Abstract
The physics of the moving contact line of an impacting droplet is widely applied in a variety of domains in rapidly advancing healthcare technology and medicine. The behavior of the dynamic contact line after impact of a biologically active droplet on a complex material surface involves complicated solid–liquid and liquid–gas interfacial interactions. Therefore, a deep understanding of such complex droplet contact line dynamics by applying the current physical models and state-of-the-art nanotechnology and artificial neural networks can be one of the ongoing promising interests in the field of interfacial physics. This review provides an overview of several scientific aspects of contact line dynamics of an impacting droplet and its influence on the current developed healthcare technology and medicine. Firstly, the potential applications in modern healthcare and personalized medicine are listed and discussed. Secondly, the theory of the moving contact line and the fundamental physical parameters related to the motion of impacting droplets are introduced. Afterwards, the current physical models of moving contact line dynamics are critically explained by emphasizing their limitations. Finally, current concerns and obstacles are summarized, and future perspectives and research directions are outlined to address poorly understood and conflicting issues.
1. Introduction
Droplet impact is a common physical phenomenon in daily life. The frequent examples in nature are rainfall on the ground and a coffee stain. Droplet impingement on a solid surface has shown numerous applications in various scientific fields, including healthcare, medicine, aerospace, electronics, food processing, smart materials, coatings, printings, microfluidics, and nanofluidics [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306,307,308,309,310,311,312,313,314].
The physics of droplet impact on various surfaces has been the center of attention in the scientific community during the pandemic for efficient prevention of the spread of the COVID-19 virus-laden droplets in society [234,245]. Rapidly advancing scientific and commercialization efforts around the world in state-of-the-art droplet-based bioprinting have revolutionized personalized medicine, covering various applications that include gene-expression analysis, single-cell printing, individualized diagnosis and drug therapy, and functional nanobiomaterials [85,92,96,101,105,126,152,170,178,194,215,217,218,247,289,307].
Furthermore, skin-interface wireless wearable electrochemical biosensors, which are an emerging technology, are fabricated by the innovative three-dimensional printing of bioelectrically conducting droplets on soft biocompatible materials for the possibility of real-time health surveillance. A popular example is electrochemical biosensors for peripheral biochemical, nutrition, and metabolite monitoring and control for every specific patient [260,261,262,263,264,265,266,267,268,312,314]. This highly precise, personalized healthcare technology is critically dependent on how accurate the active complex nano- and microbiodroplets are being deposited on the complex biocompatible and/or biodegradable elastic soft stretchable materials.
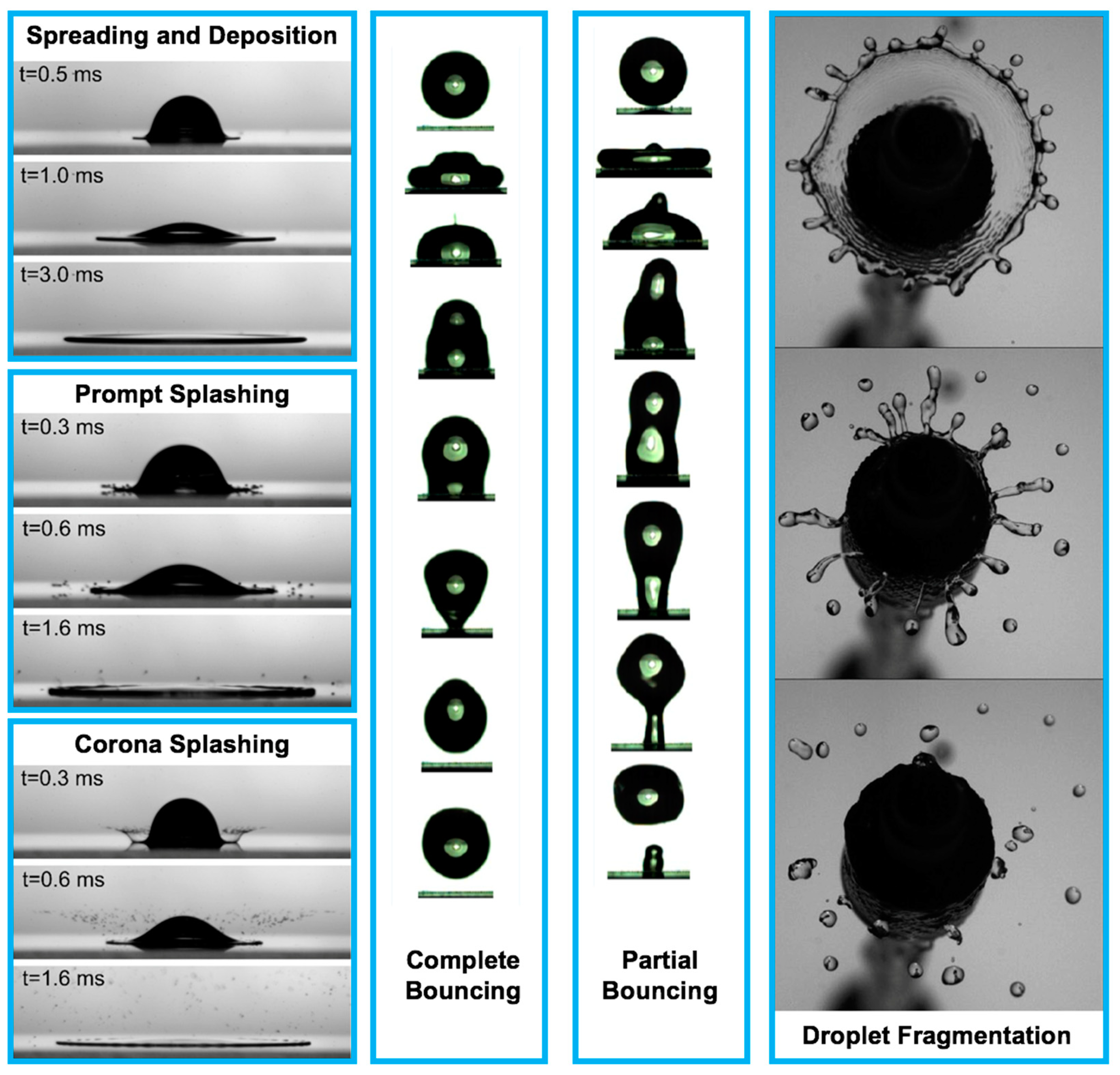
Despite more than a century of scientific study on droplet impact physics, there has not been a comprehensive understanding of this rapid physical process until the recent emergence of high-speed imaging techniques and the help of advanced computational tools [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306,307,308,309,310,311,312,313,314]. These technologies significantly facilitate better interpretation of droplet impact physics [17,68,69,70,135,137,138,223,273,274,275,281]. The droplet impact on a solid surface consists of the following physical events: spreading and deposition, receding and breakup, prompt splash, corona splash, partial bounce, and complete bounce as shown in Figure 1 [5,16,17,52,54,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130]. For numerous applications, such as printed electronics and smart materials for healthcare and medicine, a great understanding of droplet spreading after impact on a solid surface is the main concern for the purpose of high quality and precise functionality demands.
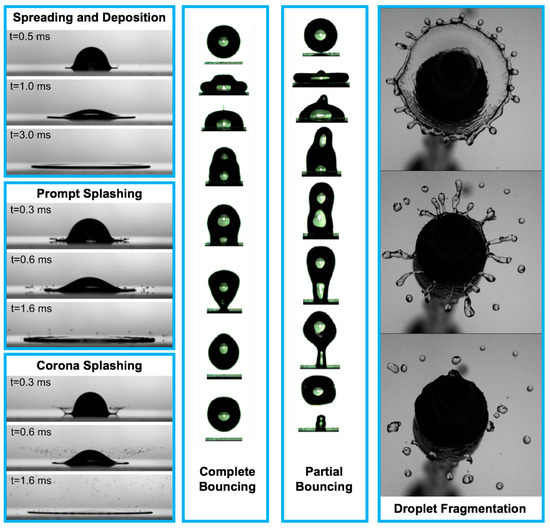
Figure 1.
The physical events after droplet impact on a solid surface [11,104,253]. Reprinted from The Journal of Fluid Mechanics, 668, Villermaux, E. and Bossa, B. “Droplet fragmentation on impact”, 412–435, Copyright (2011), with permission from Cambridge University Press. Reprinted from Langmuir, 33, Malla, L. K., Patil, N. D., Bhardwaj, R., and Neild, A. “Droplet bouncing and breakup during impact on a microgrooved surface”, 9620–9631, Copyright (2017), with permission of American Chemical Society. Reprinted from Journal of Colloid and Interface Science, 553, Almohammadi, H. and Amirfazli, A. “Droplet impact: viscosity and wettability effects on splashing”, 22–30, Copyright (2019), with permission from Elsevier. Spreading and deposition: silicone oil droplet with kinematic viscosity of 1 cSt and impact velocity of 1.4 m/s; Prompt splashing: glycerol–water droplet with kinematic viscosity of 2 cSt and impact velocity of 2.8 m/s; Corona splashing: silicone oil droplet with kinematic viscosity of 1 cSt and impact velocity of 2.5 m/s; Complete bouncing: time interval between the first and second images is 1 ms, from second image up to sixth image, the time interval is 2 ms, and the time interval between the last three images is 1.33 ms, impact velocity is 0.52 m/s, the Weber number is 6.5, and the water droplet diameter is 1.7 mm; Partial bouncing: time interval between the first and second images is 1 ms, from second image up to sixth image, the time interval is 2 ms, and the time interval between the last three images is 1.33 ms, impact velocity is 0.82 m/s, the Weber number is 16.2, and the water droplet diameter is 1.7 mm; Droplet fragmentation: time interval between the images is 10 ms, and the Weber number is 490.
This review provides the importance of this field of science (i.e., physics of droplet spreading) on the future of personalized healthcare and medicine in a wide range of areas, including bioprinting and advanced biosensors. Next, this review provides a summary of the prominent physical factors related to droplet spreading after impact. Moreover, it presents the current physical models along with their strengths and weaknesses that can be used to explain the physics of droplet spreading in healthcare and medicine. Finally, future research directions in this field are provided, considering the complexities of the droplets and solid surfaces along with recent advancements in nanotechnology, such as atomic force microscopy, cryogenic electron microscopy, and state-of-the-art neural networks.
2. Applications: Healthcare and Medicine
2.1. Three-Dimensional (3D) Bioprinting
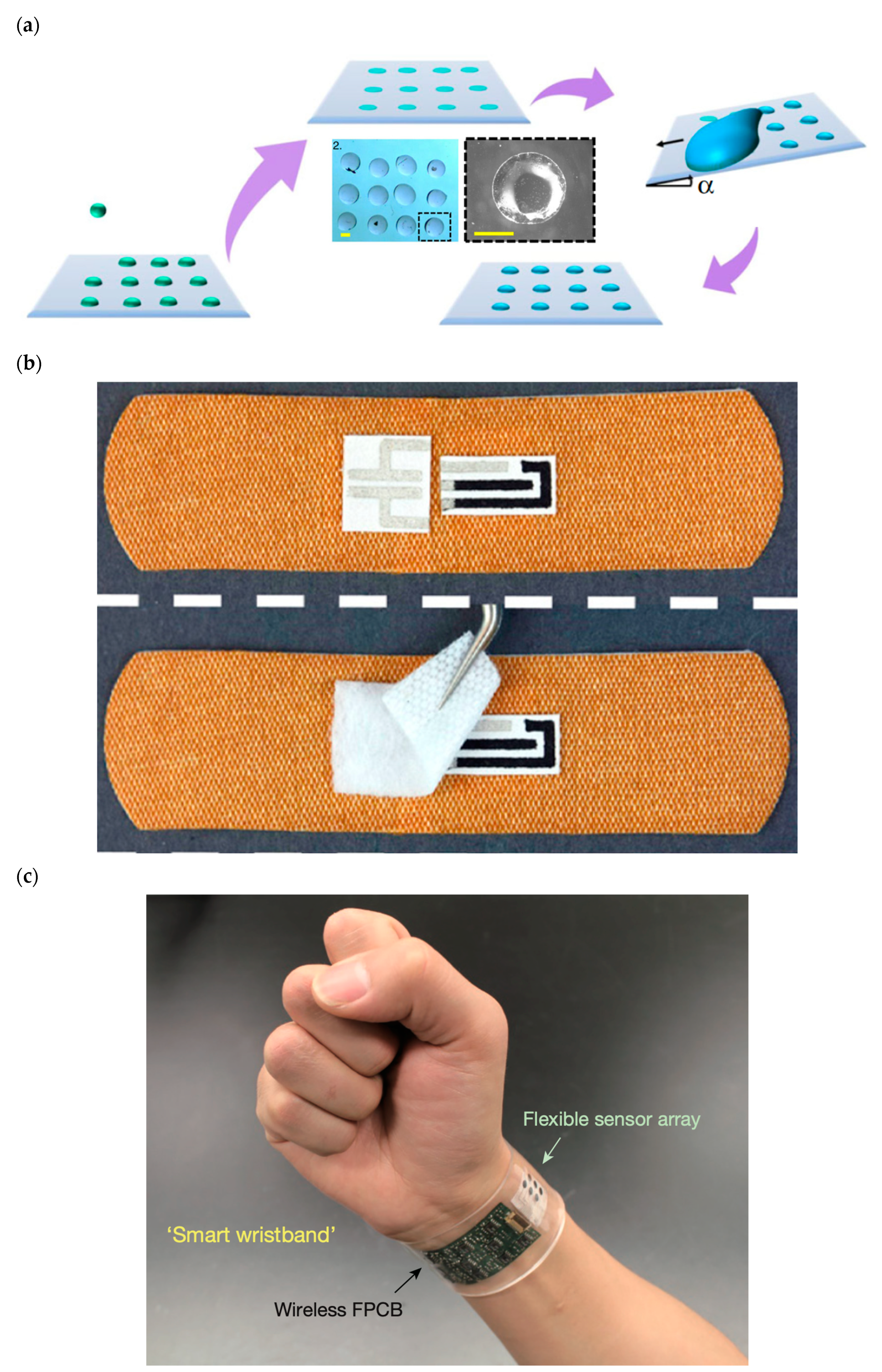
Depositing tiny specialized biological droplets onto biocompatible materials with complex geometry and advanced thermal and mechanical properties to fabricate highly sensitive advanced functional biological 3D structures has been of considerable research interest because of its wide range of applications in personalized healthcare technology and medicine (Figure 2a). Figure 2a depicts the multiple stages of printing an array of biological droplets on a substrate via applying the droplet-impact printing approach. The biological droplets can contain various biological solutions, such as live cells, DNA, virus, and bacteria. This technique is strongly applicable for various biomedical research, including cell culture-based studies. The coated substrate can have various wettability ranging from superhydrophilic to superhydrophobic.
It is important to emphasize that, in bioprinting technology, the size of the biological droplets is commonly in the order of tens of micrometers in diameter. This experimental procedure is known as 3D bioprinting in which the biological droplets are commonly made of active biochemicals, living cells, proteins, peptides, enzymes, hormones, etc. [69,76,78,79,87,88,89,90,94,95,111,112,113,114,158,187,188,189,190,191,192,193,194,197,198,199,200,201,203,204,205,206,207,208,209,210,211,212,213,214,222,223,224,232,233,255,256,257,258,259]. In biological research, room temperature bioprinting of arrays of droplets made of bacteria, DNA, cells, various biopolymers, and proteins for gene expression studies and single-cell bioprinting for fundamental cellular biology are of high interest for the scientific community [204,205,206,225,255,256,257,258,259].
There are various methods of 3D bioprinting: droplet-based, extrusion-based, laser-based, and polymerization [69,70,71,72,73,74,75,76,78,79,204,205,206,207,208,209,210,211,212,213,214,225]. However, the high demand for precision, stretch ability, financial affordability, biocompatibility, reliability, and high-throughput fabrication of printed biological droplets strengthens the 3D droplet-based bioprinting [69,70,71,72,73,74,75,158,200,204,205,206,207,208,209,210,211,212,213,214,225].
Three-dimensional droplet-based bioprinting is an innovative and rapid technique to generate functional biological materials, such as cell laden 3D conformations for various clinical and healthcare applications [69]. The need for the 3D droplet-based bioprinting technique is supported by the numerous emerging medical application fields, including patient-specific artificial organs and tissues, regenerative medicine, high-throughput gene sequencing, pharmaceutical drug testing, 3D dental prostheses with specific architectures, 3D single-cell laden printing on stretchable and elastic materials, and physiological and pathological modeling [69,70,71,72,73,74,75,76,78,79,158,200,204,205,206,207,208,209,210,211,212,213,214,255,256,257,258,259,260,261,262,263,264,265,266,267,268].
It is extremely vital that the printed impacting biological droplet on a biocompatible material is perfectly isolated from other droplets to create a high bioprinting resolution and precision [69,76,78,79,158,200,204,205,206,207,208,209,210,211,212,213,214,255,256,257,258,259,260,261,262,263,264,265,266,267,268]. Therefore, it is of great interest to gain deep insight on the physical mechanism and criteria that fully control the droplet spreading dynamics after impact and avoid any undesired splashing to maximize the bioprinting performance [69,76,78,79,158,200,204,205,206,207,208,209,210,211,212,213,214,225,255,256,257,258,259,260,261,262,263,264,265,266,267,268]. When spreading is not controllable in a process, barrier structures are often placed on the substrate via techniques such as lithography or stamping.
The essential requirement for 3D droplet-based bioprinting is to print biological ink droplets with detailed control to generate an error-free 3D pattern and shape of the biological sample on a biocompatible material. Thus, a detailed understanding of the physics of droplet spreading after impact on the material is crucial as it provides insights regarding 3D droplet-based bioprinting resolution and structural integrity and reliability [69,76,78,79,158,204,205,206,207,208,209,210,211,212,213,214,225,255,256,257,258,259,260,261,262,263,264,265,266,267,268].
2.2. Wearable/Portable/Implantable Biosensors
Wearable and portable biosensors have tremendous roles on numerous areas of personalized and precision medicine (Figure 2b,c) [58,59,69,70,71,72,195,234,235,236,237,238,239,240,241,242,243,244,245]. They can be used for the following: (1) medical diagnostic and monitoring on-chip devices; (2) medical diagnostic, monitoring, and therapeutic implants; (3) remote medical tracking systems; and (4) nanoscale-engineered smart and biocompatible medical materials.
More detailed examples of the personalized biomedical applications are as follows: (i) epidermal biosensors made of electrodes on flexible or stretchable substrates for energy harvesting and storage [315,316,317,318,319]; (ii) wearable integrated, real-time sweat monitoring biosensors that provide electrochemical swart information, such as lactate, uric acid, tyrosine, sodium, potassium, chloride, ammonia, glucose, and pH of the patient for the medical specialist [261,320,321,322,323,324,325,326,327,328]; and (iii) wearable biosensors that are applicable for monitoring tears and saliva of patients for healthcare and/or biomedical research to detect glucose and lactate [329,330,331,332,333,334,335,336,337,338].
The localization, tracking, and monitoring of smart ingestible biocompatible pills equipped with printed microdevices with nanobioelectronic ink droplets in the gastrointestinal tract are extremely precious for the diagnostics and treatment of gastrointestinal tract complications [58,59,69,70,71,72,73,74,75,76,234,235,236,237,238,239,240,241,242,243,244,245,255,256,257,258,259,260,261,262,263,264,265,266,267,268]. Such devices require high resolution and extremely controllable and precise nanoprinting of the active complex droplets in the wireless monitoring microdevice.
