The Effect of Concentration, Temperature, and pH on the Formation of Hyaluronic Acid–Surfactant Nanohydrogels
Abstract
:1. Introduction
2. Results and Discussion
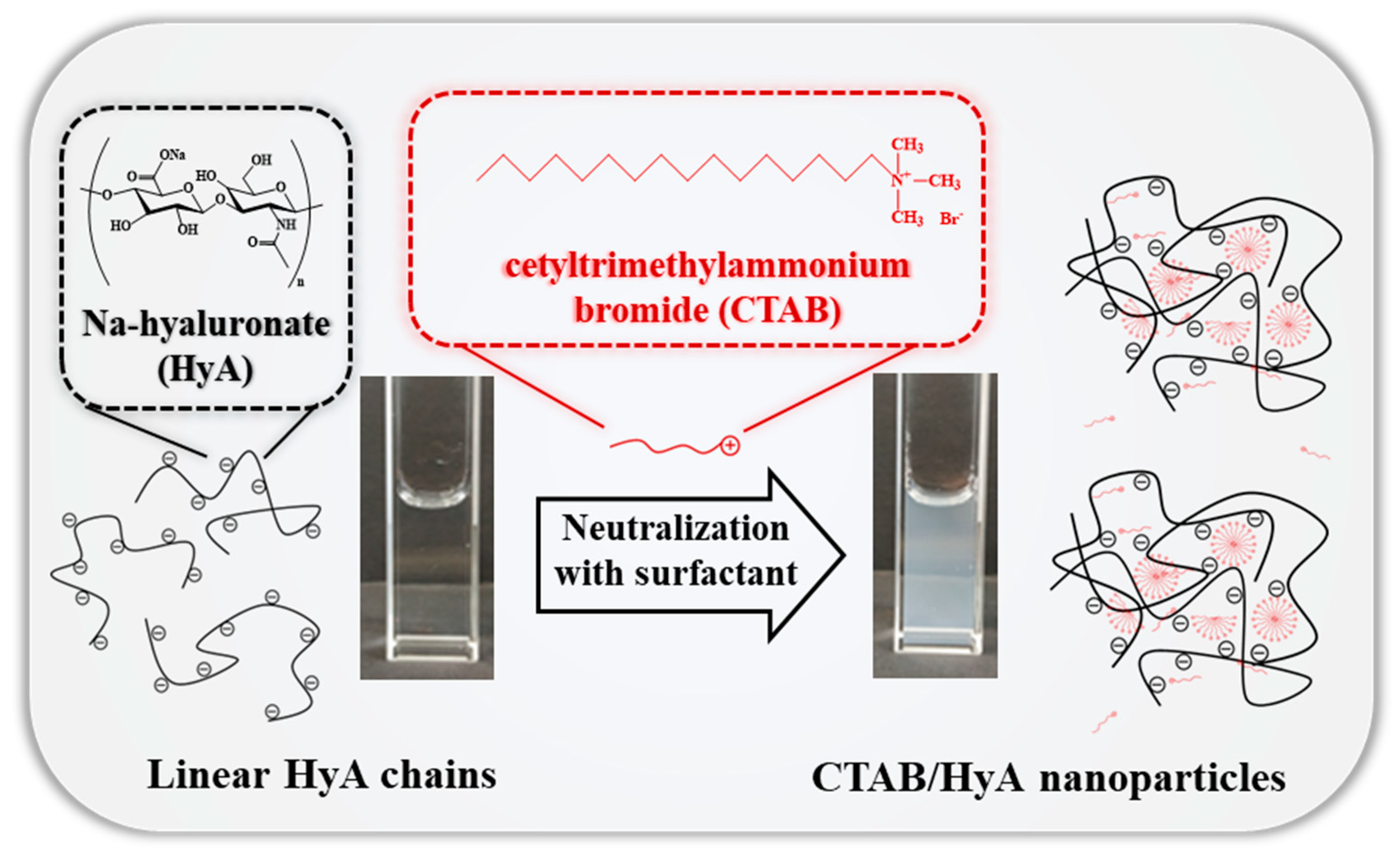
2.1. Size and Structural Characterization of HyA-CTAB Particles
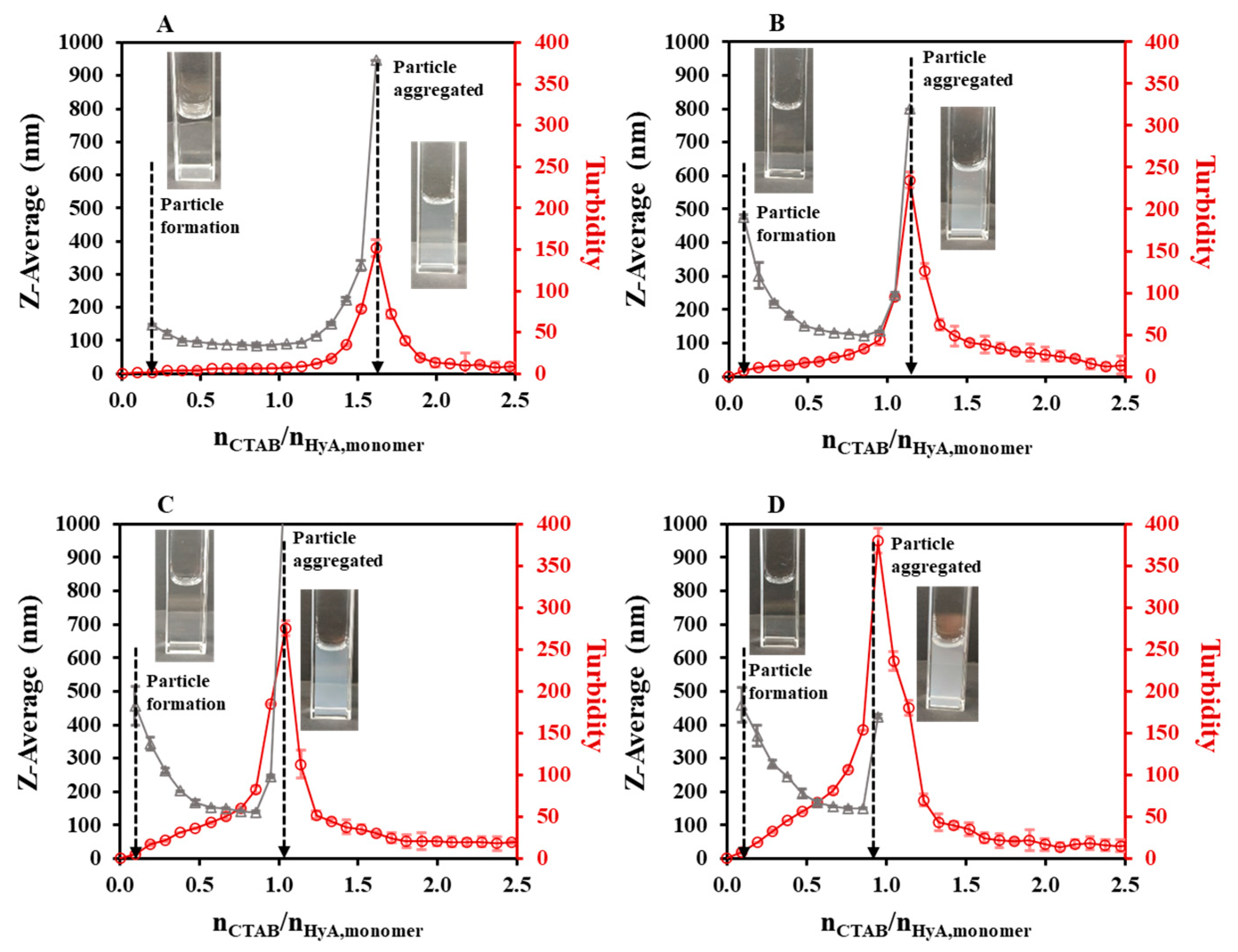
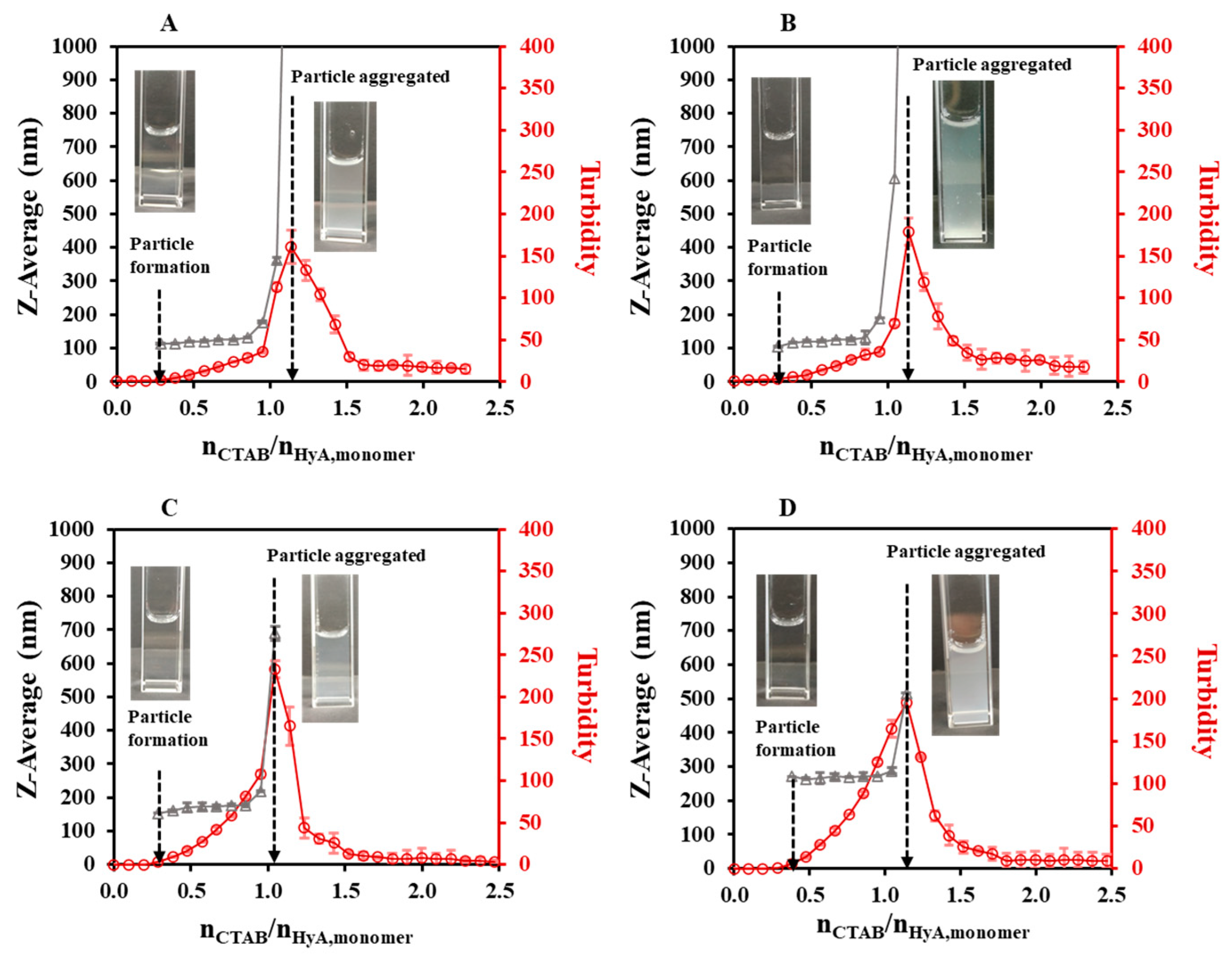
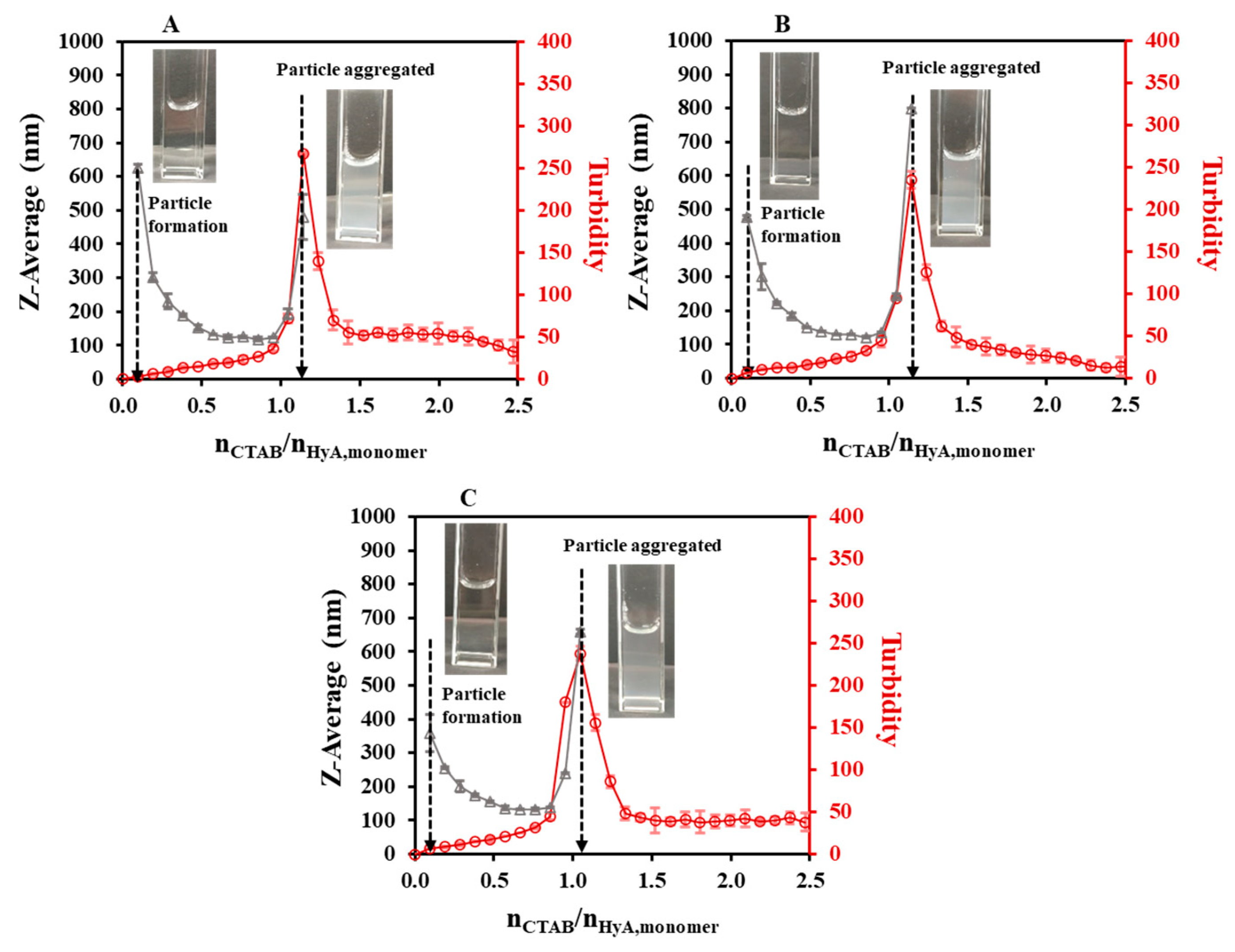
2.1.1. Effect of the Synthesis Conditions on the HyA-Based Particles
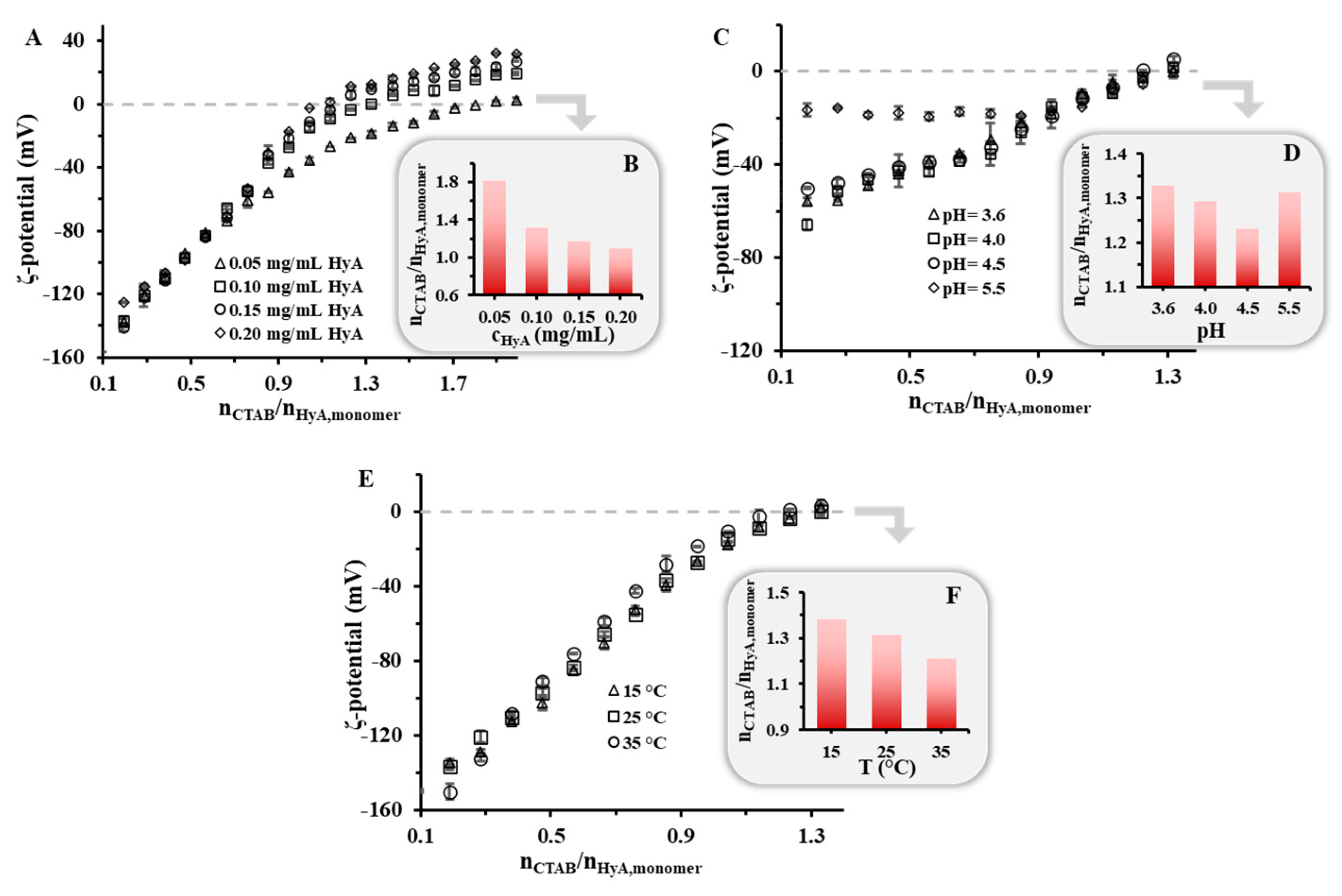
2.1.2. Zeta-Potential of HyA-CTAB Particles
2.2. Study of the HyA-CTAB Interaction
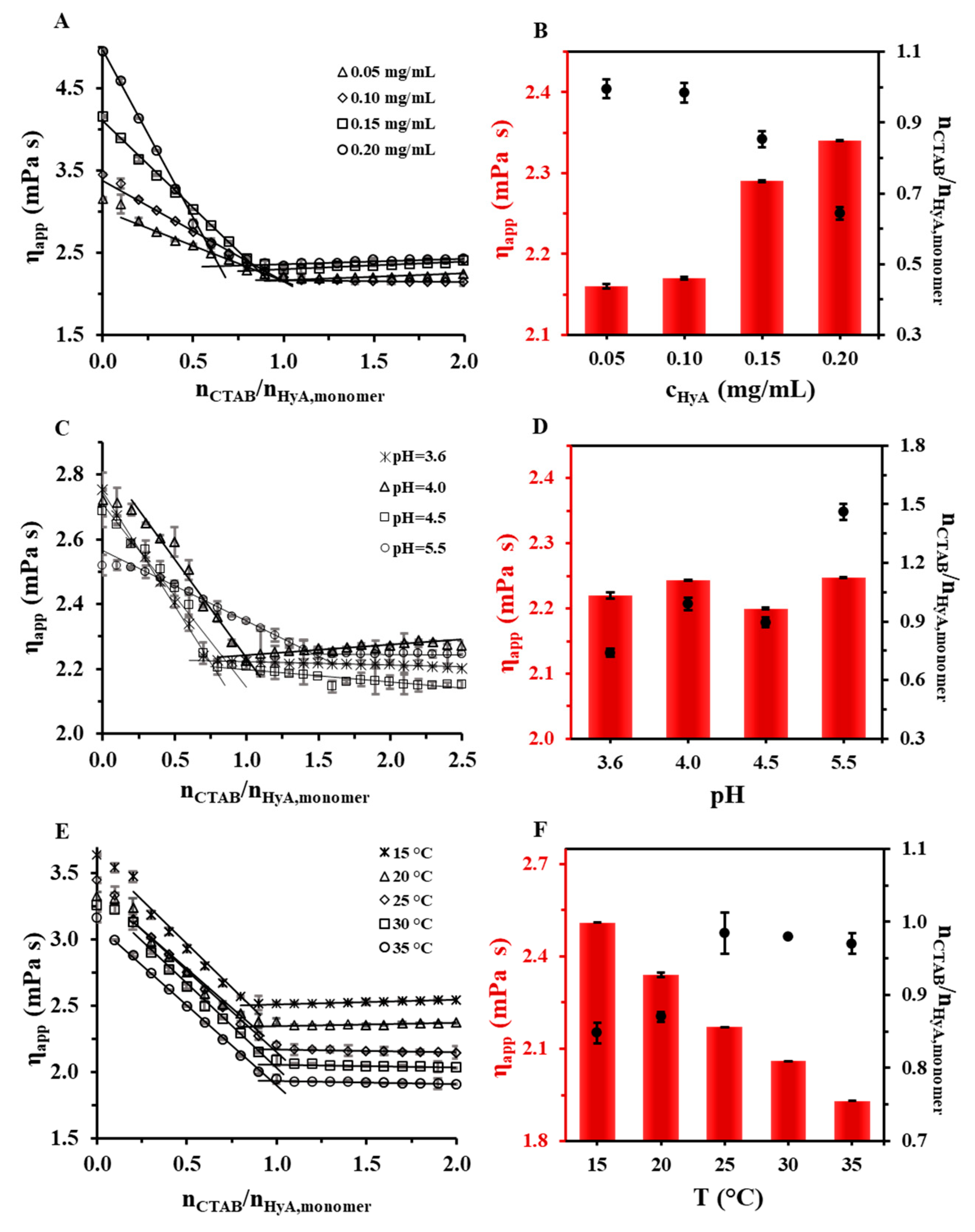
2.2.1. Rheology
2.2.2. Charge Titration
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of the CTAB-Modified HyA (HyA/CTAB) Particles
4.2.2. Rheological Measurements
4.2.3. Particle Charge Detector (PCD)
4.2.4. Characterization of the HyA/CTAB NPs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Wang, S. Polysaccharide-Based Multifunctional Hydrogel Bio-Adhesives for Wound Healing: A Review. Gels 2023, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Knopf-Marques, H.; Pravda, M.; Wolfova, L.; Velebny, V.; Schaaf, P.; Vrana, N.E.; Lavalle, P. Hyaluronic Acid and Its Derivatives in Coating and Delivery Systems: Applications in Tissue Engineering, Regenerative Medicine and Immunomodulation. Adv. Healthc. Mater. 2016, 5, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.E.; Heatley, F. Hyaluronan forms specific stable tertiary structures in aqueous solution: A 13C NMR study. Proc. Natl. Acad. Sci. USA 1999, 96, 4850–4855. [Google Scholar] [CrossRef] [Green