Quality-by-Design-Assisted Optimization of Carvacrol Oil-Loaded Niosomal Gel for Anti-Inflammatory Efficacy by Topical Route
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Carvacrol-Loaded Niosomes (CVC-Ns)
2.2. Design Validation
2.3. Optimized Composition
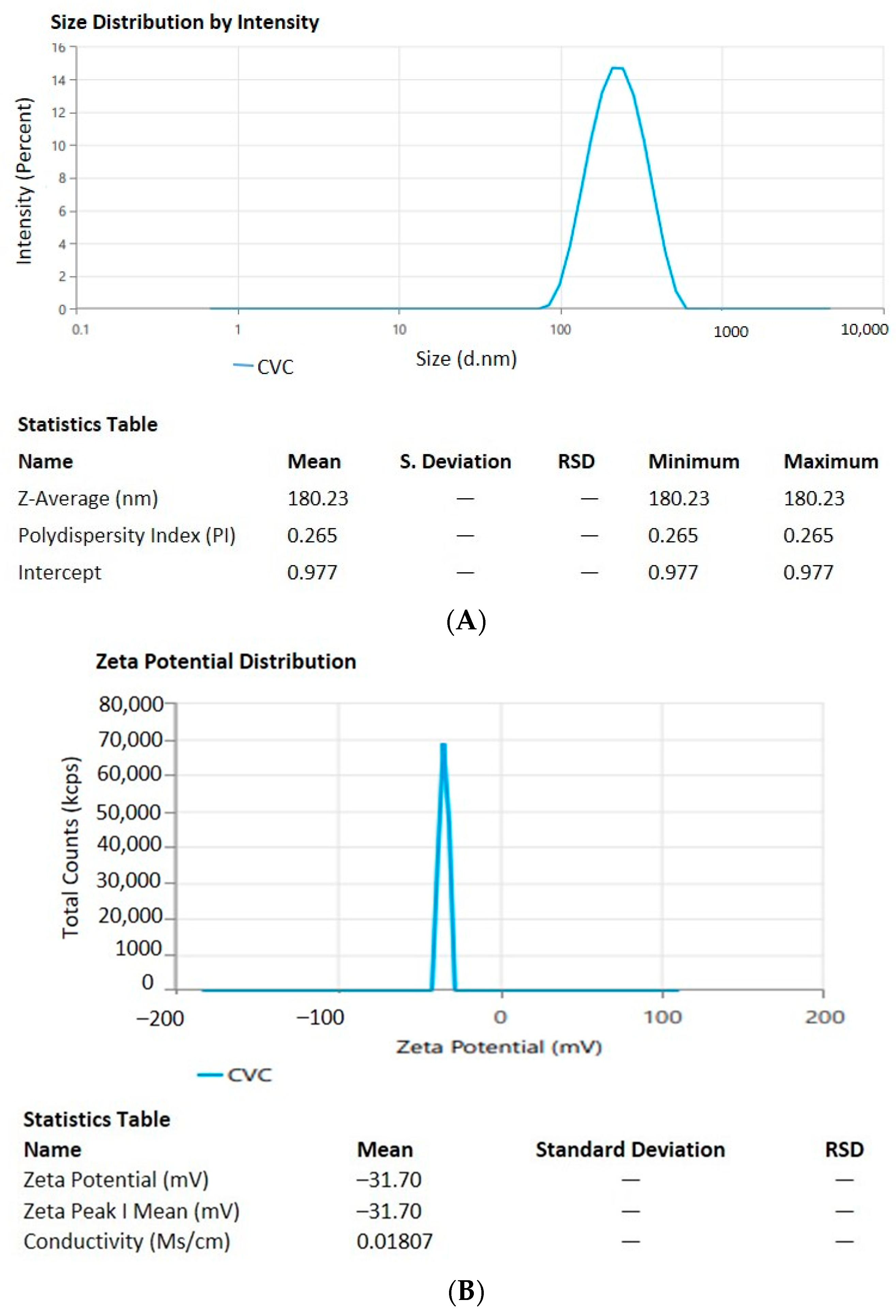
2.4. Characterization of CVC-Ns
Entrapment Efficiency and Drug Loading
2.5. Morphology of CVC-Ns
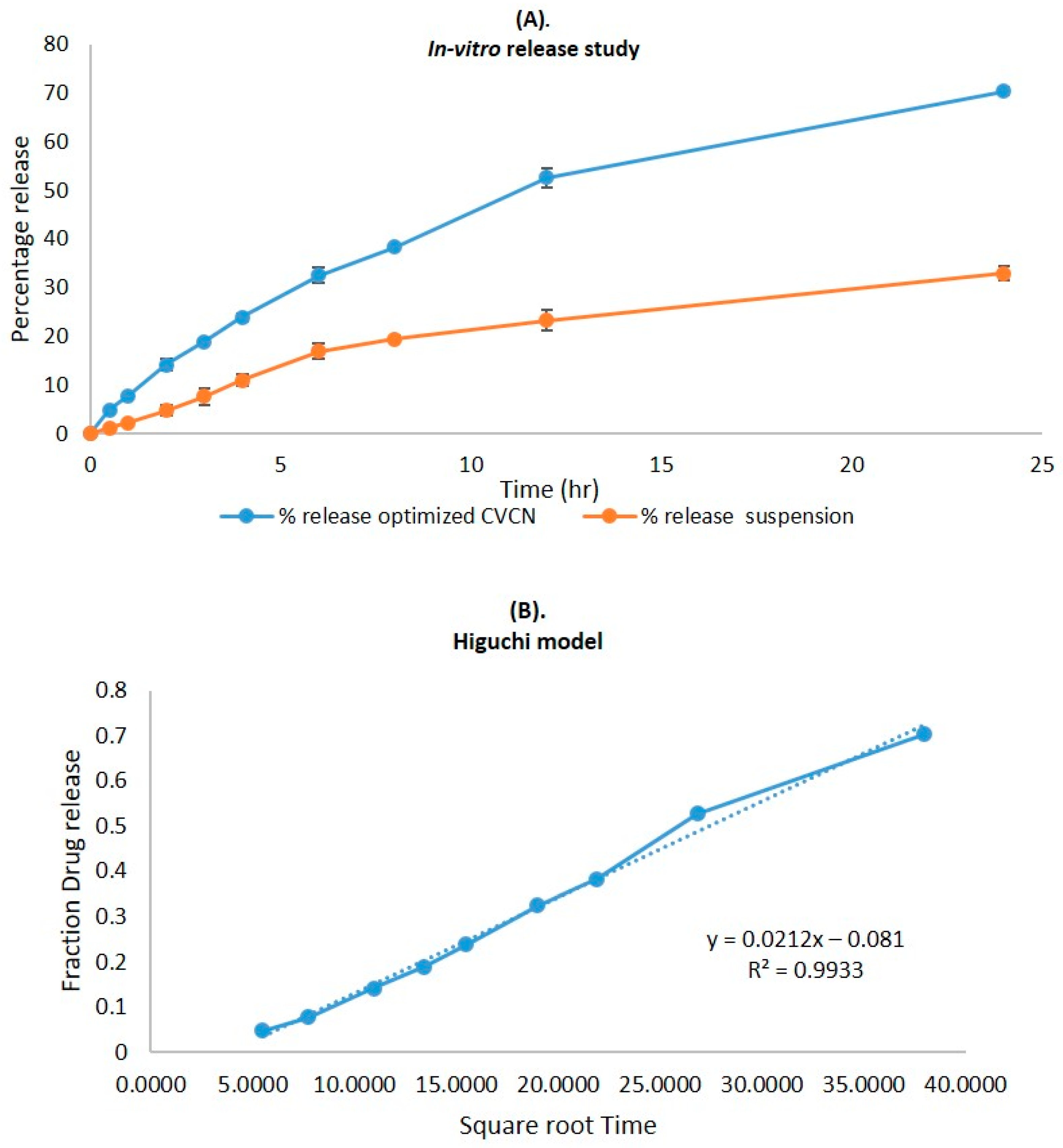
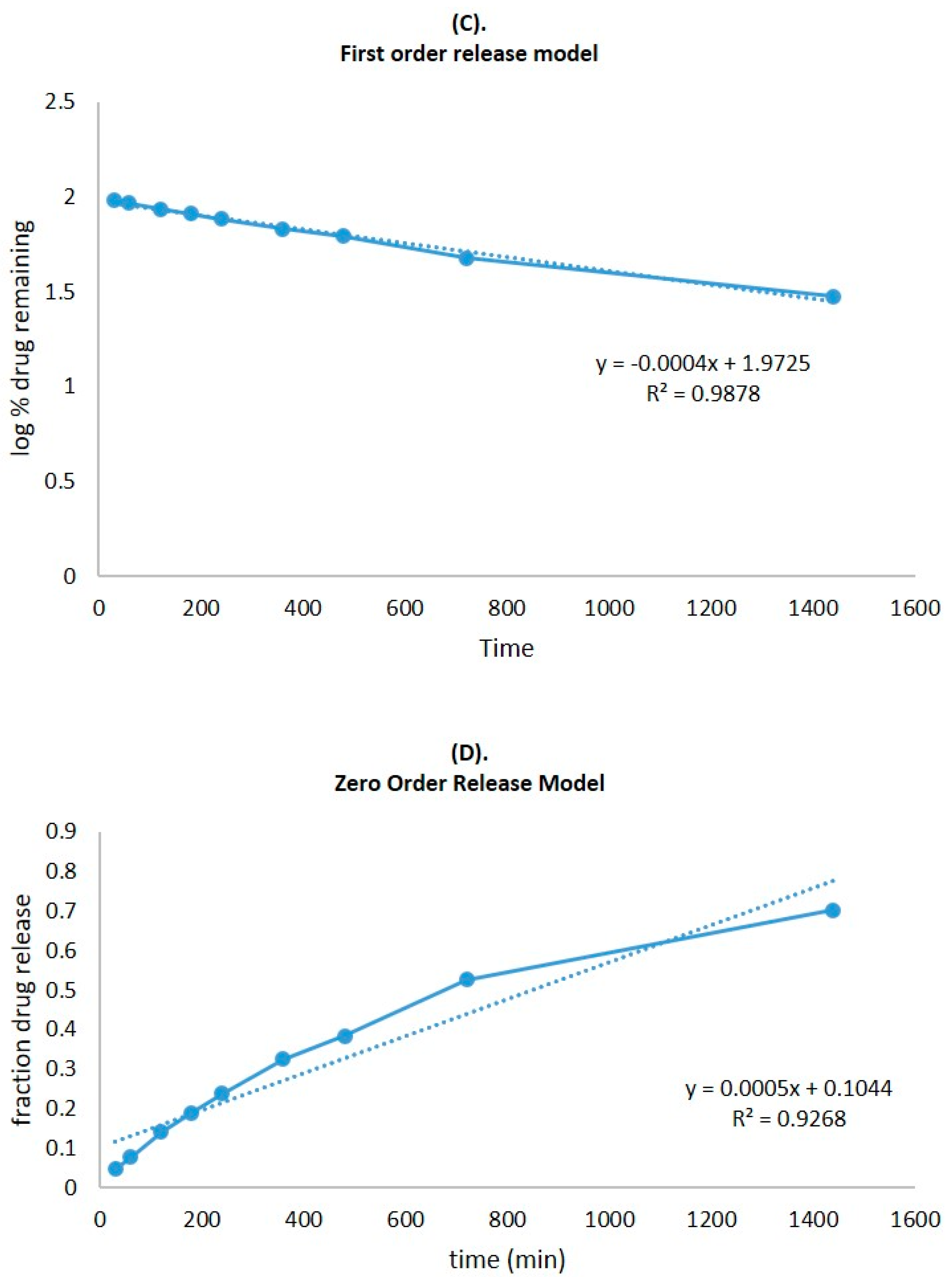
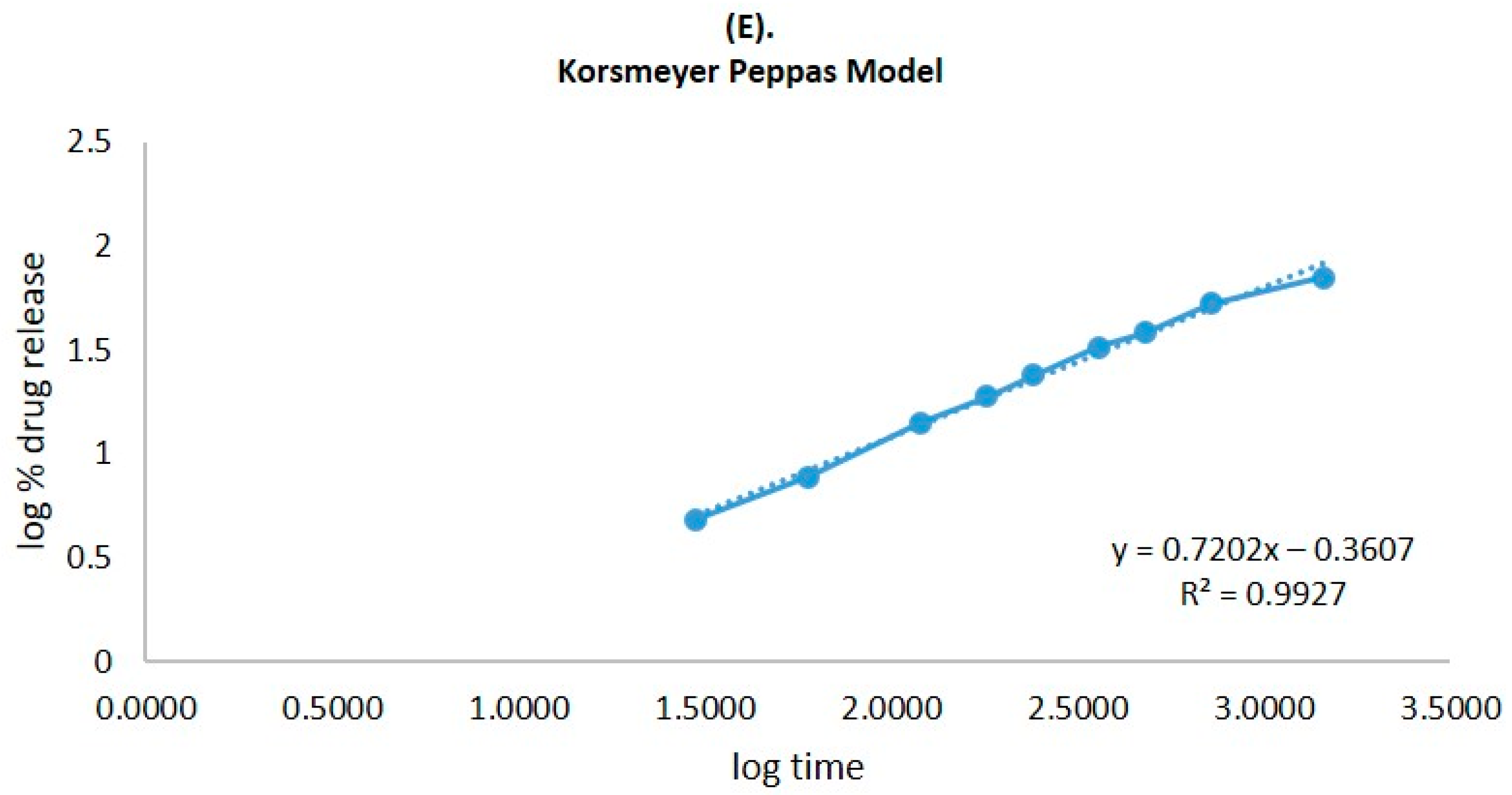
2.6. In Vitro Release Study
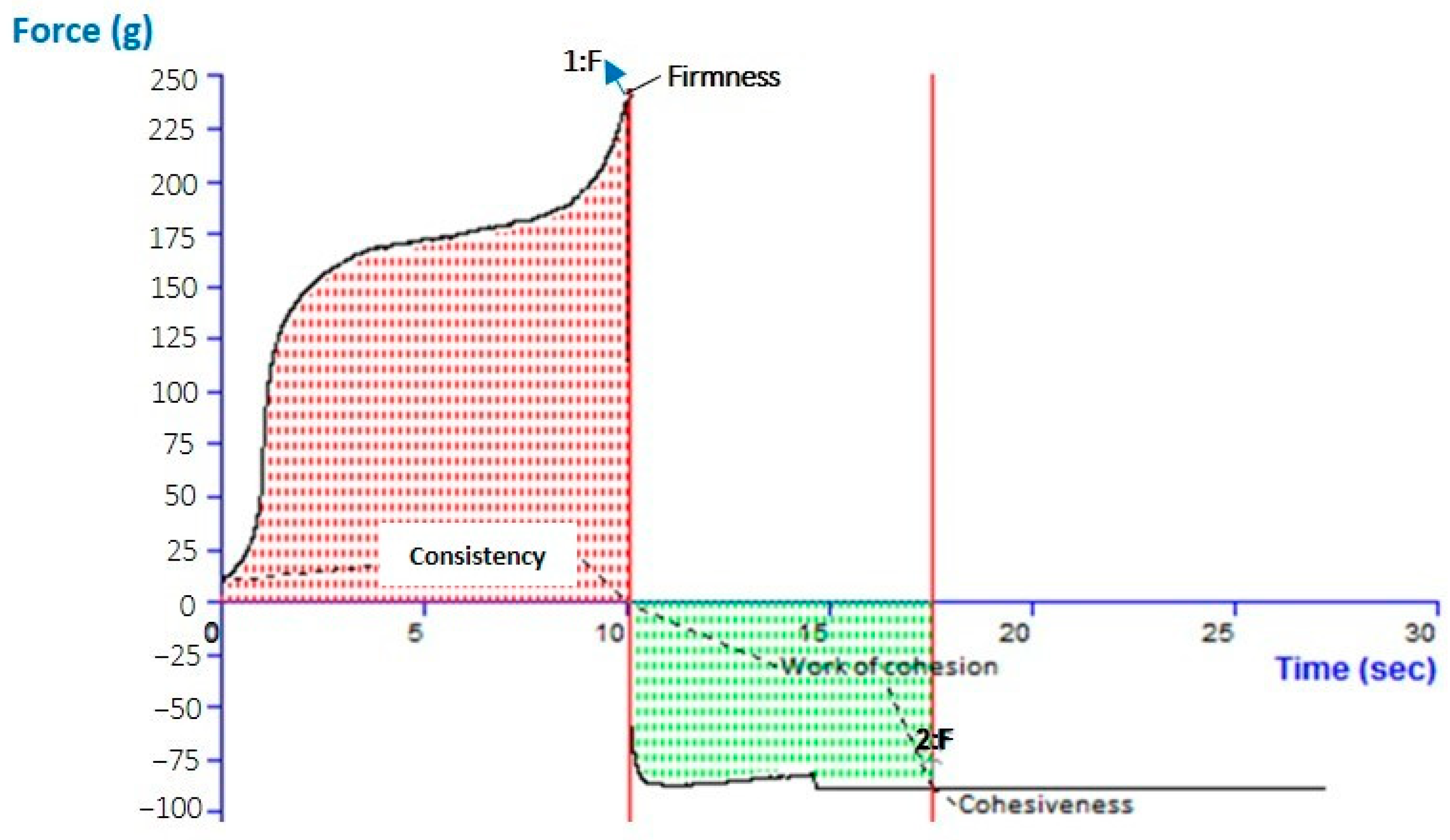
2.7. Analysis of the Gel and Texture of the Optimized CVC-N Gel
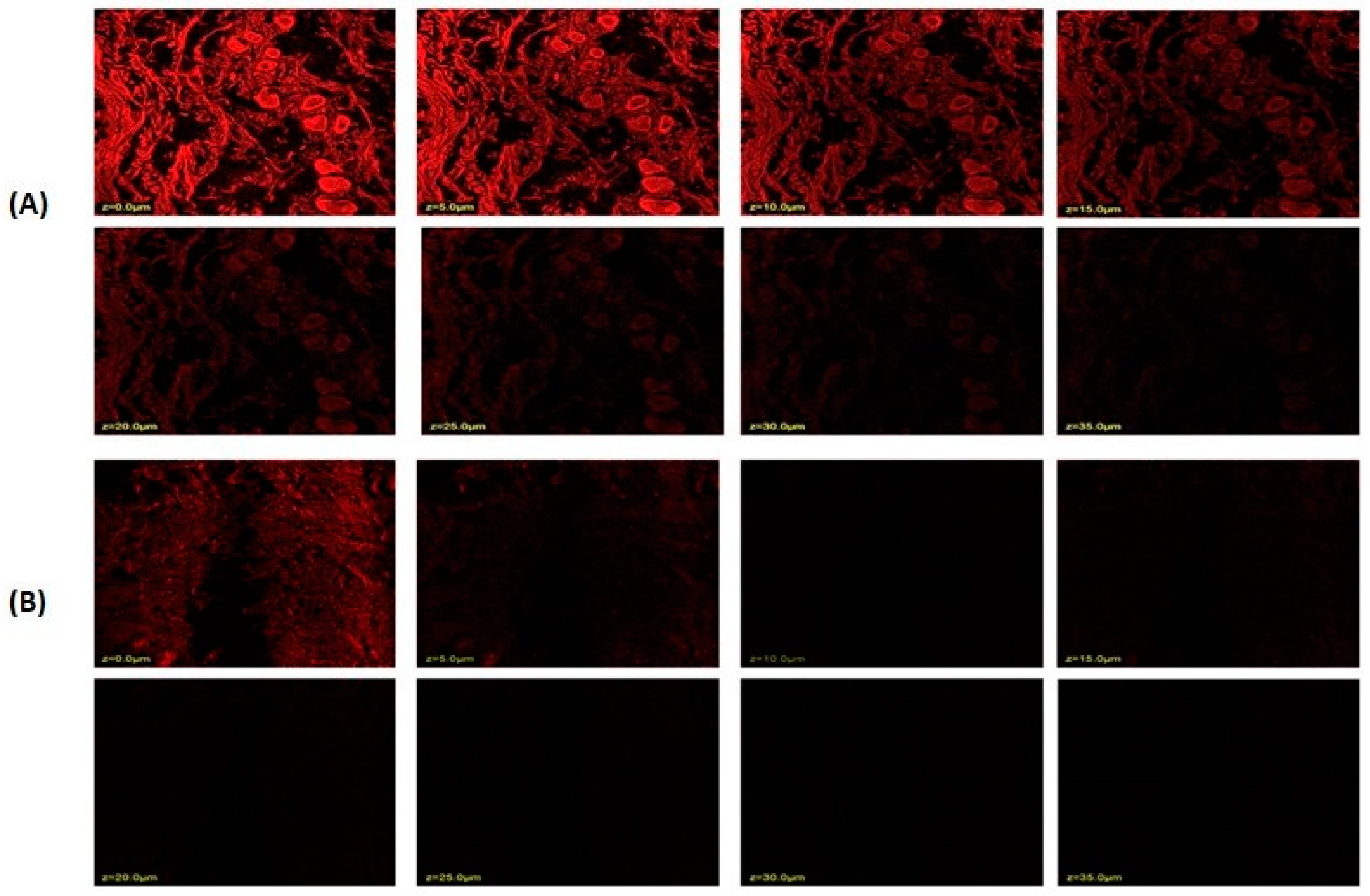
2.8. Confocal Laser Scanning Microscopy
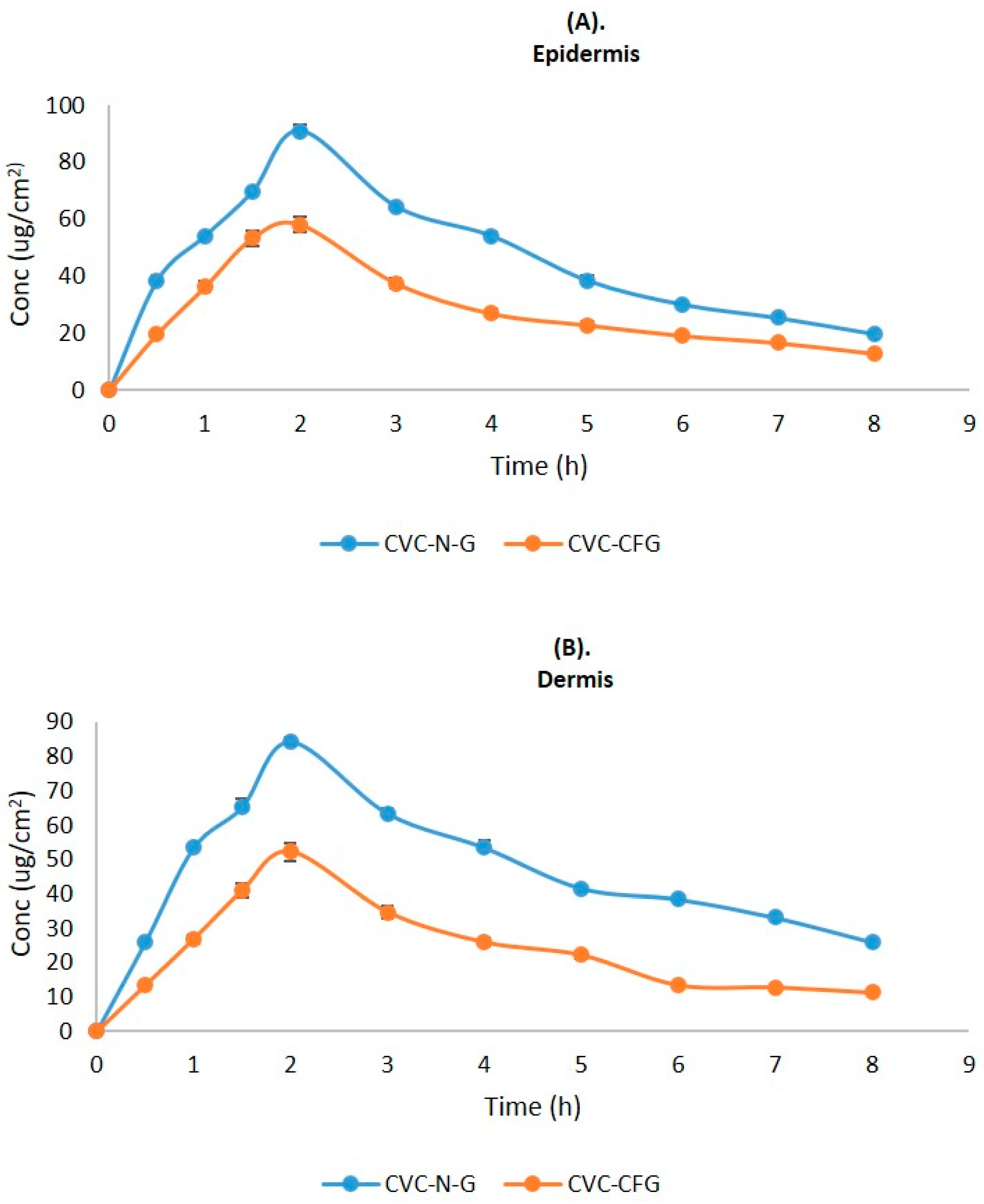
2.9. Dermatokinetic Studies
2.10. Ferric-Reducing Antioxidant Power (FRAP)
2.11. Stability Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Method
4.2.1. Preparation of CVC-Loaded Niosomes (CVC-Ns)
4.2.2. CVC-Loaded Niosome Optimization through Experimental Design
4.3. Characterization of CVC-Ns
4.3.1. Determination of Globule Size and Zeta Potential
4.3.2. Measurement of % Entrapment Efficiency and % Drug Loading
4.4. Morphological Studies
4.5. Formulation of CVC Niosomal Gel (CVCNG)
4.6. In Vitro Drug Release Study
4.7. Characterization of Gel
Evaluation of pH and Texture of CVC-N Gel
4.8. Spreadability Studies
4.9. Permeation Depth Study by Loading Rhodamine B Dye
4.10. Dermatokinetics
4.11. Ferric-Reducing Antioxidant Power (FRAP)
4.12. Stability Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schrooyen, P.M.M.; van der Meer, R.; Kruif, C.G. De Microencapsulation: Its Application in Nutrition. Proc. Nutr. Soc. 2001, 60, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Fernandes, A.R.; Martins-Gomes, C.; Coutinho, T.E.; Durazzo, A.; Lucarini, M.; Souto, S.B.; Silva, A.M.; Santini, A. Nanomaterials for Skin Delivery of Cosmeceuticals and Pharmaceuticals. Appl. Sci. 2020, 10, 1594. [Google Scholar] [CrossRef]
- Sindle, A.; Martin, K. Art of prevention: Essential oils-natural products not necessarily safe. Int. J. Women’s Dermatol. 2021, 7, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Khatibi, S.A.; Misaghi, A.; Moosavy, M.-H.; Amoabediny, G.; Basti, A.A. Effect of Preparation Methods on the Properties of Zataria Multiflora Boiss. Essential Oil Loaded Nanoliposomes: Characterization of Size, Encapsulation Efficiency and Stability. Pharm. Sci. 2015, 20, 141–148. [Google Scholar]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-Step Method for Encapsulation of Oregano Essential Oil in Chitosan Nanoparticles: Preparation, Characterization and in Vitro Release Study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Hassan Aubais-aljelehawy, Q.; Mohammadi, S.; Mohamadian, E.; Raji Mal Allah, O.; Mirzaei, A.; Ghahremanlou, M. Antimicrobial, anticancer, antidiabetic, antineurodegenerative, and antirheumatic activities of thymol: Clarification of mechanisms. Micro Nano Bio Asp. 2023, 2, 1–7. [Google Scholar]