Considering the recent space race between countries, significant research started to participate in studies of human health during long-duration deep-space missions. Instantaneous and precise detection of stress and anxiety is crucial in human performance by controlling the mental health of the space passengers. Therefore, the development of wearable biosensors that have the potential of collecting physiological data such as deep-space stress and anxiety from the astronauts is extremely vital [195]. These advanced wearable biosensors are produced by printing biopolymer ink micro- or nanodroplets with electrical properties on flexible and biocompatible copolymer substrates.
One of the very popular areas of medical research is 3D-printed epifluidic electronic skin devices, which operate via the power of neural networks, for real-time health monitoring of each patient’s physiological conditions, such as temperature, pulse, sweat, etc. [69]. These wearable sensors are produced by printing complex nanodroplets that are composed of various chemical components, such as numerous polymers, inorganic compounds, and numerous composites, on stretchable biocompatible substrates. These devices are equipped with a wireless electronic module, which is printed by nanoelectronic droplets, to remotely operate.
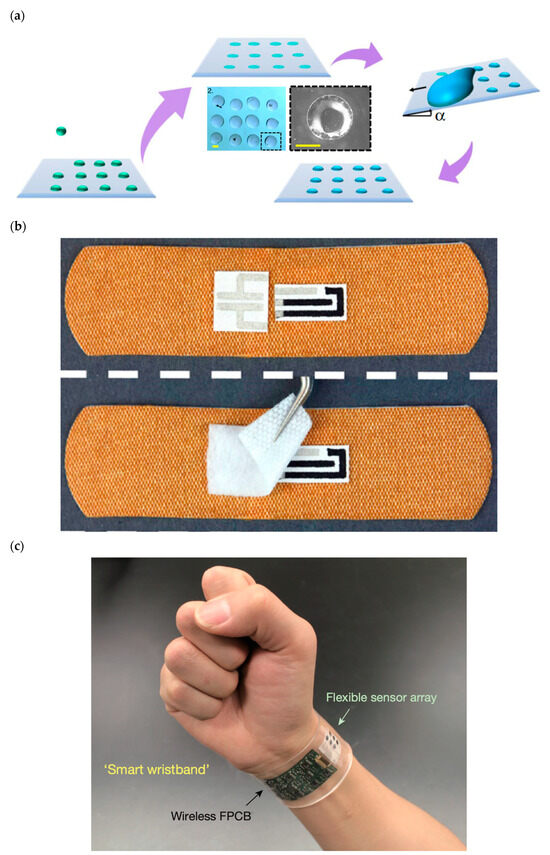
Figure 2.
(a) State-of-the-art bioprinting technique based on the bioinspired droplet impact on various materials [225]. (b) Smart wireless wearable, flexible, integrated biosensing array on an individual’s wrist for monitoring sweat data [319]. (c) Omniphobic paper-based smart bandages that monitor uric acid and pH level in the location of injury of the patient [261]. Reprinted from Nature Communications, 11, Modak, C. D., Kumar, A., Tripathy, A., and Sen, P. “Drop impact printing”, 4327, Copyright (2020), with permission of Springer Nature. Reprinted from Biosensors and Bioelectronics, 117, Pal, A., Goswami, D., Cuellar, H.E., Castro, B., Kuang, S., Martinez, R.V. “Early detection and monitoring of chronic wounds using low-cost, omniphobic paper-based smart bandages”, 696–705, Copyright (2018), with permission of Elsevier. Reprinted from Nature, 529, Gao, W., Emaminejad, S., Nyein, H.Y.Y., Challa, S., Chen, K., Peck, A., Fahad, H.M., Ota, H., Shiraki, H., Kiriya, D., Lien, D.H., Brooks, G.A., Davis, R.W., Javey, A. “ Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis”, 509–514, Copyright (2016), with permission of Springer Nature.
Figure 2.
(a) State-of-the-art bioprinting technique based on the bioinspired droplet impact on various materials [225]. (b) Smart wireless wearable, flexible, integrated biosensing array on an individual’s wrist for monitoring sweat data [319]. (c) Omniphobic paper-based smart bandages that monitor uric acid and pH level in the location of injury of the patient [261]. Reprinted from Nature Communications, 11, Modak, C. D., Kumar, A., Tripathy, A., and Sen, P. “Drop impact printing”, 4327, Copyright (2020), with permission of Springer Nature. Reprinted from Biosensors and Bioelectronics, 117, Pal, A., Goswami, D., Cuellar, H.E., Castro, B., Kuang, S., Martinez, R.V. “Early detection and monitoring of chronic wounds using low-cost, omniphobic paper-based smart bandages”, 696–705, Copyright (2018), with permission of Elsevier. Reprinted from Nature, 529, Gao, W., Emaminejad, S., Nyein, H.Y.Y., Challa, S., Chen, K., Peck, A., Fahad, H.M., Ota, H., Shiraki, H., Kiriya, D., Lien, D.H., Brooks, G.A., Davis, R.W., Javey, A. “ Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis”, 509–514, Copyright (2016), with permission of Springer Nature.
3. Droplet Motion on a Solid Surface
3.1. Contact Line
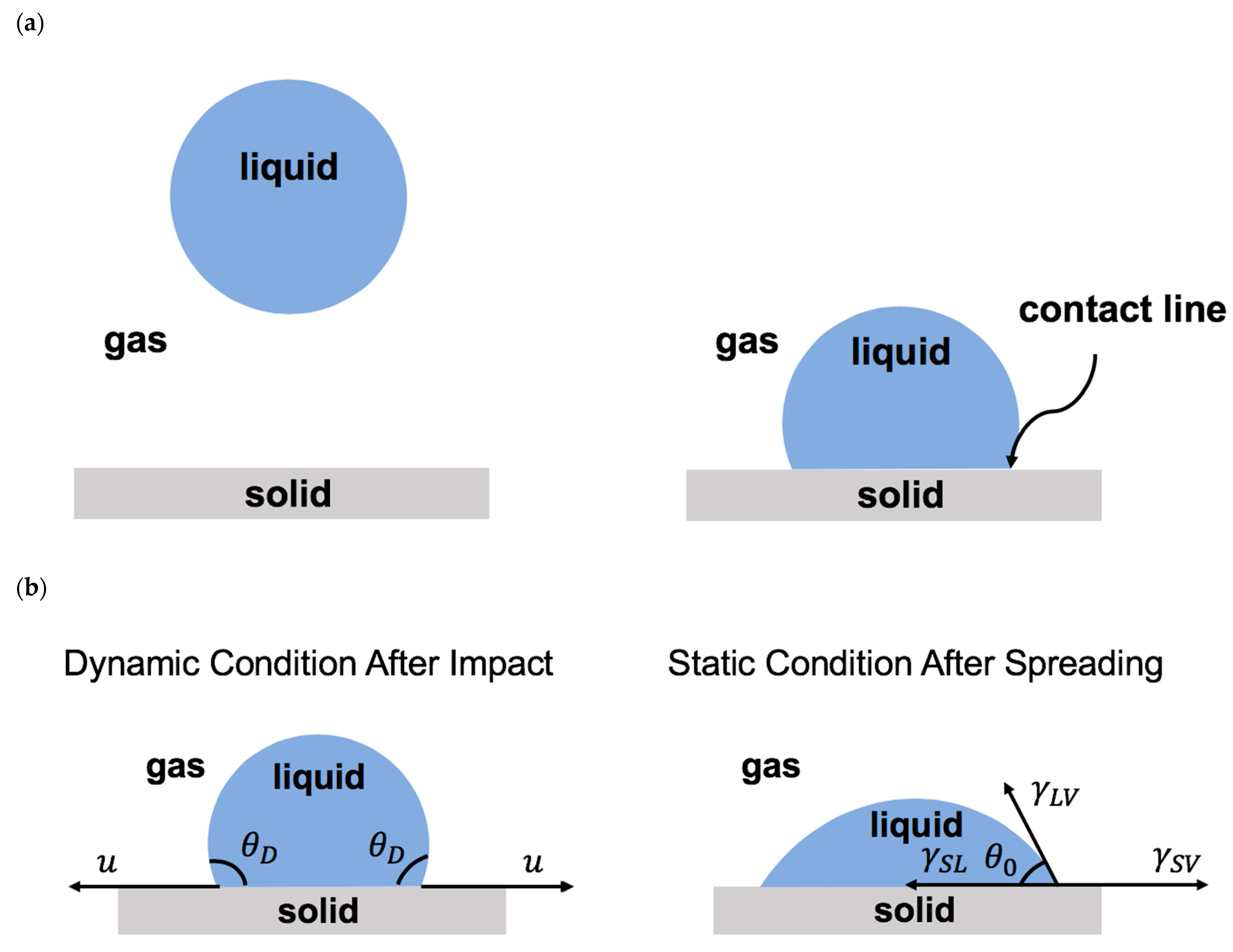
Once a droplet touches a solid surface, the contact line forms where the solid, liquid, and gas phases coexist (Figure 3a). After impact, the contact line moves on the solid surface. The level of contact line advancement on the solid surface is known as wettability. Wettability depends on the relative significance of the adhesive and the cohesive forces along the contact line [2,4,6,9,10,12,14,15,32,33,45,50,199,249,250]. The adhesive force promotes droplet advancement, whereas the cohesive forces resist the contact line motion. The physics of the moving contact line is important in various scientific fields, such as coatings, printings, microfluidics, energy, healthcare, and medicine [2,4,6,9,10,12,14,15,32,33,45,50,199,249,250,255,256,257,258,259,260,261,262,263,264,265,266,267,268].
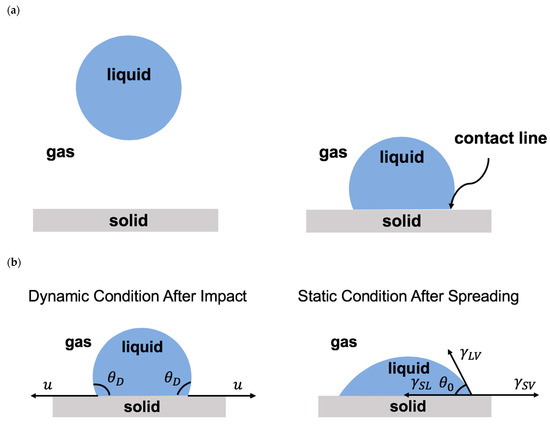
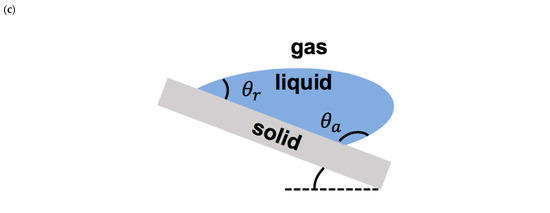
Figure 3.
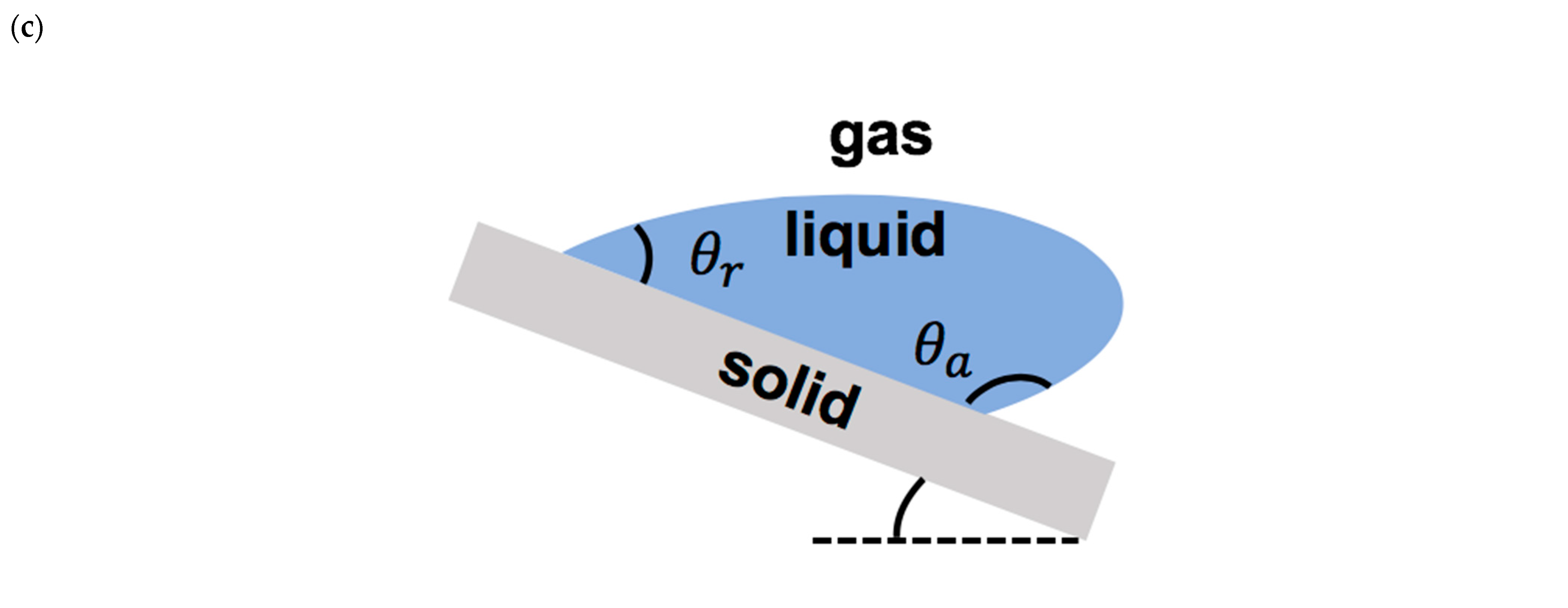
(a) Schematics of droplet falling onto a solid surface and forming a contact line. (b) Schematics of droplet on a solid surface during spreading (after impact) and at the equilibrium (after complete spreading). (c) Schematics of a moving droplet on an inclined surface with advancing and receding regions.
3.2. Contact Angle: Static and Dynamic
The dynamic contact angle () is the angle between the liquid–air interface and the solid–liquid interface formed at the moving contact line [2,4,6,9,10,12,14,15,32,33,45,50]. Once the contact line becomes stationary, the contact angle reaches equilibrium and becomes static. The static contact angle () is determined by Young’s law, which is about the balance of the interfacial forces along the surface, as shown by Equation (1) [63]:
in which denotes the liquid–air surface tension, presents the solid–air interfacial tension, and is the solid–liquid interfacial tension (Figure 3b).
The static contact angle less than 90° represents a hydrophilic surface, the static contact angle between 90° and 150° presents a hydrophobic surface, and the static contact angle larger than 150° denotes a superhydrophobic surface. Depending on the direction of the motion of the moving contact line, such as droplet impact on an inclined surface, there are two types of contact angles: the advancing contact angle and the receding contact angle (Figure 3c). The difference between these two contact angles represents the contact angle hysteresis, which presents the geometric/chemical heterogeneity of the solid surface [35].
3.3. Contact Angle Modes: Wenzel, Cassie–Baxter, and Mixed
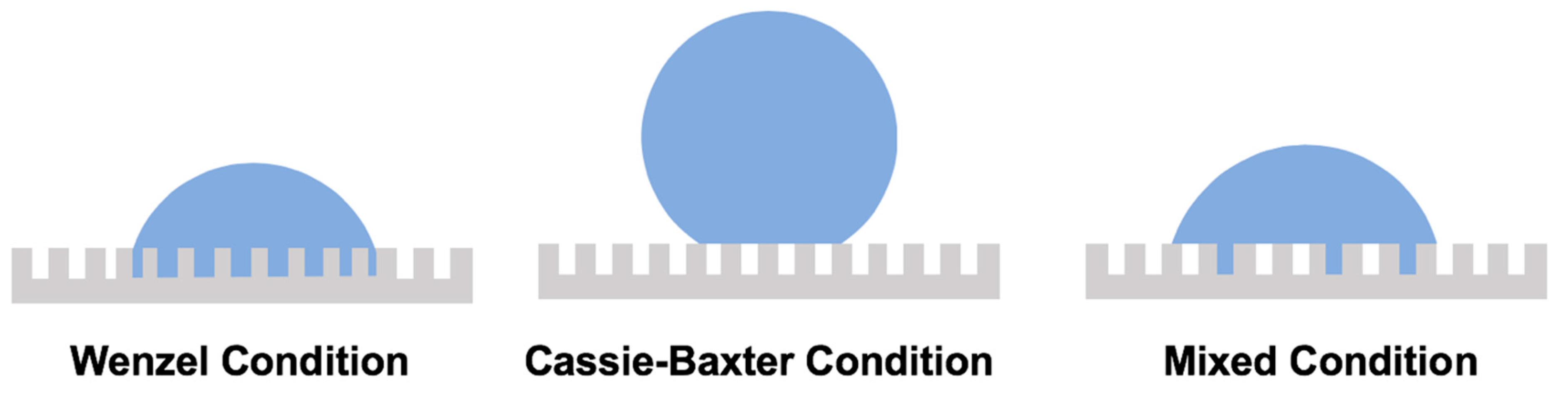
When a droplet impacts on a rough solid surface, one of the following theoretical models are valid: the Wenzel model and Cassie–Baxter model [160,161,162]. The Wenzel model states that the roughness on the solid surface increases the liquid–solid contact area by filling air pockets with liquid (Figure 4). Therefore, the contact angle in the Wenzel mode is determined by Equation (2):

Figure 4.
Schematics of droplet situation according to three physical models: Wenzel model, Cassie–Baxter model, and mixed model (i.e., combination of Wenzel and Cassie–Baxter models).
is the Wenzel static contact angle, and denotes the static contact angle on the horizontal smooth solid surface defined by Young’s equation. presents the surface roughness, which signifies the ratio of the effective solid–liquid contact area to the projected droplet surface area on the rough surface ().
In the Cassie and Baxter mode, air pockets are presented between the droplet and the rough surface [161,162], as described by Equation (3) (Figure 4):
denotes the Cassie–Baxter static contact angle, and is the fraction of the solid surface in contact with the droplet. The Cassie–Baxter model is suitable for the droplet impact on a solid surface with chemical and/or geometric heterogeneity [68,69,72,133,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196].
The existence of an alternative mixed mode has also been proposed in which part of the impacting droplet is in direct contact with the rough surface and its rest is held totally or partially on top of the air pockets (Figure 4) [197,198,199]. The mixed contact angle is defined by Equation (4):
in which represents the percentage of the droplet-surface contact area.
