More than the SRY: The Non-Coding Landscape of the Y Chromosome and Its Importance in Human Disease
Abstract
:1. Introduction
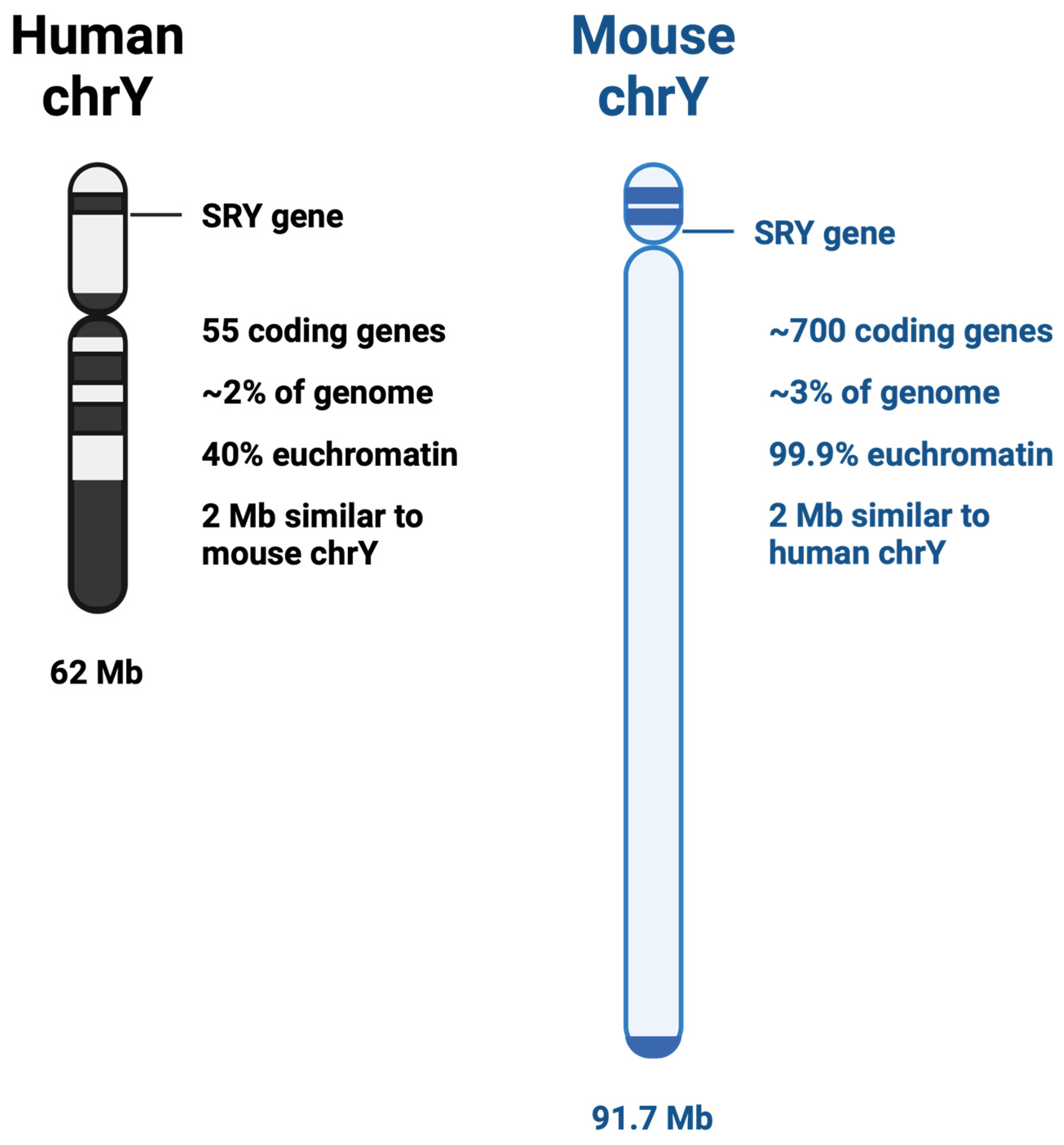
2. Y vs. X Chromosome
3. Non-Coding RNAs
3.1. Long Non-Coding RNAs from the Y Chromosome
3.2. MicroRNAs from the Y Chromosome
3.3. circRNA from the Y Chromosome
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reddy, K.D.; Oliver, B.G.G. Sexual Dimorphism in Chronic Respiratory Diseases. Cell Biosci. 2023, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Stabellini, N.; Bruno, D.S.; Dmukauskas, M.; Barda, A.J.; Cao, L.; Shanahan, J.; Waite, K.; Montero, A.J.; Barnholtz-Sloan, J.S. Sex Differences in Lung Cancer Treatment and Outcomes at a Large Hybrid Academic-Community Practice. JTO Clin. Res. Rep. 2022, 3, 100307. [Google Scholar] [CrossRef] [PubMed]
- Zang, E.A.; Wynder, E.L. Differences in Lung Cancer Risk Between Men and Women: Examination of the Evidence. JNCI J. Natl. Cancer Inst. 1996, 88, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.B.; McGlynn, K.A.; Devesa, S.S.; Freedman, N.D.; Anderson, W.F. Sex Disparities in Cancer Mortality and Survival. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Kharroubi, S.A.; Diab-El-Harake, M. Sex-Differences in COVID-19 Diagnosis, Risk Factors and Disease Comorbidities: A Large US-Based Cohort Study. Front. Public Health 2022, 10, 1029190. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Soto, M.C.; Ortega-Cáceres, G.; Arroyo-Hernández, H. Sex Differences in COVID-19 Fatality Rate and Risk of Death: An Analysis in 73 Countries, 2020–2021. Infez. Med. 2021, 29, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, S.V.; Rusu, R.; Chan, B.; Bellows, M.; O’Keefe, C.; Nicholson, S. Sex Differences in Sequelae from COVID-19 Infection and in Long COVID Syndrome: A Review. Curr. Med. Res. Opin. 2022, 38, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- SRY Sex Determining Region Y [Homo Sapiens (Human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/6736 (accessed on 7 June 2023).
- Svechnikov, K.; Söder, O. Ontogeny of Gonadal Sex Steroids. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Lauretta, R.; Sansone, M.; Sansone, A.; Romanelli, F.; Appetecchia, M. Gender in Endocrine Diseases: Role of Sex Gonadal Hormones. Int. J. Endocrinol. 2018, 2018, 4847376. [Google Scholar] [CrossRef]
- Quintero, O.L.; Amador-Patarroyo, M.J.; Montoya-Ortiz, G.; Rojas-Villarraga, A.; Anaya, J.-M. Autoimmune Disease and Gender: Plausible Mechanisms for the Female Predominance of Autoimmunity. J. Autoimmun. 2012, 38, J109–J119. [Google Scholar] [CrossRef]
- Merrill, S.J.; Mu, Y. Thyroid Autoimmunity as a Window to Autoimmunity: An Explanation for Sex Differences in the Prevalence of Thyroid Autoimmunity. J. Theor. Biol. 2015, 375, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Grazuleviciene, R.; Andrusaityte, S.; Rapalavicius, A.; Dėdelė, A. Environmentally Related Gender Health Risks: Findings from Citizen Science Cross-Sectional Study. BMC Public Health 2022, 22, 1426. [Google Scholar] [CrossRef]
- Sorensen, C.; Murray, V.; Lemery, J.; Balbus, J. Climate Change and Women’s Health: Impacts and Policy Directions. PLoS Med. 2018, 15, e1002603. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Harbin, S.; Irvin, E.; Johnston, H.; Begum, M.; Tiong, M.; Apedaile, D.; Koehoorn, M.; Smith, P. Differences between Men and Women in Their Risk of Work Injury and Disability: A Systematic Review. Am. J. Ind. Med. 2022, 65, 576–588. [Google Scholar] [CrossRef] [PubMed]
- McCann, J. Gender Differences in Cancer That Don’t Make Sense—Or Do They? JNCI J. Natl. Cancer Inst. 2000, 92, 1560–1562. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Moderna’s COVID-19 Vaccine Candidate Meets Its Primary Efficacy Endpoint in the First Interim Analysis of the Phase 3 COVE Study. Available online: https://investors.modernatx.com/news/news-details/2020/Modernas-COVID-19-Vaccine-Candidate-Meets-its-Primary-Efficacy-Endpoint-in-the-First-Interim-Analysis-of-the-Phase-3-COVE-Study/default.aspx (accessed on 28 March 2024).