- Aljelehawy, Q.; Maroufi, Y.; Javid, H.; Mohammadi, M.R.; Raji Mal Allah, O.; Taheri, S.V.; Mohammadzade, H. Anticancer, antineurodegenerative, antimicrobial, and antidiabetic activities of carvacrol: Recent advances and limitations for effective formulations. Nano Micro Biosyst. 2023, 2, 1–10. [Google Scholar]
- Guimarães, A.G.; Xavier, M.A.; De Santana, M.T.; Camargo, E.A.; Santos, C.A.; Brito, F.A.; Barreto, E.O.; Cavalcanti, S.C.H.; Antoniolli, Â.R.; Oliveira, R.C.M.; et al. Carvacrol Attenuates Mechanical Hypernociception and Inflammatory Response. Naunyn. Schmiedebergs. Arch. Pharmacol. 2012, 385, 253–263. [Google Scholar] [CrossRef]
- Guimarães, A.G.; Silva, F.V.; Xavier, M.A.; Santos, M.R.V.; Oliveira, R.C.M.; Oliveira, M.G.B.; Oliveira, A.P.; De Souza, C.C.; Quintans-Júnior, L.J. Orofacial Analgesic-like Activity of Carvacrol in Rodents. Z. Naturforsch. Sect. C J. Biosci. 2012, 67C, 481–485. [Google Scholar] [CrossRef]
- Lima, M.D.S.; Quintans-Júnior, L.J.; De Santana, W.A.; Martins Kaneto, C.; Pereira Soares, M.B.; Villarreal, C.F. Anti-Inflammatory Effects of Carvacrol: Evidence for a Key Role of Interleukin-10. Eur. J. Pharmacol. 2013, 699, 112–117. [Google Scholar] [CrossRef]
- Guimarães, A.G.; Scotti, L.; Scotti, M.T.; Mendonça, F.J.B.; Melo, N.S.R.; Alves, R.S.; De Lucca, W.; Bezerra, D.P.; Gelain, D.P.; Quintans, L.J. Evidence for the Involvement of Descending Pain-Inhibitory Mechanisms in the Attenuation of Cancer Pain by Carvacrol Aided through a Docking Study. Life Sci. 2014, 116, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.G.; Oliveira, M.A.; Alves, R.D.S.; Menezes, P.D.P.; Serafini, M.R.; De Souza Araújo, A.A.; Bezerra, D.P.; Quintans, L.J. Encapsulation of Carvacrol, a Monoterpene Present in the Essential Oil of Oregano, with β-Cyclodextrin, Improves the Pharmacological Response on Cancer Pain Experimental Protocols. Chem. Biol. Interact. 2015, 227, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Arunasree, K.M. Anti-Proliferative Effects of Carvacrol on a Human Metastatic Breast Cancer Cell Line, MDA-MB 231. Phytomedicine 2010, 17, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante Melo, F.H.; Rios, E.R.V.; Rocha, N.F.M.; Citõ, M.D.C.D.O.; Fernandes, M.L.; De Sousa, D.P.; De Vasconcelos, S.M.M.; De Sousa, F.C.F. Antinociceptive Activity of Carvacrol (5-Isopropyl-2-Methylphenol) in Mice. J. Pharm. Pharmacol. 2012, 64, 1722–1729. [Google Scholar] [CrossRef]
- Guimarães, A.G.; Oliveira, G.F.; Melo, M.S.; Cavalcanti, S.C.H.; Antoniolli, A.R.; Bonjardim, L.R.; Silva, F.A.; Santos, J.P.A.; Rocha, R.F.; Moreira, J.C.F.; et al. Bioassay-Guided Evaluation of Antioxidant and Antinociceptive Activities of Carvacrol. Basic Clin. Pharmacol. Toxicol. 2010, 107, 949–957. [Google Scholar] [CrossRef]
- Lee, B.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.H. Inhibitory Effect of Carvacrol on Lipopolysaccharide-Induced Memory Impairment in Rats. Korean J. Physiol. Pharmacol. 2019, 24, 27–37. [Google Scholar] [CrossRef]
- Gunal, M.Y.; Heper, A.O.; Zaloglu, N. The Effects of Topical Carvacrol Application on Wound Healing Process in Male Rats. Pharmacogn. J. 2014, 6, 10–14. [Google Scholar] [CrossRef]