Version]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepulveda, A.F.; Kumpgdee-Vollrath, M.; Franco, M.K.; Yokaichiya, F.; de Araujo, D.R. Supramolecular structure organization and rheological properties modulate the performance of hyaluronic acid-loaded thermosensitive hydrogels as drug-delivery systems. J. Colloid Interface Sci. 2023, 630, 328–340. [Google Scholar] [CrossRef]
- Laurano, R.; Boffito, M.; Cassino, C.; Liberti, F.; Ciardelli, G.; Chiono, V. Design of Injectable Bioartificial Hydrogels by Green Chemistry for Mini-Invasive Applications in the Biomedical or Aesthetic Medicine Fields. Gels 2023, 9, 59. [Google Scholar] [CrossRef]
- Melnik, T.; Porcello, A.; Saucy, F.; Delie, F.; Jordan, O. Bioadhesive Perivascular Microparticle-Gel Drug Delivery System for Intimal Hyperplasia Prevention: In Vitro Evaluation and Preliminary Biocompatibility Assessment. Gels 2022, 8, 776. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Yao, C.H.; Luo, L.J.; Chen, H.C.; Hsueh, Y.J.; Ma, D.H.K.; Lai, J.Y. Oxidation-mediated scaffold engineering of hyaluronic acid-based microcarriers enhances corneal stromal regeneration. Carbohydr. Polym. 2022, 292, 119668. [Google Scholar] [CrossRef]
- Montanari, E.; Capece, S.; Di Meo, C.; Meringolo, M.; Coviello, T.; Agostinelli, E.; Matricardi, P. Hyaluronic Acid Nanohydrogels as a Useful Tool for BSAO Immobilization in the Treatment of Melanoma Cancer Cells. Macromol. Biosci. 2013, 13, 1185–1194. [Google Scholar] [CrossRef]
- Jarzębski, M.; Smułek, W.; Siejak, P.; Rezler, R.; Pawlicz, J.; Trzeciak, T.; Jarzębska, M.; Majchrzak, O.; Kaczorek, E.; Kazemian, P.; et al. Aesculus hippocastanum L. as a Stabilizer in Hemp Seed Oil Nanoemulsions for Potential Biomedical and Food Applications. Int. J. Mol. Sci. 2021, 22, 887. [Google Scholar] [CrossRef]