- Rhie, A.; Nurk, S.; Cechova, M.; Hoyt, S.J.; Taylor, D.J.; Altemose, N.; Hook, P.W.; Koren, S.; Rautiainen, M.; Alexandrov, I.A.; et al. The Complete Sequence of a Human Y Chromosome. Nature 2023, 621, 344–354. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information (US). Chromosome Map. In Genes and Disease [Internet]; National Center for Biotechnology Information (US): Bethesda, MD, USA, 1998. [Google Scholar]
- Y Chromosome. Available online: https://www.genome.gov/about-genomics/fact-sheets/Y-Chromosome-facts (accessed on 7 June 2023).
- Bellott, D.W.; Hughes, J.F.; Skaletsky, H.; Brown, L.G.; Pyntikova, T.; Cho, T.-J.; Koutseva, N.; Zaghlul, S.; Graves, T.; Rock, S.; et al. Mammalian Y Chromosomes Retain Widely Expressed Dosage-Sensitive Regulators. Nature 2014, 508, 494–499. [Google Scholar] [CrossRef]
- Soh, Y.Q.S.; Alföldi, J.; Pyntikova, T.; Brown, L.G.; Graves, T.; Minx, P.J.; Fulton, R.S.; Kremitzki, C.; Koutseva, N.; Mueller, J.L.; et al. Sequencing the Mouse Y Chromosome Reveals Convergent Gene Acquisition and Amplification on Both Sex Chromosomes. Cell 2014, 159, 800–813. [Google Scholar] [CrossRef]
- XIST X Inactive Specific Transcript [Homo Sapiens (Human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/7503 (accessed on 26 February 2024).
- Brown, J.; Hendrich, B.D.; Rupert, J.L. The Human XIST Gene: Analysis of a 17 Kb Inactive X-Specific RNA That Contains Conserved Repeats and Is Highly Localized within the Nucleus. Cell 1992, 71, 527–542. [Google Scholar] [CrossRef]
- X-Inactivation Profile Reveals Extensive Variability in X-Linked Gene Expression in Females|Nature. Available online: https://www.nature.com/articles/nature03479 (accessed on 26 February 2024).
- Posynick, B.J.; Brown, C.J. Escape From X-Chromosome Inactivation: An Evolutionary Perspective. Front. Cell Dev. Biol. 2019, 7, 241. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial Sequencing and Analysis of the Human Genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef]
- Chinwalla, A.T.; Cook, L.L.; Delehaunty, K.D.; Fewell, G.A.; Fulton, L.A.; Fulton, R.S.; Graves, T.A.; Hillier, L.W.; Mardis, E.R.; McPherson, J.D.; et al. Initial Sequencing and Comparative Analysis of the Mouse Genome. Nature 2002, 420, 520–562. [Google Scholar] [CrossRef]
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F.; et al. Landscape of Transcription in Human Cells. Nature 2012, 489, 101–108. [Google Scholar] [CrossRef] [PubMed]
- International Human Genome Sequencing Consortium. Finishing the Euchromatic Sequence of the Human Genome. Nature 2004, 431, 931–945. [Google Scholar] [CrossRef]
- Skaletsky, H.; Kuroda-Kawaguchi, T.; Minx, P.J.; Cordum, H.S.; Hillier, L.; Brown, L.G.; Repping, S.; Pyntikova, T.; Ali, J.; Bieri, T.; et al. The Male-Specific Region of the Human Y Chromosome Is a Mosaic of Discrete Sequence Classes. Nature 2003, 423, 825–837. [Google Scholar] [CrossRef]
- Schneider, V.A.; Graves-Lindsay, T.; Howe, K.; Bouk, N.; Chen, H.-C.; Kitts, P.A.; Murphy, T.D.; Pruitt, K.D.; Thibaud-Nissen, F.; Albracht, D.; et al. Evaluation of GRCh38 and de Novo Haploid Genome Assemblies Demonstrates the Enduring Quality of the Reference Assembly. Genome Res. 2017, 27, 849–864. [Google Scholar] [CrossRef]
- Nurk, S.; Koren, S.; Rhie, A.; Rautiainen, M.; Bzikadze, A.V.; Mikheenko, A.; Vollger, M.R.; Altemose, N.; Uralsky, L.; Gershman, A.; et al. The Complete Sequence of a Human Genome. Science 2022, 376, 44–53. [Google Scholar] [CrossRef]
- Altemose, N.; Miga, K.H.; Maggioni, M.; Willard, H.F. Genomic Characterization of Large Heterochromatic Gaps in the Human Genome Assembly. PLoS Comput. Biol. 2014, 10, e1003628. [Google Scholar] [CrossRef]
- Betrán, E.; Demuth, J.P.; Williford, A. Why Chromosome Palindromes? Int. J. Evol. Biol. 2012, 2012, 207958. [Google Scholar] [CrossRef]
- Segmental Duplications and Their Variation in a Complete Human Genome. Available online: https://www.science.org/doi/10.1126/science.abj6965 (accessed on 24 February 2024).