- Alagawany, M. Biological Effects and Modes of Action of Carvacrol in Animal and Poultry Production and Health—A Review. Adv. Anim. Vet. Sci. 2015, 3, 73–84. [Google Scholar] [CrossRef]
- Wolf, A.M.; Wolf, D.; Rumpold, H.; Enrich, B.; Tilg, H. Adiponectin Induces the Anti-Inflammatory Cytokines IL-10 and IL-1RA in Human Leukocytes. Biochem. Biophys. Res. Commun. 2004, 323, 630–635. [Google Scholar] [CrossRef]
- Laothaweerungsawat, N.; Neimkhum, W.; Anuchapreeda, S.; Sirithunyalug, J.; Chaiyana, W. Transdermal Delivery Enhancement of Carvacrol from Origanum Vulgare L. Essential Oil by Microemulsion. Int. J. Pharm. 2020, 579, 119052. [Google Scholar] [CrossRef]
- Mufamadi, M.S.; Pillay, V.; Choonara, Y.E.; Du Toit, L.C.; Modi, G.; Naidoo, D.; Ndesendo, V.M.K. A Review on Composite Liposomal Technologies for Specialized Drug Delivery. J. Drug Deliv. 2011, 2011, 939851. [Google Scholar] [CrossRef] [PubMed]
- Hashemi Dehaghi, M.; Dadash Zadeh, S.; Keshvari, H.; Abasian, P. Preparation and Characterization of Dorzolamide HCl Loaded in Niosome in Order to Study the Amount of Its Realese. Res. Pharm. Sci. 2012, 7, 290. [Google Scholar]
- Hashemi, M.; Omidi, M.; Mohammadi, J.; Shalbaf, M.; Shayeh, J.S.; Mohagheghi, M.A. Nanohybrid Platform of Functionalized Graphene Oxide for Chemo-Photothermal Therapy. Basic Clin. Cancer Res. 2018, 10, 1–8. [Google Scholar]
- Mullaicharam, A.R.; Murthy, R.S.R. Lung Accumulation of Niosome-Entrapped Rifampicin Following Intravenous and Intratracheal Administration in the Rat. J. Drug Deliv. Sci. Technol. 2004, 14, 99–104. [Google Scholar] [CrossRef]
- Akbarzadeh, I.; Tavakkoli Yaraki, M.; Bourbour, M.; Noorbazargan, H.; Lajevardi, A.; Sadat Shilsar, S.M.; Heidari, F.; Mousavian, S.M. Optimized Doxycycline-Loaded Niosomal Formulation for Treatment of Infection-Associated Prostate Cancer: An in-Vitro Investigation. J. Drug Deliv. Sci. Technol. 2020, 57, 101715. [Google Scholar] [CrossRef]
- Dehaghi, M.H.; Haeri, A.; Keshvari, H.; Abbasian, Z.; Dadashzadeh, S. Dorzolamide Loaded Niosomal Vesicles: Comparison of Passive and Remote Loading Methods. Iran. J. Pharm. Res. IJPR 2017, 16, 413. [Google Scholar]
- Budhiraja, A.; Dhingra, G. Development and Characterization of a Novel Antiacne Niosomal Gel of Rosmarinic Acid. Drug Deliv. 2014, 22, 723–730. [Google Scholar] [CrossRef]
- Patel, J.; Ketkar, S.; Patil, S.; Fearnley, J.; Mahadik, K.R.; Paradkar, A.R. Potentiating Antimicrobial Efficacy of Propolis through Niosomal-Based System for Administration. Integr. Med. Res. 2015, 4, 94–101. [Google Scholar] [CrossRef]
- Juneja, R.; Roy, I. Iron Oxide-Doped Niosomes as Drug Carriers for Magnetically Targeted Drug Delivery. Int. J. Nanomed. 2018, 13, 7–9. [Google Scholar] [CrossRef]
- Ghafelehbashi, R.; Akbarzadeh, I.; Tavakkoli Yaraki, M.; Lajevardi, A.; Fatemizadeh, M.; Heidarpoor Saremi, L. Preparation, Physicochemical Properties, in Vitro Evaluation and Release Behavior of Cephalexin-Loaded Niosomes. Int. J. Pharm. 2019, 569, 118580. [Google Scholar] [CrossRef]
