miR-125 in Breast Cancer Etiopathogenesis: An Emerging Role as a Biomarker in Differential Diagnosis, Regenerative Medicine, and the Challenges of Personalized Medicine
Abstract
:1. Introduction
2. Clinical Features of BC
2.1. Main Risk Factors of BC
2.2. BC Characterization and Patient Management
3. Epigenetics of BC and the Role of miR-125
3.1. microRNA Nomenclature
3.2. Role of miR in BC
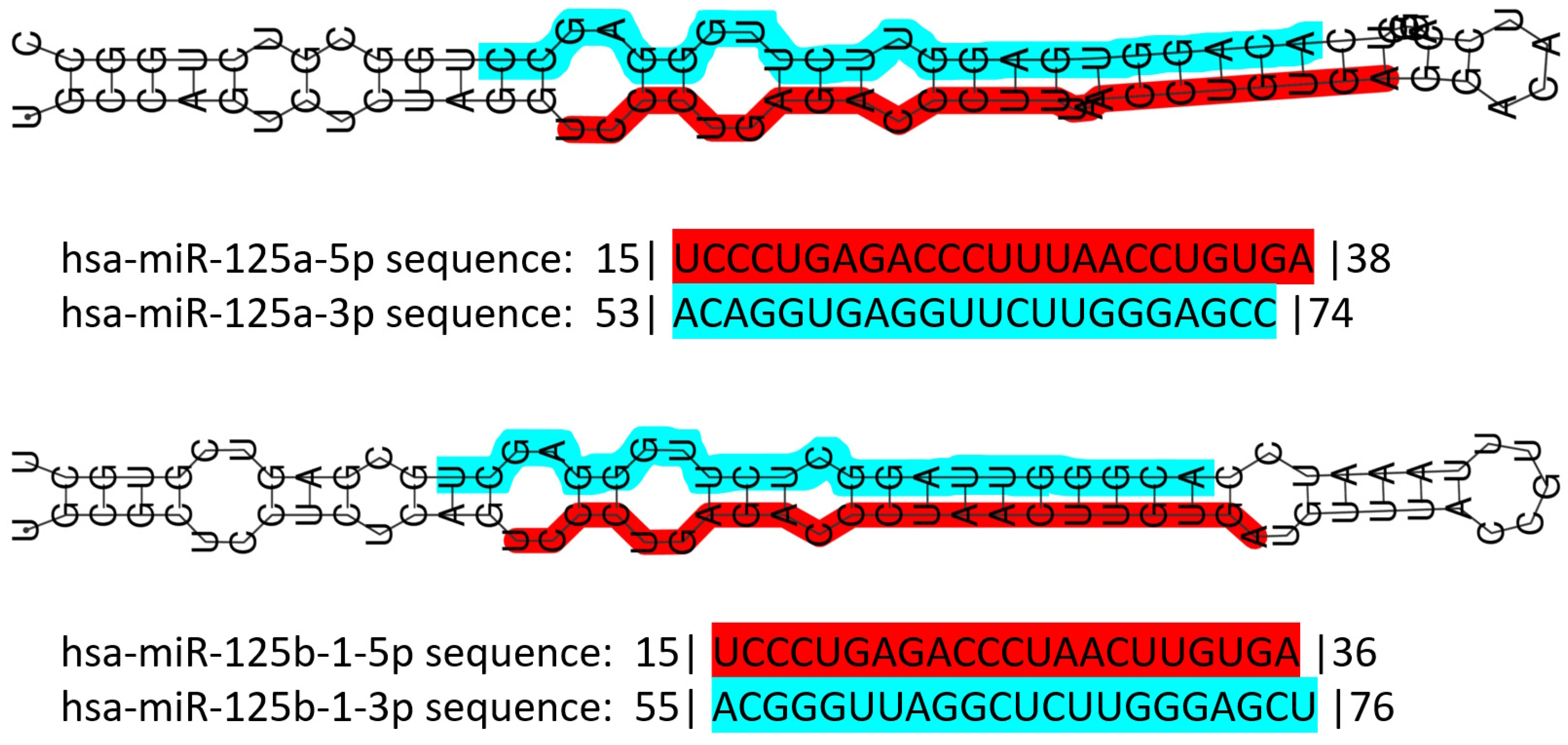
3.3. The miR-125 Family: Molecular Organization and Roles in Human Pathology
3.4. miR-125 and Cancer
3.5. Role of miR-125 in BC
3.6. Further Mining miR-125 Function in BC: Competing Endogenous RNA Networks (ceRNET)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Winters, S.; Martin, C.; Murphy, D.; Shokar, N.K. Breast Cancer Epidemiology, Prevention, and Screening. Prog. Mol. Biol. Transl. Sci. 2017, 151, 1–32. [Google Scholar] [PubMed]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R. Global, Regional, National Burden of Breast Cancer in 185 Countries: Evidence from GLOBOCAN 2018. Breast Cancer Res. Treat. 2021, 187, 557–567. [Google Scholar] [CrossRef]
- Jung, S.; Wang, M.; Anderson, K.; Baglietto, L.; Bergkvist, L.; Bernstein, L.; van den Brandt, P.A.; Brinton, L.; Buring, J.E.; Heather Eliassen, A.; et al. Alcohol Consumption and Breast Cancer Risk by Estrogen Receptor Status: In a Pooled Analysis of 20 Studies. Int. J. Epidemiol. 2016, 45, 916–928. [Google Scholar] [CrossRef]
- Key, T. Sex Hormones and Risk of Breast Cancer in Premenopausal Women: A Collaborative Reanalysis of Individual Participant Data from Seven Prospective Studies. Lancet Oncol. 2013, 14, 1009–1019. [Google Scholar] [CrossRef]
- Coughlin, S.S. Epidemiology of Breast Cancer in Women. Adv. Exp. Med. Biol. 2019, 1152, 9–29. [Google Scholar] [PubMed]
- Galati, F.; Magri, V.; Arias-Cadena, P.A.; Moffa, G.; Rizzo, V.; Pasculli, M.; Botticelli, A.; Pediconi, F. Pregnancy-Associated Breast Cancer: A Diagnostic and Therapeutic Challenge. Diagnostics 2023, 13, 604. [Google Scholar] [CrossRef]
- Bodewes, F.T.H.; van Asselt, A.A.; Dorrius, M.D.; Greuter, M.J.W.; de Bock, G.H. Mammographic Breast Density and the Risk of Breast Cancer: A Systematic Review and Meta-Analysis. Breast 2022, 66, 62–68. [Google Scholar] [CrossRef]
- Majeed, W.; Aslam, B.; Javed, I.; Khaliq, T.; Muhammad, F.; Ali, A.; Raza, A. Breast Cancer: Major Risk Factors and Recent Developments in Treatment. Asian Pac. J. Cancer Prev. 2014, 15, 3353–3358. [Google Scholar] [CrossRef]
- Petrucelli, N.; Daly, M.B.; Pal, T. BRCA1- and BRCA2-Associated Hereditary Breast and Ovarian Cancer. 4 September 1998 [Updated 21 September 2023]. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993–2024; Available online: https://www.ncbi.nlm.nih.gov/books/NBK1247/ (accessed on 15 October 2023).
- Carbognin, L.; Miglietta, F.; Paris, I.; Dieci, M.V. Prognostic and Predictive Implications of PTEN in Breast Cancer: Unfulfilled Promises but Intriguing Perspectives. Cancers 2019, 11, 1401. [Google Scholar] [CrossRef] [PubMed]
- Shahbandi, A.; Nguyen, H.D.; Jackson, J.G. TP53 Mutations and Outcomes in Breast Cancer: Reading beyond the Headlines. Trends Cancer 2020, 6, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Veronesi, P.; Sacchini, V.; Galimberti, V. Prognosis and Outcome in CDH1-Mutant Lobular Breast Cancer. Eur. J. Cancer Prev. 2018, 27, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Beggs, A.D.; Latchford, A.R.; Vasen, H.F.A.; Moslein, G.; Alonso, A.; Aretz, S.; Bertario, L.; Blanco, I.; Bülow, S.; Burn, J.; et al. Peutz–Jeghers Syndrome: A Systematic Review and Recommendations for Management. Gut 2010, 59, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Apostolou, P.; Papasotiriou, I. Current Perspectives on CHEK2 Mutations in Breast Cancer. Breast Cancer Targets Ther. 2017, 9, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Nepomuceno, T.C.; Carvalho, M.A.; Rodrigue, A.; Simard, J.; Masson, J.Y.; Monteiro, A.N.A. PALB2 Variants: Protein Domains and Cancer Susceptibility. Trends Cancer 2021, 7, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Stucci, L.S.; Internò, V.; Tucci, M.; Perrone, M.; Mannavola, F.; Palmirotta, R.; Porta, C. The ATM Gene in Breast Cancer: Its Relevance in Clinical Practice. Genes 2021, 12, 727. [Google Scholar] [CrossRef]
- Li, N.; McInerny, S.; Zethoven, M.; Cheasley, D.; Lim, B.W.X.; Rowley, S.M.; Devereux, L.; Grewal, N.; Ahmadloo, S.; Byrne, D.; et al. Combined Tumor Sequencing and Case-Control Analyses of RAD51C in Breast Cancer. J. Natl. Cancer Inst. 2019, 111, 1332–1338. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Ouyang, T.; Li, J.; Wang, T.; Fan, Z.; Fan, T.; Lin, B.; Xie, Y. Associations between RAD51D Germline Mutations and Breast Cancer Risk and Survival in BRCA1/2-Negative Breast Cancers. Ann. Oncol. 2018, 29, 2046–2051. [Google Scholar] [CrossRef]
- Śniadecki, M.; Brzeziński, M.; Darecka, K.; Klasa-Mazurkiewicz, D.; Poniewierza, P.; Krzeszowiec, M.; Kmieć, N.; Wydra, D. BARD1 and Breast Cancer: The Possibility of Creating Screening Tests and New Preventive and Therapeutic Pathways for Predisposed Women. Genes 2020, 11, 1251. [Google Scholar] [CrossRef]
- Suarez-Kelly, L.P.; Yu, L.; Kline, D.; Schneider, E.B.; Agnese, D.M.; Carson, W.E. Increased Breast Cancer Risk in Women with Neurofibromatosis Type 1: A Meta-Analysis and Systematic Review of the Literature. Hered. Cancer Clin. Pract. 2019, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.; Khan, M.S. Prognostic Value Estimation of BRIP1 in Breast Cancer by Exploiting Transcriptomics Data Through Bioinformatics Approaches. Bioinform. Biol. Insights 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Beeghly-Fadiel, A.; Long, J.; Zheng, W. Genetic Variants Associated with Breast-Cancer Risk: Comprehensive Research Synopsis, Meta-Analysis, and Epidemiological Evidence. Lancet Oncol. 2011, 12, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Shiovitz, S.; Korde, L.A. Genetics of Breast Cancer: A Topic in Evolution. Ann. Oncol. 2015, 26, 1291. [Google Scholar] [CrossRef]
- Ciriello, G.; Sinha, R.; Hoadley, K.A.; Jacobsen, A.S.; Reva, B.; Perou, C.M.; Sander, C.; Schultz, N. The Molecular Diversity of Luminal A Breast Tumors. Breast Cancer Res. Treat. 2013, 141, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Cornen, S.; Guille, A.; Adélaïde, J.; Addou-Klouche, L.; Finetti, P.; Saade, M.R.; Manai, M.; Carbuccia, N.; Bekhouche, I.; Letessier, A.; et al. Candidate Luminal B Breast Cancer Genes Identified by Genome, Gene Expression and DNA Methylation Profiling. PLoS ONE 2014, 9, 81843. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Brady, A.F.; Frayling, I.M.; Hanson, H.; Tischkowitz, M.; Turnbull, C.; Side, L. Clinical Guidelines: Consensus for Genes to Be Included on Cancer Panel Tests Offered by UK Genetics Services: Guidelines of the UK Cancer Genetics Group. J. Med. Genet. 2018, 55, 1–6. [Google Scholar] [CrossRef]
- Niell, B.L.; Freer, P.E.; Weinfurtner, R.J.; Arleo, E.K.; Drukteinis, J.S. Screening for Breast Cancer. Radiol. Clin. N. Am. 2017, 55, 1145–1162. [Google Scholar] [CrossRef]
- Andreea, G.I.; Pegza, R.; Lascu, L.; Bondari, S.; Stoica, Z.; Bondari, A. The Role of Imaging Techniques in Diagnosis of Breast Cancer. 2012. Available online: https://www.semanticscholar.org/paper/The-Role-of-Imaging-Techniques-in-Diagnosis-of-Andreea-Pegza/956784e90c8472b7d877e661201c4881034cc013 (accessed on 3 September 2023).