3.4. Contact Line Dynamics
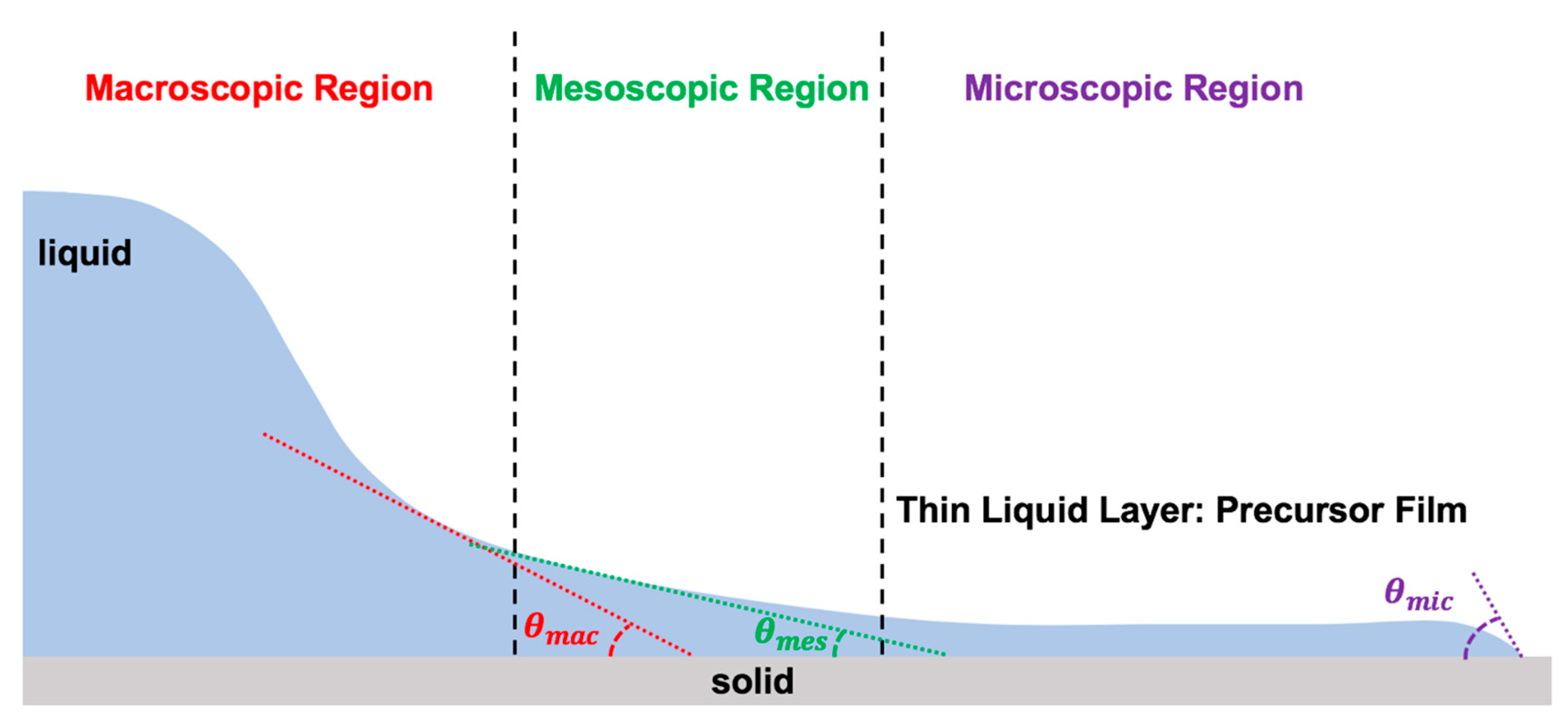
The dynamics of the moving contact line are defined by the complex relation between the dynamic contact angle (), the moving contact line velocity (), the equilibrium receding contact angle (), the equilibrium advancing contact angle (), and several other physical parameters (): [3,7,8,23,33,39]. To explain the physics of the moving contact line, one must divide the vicinity of the contact line into three regions: macroscopic (outer region), mesoscopic (intermediate region), and microscopic (inner region), as illustrated in Figure 5 [2,3,4,6,7,8,9,10,12,14,15,23,32,33,39,45,50,113,114,115,117,118,119,199,249,250,255,256,257,258,259,260,261,262,263,264,265,266,267,268].
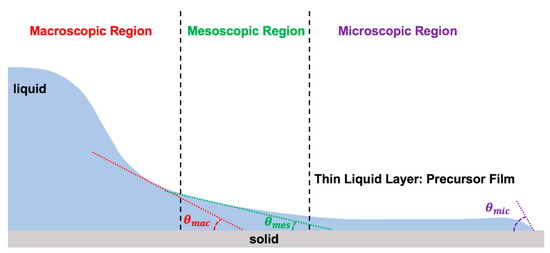
Figure 5.
Three regions of the moving contact line with zoom in condition.
3.5. Physical Forces and Non-Dimensional Parameters
The physics of droplet impact on a solid surface is governed by three factors: the physical properties of the droplet and gaseous environment as well as the wettability of the solid surface. These factors can be defined by four physical forces: inertia, viscous, interfacial, and gravity [17,55,56,57,120,144,157,201,208,218,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271]. Viscous force resists the droplet motion on the substrate through the viscous dissipation process. The interfacial tension denotes the droplet elasticity. The importance of gravity depends on the ratio of the droplet size and the capillary length () in which denotes droplet density, presents the surface tension, and is the gravitational acceleration. Depending on the physical system, some external forces may be important to consider, such as the electric and magnetic fields and acoustic waves.
These physical forces can identify the physics of the droplet contact line after impact on a solid surface via the appropriate dimensionless parameters. These dimensionless parameters are the Reynolds number (), the Weber number (), the Ohnesorge number (), the capillary number (), the Stokes number (), the Bond number (), the Froude number (), the Weissenberg number (), the Deborah number (), and the elasto-capillary number () [17,68,69,253,254,272,273,274,275,276,277,278,279]. If any external force presents in the problem, additional dimensionless numbers can be introduced in the physics of the droplet contact line [8,11,17,33,54,68,69,104,105,253,254,272,273,274,275,276,277,278,279].
The Reynolds number is defined as the ratio of the inertia over the viscous force: . The Weber number describes the relative significance of inertia over the capillary force: . The Ohnesorge number shows the balance between three physical forces (inertia, viscous force, and capillary force): . The capillary number signifies the relative importance of viscous force over capillary force: . The Stokes number is defined as the relative importance of gas viscosity () over the inertia: . The Bond number determines the relative effect of gravity over the capillary force: . The Froude number defines the ratio of the inertia over gravity: . The Weissenberg number highlights the relative importance of elasticity over viscosity: , where presents the shear rate of strain and denotes the relaxation time. The Deborah number defines the ratio of the relaxation time over characteristic time scale of the physical problem (): . The elasto-capillary number defines the relative significance of . In these equations, which define the dimensionless numbers, is the droplet viscosity, denotes the droplet density, presents the droplet diameter, and is the droplet contact line velocity. is the droplet surface tension.
The non-dimensional parameters are commonly used to categorize physical phenomena. The capillary length is used to distinguish between small and large droplets by providing physical meaning to small and large size in the droplet impact dynamics. The Reynolds number and Weber number are applied to verify the possibility of a splashing event in the droplet impact mechanism. The Bond number provides an insight to whether gravity is important in the physical situation. The Weissenberg number, Deborah number, and elasto-capillary number signify the viscoelasticity effect in the droplet impact physics.
3.6. Maximum Spreading Parameter
The maximum area over which the impacting droplet covers on a solid surface is denoted by a non-dimensional parameter known as the maximum spreading parameter (). The maximum spreading is defined as the maximum area that can be covered by the impacting droplet when the droplet reaches equilibrium on the solid surface and does not retract or leave the surface afterwards. The maximum spreading parameter has commonly been used to characterize the dynamics of the spreading of the impacting droplet [55,56,57,120,144,218,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269]. The maximum spreading parameter is defined as the ratio of the droplet size at the end of spreading, , over the initial droplet size, .
Previous studies have attempted to formulate the maximum spreading parameter via other non-dimensional numbers. Laan et al. (2014) provided a list of the previous studies in which they attempted to identify the maximum spreading parameter [75]. All these formulas were obtained based on theoretical analyses or experimental observations and by applying the three conservation laws: mass, momentum, and energy. They all concluded that the maximum spreading parameter depends on three non-dimensional parameters (i.e., Reynolds number, Weber number, and Ohnesorge number) as well as the Young static and dynamic contact angles. Furthermore, they found that these dependencies are in the power law: . It is important to note that, in all these studies, the droplet is assumed to not splash after impact on the solid surface. It is worth emphasizing that the calculated values of the maximum spreading parameter from all previous studies might differ from what is observed in the real physical situation. The formulas, which were obtained from all previous works, are only valid within certain limits and cannot be applied for complex droplets on real substrates.
It is important to mention that these findings only provide approximations for the maximum spreading parameter, since the level of accuracy is limited due to the limitation in observations caused by constraints in speed and resolution of imaging. Nevertheless, in healthcare and medicine, these formulas will not work as it is vital to have a high precision and accuracy to control the degree of spreading. Moreover, these findings are all applied for droplet impact on a rigid solid surface. In real applications, including healthcare and medicine, the solid is mostly flexible and heterogenous in terms of chemistry and geometry. Therefore, all these formulas need to be revisited by consideration of more practical surfaces and ultra-fast imaging techniques with very high resolutions in subangstrom, such as cryogenic electron microscopy. When high precision is required, one needs to add geometrical features on the substrate to control spreading. These features can be added by means of several techniques, including lithography or stamping [73].
4. Physical Models of Contact Line Dynamics
The physics of the moving contact line in various situations has been studied through a large number of theoretical, experimental, and numerical efforts [2,3,4,6,7,8,9,10,12,14,15,23,32,33,39,45,50,72,113,114,115,117,118,119,133,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,199,249,250,255,256,257,258,259,260,261,262,263,264,265,266,267,268]. The contact line dynamics involve the fluid dynamics and the interfacial physics [3,7,8,23,33,39]. The physics of the moving contact line has been commonly explained through the dependency of the dynamic contact angle to the contact line velocity along with the advancing and receding static contact angles [68,69,72,133,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196]. There are six main physical models that conventionally explain the physics of the moving contact line: molecular kinetic (MK), hydrodynamics (HD), combined (MK–HD), precursor film, Shikhmurzaev, and Cahn–Hilliard–van der Waals.
4.1. Molecular Kinetic Model
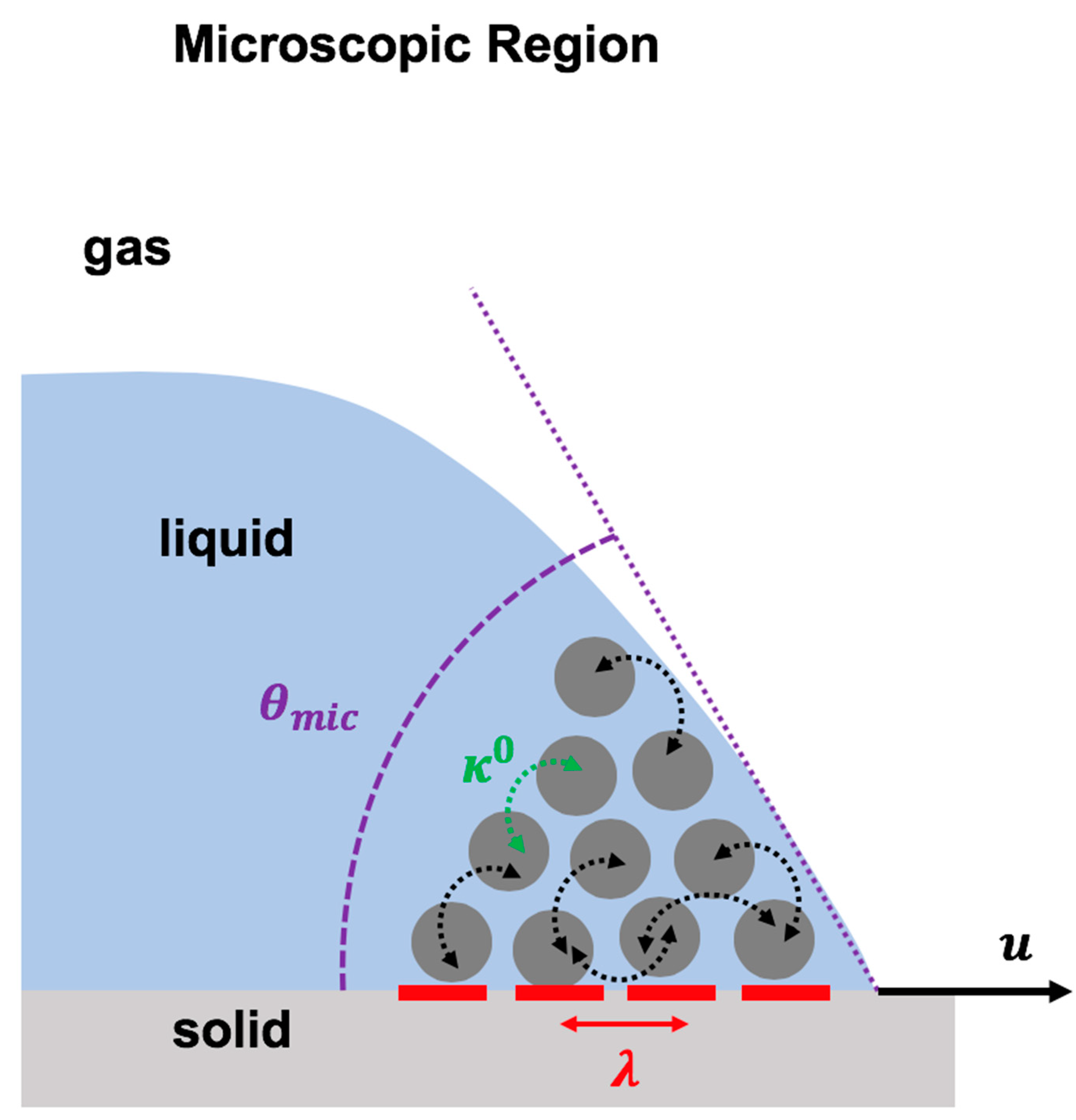
Molecular kinetic theory explains the molecular dynamics at the vicinity of the dynamic contact in the microscopic region [3,114,115]. In molecular kinetic theory, it is postulated that liquid molecules in the microscopic region jump on the adsorption sites situated on the solid surface at the vicinity of the contact line (Figure 6). These molecular activities are controlled by the power of the energy barriers, which resist the contact line motion. In the molecular kinetic model, the physics of the moving contact line is formulated by the dependency of the contact line velocity to two molecular parameters, the frequency of the molecular dynamics () and the distance between the adsorption sites () at the contact line, as shown in Equation (5) [3,114,115]:
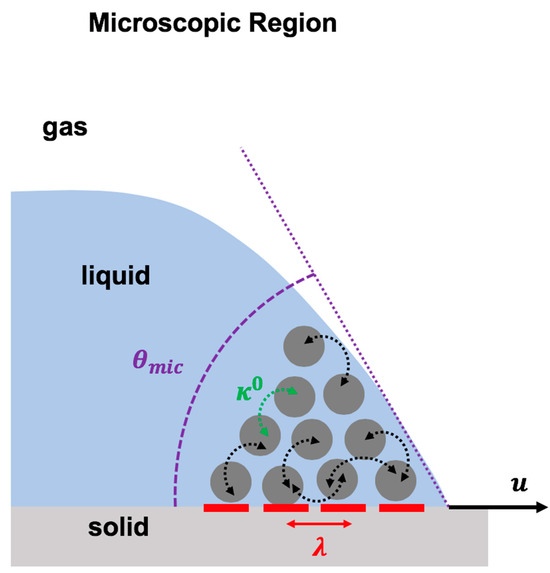
Figure 6.
Schematic representation of the molecular kinetic theory to describe the physics of the moving contact line based on two molecular dynamic parameters: the frequency of molecular vibration in the microscopic region () and the average distance between molecular adsorption sites on the solid surface ().
In Equation (5), denotes the Boltzmann constant, and represents the environment temperature with the unit in Kelvin.
As shown in Figure 6, presents the average distance between two adsorption sites that are located next to each other. These adsorption sites are the sites on the solid surface at which the liquid molecules attach or detach. highlights the mean frequency of the molecular activity (molecular attachment/detachment on these adsorption sites) at the equilibrium condition.
4.2. Hydrodynamics Model
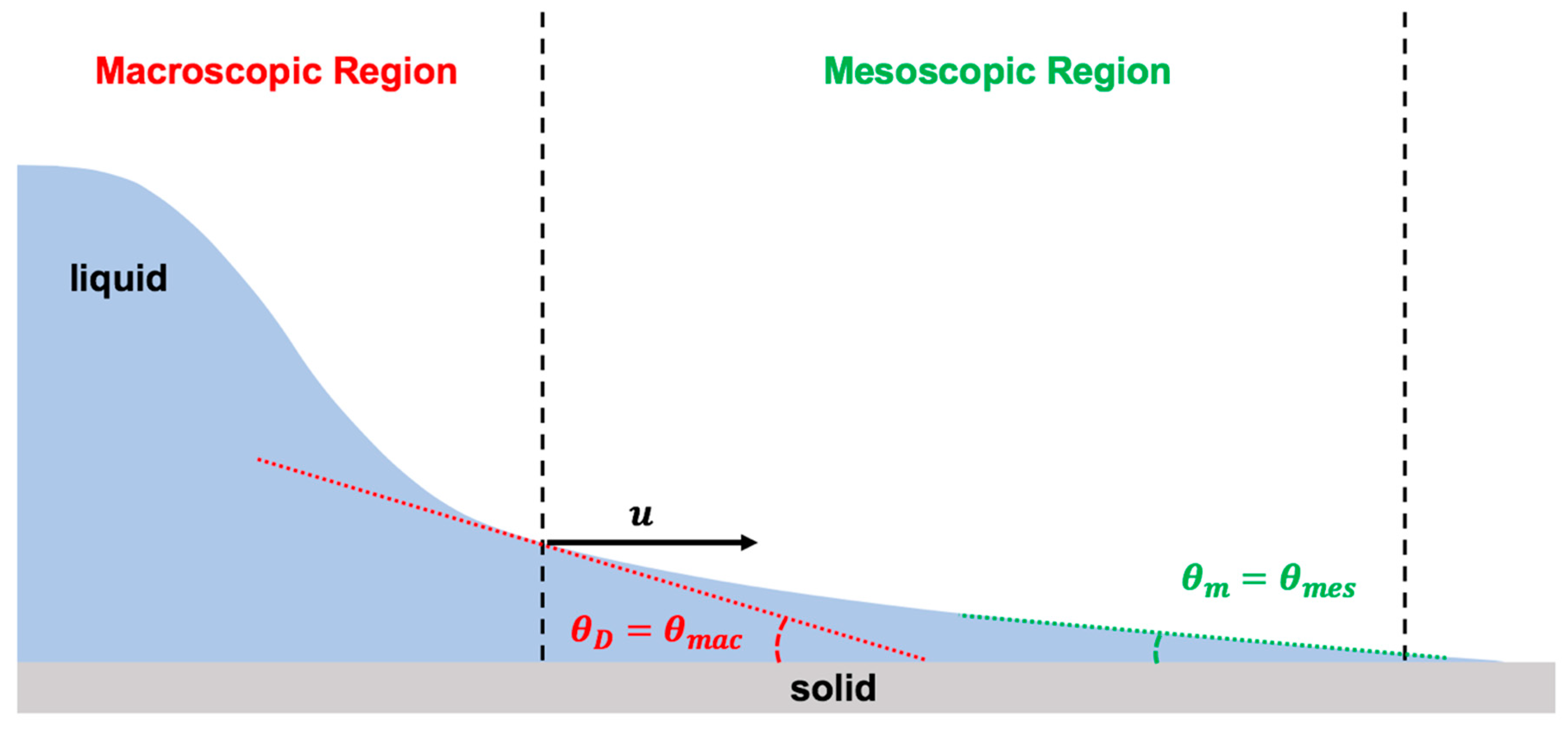
The hydrodynamics model explains the fluid dynamics near the moving contact line in the macroscopic region using Navier–Stokes equations for two-dimensional steady state flow based on lubrication assumption. Moreover, the hydrodynamics model applies the matched asymptotic expansions approach on the viscous bending of the liquid-free interface in the mesoscopic region of the flow (Figure 7). To resolve the issue of the violating boundary condition along the solid–liquid interface, at the moving contact line, it is assumed that the conventional no-slip boundary condition at the solid–liquid interface in the microscopic region must be relaxed over a distance known as the slip length () [7,8,23,39,113,117,118,119].