- Khan, D.H.; Bashir, S.; Figueiredo, P.; Santos, H.A.; Khan, M.I.; Peltonen, L. Process Optimization of Ecological Probe Sonication Technique for Production of Rifampicin Loaded Niosomes. J. Drug Deliv. Sci. Technol. 2019, 50, 27–33. [Google Scholar] [CrossRef]
- Wahyuni, S.T.; Rahmasari, D.; Nugroho, R.S.; Agusta, I.; Daminda, R.D.K.; Sundugesti, R.V.; Ermawati, D. Enhanced Antibacterial Activity of Piper Betle Extract Niosome Serum Gel and Its Irritation Effects. KnE Med. 2023, 2023, 178–188. [Google Scholar] [CrossRef]
- Jufri, M.; Muthaharrah, M.; Humairah, E.; Purwaningsih, E.H. Stability of Anti-Acne Niosome Gels Containing Betel Leaf (Piper Betle L.) Essential Oil. Int. J. Appl. Pharm. 2017, 9, 130–134. [Google Scholar] [CrossRef] [PubMed]
- García-Díaz, M.; Patiño, B.; Vázquez, C.; Gil-Serna, J. A Novel Niosome-Encapsulated Essential Oil Formulation to Prevent Aspergillus Flavus Growth and Aflatoxin Contamination of Maize Grains During Storage. Toxins 2019, 11, 646. [Google Scholar] [CrossRef] [PubMed]
- Trinh, L.H.; Takzare, A.; Ghafoor, D.D.; Siddiqi, A.F.; Ravali, S.; Shalbaf, M.; Bakhtiar, M. Trachyspermum Copticum Essential Oil Incorporated Niosome for Cancer Treatment. J. Drug Deliv. Sci. Technol. 2019, 52, 818–824. [Google Scholar] [CrossRef]
- Abdelhamed, F.M.; Abdeltawab, N.F.; ElRakaiby, M.T.; Shamma, R.N.; Moneib, N.A. Antibacterial and Anti-Inflammatory Activities of Thymus Vulgaris Essential Oil Nanoemulsion on Acne Vulgaris. Microorganisms 2022, 10, 1874. [Google Scholar] [CrossRef]
- Shah, P.; Goodyear, B.; Haq, A.; Puri, V.; Michniak-Kohn, B. Evaluations of Quality by Design (QbD) Elements Impact for Developing Niosomes as a Promising Topical Drug Delivery Platform. Pharmaceutics 2020, 12, 246. [Google Scholar] [CrossRef]
- Gurumukhi, V.C.; Bari, S.B. Fabrication of Efavirenz Loaded Nano-Formulation Using Quality by Design (QbD) Based Approach: Exploring Characterizations and in Vivo Safety. J. Drug Deliv. Sci. Technol. 2020, 56, 101545. [Google Scholar] [CrossRef]
- Yaghoobian, M.; Haeri, A.; Bolourchian, N.; Shahhosseni, S.; Dadashzadeh, S. The Impact of Surfactant Composition and Surface Charge of Niosomes on the Oral Absorption of Repaglinide as a BCS II Model Drug. Int. J. Nanomed. 2020, 15, 8767–8781. [Google Scholar] [CrossRef]
- Nadzir, M.M.; Fen, T.W.; Mohamed, A.R.; Hisham, S.F. Size and Stability of Curcumin Niosomes from Combinations of Tween 80 and Span 80. Sains Malays. 2020, 46, 2455–2460. [Google Scholar] [CrossRef]
- Shekhawat, P.; Pokharkar, V. Risk Assessment and QbD Based Optimization of an Eprosartan Mesylate Nanosuspension: In-Vitro Characterization, PAMPA and in-Vivo Assessment. Int. J. Pharm. 2019, 567, 118415. [Google Scholar] [CrossRef] [PubMed]
- Shehata, T.M.; Ibrahim, M.M.; Elsewedy, H.S. Curcumin Niosomes Prepared from Proniosomal Gels: In Vitro Skin Permeability, Kinetic and In Vivo Studies. Polymers 2021, 13, 791. [Google Scholar] [CrossRef]
