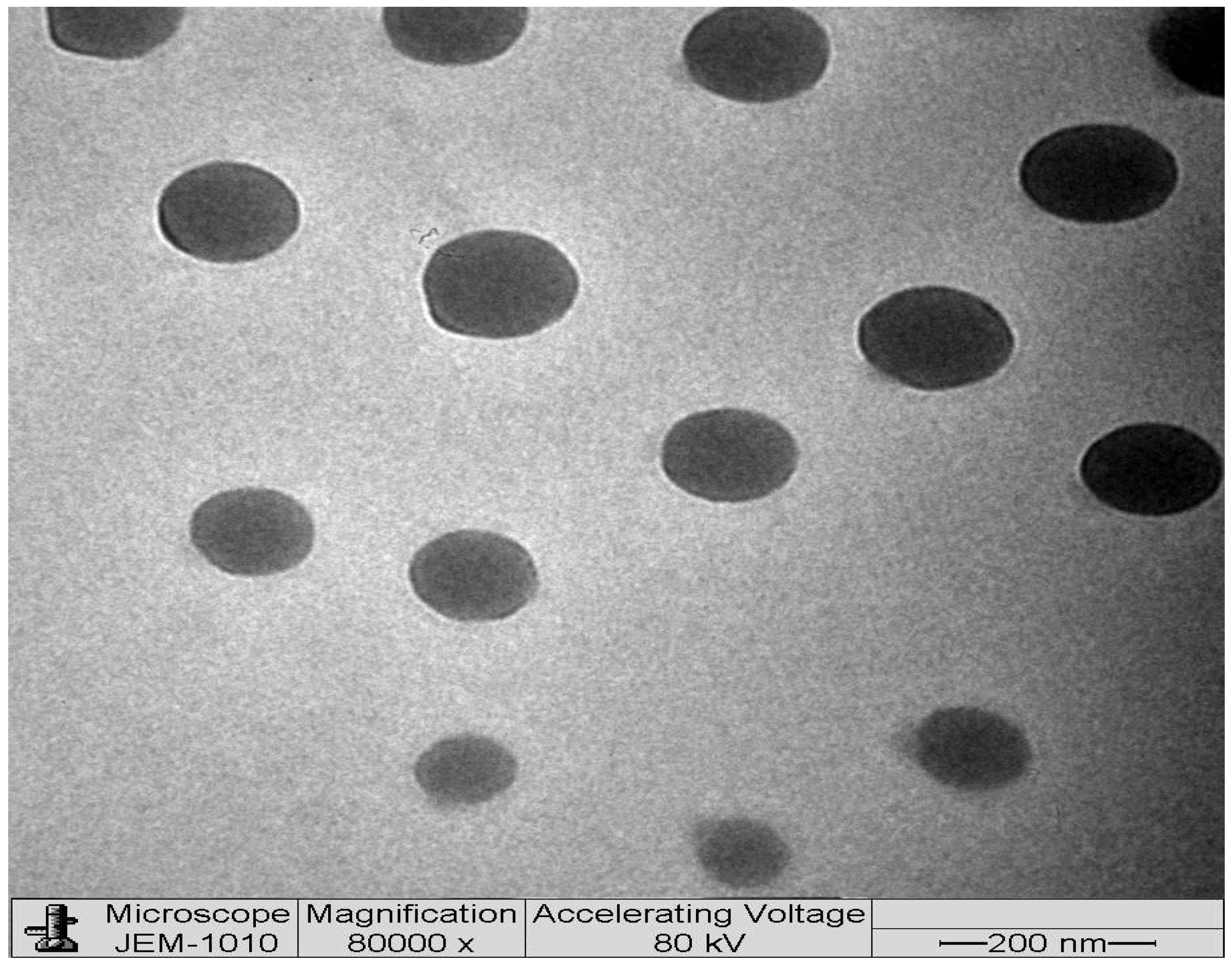

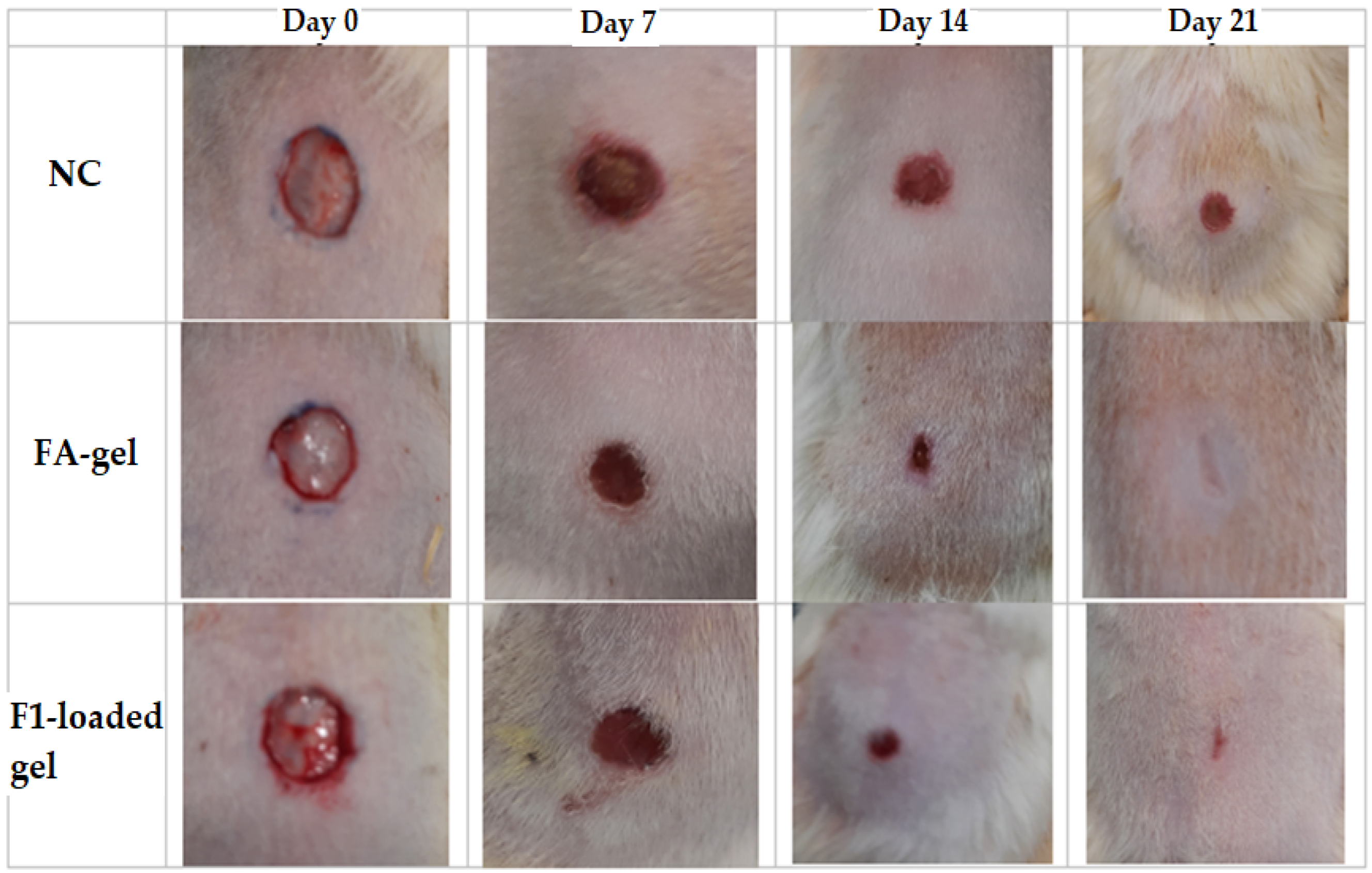

| Runs | A:Oil %w/w | B:Surfactant %w/w | C:Co-Surfactant %w/w | Globule Size nm (Y1) | PDI (Y2) | EE % (Y3) |
|---|---|---|---|---|---|---|
| F1 | 25 | 20 | 20 | 41 ± 0.07 | 0.073 ± 0.008 | 87 ± 0.81 |
| F2 | 25 | 10 | 10 | 50 ± 1.22 | 0.095 ± 0.007 | 77 ± 0.79 |
| F3 | 40 | 20 | 30 | 43 ± 0.91 | 0.089 ± 0.001 | 94 ± 1.61 |
| F4 | 10 | 30 | 20 | 52 ± 0.99 | 0.077 ± 0.004 | 67 ± 0.93 |
| F5 | 25 | 30 | 10 | 63 ± 1.42 | 0.099 ± 0.001 | 65 ± 1.09 |
| F6 | 10 | 10 | 20 | 53 ± 0.82 | 0.097 ± 0.005 | 65 ± 0.14 |
| F7 | 25 | 10 | 30 | 51 ± 0.07 | 0.114 ± 0.011 | 70 ± 1.05 |
| F8 | 25 | 20 | 20 | 38 ± 0.69 | 0.08 ± 0.003 | 88 ± 2.06 |
| F9 | 25 | 20 | 20 | 43 ± 1.05 | 0.079 ± 0.001 | 87 ± 1.53 |
| F10 | 40 | 30 | 20 | 42 ± 0.78 | 0.055 ± 0.002 | 78 ± 0.72 |
| F11 | 25 | 20 | 20 | 40 ± 0.92 | 0.078 ± 0.001 | 88 ± 0.76 |
| F12 | 40 | 20 | 10 | 65 ± 1.21 | 0.072 ± 0.004 | 67 ± 0.67 |
| F13 | 10 | 20 | 30 | 59 ± 1.10 | 0.103 ± 0.009 | 64 ± 1.24 |
| F14 | 10 | 20 | 10 | 63 ± 0.55 | 0.128 ± 0.022 | 78 ± 2.54 |
| F15 | 40 | 10 | 20 | 45 ± 0.74 | 0.076 ± 0.001 | 76 ± 0.83 |
| F16 | 25 | 20 | 20 | 39 ± 0.06 | 0.089 ± 0.012 | 89 ± 1.44 |
| F17 | 25 | 30 | 30 | 34 ± 0.43 | 0.061 ± 0.002 | 83 ± 2.41 |