- Porubsky, D.; Höps, W.; Ashraf, H.; Hsieh, P.; Rodriguez-Martin, B.; Yilmaz, F.; Ebler, J.; Hallast, P.; Maria Maggiolini, F.A.; Harvey, W.T.; et al. Recurrent Inversion Polymorphisms in Humans Associate with Genetic Instability and Genomic Disorders. Cell 2022, 185, 1986–2005. [Google Scholar] [CrossRef] [PubMed]
- Nakahori, Y.; Mitani, K.; Yamada, M.; Nakagome, Y. A Human Y-Chromosome Specific Repeated DNA Family (DYZ1) Consists of a Tandem Array of Pentanucleotides. Nucleic Acids Res. 1986, 14, 7569–7580. [Google Scholar] [CrossRef] [PubMed]
- Jarmuż, M.; Glotzbach, C.D.; Bailey, K.A.; Bandyopadhyay, R.; Shaffer, L.G. The Evolution of Satellite III DNA Subfamilies among Primates. Am. J. Hum. Genet. 2007, 80, 495–501. [Google Scholar] [CrossRef]
- Cooke, H.J.; Hindley, J. Cloning of Human Satellite III DNA: Different Components Are on Different Chromosomes. Nucleic Acids Res. 1979, 6, 3177–3198. [Google Scholar] [CrossRef]
- Fonseca-Carvalho, M.; Veríssimo, G.; Lopes, M.; Ferreira, D.; Louzada, S.; Chaves, R. Answering the Cell Stress Call: Satellite Non-Coding Transcription as a Response Mechanism. Biomolecules 2024, 14, 124. [Google Scholar] [CrossRef] [PubMed]
- Valgardsdottir, R.; Chiodi, I.; Giordano, M.; Rossi, A.; Bazzini, S.; Ghigna, C.; Riva, S.; Biamonti, G. Transcription of Satellite III Non-Coding RNAs Is a General Stress Response in Human Cells. Nucleic Acids Res. 2008, 36, 423–434. [Google Scholar] [CrossRef]
- Porokhovnik, L.N.; Veiko, N.N.; Ershova, E.S.; Kostyuk, S.V. The Role of Human Satellite III (1q12) Copy Number Variation in the Adaptive Response during Aging, Stress, and Pathology: A Pendulum Model. Genes 2021, 12, 1524. [Google Scholar] [CrossRef]
- Jehan, Z.; Vallinayagam, S.; Tiwari, S.; Pradhan, S.; Singh, L.; Suresh, A.; Reddy, H.M.; Ahuja, Y.R.; Jesudasan, R.A. Novel Noncoding RNA from Human Y Distal Heterochromatic Block (Yq12) Generates Testis-Specific Chimeric CDC2L2. Genome Res. 2007, 17, 433–440. [Google Scholar] [CrossRef]
- Hallast, P.; Ebert, P.; Loftus, M.; Yilmaz, F.; Audano, P.A.; Logsdon, G.A.; Bonder, M.J.; Zhou, W.; Höps, W.; Kim, K.; et al. Assembly of 43 Human Y Chromosomes Reveals Extensive Complexity and Variation. Nature 2023, 621, 355–364. [Google Scholar] [CrossRef]
- Zhou, W.; Machiela, M.J.; Freedman, N.D.; Rothman, N.; Malats, N.; Dagnall, C.; Caporaso, N.; Teras, L.T.; Gaudet, M.M.; Gapstur, S.M.; et al. Mosaic Loss of Chromosome Y Is Associated with Common Variation near TCL1A. Nat. Genet. 2016, 48, 563–568. [Google Scholar] [CrossRef]
- Dumanski, J.P.; Rasi, C.; Lönn, M.; Davies, H.; Ingelsson, M.; Giedraitis, V.; Lannfelt, L.; Magnusson, P.K.; Lindgren, C.M.; Morris, A.P.; et al. Smoking Is Associated with Mosaic Loss of Chromosome Y. Science 2014, 347, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Dumanski, J.P.; Lambert, J.-C.; Rasi, C.; Giedraitis, V.; Davies, H.; Grenier-Boley, B.; Lindgren, C.M.; Campion, D.; Dufouil, C.; Pasquier, F.; et al. Mosaic Loss of Chromosome Y in Blood Is Associated with Alzheimer Disease. Am. J. Hum. Genet. 2016, 98, 1208–1219. [Google Scholar] [CrossRef] [PubMed]
- Sano, S.; Horitani, K.; Ogawa, H.; Halvardson, J.; Chavkin, N.W.; Wang, Y.; Sano, M.; Mattisson, J.; Hata, A.; Danielsson, M.; et al. Hematopoietic Loss of Y Chromosome Leads to Cardiac Fibrosis and Heart Failure Mortality. Science 2022, 377, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Mattisson, J.; Danielsson, M.; Hammond, M.; Davies, H.; Gallant, C.J.; Nordlund, J.; Raine, A.; Edén, M.; Kilander, L.; Ingelsson, M.; et al. Leukocytes with Chromosome Y Loss Have Reduced Abundance of the Cell Surface Immunoprotein CD99. Sci. Rep. 2021, 11, 15160. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, L.A.; Rasi, C.; Malmqvist, N.; Davies, H.; Pasupulati, S.; Pakalapati, G.; Sandgren, J.; de Ståhl, T.D.; Zaghlool, A.; Giedraitis, V.; et al. Mosaic Loss of Chromosome Y in Peripheral Blood Is Associated with Shorter Survival and Higher Risk of Cancer. Nat. Genet. 2014, 46, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Velazquez Camacho, O.; Yazbeck, A.M.; Wölwer, C.; Zhai, W.; Schumacher, J.; Heider, D.; Buettner, R.; Quaas, A.; Hillmer, A.M. Why Loss of Y? A Pan-Cancer Genome Analysis of Tumors with Loss of Y Chromosome. Comput. Struct. Biotechnol. J. 2023, 21, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Hollows, R.; Wei, W.; Cazier, J.; Mehanna, H.; Parry, G.; Halford, G.; Murray, P. Association between Loss of Y Chromosome and Poor Prognosis in Male Head and Neck Squamous Cell Carcinoma. Head Neck 2019, 41, 993–1006. [Google Scholar] [CrossRef] [PubMed]
- Palade, G.E. A Small Particulate Component of the Cytoplasm. J. Biophys. Biochem. Cytol. 1955, 1, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, M.B.; Stephenson, M.L.; Scott, J.F.; Hecht, L.I.; Zamecnik, P.C. A Soluble Ribonucleic Acid Intermediate in Protein Synthesis. J. Biol. Chem. 1958, 231, 241–257. [Google Scholar] [CrossRef]
- Chi, K.R. The Dark Side of the Human Genome. Nature 2016, 538, 275–277. [Google Scholar] [CrossRef]
- de Koning, A.P.J.; Gu, W.; Castoe, T.A.; Batzer, M.A.; Pollock, D.D. Repetitive Elements May Comprise Over Two-Thirds of the Human Genome. PLoS Genet. 2011, 7, e1002384. [Google Scholar] [CrossRef] [PubMed]
- Kung, J.T.Y.; Colognori, D.; Lee, J.T. Long Noncoding RNAs: Past, Present, and Future. Genetics 2013, 193, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, M.; Mohan, M. MicroRNAs: History, Biogenesis, and Their Evolving Role in Animal Development and Disease. Vet. Pathol. 2014, 51, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Nigro, J.M.; Cho, K.R.; Fearon, E.R.; Kern, S.E.; Ruppert, J.M.; Oliner, J.D.; Kinzler, K.W.; Vogelstein, B. Scrambled Exons. Cell 1991, 64, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, W.; Chen, Q.; Chen, M. Non-Coding RNAs and Their Integrated Networks. J. Integr. Bioinform. 2019, 16, 20190027. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Hirochika, H. Silencing of Transposable Elements in Plants. Trends Plant Sci. 2001, 6, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Nishida, K.M.; Mori, T.; Kawamura, Y.; Miyoshi, K.; Nagami, T.; Siomi, H.; Siomi, M.C. Specific Association of Piwi with rasiRNAs Derived from Retrotransposon and Heterochromatic Regions in the Drosophila Genome. Genes Dev. 2006, 20, 2214–2222. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Maji, R.K.; Saha, S.; Ghosh, Z. piRNAQuest: Searching the piRNAome for Silencers. BMC Genom. 2014, 15, 555. [Google Scholar] [CrossRef] [PubMed]
- Özata, D.M.; Yu, T.; Mou, H.; Gainetdinov, I.; Colpan, C.; Cecchini, K.; Kaymaz, Y.; Wu, P.-H.; Fan, K.; Kucukural, A.; et al. Evolutionarily Conserved Pachytene piRNA Loci Are Highly Divergent among Modern Humans. Nat. Ecol. Evol. 2020, 4, 156–168. [Google Scholar] [CrossRef]
- Ha, H.; Song, J.; Wang, S.; Kapusta, A.; Feschotte, C.; Chen, K.C.; Xing, J. A Comprehensive Analysis of piRNAs from Adult Human Testis and Their Relationship with Genes and Mobile Elements. BMC Genom. 2014, 15, 545. [Google Scholar] [CrossRef]
- Tang, H.; Wu, Z.; Zhang, Y.; Xia, T.; Liu, D.; Cai, J.; Ye, Q. Identification and Function Analysis of a Five-Long Noncoding RNA Prognostic Signature for Endometrial Cancer Patients. DNA Cell Biol. 2019, 38, 1480–1498. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, A.F.; Perelli, F.; Zaami, S.; Piergentili, R.; Mattei, A.; Vizzielli, G.; Scambia, G.; Straface, G.; Restaino, S.; Signore, F. Towards Personalized Medicine: Non-Coding RNAs and Endometrial Cancer. Healthcare 2021, 9, 965. [Google Scholar] [CrossRef] [PubMed]
- Perakis, S.O.; Thomas, J.E.; Pichler, M. Non-Coding RNAs Enabling Prognostic Stratification and Prediction of Therapeutic Response in Colorectal Cancer Patients. Adv. Exp. Med. Biol. 2016, 937, 183–204. [Google Scholar] [CrossRef] [PubMed]
- Gulìa, C.; Signore, F.; Gaffi, M.; Gigli, S.; Votino, R.; Nucciotti, R.; Bertacca, L.; Zaami, S.; Baffa, A.; Santini, E.; et al. Y RNA: An Overview of Their Role as Potential Biomarkers and Molecular Targets in Human Cancers. Cancers 2020, 12, 1238. [Google Scholar] [CrossRef]
- Piergentili, R.; Basile, G.; Nocella, C.; Carnevale, R.; Marinelli, E.; Patrone, R.; Zaami, S. Using ncRNAs as Tools in Cancer Diagnosis and Treatment—The Way towards Personalized Medicine to Improve Patients’ Health. Int. J. Mol. Sci. 2022, 23, 9353. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef]
- Johnsson, P.; Lipovich, L.; Grandér, D.; Morris, K.V. Evolutionary Conservation of Long Noncoding RNAs; Sequence, Structure, Function. Biochim. Biophys. Acta 2014, 1840, 1063–1071. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, X.; Liu, L.; Deng, H.; Zhang, J.; Xu, Q.; Cen, B.; Ji, A. Regulation of lncRNA Expression. Cell Mol. Biol. Lett. 2014, 19, 561–575. [Google Scholar] [CrossRef]
- Ma, L.; Bajic, V.B.; Zhang, Z. On the Classification of Long Non-Coding RNAs. RNA Biol. 2013, 10, 924–933. [Google Scholar] [CrossRef]
- ASMTL-AS1 ASMTL Antisense RNA 1 [Homo Sapiens (Human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/80161 (accessed on 15 June 2023).