- Dabbagh Moghaddam, F.; Akbarzadeh, I.; Marzbankia, E.; Farid, M.; Khaledi, L.; Reihani, A.H.; Javidfar, M.; Mortazavi, P. Delivery of Melittin-Loaded Niosomes for Breast Cancer Treatment: An in Vitro and in Vivo Evaluation of Anti-Cancer Effect. Cancer Nanotechnol. 2021, 12, 1–35. [Google Scholar] [CrossRef]
- Mohanty, D.; Rani, M.J.; Haque, M.A.; Bakshi, V.; Jahangir, M.A.; Imam, S.S.; Gilani, S.J. Preparation and Evaluation of Transdermal Naproxen Niosomes: Formulation Optimization to Preclinical Anti-Inflammatory Assessment on Murine Model. J. Liposome Res. 2019, 30, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Jadupati, M.; Kumar, N.A.; Amites, G. Transferosome: An Opportunistic Carrier for Transdermal. Int. Res. J. Pharm. 2012, 3, 35–38. [Google Scholar]
- Hajimehdipoor, H.; Shekarchi, M.; Khanavi, M.; Adib, N.; Amri, M. A Validated High Performance Liquid Chromatography Method for the Analysis of Thymol and Carvacrol in Thymus Vulgaris L. Volatile Oil. Pharmacogn. Mag. 2010, 6, 154. [Google Scholar] [CrossRef]
- Dey, S.; Pramanik, S.; Malgope, A. Formulation and Optimization of Sustained Release Stavudine Microspheres Using Response Surface Methodology. ISRN Pharm. 2011, 2011, 627623. [Google Scholar] [CrossRef] [PubMed]
- Waghule, T.; Rapalli, V.K.; Singhvi, G.; Gorantla, S.; Khosa, A.; Dubey, S.K.; Saha, R.N. Design of Temozolomide-Loaded Proliposomes and Lipid Crystal Nanoparticles with Industrial Feasible Approaches: Comparative Assessment of Drug Loading, Entrapment Efficiency, and Stability at Plasma PH. J. Liposome Res. 2020, 31, 158–168. [Google Scholar] [CrossRef]
- Das, P.; Das, M.K. Production and physicochemical characterization of nanocosmeceuticals. In Nanocosmeceuticals; Academic Press: Cambridge, MA, USA, 2022; pp. 95–138. [Google Scholar]
- Mohammad, E. Anti-aging Effect of Free Curcumin and Niosome Entrapping Curcumin in H2O2-induced Aging in Human Fibroblast Cell Lines. J. Adv. Phys. 2019, 16, 237–246. [Google Scholar] [CrossRef]
- Malatesta, M. Transmission Electron Microscopy for Nanomedicine: Novel Applications for Long-Established Techniques. Eur. J. Histochem. 2016, 60, 8–12. [Google Scholar] [CrossRef]
- Kapoor, H.; Aqil, M.; Imam, S.S.; Sultana, Y.; Ali, A. Formulation of Amlodipine Nano Lipid Carrier: Formulation Design, Physicochemical and Transdermal Absorption Investigation. J. Drug Deliv. Sci. Technol. 2019, 49, 209–218. [Google Scholar] [CrossRef]
- Balasubramaniam, A.; Kumar, V.A.; Pillai, K.S. Formulation and In Vivo Evaluation of Niosome-Encapsulated Daunorubicin Hydrochloride. Drug Dev. Ind. Pharm. 2002, 28, 1181–1193. [Google Scholar] [CrossRef]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Fisetin Loaded Binary Ethosomes for Management of Skin Cancer by Dermal Application on UV Exposed Mice. Int. J. Pharm. 2019, 560, 78–91. [Google Scholar] [CrossRef]
- Jain, A.; Deveda, P.; Vyas, N.; Chauhan, J.; Khambete, H.; Jain, S. Development of Antifungal Emulsion Based Gel for Topical Fungal Infection. Available online: https://www.researchgate.net/publication/284789522_Development_of_antifungal_emulsion_based_gel_for_topical_fungal_infection (accessed on 6 April 2023).