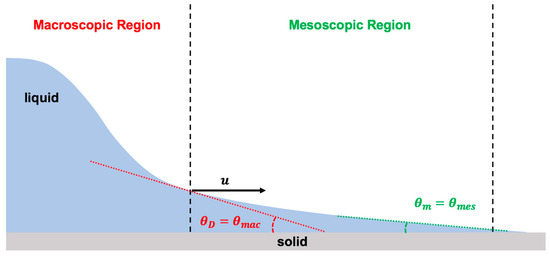
Figure 7.
Schematic illustration of the physics of the moving contact line using hydrodynamics theory, which is based on bending of the liquid-free interface due to viscous effect at the vicinity of the moving contact line. Hydrodynamics theory considers two regions of the contact line: macroscopic (viscous bending) and mesoscopic (assumes Young static contact angle).
The conventional stress singularity at the moving contact line is relaxed within a distance called the slip length (), which is in the order of a few angstroms [4]. The slip length is related to the contact line speed and the liquid physical properties [4]. Accordingly, the slip length is defined by the capillary length () and the capillary number (): [4]. The capillary length shows the relative significance of the surface tension over gravity: .
The hydrodynamics model depends on droplet size. For a large droplet (radius is larger than the capillary length), indeed there are two regions of interest in the contact line dynamics. However, for a small droplet, it is all about the mesoscopic region. Therefore, the hydrodynamics model determines the relation between the macroscopic dynamic contact angle () to the capillary number () and the mesoscopic static contact angle () in the inner region based on the viscous bending of the liquid-free interface in the mesoscopic region (Equations (6) and (7)) [7,8,23,39,113,117,118,119]:
where denotes the macroscopic characteristic length, presents the microscopic characteristic length (slip length), and the capillary number is the ratio of the viscous force over interfacial force: in which is the viscosity and is the surface tension.
4.3. Combined Model: Molecular Kinetic Model + Hydrodynamics Model
The combined model involves molecular kinetic theory and hydrodynamics theory to replace the constant mesoscopic static angle with the non-hydrodynamic variable static contact angle in the hydrodynamics model [3,114,181,293] as formulated in Equation (8):
The combined model claims that, by assigning a proper value for the three experimentally fitting parameters (, , ), the physics of the contact line can be fairly estimated by the combination of the hydrodynamics and molecular kinetic theories [3,114,181].
The accuracy of the combined model strongly depends on the level of precision in the fitting analyses for determining the molecular kinetic and hydrodynamics parameters. There is a high possibility of overfitting, which leads to a significant weakness in its reliability. As a result, this model might not be a suitable model for applications that require strong precision and accuracy, such as healthcare, medicine, and biosensors.
4.4. Precursor Film Model
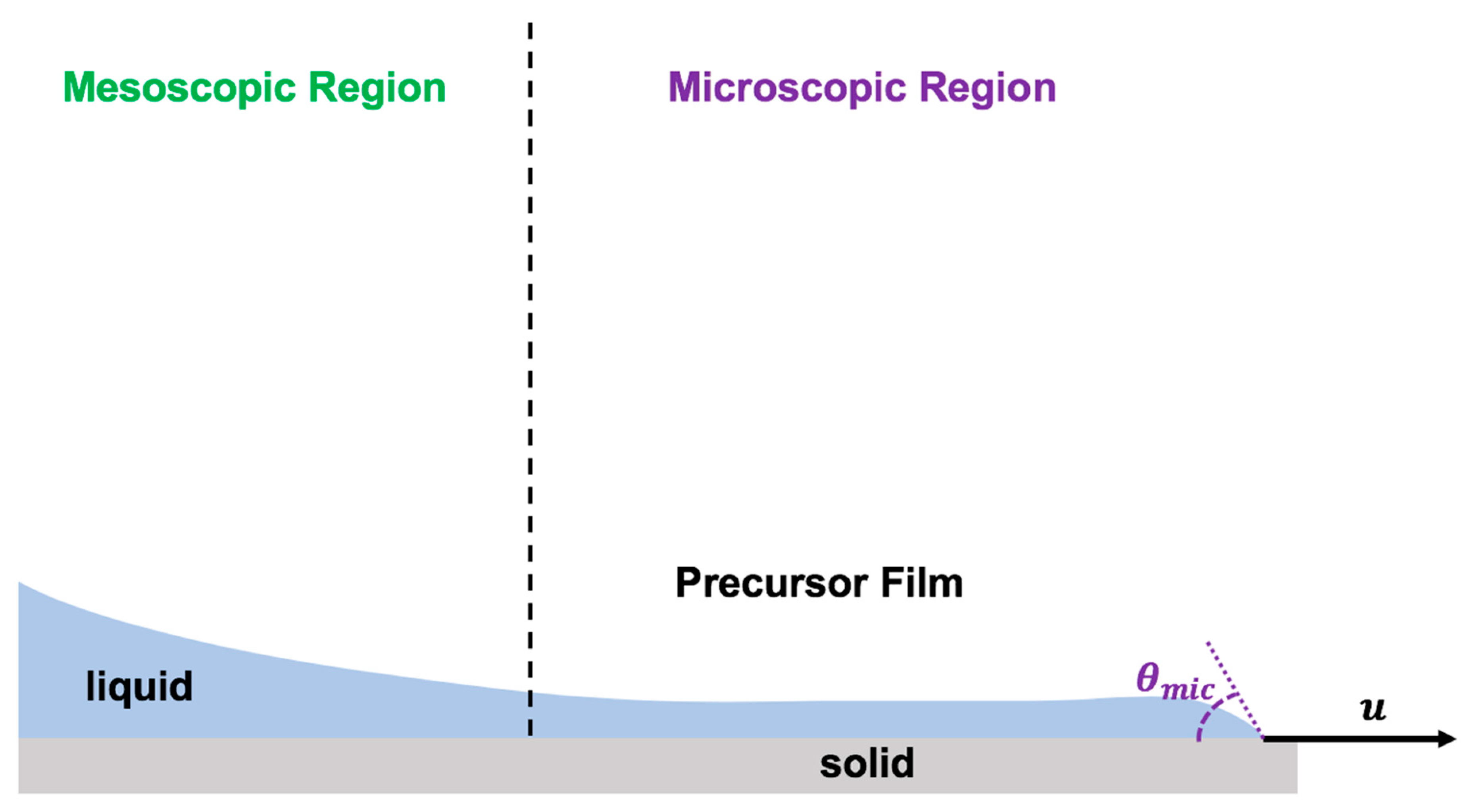
The physics of the dynamic contact line has been formulated by assuming the presence of a very thin layer of liquid well ahead of the contact line moving much faster than the apparent moving contact line [50,106,155] (Figure 8). The moving contact line dynamics were derived based on the precursor film by applying the absolute speed of molecular reactions based on molecular kinetic theory [60,106].
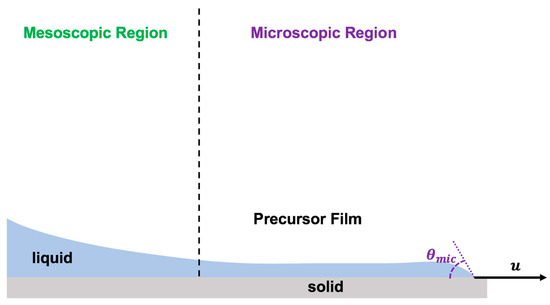
Figure 8.
Schematics of the precursor film model to describe the physics of the moving contact line in the forefront of the advancing thin layer of droplet spreading.
In this model, the speed of the moving contact line was claimed to be driven by the sum of works done by van der Waals forces (), polar molecular forces (), geometric forces (), and any other type of forces, such as electric and/or magnetic forces (). Therefore, the moving contact line velocity was formulated by Equation (9):
It is important to note that the precursor film model requires very advanced computational tools with the help of supercomputers and state-of-the-art quantum computations to explain the physics of the moving contact line.
4.5. Shikhmurzaev Model
Shikhmurzaev considered the changes in interfacial tension forces in the microscopic region as the contact line moves on the solid surface and modeled the physics of the moving contact line by applying the Navier–Stokes equations and the generalized Navier boundary conditions [269,270,271]. In the Shikhmurzaev model, the dynamic contact angle is postulated to be deviated from the classical Young static contact angle due to changes in the interfacial tensions from their equilibrium values at the static condition in the microscopic region [269,270,271]. The current Shikhmurzaev model is only applicable for smooth, chemically homogeneous solid surfaces.
4.6. Cahn–Hilliard–van der Waals Model
Cahn–Hilliard theory and van der Waals theory together explain the physics of the moving contact line via determining the dependency of the mesoscopic dynamic contact angle to the speed of the moving contact line [81,82,83,84,85,113]. In this model, the liquid–gas interface is an ultrathin layer (i.e., order of a few nanometer thickness) through which liquid properties vary significantly. They formulated their model by assuming the diffusion of the molecules across the interface. This model could resolve the long-standing challenging stress singularity boundary condition at the moving contact line, which was presented in the hydrodynamics model. This model can only be applied explicitly by powerful computational tools.
This model is suitable for liquids with multiple phases. Therefore, it has a promising potential application in healthcare and medicine in which the liquid droplets are composed of heterogeneous constituents, such as various proteins, hormones, enzymes, antibodies, etc. However, for its application on flexible biocompatible–biodegradable solid surfaces, it needs very serious modifications, as this model does not take into account the influence of the outer and inner regions on the dynamics of the moving contact line. The integral mechanical energy balance formula, derived from the generalized transport theorem as proposed by Slattery et al. 2007, could be the starting point to enhance the applicability of the Cahn–Hilliard–van der Waals model for biological droplet impact on biomaterials [74].
5. Future Directions
The printing of micro- and nanobiological droplets on wearable and biocompatible materials has been the center of attention in numerous modern technologies, such as smart biomaterials and advanced biosensors [200,225,226,227,228,229,230,231,232]. Droplets can contain biological samples and a suspension of micro- and nanosolid particles. In the bioprinting technique, it is extremely important for precise and controllable dispersion of micro- and nanodroplets on a solid surface. The state-of-the-art bioprinting approach can be applied for printing of numerous types of active complex biological droplets with desired sizes and components on biocompatible–wearable materials and elastic surfaces [200,225,226,227,228,229].
5.1. Future of Personalized Healthcare and Medicine by Wearable Biosensors
Promptly advancing research in personalized healthcare and medicine has shed light on the transformation of conventional healthcare and medicine. Wearable biosensors, which can monitor and control the physiological conditions of an individual and possibly treat lift-threatening diseases, present an extremely popular research topic for modern society.
Highly error-free biosensors with flexible and elastic biocompatible and biodegradable materials in healthcare demand extremely accurate formation of droplets that are composed of active biological macromolecules, such as proteins, enzymes, living cells, hormones, bacteria, viruses, and antibodies, onto the complex materials. Therefore, the previous findings for the maximum spreading parameter and the conventional theoretical–experimental–numerical approximations for simple liquids and solids are not appropriate for such highly demanding applications in healthcare and medicine.
It is important to expand the research in this field by utilizing multidisciplinary techniques covering various scientific disciplines, including biology, biochemistry, biophysics, interfacial physics, and electromagnetic physics, along with the use of state-of-the-art techniques, including deep neural networks, cryogenic electron microscopy, and quantum computing tools to harness the application of all aspects of the moving contact line physics in all three regions of interest (macroscopic, mesoscopic, microscopic) as all regions equivalently contribute to the droplet motion for such complex systems.
Furthermore, it is extremely vital to simultaneously apply all physical models: molecular kinetic, hydrodynamics, precursor film, Shikhmurzaev, and Chan–Hilliard–van der Waals. Molecular kinetic describes activity in the microscopic region, hydrodynamics explains the macroscopic region with a connection with the mesoscopic zone, precursor film theory explicitly describes the molecular structure of the advanced microscopic region well ahead of the apparent contact line, Shikhmurzaev focuses on the changes in interfacial tensions due to activity of the molecules in the microscopic–mesoscopic zone, and Chan–Hilliard–van der Waals specifies the inter- and intra-molecular interactions via interface diffusion in the mesoscopic region. All three regions simultaneously interact with each other and subsequently influence the moving contact line for complex active biological droplets on an elaborate biocompatible–biodegradable solid surface.
Moreover, this highly demanding application becomes more complex when considering the application for personalized medicine. In personalized medicine, it is vital to consider all various physiological conditions of each specific individual. Therefore, the current physical models of the droplet moving contact line need to be revisited and modified to be applicable for complex physical situations in healthcare and medicine. Almost all these contact line dynamics models are explicitly suitable for simple conditions.
5.2. Nanodroplet Impact
Nanobioprinting is an innovative method for advancements in healthcare and medicine. In nanobioprinting, droplets with nanometer diameters are deposited on biocompatible materials. Consequently, it requires a considerably high level of precision and accuracy. This can be accomplished by controlling the contact line motion with high resolution on the surface. In nanobioprinting, it is vital to look deeply in the microscopic region and the influence of the mesoscopic region on the microscopic space. Therefore, connecting the multiple models of contact line dynamics is very crucial. Achievement of this goal needs ultra-fast imaging with extremely high resolution in sub-angstrom, such as the cryogenic electron microscope and the atomic force microscope. For validation of the experimental results, one needs to utilize high-performance computational tools and state-of-the-art neural networks and artificial intelligence on the large pool of data extracted from the experiments.
5.3. Physical Models of the Moving Contact Line: Limitations
Previous works that applied the energy equation reported approximate relations between the maximum spreading parameter and other non-dimensional parameters by only focusing on the mechanical energy. This is not a correct approach from energy conservation as the other forms of energy, including internal energy due to chemical/physical interactions between molecules, the thermal energy, as well as the entropy of the system, must be taken into account.
All current contact line models only look at the macroscopic region and/or mesoscopic region. This is not sufficient for applications in healthcare and medicine as they require high precision and accuracy in controlling the biological droplet contact line motion on an elastic stretchable biocompatible solid surface. Especially for nanobioprinting, tissue engineering, and artificial organs, it is extremely vital to look at the biological droplet contact line in nanoscale within the wide area of the microscopic region.
The current form of the molecular kinetic model is suitable to qualitatively describe the contact line dynamics. This limitation can be resolved by applying the power of neural networks and machine learning on the large pool of experimental observations obtained from the case of biological droplet impact dynamics to generate a quantitatively analytical formulation for the molecular kinetics parameters ( and ).
The conventional hydrodynamics model is generally limited to the case of Newtonian solutions. The biological solutions are non-Newtonian. The hydrodynamics model needs to be revisited and modified to incorporate the role of non-Newtonian rheological characteristics of the droplet in the model. This can be achieved by conducting experiments on impacting of biological droplets with various components, such as proteins, hormones, live cells, antibodies, live bacteria and viruses, etc., and taking advantage of the observations and state-of-the-art machine learning to revise the current model of hydrodynamics.
The credibility of the presence of an advancing thin layer ahead of the apparent moving contact line must be widely studied. The concept of precursor film is very critical for the case of bioprinting as it requires high precision and accuracy in nanoscale. The investigation in this area can be conducted by applying state-of-the-art nanotechnology and high-resolution imaging techniques, including atomic force microscopy and cryogenic electron microscopy.
Furthermore, almost all previous works only considered Newtonian or homogeneous droplets. In healthcare and medicine, droplets are non-Newtonian with active chemical/physical/biological interactions between biomacromolecules, such as living cells, bacteria, peptides, proteins, enzymes, and hormones, with very long complex molecular or self-assembled structures whose chemical and biophysical interactions with each other and the solid surface significantly influence the droplet contact line motion. These factors must be monitored by ultra-fast imaging techniques such as AFM with sub-angstrom resolution to resolve the chemical binding between biomacromolecules.
5.4. Evaporative Droplets
The contact line motion of evaporative biological droplets on biocompatible materials has significant interest in advanced biosensors for personalized healthcare. The coffee ring formation during the drying stage of the printed biological droplets could tremendously influence the reliability, precision, and accuracy of the bioprinting performance. The power of control on the dynamics of the moving contact line on such surfaces is extremely vital. Therefore, the volatility of the biological droplets must be taken into account and controlled during spreading. This can only be achieved by considering multiple physical models of the contact line that consider the influence of hydrodynamics in the macroscopic level on the drying events within the microscopic region. As a result, this requires the application of molecular kinetic, hydrodynamics, Chan–Hilliard–van der Waals, Shikhmurzaev, and, especially, precursor film. The inner region of the contact line, including the thin layer ahead of the apparent moving contact line, should be monitored using atomic force microscopy.
5.5. Heterogenous Biological Droplets
Biological droplets used in bioprinting and biosensors are commonly composed of numerous suspended biomacromolecules of different sizes, shapes, and chemical and physical properties. To provide high reliability, precision, and control of bioprinting techniques and biosensors, it is important to consider these factors during spreading of the impacting biological droplets. This challenge needs to be addressed by applying state-of-the-art technologies such as atomic force microscopy to monitor the contact line motion of such complex droplets and model the physics of such events by applying molecular kinetic, modified hydrodynamics, precursor film, Cahn–Hilliard–van der Waals, and Shikhmurzaev theories.
5.6. Future Perspective: Multi-Disciplinary Research
The innovative 3D bioprinting has the astonishing advantage to produce a wide range of functional biomaterials. This fascinating bioprinting technique can be utilized for numerous biomedical purposes, such as gene expression analysis, state-of-the-art stem cell research, artificial living organs, tissue engineering, advanced biosensors, and production of printed advanced biomaterials, which consist of living cells, DNA, bacteria, viruses, and proteins [200,225,226,227,228,229].
Considering the serious necessity for rapidly advancing bioprinting and biosensor technology, future research is expected to focus on applying the emerging technologies in electron microscopy, such as cryogenic electron microscopy, which is suitable for active biological droplets, high-resolution atomic force microscopy, and a physics-informed data-driven approach for the science of droplet contact line dynamics after impact on materials (i.e., biocompatible, biodegradable, wearable) (Figure 9). It is important to apply all five current physical models of the moving contact line, as all three regions of the dynamic contact line need to be investigated for healthcare applications.
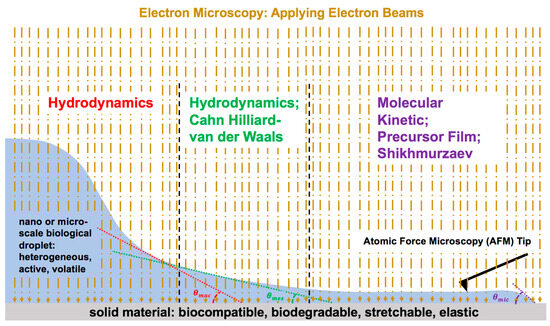
Figure 9.
Schematic representation of future research direction: multi-disciplinary research combining nanotechnology, biology, materials science, and interfacial physics along with the combination of all physical models of the moving contact line in modified version.