| Source | Sum of Square | DF | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Globule size | ||||||
| Model | 1480.23 | 9 | 164.47 | 66.55 | <0.0001 | Significant |
| A—Oil | 128.00 | 1 | 128.00 | 51.79 | 0.0002 | Significant |
| B—Surfactant | 8.00 | 1 | 8.00 | 3.24 | 0.1150 | Not significant |
| C—Co-surfactant | 364.50 | 1 | 364.50 | 147.49 | <0.0001 | Significant |
| AB | 1.0000 | 1 | 1.0000 | 0.4046 | 0.5449 | Not significant |
| AC | 81.00 | 1 | 81.00 | 32.77 | 0.0007 | Significant |
| BC | 225.00 | 1 | 225.00 | 91.04 | <0.0001 | Significant |
| A2 | 262.78 | 1 | 262.78 | 106.33 | <0.0001 | Significant |
| B2 | 0.0421 | 1 | 0.0421 | 0.0170 | 0.8998 | Not significant |
| C2 | 372.04 | 1 | 372.04 | 150.54 | <0.0001 | Significant |
| Residual | 17.30 | 7 | 2.47 | |||
| Lack of Fit | 2.50 | 3 | 0.8333 | 0.2252 | 0.8746 | Not significant |
| Pure Error | 14.80 | 4 | 3.70 | |||
| Cor Total | 1497.53 | 16 | ||||
| PDI | ||||||
| Model | 0.0053 | 9 | 0.0006 | 16.42 | 0.0006 | Significant |
| A—Oil | 0.0016 | 1 | 0.0016 | 44.86 | 0.0003 | Significant |
| B—Surfactant | 0.0010 | 1 | 0.0010 | 28.46 | 0.0011 | Significant |
| C—Co-surfactant | 0.0001 | 1 | 0.0001 | 2.56 | 0.1535 | Not significant |
| AB | 2.500 × 10−7 | 1 | 2.500 × 10−7 | 0.0070 | 0.9355 | Not significant |
| AC | 0.0004 | 1 | 0.0004 | 12.40 | 0.0097 | Significant |