- Fabozzi, A.; Della Sala, F.; di Gennaro, M.; Borzacchiello, A. Synthesis of hyaluronic acid core–shell nanoparticles via simple microfluidic-assisted nanoprecipitation method for active tumor targeting. New J. Chem. 2022, 46, 19763–19772. [Google Scholar] [CrossRef]
- Sun, C.Y.; Zhang, B.B.; Zhou, J.Y. Light-activated drug release from a hyaluronic acid targeted nanoconjugate for cancer therapy. J. Mater. Chem. B 2019, 7, 4843–4853. [Google Scholar] [CrossRef]
- Csapó, E.; Szokolai, H.; Juhász, Á.; Varga, N.; Janovák, L.; Dékány, I. Cross-linked and hydrophobized hyaluronic acid-based controlled drug release systems. Carbohydr. Polym. 2018, 195, 99–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hintze, V.; Schnabelrauch, M.; Rother, S. Chemical Modification of Hyaluronan and Their Biomedical Applications. Front. Chem. 2022, 10, 39. [Google Scholar] [CrossRef]
- Varga, N.; Seres, L.; Kovács, N.A.; Turcsányi, Á.; Juhász, Á.; Csapó, E. Serum albumin/hyaluronic acid nanoconjugate: Evaluation of concentration-dependent structural changes to form an efficient drug carrier particle. Int. J. Biol. Macromol. 2022, 220, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Raghav, N.; Mor, N.; Gupta, R.D.; Kaur, R.; Sharma, M.R.; Arya, P. Some cetyltrimethylammonium bromide modified polysaccharide supports as sustained release systems for curcumin. Int. J. Biol. Macromol. 2020, 154, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Le, H.V.; Le Cerf, D. Colloidal Polyelectrolyte Complexes from Hyaluronic Acid: Preparation and Biomedical Applications. Small 2022, 18, 2204283. [Google Scholar] [CrossRef]