Cell culture technique is a very common technique in biomedical research. In cell culture, the deposition of a precise amount of a cell-media droplet, such as human mitral valve interstitial cells diluted in full serum media on a collagen-coated plate for biomechanics studies of mitral valve diseases, is extremely critical for complete prevention of evaporation of the media on the cell culture plate and perfectly regulates the number of cells on the collagen-coated plate for gene analysis. Therefore, it is strongly vital to consider all theoretical models, including molecular kinetic theory, hydrodynamics theory, and precursor film, to evaluate the exact amount of live cell deposition on the collagen-coated plate via considering the evaporation of the precursor film of the cell-media solution droplet. These considerations have not yet been attempted.
This review article aims to highlight the drawbacks and limitations of the capabilities of the current physical models of droplet contact line dynamics upon impact in regard to its applicability and importance in biomedical research. The goal of this review article is to provide future road maps for research scientists in complex biological droplet impact upon a flexible plate and its contact line dynamics in various subjects of biomedicine and biomedical research, which have not been studied. The achievement of this goal requires revisiting the current physical models of the droplet liquid contact line upon impact on various solid surfaces. As of now, most works were experimental. The experimental data provide insights on the reality of each specific biophysical problem in the topic of the intersection of droplet impact dynamics and biomedicine. However, the experimental attempts do not provide a general thorough understanding of the various cases of biological droplet impact dynamics even for similar cases in biomedicine. Therefore, this review article emphasizes urgent advancements of the current versions of the physical models of contact line dynamics of the biological droplets upon impact on solid plates by considering major revisions on all five physical models of the droplet contact line and combining them together to create a global physical model for droplet contact line dynamics to be applicable on the complex biofluid droplets in various topics of biomedical research.
Funding
This research received no external funding.
Data Availability Statement
There are no new data in this review article.
Conflicts of Interest
The author declares no conflict of interest.
References
- Bird, J.C.; Dhiman, R.; Kwon, H.M.; Varanasi, K.K. Reducing the contact time of a bouncing drop. Nature 2013, 503, 385. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Weinan, E. Contact Line Dynamics on Heterogeneous Surfaces. Phys. Fluids 2011, 23, 072103. [Google Scholar] [CrossRef]
- Blake, T.D. The Physics of Moving Wetting Lines. J. Colloid Interface Sci. 2006, 299, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Eggers, J.; Stone, H.A. Characteristic Lengths at Moving Contact Lines for a Perfectly Wetting Fluid: The Influence of Speed on The Dynamic Contact Angle. J. Fluid Mech. 2004, 505, 309–321. [Google Scholar] [CrossRef]
- Yarin, A.L. Drop Impact Dynamics: Splashing, Spreading, Receding, Bouncing. Annu. Rev. Fluid Mech. 2006, 38, 159–192. [Google Scholar] [CrossRef]
- Diaz, M.E.; Cerro, R.L. A General Solution of Dewetting Flow with a Moving Contact Line. Phys. Fluids 2021, 33, 103601. [Google Scholar] [CrossRef]
- Cox, R.G. The Dynamics of the Spreading of Liquids on a Solid Surface. Part 1. Viscous Flow. J. Fluid Mech. 1986, 168, 169–194. [Google Scholar] [CrossRef]
- Huh, C.; Scriven, J.L.E. Hydrodynamic Model of Steady Movement of a Solid/Liquid/Fluid Contact Line. J. Colloid Interface Sci. 1971, 35, 85–101. [Google Scholar] [CrossRef]
- Harikrishnan, A.R.; Dhar, P.; Agnihotri, P.K.; Gedupudi, S.; Das, S.K. Correlating Contact Line Capillarity and Dynamic Contact Angle Hysteresis in Surfactant-Nanoparticle Based Complex Fluids. Phys. Fluids 2018, 30, 042006. [Google Scholar] [CrossRef]
- Liu, M.; Chen, X.-P. Numerical Study on the Stick-Slip Motion of Contact Line Moving on Heterogeneous Surfaces. Phys. Fluids 2017, 29, 082102. [Google Scholar] [CrossRef]
- Villermaux, E.; Bossa, B. Drop fragmentation on impact. J. Fluid Mech. 2011, 668, 412–435. [Google Scholar] [CrossRef]
- Ding, H.; Spelt, P.D.M. Inertial Effects in Droplet Spreading: A Comparison between Diffuse-Interface and Level-Set Simulations. J. Fluid Mech. 2007, 576, 287–296. [Google Scholar] [CrossRef]
- Kavehpour, H.P.; Mohammad Karim, A.; Rothstein, J.P.; Davis, S. Laws of Spreading: When Hydrodynamic Equations Are Not Enough. In Proceedings of the 70th Annual Meeting of the Fluid Dynamics, Denver, CO, USA, 19–21 November 2017; Division of the American Physical Society: College Park, MD, USA, 2017; Volume 62. [Google Scholar]
- Biance, A.-L.; Clanet, C.; Quéré, D. First Steps in the Spreading of a Liquid Droplet. Phys. Rev. E 2004, 69, 016301. [Google Scholar] [CrossRef] [PubMed]
- Foister, R.T. The Kinetics of Displacement Wetting in Liquid/Liquid/Solid Systems. J. Colloid Interface Sci. 1990, 136, 266–282. [Google Scholar] [CrossRef]
- Marengo, M.; Antonini, C.; Roisman, I.V.; Tropea, C. Drop collisions with simple and complex surfaces. Curr. Opin. Colloid Interface Sci. 2011, 16, 292–302. [Google Scholar] [CrossRef]
- Josserand, C.; Thoroddsen, S.T. Drop impact on a solid surface. Annu. Rev. Fluid Mech. 2016, 48, 365–391. [Google Scholar] [CrossRef]
- Yarin, A.L.; Roisman, I.V.; Tropea, C. Collision Phenomena in Liquids and Solids; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Pierzyna, M.; Burzynski, D.A.; Bansmer, S.E.; Semaan, R. Data-driven splashing threshold model for drop impact on dry smooth surfaces. Phys. Fluids 2021, 33, 123317. [Google Scholar] [CrossRef]
- Mohammad Karim, A.; Rothstein, J.P.; Kavehpour, H.P. Dynamics of Spreading on Micro-Textured Surfaces. In Proceedings of the 68th Annual Meeting of the Fluid Dynamics, Boston, MA, USA, 22–24 November 2015; Division of the American Physical Society: College Park, MD, USA, 2015; Volume 60. [Google Scholar]
- Wijshoff, H. Drop dynamics in the inkjet printing process. Curr. Opin. Colloid Interface Sci. 2018, 36, 20–27. [Google Scholar] [CrossRef]
- Mohammad Karim, A.; Rothstein, J.P.; Kavehpour, H.P. Experimental Study of Dynamic Contact Angles on Rough Hydrophobic Surfaces. J. Colloid Interface Sci. 2018, 513, 658–665. [Google Scholar] [CrossRef]
- Voinov, O.V. Hydrodynamics of Wetting. Fluid Dyn. 1976, 11, 714–721. [Google Scholar] [CrossRef]
- Mohammad Karim, A.; Kavehpour, H.P. Dynamics of Spreading on Ultra-hydrophobic Surfaces. J. Coat. Technol. Res. 2015, 12, 959–964. [Google Scholar] [CrossRef]
- Wibowo, C.; Ng, K.M. Product-oriented process synthesis and development: Creams and pastes. AIChE J. 2001, 47, 2746–2767. [Google Scholar] [CrossRef]
- Skurtys, O.; Aguilera, J.M. Applications of microfluidic devices in food engineering. Food Biophys. 2008, 3, 1–15. [Google Scholar] [CrossRef]
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet printing process and its applications. Adv. Mater. 2010, 22, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Roach, L.S.; Ismagilov, R.F. Screening of protein crystallization conditions on a microfluidic chip using nanoliter-size droplets. J. Am. Chem. Soc. 2003, 125, 11170–11171. [Google Scholar] [CrossRef]
- Suea-Ngam, A.; Rattanarat, P.; Chailapakul, O.; Srisa-Art, M. Electrochemical droplet-based microfluidics using chip-based carbon paste electrodes for high-throughput analysis in pharmaceutical applications. Anal. Chim. Acta 2015, 883, 45–54. [Google Scholar] [CrossRef]
- Mohammad Karim, A.; Suszynski, W.J.; Griffith, W.B.; Pujari, S.; Francis, L.F.; Carvalho, M.S. Effect of rheological properties of shear thinning liquids on curtain stability. J. Non-Newton. Fluid Mech. 2019, 263, 69–76. [Google Scholar] [CrossRef]
- Singh, R.; Bahga, S.S.; Gupta, A. Electrohydrodynamic droplet formation in a T-junction microfluidic device. J. Fluid Mech. 2020, 905, A29. [Google Scholar] [CrossRef]
- Spelt, P.D. A level-Set Approach for Simulations of Flows with Multiple Moving Contact Lines with Hysteresis. J. Comput. Phys. 2005, 207, 389–404. [Google Scholar] [CrossRef]
- Wang, H. From Contact Line Structures to Wetting Dynamics. Langmuir 2019, 35, 10233–10245. [Google Scholar] [CrossRef]
- Mohammad Karim, A.; Suszynski, W.J.; Pujari, S.; Francis, L.F.; Carvalho, M.S. Delaying Breakup and Avoiding Air Entrainment in Curtain Coating Using a Two-Layer Liquid Structure. Chem. Eng. Sci. 2020, 213, 115376. [Google Scholar] [CrossRef]
- Brandon, S.; Marmur, A. Simulation of Contact Angle Hysteresis on Chemically Heterogeneous Surfaces. J. Colloid Interface Sci. 1996, 183, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Ng, A.H.; Fobel, R.; Wheeler, A.R. Digital microfluidics. Annu. Rev. Anal. Chem. 2012, 5, 413–440. [Google Scholar] [CrossRef]
- Mashaghi, S.; Abbaspourrad, A.; Weitz, D.A.; van Oijen, A.M. Droplet microfluidics: A tool for biology, chemistry and nanotechnology. TrAC Trends Anal. Chem. 2016, 82, 118–125. [Google Scholar] [CrossRef]
- Mohammad Karim, A.; Suszynski, W.J.; Pujari, S.; Francis, L.F.; Carvalho, M.S. Stability of Two-Layer Curtain Coating. In Proceedings of the ICR 2020—18th International Congress on Rheology, Rio de Janeiro, Brazil, 2–7 August 2020. [Google Scholar]
- De Gennes, P.G. Deposition of Langmuir-Blodgett Layers. Colloid Polym. Sci. 1986, 264, 463–465. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Hejazian, M.; Ooi, C.; Kashaninejad, N. Recent advances and future perspectives on microfluidic liquid handling. Micromachines 2017, 8, 186. [Google Scholar] [CrossRef]
- Song, H.; Chen, D.L.; Ismagilov, R.F. Reactions in droplets in microfluidic channels. Angew. Chem. Int. Ed. Engl. 2006, 45, 7336–7356. [Google Scholar] [CrossRef]
- Teh, S.Y.; Lin, R.; Hung, L.H.; Lee, A.P. Droplet microfluidics. Lab Chip 2008, 8, 198–220. [Google Scholar] [CrossRef]
- Weigl, B.H.; Bardell, R.L.; Cabrera, C.R. Lab-on-a-chip for drug development. Adv. Drug Deliv. Rev. 2003, 55, 349–377. [Google Scholar] [CrossRef]
- Tao, X.; Chakrabarty, K. Parallel scan-like test and multiple-defect diagnosis for digital microfluidic biochips. IEEE Trans. Biomed. Circuits Syst. 2007, 1, 148–158. [Google Scholar]
- Mohammad Karim, A.; Suszynski, W.J.; Pujari, S.; Francis, L.F.; Carvalho, M.S. Contact Line Dynamics in Curtain Coating of Non-Newtonian Liquids. Phys. Fluids 2021, 33, 103103. [Google Scholar] [CrossRef]
- Mohammad Karim, A. Experimental Dynamics of Newtonian Non-elastic and Viscoelastic Droplets Impacting Immiscible Liquid Surface. AIP Adv. 2019, 9, 125141. [Google Scholar] [CrossRef]
- Mohammad Karim, A. Experimental Dynamics of Newtonian and Non-Newtonian Droplets Impacting Liquid Surface with Different Rheology. Phys. Fluids 2020, 32, 043102. [Google Scholar] [CrossRef]
- Joung, Y.S.; Buie, C.R. Aerosol generation by raindrop impact on soil. Nat. Commun. 2015, 6, 6083. [Google Scholar] [CrossRef]
- Bourouiba, L. The fluid dynamics of disease transmission. Annu. Rev. Fluid Mech. 2021, 53, 473–508. [Google Scholar] [CrossRef]
- Pham, T.; Kumar, S. Imbibition and Evaporation of Droplets of Colloidal Suspensions on Permeable Substrates. Phys. Rev. Fluids 2019, 4, 034004. [Google Scholar] [CrossRef]
- Riboux, G.; Gordillo, J.M. Experiments of drops impacting a smooth solid surface: A model of the critical impact speed for drop splashing. Phys. Rev. Lett. 2014, 113, 024507. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, W.W.; Nagel, S.R. Drop splashing on a dry smooth surface. Phys. Rev. Lett. 2005, 94, 184505. [Google Scholar] [CrossRef]
- Lee, M.; Chang, Y.S.; Kim, H.-Y. Drop impact on microwetting patterned surfaces. Phys. Fluids 2010, 22, 072101. [Google Scholar] [CrossRef]
- Burzynski, D.A.; Roisman, I.V.; Bansmer, S.E. On the splashing of high-speed drops impacting a dry surface. J. Fluid Mech. 2020, 892, A2. [Google Scholar] [CrossRef]
- Piskunov, M.; Semyonova, A.; Khomutov, N.; Ashikhmin, A.; Yanovsky, V. Effect of rheology and interfacial tension on spreading of emulsion drops impacting a solid surface. Phys. Fluids 2021, 33, 083309. [Google Scholar] [CrossRef]
- Pasandideh-Fard, M.; Qiao, Y.M.; Chandra, S.; Mostaghimi, J. Capillary effects during droplet impact on a solid surface. Phys. Fluids 1996, 8, 650–659. [Google Scholar] [CrossRef]
- Ukiwe, C.; Kwok, D. On the maximum spreading diameter of impacting droplets on well-prepared solid surfaces. Langmuir 2005, 21, 666–673. [Google Scholar] [CrossRef] [PubMed]
- De la Paz, E.; Maganti, N.H.; Trifonov, A.; Jeerapan, I.; Mahato, K.; Yin, L.; Sonsa-Ard, T.; Ma, N.; Jung, W.; Burns, R.; et al. A self-powered ingestible wireless biosensing system for real-time in situ monitoring of gastrointestinal tract metabolites. Nat. Commun. 2022, 13, 7405. [Google Scholar] [CrossRef]
- Sharma, S.; Ramadi, K.B.; Poole, N.H.; Srinivasan, S.S.; Ishida, K.; Kuosmanen, J.; Jenkins, J.; Aghlmand, F.; Swift, M.B.; Shapiro, M.G.; et al. Location-aware ingestible microdevices for wireless monitoring of gastrointestinal dynamics. Nat. Electron. 2023, 6, 242–256. [Google Scholar] [CrossRef]
- Glasstone, S.; Laidler, K.J.; Eyring, H.J. The Theory of Rate Processes; McGraw-Hill: New York, NY, USA, 1941. [Google Scholar]
- Jung, S.; Tiwari, M.K.; Doan, N.V.; Poulikakos, D. Mechanism of supercooled droplet freezing on surfaces. Nat. Commun. 2012, 3, 615. [Google Scholar] [CrossRef]
- Mishchenko, L.; Hatton, B.; Bahadur, V.; Taylor, J.A.; Krupenkin, T.; Aizenberg, J. Design of ice-free nanostructured surfaces based on repulsion of impacting water droplets. ACS Nano 2010, 4, 7699–7707. [Google Scholar] [CrossRef]
- Young, T. III. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Chen, L.; Wu, J.; Li, Z.; Yao, S. Evolution of entrapped air under bouncing droplets on viscoelastic surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 726–732. [Google Scholar] [CrossRef]
- Blossey, R. Self-cleaning surfaces_virtual realities. Nat. Mater. 2003, 2, 301–306. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing superoleophobic surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef]
- Deng, X.; Mammen, L.; Butt, H.J.; Vollmer, D. Candle soot as a template for a transparent robust superamphiphobic coating. Science 2012, 335, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; ÓMahony, A.P.; Barber, T.J. Effect of 3D-bioprinted droplet impact dynamics on a pre-printed soft hydrogel matrix. Exp. Fluids 2023, 64, 60. [Google Scholar] [CrossRef]
- Song, Y.; Tay, R.Y.; Li, J.; Xu, C.; Min, J.; Sani, E.S.; Kim, G.; Heng, W.; Kim, I.; Gao, W. 3D-printed epifluidic electronic skin for machine learning-powered multimodal health surveillance. Sci. Adv. 2023, 9, eadi6492. [Google Scholar] [CrossRef]
- Yu, Y.; Li, J.; Solomon, S.A.; Min, J.; Tu, J.; Guo, W.; Xu, C.; Song, Y.; Gao, W. All-printed soft human-machine interface for robotic physicochemical sensing. Sci. Robot. 2022, 7, eabn0495. [Google Scholar] [CrossRef] [PubMed]
- Bariya, M.; Shahpar, Z.; Park, H.; Sun, J.; Jung, Y.; Gao, W.; Nyein, H.Y.Y.; Liaw, T.S.; Tai, L.-C.; Ngo, Q.P.; et al. Roll-to-roll gravure printed electrochemical sensors for wearable and medical devices. ACS Nano 2018, 12, 6978–6987. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Chao, M.; Gao, Y.; Wu, E.; Tai, L.-C.; Chen, K.; Matsuoka, Y.; Iwai, K.; Fahad, H.M.; Gao, W.; et al. 3D printed earable smart devices for real-time detection of core body temperature. ACS Sens. 2017, 2, 990. [Google Scholar] [CrossRef]
- Fang, A.; Dujardin, E.; Ondarçuhu, T. Control of droplet size in liquid nanodispensing. Nano Lett. 2006, 6, 2368–2374. [Google Scholar] [CrossRef]
- Slattery, J.C.; Sagis, L.; Oh, E.S. Interfacial Transport Phenomena; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Laan, N.; de Bruin, K.G.; Bartolo, D.; Josserand, C.; Bonn, D. Maximum diameter of impacting liquid droplets. Phys. Rev. Appl. 2014, 2, 044018. [Google Scholar] [CrossRef]
- Yilmaz, B.; Al Rashid, A.; Mou, Y.A.; Evis, Z.; Koç, M. Bioprinting: A review of processes, materials and applications. Bioprinting 2021, 23, 00148. [Google Scholar] [CrossRef]