- Perez, C.A.G.; Adachi, S.; Nong, Q.D.; Adhitama, N.; Matsuura, T.; Natsume, T.; Wada, T.; Kato, Y.; Watanabe, H. Sense-Overlapping lncRNA as a Decoy of Translational Repressor Protein for Dimorphic Gene Expression. PLoS Genet. 2021, 17, e1009683. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, J.D.; Wei, Y.; Khavari, P.A. The Functions and Unique Features of Long Intergenic Non-Coding RNA. Nat. Rev. Mol. Cell Biol. 2018, 19, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Brownmiller, T.; Juric, J.A.; Ivey, A.D.; Harvey, B.M.; Westemeier, E.S.; Winters, M.T.; Stevens, A.M.; Stanley, A.N.; Hayes, K.E.; Sprowls, S.A.; et al. Y Chromosome LncRNA Are Involved in Radiation Response of Male Non–Small Cell Lung Cancer Cells. Cancer Res. 2020, 80, 4046–4057. [Google Scholar] [CrossRef] [PubMed]
- Cerase, A.; Pintacuda, G.; Tattermusch, A.; Avner, P. Xist Localization and Function: New Insights from Multiple Levels. Genome Biol. 2015, 16, 166. [Google Scholar] [CrossRef] [PubMed]
- Leisegang, M.S.; Fork, C.; Josipovic, I.; Richter, F.M.; Preussner, J.; Hu, J.; Miller, M.J.; Epah, J.; Hofmann, P.; Günther, S.; et al. Long Noncoding RNA MANTIS Facilitates Endothelial Angiogenic Function. Circulation 2017, 136, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, V.; Ellis, J.D.; Shen, Z.; Song, D.Y.; Pan, Q.; Watt, A.T.; Freier, S.M.; Bennett, C.F.; Sharma, A.; Bubulya, P.A.; et al. The Nuclear-Retained Noncoding RNA MALAT1 Regulates Alternative Splicing by Modulating SR Splicing Factor Phosphorylation. Mol. Cell 2010, 39, 925–938. [Google Scholar] [CrossRef]
- Brown, J.A.; Kinzig, C.G.; DeGregorio, S.J.; Steitz, J.A. Methyltransferase-like Protein 16 Binds the 3′-Terminal Triple Helix of MALAT1 Long Noncoding RNA. Proc. Natl. Acad. Sci. USA 2016, 113, 14013–14018. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, T.; Zhang, Z.; Lu, M.; Zhao, W.; Zeng, X.; Zhang, W. Long Non-Coding RNA TUG1 Promotes Migration and Invasion by Acting as a ceRNA of miR-335-5p in Osteosarcoma Cells. Cancer Sci. 2017, 108, 859–867. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, Y.; Xu, X.; Su, X.; Liu, Y.; Ma, Y.; Zhao, Y.; Shen, Z.; Huang, B.; Cao, X. Inducible Degradation of lncRNA Sros1 Promotes IFN-γ-Mediated Activation of Innate Immune Responses by Stabilizing Stat1 mRNA. Nat. Immunol. 2019, 20, 1621–1630. [Google Scholar] [CrossRef]
- Feng, Y.; Gao, L.; Cui, G.; Cao, Y. LncRNA NEAT1 Facilitates Pancreatic Cancer Growth and Metastasis through Stabilizing ELF3 mRNA. Am. J. Cancer Res. 2020, 10, 237–248. [Google Scholar]
- Rossi, M.; Bucci, G.; Rizzotto, D.; Bordo, D.; Marzi, M.J.; Puppo, M.; Flinois, A.; Spadaro, D.; Citi, S.; Emionite, L.; et al. LncRNA EPR Controls Epithelial Proliferation by Coordinating Cdkn1a Transcription and mRNA Decay Response to TGF-β. Nat. Commun. 2019, 10, 1969. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Chen, H.; Ma, Z.; Han, C.; Yin, W.; Wang, S.; Zhu, H.; Xia, W.; Wang, J.; Xu, L.; et al. LncRNA LINC00525 Suppresses P21 Expression via mRNA Decay and Triplex-Mediated Changes in Chromatin Structure in Lung Adenocarcinoma. Cancer Commun. 2021, 41, 596–614. [Google Scholar] [CrossRef] [PubMed]
- Grinman, E.; Nakahata, Y.; Avchalumov, Y.; Espadas, I.; Swarnkar, S.; Yasuda, R.; Puthanveettil, S.V. Puthanveettil Activity-Regulated Synaptic Targeting of lncRNA ADEPTR Mediates Structural Plasticity by Localizing Sptn1 and AnkB in Dendrites|Science Advances. Sci. Adv. 2021, 7, eabf0605. [Google Scholar] [CrossRef] [PubMed]
- Sirey, T.M.; Roberts, K.; Haerty, W.; Bedoya-Reina, O.; Rogatti-Granados, S.; Tan, J.Y.; Li, N.; Heather, L.C.; Carter, R.N.; Cooper, S.; et al. The Long Non-Coding RNA Cerox1 Is a Post Transcriptional Regulator of Mitochondrial Complex I Catalytic Activity. eLife 2019, 8, e45051. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Orera, J.; Villanueva-Cañas, J.L.; Albà, M.M. Evolution of New Proteins from Translated sORFs in Long Non-Coding RNAs. Exp. Cell Res. 2020, 391, 111940. [Google Scholar] [CrossRef] [PubMed]
- Bánfai, B.; Jia, H.; Khatun, J.; Wood, E.; Risk, B.; Gundling, W.E.; Kundaje, A.; Gunawardena, H.P.; Yu, Y.; Xie, L.; et al. Long Noncoding RNAs Are Rarely Translated in Two Human Cell Lines. Genome Res. 2012, 22, 1646–1657. [Google Scholar] [CrossRef] [PubMed]
- Volders, P.-J.; Anckaert, J.; Verheggen, K.; Nuytens, J.; Martens, L.; Mestdagh, P.; Vandesompele, J. LNCipedia 5: Towards a Reference Set of Human Long Non-Coding RNAs. Nucleic Acids Res. 2019, 47, D135–D139. [Google Scholar] [CrossRef] [PubMed]
- Yapijakis, C.; Serefoglou, Z.; Papadimitriou, K.; Makrinou, E. High Frequency of TTTY2-like Gene-Related Deletions in Patients with Idiopathic Oligozoospermia and Azoospermia. Andrologia 2015, 47, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.; Chew, G.S.; Myers, S.A.; Clarence, E.M.; Eales, J.M.; Tomaszewski, M.; Charchar, F.J. A Novel Y-Specific Long Non-Coding RNA Associated with Cellular Lipid Accumulation in HepG2 Cells and Atherosclerosis-Related Genes. Sci. Rep. 2017, 7, 16710. [Google Scholar] [CrossRef]
- Wicik, Z.; Jales Neto, L.H.; Guzman, L.E.F.; Pavão, R.; Takayama, L.; Caparbo, V.F.; Lopes, N.H.M.; Pereira, A.C.; Pereira, R.M.R. The Crosstalk between Bone Metabolism, lncRNAs, microRNAs and mRNAs in Coronary Artery Calcification. Genomics 2021, 113, 503–513. [Google Scholar] [CrossRef]