- Albert, U.S.; Altland, H.; Duda, V.; Engel, J.; Geraedts, M.; Heywang-Köbrunner, S.; Hölzel, D.; Kalbheim, E.; Koller, M.; König, K.; et al. 2008 Update of the Guideline: Early Detection of Breast Cancer in Germany. J. Cancer Res. Clin. Oncol. 2009, 135, 339–354. [Google Scholar] [CrossRef]
- Lima, Z.S.; Ebadi, M.R.; Amjad, G.; Younesi, L. Application of Imaging Technologies in Breast Cancer Detection: A Review Article. Open Access Maced. J. Med. Sci. 2019, 7, 838–848. [Google Scholar] [CrossRef]
- Gerami, R.; Joni, S.S.; Akhondi, N.; Etemadi, A.; Fosouli, M.; Eghbal, A.F.; Souri, Z. A Literature Review on the Imaging Methods for Breast Cancer. Int. J. Physiol. Pathophysiol. Pharmacol. 2022, 14, 171–176. [Google Scholar]
- Zeng, Z.; Amin, A.; Roy, A.; Pulliam, N.E.; Karavites, L.C.; Espino, S.; Helenowski, I.; Li, X.; Luo, Y.; Khan, S.A. Preoperative Magnetic Resonance Imaging Use and Oncologic Outcomes in Premenopausal Breast Cancer Patients. NPJ Breast Cancer 2020, 6, 49. [Google Scholar] [CrossRef]
- El Bairi, K.; Haynes, H.R.; Blackley, E.; Fineberg, S.; Shear, J.; Turner, S.; de Freitas, J.R.; Sur, D.; Amendola, L.C.; Gharib, M.; et al. The Tale of TILs in Breast Cancer: A Report from The International Immuno-Oncology Biomarker Working Group. NPJ Breast Cancer 2021, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Chong, A.; Weinstein, S.P.; McDonald, E.S.; Conant, E.F. Digital Breast Tomosynthesis: Concepts and Clinical Practice. Radiology 2019, 292, 1–14. [Google Scholar] [CrossRef]
- Dromain, C.; Balleyguier, C. Contrast-Enhanced Digital Mammography. In Digital Mammography; Springer: Berlin/Heidelberg, Germany, 2010; pp. 187–198. [Google Scholar] [CrossRef]
- Heywang-Köbrunner, S.H.; Hacker, A.; Sedlacek, S. Advantages and Disadvantages of Mammography Screening. Breast Care 2011, 6, 199–207. [Google Scholar] [CrossRef]
- Grigoryants, N.F.; Sass, S.; Alexander, J. Novel Technologies in Breast Imaging: A Scoping Review. Cureus 2023, 15, e44061. [Google Scholar] [CrossRef]
- Abdul Halim, A.A.; Andrew, A.M.; Mohd Yasin, M.N.; Abd Rahman, M.A.; Jusoh, M.; Veeraperumal, V.; Rahim, H.A.; Illahi, U.; Abdul Karim, M.K.; Scavino, E. Existing and Emerging Breast Cancer Detection Technologies and Its Challenges: A Review. Appl. Sci. 2021, 11, 10753. [Google Scholar] [CrossRef]
- Iranmakani, S.; Mortezazadeh, T.; Sajadian, F.; Ghaziani, M.F.; Ghafari, A.; Khezerloo, D.; Musa, A.E. A Review of Various Modalities in Breast Imaging: Technical Aspects and Clinical Outcomes. Egypt. J. Radiol. Nucl. Med. 2020, 51, 57. [Google Scholar] [CrossRef]
- Veronesi, U.; Viale, G.; Rotmensz, N.; Goldhirsch, A. Rethinking TNM: Breast Cancer TNM Classification for Treatment Decision-Making and Research. Breast 2006, 15, 3–8. [Google Scholar] [CrossRef]
- Eliyatkin, N.; Yalcin, E.; Zengel, B.; Aktaş, S.; Vardar, E. Molecular Classification of Breast Carcinoma: From Traditional, Old-Fashioned Way to A New Age, and A New Way. J. Breast Health 2015, 11, 59–66. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørile, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Ress, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular Portraits of Human Breast Tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Sinn, H.P.; Kreipe, H. A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition. Breast Care 2013, 8, 149–154. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Connolly, J.L.; Edge, S.B.; Mittendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Hortobagyi, G.N. Breast Cancer-Major Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J. Clin. 2017, 67, 290–303. [Google Scholar] [CrossRef]
- Fisusi, F.A.; Akala, E.O. Drug Combinations in Breast Cancer Therapy. Pharm. Nanotechnol. 2019, 7, 3–23. [Google Scholar] [CrossRef]
- Burstein, H.J.; Curigliano, G.; Thürlimann, B.; Weber, W.P.; Poortmans, P.; Regan, M.M.; Senn, H.J.; Winer, E.P.; Gnant, M.; Aebi, S.; et al. Customizing Local and Systemic Therapies for Women with Early Breast Cancer: The St. Gallen International Consensus Guidelines for Treatment of Early Breast Cancer 2021. Ann. Oncol. 2021, 32, 1216–1235. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dai, A.; Tran, R.; Wang, J. Identifying MiRNA Biomarkers for Breast Cancer and Ovarian Cancer: A Text Mining Perspective. Breast Cancer Res. Treat. 2023, 201, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H.; Gao, F. Identification of MiRNA Biomarkers for Breast Cancer by Combining Ensemble Regularized Multinomial Logistic Regression and Cox Regression. BMC Bioinform. 2022, 23, 434. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.G.; Davies, M.; Lowery, A.J.; Miller, N.; Kerin, M.J. The Role of MicroRNA as Clinical Biomarkers for Breast Cancer Surgery and Treatment. Int. J. Mol. Sci. 2021, 22, 8290. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Kim, Y.S.; Kang, K.N.; Kim, K.H.; Park, Y.J.; Kim, C.W. Multiple MicroRNAs as Biomarkers for Early Breast Cancer Diagnosis. Mol. Clin. Oncol. 2021, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Palacios, A.; Rojas Carvajal, A.M.; Núñez-Negrillo, A.M.; Cortés-Martín, J.; Sánchez-García, J.C.; Aguilar-Cordero, M.J. MicroRNA Dysregulation in Early Breast Cancer Diagnosis: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 8270. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, Z.; Talkhabi, M.; Taleahmad, S. Identification of Potential MicroRNA Diagnostic Panels and Uncovering Regulatory Mechanisms in Breast Cancer Pathogenesis. Sci. Rep. 2022, 12, 20135. [Google Scholar] [CrossRef]
- Khadka, V.S.; Nasu, M.; Deng, Y.; Jijiwa, M. Circulating MicroRNA Biomarker for Detecting Breast Cancer in High-Risk Benign Breast Tumors. Int. J. Mol. Sci. 2023, 24, 7553. [Google Scholar] [CrossRef]
- Nguyen, T.H.N.; Nguyen, T.T.N.; Nguyen, T.T.M.; Nguyen, L.H.M.; Huynh, L.H.; Phan, H.N.; Nguyen, H.T. Panels of Circulating MicroRNAs as Potential Diagnostic Biomarkers for Breast Cancer: A Systematic Review and Meta-Analysis. Breast Cancer Res. Treat. 2022, 196, 1–15. [Google Scholar] [CrossRef]
- Huynh, K.Q.; Le, A.T.; Phan, T.T.; Ho, T.T.; Pho, S.P.; Nguyen, H.T.; Le, B.T.; Nguyen, T.T.; Nguyen, S.T. The Diagnostic Power of Circulating MiR-1246 in Screening Cancer: An Updated Meta-Analysis. Oxid. Med. Cell. Longev. 2023, 2023, 8379231. [Google Scholar] [CrossRef]
- Tiberio, P.; Gaudio, M.; Belloni, S.; Pindilli, S.; Benvenuti, C.; Jacobs, F.; Saltalamacchia, G.; Zambelli, A.; Santoro, A.; De Sanctis, R. Unlocking the Potential of Circulating MiRNAs in the Breast Cancer Neoadjuvant Setting: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 3424. [Google Scholar] [CrossRef]
- Naeli, P.; Winter, T.; Hackett, A.P.; Alboushi, L.; Jafarnejad, S.M. The Intricate Balance between MicroRNA-Induced MRNA Decay and Translational Repression. FEBS J. 2023, 290, 2508–2524. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.C.; Farh, K.K.H.; Burge, C.B.; Bartel, D.P. Most Mammalian MRNAs Are Conserved Targets of MicroRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V.; Bartel, B.; Bartel, D.P.; Burge, C.B.; Carrington, J.C.; Chen, X.; Dreyfuss, G.; Eddy, S.R.; Griffiths-Jones, S.; Marshall, M.; et al. A Uniform System for MicroRNA Annotation. RNA 2003, 9, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S.; Grocock, R.J.; van Dongen, S.; Bateman, A.; Enright, A.J. MiRBase: MicroRNA Sequences, Targets and Gene Nomenclature. Nucleic Acids Res. 2006, 34, D140–D144. [Google Scholar] [CrossRef] [PubMed]
- Dziechciowska, I.; Dąbrowska, M.; Mizielska, A.; Pyra, N.; Lisiak, N.; Kopczyński, P.; Jankowska-Wajda, M.; Rubiś, B. MiRNA Expression Profiling in Human Breast Cancer Diagnostics and Therapy. Curr. Issues Mol. Biol. 2023, 45, 9500–9525. [Google Scholar] [CrossRef] [PubMed]
- Loh, H.Y.; Norman, B.P.; Lai, K.S.; Rahman, N.M.A.N.A.; Alitheen, N.B.M.; Osman, M.A. The Regulatory Role of MicroRNAs in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 4940. [Google Scholar] [CrossRef] [PubMed]
- Najjary, S.; Mohammadzadeh, R.; Mokhtarzadeh, A.; Mohammadi, A.; Kojabad, A.B.; Baradaran, B. Role of MiR-21 as an Authentic Oncogene in Mediating Drug Resistance in Breast Cancer. Gene 2020, 738, 144453. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Lu, B.B.; Wang, H.; Cheng, Z.X.; Yin, Y.M. MicroRNA-21 Modulates Chemosensitivity of Breast Cancer Cells to Doxorubicin by Targeting PTEN. Arch. Med. Res. 2011, 42, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tan, Z.; Hu, H.; Liu, H.; Wu, T.; Zheng, C.; Wang, X.; Luo, Z.; Wang, J.; Liu, S.; et al. MicroRNA-21 Promotes Breast Cancer Proliferation and Metastasis by Targeting LZTFL1. BMC Cancer 2019, 19, 738. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ye, P.; Long, X. Differential Expression Profiles of the Transcriptome in Breast Cancer Cell Lines Revealed by Next Generation Sequencing. Cell. Physiol. Biochem. 2017, 44, 804–816. [Google Scholar] [CrossRef]
- Mohmmed, E.A.; Shousha, W.G.; EL-Saiid, A.S.; Ramadan, S.S. A Clinical Evaluation of Circulating MiR-106a and Raf-1 as Breast Cancer Diagnostic and Prognostic Markers. Asian Pac. J. Cancer Prev. 2021, 22, 3513–3520. [Google Scholar] [CrossRef]
- You, F.; Luan, H.; Sun, D.; Cui, T.; Ding, P.; Tang, H.; Sun, D. MiRNA-106a Promotes Breast Cancer Cell Proliferation, Clonogenicity, Migration, and Invasion Through Inhibiting Apoptosis and Chemosensitivity. DNA Cell Biol. 2019, 38, 198–207. [Google Scholar] [CrossRef]
- You, F.; Li, J.; Zhang, P.; Zhang, H.; Cao, X. MiR106a Promotes the Growth of Transplanted Breast Cancer and Decreases the Sensitivity of Transplanted Tumors to Cisplatin. Cancer Manag. Res. 2020, 12, 233–246. [Google Scholar] [CrossRef]
- Dinami, R.; Ercolani, C.; Petti, E.; Piazza, S.; Ciani, Y.; Sestito, R.; Sacconi, A.; Biagioni, F.; Le Sage, C.; Agami, R.; et al. MiR-155 Drives Telomere Fragility in Human Breast Cancer by Targeting TRF1. Cancer Res. 2014, 74, 4145–4156. [Google Scholar] [CrossRef]