- Yang, S.; Hou, Y.; Shang, Y.; Zhong, X. BPNN and CNN-based AI modeling of spreading and icing pattern of a water droplet impact on a supercooled surface. AIP Adv. 2022, 12, 045209. [Google Scholar] [CrossRef]
- Ng, W.L.; Lee, J.M.; Zhou, M.; Chen, Y.W.; Lee, K.X.A.; Yeong, W.Y.; Shen, Y.F. Vat polymerization-based bioprinting-process, materials, applications and regulatory challenges. Biofabrication 2020, 12, 022001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kumar, P.; Lv, S.; Xiong, D.; Zhao, H.; Cai, Z.; Zhao, X. Recent advances in 3D bioprinting of vascularized tissues. Mater. Des. 2021, 199, 109398. [Google Scholar] [CrossRef]
- Sun, L.; Pan, J.; Wang, X.; Jing, D. Molecular dynamics study of nanoscale droplets impacting on textured substrates of variable wettability. Phys. Fluids 2022, 34, 012005. [Google Scholar] [CrossRef]
- Yue, P.; Zhou, C.; Feng, J.J. Sharp-Interface Limit of the Cahn–Hilliard Model for Moving Contact Lines. J. Fluid Mech. 2010, 645, 279–294. [Google Scholar] [CrossRef]
- Yue, P.; Zhou, C.; Feng, J.J.; Ollivier-Gooch, C.F.; Hu, H.H. Phase-Field Simulations of Interfacial Dynamics in Viscoelastic Fluids Using Finite Elements with Adaptive Meshing. J. Comput. Phys. 2006, 219, 47–67. [Google Scholar] [CrossRef]
- Van Der Waals, J.D. The Thermodynamic Theory of Capillarity Under the Hypothesis of a Continuous Variation of Density. J. Stat. Phys. 1979, 20, 200–244. [Google Scholar] [CrossRef]
- Cahn, J.W. Critical Point Wetting. J. Chem. Phys. 1977, 66, 3667–3672. [Google Scholar] [CrossRef]
- Cahn, J.W.; Hilliard, J.E. Free Energy of a Non-Uniform System. Part I. Interfacial Free Energy. J. Chem. Phys. 1958, 28, 258–267. [Google Scholar]
- Quéré, D. Wetting and roughness. Annu. Rev. Mater. Res. 2008, 38, 71. [Google Scholar] [CrossRef]
- Howland, C.J.; Antkowiak, A.; Castrejón-Pita, J.R.; Howison, S.D.; Oliver, J.M.; Style, R.W.; Castrejón-Pita, A.A. It’s harder to splash on soft solids. Phys. Rev. Lett. 2016, 117, 184502. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Huang, X.; Shkolnikov, V.; Goh, G.L.; Suntornnond, R.; Yeong, W.Y. Controlling droplet impact velocity and droplet volume: Key factors to achieving high cell viability in sub-nanoliter droplet-based bioprinting. Int. J. Bioprinting 2022, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Basso, B.C.; Bostwick, J.B. Splashing on soft elastic substrates. Langmuir 2020, 36, 15010–15017. [Google Scholar] [CrossRef] [PubMed]
- Saile, D.; Kühl, V.; Gülhan, A. On the subsonic near-wake of a space launcher configuration without exhaust jet. Exp. Fluids 2019, 60, 50. [Google Scholar] [CrossRef]
- Nilsson, M.A.; Rothstein, J.P. The effect of contact angle hysteresis on droplet coalescence and mixing. J. Colloid Interface Sci. 2011, 363, 646. [Google Scholar] [CrossRef]
- Makrygianni, M.; Milionis, A.; Kryou, C.; Trantakis, I. On-demand laser printing of picoliter-sized, highly viscous, adhesive fluids: Beyond inkjet limitations. Adv. Mater. Interfaces 2018, 5, 1800440. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2020, 18, 100479. [Google Scholar] [CrossRef]
- Catros, S.; Guillotin, B.; Bačáková, M.; Fricain, J.-C.; Guillemot, F. Effect of laser energy, substrate film thickness and bioink viscosity on viability of endothelial cells printed by laser-assisted bioprinting. Appl. Surf. Sci. 2011, 257, 5142–5147. [Google Scholar] [CrossRef]
- Ferraro, P.; Coppola, S.; Grilli, S.; Paturzo, M.; Vespini, V. Dispensing nano–pico droplets and liquid patterning by pyroelectrodynamic shooting. Nat. Nanotechnol. 2010, 5, 429. [Google Scholar] [CrossRef]
- Srinivasan, S.; Choi, W.; Park, K.-C.; Chhatre, S.S.; Cohen, R.E.; McKinley, G.H. Drag reduction for viscous laminar flow on spray coated non-wetting surfaces. Soft Matter 2013, 9, 5691. [Google Scholar] [CrossRef]
- Quéré, E. Non-sticking drops. Rep. Prog. Phys. 2005, 68, 2495. [Google Scholar] [CrossRef]
- Rioboo, R.; Tropea, C.; Marengo, M. Outcomes from a drop impact on solid surfaces. At. Sprays 2001, 11, 155–165. [Google Scholar] [CrossRef]
- Rein, M. Phenomena of liquid drop impact on solid and liquid surfaces. Fluid Dyn. Res. 1993, 12, 61. [Google Scholar] [CrossRef]
- Li, J.; Rossignol, F.; Macdonald, J. Inkjet printing for biosensor fabrication: Combining chemistry and technology for advanced manufacturing. Lab Chip 2015, 15, 2538–2558. [Google Scholar] [CrossRef]
- Bange, P.G.; Patil, N.D.; Bhardwaj, R. Impact Dynamics of a Droplet on a Heated Surface. In Proceedings of the 5th International Conference of Fluid Flow, Heat and Mass Transfer (FFHMT‘18), Niagara Falls, ON, Canada, 7–9 June 2018; Volume 190, pp. 232–247. [Google Scholar]
- Guo, Y.; Shen, S.; Yang, Y.; Liang, G.; Zhen, N. Rebound and spreading during a drop impact on wetted cylinders. Exp. Therm. Fluid Sci. 2013, 52, 97–103. [Google Scholar]
- Malla, L.K.; Patil, N.D.; Bhardwaj, R.; Neild, A. Droplet bouncing and breakup during impact on a microgrooved surface. Langmuir 2017, 33, 9620–9631. [Google Scholar] [CrossRef]
- Hamazaki, T.; Morita, N. Ejection characteristics and drop modulation of acoustic inkjet printing using fresnel lens. J. Fluid Sci. Technol. 2009, 4, 25–36. [Google Scholar] [CrossRef][Green Version]
- Derjaguin, B.; Churaev, N. Structural Component of Disjoining Pressure. J. Colloid Interface Sci. 1974, 49, 249–255. [Google Scholar] [CrossRef]
- Andreotti, B.; Baumchen, O.; Boulogne, F.; Daniels, K.E.; Dufresne, E.R.; Perrin, H.; Salez, T.; Snoeijer, J.H.; Style, R.W. Solid capillarity: When and how does surface tension deform soft solids? Soft Matter 2016, 12, 2993. [Google Scholar] [CrossRef]
- Boyer, F.; Sandoval-Nava, E.; Snoeijer, J.H.; Dijksman, J.F.; Lohse, D. Drop impact of shear thickening liquids. Phys. Rev. Fluids 2016, 1, 013901. [Google Scholar] [CrossRef]
- Aytouna, M.; Bartolo, D.; Wegdam, G.; Bonn, D.; Rafaï, S. Impact dynamics of surfactant laden drops: Dynamic surface tension effects. Exp. Fluids 2010, 48, 49–57. [Google Scholar] [CrossRef]
- Izbassarov, D.; Muradoglu, M. Effects of viscoelasticity on drop impact and spreading on a solid surface. Phys. Rev. Fluids 2016, 1, 023302. [Google Scholar] [CrossRef]
- Utama, R.H.; Tan, V.T.G.; Tjandra, K.C.; Sexton, A.; Nguyen, D.H.T.; O’Mahony, A.P.; Du, E.Y.; Tian, P.; Ribeiro, J.C.C.; Kavallaris, M.; et al. A covalently crosslinked ink for multimaterials drop-on-demand 3D bioprinting of 3D cell cultures. Macromol. Biosci. 2021, 21, 2100125. [Google Scholar] [CrossRef] [PubMed]
- Utama, R.H.; Atapattu, L.; O’Mahony, A.P.; Fife, C.M.; Baek, J.; Allard, T.; O’Mahony, K.J.; Ribeiro, J.; Gaus, K.; Kavallaris, M.; et al. Precise, high-throughput production of multicellular spheroids with a bespoke 3D bioprinter. bioRxiv 2020. [Google Scholar] [CrossRef]
- Dussan, V.E.B. On the Spreading of Liquids on Solid Surfaces: Static and Dynamic Contact Lines. Annu. Rev. Fluid Mech. 1979, 11, 371–400. [Google Scholar] [CrossRef]
- Blake, T.D.; Berg, J.C. Wettability: Dynamic Contact Angles and Wetting Kinetics; Marcel Dekker: New York, NY, USA, 1993; Volume 49, pp. 251–309. [Google Scholar]
- Blake, T.D.; Haynes, J.M. Kinetics of Liquid/Liquid Displacement. J. Colloid Interf. Sci. 1969, 30, 421–423. [Google Scholar] [CrossRef]
- Wu, Z.; Cao, Y. Dynamics of initial drop splashing on a dry smooth surface. PLoS ONE 2017, 12, e0177390. [Google Scholar] [CrossRef]
- Huh, C.; Mason, S.G. The Steady Movement of a Liquid Meniscus in a Capillary Tube. J. Fluid Mech. 1977, 81, 401–419. [Google Scholar] [CrossRef]
- Dussan, V.E.B. The Moving Contact Line: The Slip Boundary Condition. J. Fluid Mech. 1976, 77, 665–684. [Google Scholar] [CrossRef]
- Ngan, C.G.; Dussan, V.E.B. On the Dynamics of Liquid Spreading on Solid Surfaces. J. Fluid Mech. 1989, 209, 191–226. [Google Scholar] [CrossRef]
- Schroll, R.D.; Josserand, C.; Zaleski, S.; Zhang, W.W. Impact of a viscous liquid drop. Phys. Rev. Lett. 2010, 104, 034504. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Rocco, G.; de Luca, L. Insights on the impact of a plane drop on a thin liquid film. Phys. Fluids 2011, 23, 022105. [Google Scholar] [CrossRef]
- Josserand, C.; Ray, P.; Zaleski, S. Droplet impact on a thin liquid film: Anatomy of the splash. arXiv 2015, 1511, 09395. [Google Scholar] [CrossRef]
- Mahady, K.; Afkhami, S.; Kondic, L. A volume of fluid method for simulating fluid/fluid interfaces in contact with solid boundaries. J. Comput. Phys. 2015, 294, 243–257. [Google Scholar] [CrossRef]
- Couder, Y.; Protière, S.; Fort, E.; Boudaoud, A. Walking and orbiting droplets. Nature 2005, 437, 208. [Google Scholar] [CrossRef]
- Couder, Y.; Fort, E.; Gautier, C.-H.; Boudaoud, A. From bouncing to floating: Nanocoalescence of drops on a fluid bath. Phys. Rev. Lett. 2005, 94, 177801. [Google Scholar] [CrossRef]
- Lee, A.; Jin, H.; Dang, H.-W.; Choi, K.-H.; Ahn, K.H. Optimization of experimental parameters to determine the jetting regimes in electrohydrodynamic printing. Langmuir 2013, 29, 13630–13639. [Google Scholar] [CrossRef]
- Šikalo, Š.; Wilhelm, H.-D.; Roisman, I.V.; Jakirlić, S.; Tropea, C. Dynamic contact angle of spreading droplets: Experiments and simulations. Phys. Fluids 2005, 17, 062103. [Google Scholar] [CrossRef]
- Gunjal, P.R.; Ranade, V.V.; Chaudhari, R.V. Dynamics of drop impact on solid surface: Experiments and VOF simulations. AIChE J. 2005, 51, 59–78. [Google Scholar] [CrossRef]
- Sahoo, N.; Khurana, G.; Harikrishnan, A.R.; Samanta, D.; Dhar, P. Post impact droplet hydrodynamics on inclined planes of variant wettabilities. Eur. J. Mech. B Fluids 2020, 79, 27–37. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, Y.; Hou, Y.; Bai, B.; Zhong, X. Effects of surface subcooling on the spreading dynamics of an impact water droplet. Phys. Fluids 2020, 32, 123309. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, W.; Guo, L.; Liu, Z.; Cai, P.; Cui, Y.; Wang, T.; Wang, C.; Zhu, M.; Zhou, Y.; et al. Haptically quantifying Young’s modulus of soft materials using a self-locked stretchable strain sensor. Adv. Mater. 2021, 34, 2104078. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Karim, A.; Davis, S.H.; Kavehpour, H.P. Forced versus Spontaneous Spreading of Liquids. Langmuir 2016, 32, 10153–10158. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Karim, A.; Suszynski, W.J.; Francis, L.F.; Carvalho, M.S. Effect of Viscosity on Liquid Curtain Stability. AIChE J. 2018, 64, 1448–1457. [Google Scholar] [CrossRef]
- Mohammad Karim, A.; Kavehpour, H.P. Effect of Viscous Force on Dynamic Contact Angle Measurement Using Wilhelmy Plate Method. Colloids Surf. A 2018, 548, 54–60. [Google Scholar] [CrossRef]
- Li, E.Q.; Thoroddsen, S.T. Time-resolved imaging of a compressible air-disc under a drop impacting on a solid surface. J. Fluid Mech. 2015, 780, 636–648. [Google Scholar] [CrossRef]
- Driscoll, M.M.; Stevens, C.S.; Nagel, S.R. Thin film formation during splashing of viscous liquids. Phys. Rev. E 2010, 83, 036302. [Google Scholar] [CrossRef]
- Thoroddsen, S.T.; Takehara, K.; Etoh, T.G. Micro-splashing by drop impacts. J. Fluid Mech. 2012, 706, 560–570. [Google Scholar] [CrossRef]
- Thoroddsen, S.T.; Sakakibara, J. Evolution of the fingering pattern of an impacting drop. Phys. Fluids 1998, 10, 1359–1374. [Google Scholar] [CrossRef]
- Bischofberger, I.; Mauser, K.W.; Nagel, S.R. Seeing the invisible-air vortices around a splashing drop. Phys. Fluids 2013, 25, 091110. [Google Scholar] [CrossRef]
- Abouelsoud, M.; Bai, B. Bouncing and coalescence dynamics during the impact of a falling drop with a sessile drop on different solid surfaces. Phys. Fluids 2021, 33, 063309. [Google Scholar] [CrossRef]
- Dalgamoni, H.N.; Yong, X. Numerical and theoretical modeling of droplet impact on spherical surfaces. Phys. Fluids 2021, 33, 052112. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.; Su, J.; Jing, D.; Mohamad, A.A. Impact on mechanical robustness of water droplet due to hydrophilic nanoparticles. Phys. Fluids 2020, 32, 122110. [Google Scholar] [CrossRef]
- Yee, J.; Yamanaka, A.; Tagawa, Y. Image features of a splashing drop on a solid surface extracted using a feedforward neural network. Phys. Fluids 2022, 34, 013317. [Google Scholar] [CrossRef]
- Vadillo, D.; Soucemarianadin, A.; Delattre, C.; Roux, D. Dynamic contact angle effects onto the maximum drop impact spreading on solid surfaces. Phys. Fluids 2009, 21, 122002. [Google Scholar] [CrossRef]
- Mongruel, A.; Daru, V.; Feuillebois, F.; Tabakova, S. Early post-impact time dynamics of viscous drops onto a solid dry surface. Phys. Fluids 2009, 21, 032101. [Google Scholar] [CrossRef]
- Stevens, C.S.; Latka, A.; Nagel, S.R. Comparison of splashing in high- and low-viscosity liquids. Phys. Rev. E 2014, 89, 063006. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, P.; Xu, L. Kelvin-Helmholtz instability in an ultrathin air film causes drop splashing on smooth surfaces. Proc. Natl. Acad. Sci. USA 2015, 112, 3280–3284. [Google Scholar] [CrossRef]
- Mishra, N.K.; Zhang, Y.; Ratner, A. Effect of chamber pressure on spreading and splashing of liquid drops upon impact on a dry smooth stationary surface. Exp. Fluids 2011, 51, 483–491. [Google Scholar] [CrossRef]
- Fabmann, B.W.; Bansmer, S.E.; Möller, T.J.; Radespiel, R.; Hartmann, M. High velocity impingement of single droplets on a dry smooth surface. Exp. Fluids 2013, 54, 1516. [Google Scholar]
- Palacios, J.; Hernández, J.; Gómez, P.; Zanzi, C.; López, J. On the impact of viscous drops onto dry smooth surfaces. Exp. Fluids 2012, 52, 1449–1463. [Google Scholar] [CrossRef]
- Kittel, H.M.; Roisman, I.V.; Tropea, C. Splash of a drop impacting onto a solid substrate wetted by a thin film of another liquid. Phys. Rev. Fluids 2018, 3, 073601. [Google Scholar] [CrossRef]
- Reiser, A.; Lindén, M.; Rohner, P.; Marchand, A.; Galinski, H.; Sologubenko, A.S.; Wheeler, J.M.; Zenobi, R.; Poulikakos, D. Multi-metal electrohydrodynamic redox 3D printing at the submicron scale. Nat. Commun. 2019, 10, 1853. [Google Scholar] [CrossRef]
- Mohammad Karim, A.; Kim, J.; Rothstein, J.P.; Kavehpour, H.P. Dynamics of Wetting of Ultra-hydrophobic Surfaces. In Proceedings of the 66th Annual Meeting of the Fluid Dynamics, Pittsburgh, PA, USA, 24–26 November 2013; Division of the American Physical Society: College Park, MD, USA, 2013; Volume 58. [Google Scholar]
- Mohammad Karim, A.; Kavehpour, H.P. Spreading of Emulsions on Glass Substrates. In Proceedings of the 65th Annual Meeting of the Fluid Dynamics, San Diego, CA, USA, 18–20 November 2012; Division of the American Physical Society: College Park, MD, USA, 2012; Volume 57. [Google Scholar]
- Yuan, Q.; Zhao, Y.-P. Precursor Film in Dynamic Wetting, Electrowetting, and Electro-Elasto-Capillarity. Phys. Rev. Lett. 2010, 104, 246101. [Google Scholar] [CrossRef]
- Mohammad Karim, A.; Kavehpour, H.P. Laws of spreading: Why Tanner, Hoffman, Voinov, Cox and de Gennes were wrong, generally speaking. In Proceedings of the 67th Annual Meeting of the Fluid Dynamics, San Francisco, CA, USA, 23–25 November 2014; Division of the American Physical Society: College Park, MD, USA, 2014; Volume 59. [Google Scholar]
- Mohammad Karim, A.; Kavehpour, H.P. Spreading of Emulsions on a Solid Substrate. J. Coat. Technol. Res. 2014, 11, 103–108. [Google Scholar] [CrossRef]
- Vanaei, S.; Parizi, M.S.; Vanaei, S.; Salemizadehparizi, F.; Vanaei, H.R. An overview on materials and techniques in 3D bioprinting toward biomedical application. Eng. Regen. 2021, 2, 1–18. [Google Scholar] [CrossRef]
- Rao, D.N. The concept, characterization, concerns and consequences of contact angles in solid-liquid-liquid systems. In Proceedings of the 3rd International Symposium on Contact Angle, Wettability and Adhesion, Providence, RI, USA, 20–23 May 2002; Volume 3, pp. 191–210. [Google Scholar]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Cassie, A.B.D. Contact angles. Discuss Faraday Soc. 1948, 3, 11–16. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Gee, M.L. Contact angles on chemically heterogeneous surfaces. Langmuir 1989, 5, 288–289. [Google Scholar] [CrossRef]
- Gao, L.C.; McCarthy, T.J. How Wenzel and Cassie were wrong? Langmuir 2007, 23, 3762–3765. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.T.; Taboryski, R.A. Cassie-like law using triple phase boundary line fractions for faceted droplets on chemically heterogeneous surfaces. Langmuir 2009, 25, 1282–1284. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Tuteja, A.; Mabry, J.M.; Cohen, R.E.; McKinley, G.H. A modified Cassie-Baxter relationship to explain contact angle hysteresis and anisotropy on non-wetting textured surfaces. J. Colloid Interface Sci. 2009, 339, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Milne, A.J.B.; Amirfazli, A. The Cassie equation: How it is meant to be used. Adv. Colloid Interface Sci. 2012, 170, 48–55. [Google Scholar] [CrossRef]