- Aodah, A.H.; Hashmi, S.; Akhtar, N.; Ullah, Z.; Zafar, A.; Zaki, R.M.; Khan, S.; Ansari, M.J.; Jawaid, T.; Alam, A.; et al. Formulation Development, Optimization by Box–Behnken Design, and In Vitro and Ex Vivo Characterization of Hexatriacontane-Loaded Transethosomal Gel for Antimicrobial Treatment for Skin Infections. Gels 2023, 9, 322. [Google Scholar] [CrossRef]
- Jahan, S.; Aqil, M.; Ahad, A.; Imam, S.S.; Waheed, A.; Qadir, A.; Ali, A. Nanostructured Lipid Carrier for Transdermal Gliclazide Delivery: Development and Optimization by Box-Behnken Design. Inorg. Nano-Met. Chem. 2022, 2022, 1–14. [Google Scholar] [CrossRef]
- Gupta, D.K.; Aqil, M.; Ahad, A.; Imam, S.S.; Waheed, A.; Qadir, A.; Iqubal, M.K.; Sultana, Y. Tailoring of Berberine Loaded Transniosomes for the Management of Skin Cancer in Mice. J. Drug Deliv. Sci. Technol. 2020, 60, 102051. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.H.; Basri, M.; Ismail, R.; Lau, H.L.N.; Tejo, B.A.; Kanthimathi, M.S.; Hassan, H.A.; Choo, Y.M. Effect of Compositions in Nanostructured Lipid Carriers (NLC) on Skin Hydration and Occlusion. Int. J. Nanomed. 2013, 8, 13–22. [Google Scholar] [CrossRef]
- Chaubey, P.; Patel, R.R.; Mishra, B. Development and Optimization of Curcumin-Loaded Mannosylated Chitosan Nanoparticles Using Response Surface Methodology in the Treatment of Visceral Leishmaniasis. Expert Opin. Drug Deliv. 2014, 11, 1163–1181. [Google Scholar] [CrossRef]
- Jianxian, C.; Saleem, K.; Ijaz, M.; Ur-Rehman, M.; Murtaza, G.; Asim, M.H. Development and in vitro Evaluation of Gastro-protective Aceclofenac-loaded Self-emulsifying Drug Delivery System. Int. J. Nanomed. 2020, 15, 5217–5226. [Google Scholar] [CrossRef]
- Sarheed, O.; Dibi, M.; Ramesh, K.V.R.N.S. Studies on the Effect of Oil and Surfactant on the Formation of Alginate-Based O/W Lidocaine Nanocarriers Using Nanoemulsion Template. Pharmaceutics 2020, 12, 1223. [Google Scholar] [CrossRef] [PubMed]
- Iqubal, M.K.; Iqubal, A.; Imtiyaz, K.; Rizvi, M.M.A.; Gupta, M.M.; Ali, J.; Baboota, S. Combinatorial Lipid-Nanosystem for Dermal Delivery of 5-Fluorouracil and Resveratrol against Skin Cancer: Delineation of Improved Dermatokinetics and Epidermal Drug Deposition Enhancement Analysis. Eur. J. Pharm. Biopharm. 2021, 163, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Emami, J.; Yousefian, H.; Sadeghi, H. Targeted Nanostructured Lipid Carrier for Brain Delivery of Artemisinin: Design, Preparation, Characterization, Optimization and Cell Toxicity. J. Pharm. Pharm. Sci. 2018, 21, 225s–241s. [Google Scholar] [CrossRef]
- Qamar, Z.; Ashhar, M.U.; Annu; Qizilibash, F.F.; Sahoo, P.K.; Ali, A.; Ali, J.; Baboota, S. Lipid Nanocarrier of Selegiline Augmented Anti-Parkinson’s Effect via P-Gp Modulation Using Quercetin. Int. J. Pharm. 2021, 609, 121131. [Google Scholar] [CrossRef] [PubMed]