- Roth, C.; Rack, B.; Müller, V.; Janni, W.; Pantel, K.; Schwarzenbach, H. Circulating MicroRNAs as Blood-Based Markers for Patients with Primary and Metastatic Breast Cancer. Breast Cancer Res. 2010, 12, R90. [Google Scholar] [CrossRef]
- Li, P.; Xu, T.; Zhou, X.; Liao, L.; Pang, G.; Luo, W.; Han, L.; Zhang, J.; Luo, X.; Xie, X.; et al. Downregulation of MiRNA-141 in Breast Cancer Cells Is Associated with Cell Migration and Invasion: Involvement of ANP32E Targeting. Cancer Med. 2017, 6, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Ye, L.; Zhenyu, H.; Li, F.; Xiong, Y.; Lin, C.; Wu, X.; Deng, G.; Shi, W.; Song, L.; et al. ANP32E Induces Tumorigenesis of Triple-Negative Breast Cancer Cells by Upregulating E2F1. Mol. Oncol. 2018, 12, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Mitwally, N.; Soliman, A.S.; Yousef, E. Potential Diagnostic and Prognostic Utility of MiR-141, MiR-181b1, and MiR-23b in Breast Cancer. Int. J. Mol. Sci. 2020, 21, 8589. [Google Scholar] [CrossRef]
- Li, X.X.; Gao, S.Y.; Wang, P.Y.; Zhou, X.; Li, Y.J.; Yu, Y.; Yan, Y.F.; Zhang, H.H.; Lv, C.J.; Zhou, H.H.; et al. Reduced Expression Levels of Let-7c in Human Breast Cancer Patients. Oncol. Lett. 2015, 9, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Mao, X.; Wang, Y.; Ding, X.; Li, Y. Let-7c-5p Inhibits Cell Proliferation and Induces Cell Apoptosis by Targeting ERCC6 in Breast Cancer. Oncol. Rep. 2017, 38, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Swellam, M.; Mahmoud, M.S.; Hashim, M.; Hassan, N.M.; Sobeih, M.E.; Nageeb, A.M. Clinical Aspects of Circulating MiRNA-335 in Breast Cancer Patients: A Prospective Study. J. Cell. Biochem. 2019, 120, 8975–8982. [Google Scholar] [CrossRef]
- Heyn, H.; Engelmann, M.; Schreek, S.; Ahrens, P.; Lehmann, U.; Kreipe, H.; Schlegelberger, B.; Beger, C. MicroRNA MiR-335 Is Crucial for the BRCA1 Regulatory Cascade in Breast Cancer Development. Int. J. Cancer 2011, 129, 2797–2806. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zeng, F.; Wu, J.Y.; Li, H.Y.; Fan, J.J.; Mai, L.; Zhang, J.; Ma, D.M.; Li, Y.; Song, F.Z. MiR-335 Inhibits Migration of Breast Cancer Cells through Targeting Oncoprotein c-Met. Tumour Biol. 2015, 36, 2875–2883. [Google Scholar] [CrossRef]
- Soofiyani, S.R.; Hosseini, K.; Ebrahimi, T.; Forouhandeh, H.; Sadeghi, M.; Beirami, S.M.; Ghasemnejad, T.; Tarhriz, V.; Montazersaheb, S. Prognostic Value and Biological Role of MiR-126 in Breast Cancer. MicroRNA 2022, 11, 95–103. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Xie, H.; Zhou, Z.; Chen, H.; Hu, T.; Bai, Y.; Shen, Y.; Yuan, W.; Jing, Q.; et al. Endothelial-Specific Intron-Derived MiR-126 Is down-Regulated in Human Breast Cancer and Targets Both VEGFA and PIK3R2. Mol. Cell. Biochem. 2011, 351, 157–164. [Google Scholar] [CrossRef]
- Fu, R.; Tong, J.S. MiR-126 Reduces Trastuzumab Resistance by Targeting PIK3R2 and Regulating AKT/MTOR Pathway in Breast Cancer Cells. J. Cell. Mol. Med. 2020, 24, 7600–7608. [Google Scholar] [CrossRef]
- Wang, C.Z.; Yuan, P.; Li, Y. MiR-126 Regulated Breast Cancer Cell Invasion by Targeting ADAM9. Int. J. Clin. Exp. Pathol. 2015, 8, 6547–6553. [Google Scholar]
- Li, S.Q.; Wang, Z.H.; Mi, X.G.; Liu, L.; Tan, Y. MiR-199a/b-3p Suppresses Migration and Invasion of Breast Cancer Cells by Downregulating PAK4/MEK/ERK Signaling Pathway. IUBMB Life 2015, 67, 768–777. [Google Scholar] [CrossRef]
- Qattan, A.; Al-Tweigeri, T.; Alkhayal, W.; Suleman, K.; Tulbah, A.; Amer, S. Clinical Identification of Dysregulated Circulating MicroRNAs and Their Implication in Drug Response in Triple Negative Breast Cancer (TNBC) by Target Gene Network and Meta-Analysis. Genes 2021, 12, 549. [Google Scholar] [CrossRef]
- Fan, X.; Zhou, S.; Zheng, M.; Deng, X.; Yi, Y.; Huang, T. MiR-199a-3p Enhances Breast Cancer Cell Sensitivity to Cisplatin by Downregulating TFAM (TFAM). Biomed. Pharmacother. 2017, 88, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Qu, C.; Tian, Y.; Wen, Y.; Xia, S.; Ma, M. The HIF-1/SNHG1/MiR-199a-3p/TFAM Axis Explains Tumor Angiogenesis and Metastasis under Hypoxic Conditions in Breast Cancer. Biofactors 2021, 47, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Fuso, P.; Di Salvatore, M.; Santonocito, C.; Guarino, D.; Autilio, C.; Mulè, A.; Arciuolo, D.; Rinninella, A.; Mignone, F.; Ramundo, M.; et al. Let-7a-5p, MiR-100-5p, MiR-101-3p, and MiR-199a-3p Hyperexpression as Potential Predictive Biomarkers in Early Breast Cancer Patients. J. Pers. Med. 2021, 11, 816. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Deng, F.; Li, H.; Wang, D.D.; Zhang, W.; Ding, L.; Tang, J.H. MiR-101: A Potential Therapeutic Target of Cancers. Am. J. Transl. Res. 2018, 10, 3310–3321. [Google Scholar] [PubMed]
- Harati, R.; Mohammad, M.G.; Tlili, A.; El-Awady, R.A.; Hamoudi, R. Loss of MiR-101-3p Promotes Transmigration of Metastatic Breast Cancer Cells through the Brain Endothelium by Inducing COX-2/MMP1 Signaling. Pharmaceuticals 2020, 13, 144. [Google Scholar] [CrossRef]
- Jiang, H.; Li, L.; Zhang, J.; Wan, Z.; Wang, Y.; Hou, J.; Yu, Y. MiR-101-3p and Syn-Cal14.1a Synergy in Suppressing EZH2-Induced Progression of Breast Cancer. Onco Targets Ther. 2020, 13, 9599–9609. [Google Scholar] [CrossRef]
- Toda, H.; Seki, N.; Kurozumi, S.; Shinden, Y.; Yamada, Y.; Nohata, N.; Moriya, S.; Idichi, T.; Maemura, K.; Fujii, T.; et al. RNA-sequence-based MicroRNA Expression Signature in Breast Cancer: Tumor-suppressive MiR-101-5p Regulates Molecular Pathogenesis. Mol. Oncol. 2020, 14, 426–446. [Google Scholar] [CrossRef]
- Piasecka, D.; Braun, M.; Kordek, R.; Sadej, R.; Romanska, H. MicroRNAs in Regulation of Triple-Negative Breast Cancer Progression. J. Cancer Res. Clin. Oncol. 2018, 144, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Sporn, J.C.; Katsuta, E.; Yan, L.; Takabe, K. Expression of MicroRNA-9 Is Associated with Overall Survival in Breast Cancer Patients. J. Surg. Res. 2019, 233, 426–435. [Google Scholar] [CrossRef]
- Gwak, J.M.; Kim, H.J.; Kim, E.J.; Chung, Y.R.; Yun, S.; Seo, A.N.; Lee, H.J.; Park, S.Y. MicroRNA-9 Is Associated with Epithelial-Mesenchymal Transition, Breast Cancer Stem Cell Phenotype, and Tumor Progression in Breast Cancer. Breast Cancer Res. Treat. 2014, 147, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Dong, C.; Ruan, X.; Yan, W.; Cao, M.; Pizzo, D.; Wu, X.; Yang, L.; Liu, L.; Ren, X.; et al. Chemotherapy-Induced Extracellular Vesicle miRNAs Promote Breast Cancer Stemness by Targeting ONECUT2. Cancer Res. 2019, 79, 3608–3621. [Google Scholar] [CrossRef]
- Liu, D.Z.; Chang, B.; Li, X.D.; Zhang, Q.H.; Zou, Y.H. MicroRNA-9 Promotes the Proliferation, Migration, and Invasion of Breast Cancer Cells via down-Regulating FOXO1. Clin. Transl. Oncol. 2017, 19, 1133–1140. [Google Scholar] [CrossRef]
- Li, X.; Zeng, Z.; Wang, J.; Wu, Y.; Chen, W.; Zheng, L.; Xi, T.; Wang, A.; Lu, Y. MicroRNA-9 and Breast Cancer. Biomed. Pharmacother. 2020, 122, 109687. [Google Scholar] [CrossRef]
- Chen, D.; Sun, Y.; Wei, Y.; Zhang, P.; Rezaeian, A.H.; Teruya-Feldstein, J.; Gupta, S.; Liang, H.; Lin, H.K.; Hung, M.C.; et al. LIFR Is a Breast Cancer Metastasis Suppressor Upstream of the Hippo-YAP Pathway and a Prognostic Marker. Nat. Med. 2012, 18, 1511–1517. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, M.; Zheng, X.; Zheng, L.; Liu, H.; Lu, J.; Liu, Y.; Chen, W. Interactions Between LncRNA TUG1 and MiR-9-5p Modulate the Resistance of Breast Cancer Cells to Doxorubicin by Regulating EIF5A2. OncoTargets Ther. 2020, 13, 13159–13170. [Google Scholar] [CrossRef]
- D’Ippolito, E.; Plantamura, I.; Bongiovanni, L.; Casalini, P.; Baroni, S.; Piovan, C.; Orlandi, R.; Gualeni, A.V.; Gloghini, A.; Rossini, A.; et al. MiR-9 and MiR-200 Regulate PDGFRβ-Mediated Endothelial Differentiation of Tumor Cells in Triple-Negative Breast Cancer. Cancer Res. 2016, 76, 5562–5572. [Google Scholar] [CrossRef]
- Khew-Goodall, Y.; Goodall, G.J. Myc-Modulated MiR-9 Makes More Metastases. Nat. Cell Biol. 2010, 12, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Young, J.; Prabhala, H.; Pan, E.; Mestdagh, P.; Muth, D.; Teruya-Feldstein, J.; Reinhardt, F.; Onder, T.T.; Valastyan, S.; et al. MiR-9, a MYC/MYCN-Activated MicroRNA, Regulates E-Cadherin and Cancer Metastasis. Nat. Cell Biol. 2010, 12, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans Heterochronic Gene Lin-4 Encodes Small RNAs with Antisense Complementarity to Lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Pak, C.H.; Jin, P. Single Nucleotide Polymorphism Associated with Mature MiR-125a Alters the Processing of Pri-MiRNA. Hum. Mol. Genet. 2007, 16, 1124–1131. [Google Scholar] [CrossRef]
- Shaham, L.; Binder, V.; Gefen, N.; Borkhardt, A.; Izraeli, S. MiR-125 in Normal and Malignant Hematopoiesis. Leukemia 2012, 26, 2011–2018. [Google Scholar] [CrossRef]
- Ciafrè, S.A.; Galardi, S.; Mangiola, A.; Ferracin, M.; Liu, C.G.; Sabatino, G.; Negrini, M.; Maira, G.; Croce, C.M.; Farace, M.G. Extensive Modulation of a Set of MicroRNAs in Primary Glioblastoma. Biochem. Biophys. Res. Commun. 2005, 334, 1351–1358. [Google Scholar] [CrossRef]
- Emmrich, S.; Streltsov, A.; Schmidt, F.; Thangapandi, V.R.; Reinhardt, D.; Klusmann, J.H. LincRNAs MONC and MIR100HG Act as Oncogenes in Acute Megakaryoblastic Leukemia. Mol. Cancer 2014, 13, 171. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of Tissue-Specific MicroRNAs from Mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef]
- Huang, H.Y.; Lin, Y.C.D.; Cui, S.; Huang, Y.; Tang, Y.; Xu, J.; Bao, J.; Li, Y.; Wen, J.; Zuo, H.; et al. MiRTarBase Update 2022: An Informative Resource for Experimentally Validated MiRNA-Target Interactions. Nucleic Acids Res. 2022, 50, D222–D230. [Google Scholar] [CrossRef]
- MiRTarBase: The Experimentally Validated MicroRNA-Target Interactions Database. Available online: https://mirtarbase.cuhk.edu.cn/~miRTarBase/miRTarBase_2022/php/index.php (accessed on 24 August 2023).