- Winsvold, B.S.; Palta, P.; Eising, E.; Page, C.M.; van den Maagdenberg, A.M.; Palotie, A.; Zwart, J.-A. Epigenetic DNA Methylation Changes Associated with Headache Chronification: A Retrospective Case-Control Study. Cephalalgia 2018, 38, 312–322. [Google Scholar] [CrossRef]
- Zhou, M.; Zhao, H.; Wang, Z.; Cheng, L.; Yang, L.; Shi, H.; Yang, H.; Sun, J. Identification and Validation of Potential Prognostic lncRNA Biomarkers for Predicting Survival in Patients with Multiple Myeloma. J. Exp. Clin. Cancer Res. 2015, 34, 102. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, L.; Deng, J.; Guo, B.; Li, F.; Wang, Y.; Wu, R.; Zhang, S.; Lu, J.; Zhou, Y. A Novel Micropeptide Encoded by Y-Linked LINC00278 Links Cigarette Smoking and AR Signaling in Male Esophageal Squamous Cell Carcinoma. Cancer Res. 2020, 80, 2790–2803. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yu, J.; Zhu, H.; Guo, Y.; Feng, S. Identification of Key lncRNAs in Colorectal Cancer Progression Based on Associated Protein–Protein Interaction Analysis. World J. Surg. Oncol. 2017, 15, 153. [Google Scholar] [CrossRef]
- Wen, X.; Han, W.; Liu, C. Long Non-Coding RNA TTTY15 Silencing Inhibits Gastric Cancer Progression by Sponging microRNA-98-5p to down-Regulate Cyclin D2 Expression. Bioengineered 2022, 13, 7380–7391. [Google Scholar] [CrossRef]
- Wang, W.; Yang, J. Long Noncoding RNA TTTY15 Promotes Growth and Metastasis of Esophageal Squamous Cell Carcinoma by Sponging microRNA-337-3p to Upregulate the Expression of JAK2. Anti-Cancer Drugs 2020, 31, 1038. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Yao, J.; Kong, D.; Ye, C.; Chen, R.; Li, L.; Zeng, T.; Wang, L.; Zhang, W.; Shi, X.; et al. The Long Noncoding RNA TTTY15, Which Is Located on the Y Chromosome, Promotes Prostate Cancer Progression by Sponging Let-7. Eur. Urol. 2019, 76, 315–326. [Google Scholar] [CrossRef]
- Ma, D.; Gao, X.; Liu, Z.; Lu, X.; Ju, H.; Zhang, N. Exosome-Transferred Long Non-Coding RNA ASMTL-AS1 Contributes to Malignant Phenotypes in Residual Hepatocellular Carcinoma after Insufficient Radiofrequency Ablation. Cell Prolif. 2020, 53, e12795. [Google Scholar] [CrossRef]
- Liang, W.; Wang, Y.; Zhang, Q.; Gao, M.; Zhou, H.; Wang, Z. M6A-Mediated Upregulation of LINC00106 Promotes Stemness and Metastasis Properties of Hepatocellular Carcinoma via Sponging Let7f. Front. Cell Dev. Biol. 2021, 9, 781867. [Google Scholar] [CrossRef]
- Li, Z.-B.; Shi, L.-Y.; Han, Y.-S.; Chen, J.; Zhang, S.-Q.; Chen, J.-X.; Liu, J.; Tu, H.-H.; Lu, Q.-Q.; Yu, Y.; et al. Pyridoxal Phosphate, Pyridoxamine Phosphate, and Folic Acid Based on ceRNA Regulatory Network as Potential Biomarkers for the Diagnosis of Pulmonary Tuberculosis. Infect. Genet. Evol. 2022, 99, 105240. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V.; Bartel, B.; Bartel, D.P.; Burge, C.B.; Carrington, J.C.; Chen, X.; Dreyfuss, G.; Eddy, S.R.; Griffiths-Jones, S.; Marshall, M.; et al. A Uniform System for microRNA Annotation. RNA 2003, 9, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Vidigal, J.A.; Ventura, A. The Biological Functions of miRNAs: Lessons from in Vivo Studies. Trends Cell Biol. 2015, 25, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.I.; Yan, K.; Amuthan, G.; Chendrimada, T.; Doratotaj, B.; Cooch, N.; Shiekhattar, R. The Microprocessor Complex Mediates the Genesis of microRNAs. Nature 2004, 432, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microRNA Biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Cullen, B.R. Transcription and Processing of Human microRNA Precursors. Mol. Cell 2004, 16, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jeon, K.; Lee, J.-T.; Kim, S.; Kim, V.N. MicroRNA Maturation: Stepwise Processing and Subcellular Localization. EMBO J. 2002, 21, 4663–4670. [Google Scholar] [CrossRef]
- Alarcón, C.R.; Lee, H.; Goodarzi, H.; Halberg, N.; Tavazoie, S.F. N6-Methyladenosine Marks Primary microRNAs for Processing. Nature 2015, 519, 482–485. [Google Scholar] [CrossRef]
- Landthaler, M.; Yalcin, A.; Tuschl, T. The Human DiGeorge Syndrome Critical Region Gene 8 and Its D. Melanogaster Homolog Are Required for miRNA Biogenesis. Curr. Biol. 2004, 14, 2162–2167. [Google Scholar] [CrossRef] [PubMed]
- Okada, C.; Yamashita, E.; Lee, S.J.; Shibata, S.; Katahira, J.; Nakagawa, A.; Yoneda, Y.; Tsukihara, T. A High-Resolution Structure of the Pre-microRNA Nuclear Export Machinery. Science 2009, 326, 1275–1279. [Google Scholar] [CrossRef]
- Yoshida, T.; Asano, Y.; Ui-Tei, K. Modulation of MicroRNA Processing by Dicer via Its Associated dsRNA Binding Proteins. Noncoding RNA 2021, 7, 57. [Google Scholar] [CrossRef]
- Yoda, M.; Kawamata, T.; Paroo, Z.; Ye, X.; Iwasaki, S.; Liu, Q.; Tomari, Y. ATP-Dependent Human RISC Assembly Pathways. Nat. Struct. Mol. Biol. 2010, 17, 17–23. [Google Scholar] [CrossRef]
- Huntzinger, E.; Izaurralde, E. Gene Silencing by microRNAs: Contributions of Translational Repression and mRNA Decay. Nat. Rev. Genet. 2011, 12, 99–110. [Google Scholar] [CrossRef]
- Babiarz, J.E.; Ruby, J.G.; Wang, Y.; Bartel, D.P.; Blelloch, R. Mouse ES Cells Express Endogenous shRNAs, siRNAs, and Other Microprocessor-Independent, Dicer-Dependent Small RNAs. Genes Dev. 2008, 22, 2773–2785. [Google Scholar] [CrossRef] [PubMed]
- A Dicer-Independent miRNA Biogenesis Pathway That Requires Ago Catalysis|Nature. Available online: https://www.nature.com/articles/nature09092 (accessed on 29 February 2024).