| BC | 0.0008 | 1 | 0.0008 | 22.83 | 0.0020 | Significant |
| A2 | 5.095 × 10−6 | 1 | 5.095 × 10−6 | 0.1432 | 0.7163 | Not significant |
| B2 | 0.0001 | 1 | 0.0001 | 2.56 | 0.1537 | Not significant |
| C2 | 0.0012 | 1 | 0.0012 | 34.61 | 0.0006 | Significant |
| Residual | 0.0002 | 7 | 0.0000 | |||
| Lack of Fit | 0.0001 | 3 | 0.0000 | 1.13 | 0.4370 | Not significant |
| Pure Error | 0.0001 | 4 | 0.0000 | |||
| Cor Total | 0.0055 | 16 | ||||
| %EE | ||||||
| Model | 1602.92 | 9 | 178.10 | 224.63 | <0.0001 | Significant |
| A—Oil | 210.13 | 1 | 210.13 | 265.02 | <0.0001 | Significant |
| B—Surfactant | 3.12 | 1 | 3.12 | 3.94 | 0.0875 | Not significant |
| C—Co-surfactant | 72.00 | 1 | 72.00 | 90.81 | <0.0001 | Significant |
| AB | 0.0000 | 1 | 0.0000 | 0.0000 | 1.0000 | Not significant |
| AC | 420.25 | 1 | 420.25 | 530.05 | <0.0001 | Significant |
| BC | 156.25 | 1 | 156.25 | 197.07 | <0.0001 | Significant |
| A2 | 215.25 | 1 | 215.25 | 271.49 | <0.0001 | Significant |
| B2 | 352.52 | 1 | 352.52 | 444.61 | <0.0001 | Significant |
| C2 | 101.09 | 1 | 101.09 | 127.51 | <0.0001 | Significant |
| Residual | 5.55 | 7 | 0.7929 | |||
| Lack of Fit | 2.75 | 3 | 0.9167 | 1.31 | 0.3873 | Not significant |
| Pure Error | 2.80 | 4 | 0.7000 | |||
| Cor Total | 1608.47 | 16 | ||||