- Sun, Y.M.; Lin, K.Y.; Chen, Y.Q. Diverse Functions of MiR-125 Family in Different Cell Contexts. J. Hematol. Oncol. 2013, 6, 6. [Google Scholar] [CrossRef]
- Ji, X.; Lu, Y.; Tian, H.; Meng, X.; Wei, M.; Cho, W.C. Chemoresistance Mechanisms of Breast Cancer and Their Countermeasures. Biomed. Pharmacother. 2019, 114, 108800. [Google Scholar] [CrossRef]
- Ge, Y.; Sun, Y.; Chen, J. IGF-II Is Regulated by MicroRNA-125b in Skeletal Myogenesis. J. Cell Biol. 2011, 192, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhu, J.; Zhang, R.; Liang, W.; Ma, W.; Zhang, Q.; Huang, Z.; Ding, F.; Sun, H. MiR-125b-5p Targeting TRAF6 Relieves Skeletal Muscle Atrophy Induced by Fasting or Denervation. Ann. Transl. Med. 2019, 7, 456. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, S.; Gao, Y.; Yu, C.; Nie, Z.; Lu, R.; Sun, Y.; Guan, Z. MicroRNA-125b Inhibits the Proliferation of Vascular Smooth Muscle Cells Induced by Platelet-Derived Growth Factor BB. Exp. Ther. Med. 2021, 22, 791. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tan, J.; Wang, L.; Pei, G.; Cheng, H.; Zhang, Q.; Wang, S.; He, C.; Fu, C.; Wei, Q. MiR-125 Family in Cardiovascular and Cerebrovascular Diseases. Front. Cell Dev. Biol. 2021, 9, 799049. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Velasco, E.; Galiano-Torres, J.; Jodar-Garcia, A.; Aranega, A.E.; Franco, D. MiR-27 and MiR-125 Distinctly Regulate Muscle-Enriched Transcription Factors in Cardiac and Skeletal Myocytes. BioMed Res. Int. 2015, 2015, 391306. [Google Scholar] [CrossRef]
- Li, L.; Wang, Q.; Yuan, Z.; Chen, A.; Liu, Z.; Wang, Z.; Li, H. LncRNA-MALAT1 Promotes CPC Proliferation and Migration in Hypoxia by up-Regulation of JMJD6 via Sponging MiR-125. Biochem. Biophys. Res. Commun. 2018, 499, 711–718. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Chen, W.; Wang, R.; Ye, Z.; Wang, Y.; Li, X.; Cai, C. LncRNA-HOTAIR Inhibition Aggravates Oxidative Stress-Induced H9c2 Cells Injury through Suppression of MMP2 by MiR-125. Acta Biochim. Biophys. Sin. 2018, 50, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Lee, D.S.; Choong, O.K.; Chang, S.K.; Hsu, T.; Nicholson, M.W.; Liu, L.W.; Lin, P.J.; Ruan, S.C.; Lin, S.W.; et al. Cardiac-Specific MicroRNA-125b Deficiency Induces Perinatal Death and Cardiac Hypertrophy. Sci. Rep. 2021, 11, 2377. [Google Scholar] [CrossRef]
- Le, M.T.N.; Xie, H.; Zhou, B.; Chia, P.H.; Rizk, P.; Um, M.; Udolph, G.; Yang, H.; Lim, B.; Lodish, H.F. MicroRNA-125b Promotes Neuronal Differentiation in Human Cells by Repressing Multiple Targets. Mol. Cell. Biol. 2009, 29, 5290–5305. [Google Scholar] [CrossRef]
- Dash, S.; Balasubramaniam, M.; Rana, T.; Godino, A.; Peck, E.G.; Goodwin, J.S.; Villalta, F.; Calipari, E.S.; Nestler, E.J.; Dash, C.; et al. Poly (ADP-Ribose) Polymerase-1 (PARP-1) Induction by Cocaine Is Post-Transcriptionally Regulated by MiR-125b. eNeuro 2017, 4, ENEURO.0089-17.2017. [Google Scholar] [CrossRef]
- Edbauer, D.; Neilson, J.R.; Foster, K.A.; Wang, C.F.; Seeburg, D.P.; Batterton, M.N.; Tada, T.; Dolan, B.M.; Sharp, P.A.; Sheng, M. Regulation of Synaptic Structure and Function by FMRP-Associated MicroRNAs MiR-125b and MiR-132. Neuron 2010, 65, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Åkerblom, M.; Petri, R.; Sachdeva, R.; Klussendorf, T.; Mattsson, B.; Gentner, B.; Jakobsson, J. MicroRNA-125 Distinguishes Developmentally Generated and Adult-Born Olfactory Bulb Interneurons. Development 2014, 141, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Gioia, U.; Di Carlo, V.; Caramanica, P.; Toselli, C.; Cinquino, A.; Marchioni, M.; Laneve, P.; Biagioni, S.; Bozzoni, I.; Cacci, E.; et al. Mir-23a and Mir-125b Regulate Neural Stem/Progenitor Cell Proliferation by Targeting Musashi1. RNA Biol. 2014, 11, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Pogue, A.I.; Cui, J.G.; Li, Y.Y.; Zhao, Y.; Culicchia, F.; Lukiw, W.J. Micro RNA-125b (MiRNA-125b) Function in Astrogliosis and Glial Cell Proliferation. Neurosci. Lett. 2010, 476, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Da Silva, A.C.A.L.; Arnold, A.; Okeke, L.; Ames, H.; Correa-Cerro, L.S.; Vizcaino, M.A.; Ho, C.Y.; Eberhart, C.G.; Rodriguez, F.J. MicroRNA (MiR) 125b Regulates Cell Growth and Invasion in Pediatric Low Grade Glioma. Sci. Rep. 2018, 8, 12506. [Google Scholar] [CrossRef]
- Laneve, P.; Di Marcotullio, L.; Gioia, U.; Fiori, M.E.; Ferretti, E.; Gulino, A.; Bozzoni, I.; Caffarelli, E. The Interplay between MicroRNAs and the Neurotrophin Receptor Tropomyosin-Related Kinase C Controls Proliferation of Human Neuroblastoma Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 7957–7962. [Google Scholar] [CrossRef]
- Ferretti, E.; De Smaele, E.; Po, A.; Marcotullio, L.D.; Tosi, E.; Espinola, M.S.B.; Rocco, C.D.; Riccardi, R.; Giangaspero, F.; Farcomeni, A.; et al. MicroRNA Profiling in Human Medulloblastoma. Int. J. Cancer 2009, 124, 568–577. [Google Scholar] [CrossRef]
- Wu, N.; Lin, X.; Zhao, X.; Zheng, L.; Xiao, L.; Liu, J.; Ge, L.; Cao, S. MiR-125b Acts as an Oncogene in Glioblastoma Cells and Inhibits Cell Apoptosis through P53 and P38MAPK-Independent Pathways. Br. J. Cancer 2013, 109, 2853–2863. [Google Scholar] [CrossRef]
- Xia, H.F.; He, T.Z.; Liu, C.M.; Cui, Y.; Song, P.P.; Jin, X.H.; Ma, X. MiR-125b Expression Affects the Proliferation and Apoptosis of Human Glioma Cells by Targeting Bmf. Cell. Physiol. Biochem. 2009, 23, 347–358. [Google Scholar] [CrossRef]
- Wojtowicz, E.E.; Lechman, E.R.; Hermans, K.G.; Schoof, E.M.; Wienholds, E.; Isserlin, R.; van Veelen, P.A.; Broekhuis, M.J.C.; Janssen, G.M.C.; Trotman-Grant, A.; et al. Ectopic MiR-125a Expression Induces Long-Term Repopulating Stem Cell Capacity in Mouse and Human Hematopoietic Progenitors. Cell Stem Cell 2016, 19, 383–396. [Google Scholar] [CrossRef]
- Guo, S.; Lu, J.; Schlanger, R.; Zhang, H.; Wang, J.Y.; Fox, M.C.; Purton, L.E.; Fleming, H.H.; Cobb, B.; Merkenschlager, M.; et al. MicroRNA MiR-125a Controls Hematopoietic Stem Cell Number. Proc. Natl. Acad. Sci. USA 2010, 107, 14229–14234. [Google Scholar] [CrossRef] [PubMed]
- Emmrich, S.; Rasche, M.; Schöning, J.; Reimer, C.; Keihani, S.; Maroz, A.; Xie, Y.; Li, Z.; Schambach, A.; Reinhardt, D.; et al. MiR-99a/100~125b Tricistrons Regulate Hematopoietic Stem and Progenitor Cell Homeostasis by Shifting the Balance between TGFβ and Wnt Signaling. Genes Dev. 2014, 28, 858–874. [Google Scholar] [CrossRef] [PubMed]
- Allantaz, F.; Cheng, D.T.; Bergauer, T.; Ravindran, P.; Rossier, M.F.; Ebeling, M.; Badi, L.; Reis, B.; Bitter, H.; D’Asaro, M.; et al. Expression Profiling of Human Immune Cell Subsets Identifies MiRNA-MRNA Regulatory Relationships Correlated with Cell Type Specific Expression. PLoS ONE 2012, 7, e29979. [Google Scholar] [CrossRef]
- Yao, D.; Zhou, Z.; Wang, P.; Zheng, L.; Huang, Y.; Duan, Y.; Liu, B.; Li, Y. MiR-125-5p/IL-6R Axis Regulates Macrophage Inflammatory Response and Intestinal Epithelial Cell Apoptosis in Ulcerative Colitis through JAK1/STAT3 and NF-ΚB Pathway. Cell Cycle 2021, 20, 2547–2564. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Tang, W.; Lu, R.; Tao, Y.; Ren, T.; Gao, Y. Human Adipose-Derived Mesenchymal Stem Cells Promote Lymphocyte Apoptosis and Alleviate Atherosclerosis via MiR-125b-1-3p/BCL11B Signal Axis. Ann. Palliat. Med. 2021, 10, 2123–2133. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, S.; Ma, X. Prognostic Value of MicroRNA-125 in Various Human Malignant Neoplasms: A Meta-Analysis. Clin. Lab. 2015, 61, 1667–1674. [Google Scholar] [CrossRef]
- Testa, U.; Pelosi, E. MicroRNAs Expressed in Hematopoietic Stem/Progenitor Cells Are Deregulated in Acute Myeloid Leukemias. Leuk. Lymphoma 2015, 56, 1466–1474. [Google Scholar] [CrossRef]
- Alemdehy, M.F.; Erkeland, S.J. MicroRNAs: Key Players of Normal and Malignant Myelopoiesis. Curr. Opin. Hematol. 2012, 19, 261–267. [Google Scholar] [CrossRef]
- Cowden Dahl, K.D.; Dahl, R.; Kruichak, J.N.; Hudson, L.G. The Epidermal Growth Factor Receptor Responsive MiR-125a Represses Mesenchymal Morphology in Ovarian Cancer Cells. Neoplasia 2009, 11, 1208–1215. [Google Scholar] [CrossRef]