- Turcsányi, Á.; Varga, N.; Csapó, E. Chitosan-modified hyaluronic acid-based nanosized drug carriers. Int. J. Biol. Macromol. 2020, 148, 218–225. [Google Scholar] [CrossRef]
- Wang, J.; Asghar, S.; Yang, L.; Gao, S.; Chen, Z.; Huang, L.; Zong, L.; Ping, Q.; Xiao, Y. Chitosan hydrochloride/hyaluronic acid nanoparticles coated by mPEG as long-circulating nanocarriers for systemic delivery of mitoxantrone. Int. J. Biol. Macromol. 2018, 113, 345–353. [Google Scholar] [CrossRef]
- Shabani Ravari, N.; Goodarzi, N.; Alvandifar, F.; Amini, M.; Souri, E.; Khoshayand, M.R.; Hadavand Mirzaie, Z.; Atyabi, F.; Dinarvand, R. Fabrication and biological evaluation of chitosan coated hyaluronic acid-docetaxel conjugate nanoparticles in CD44+ cancer cells. DARU J. Pharm. Sci. 2016, 24, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.X.; Wang, M.; Tian, H.H.; Chen, J.H. Hyaluronic acid and polyethylenimine self-assembled polyion complexes as pH-sensitive drug carrier for cancer therapy. Colloids Surf. B Biointerfaces 2015, 134, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.Y.; Rejinold, N.S.; Lekshmi, K.M.; Cherukula, K.; Park, I.K.; Kim, Y.C. CD44 targeting biocompatible and biodegradable hyaluronic acid cross-linked zein nanogels for curcumin delivery to cancer cells: In vitro and in vivo evaluation. J. Control. Release 2018, 280, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.N.; Katona, G.; Juhász, Á.; Balogh, G.T.; Csapó, E. Albumin-hyaluronic acid colloidal nanocarriers: Effect of human and bovine serum albumin for intestinal ibuprofen release enhancement. J. Mol. Liq. 2022, 351, 118614. [Google Scholar] [CrossRef]