| Code | Zero Order | First Order | Higuchi Model | Korsmeyer Peppas | Release Exponents |
|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | n | |
| F1 | 0.6013 | 0.8148 | 0.9825 | 0.9890 | 0.442 |
| F1-gel | 0.6119 | 0.8625 | 0.9751 | 0.9793 | 0.452 |
| Groups | Percent Swelling | Percent Inhibition |
|---|---|---|
| NC | 38.47 ± 1.51 | - |
| FA gel | 22.11 ± 1.90 *** | 42.51 |
| F1-loaded gel | 18.97 ± 2.20 *** | 50.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anwer, M.K.; Aldawsari, M.F.; Iqbal, M.; Almutairy, B.K.; Soliman, G.A.; Aboudzadeh, M.A. Diosmin-Loaded Nanoemulsion-Based Gel Formulation: Development, Optimization, Wound Healing and Anti-Inflammatory Studies. Gels 2023, 9, 95. https://doi.org/10.3390/gels9020095
Anwer MK, Aldawsari MF, Iqbal M, Almutairy BK, Soliman GA, Aboudzadeh MA. Diosmin-Loaded Nanoemulsion-Based Gel Formulation: Development, Optimization, Wound Healing and Anti-Inflammatory Studies. Gels. 2023; 9(2):95. https://doi.org/10.3390/gels9020095
Chicago/Turabian StyleAnwer, Md. Khalid, Mohammed F. Aldawsari, Muzaffar Iqbal, Bjad K. Almutairy, Gamal A. Soliman, and M. Ali Aboudzadeh. 2023. "Diosmin-Loaded Nanoemulsion-Based Gel Formulation: Development, Optimization, Wound Healing and Anti-Inflammatory Studies" Gels 9, no. 2: 95. https://doi.org/10.3390/gels9020095