- Guan, Y.; Yao, H.; Zheng, Z.; Qiu, G.; Sun, K. MiR-125b Targets BCL3 and Suppresses Ovarian Cancer Proliferation. Int. J. Cancer 2011, 128, 2274–2283. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, X.; Sun, S.; Lu, C.; Wang, L. Serum MiR-125b Levels Associated with Epithelial Ovarian Cancer (EOC) Development and Treatment Responses. Bioengineered 2020, 11, 311–317. [Google Scholar] [CrossRef]
- Huang, L.; Luo, J.; Cai, Q.; Pan, Q.; Zeng, H.; Guo, Z.; Dong, W.; Huang, J.; Lin, T. MicroRNA-125b Suppresses the Development of Bladder Cancer by Targeting E2F3. Int. J. Cancer 2011, 128, 1758–1769. [Google Scholar] [CrossRef]
- Pospisilova, S.; Pazzourkova, E.; Horinek, A.; Brisuda, A.; Svobodova, I.; Soukup, V.; Hrbacek, J.; Capoun, O.; Hanus, T.; Mares, J.; et al. MicroRNAs in Urine Supernatant as Potential Non-Invasive Markers for Bladder Cancer Detection. Neoplasma 2016, 63, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Blick, C.; Ramachandran, A.; Mccormick, R.; Wigfield, S.; Cranston, D.; Catto, J.; Harris, A.L. Identification of a Hypoxia-Regulated MiRNA Signature in Bladder Cancer and a Role for MiR-145 in Hypoxia-Dependent Apoptosis. Br. J. Cancer 2015, 113, 634–644. [Google Scholar] [CrossRef]
- Zhou, H.; Tang, K.; Xiao, H.; Zeng, J.; Guan, W.; Guo, X.; Xu, H.; Ye, Z. A Panel of Eight-MiRNA Signature as a Potential Biomarker for Predicting Survival in Bladder Cancer. J. Exp. Clin. Cancer Res. 2015, 34, 53. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.; Tang, S.; Xia, L.; Du, R.; Fan, R.; Gao, L.; Jin, J.; Liang, S.; Chen, Z.; Xu, G.; et al. Ectopic Expression of MiR-125a Inhibits the Proliferation and Metastasis of Hepatocellular Carcinoma by Targeting MMP11 and VEGF. PLoS ONE 2012, 7, e40169. [Google Scholar] [CrossRef]
- Jia, H.Y.; Wang, Y.X.; Yan, W.T.; Li, H.Y.; Tian, Y.Z.; Wang, S.M.; Zhao, H.L. MicroRNA-125b Functions as a Tumor Suppressor in Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2012, 13, 8762–8774. [Google Scholar] [CrossRef]
- Liang, L.; Wong, C.M.; Ying, Q.; Fan, D.N.Y.; Huang, S.; Ding, J.; Yao, J.; Yan, M.; Li, J.; Yao, M.; et al. MicroRNA-125b Suppressesed Human Liver Cancer Cell Proliferation and Metastasis by Directly Targeting Oncogene LIN28B2. Hepatology 2010, 52, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Liu, X.; Li, X.; Wu, J.; Wu, N.; Chen, J.; Fang, F. MiR-125/Pokemon Auto-Circuit Contributes to the Progression of Hepatocellular Carcinoma. Tumour Biol. 2016, 37, 511–519. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, L.Z.; Chen, Z.L.; Zhong, W.J.; Fang, J.H.; Zhu, Y.; Xiao, M.H.; Guo, Z.W.; Zhao, N.; He, X.; et al. A HMTR4-PDIA3P1-MiR-125/124-TRAF6 Regulatory Axis and Its Function in NF Kappa B Signaling and Chemoresistance. Hepatology 2020, 71, 1660–1677. [Google Scholar] [CrossRef]
- Jiang, J.X.; Gao, S.; Pan, Y.Z.; Yu, C.; Sun, C.Y. Overexpression of MicroRNA-125b Sensitizes Human Hepatocellular Carcinoma Cells to 5-Fluorouracil through Inhibition of Glycolysis by Targeting Hexokinase II. Mol. Med. Rep. 2014, 10, 995–1002. [Google Scholar] [CrossRef]
- Xu, Z.; Pei, C.; Cheng, H.; Song, K.; Yang, J.; Li, Y.; He, Y.; Liang, W.; Liu, B.; Tan, W.; et al. Comprehensive Analysis of FOXM1 Immune Infiltrates, M6a, Glycolysis and CeRNA Network in Human Hepatocellular Carcinoma. Front. Immunol. 2023, 14, 1138524. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, T.; Li, W.; Yin, S. MiR-125b Acts as a Tumor Suppressor of Melanoma by Targeting NCAM. JBUON 2021, 26, 182–188. [Google Scholar]
- Kappelmann, M.; Kuphal, S.; Meister, G.; Vardimon, L.; Bosserhoff, A.K. MicroRNA MiR-125b Controls Melanoma Progression by Direct Regulation of c-Jun Protein Expression. Oncogene 2013, 32, 2984–2991. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, L.; Meisgen, F.; Harada, M.; Heilborn, J.; Homey, B.; Grandér, D.; Ståhle, M.; Sonkoly, E.; Pivarcsi, A. MicroRNA-125b down-Regulates Matrix Metallopeptidase 13 and Inhibits Cutaneous Squamous Cell Carcinoma Cell Proliferation, Migration, and Invasion. J. Biol. Chem. 2012, 287, 29899–29908. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Liu, W.; Zhang, J.; Fan, X.; Liu, J.; Zhao, N.; Yao, C.; Miao, G. MicroRNA-125b Exerts Antitumor Functions in Cutaneous Squamous Cell Carcinoma by Targeting the STAT3 Pathway. Cell. Mol. Biol. Lett. 2020, 25, 12. [Google Scholar] [CrossRef]
- Sand, M.; Skrygan, M.; Sand, D.; Georgas, D.; Hahn, S.A.; Gambichler, T.; Altmeyer, P.; Bechara, F.G. Expression of MicroRNAs in Basal Cell Carcinoma. Br. J. Dermatol. 2012, 167, 847–855. [Google Scholar] [CrossRef]
- Liu, L.H.; Li, H.; Li, J.P.; Zhong, H.; Zhang, H.C.; Chen, J.; Xiao, T. MiR-125b Suppresses the Proliferation and Migration of Osteosarcoma Cells through down-Regulation of STAT3. Biochem. Biophys. Res. Commun. 2011, 416, 31–38. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Liu, J.; Zhang, B.; Yang, L.; Xue, J.; Zhang, Z.; Qin, L.; Bian, R. MiR-125b-5p/STAT3 Axis Regulates Drug Resistance in Osteosarcoma Cells by Acting on ABC Transporters. Stem Cells Int. 2023, 2023, 9997676. [Google Scholar] [CrossRef]
- Tang, X.Y.; Zheng, W.; Ding, M.; Guo, K.J.; Yuan, F.; Feng, H.; Deng, B.; Sun, W.; Hou, Y.; Gao, L. MiR-125b Acts as a Tumor Suppressor in Chondrosarcoma Cells by the Sensitization to Doxorubicin through Direct Targeting the ErbB2-Regulated Glucose Metabolism. Drug Des. Dev. Ther. 2016, 10, 571–583. [Google Scholar] [CrossRef]
- Gao, S.; Sun, H.; Cheng, C.; Wang, G. BRCA1-Associated Protein-1 Suppresses Osteosarcoma Cell Proliferation and Migration Through Regulation PI3K/Akt Pathway. DNA Cell Biol. 2017, 36, 386–393. [Google Scholar] [CrossRef]
- Wu, S.; Shen, W.; Yang, L.; Zhu, M.; Zhang, M.; Zong, F.; Geng, L.; Wang, Y.; Huang, T.; Pan, Y.; et al. Genetic Variations in MiR-125 Family and the Survival of Non-Small Cell Lung Cancer in Chinese Population. Cancer Med. 2019, 8, 2636–2645. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Mao, W.; Zheng, S.; Ye, J. Epidermal Growth Factor Receptor-Regulated MiR-125a-5p--a Metastatic Inhibitor of Lung Cancer. FEBS J. 2009, 276, 5571–5578. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, S.; Fujita, Y.; Kitazono, S.; Ko, R.; Nakadate, Y.; Sawada, T.; Kitamura, Y.; Shimoyama, T.; Maeda, Y.; Takahashi, F.; et al. Chemotherapy-Regulated MicroRNA-125-HER2 Pathway as a Novel Therapeutic Target for Trastuzumab-Mediated Cellular Cytotoxicity in Small Cell Lung Cancer. Mol. Cancer Ther. 2015, 14, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wei, F.; Yu, J.; Zhao, H.; Jia, L.; Ye, Y.; Du, R.; Ren, X.; Li, H. Matrix Metalloproteinase 13: A Potential Intermediate between Low Expression of MicroRNA-125b and Increasing Metastatic Potential of Non-Small Cell Lung Cancer. Cancer Genet. 2015, 208, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Bloomston, M.; Frankel, W.L.; Petrocca, F.; Volinia, S.; Alder, H.; Hagan, J.P.; Liu, C.G.; Bhatt, D.; Taccioli, C.; Croce, C.M. MicroRNA Expression Patterns to Differentiate Pancreatic Adenocarcinoma from Normal Pancreas and Chronic Pancreatitis. JAMA 2007, 297, 1901–1908. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Paris, P.L.; Chen, J.; Ngo, V.; Yao, H.; Frazier, M.L.; Killary, A.M.; Liu, C.G.; Liang, H.; Mathy, C.; et al. Next Generation Sequencing of Pancreatic Cyst Fluid MicroRNAs from Low Grade-Benign and High Grade-Invasive Lesions. Cancer Lett. 2015, 356, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhong, Y.; Wu, T.; Sheng, Y.; Dai, Y.; Xu, L.; Bao, C. Anti-Proliferative and Apoptosis-Promoting Effect of MicroRNA-125b on Pancreatic Cancer by Targeting NEDD9 via PI3K/AKT Signaling. Cancer Manag. Res. 2020, 12, 7363–7373. [Google Scholar] [CrossRef]
- Walter, B.A.; Valera, V.A.; Pinto, P.A.; Merino, M.J. Comprehensive MicroRNA Profiling of Prostate Cancer. J. Cancer 2013, 4, 350–357. [Google Scholar] [CrossRef]
- Li, W.; Dong, Y.; Wang, K.J.; Deng, Z.; Zhang, W.; Shen, H.F. Plasma Exosomal MiR-125a-5p and MiR-141-5p as Non-Invasive Biomarkers for Prostate Cancer. Neoplasma 2020, 67, 1314–1318. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Bryzgunova, O.E.; Zaporozhchenko, I.A.; Yarmoschuk, S.V.; Pashkovskaya, O.A.; Pak, S.V.; Laktionov, P.P. The Panel of 12 Cell-Free MicroRNAs as Potential Biomarkers in Prostate Neoplasms. Diagnostics 2020, 10, 38. [Google Scholar] [CrossRef]