- Sato, T.; Nakata, M.; Yang, Z.; Torizuka, Y.; Kishimoto, S.; Ishihara, M. In vitro and in vivo gene delivery using chitosan/hyaluronic acid nanoparticles: Influences of molecular mass of hyaluronic acid and lyophilization on transfection efficiency. J. Gene Med. 2017, 19, e2968. [Google Scholar] [CrossRef] [PubMed]
- Djafari, J.; Fernández-Lodeiro, J.; Santos, H.M.; Lorenzo, J.; Rodriguez-Calado, S.; Bértolo, E.; Capelo-Martínez, J.L.; Lodeiro, C. Study and Preparation of Multifunctional Poly(L-Lysine)@Hyaluronic Acid Nanopolyplexes for the Effective Delivery of Tumor Suppressive MiR-34a into Triple-Negative Breast Cancer Cells. Materials 2020, 13, 5309. [Google Scholar] [CrossRef] [PubMed]
- Holínková, P.; Mravec, F.; Venerová, T.; Chang, C.H.; Pekař, M. Hyaluronan interactions with cationic surfactants—Insights from fluorescence resonance energy transfer and anisotropy techniques. Int. J. Biol. Macromol. 2022, 211, 107–115. [Google Scholar] [CrossRef]
- Sharma, M.R.; Arya, P.; Kaur, R.; Kennedy, J.F.; Raghav, N. Differential binding of piperine & curcumin with modified cellulose, alginate and pectin supports: In-vitro & in-silico studies. Int. J. Biol. Macromol. 2023, 233, 123508. [Google Scholar] [CrossRef]
- Kayitmazer, A.B.; Seyrek, E.; Dubin, P.L.; Staggemeier, B.A. Influence of chain stiffness on the interaction of polyelectrolytes with oppositely charged micelles and proteins. J. Phys. Chem. B 2003, 107, 8158–8165. [Google Scholar] [CrossRef] [Green Version]