- Ruby, J.G.; Jan, C.H.; Bartel, D.P. Intronic microRNA Precursors That Bypass Drosha Processing. Nature 2007, 448, 83–86. [Google Scholar] [CrossRef]
- Rorbach, G.; Unold, O.; Konopka, B.M. Distinguishing Mirtrons from Canonical miRNAs with Data Exploration and Machine Learning Methods. Sci. Rep. 2018, 8, 7560. [Google Scholar] [CrossRef]
- Yang, J.-S.; Maurin, T.; Lai, E.C. Functional Parameters of Dicer-Independent microRNA Biogenesis. RNA 2012, 18, 945–957. [Google Scholar] [CrossRef]
- Broughton, J.P.; Lovci, M.T.; Huang, J.L.; Yeo, G.W.; Pasquinelli, A.E. Pairing beyond the Seed Supports MicroRNA Targeting Specificity. Mol. Cell 2016, 64, 320–333. [Google Scholar] [CrossRef]
- Riolo, G.; Cantara, S.; Marzocchi, C.; Ricci, C. miRNA Targets: From Prediction Tools to Experimental Validation. Methods Protoc. 2021, 4, 1. [Google Scholar] [CrossRef]
- Yang, M.; Woolfenden, H.C.; Zhang, Y.; Fang, X.; Liu, Q.; Vigh, M.L.; Cheema, J.; Yang, X.; Norris, M.; Yu, S.; et al. Intact RNA Structurome Reveals mRNA Structure-Mediated Regulation of miRNA Cleavage in Vivo. Nucleic Acids Res. 2020, 48, 8767–8781. [Google Scholar] [CrossRef]
- Zheng, Z.; Reichel, M.; Deveson, I.; Wong, G.; Li, J.; Millar, A.A. Target RNA Secondary Structure Is a Major Determinant of miR159 Efficacy. Plant Physiol. 2017, 174, 1764–1778. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, R.; Van Roosbroeck, K.; Calin, G.A. Cell-to-Cell Communication: microRNAs as Hormones. Mol. Oncol. 2017, 11, 1673–1686. [Google Scholar] [CrossRef]
- Fu, H.; Zhou, F.; Yuan, Q.; Zhang, W.; Qiu, Q.; Yu, X.; He, Z. miRNA-31-5p Mediates the Proliferation and Apoptosis of Human Spermatogonial Stem Cells via Targeting JAZF1 and Cyclin A2. Mol. Ther. Nucleic Acids 2019, 14, 90–100. [Google Scholar] [CrossRef]
- Yu, T.; Zuo, Q.-F.; Gong, L.; Wang, L.-N.; Zou, Q.-M.; Xiao, B. MicroRNA-491 Regulates the Proliferation and Apoptosis of CD8+ T Cells. Sci. Rep. 2016, 6, 30923. [Google Scholar] [CrossRef]
- Chen, W.; Cai, G.; Liao, Z.; Lin, K.; Li, G.; Li, Y. miRNA-766 Induces Apoptosis of Human Colon Cancer Cells through the P53/Bax Signaling Pathway by MDM4. Exp. Ther. Med. 2019, 17, 4100–4108. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, Y.; Zhao, J.; Liu, Q.; Feng, W.; Fan, J.; Wang, P. miR-367 Promotes Epithelial-to-Mesenchymal Transition and Invasion of Pancreatic Ductal Adenocarcinoma Cells by Targeting the Smad7-TGF-β Signalling Pathway. Br. J. Cancer 2015, 112, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Tanahashi, T.; Okada, R.; Toyoda, H.; Kumada, T.; Enomoto, M.; Tamori, A.; Kawada, N.; Taguchi, Y.-H.; Azuma, T. Comparison of Hepatocellular Carcinoma miRNA Expression Profiling as Evaluated by Next Generation Sequencing and Microarray. PLoS ONE 2014, 9, e106314. [Google Scholar] [CrossRef]
- Yuan, Z.; Sun, X.; Liu, H.; Xie, J. MicroRNA Genes Derived from Repetitive Elements and Expanded by Segmental Duplication Events in Mammalian Genomes. PLoS ONE 2011, 6, e17666. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA Sequences to Function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Griffiths-Jones, S. The microRNA Registry. Nucleic Acids Res. 2004, 32, D109–D111. [Google Scholar] [CrossRef]
- Griffiths-Jones, S.; Grocock, R.J.; van Dongen, S.; Bateman, A.; Enright, A.J. miRBase: microRNA Sequences, Targets and Gene Nomenclature. Nucleic Acids Res. 2006, 34, D140–D144. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S.; Saini, H.K.; van Dongen, S.; Enright, A.J. miRBase: Tools for microRNA Genomics. Nucleic Acids Res. 2008, 36, D154–D158. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating High Confidence microRNAs Using Deep Sequencing Data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Integrating microRNA Annotation and Deep-Sequencing Data. Nucleic Acids Res. 2011, 39, D152–D157. [Google Scholar] [CrossRef]
- Loss of miR-514a-3p Regulation of PEG3 Activates the NF-Kappa B Pathway in Human Testicular Germ Cell Tumors—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/28471449/ (accessed on 29 February 2024).