- Shi, X.B.; Xue, L.; Yang, J.; Ma, A.H.; Zhao, J.; Xu, M.; Tepper, C.G.; Evans, C.P.; Kung, H.J.; White, R.W.D.V. An Androgen-Regulated MiRNA Suppresses Bak1 Expression and Induces Androgen-Independent Growth of Prostate Cancer Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 19983–19988. [Google Scholar] [CrossRef]
- Shi, X.B.; Xue, L.; Ma, A.H.; Tepper, C.G.; Kung, H.J.; White, R.W.D. MiR-125b Promotes Growth of Prostate Cancer Xenograft Tumor through Targeting pro-Apoptotic Genes. Prostate 2011, 71, 538–549. [Google Scholar] [CrossRef]
- Wang, S.; Wu, J.; Ren, J.; Vlantis, A.C.; Li, M.-y.; Liu, S.Y.W.; Ng, E.K.W.; Chan, A.B.W.; Luo, D.C.; Liu, Z.; et al. MicroRNA-125b Interacts with Foxp3 to Induce Autophagy in Thyroid Cancer. Mol. Ther. 2018, 26, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.G.; Wang, J.J.; Jiang, X.; Lan, J.P.; He, X.J.; Wang, H.J.; Ma, Y.Y.; Xia, Y.J.; Ru, G.Q.; Ma, J.; et al. MiR-125b Promotes Cell Migration and Invasion by Targeting PPP1CA-Rb Signal Pathways in Gastric Cancer, Resulting in a Poor Prognosis. Gastric Cancer 2015, 18, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Liu, N.; Lin, L.; Guo, X.; Yang, D.; Zhang, Q. MiR-125a-5p Inhibits Cell Proliferation and Induces Apoptosis in Colon Cancer via Targeting BCL2, BCL2L12 and MCL1. Biomed. Pharmacother. 2015, 75, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Liu, Z.; Pan, D.; Zhang, W.; Xu, L.; Zhu, Y.; Liu, H.; Xu, J. Tumor MiR-125b Predicts Recurrence and Survival of Patients with Clear-Cell Renal Cell Carcinoma after Surgical Resection. Cancer Sci. 2014, 105, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Mattie, M.D.; Benz, C.C.; Bowers, J.; Sensinger, K.; Wong, L.; Scott, G.K.; Fedele, V.; Ginzinger, D.; Getts, R.; Haqq, C. Optimized High-Throughput MicroRNA Expression Profiling Provides Novel Biomarker Assessment of Clinical Prostate and Breast Cancer Biopsies. Mol. Cancer 2006, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.V.; Ferracin, M.; Liu, C.G.; Veronese, A.; Spizzo, R.; Sabbioni, S.; Magri, E.; Pedriali, M.; Fabbri, M.; Campiglio, M.; et al. MicroRNA Gene Expression Deregulation in Human Breast Cancer. Cancer Res. 2005, 65, 7065–7070. [Google Scholar] [CrossRef] [PubMed]
- Mar-Aguilar, F.; Luna-Aguirre, C.M.; Moreno-Rocha, J.C.; Araiza-Chávez, J.; Trevino, V.; Rodríguez-Padilla, C.; Reséndez-Pérez, D. Differential Expression of MiR-21, MiR-125b and MiR-191 in Breast Cancer Tissue. Asia Pac. J. Clin. Oncol. 2013, 9, 53–59. [Google Scholar] [CrossRef]
- Liang, F.; Yang, M.; Tong, N.; Fang, J.; Pan, Y.; Li, J.; Zhang, X. Identification of Six Key MiRNAs Associated with Breast Cancer through Screening Large-Scale Microarray Data. Oncol. Lett. 2018, 16, 4159–4168. [Google Scholar] [CrossRef]
- Braicu, C.; Raduly, L.; Morar-Bolba, G.; Cojocneanu, R.; Jurj, A.; Pop, L.A.; Pileczki, V.; Ciocan, C.; Moldovan, A.; Irimie, A.; et al. Aberrant MiRNAs Expressed in HER-2 Negative Breast Cancers Patient. J. Exp. Clin. Cancer Res. 2018, 37, 257. [Google Scholar] [CrossRef]
- Incoronato, M.; Grimaldi, A.M.; Mirabelli, P.; Cavaliere, C.; Parente, C.A.; Franzese, M.; Staibano, S.; Ilardi, G.; Russo, D.; Soricelli, A.; et al. Circulating MiRNAs in Untreated Breast Cancer: An Exploratory Multimodality Morpho-Functional Study. Cancers 2019, 11, 876. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.K.; Goga, A.; Bhaumik, D.; Berger, C.E.; Sullivan, C.S.; Benz, C.C. Coordinate Suppression of ERBB2 and ERBB3 by Enforced Expression of Micro-RNA MiR-125a or MiR-125b. J. Biol. Chem. 2007, 282, 1479–1486. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, L.X.; Wu, Q.N.; Du, Z.M.; Chen, J.; Liao, D.Z.; Huang, M.Y.; Hou, J.H.; Wu, Q.L.; Zeng, M.S.; et al. MiR-125b Is Methylated and Functions as a Tumor Suppressor by Regulating the ETS1 Proto-Oncogene in Human Invasive Breast Cancer. Cancer Res. 2011, 71, 3552–3562. [Google Scholar] [CrossRef]
- Rajabi, H.; Jin, C.; Ahmad, R.; McClary, A.C.; Joshi, M.D.; Kufe, D. Mucin 1 Oncoprotein Expression Is Suppressed by the miR-125b Oncomir. Genes Cancer 2010, 1, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Zhang, R.; He, Y.; Zou, M.; Guo, L.; Xi, T. MicroRNA-125b Induces Metastasis by Targeting STARD13 in MCF-7 and MDA-MB-231 Breast Cancer Cells. PLoS ONE 2012, 7, e35435. [Google Scholar] [CrossRef]
- Metheetrairut, C.; Adams, B.D.; Nallur, S.; Weidhaas, J.B.; Slack, F.J. Cel-Mir-237 and Its Homologue, Hsa-MiR-125b, Modulate the Cellular Response to Ionizing Radiation. Oncogene 2017, 36, 512–524. [Google Scholar] [CrossRef]
- Wang, H.; Tan, G.; Dong, L.; Cheng, L.; Li, K.; Wang, Z.; Luo, H. Circulating MiR-125b as a Marker Predicting Chemoresistance in Breast Cancer. PLoS ONE 2012, 7, e34210. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, Z.; Zhao, Y.; Ding, Y.; Liu, H.; Xi, Y.; Xiong, W.; Li, G.; Lu, J.; Fodstad, O.; et al. MicroRNA-125b Confers the Resistance of Breast Cancer Cells to Paclitaxel through Suppression of pro-Apoptotic Bcl-2 Antagonist Killer 1 (Bak1) Expression. J. Biol. Chem. 2010, 285, 21496–21507. [Google Scholar] [CrossRef]
- He, H.; Xu, F.; Huang, W.; Luo, S.Y.; Lin, Y.T.; Zhang, G.H.; Du, Q.; Duan, R.H. MiR-125a-5p Expression Is Associated with the Age of Breast Cancer Patients. Genet. Mol. Res. 2015, 14, 17927–17933. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Y.; Fan, X.; Zhang, P.; Wang, P.; Cheng, S.; Zhang, J. MicroRNA-125b as a Tumor Suppressor by Targeting MMP11 in Breast Cancer. Thorac. Cancer 2020, 11, 1613–1620. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 388354. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzadeh, R.; Daraei, A.; Mansoori, Y.; Sepahvand, M.; Amoli, M.M.; Tavakkoly-Bazzaz, J. Competing Endogenous RNA (CeRNA) Cross Talk and Language in CeRNA Regulatory Networks: A New Look at Hallmarks of Breast Cancer. J. Cell. Physiol. 2019, 234, 10080–10100. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.D.; Baran-Gale, J.; Perou, C.M.; Sethupathy, P.; Prins, J.F. Pseudogenes Transcribed in Breast Invasive Carcinoma Show Subtype-Specific Expression and CeRNA Potential. BMC Genom. 2015, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Wang, Q.; Wang, L. Analysis of Competitive Endogenous RNA Regulatory Network of Exosomal Breast Cancer Based on ExoRBase. Evol. Bioinform. Online 2022, 18, 11769343221113286. [Google Scholar] [CrossRef] [PubMed]
- Rieger, M.A.; Ebner, R.; Bell, D.R.; Kiessling, A.; Rohayem, J.; Schmitz, M.; Temme, A.; Rieber, E.P.; Weigle, B. Identification of a Novel Mammary-Restricted Cytochrome P450, CYP4Z1, with Overexpression in Breast Carcinoma. Cancer Res. 2004, 64, 2357–2364. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Chai, H.; Li, Y.; Zhao, H.; Xie, X.; Zheng, H.; Wang, C.; Wang, X.; Yang, G.; Cai, X.; et al. Increased Expression of CYP4Z1 Promotes Tumor Angiogenesis and Growth in Human Breast Cancer. Toxicol. Appl. Pharmacol. 2012, 264, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, X.; Gu, Y.; Ma, Y.; Xi, T. Pseudogene CYP4Z2P 3′UTR Promotes Angiogenesis in Breast Cancer. Biochem. Biophys. Res. Commun. 2014, 453, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, X.; Gu, Y.; Lv, X.; Xi, T. The 3′UTR of the Pseudogene CYP4Z2P Promotes Tumor Angiogenesis in Breast Cancer by Acting as a CeRNA for CYP4Z1. Breast Cancer Res. Treat. 2015, 150, 105–118, Erratum in Breast Cancer Res. Treat. 2020, 179, 521–522. [Google Scholar] [CrossRef]