- Pardakhty, A.; Varshosaz, J.; Rouholamini, A. In Vitro Study of Polyoxyethylene Alkyl Ether Niosomes for Delivery of Insulin. Int. J. Pharm. 2007, 328, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Farmoudeh, A.; Akbari, J.; Saeedi, M.; Ghasemi, M.; Asemi, N.; Nokhodchi, A. Methylene Blue-Loaded Niosome: Preparation, Physicochemical Characterization, and in Vivo Wound Healing Assessment. Drug Deliv. Transl. Res. 2020, 10, 1428–1441. [Google Scholar] [CrossRef]
- Qadir, A.; Aqil, M.; Ali, A.; Warsi, M.H.; Mujeeb, M.; Ahmad, F.J.; Ahmad, S.; Beg, S. Nanostructured Lipidic Carriers for Dual Drug Delivery in the Management of Psoriasis: Systematic Optimization, Dermatokinetic and Preclinical Evaluation. J. Drug Deliv. Sci. Technol. 2020, 57, 101775. [Google Scholar] [CrossRef]
- Pande, V.; Patel, S.; Patil, V.; Sonawane, R. Design Expert Assisted Formulation of Topical Bioadhesive Gel of Sertaconazole Nitrate. Adv. Pharm. Bull. 2014, 4, 121. [Google Scholar] [CrossRef]
- Ahmad, N.; Afzali, R.; Neupane, Y.; Amin, S.; Kohli, K. Optimization of Gel Based System of Lercanidipine by Statistical Design for Transdermal Delivery; Histopathological Examination and Rheological Characterization. J. Biopharm. Sci. 2014, 2, 15–25. [Google Scholar]
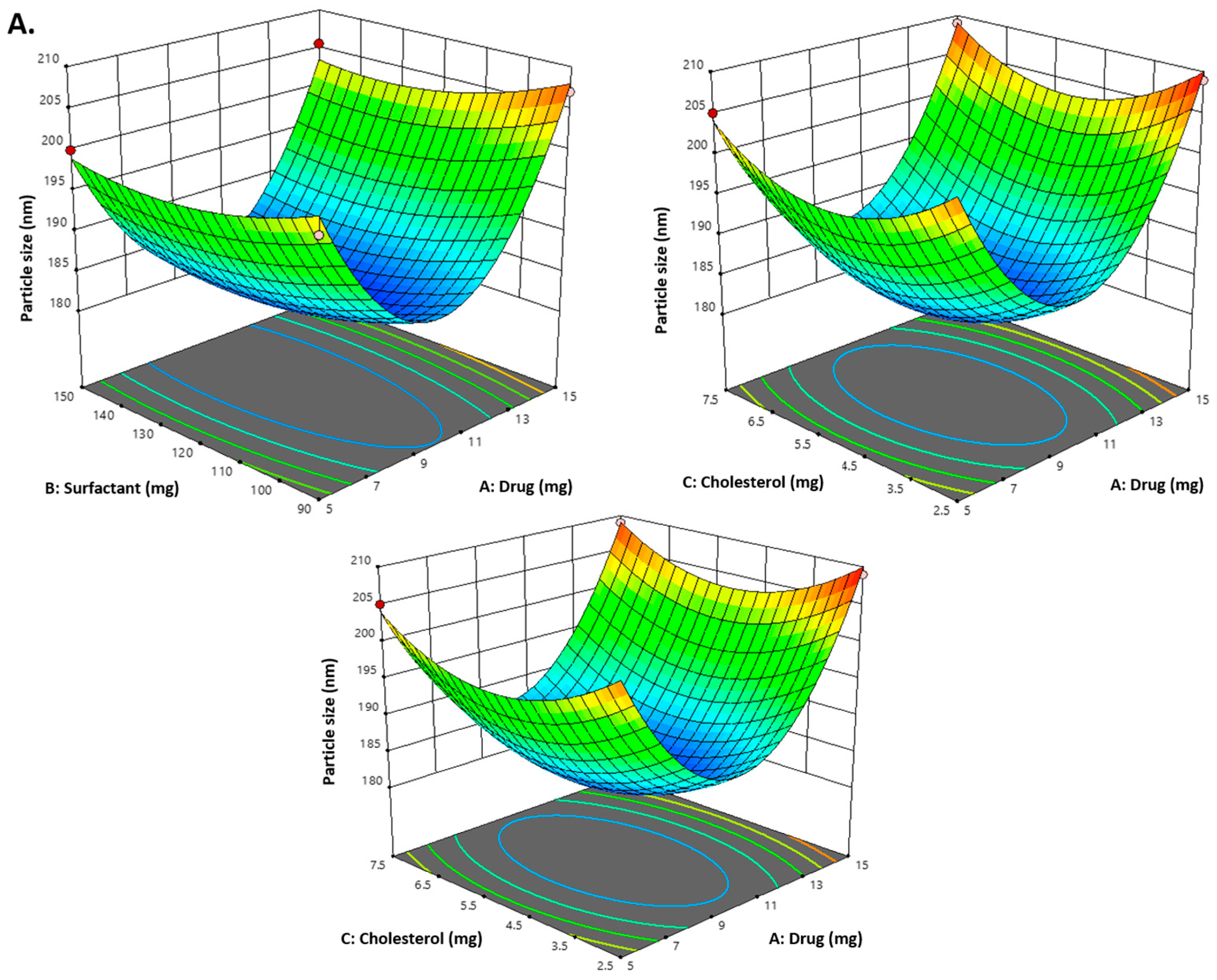
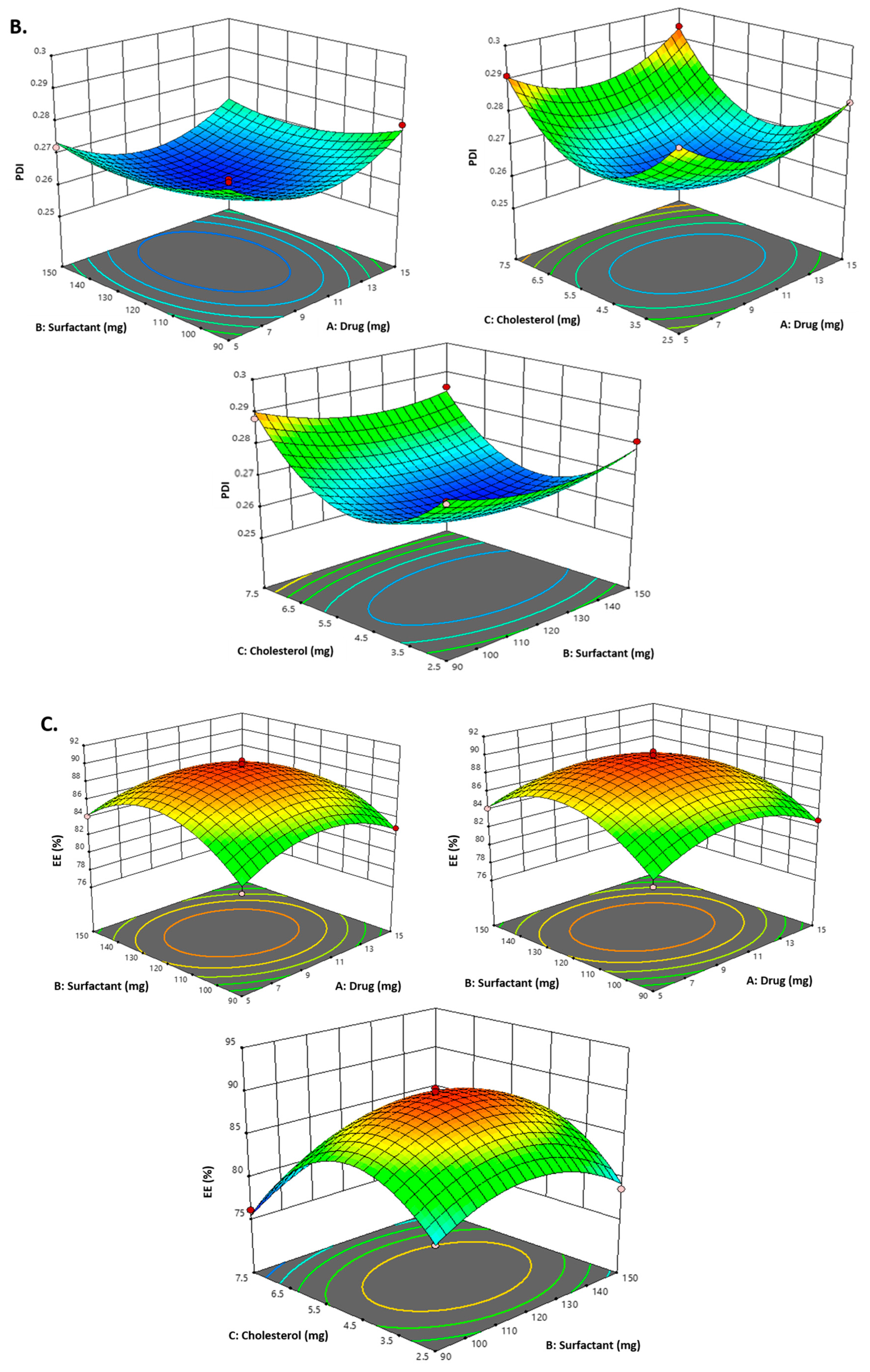
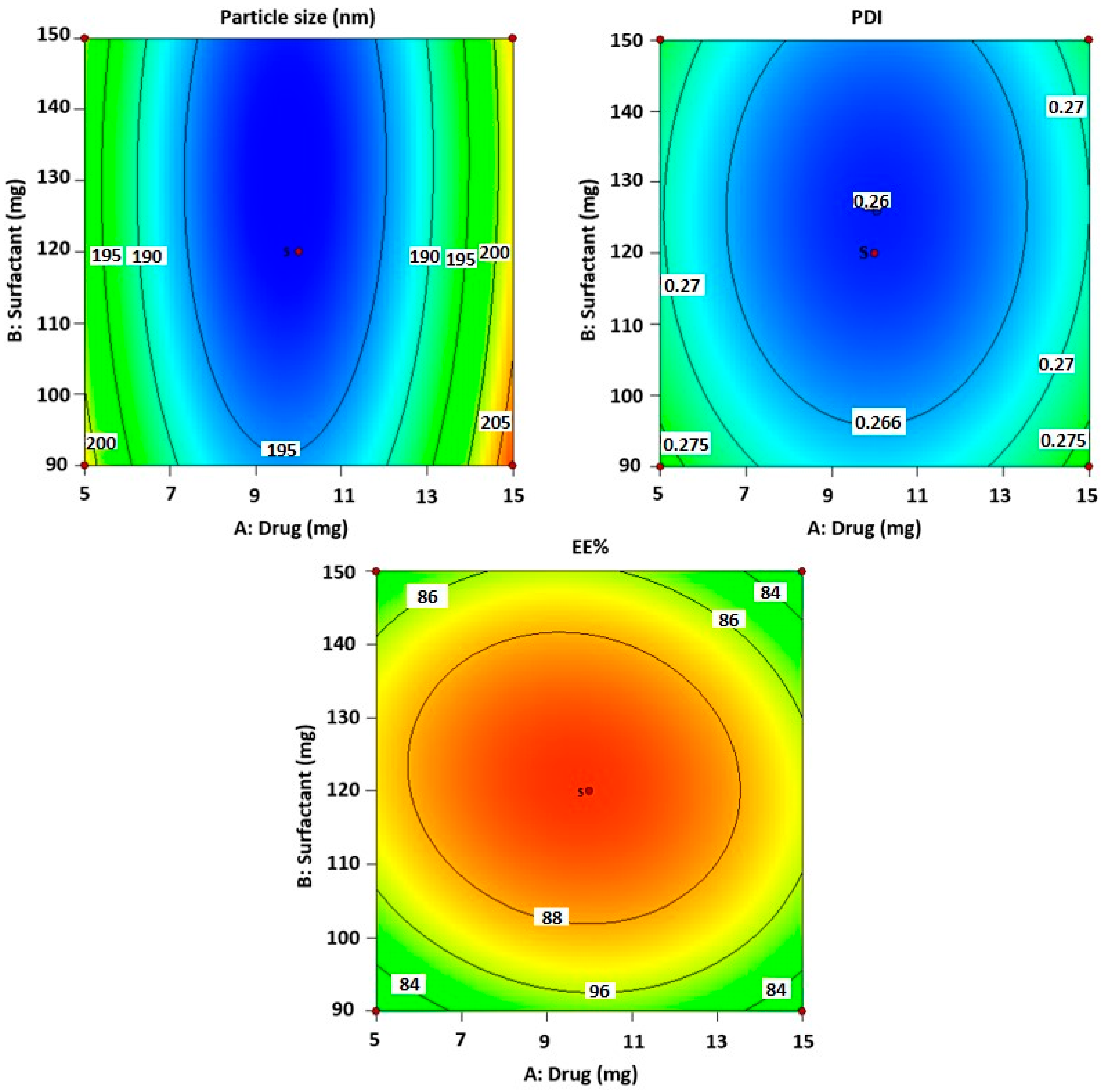

| Formulations | Independent Variables | Dependent Variables | ||||
|---|---|---|---|---|---|---|
| A | B | C | Y1 | Y2 | Y3 | |
| 1 | 5 | 120 | 7.5 | 205.05 | 0.291 | 78.49 |
| 2 | 10 | 120 | 5 | 180.98 | 0.259 | 88.97 |
| 3 | 10 | 150 | 7.5 | 186.98 | 0.285 | 77.95 |
| 4 | 10 | 120 | 5 | 180.23 | 0.265 | 90.41 |
| 5 | 10 | 120 | 5 | 181.07 | 0.261 | 89.48 |
| 6 | 10 | 150 | 2.5 | 185.89 | 0.281 | 78.62 |
| 7 | 15 | 120 | 7.5 | 208.95 | 0.294 | 76.38 |
| 8 | 5 | 120 | 2.5 | 204.62 | 0.286 | 81.64 |
| 9 | 15 | 90 | 5 | 206.99 | 0.279 | 82.84 |
| 10 | 15 | 120 | 2.5 | 208.95 | 0.283 | 80.51 |
| 11 | 15 | 120 | 5 | 181.28 | 0.262 | 89.55 |
| 12 | 5 | 150 | 5 | 200.01 | 0.272 | 84.18 |
| 13 | 10 | 90 | 2.5 | 195.54 | 0.279 | 79.98 |
| 14 | 5 | 90 | 5 | 199.94 | 0.279 | 81.55 |
| 15 | 10 | 120 | 5 | 181.08 | 0.26 | 90.01 |
| 16 | 10 | 90 | 7.5 | 191.08 | 0.288 | 76.08 |
| 17 | 15 | 150 | 5 | 205.54 | 0.271 | 83.17 |
| Quadratic Model | R2 | Adjusted R2 | Predicted R2 | SD | %CV | p-Value |
|---|---|---|---|---|---|---|
| Response (Y1) | 0.9882 | 0.9731 | 0.8162 | 1.85 | 0.9395 | <0.0001 |
| Response (Y2) | 0.9777 | 0.9490 | 0.7883 | 0.0026 | 0.7818 | <0.0001 |
| Response (Y3) | 0.9890 | 0.9749 | 0.8673 | 0.7946 | 0.9582 | <0.0001 |
| Kinetic Models | X-Axis | Y-Axis | CVC-Ns (R2) |
|---|---|---|---|
| Zero-order | Fraction of drug released | Time | 0.9268 |
| First order | Log % drug remaining | Time | 0.9878 |
| Higuchi matrix | Fraction of drug release | Square root time | 0.9933 |
| Korsmeyer–Peppas | Log fraction of drug released | Log time | 0.9927 |
| Homogeneity | Appearance | Washability | Separation of Phase | Odour |
|---|---|---|---|---|
| Homogeneous | Translucent | Yes | No | odourless |
| Colour | Content of Drug (%) | pH | Spreadability (gm. cm/sec) | |
| off-white | 90.11 ± 0.98 | 6.01 ± 1.01 | 18.27 ± 1.24 | |