- Mohammad Karim, A.; Suszynski, W.J.; Griffith, W.B.; Pujari, S.; Francis, L.F.; Carvalho, M.S. Effect of Viscoelasticity on Stability of Liquid Curtain. J. Non-Newton. Fluid Mech. 2018, 257, 83–94. [Google Scholar] [CrossRef]
- Wang, X.; Xu, B.; Chen, Z.; Yang, Y.; Cao, Q. Effects of gravitational force and surface orientation on the jumping velocity and energy conversion efficiency of coalesced droplets. Microgravity Sci. Tec. 2020, 32, 1185–1197. [Google Scholar] [CrossRef]
- Hail, C.U.; Höller, C.; Matsuzaki, K.; Rohner, P.; Renger, J.; Sandoghdar, V.; Poulikakos, D.; Eghlidi, H. Nanoprinting organic molecules at the quantum level. Nat. Commun. 2019, 10, 1880. [Google Scholar] [CrossRef]
- Eggers, J.; Lister, J.R.; Stone, H.A. Coalescence of liquid drops. J. Fluid Mech. 1999, 401, 293–310. [Google Scholar] [CrossRef]
- Joseph, D.P.; Justin, C.B.; Sidney, R.N.; Santosh, A.; Michael, T.H.; Osman, A.B. The inexorable resistance of inertia determines the initial regime of drop coalescence. Proc. Natl. Acad. Sci. USA 2012, 109, 6857–6861. [Google Scholar]
- Wang, X.Y.; Jia, L. Experimental study on heat transfer performance of pulsating heat pipe with refrigerants. J. Therm. Sci. 2016, 25, 449–453. [Google Scholar] [CrossRef]
- Wiedenheft, K.F.; Guo, H.A.; Qu, X.P.; Boreyko, J.B.; Liu, F.J.; Zhang, K.G.; Eid, F.; Choudhury, A.; Li, Z.H.; Chen, C.H. Hotspot cooling with jumping-drop vapor chambers. Appl. Phys. Lett. 2017, 110, 141601. [Google Scholar] [CrossRef]
- McNeil, D.A.; Burnside, B.M.; Cuthbertson, G. Dropwise condensation of steam on a small tube bundle at turbine condenser conditions. Exp. Heat Transf. 2000, 13, 89–105. [Google Scholar]
- Song, K.; Kim, G.; Oh, S.; Lim, H. Enhanced water collection through a periodic array of tiny holes in dropwise condensation. Appl. Phys. Lett. 2018, 112, 071602. [Google Scholar] [CrossRef]
- Humplik, T.; Lee, J.; O’Hern, S.C.; Fellman, B.A.; Baig, M.A.; Hassan, S.F.; Atieh, M.A.; Rahman, F.; Laoui, T.; Karnik, R.; et al. Nanostructured materials for water desalination. Nanotechnology 2011, 22, 292001. [Google Scholar] [CrossRef] [PubMed]
- Delrot, P.; Modestino, M.A.; Gallaire, F.; Psaltis, D.; Moser, C. Inkjet printing of viscous monodisperse microdroplets by laser-induced flow focusing. Phys. Rev. Appl. 2016, 6, 24003. [Google Scholar] [CrossRef]
- Min, J.; Sempionatto, J.R.; Teymourian, H.; Wang, J.; Gao, W. Wearable electrochemical biosensors in North America. Biosens. Bioelectron. 2021, 172, 112750. [Google Scholar] [CrossRef]
- Ju, J.; Bai, H.; Zheng, Y.; Zhao, T.; Fang, R.; Jiang, L. A multi-structural and multi-functional integrated fog collection system in cactus. Nat. Commun. 2012, 3, 1247. [Google Scholar] [CrossRef]
- Petrov, P.G.; Petrov, J.G. A Combined Molecular-Hydrodynamic Approach to Wetting Kinetics. Langmuir 1992, 8, 1762–1767. [Google Scholar] [CrossRef]
- Ghosh, A.; Beaini, S.; Zhang, B.J.; Ganguly, R.; Megaridis, C.M. Enhancing dropwise condensation through bioinspired wettability patterning. Langmuir 2014, 30, 13103–13115. [Google Scholar] [CrossRef]
- Parker, A.R.; Lawrence, C.R. Water capture by a desert beetle. Nature 2001, 414, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Delannoy, J.; Malod, A.; Zheng, H.; Quéré, D.; Wang, Z. Tip-induced flipping of droplets on Janus pillars: From local reconfiguration to global transport. Sci. Adv. 2020, 6, eabb4540. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yu, M.; Chen, X.; Wang, Z.; Yao, S. Recurrent filmwise and dropwise condensation on a beetle mimetic surface. ACS Nano 2015, 9, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, Z.; Frechette, J. Dynamic adhesion due to fluid infusion. Curr. Opin. Colloid Interface Sci. 2020, 50, 101397. [Google Scholar] [CrossRef]
- Klinger, M.; Laske, C.; Graeve, M.; Thoma, M.; Traube, A. A Sensor for the In-Flight Detection of Single Fluorescent Microbodies in Nanoliter Droplets. IEEE Sens. J. 2020, 20, 5809–5817. [Google Scholar] [CrossRef]
- Chen, X.; O’Mahony, A.P.; Barber, T. The characterization of particle number and distribution inside in-flight 3D printed droplets using a high speed droplet imaging system. J. Appl. Phys. 2021, 130, 044701. [Google Scholar] [CrossRef]
- Chen, X.; O’Mahony, A.P.; Barber, T. The assessment of average cell number inside in-flight 3D printed droplets in microvalvebased bioprinting. J. Appl. Phys. 2022, 131, 224701. [Google Scholar] [CrossRef]
- Engel, M.; Belfiore, L.; Aghaei, B.; Sutija, M. Enabling high throughput drug discovery in 3D cell cultures through a novel bioprinting workflow. SLAS Technol. 2022, 27, 32–38. [Google Scholar] [CrossRef]
- Mallinson, S.; Barber, T.; Yeoh, G.; McBain, G. Simulation of droplet impact and spreading using a simple dynamic contact angle model. Simulation 2018, 10, 13. [Google Scholar]
- Kang, S.H.; Kim, S.; Sohn, D.K.; Ko, H.S. Analysis of drop-ondemand piezo inkjet performance. Phys. Fluids 2020, 32, 022007. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, M.; Huang, Y.; Ogale, A.; Fu, J.; Markwald, R.R. Study of droplet formation process during drop-on-demand inkjetting of living cell-laden bioink. Langmuir 2014, 30, 9130–9138. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Yeong, W.Y.; Naing, M.W. Polyvinylpyrrolidone-based bio-ink improves cell viability and homogeneity during drop-ondemand printing. Materials 2017, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Torrente-Rodríguez, R.M.; Tu, J.; Yang, Y.; Min, J.; Wang, M.; Song, Y.; Yu, Y.; Xu, C.; Ye, C.; IsHak, W.W.; et al. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mhealth system. Matter 2020, 2, 921–937. [Google Scholar] [CrossRef]
- Cen, C.; Wu, H.; Lee, C.-F.; Fan, L.; Liu, F. Experimental investigation on the sputtering and micro-explosion of emulsion fuel droplets during impact on a heated surface. Int. J. Heat Mass Transf. 2019, 132, 130–137. [Google Scholar] [CrossRef]
- Mahmodi, H.; Piloni, A.; Utama, R.H.; Kabakova, I. Mechanical mapping of bioprinted hydrogel models by brillouin microscopy. Bioprinting 2021, 23, 00151. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, X.; Lin, Y.; Ma, J.; Xiao, J.; Wu, Y.; Wang, J. Effects of surface roughness on splashing characteristics of large droplets with digital inline holographic imaging. Cold Reg. Sci. Technol. 2021, 191, 103373. [Google Scholar] [CrossRef]
- Quetzeri-Santiago, M.A.; Castrejón-Pita, A.A.; Castrejón-Pita, J.R. The effect of surface roughness on the contact line and splashing dynamics of impacting droplets. Sci. Rep. 2019, 9, 15030. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Chen, N.; Chen, H.; Amirfazli, A. Drop impact onto a thin film: Miscibility effect. Phys. Fluids 2017, 29, 092106. [Google Scholar] [CrossRef]
- Yokoi, K. Numerical studies of droplet splashing on a dry surface: Triggering a splash with the dynamic contact angle. Soft Matter 2011, 7, 5120. [Google Scholar] [CrossRef]
- Mundo, C.; Sommerfeld, M.; Tropea, C. Droplet-wall collisions: Experimental studies of the deformation and breakup process. Int. J. Multiph. Flow 1995, 21, 151–173. [Google Scholar] [CrossRef]
- Gudapati, H.; Dey, M.; Ozbolat, I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials 2016, 102, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Saygili, E.; Dogan-Gurbuz, A.A.; Yesil-Celiktas, O.; Draz, M.S. 3D bioprinting: A powerful tool to leverage tissue engineering and microbial systems. Bioprinting 2020, 18, 00071. [Google Scholar] [CrossRef]
- Machekposhti, S.A.; Movahed, S.; Narayan, R.J. Physicochemical parameters that underlie inkjet printing for medical applications. Biophys. Rev. 2020, 1, 011301. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet bioprinting of biomaterials. Chem. Rev. 2020, 120, 10793–10833. [Google Scholar] [CrossRef]
- Bejoy, A.M.; Makkithaya, K.N.; Hunakunti, B.B.; Hegde, A.; Krishnamurthy, K.; Sarkar, A.; Lobo, C.F.; Keshav, D.V.S.; Dharshini, G.; Mascarenhas, S.; et al. An insight on advances and applications of 3d bioprinting: A review. Bioprinting 2021, 24, 00176. [Google Scholar] [CrossRef]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef]
- Ng, W.L.; Lee, J.M.; Yeong, W.Y.; Win Naing, M. Microvalvebased bioprinting-process, bio-inks and applications. Biomater. Sci. 2017, 5, 632–647. [Google Scholar] [CrossRef]
- Xu, H.; Casillas, J.; Xu, C. Effects of printing conditions on cell distribution within microspheres during inkjet-based bioprinting. AIP Adv. 2019, 9, 095055. [Google Scholar] [CrossRef]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater 2018, 3, 144–156. [Google Scholar] [CrossRef]
- Chameettachal, S.; Yeleswarapu, S.; Sasikumar, S.; Shukla, P.; Hibare, P.; Bera, A.K.; Bojedla, S.S.R.; Pati, F. 3D Bioprinting: Recent trends and challenges. J. Indian Inst. Sci. 2019, 99, 375–403. [Google Scholar] [CrossRef]
- Mobaraki, M.; Ghaffari, M.; Yazdanpanah, A.; Luo, Y.; Mills, D.K. Bioinks and bioprinting: A focused review. Bioprinting 2020, 18, e00080. [Google Scholar] [CrossRef]
- Tan, Y.-C.; Cristini, V.; Lee, A.P. Monodispersed microfluidic droplet generation by shear focusing microfluidic device. Sens. Actuators B Chem. 2006, 114, 350–356. [Google Scholar] [CrossRef]
- Motornov, M.; Minko, S.; Eichhorn, K.-J.; Nitschke, M.; Simon, F.; Stamm, M. Reversible tuning of wetting behaviour of polymer surface with responsive polymer brushes. Langmuir 2003, 19, 8077–8085. [Google Scholar] [CrossRef]
- Galliker, P.; Schneider, J.; Eghlidi, H.; Kress, S.; Sandoghdar, V.; Poulikakos, D. Direct printing of nanostructures by electrostatic autofocussing of ink nanodroplets. Nat. Commun. 2012, 3, 890. [Google Scholar] [CrossRef]
- Castrejon-Pita, J.R.; Baxter, W.R.S.; Morgan, J.; Temple, S.; Martin, G.D.; Hutchings, I.M. Future, opportunities and challenges of inkjet technologies. At. Sprays 2013, 23, 541–565. [Google Scholar] [CrossRef]
- Bormashenko, E. Wetting of Real Surfaces; De Gruyter: Berlin, Germany, 2013. [Google Scholar]
- Neumann, A.W.; Good, R.J. Thermodynamics of contact angles. I. Heterogeneous solid surfaces. J. Colloid Interface Sci. 1972, 38, 341–358. [Google Scholar] [CrossRef]
- Marmur, A. Soft contact: Measurement and interpretation of contact angles. Soft Matter 2006, 2, 12–17. [Google Scholar] [CrossRef]
- Calvert, P. Inkjet Printing for Materials and Devices. Chem. Mater. 2001, 13, 3299–3305. [Google Scholar] [CrossRef]
- Gili, E.; Caironi, M.; Sirringhaus, H. Picoliter printing. In Handbook of Nanofabrication; Wiederrecht, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 183–196. [Google Scholar]
- Park, E.S. Application of Inkjet-Printing Technology to Micro-Electro-Mechanical Systems; University of California: Berkeley, CA, USA, 2014. [Google Scholar]
- Modak, C.D.; Kumar, A.; Tripathy, A.; Sen, P. Drop impact printing. Nat. Commun. 2020, 11, 4327. [Google Scholar] [CrossRef]
- Starly, B.; Shirwaiker, R. 3D bioprinting techniques. In 3D Bioprinting Nanotechnology in Tissue Engineering Regenerative Medicine; Zhang, L.G., Fisher, J.P., Leong, K.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 57–77. [Google Scholar]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, Q.; Black, L.D. A fresh slate for 3D bioprinting. Science 2019, 365, 446. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Dou, C.; Xu, B.; Li, J.; Rao, Z.; Tsin, A. Printing quality improvement for laser-induced forward transfer bioprinting: Numerical modeling and experimental validation. Phys. Fluids 2021, 33, 071906. [Google Scholar] [CrossRef]
- Shin, P.; Sung, J.; Lee, M.H. Control of droplet formation for low viscosity fluid by double waveforms applied to a piezoelectric inkjet nozzle. Microelectron. Reliab. 2011, 51, 797–804. [Google Scholar] [CrossRef]
- Lorenceau, É.; Quéré, D. Drops impacting a sieve. J. Colloid Interface Sci. 2003, 263, 244–249. [Google Scholar] [CrossRef]
- Guo, Y.; Patanwala, H.S.; Bognet, B.; Ma, A.W.K. Inkjet and inkjet-based 3D printing: Connecting fluid properties and printing performance. Rapid Prototyp. J. 2017, 23, 562–576. [Google Scholar] [CrossRef]
- Gao, M.; Li, L.; Song, Y. Inkjet printing wearable electronic devices. J. Mater. Chem. C 2017, 5, 2971–2993. [Google Scholar] [CrossRef]
- Stadnytskyi, V.; Bax, C.E.; Bax, A.; Anfinrud, P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl. Acad. Sci. USA 2020, 117, 11875–11877. [Google Scholar] [CrossRef]
- Jayaweera, M.; Perera, H.; Gunawardana, B.; Manatunge, J. Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environ. Res. 2020, 188, 109819. [Google Scholar] [CrossRef]
- Melayil, K.R.; Mitra, S.K. Wetting, adhesion, and droplet impact on face masks. Langmuir 2021, 37, 2810–2815. [Google Scholar] [CrossRef]
- World Health Organization. Advice on the Use of Masks in the Context of COVID-19; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Katre, P.; Banerjee, S.; Balusamy, S.; Sahu, K.C. Fluid dynamics of respiratory droplets in the context of COVID-19: Airborne and surfaceborne transmissions. Phys. Fluids 2021, 33, 081302. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, R.; Hasan, A.B.M.T.; Khan, A.; Roy, D.; Salehin, M.; Wadud, Z. Exposure risk analysis of COVID-19 for a ride-sharing motorbike taxi. Phys. Fluids 2021, 33, 113319. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.; Agrawal, A. Likelihood of survival of coronavirus in a respiratory droplet deposited on a solid surface. Phys. Fluids 2020, 32, 061704. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Chatterjee, S.; Agrawal, A.; Bhardwaj, R. Evaluating a transparent coating on a face shield for repelling airborne respiratory droplets. Phys. Fluids 2021, 33, 111705. [Google Scholar] [CrossRef] [PubMed]
- Shafaghi, A.H.; Talabazar, F.R.; Kosar, A.; Ghorbani, M. On the effect of the respiratory droplet generation condition on COVID-19 transmission. Fluids 2020, 5, 113. [Google Scholar] [CrossRef]
- Mittal, R.; Ni, R.; Seo, J.-H. The flow physics of COVID-19. J. Fluid Mech. 2020, 894, F2. [Google Scholar] [CrossRef]
- Poon, W.C.K.; Brown, A.T.; Direito, S.O.L.; Hodgson, D.J.M.; Nagard, L.L.; Lips, A.; MacPhee, C.E.; Marenduzzo, D.; Royer, J.R.; Silva, A.F.; et al. Soft matter science and the COVID-19 pandemic. Soft Matter 2020, 16, 8310–8324. [Google Scholar] [CrossRef]
- Liao, M.; Liu, H.; Wang, X.; Hu, X.; Huang, Y.; Liu, X.; Brenan, K.; Mecha, J.; Nirmalan, M.; Lu, J.R. A technical review of face mask wearing in preventing respiratory COVID-19 transmission. Curr. Opin. Colloid Interface Sci. 2021, 52, 101417. [Google Scholar] [CrossRef]
- Meier, W.; Greune, G.; Meyboom, A.; Hofmann, K.P. Surface tension and viscosity of surfactant from the resonance of an oscillating drop. Eur. Biophys. J. 2000, 29, 113–124. [Google Scholar] [CrossRef]
- Wijshoff, H. The dynamics of the piezo inkjet printhead operation. Phys. Rep. 2010, 491, 77–177. [Google Scholar] [CrossRef]
- Bostwick, J.B.; Steen, P.H. Dynamics of sessile drops. Part 1. Inviscid theory. J. Fluid Mech. 2014, 760, 5–38. [Google Scholar] [CrossRef]
- Mohammad Karim, A. Parametric Study of Liquid Contact Line Dynamics: Adhesion vs. Hydrodynamics. Ph.D. Thesis, UCLA, Los Angeles, CA, USA, 2015. [Google Scholar]
- Mohammad Karim, A.; Fujii, K.; Kavehpour, H.P. Contact line dynamics of gravity driven spreading of liquids. Fluid Dyn. Res. 2021, 53, 035503. [Google Scholar] [CrossRef]
- Mohammad Karim, A.; Suszynski, W.J.; Pujari, S. Liquid Film Stability and Contact Line Dynamics of Emulsion Liquid Films in Curtain Coating Process. J. Coat. Technol. Res. 2021, 18, 1531–1541. [Google Scholar] [CrossRef]
- Abe, Y.; Zhang, B.; Gordillo, L.; Mohammad Karim, A.; Francis, L.F.; Cheng, X. Dynamic self-assembly of charged colloidal strings and walls in simple fluid flows. Soft Matter 2017, 13, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Almohammadi, H.; Amirfazli, A. Droplet impact: Viscosity and wettability effects on splashing. J. Colloid Interf. Sci. 2019, 553, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Kavehpour, H.P.; Mohammad Karim, A.; Davis, S.H. Inconsistencies in the experimental study of spontaneous spreading vs. forced spreading. In Proceedings of the Droplets 2015; University of Twente: Enschede, The Netherlands, 2015. [Google Scholar]