- Zheng, L.; Li, X.; Meng, X.; Chou, J.; Hu, J.; Zhang, F.; Zhang, Z.; Xing, Y.; Liu, Y.; Xi, T. Competing Endogenous RNA Networks of CYP4Z1 and Pseudogene CYP4Z2P Confer Tamoxifen Resistance in Breast Cancer. Mol. Cell. Endocrinol. 2016, 427, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zheng, L.; Xin, Y.; Tan, Z.; Zhang, Y.; Meng, X.; Wang, Z.; Xi, T. The Competing Endogenous RNA Network of CYP4Z1 and Pseudogene CYP4Z2P Exerts an Anti-Apoptotic Function in Breast Cancer. FEBS Lett. 2017, 591, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Guo, Q.; Xiang, C.; Liu, S.; Jiang, Y.; Gao, L.; Ni, H.; Wang, T.; Zhao, Q.; Liu, H.; et al. Transcriptional Factor Six2 Promotes the Competitive Endogenous RNA Network between CYP4Z1 and Pseudogene CYP4Z2P Responsible for Maintaining the Stemness of Breast Cancer Cells. J. Hematol. Oncol. 2019, 12, 23, Erratum in J. Hematol. Oncol. 2019, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Ching, Y.P.; Wong, C.M.; Chan, S.F.; Leung, T.H.Y.; Ng, D.C.H.; Jin, D.Y.; Ng, I.O.L. Deleted in Liver Cancer (DLC) 2 Encodes a RhoGAP Protein with Growth Suppressor Function and Is Underexpressed in Hepatocellular Carcinoma. J. Biol. Chem. 2003, 278, 10824–10830. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, N.T.; Shih, Y.P.; Liao, Y.C.; Xue, L.; Lo, S.H. DLC2 Modulates Angiogenic Responses in Vascular Endothelial Cells by Regulating Cell Attachment and Migration. Oncogene 2010, 29, 3010–3016. [Google Scholar] [CrossRef]
- Ullmannova, V.; Popescu, N.C. Expression Profile of the Tumor Suppressor Genes DLC-1 and DLC-2 in Solid Tumors. Int. J. Oncol. 2006, 29, 1127–1132. [Google Scholar] [CrossRef]
- Hanna, S.; Khalil, B.; Nasrallah, A.; Saykali, B.A.; Sobh, R.; Nasser, S.; El-Sibai, M. StarD13 Is a Tumor Suppressor in Breast Cancer That Regulates Cell Motility and Invasion. Int. J. Oncol. 2014, 44, 1499–1511. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Guo, X.; Guo, Q.; Xiang, C.; Zhang, Z.; Xing, Y.; Xi, T.; Zheng, L. The CCR2 3′UTR Functions as a Competing Endogenous RNA to Inhibit Breast Cancer Metastasis. J. Cell Sci. 2017, 130, 3399–3413. [Google Scholar] [CrossRef]
- Basak, P.; Leslie, H.; Dillon, R.L.; Muller, W.J.; Raouf, A.; Mowat, M.R.A. In Vivo Evidence Supporting a Metastasis Suppressor Role for Stard13 (Dlc2) in ErbB2 (Neu) Oncogene Induced Mouse Mammary Tumors. Genes Chromosomes Cancer 2018, 57, 182–191. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, X.; Xiong, B.; Sun, Y. Homeobox B4 Inhibits Breast Cancer Cell Migration by Directly Binding to StAR-Related Lipid Transfer Domain Protein 13. Oncol. Lett. 2017, 14, 4625–4632. [Google Scholar] [CrossRef]
- Guo, X.; Xiang, C.; Zhang, Z.; Zhang, F.; Xi, T.; Zheng, L. Displacement of Bax by BMF Mediates STARD13 3′UTR-Induced Breast Cancer Cells Apoptosis in an MiRNA-Depedent Manner. Mol. Pharm. 2018, 15, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Zhao, Q.; Xie, T.; Xiang, C.; Guo, Q.; Zhang, W.; Zhou, Y.; Yuan, Y.; Zhang, Y.; et al. A Positive TGF-β/MiR-9 Regulatory Loop Promotes the Expansion and Activity of Tumour-Initiating Cells in Breast Cancer. Br. J. Pharmacol. 2023, 180, 2280–2297. [Google Scholar] [CrossRef] [PubMed]
- Amirfallah, A.; Knutsdottir, H.; Arason, A.; Hilmarsdottir, B.; Johannsson, O.T.; Agnarsson, B.A.; Barkardottir, R.B.; Reynisdottir, I. Hsa-MiR-21-3p Associates with Breast Cancer Patient Survival and Targets Genes in Tumor Suppressive Pathways. PLoS ONE 2021, 16, e260327. [Google Scholar] [CrossRef]
- Zheng, L.; Xiang, C.; Li, X.; Guo, Q.; Gao, L.; Ni, H.; Xia, Y.; Xi, T. STARD13-Correlated CeRNA Network-Directed Inhibition on YAP/TAZ Activity Suppresses Stemness of Breast Cancer via Co-Regulating Hippo and Rho-GTPase/F-Actin Signaling. J. Hematol. Oncol. 2018, 11, 72. [Google Scholar] [CrossRef]
- Li, X.; Zheng, L.; Zhang, F.; Hu, J.; Chou, J.; Liu, Y.; Xing, Y.; Xi, T. STARD13-Correlated CeRNA Network Inhibits EMT and Metastasis of Breast Cancer. Oncotarget 2016, 7, 23197–23211. [Google Scholar] [CrossRef] [PubMed]
- Seillier, M.; Peuget, S.; Gayet, O.; Gauthier, C.; N’Guessan, P.; Monte, M.; Carrier, A.; Iovanna, J.L.; Dusetti, N.J. TP53INP1, a Tumor Suppressor, Interacts with LC3 and ATG8-Family Proteins through the LC3-Interacting Region (LIR) and Promotes Autophagy-Dependent Cell Death. Cell Death Differ. 2012, 19, 1525–1535. [Google Scholar] [CrossRef]
- Seux, M.; Peuget, S.; Montero, M.P.; Siret, C.; Rigot, V.; Clerc, P.; Gigoux, V.; Pellegrino, E.; Pouyet, L.; N’Guessan, P.; et al. TP53INP1 Decreases Pancreatic Cancer Cell Migration by Regulating SPARC Expression. Oncogene 2011, 30, 3049–3061. [Google Scholar] [CrossRef]
- Zheng, L.; Li, X.; Chou, J.; Xiang, C.; Guo, Q.; Zhang, Z.; Guo, X.; Gao, L.; Xing, Y.; Xi, T. StarD13 3′-Untranslated Region Functions as a CeRNA for TP53INP1 in Prohibiting Migration and Invasion of Breast Cancer Cells by Regulating MiR-125b Activity. Eur. J. Cell Biol. 2018, 97, 23–31. [Google Scholar] [CrossRef]
- Puthalakath, H.; Villunger, A.; O’Reilly, L.A.; Beaumont, J.G.; Coultas, L.; Cheney, R.E.; Huang, D.C.S.; Strasser, A. Bmf: A Proapoptotic BH3-Only Protein Regulated by Interaction with the Myosin V Actin Motor Complex, Activated by Anoikis. Science 2001, 293, 1829–1832. [Google Scholar] [CrossRef]
- Li, X.; Jia, Q.; Zhou, Y.; Jiang, X.; Song, L.; Wu, Y.; Wang, A.; Chen, W.; Wang, S.; Lu, Y. Tanshinone IIA Attenuates the Stemness of Breast Cancer Cells via Targeting the MiR-125b/STARD13 Axis. Exp. Hematol. Oncol. 2022, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Pashayan, N.; Antoniou, A.C.; Ivanus, U.; Esserman, L.J.; Easton, D.F.; French, D.; Sroczynski, G.; Hall, P.; Cuzick, J.; Evans, D.G.; et al. Personalized Early Detection and Prevention of Breast Cancer: ENVISION Consensus Statement. Nat. Rev. Clin. Oncol. 2020, 17, 687–705. [Google Scholar] [CrossRef] [PubMed]
- Larijani, B.; Salari, P.; Larijani, B. Ethical Issues Surrounding Personalized Medicine: A Literature Review. Acta Med. Iran. 2017, 55, 209–217. [Google Scholar]
- Cavaliere, A.F.; Perelli, F.; Zaami, S.; Piergentili, R.; Mattei, A.; Vizzielli, G.; Scambia, G.; Straface, G.; Restaino, S.; Signore, F. Towards Personalized Medicine: Non-Coding Rnas and Endometrial Cancer. Healthcare 2021, 9, 965. [Google Scholar] [CrossRef]
- Zaami, S.; Melcarne, R.; Patrone, R.; Gullo, G.; Negro, F.; Napoletano, G.; Monti, M.; Aceti, V.; Panarese, A.; Borcea, M.C.; et al. Oncofertility and Reproductive Counseling in Patients with Breast Cancer: A Retrospective Study. J. Clin. Med. 2022, 11, 1311. [Google Scholar] [CrossRef] [PubMed]
- Niida, A.; Iwasaki, W.M.; Innan, H. Neutral Theory in Cancer Cell Population Genetics. Mol. Biol. Evol. 2018, 35, 1316–1321. [Google Scholar] [CrossRef]
- Ben-David, U.; Amon, A. Context Is Everything: Aneuploidy in Cancer. Nat. Rev. Genet. 2020, 21, 44–62. [Google Scholar] [CrossRef]
- Newburger, D.E.; Kashef-Haghighi, D.; Weng, Z.; Salari, R.; Sweeney, R.T.; Brunner, A.L.; Zhu, S.X.; Guo, X.; Varma, S.; Troxell, M.L.; et al. Genome Evolution during Progression to Breast Cancer. Genome Res. 2013, 23, 1097–1108. [Google Scholar] [CrossRef]
- Murakami, F.; Tsuboi, Y.; Takahashi, Y.; Horimoto, Y.; Mogushi, K.; Ito, T.; Emi, M.; Matsubara, D.; Shibata, T.; Saito, M.; et al. Short Somatic Alterations at the Site of Copy Number Variation in Breast Cancer. Cancer Sci. 2021, 112, 444–453. [Google Scholar] [CrossRef]
- Soysal, S.D.; Tzankov, A.; Muenst, S.E. Role of the Tumor Microenvironment in Breast Cancer. Pathobiology 2015, 82, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4567. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Kan, C.; Sun, M.; Yang, F.; Wong, M.; Wang, S.; Zheng, H. Mapping Breast Cancer Microenvironment Through Single-Cell Omics. Front. Immunol. 2022, 13, 868813. [Google Scholar] [CrossRef] [PubMed]
- Boo, L.; Ho, W.Y.; Ali, N.M.; Yeap, S.K.; Ky, H.; Chan, K.G.; Yin, W.F.; Satharasinghe, D.A.; Liew, W.C.; Tan, S.W.; et al. Phenotypic and MicroRNA Transcriptomic Profiling of the MDA-MB-231 Spheroid-Enriched CSCs with Comparison of MCF-7 MicroRNA Profiling Dataset. PeerJ 2017, 5, e3551. [Google Scholar] [CrossRef] [PubMed]
- Ahram, M.; Mustafa, E.; Zaza, R.; Abu Hammad, S.; Alhudhud, M.; Bawadi, R.; Zihlif, M. Differential Expression and Androgen Regulation of MicroRNAs and Metalloprotease 13 in Breast Cancer Cells. Cell Biol. Int. 2017, 41, 1345–1355. [Google Scholar] [CrossRef]
- Search for: Breast Cancer, Other Terms: Micro RNA, Micro RNA|Card Results|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/search?cond=Breast%20Cancer&term=Micro%20RNA&intr=micro%20RNA (accessed on 2 November 2023).