- Bao, H.; Li, L.; Gan, L.H.; Zhang, H. Interactions between ionic surfactants and polysaccharides in aqueous solutions. Macromolecules 2008, 41, 9406–9412. [Google Scholar] [CrossRef]
- Appel, E.A.; Tibbitt, M.W.; Greer, J.M.; Fenton, O.S.; Kreuels, K.; Anderson, D.G.; Langer, R. Exploiting Electrostatic Interactions in Polymer-Nanoparticle Hydrogels. ACS Macro Lett. 2015, 4, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Tolentino, A.; Alla, A.; de Ilarduya, A.M.; Muñoz-Guerra, S. Comb-like ionic complexes of hyaluronic acid with alkyltrimethylammonium surfactants. Carbohydr. Polym. 2013, 92, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Kalbáčová, M.; Verdánová, M.; Mravec, F.; Halasová, T.; Pekař, M. Effect of CTAB and CTAB in the presence of hyaluronan on selected human cell types. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 204–208. [Google Scholar] [CrossRef]
- Sauerová, P.; Verdánová, M.; Mravec, F.; Pilgrová, T.; Venerová, T.; Kalbáčová, M.H.; Pekař, M. Hyaluronic acid as a modulator of the cytotoxic effects of cationic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 155–161. [Google Scholar] [CrossRef]
- Sauerová, P.; Pilgrová, T.; Pekař, M.; Kalbáčová, M.H. Hyaluronic acid in complexes with surfactants: The efficient tool for reduction of the cytotoxic effect of surfactants on human cell types. Int. J. Biol. Macromol. 2017, 103, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.N.; Varga, N.; Juhász, Á.; Csapó, E. Serum protein-hyaluronic acid complex nanocarriers: Structural characterisation and encapsulation possibilities. Carbohydr. Polym. 2021, 251, 117047. [Google Scholar] [CrossRef]