- Vaz, C.; Ahmad, H.M.; Sharma, P.; Gupta, R.; Kumar, L.; Kulshreshtha, R.; Bhattacharya, A. Analysis of microRNA Transcriptome by Deep Sequencing of Small RNA Libraries of Peripheral Blood. BMC Genom. 2010, 11, 288. [Google Scholar] [CrossRef]
- Yoo, J.K.; Kim, J.; Choi, S.-J.; Noh, H.M.; Kwon, Y.D.; Yoo, H.; Yi, H.S.; Chung, H.M.; Kim, J.K. Discovery and Characterization of Novel microRNAs during Endothelial Differentiation of Human Embryonic Stem Cells. Stem Cells Dev. 2012, 21, 2049–2057. [Google Scholar] [CrossRef]
- Song, J.; Widen, S.G.; Wolf, S.E.; EI Ayadi, A. Skeletal Muscle Transcriptome Is Affected by Age in Severely Burned Mice. Sci. Rep. 2022, 12, 21584. [Google Scholar] [CrossRef]
- Yin, M.; Zhou, L.; Ji, Y.; Lu, R.; Ji, W.; Jiang, G.; Ma, J.; Song, X. In Silico Identification and Verification of Ferroptosis-Related Genes in Type 2 Diabetic Islets. Front. Endocrinol. 2022, 13, 946492. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, Z.; Guo, Y.; Wang, D. Long Noncoding RNA SNHG10 Promotes Colorectal Cancer Cells Malignant Progression by Targeting miR-3690. Bioengineered 2021, 12, 6010–6020. [Google Scholar] [CrossRef]
- Shen, F.; Gan, X.-X.; Deng, X.-Y.; Feng, J.-H.; Cai, W.-S.; Shen, L.; Xiao, H.-Q.; Xu, B. MicroRNA-3690 Promotes Cell Proliferation and Cell Cycle Progression by Altering DKK3 Expression in Human Thyroid Cancer. Oncol. Lett. 2020, 20, 223. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-C.; Pang, F.-X.; Ou, S.-S.; Wei, X.-J.; Xu, Y.-J.; Lai, Y.-H. Risk Score Based on Two microRNAs as a Prognostic Marker of Hepatocellular Carcinoma and the Corresponding Competitive Endogenous RNA Network. IJGM 2021, 14, 3377–3385. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cheng, M.; Gu, B.; Wang, J.; Yan, S.; Xu, D. CircRNA_09505 Aggravates Inflammation and Joint Damage in Collagen-Induced Arthritis Mice via miR-6089/AKT1/NF-κB Axis. Cell Death Dis. 2020, 11, 833. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Song, M.; Chai, C.; Wang, J.; Jin, C.; Wang, X.; Cheng, M.; Yan, S. Exosome-encapsulated miR-6089 Regulates Inflammatory Response via Targeting TLR4. J. Cell. Physiol. 2019, 234, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wang, S.; Zhang, Z.; Lu, Y.; Yang, M.; Chen, P.; Chen, L.; Wang, M. MiRNA-6089 Inhibits Rheumatoid Arthritis Fibroblast-like Synoviocytes Proliferation and Induces Apoptosis by Targeting CCR4. Arch. Physiol. Biochem. 2020, 128, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wang, P.; Wang, J.; Yang, J.; Lu, H.; Jin, C.; Cheng, M.; Xu, D. Long Non-Coding RNA HIX003209 Promotes Inflammation by Sponging miR-6089 via TLR4/NF-κB Signaling Pathway in Rheumatoid Arthritis. Front. Immunol. 2019, 10, 2218. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Shi, A.-J.; Li, J.; Yuan, H.-F. Plasma miR-6089 as Potential Diagnostic Biomarker for Retinoblastoma. Int. Ophthalmol. 2021, 41, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- Fulzele, S.; Sahay, B.; Yusufu, I.; Lee, T.J.; Sharma, A.; Kolhe, R.; Isales, C.M. COVID-19 Virulence in Aged Patients Might Be Impacted by the Host Cellular MicroRNAs Abundance/Profile. Aging Dis. 2020, 11, 509–522. [Google Scholar] [CrossRef]
- Hsu, M.-T.; Coca-Prados, M. Electron Microscopic Evidence for the Circular Form of RNA in the Cytoplasm of Eukaryotic Cells. Nature 1979, 280, 339–340. [Google Scholar] [CrossRef]
- Jeck, W.R.; Sharpless, N.E. Detecting and Characterizing Circular RNAs. Nat. Biotechnol. 2014, 32, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Cocquerelle, C.; Mascrez, B.; Hétuin, D.; Bailleul, B. Mis-Splicing Yields Circular RNA Molecules. FASEB J. 1993, 7, 155–160. [Google Scholar] [CrossRef]
- Salzman, J.; Gawad, C.; Wang, P.L.; Lacayo, N.; Brown, P.O. Circular RNAs Are the Predominant Transcript Isoform from Hundreds of Human Genes in Diverse Cell Types. PLoS ONE 2012, 7, e30733. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs Are a Large Class of Animal RNAs with Regulatory Potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA Circles Function as Efficient microRNA Sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Duan, J.-L.; Chen, W.; Xie, J.-J.; Zhang, M.-L.; Nie, R.-C.; Liang, H.; Mei, J.; Han, K.; Xiang, Z.-C.; Wang, F.-W.; et al. A Novel Peptide Encoded by N6-Methyladenosine Modified circMAP3K4 Prevents Apoptosis in Hepatocellular Carcinoma. Mol. Cancer 2022, 21, 93. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, C.; Bao, C.; Chen, L.; Lin, M.; Wang, X.; Zhong, G.; Yu, B.; Hu, W.; Dai, L.; et al. Exon-Intron Circular RNAs Regulate Transcription in the Nucleus. Nat. Struct. Mol. Biol. 2015, 22, 256–264. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Wu, S.; Zhou, Z.; Ding, X.; Shi, R.; Thorne, R.F.; Zhang, X.D.; Hu, W.; Wu, M. CircACC1 Regulates Assembly and Activation of AMPK Complex under Metabolic Stress. Cell Metab. 2019, 30, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Capel, B.; Swain, A.; Nicolis, S.; Hacker, A.; Walter, M.; Koopman, P.; Goodfellow, P.; Lovell-Badge, R. Circular Transcripts of the Testis-Determining Gene Sry in Adult Mouse Testis. Cell 1993, 73, 1019–1030. [Google Scholar] [CrossRef]
- Dong, R.; Ma, X.-K.; Li, G.-W.; Yang, L. CIRCpedia v2: An Updated Database for Comprehensive Circular RNA Annotation and Expression Comparison. Genom. Proteom. Bioinform. 2018, 16, 226–233. [Google Scholar] [CrossRef]