- Ebert, J.; Özkol, E.; Zeichner, A.; Uibel, K.B.; Weiss, Ö.; Koops, U.; Telle, R.; Fischer, H. Direct inkjet printing of dental prostheses made of zirconia. J. Dent. Res. 2009, 88, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Jonczyk, R.; Kurth, T.; Lavrentieva, A.; Walter, J.-G.; Scheper, T.; Stahl, F. Living cell microarrays: An overview of concepts. Microarrays 2016, 5, 11. [Google Scholar] [CrossRef]
- Liu, Y.; Derby, B. Experimental study of the parameters for stable drop-on-demand inkjet performance. Phys. Fluids 2019, 31, 032004. [Google Scholar] [CrossRef]
- Derby, B. Inkjet printing of functional and structural materials: Fluid property requirements, feature stability, and resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- Gong, Y.; Bi, Z.; Bian, X.; Ge, A.; He, J.; Li, W.; Shao, H.; Chen, G.; Zhang, X. Study on linear bio-structure print process based on alginate bio-ink in 3D bio-fabrication. Bio-Des. Manuf. 2020, 3, 109–121. [Google Scholar] [CrossRef]
- Libanori, A.; Chen, G.; Zhao, X.; Zhou, Y.; Chen, J. Smart textiles for personalized healthcare. Nat. Electron. 2022, 5, 142–156. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Campbell, A.; de Ávila, B.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Ates, H.; Nguyen, P.; Gonzalez-Macia, L.; Morales-Narvez, E.; Güder, F.; Collins, J.; Dincer, C. End-to-end design of wearable sensors. Nat. Rev. Mater. 2022, 7, 887–907. [Google Scholar] [CrossRef]
- Xu, C.; Yang, Y.; Gao, W. Skin-interfaced sensors in digital medicine: From materials to applications. Matter 2020, 2, 1414–1445. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Feldman, B.; Granger, S.; Gaitonde, S.; Begtrup, G.; Katchman, B. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 2019, 37, 407–419. [Google Scholar] [CrossRef]
- Someya, T.; Bao, Z.; Malliaras, G.G. The rise of plastic bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jeang, W.J.; Ghaffari, R.; Rogers, J.A. Wearable sensors for biochemical sweat analysis. Annu. Rev. Anal. Chem. 2019, 12, 1–22. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Min, J.; Song, Y.; Tu, J.; Mukasa, D.; Ye, C.; Xu, C.; Heflin, N.; McCune, J.; et al. Awearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 2022, 6, 1225–1235. [Google Scholar] [CrossRef]
- Shikhmurzaev, Y. The Moving Contact Line on a Smooth Solid Surface. Int. J. Multiph. Flow 1993, 19, 589–610. [Google Scholar] [CrossRef]
- Shikhmurzaev, Y.D. Mathematical Modeling of Wetting Hydrodynamics. Fluid Dyn. Res. 1994, 13, 45–64. [Google Scholar] [CrossRef]
- Shikhmurzaev, Y.D. Moving Contact Lines in Liquid/Liquid/Solid Systems. J. Fluid Mech. 1997, 334, 211–249. [Google Scholar] [CrossRef]
- Moghtadernejad, S.; Lee, C.; Jadidi, M. An introduction of droplet impact dynamics to engineering students. Fluids 2020, 5, 107. [Google Scholar] [CrossRef]
- Thoroddsen, S.T.; Etoh, T.G.; Takehara, K. High-speed imaging of drops and bubbles. Annu. Rev. Fluid Mech. 2008, 40, 257–285. [Google Scholar] [CrossRef]
- Worthington, A.M. On the forms assumed by drops of liquids falling vertically on a horizontal plate. Proc. R. Soc. 1876, 25, 261–272. [Google Scholar]
- Cheng, X.; Sun, T.-P.; Gordillo, L. Drop impact dynamics: Impact force and stress distributions. Annu. Rev. Fluid Mech. 2022, 54, 57–81. [Google Scholar] [CrossRef]
- Chen, L.; Bonaccurso, E.; Deng, P.; Zhang, H. Droplet impact on soft viscoelastic surfaces. Phys. Rev. E 2016, 94, 063117. [Google Scholar] [CrossRef]
- Saiki, Y.; Prestidge, C.A.; Horn, R.G. Effects of droplet deformability on emulsion rheology. Colloids Surf. A 2006, 299, 65. [Google Scholar] [CrossRef]
- Lester, G.R. Contact angles of liquids at deformable solid surfaces. J. Colloid Sci. 1961, 16, 315. [Google Scholar] [CrossRef]
- Blanken, N.; Saleem, M.S.; Thoraval, M.-J.; Antonini, C. Impact of compound drops: A perspective. Curr. Opin. Colloid Interface Sci. 2021, 51, 101389. [Google Scholar] [CrossRef]
- Lee, J.B.; dos Santos, S.; Antonini, C. Water touch-and-bounce from a soft viscoelastic substrate: Wetting, dewetting, and rebound on bitumen. Langmuir 2016, 32, 8245–8254. [Google Scholar] [CrossRef] [PubMed]
- Langley, K.R.; Castrejón-Pita, A.A.; Thoroddsen, S.T. Droplet impacts onto soft solids entrap more air. Soft Matter 2020, 16, 5702–5710. [Google Scholar] [CrossRef]
- Chen, S.; Bertola, V. Drop impact on spherical soft surfaces. Phys. Fluids 2017, 29, 082106. [Google Scholar] [CrossRef]
- Poulain, S.; Carlson, A. Droplet settling on solids coated with a soft layer. J. Fluid Mech. 2022, 934, A25. [Google Scholar] [CrossRef]
- Skotheim, J.M.; Mahadevan, L. Soft lubrication: The elastohydrodynamics of nonconforming and conforming contacts. Phys. Fluids 2005, 17, 092101. [Google Scholar] [CrossRef]
- Essink, M.H.; Pandey, A.; Karpitschka, S.; Venner, C.H.; Snoeijer, J.H. Regimes of soft lubrication. J. Fluid Mech. 2021, 915, A49. [Google Scholar] [CrossRef]
- Wang, Y.; Pilkington, G.A.; Dhong, C.; Frechette, J. Elastic deformation during dynamic force measurements in viscous fluids. Curr. Opin. Colloid Interface Sci. 2017, 27, 43–49. [Google Scholar] [CrossRef]
- Chan, T.S.; Carlson, A. Physics of adhesive organs in animals. Eur. Phys. J. Spec. Top. 2019, 227, 2501–2512. [Google Scholar] [CrossRef]
- Pepper, R.E.; Courbin, L.; Stone, H.A. Splashing on elastic membranes: The importance of early-time dynamics. Phys. Fluids 2008, 20, 082103. [Google Scholar] [CrossRef]
- Arai, K.; Iwanaga, S.; Toda, H.; Genci, C.; Nishiyama, Y.; Nakamura, M. Three-dimensional inkjet biofabrication based on designed images. Biofabrication 2011, 3, 34113. [Google Scholar] [CrossRef]
- Andreotti, B.; Snoeijer, J.H. Statics and dynamics of soft wetting. Annu. Rev. Fluid Mech. 2020, 52, 285–308. [Google Scholar] [CrossRef]
- Dervaux, J.; Roché, M.; Limat, L. Nonlinear theory of wetting on deformable substrates. Soft Matter 2020, 16, 5157–5176. [Google Scholar] [CrossRef] [PubMed]
- Rioboo, R.; Voue, M.; Adao, H.; Conti, J.; Vaillant, A.; Seveno, D.; De Coninck, J. Drop impact on soft surfaces: Beyond the static contact angles. Langmuir 2010, 26, 4873–4879. [Google Scholar] [CrossRef] [PubMed]
- Brochard-Wyart, F.; de Gennes, P. Dynamics of Partial Wetting. Adv. Colloid Interface Sci. 1992, 39, 1–11. [Google Scholar] [CrossRef]
- Alizadeh, A.; Bahadur, V.; Shang, W.; Zhu, Y.; Buckley, D.; Dhinojwala, A.; Sohal, M. Influence of substrate elasticity on droplet impact dynamics. Langmuir 2013, 29, 4520–4524. [Google Scholar] [CrossRef]
- Mangili, S.; Antonini, C.; Marengo, M.; Amirfazli, A. Understanding the drop impact phenomenon on soft PDMS substrates. Soft Matter 2012, 8, 10045. [Google Scholar] [CrossRef]
- Pericet-Camara, R.; Auernhammer, G.K.; Koynov, K.; Lorenzoni, S.; Raiteri, R.; Bonnacurso, E. Solid-supported thin elastomer films deformed by microdrops. Soft Matter 2009, 5, 3611–3617. [Google Scholar] [CrossRef]
- Kern, R.; Müller, P. Deformation of an elastic thin solid induced by a liquid droplet. Surf. Sci. 1992, 264, 467–494. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, Y. Elastic deformation of soft membrane with finite thickness induced by a sessile liquid droplet. J. Colloid Interface Sci. 2009, 339, 489–494. [Google Scholar] [CrossRef]
- Gerber, J.; Schutzius, T.M.; Poulikakos, D. Patterning of colloidal droplet deposits on soft materials. J. Fluid Mech. 2021, 907, A39. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, L.; Wang, W.; Kota, A.K.; Hu, H. An experimental study on soft PDMS materials for aircraft icing mitigation. Appl. Surf. Sci. 2018, 447, 599–609. [Google Scholar] [CrossRef]
- Dressaire, E.; Sauret, A.; Boulogne, F.; Stone, H.A. Drop impact on a flexible fiber. Soft Matter 2016, 12, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhou, Q.; Chen, X.; Xu, T.; Hui, S.; Zhang, D. Liquid drop impact on solid surface with application to water drop erosion on turbine blades, Part I: Nonlinear wave model and solution of one-dimensional impact. Int. J. Mech. Sci. 2008, 50, 1526–1542. [Google Scholar] [CrossRef]
- Adler, W.F. Waterdrop impact modeling. Wear 1995, 186–187, 341–351. [Google Scholar] [CrossRef]
- Bico, J.; Reyssat, É.; Roman, B. Elastocapillarity: When surface tension deforms elastic solids. Annu. Rev. Fluid Mech. 2018, 50, 629–659. [Google Scholar] [CrossRef]
- de Goede, T.C.; Moqaddam, A.M.; Limpens, K.C.M.; Kooij, S.A.; Derome, D.; Carmeliet, J.; Shahidzadeh, N.; Bonn, D. Droplet impact of Newtonian fluids and blood on simple fabrics: Effect of fabric pore size and underlying substrate. Phys. Fluids 2021, 33, 033308. [Google Scholar] [CrossRef]
- Banitabaei, S.A.; Amirfazli, A. Droplet impact onto a solid sphere: Effect of wettability and impact velocity. Phys. Fluids 2017, 29, 062111. [Google Scholar] [CrossRef]
- Faulkner-Jones, A.; Greenhough, S.; A King, J.; Gardner, J.; Courtney, A.; Shu, W. Development of a valve-based cell printer for the formation of human embryonic stem cell spheroid aggregates. Biofabrication 2013, 5, 15013. [Google Scholar] [CrossRef]
- Soto, D.; De Larivière, A.B.; Boutillon, X.; Clanet, C.; Quéré, D. The force of impacting rain. Soft Matter 2014, 10, 4929. [Google Scholar] [CrossRef]
- Gart, S.; Mates, J.E.; Megaridis, C.M.; Jung, S. Droplet impacting a cantilever: A leaf–raindrop system. Phys. Rev. Appl. 2015, 3, 044019. [Google Scholar] [CrossRef]
- Dong, X.; Huang, X.; Liu, J. Modeling and simulation of droplet impact on elastic beams based on SPH. Eur. J. Mech. A Solids 2019, 75, 237–257. [Google Scholar] [CrossRef]
- Huang, X.; Dong, X.; Li, J.; Liu, J. Droplet impact induced large deflection of a cantilever. Phys. Fluids 2019, 31, 062106. [Google Scholar] [CrossRef]
- Ray, T.; Choi, J.; Bandodkar, A.; Krishnan, S.; Gutruf, P.; Tian, L.; Ghaffari, R.; Rogers, J. Bio-integrated wearable systems: A comprehensive review. Chem. Rev. 2019, 119, 5461–5533. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Song, J.; Lu, Y.; Huang, S.; Liu, X.; Sun, J.; Carmalt, C.J.; Parkin, I.P.; Xu, W. Creating robust superamphiphobic coatings for both hard and soft materials. J. Mater. Chem. A. 2015, 3, 20999–21008. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, W. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 2019, 48, 1465–1491. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Bandodkar, A.J.; Vald´ es-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef]
- Jia, W.; Vald´ es-Ramírez, G.; Bandodkar, A.J.; Windmiller, J.R.; Wang, J. Epidermal Biofuel Cells: Energy Harvesting from Human Perspiration. Angew. Chem. Int. Ed. 2013, 52, 7233–7236. [Google Scholar] [CrossRef]
- Lv, J.; Jeerapan, I.; Tehrani, F.; Yin, L.; Silva-Lopez, C.A.; Jang, J.H.; Joshuia, D.; Shah, R.; Liang, Y.; Xie, L.; et al. Sweat-based wearable energy harvesting-storage hybrid textile devices. Energy Environ. Sci. 2018, 11, 3431–3442. [Google Scholar] [CrossRef]
- Yang, Y.; Song, Y.; Bo, X.; Min, J.; Pak, O.S.; Zhu, L.; Wang, M.; Tu, J.; Kogan, A.; Zhang, H.; et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 2020, 38, 217–224. [Google Scholar] [CrossRef]
- Pal, A.; Goswami, D.; Cuellar, H.E.; Castro, B.; Kuang, S.; Martinez, R.V. Early detection and monitoring of chronic wounds using low-cost, omniphobic paper-based smart bandages. Biosens. Bioelectron. 2018, 117, 696–705. [Google Scholar] [CrossRef]
- Gao, W.; Nyein, H.Y.Y.; Shahpar, Z.; Fahad, H.M.; Chen, K.; Emaminejad, S.; Gao, Y.; Tai, L.C.; Ota, H.; Wu, E.; et al. Wearable microsensor array for multiplexed heavy metal monitoring of body fluids. ACS Sens. 2016, 1, 866–874. [Google Scholar] [CrossRef]
- Yu, Y.; Nyein, H.Y.Y.; Gao, W.; Javey, A. Flexible electrochemical bioelectronics: The rise of in situ bioanalysis. Adv. Mater. 2020, 32, 1902083. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Nassar, J.; Xu, C.; Min, J.; Yang, Y.; Dai, A.; Doshi, R.; Huang, A.; Song, Y.; Gehlhar, R.; et al. Biofuel-powered soft electronic skin with multiplexed and wireless sensing for human-machine interfaces. Sci. Robot. 2020, 5, eaaz7946. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.P.; Ratterman, M.; Griffin, D.K.; Hou, L.; Kelley-Loughnane, N.; Naik, R.K.; Hagen, J.A.; Papautsky, I.; Heikenfeld, J. System-level design of an RFID sweat electrolyte sensor patch. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBC 2014, Chicago, IL, USA, 26–30 August 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 4038–4041. [Google Scholar]
- Rose, D.P.; Ratterman, M.E.; Griffin, D.K.; Hou, L.; Kelley-Loughnane, N.; Naik, R.R.; Hagen, J.A.; Papautsky, I.; Heikenfeld, J.C. Adhesive RFID sensor patch for monitoring of sweat electrolytes. IEEE Trans. Biomed. Eng. 2015, 62, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Gutruf, P.; Choi, J.; Lee, K.H.; Sekine, Y.; Reeder, J.T.; Jeang, W.J.; Aranyosi, A.J.; Lee, S.P.; Model, J.B.; et al. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 2019, 5, eaav3294. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Hung, V.W.S.; Jia, W.; Vald´ es-Ramírez, G.; Windmiller, J.R.; Martinez, A.G.; Ramírez, J.; Chan, G.; Kerman, K.; Wang, J. Tattoo-based potentiometric ion-selective sensors for epidermal pH monitoring. Analyst 2013, 138, 123–128. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jia, W.; Yardimci, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-based noninvasive glucose monitoring: A proof-of-concept study. Anal. Chem. 2015, 87, 394–398. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; O’Mahony, A.M.; Ramírez, J.; Samek, I.A.; Anderson, S.M.; Windmiller, J.R.; Wang, J. Solid-state forensic finger sensor for integrated sampling and detection of gunshot residue and explosives: Towards Lab-on-a-finger. Analyst 2013, 138, 5288–5295. [Google Scholar] [CrossRef]
- Kagie, A.; Bishop, D.K.; Burdick, J.; La Belle, J.T.; Dymond, R.; Felder, R.; Wanga, J. Flexible rolled thick-film miniaturized flow-cell for minimally invasive amperometric sensing. Electroanalysis 2008, 20, 1610–1614. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Brazaca, L.C.; García-Carmona, L.; Bolat, G.; Campbell, A.S.; Martin, A.; Tang, G.; Shah, R.; Mishra, R.K.; Kim, J.; et al. Eyeglasses-based tear biosensing system: Non-invasive detection of alcohol, vitamins and glucose. Biosens. Bioelectron. 2019, 137, 161–170. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Itthipon, I.; Krishnan, S.; Wang, J. Wearable chemical sensors: Emerging systems for on-body analytical chemistry. Anal. Chem. 2019, 92, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Sempionatto, J.R.; Khorshed, A.A.; Ahmed, A.; De Loyola E Silva, A.N.; Barfidokht, A.; Yin, L.; Goud, K.Y.; Mohamed, M.A.; Bailey, E.; May, J.; et al. Epidermal enzymatic biosensors for sweat vitamin C: Toward personalized nutrition. ACS Sens. 2020, 5, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; De Araujo, W.R.; Samek, I.A.; Bandodkar, A.J.; Jia, W.; Brunetti, B.; Paixão, T.R.L.C.; Wang, J. Wearable temporary tattoo sensor for real-time trace metal monitoring in human sweat. Electrochem. Commun. 2015, 51, 41–45. [Google Scholar] [CrossRef]
- Kim, J.; Imani, S.; de Araujo, W.R.; Warchall, J.; Valdés-Ramírez, G.; Paixão, T.R.L.C.; Mercier, P.P.; Wang, J. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef]
- García-Carmona, L.; Martín, A.; Sempionatto, J.R.; Moreto, J.R.; González, M.C.; Wang, J.; Escarpa, A. Pacifier biosensor: Toward noninvasive saliva biomarker monitoring. Anal. Chem. 2019, 91, 13883–13891. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Mishra, R.K.; Martín, A.; Tang, G.; Nakagawa, T.; Lu, X.; Campbell, A.S.; Lyu, K.M.; Wang, J. Wearable ring-based sensing platform for detecting chemical threats. ACS Sens. 2017, 2, 1531–1538. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Nakagawa, T.; Pavinatto, A.; Mensah, S.T.; Imani, S.; Mercier, P.; Wang, J. Eyeglasses based wireless electrolyte and metabolite sensor platform. Lab Chip 2017, 17, 1834–1842. [Google Scholar] [CrossRef]
- Ciui, B.; Martin, A.; Mishra, R.K.; Brunetti, B.; Nakagawa, T.; Dawkins, T.J.; Lyu, M.; Cristea, C.; Sandulescu, R.; Wang, J. Wearable wireless tyrosinase bandage and microneedle sensors: Toward melanoma screening. Adv. Healthc. Mater. 2018, 7, 1701264. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).