| Rheology | DLS | Turbidity | ζ-Potential | Charge Titration | |||||
|---|---|---|---|---|---|---|---|---|---|
| cHyA (mg/mL) | T (°C) | pH | HyA Structure Change (nCTAB/nHyA,monomer) | Starting Particle Aggregation (nCTAB/nHyA,monomer) | Particle Aggregation Max. (nCTAB/nHyA,monomer) | Starting Particle Aggregation (nCTAB/nHyA,monomer) | Particle Aggregation Max. (nCTAB/nHyA,monomer) | Neutralization (nCTAB/nHyA,monomer) | Neutralization (nCTAB/nHyA,monomer) |
| 0.05 | 25 | - | 1.00 ± 0.03 | 1.26 | 1.62 | 1.39 | 1.62 | 1.81 | 1.88 ± 0.02 |
| 0.10 | 25 | - | 0.98 ± 0.03 | 1.02 | 1.14 | 0.95 | 1.14 | 1.31 | 1.29 ± 0.01 |
| 0.15 | 25 | - | 0.85 ± 0.02 | 0.94 | 1.05 | 0.83 | 1.05 | 1.17 | 1.16 ± 0.02 |
| 0.20 | 25 | - | 0.64 ± 0.02 | 0.85 | 0.95 | 0.77 | 0.95 | 1.10 | 1.06 ± 0.01 |
| 0.10 | 25 | 3.6 | 0.74 ± 0.02 | 0.93 | 1.14 | 0.94 | 1.14 | 1.33 | 1.37 ± 0.01 |
| 0.10 | 25 | 4.0 | 0.99 ± 0.03 | 0.94 | 1.14 | 1.02 | 1.14 | 1.29 | 1.40 ± 0.02 |
| 0.10 | 25 | 4.5 | 0.90 ± 0.03 | 0.94 | 1.05 | 0.92 | 1.05 | 1.23 | 1.53 ± 0.02 |
| 0.10 | 25 | 5.5 | 1.46 ± 0.04 | 1.04 | 1.14 | 0.82 | 1.14 | 1.31 | 1.54 ± 0.03 |
| 0.10 | 15 | - | 0.85 ± 0.01 | 1.02 | 1.14 | 1.02 | 1.14 | 1.38 | - |
| 0.10 | 20 | - | 0.87 ± 0.01 | - | - | - | - | - | - |
| 0.10 | 25 | - | 0.98 ± 0.03 | 1.02 | 1.14 | 0.95 | 1.14 | 1.31 | - |
| 0.10 | 30 | - | 0.98 ± 0.02 | - | - | - | - | - | - |
| 0.10 | 35 | - | 0.97 ± 0.02 | 0.93 | 1.05 | 0.83 | 1.05 | 1.21 | - |
| T (°C) | Medium | cHyA (mg/mL) | cCTAB (mM) | |
|---|---|---|---|---|
| HyA concentration dependence | 25 | water/pH = 4.5 (acetate buffer) | 0.05 | 0.013–0.37 |