- Xu, T.; Wu, J.; Han, P.; Zhao, Z.; Song, X. Circular RNA Expression Profiles and Features in Human Tissues: A Study Using RNA-Seq Data. BMC Genom. 2017, 18, 680. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, E.B.; Just, J.; Viuff, M.H.; Okholm, T.L.H.; Pedersen, S.B.; Meyer Lauritsen, K.; Trolle, C.; Pedersen, M.G.B.; Chang, S.; Fedder, J.; et al. Sex Chromosome Aneuploidies Give Rise to Changes in the Circular RNA Profile: A Circular Transcriptome-Wide Study of Turner and Klinefelter Syndrome across Different Tissues. Front. Genet. 2022, 13, 928874. [Google Scholar] [CrossRef] [PubMed]
- Luan, R.; Tian, G.; Zhang, H.; Shi, X.; Li, J.; Zhang, R.; Lu, X. Urinary Exosomal Circular RNAs of Sex Chromosome Origin Are Associated with Gender-Related Risk Differences of Clinicopathological Features in Patients with IgA Nephropathy. J. Nephrol. 2022, 35, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Zhao, G.; Chen, Z.; Wang, X.; Lv, F.; Zhang, Y.; Yang, X.; Liang, W.; Cai, R.; Li, J.; et al. circRNA-miRNA Association for Coronary Heart Disease. Mol. Med. Rep. 2019, 19, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- Wiener, D.; Schwartz, S. The Epitranscriptome beyond m6A. Nat. Rev. Genet. 2021, 22, 119–131. [Google Scholar] [CrossRef]
- Flynn, R.A.; Pedram, K.; Malaker, S.A.; Batista, P.J.; Smith, B.A.H.; Johnson, A.G.; George, B.M.; Majzoub, K.; Villalta, P.W.; Carette, J.E.; et al. Small RNAs Are Modified with N-Glycans and Displayed on the Surface of Living Cells. Cell 2021, 184, 3109–3124. [Google Scholar] [CrossRef]
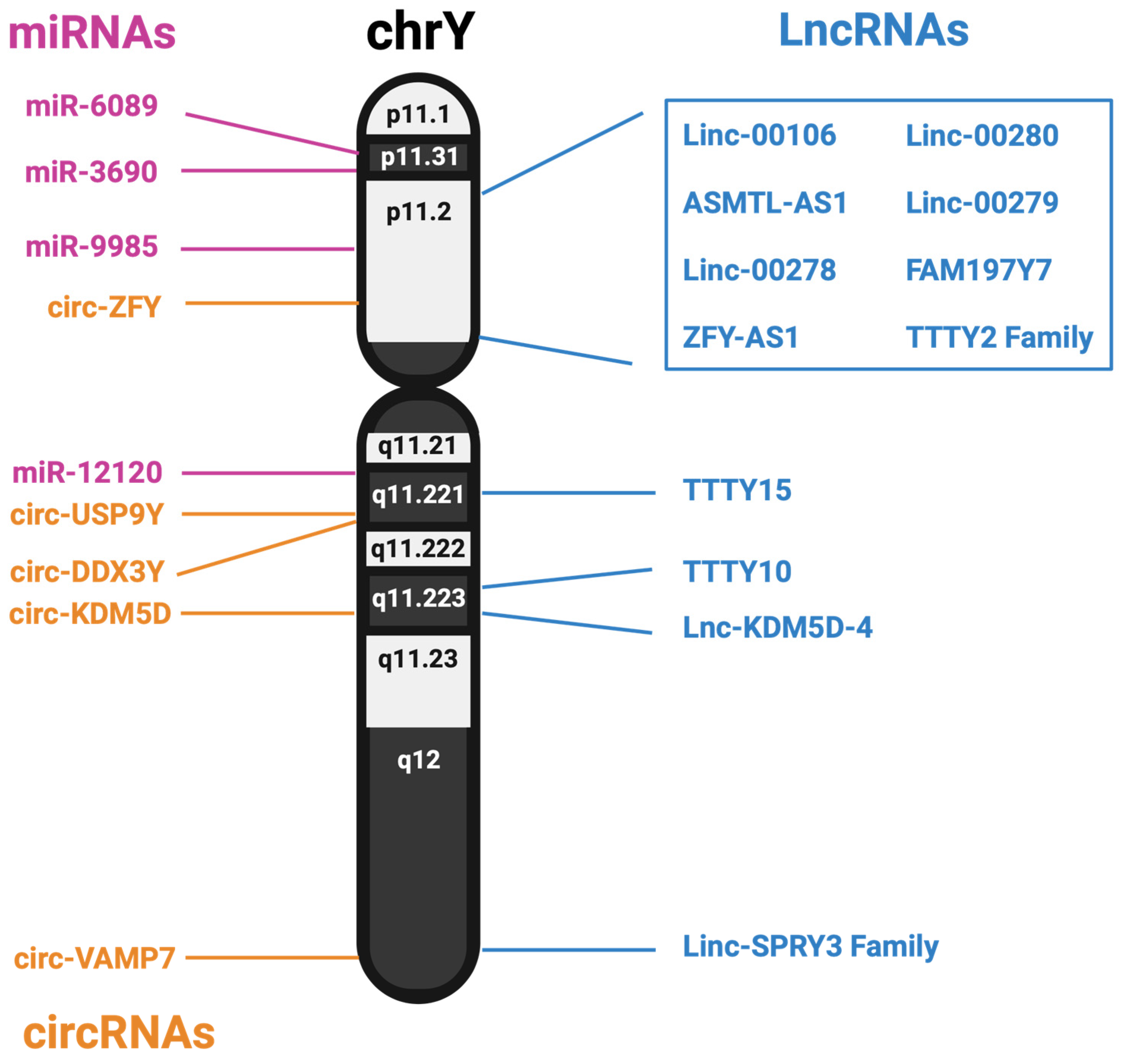
| Name of lncRNA | Genomic Location (GRCh38) | Function of Disease State Impact | Citations |
|---|---|---|---|
| Linc00106 | chrY:1,397,025–1,399,412 chrX:1,392,420–1,401,611 | Shown to sponge Let7f in hepatocellular carcinoma, increasing metastatic phenotypes | Liang et al., 2021 [107] |
| ASMTL-AS1 | chrY:1,401,769–1,403,493 chrX:1,400,531–1,415,421 | Shown to increase aggression of hepatocellular carcinoma after radiation treatment | Ma et al., 2020 [106] |
| linc00278 | chrY:2,918,373–3,590,925 | encodes Ying Yang 1 binding micropeptide, known to contribute to esophageal carcinoma | Wu et al., 2020 [101] |
| ZFY-AS1 | chrY:2,965,356–3,002,929 | Bioinformatic analysis identified as a protective biomarker in multiple myeloma | Zhou et al., 2015 [100] |
| Linc00280 | chrY:6,357,218–6,369,921 | Associated with chronic headaches when methylated | Winsvold, et al., 2018 [99] |
| Linc00279 | chrY:8,550,518–8,713,825 | Identified as a competitive endogenous RNA sponging has-mir485-5p in pulmonary tuberculosis patients | Li et al., 2022 [108] |
| FAM197Y7 | chrY:9,367,802–9,377,092 | influences bone metabolism in coronary artery calcification | Wicik, et al., 2021 [98] |
| TTTY2 Family | ChrY: 9,721,669–9,758,630 | Deletion of the TTTY2 region results in spermatogenesis problems and early developmental issues | Yapijakis et al., 2015 [96] |
| TTTY15 | chrY:12,537,650–12,860,839 | miRNA sponge in gastric cancer, esophageal squamous cell carcinoma, and prostate cancer | Wen et al., 2022, Wang and Yang 2020, Xiao et al., 2019 [103,104,105] |
| TTTY10 | chrY:20,375,319–20,824,330 | Suggested master regulator of over 350 genes in colorectal cancer progression, primarily in cell adhesion and differentiation | Zhu et al., 2017 [102] |
| lnc-KDM5D-4 | chrY: 20,519,948–20,524,433 | Potential transcription factor in coronary artery disease | Molina, et al., 2017 [97] |
| linc-SPRY3 family | chrY:56,675,832–56,678,566, chrY:56,703,707–56,707,491, chrY:56,748,794–56,752,370 | Suggested biomarker for radiation sensitivity in non-small-cell lung cancer | Brownmiller et al., 2020 [81] |
| Name of miRNA | Genomic Location (GRCh38) | Function of Disease State Impact | Citations |
|---|---|---|---|
| MiR9985 | chrY:4,606,120–4,606,228 | Differentially expressed after burn trauma and type 2 diabetes | Song et al. [149] Yin et al. [150] |
| MiR3690 | chrY:1,293,918–1,293,992 | Colorectal and thyroid cancer prognostic marker and differentially expressed in hepatocellular carcinoma | Zhang et al. [151] Shen et al. [152] Huang et al. [153] |
| MiR6089 | chrY:2,609,191–2,609,254 | Contributes to arthritis progression by increasing inflammation and acts as a prognostic marker for retinoblastoma | Yang et al. [154] Donghua et al. [155] Suxian et al. [156] Yan et al. [157] Li et al. [158] |
| MiR12120 | chrY:13,479,177–13,479,266 | SARS-CoV-2 targeting miRNA | Fulzele et al. [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westemeier-Rice, E.S.; Winters, M.T.; Rawson, T.W.; Martinez, I. More than the SRY: The Non-Coding Landscape of the Y Chromosome and Its Importance in Human Disease. Non-Coding RNA 2024, 10, 21. https://doi.org/10.3390/ncrna10020021
Westemeier-Rice ES, Winters MT, Rawson TW, Martinez I. More than the SRY: The Non-Coding Landscape of the Y Chromosome and Its Importance in Human Disease. Non-Coding RNA. 2024; 10(2):21. https://doi.org/10.3390/ncrna10020021
Chicago/Turabian StyleWestemeier-Rice, Emily S., Michael T. Winters, Travis W. Rawson, and Ivan Martinez. 2024. "More than the SRY: The Non-Coding Landscape of the Y Chromosome and Its Importance in Human Disease" Non-Coding RNA 10, no. 2: 21. https://doi.org/10.3390/ncrna10020021
APA StyleWestemeier-Rice, E. S., Winters, M. T., Rawson, T. W., & Martinez, I. (2024). More than the SRY: The Non-Coding Landscape of the Y Chromosome and Its Importance in Human Disease. Non-Coding RNA, 10(2), 21. https://doi.org/10.3390/ncrna10020021