| Cohesiveness (gm) | Consistency (gm.Sec) | Firmness (gm) | Work of cohesion g, sec | |
| −89.17 | 1587.00 | 239.09 | −645.47 | |
| Dermatokinetics Parameters | CVC-N Gel | CVC-CF Gel | ||
|---|---|---|---|---|
| Epidermis Mean ± SD | Dermis Mean ± SD | Epidermis Mean ± SD | Dermis Mean ± SD | |
| Tskin max (h) | 2 | 2 | 2 | 2 |
| Cskin max (μg/cm2) | 283.54 ± 1.01 | 262.64 ± 1.12 | 179.04 ± 0.96 | 160.13 ± 0.64 |
| AUC0-8 (μg/cm2 h) | 1135.5 ± 0.64 | 1158 ± 1.08 | 677.47 ± 0.28 | 572.23 ± 0.31 |
| Ke (h−1) | 0.171 ± 1.11 | 0.119 ± 0.91 | 0.146 ± 0.47 | 0.119 ± 0.78 |
| Evaluation Parameters | Initial | 1 Month | 3 Months | 6 Months | |||
|---|---|---|---|---|---|---|---|
| 4 ± 2 °C | 25 ± 2 °C/ 60 ± 5% RH | 4 ± 2 °C | 25 ± 2 °C/ 60 ± 5% RH | 4 ± 2 °C | 25 ± 2 °C/ 60 ± 5% RH | ||
| Appearance | +++ | +++ | +++ | ++ | ++ | ++ | ++ |
| Phase separation | NO PHASE SEPARATION | ||||||
| Shape | Spherical in shape | ||||||
| PDI | 0.259 | 0.259 | 0.264 | 0.271 | 0.277 | 0.280 | 0.284 |
| Vesicle size (nm) | 180.23 | 180.23 | 180.99 | 181.11 | 182.14 | 183.09 | 183.21 |
| Zeta potential (mV) | −31.70 | −31.70 | −32.01 | −32.45 | −33.41 | −33.39 | −33.34 |
| Evaluation Parameters | Initial | 1 Month | 3 Months | 6 Months | |||
|---|---|---|---|---|---|---|---|
| 4 ± 2 °C | 25 ± 2 °C/60 ± 5% RH | 4 ± 2 °C | 25 ± 2 °C/60 ± 5% RH | 4 ± 2 °C | 25 ± 2 °C/60 ± 5% RH | ||
| Colour | Slightly off white | ||||||
| Appearance | Translucent | ||||||
| Phase Separation | NO PHASE SEPARATION | ||||||
| Clarity | √ | √ | √ | √ | √ | √ | √ |
| pH | 6.01 | 6.01 | 6.29 | 6.31 | 6.39 | 6.55 | 6.67 |
| Homogeneity | *** | *** | ** | *** | ** | *** | * |
| Washability | Washable | ||||||
| Odour | NO | ||||||
| Factor | The Level Used, Actual Coded | ||
|---|---|---|---|
| Factors | Low (−1) | Medium (0) | High (+1) |
| A = Drug (mg) | 05 | 10 | 15 |
| B = Surfactant (mg) | 90 | 120 | 150 |
| C = Cholesterol (mg) | 2.5 | 05 | 7.5 |
| Responses | Aim | ||
| Y1 = globule size (nm) | <200 nm | ||
| Y2 = PDI | <0.3 | ||
| Y3 = Entrapment Efficiency (%) | >70% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghazwani, M.; Hani, U.; Alam, A.; Alqarni, M.H. Quality-by-Design-Assisted Optimization of Carvacrol Oil-Loaded Niosomal Gel for Anti-Inflammatory Efficacy by Topical Route. Gels 2023, 9, 401. https://doi.org/10.3390/gels9050401
Ghazwani M, Hani U, Alam A, Alqarni MH. Quality-by-Design-Assisted Optimization of Carvacrol Oil-Loaded Niosomal Gel for Anti-Inflammatory Efficacy by Topical Route. Gels. 2023; 9(5):401. https://doi.org/10.3390/gels9050401
Chicago/Turabian StyleGhazwani, Mohammed, Umme Hani, Aftab Alam, and Mohammed H. Alqarni. 2023. "Quality-by-Design-Assisted Optimization of Carvacrol Oil-Loaded Niosomal Gel for Anti-Inflammatory Efficacy by Topical Route" Gels 9, no. 5: 401. https://doi.org/10.3390/gels9050401
APA StyleGhazwani, M., Hani, U., Alam, A., & Alqarni, M. H. (2023). Quality-by-Design-Assisted Optimization of Carvacrol Oil-Loaded Niosomal Gel for Anti-Inflammatory Efficacy by Topical Route. Gels, 9(5), 401. https://doi.org/10.3390/gels9050401