- Lima, J.F.; Cerqueira, L.; Figueiredo, C.; Oliveira, C.; Azevedo, N.F. Anti-MiRNA Oligonucleotides: A Comprehensive Guide for Design. RNA Biol. 2018, 15, 338–352. [Google Scholar] [CrossRef]


| Gene | Function(s) | Estimated Risk | BC Type | Refs |
|---|---|---|---|---|
| BRCA1 | DNA repair transcription regulation cell cycle regulation chromatin remodeling | 55–65% by age 70 | TNBC luminal B | [11,25,27,28] |
| BRCA2 | DNA repair DNA replication transcription regulation cell cycle regulation mitophagy | ~45% by age 70 | TNBC luminal B | [11,25,28] |
| PALB2 | DNA repair | All women: RR 2.3, 95% CI 1.4–3.9 < 50 years: RR 3.0, 95% CI 1.4–5.5 | n/a | [17,25,28] |
| PTEN | cell survival cell growth | 85% lifetime | luminal A luminal B | [12,25,26,27,28] |
| TP53 | cell cycle regulation | 25% by age 74 | all | [13,25,26,27,28] |
| CDH1 | cell adhesion | 39% lifetime | luminal A | [14,25,26,27,28] |
| STK11 | cell cycle regulation | 32% by age 60 | n/a | [15,25,28] |
| CHEK2 | DNA repair cell cycle regulation apoptosis | Female: RR 1.70, 95% CI 1.3–2.2 Male: RR 10.3, 95% CI 3.5–30.0 | n/a | [16,25,26,27,28] |
| BRIP1 | DNA repair | All women: RR 2.0, 95% | n/a | [23,25] |
| ATM | DNA repair | RR 2.37, 95% CI 1.5–3.8 | n/a | [18,25,26,27,28] |
| Tumor | Node | Metastasis | |||
|---|---|---|---|---|---|
| Tx | no primary tumor information | Nx | not assessable | Mx | not assessed |
| T0 | no primary tumor evidence | N0 | no clinically positive nodes | M0 | no evidence |
| TIS | carcinoma in situ (primary sites) | N1 | single, ipsilateral, size < 3 cm | M1 | metastasis present at distance |
| T1 | size < 2 cm | N2a | single, ipsilateral, size 3–6 cm | ||
| T2 | size 2 to 4 cm | N2b | multiple, ipsilateral, size < 6 cm | ||
| T3 | size > 4 cm | N3 | massive/ipsilateral/bilateral/controlateral | ||
| T4 | size > 4 cm, pterygoid muscle, base of tongue or skin involved | N3a | ipsilateral node(s), one more than 6 cm | ||
| N3b | bilateral | ||||
| N4 | controlateral | ||||
| miR Name | Target Gene(s) | Affected Cellular Functions | Refs |
|---|---|---|---|
| miR-21 | PTEN | drug resistance | [65,66] |
| miR-21 | LZTFL1 | proliferation and metastasis | [67] |
| miR-21 | IGFBP3 TPM1 PCD4 TGF-β1 | proliferation, metastasis, epithelial-to-mesenchymal transition (EMT), apoptosis | [68] |
| miR-106a | RAF-1 | invasion and proliferation | [69] |
| miR-106a | P53 BAX RUNX3 Bcl-2 ABCG2 | proliferation, colony-forming capacity, migration, invasion, apoptosis, sensitivity to cisplatin | [70,71] |
| miR-155 | TRF1 | telomere fragility | [72] |
| miR-141 | ANP32E | migration and invasion | [74] |
| let-7 | ERCC6 | proliferation, apoptosis | [78] |
| miR-335 | ERα IGF1R SP1 ID4 | proliferation, apoptosis | [80] |
| miR-335 | c-Met | cell scattering, migration, and invasion | [81] |
| miR-126 | VEGFA PIK3R2 | angiogenesis, tumor genesis and growth | [83] |
| miR-126 | PIK3R2 | trastuzumab resistance | [84] |
| miR-199a/b-3p | PAK4 | migration and invasion | [86] |
| miR-199a-3p | mTOR c-Met | cell cycle progression, doxorubicin sensitivity, apoptosis | [87] |
| miR-199a-3p | TFAM | resistance to cisplatin | [88] |
| miR-199a-3p | TFAM | angiogenesis and metastasis under hypoxia | [89] |
| miR-101 | POMP Stmn1 DNMT3A EYA1 VHL SOX2 Jak2 MCL-1 | proliferation, apoptosis, angiogenesis, drug resistance, invasion, metastasis | [91] |
| miR-101-3p | COX-2 | migration, metastasis | [92] |
| miR-101-3p | EZH2 | migration, invasion, proliferation | [93] |
| miR-101-5p | GINS1 | DNA replication | [94] |
| miR-9 | FOXO1 | proliferation, migration, invasion | [99] |
| miR-9 | STARD13 | EMT, metastasis | [100] |
| miR-9 | LIFR | metastasis | [101] |
| miR-9 | elf5A2 | resistance to doxorubicin | [102] |
| miR-9 | HMGA2 EGR1 IGFBP3 | proliferation, metastasis, EMT, apoptosis | [68] |
| miR-9 | PDGFRβ | vasculogenesis | [103] |
| miR-200 | PDGFRβ | vasculogenesis | [103] |
| let-7a-5p miR-9-5p miR-10b miR-21 miR-22-3p miR-23b-3p miR-25-3p miR-29 miR-34a miR-93-5p miR-99a-5p/-3p miR-100-5p miR-101-3p miR-101-5p miR-126-5p/-3p miR-141-3p miR-143-5p/-3p miR-144-5p/-3p miR-145 miR-155 mir-181b1-5p miR-195-5p miR-199a-5p miR-200a miR-203 miR-203a-3p miR-205 miR-210-3p miR-221/222 miR-373 | n/a | biomarkers | [73,76,87,90,94,95,97,98] |
| miR | Organ | Target(s) | Notes | Refs |
|---|---|---|---|---|
| 125 | CNS | n/a | deregulated, pediatric | [130] |
| 125 | CNS | n/a | deregulated | [131,132] |
| 125 | CNS | p53, p38MAPK | none | [133] |
| 125 | CNS | BMF | none | [134] |
| 125a | ovary | n/a | EMT negative regulator | [144] |
| 125b | ovary | BCL3 | none | [145] |
| 125b | ovary | n/a | serum biomarker | [146] |
| 125b | bladder | E2F3 | none | [147] |
| 125b | bladder | n/a | urine biomarker | [148] |
| 125-3p | bladder | n/a | hypoxia regulated | [149] |
| 125 | bladder | n/a | survival predictor | [150] |
| 125a | liver | MMP11, VEGF | none | [151] |
| 125b | liver | Mcl-1, IL6R | none | [152] |
| 125b | liver | Lin28B2 | none | [153] |
| 125 | liver | Pokemon | none | [154] |
| 125 | liver | TRAF6 | none | [155] |
| 125 | liver | hexokinase II | none | [156] |
| 125 | liver | FOXM1 | none | [157] |
| 125 | skin | NCAM | none | [158] |
| 125 | skin | c-Jun | none | [159] |
| 125b | skin | MMP13 | none | [160] |
| 125b | skin | STAT3 | none | [161] |
| 125 | skin | n/a | deregulated | [162] |
| 125b | bone | STAT3 | none | [163,164] |
| 125 | bone | ErbB2 | none | [165] |
| 125 | bone | BAP1 | none | [166] |
| 125 | lung | n/a | survival predictor | [167] |
| 125 | lung | EGFR | none | [168] |
| 125 | lung | HER2 | trastuzumab resistance | [169] |
| 125 | lung | MMP13 | none | [170] |
| 125 | pancreas | n/a | deregulated | [171,172] |
| 125 | pancreas | NEDD9 | none | [173] |
| 125 | prostate | n/a | deregulated | [174,175,176] |
| 125 | prostate | BAK1 | none | [177] |
| 125 | prostate | p53, PUMA | none | [178] |
| 125b | thyroid | Foxp3 | cisplatin sensitivity | [179] |
| 125b | stomach | PPP1CA-Rb | none | [180] |
| 125a-5p | colon | BCL2, BCL2L12, MCL1 | none | [181] |
| 125b | kidney | n/a | survival predictor | [182] |
| miR | Reg. | Target | Reg. | Cellular Function | Cell Line | Ref. |
|---|---|---|---|---|---|---|
| miR-125a miR-125b | ↑ ↑ | ERBB2 ERBB3 | ↓ ↓ | migration invasion | SKBR3 | [189] |
| miR-125b | ↓ | ETS1 | ↑ | proliferation | BC samples | [190] |
| miR-125b | ↓ | MUC1 | ↑ | apoptosis | BT-549 ZR-75-1 | [191] |
| miR-125b | ↓ | STARD13 | ↑ | metastasis | MCF-7 MDA-MB-231 | [192] |
| miR-125 | ↓ | n/a | n/a | radioresistance | MCF-7 MDA-MB-231 | [193] |
| miR-125b | ↑ | n/a | n/a | chemoresistance proliferation apoptosis | blood samples | [194] |
| miR-125b | ↑ | BAK1 | ↓ | chemoresistance apoptosis | MDA-435 MDA-436 MDA-231 MCF7 SKBR3 | [195] |
| miR-125a-5p miR-125b | ↓ ↓ | n/a | n/a | age-dependent BC formation | BC samples | [196] |
| miR-125b | ↓ | MMP11 | ↑ | proliferation migration invasion | T47D SKBR3 | [197] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piergentili, R.; Marinelli, E.; Cucinella, G.; Lopez, A.; Napoletano, G.; Gullo, G.; Zaami, S. miR-125 in Breast Cancer Etiopathogenesis: An Emerging Role as a Biomarker in Differential Diagnosis, Regenerative Medicine, and the Challenges of Personalized Medicine. Non-Coding RNA 2024, 10, 16. https://doi.org/10.3390/ncrna10020016
Piergentili R, Marinelli E, Cucinella G, Lopez A, Napoletano G, Gullo G, Zaami S. miR-125 in Breast Cancer Etiopathogenesis: An Emerging Role as a Biomarker in Differential Diagnosis, Regenerative Medicine, and the Challenges of Personalized Medicine. Non-Coding RNA. 2024; 10(2):16. https://doi.org/10.3390/ncrna10020016
Chicago/Turabian StylePiergentili, Roberto, Enrico Marinelli, Gaspare Cucinella, Alessandra Lopez, Gabriele Napoletano, Giuseppe Gullo, and Simona Zaami. 2024. "miR-125 in Breast Cancer Etiopathogenesis: An Emerging Role as a Biomarker in Differential Diagnosis, Regenerative Medicine, and the Challenges of Personalized Medicine" Non-Coding RNA 10, no. 2: 16. https://doi.org/10.3390/ncrna10020016
APA StylePiergentili, R., Marinelli, E., Cucinella, G., Lopez, A., Napoletano, G., Gullo, G., & Zaami, S. (2024). miR-125 in Breast Cancer Etiopathogenesis: An Emerging Role as a Biomarker in Differential Diagnosis, Regenerative Medicine, and the Challenges of Personalized Medicine. Non-Coding RNA, 10(2), 16. https://doi.org/10.3390/ncrna10020016