| 25 | water/pH = 4.5 (acetate buffer) | 0.10 | 0.025–0.73 | |
| 25 | water/pH = 4.5 (acetate buffer) | 0.15 | 0.037–1.08 | |
| 25 | water/pH = 4.5 (acetate buffer) | 0.20 | 0.050–1.42 | |
| Temperature dependence | 15 | water | 0.10 | 0.025–0.73 |
| 20 | water | 0.10 | 0.025–0.73 | |
| 25 | water | 0.10 | 0.025–0.73 | |
| 30 | water | 0.10 | 0.025–0.73 | |
| 35 | water | 0.10 | 0.025–0.73 | |
| Medium dependence | 25 | pH = 3.6 (acetate buffer) | 0.10 | 0.025–0.73 |
| 25 | pH = 4.0 (acetate buffer) | 0.10 | 0.025–0.73 | |
| 25 | pH = 4.5 (acetate buffer) | 0.10 | 0.025–0.73 | |
| 25 | pH = 5.5 (acetate buffer) | 0.10 | 0.025–0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seres, L.; Csapó, E.; Varga, N.; Juhász, Á. The Effect of Concentration, Temperature, and pH on the Formation of Hyaluronic Acid–Surfactant Nanohydrogels. Gels 2023, 9, 529. https://doi.org/10.3390/gels9070529
Seres L, Csapó E, Varga N, Juhász Á. The Effect of Concentration, Temperature, and pH on the Formation of Hyaluronic Acid–Surfactant Nanohydrogels. Gels. 2023; 9(7):529. https://doi.org/10.3390/gels9070529
Chicago/Turabian StyleSeres, László, Edit Csapó, Norbert Varga, and Ádám Juhász. 2023. "The Effect of Concentration, Temperature, and pH on the Formation of Hyaluronic Acid–Surfactant Nanohydrogels" Gels 9, no. 7: 529. https://doi.org/10.3390/gels9070529
APA StyleSeres, L., Csapó, E., Varga, N., & Juhász, Á. (2023). The Effect of Concentration, Temperature, and pH on the Formation of Hyaluronic Acid–Surfactant Nanohydrogels. Gels, 9(7), 529. https://doi.org/10.3390/gels9070529










