Abstract
Podocytes, alternatively called glomerular epithelial cells, are terminally differentiated cells that wrap around glomerular capillaries and function as a part of the glomerular filtration barrier in the kidney. Therefore, podocyte injury with morphological alteration and detachment from glomerular capillaries leads to severe proteinuria and subsequent renal failure through glomerulosclerosis. Previous RNA sequencing analysis of primary rat podocytes exposed to puromycin aminonucleoside (PAN), a well-known experimental model of injured podocytes, identified several transcripts as being aberrantly expressed. However, how the expression of these transcripts is regulated remains unclear. MicroRNAs (miRNAs) are small noncoding RNAs that posttranscriptionally inhibit the expression of their target transcripts. In this study, using small RNA sequencing analysis, miR-217-5p was identified as the most upregulated transcript in PAN-treated rat podocytes. MiR-217-5p overexpression in E11 podocyte cells led to shrunken cells with abnormal actin cytoskeletons. Consistent with these changes in cell morphology, gene ontology (GO) enrichment analysis showed that interactive GO terms related to cell morphogenesis were enriched with the predicted targets of miR-217-5p. Of the predicted targets highly downregulated by PAN, Myosin 1d (Myo1d) is a nonmuscle myosin predicted to be involved in actin filament organization and thought to play a role in podocyte morphogenesis and injury. We demonstrated that miR-217-5p targets Myo1d by luciferase assays, qRT–PCR, and Western blotting. Furthermore, we showed that miR-217-5p was present in urine from PAN- but not saline-administrated rats. Taken together, our data suggest that miR-217-5p may serve as a therapeutic target and a biomarker for podocyte injury.
1. Introduction
Approximately 10% of the world population is affected by chronic kidney disease (CKD), defined as abnormalities in kidney structure and/or function that are present for at least 3 months, irrespective of cause [1]. Millions of patients with CKD die every year due to inadequate treatment [1]. CKD shows few symptoms in initial stages and is often detected in advanced stages when symptoms become more obvious [2,3]. For this reason, CKD is often classified as a “silent” disease. When kidney functions are practically lost, this is expressed as ‘renal failure’ [4,5]. Unfortunately, no therapeutic methods to restore the function of chronically impaired kidneys have been established. During late stages of CKD, patients with CKD require renal replacement therapies including hemodialysis and kidney transplants for survival. Although hemodialysis is generally applied in cases of renal failure, patients receiving dialysis have significantly decreased life expectancy compared to the normal population [6]. Hemodialysis also reduces patient quality of life in physiological, mental, and/or social aspect(s) [7]. Furthermore, the high cost of dialysis places an economic burden on patients and is suggested to exert a negative influence on health economics [7]. While kidney transplantation can be a therapy option for end-stage CKD patients [8,9], the outcome is controversial among health care professionals [10,11]. Furthermore, patients usually need to wait a long time for a compatible donor and may die while waiting for a kidney [12]. Finally, for a transplant to be considered successful, the donated kidney must be accepted by the patient’s immune system [13].
In the kidney, glomeruli are found in the cortex and serve as a hemofiltration device [14]. Podocytes, alternatively called glomerular epithelial cells, are highly differentiated cells that cover glomerular capillaries. Podocytes, together with endothelial cells and glomerular basal membranes, form a unit that functions as a glomerular filtration barrier in the kidney [15,16]. Podocytes have a characteristic morphology that contains many foot processes [14]. Between the foot processes, membranous structures called slit membranes form, and these membranes play an essential role as a final filtration barrier to prevent leakage of proteins into the urine during glomerular filtration [14,15,16]. The structure of the filtration barrier can be disrupted by podocyte damage, which occasionally causes podocyte detachment from the glomeruli; in these cases, severe proteinuria leading to renal failure may occur [16,17]. However, the mechanisms that underlie the structural disruption of podocyte remain unclear.
MicroRNAs (miRNAs) are small noncoding RNAs of 19–25 nucleotides in length that play a crucial role in posttranscriptional gene regulation by repressing the translation of or degrading target messenger RNAs (mRNA) [18,19]. Notably, mice with podocyte-specific deficiency in Dicer, a nuclease essential for miRNA biosynthesis, have been shown to develop severe proteinuria and die within weeks, indicating that miRNAs are indispensable for podocyte function [20,21,22]. Previous studies have also demonstrated that many miRNAs play essential roles in nephropathy-related podocyte injury [23,24]. However, the mechanisms underlying miRNA-mediated podocyte injury are not fully understood. Here, we performed an integrative analysis of miRNA and mRNA expression profiles associated with puromycin nucleoside (PAN)-induced podocyte injury. Our analysis showed that miR-217-5p upregulation in PAN-treated podocytes can lead to morphological alteration. Furthermore, we demonstrated that miR-217-5p is present in urine from PAN- but not saline-administrated rats, suggesting that miR-217-5p may serve as a therapeutic target and biomarker for podocyte injury.
2. Materials and Methods
2.1. Cell Culture and Induction of Podocyte Injury
Podocytes in primary culture were isolated from male Wistar rats at ages 7–8 weeks (SLC, Hamamatsu, Japan) and cultured as described previously [25,26]. The mouse podocyte cell line E11 was purchased from Cell Lines Service GmbH (Eppelheim, Germany) and cultured as described previously [27]. To cause cell injury, primary rat podocytes and E11 cells were treated with various concentrations of PAN (Wako, Osaka, Japan) for 24 h or 48 h.
2.2. Comprehensive Small RNA Sequencing
One μg of total RNA isolated from the rat podocytes in primary culture was subjected to small RNA sequencing to comprehensively analyze miRNA expression. Small RNA libraries were constructed using the TruSeq Small RNA Library Preparation Kits (Illumina, San Diego, CA, USA) and analyzed on a HiSeq-3000 sequencer (Illumina) at the Genome Information Research Centre of Osaka University. Raw reads obtained from the sequencing analysis were trimmed using Cutadapt v1.9.2 and subjected to miRNA-derived read counting and annotation using CLC Genomics Workbench 11.0.1 (Qiagen, Venlo, The Netherlands).
The reads obtained in this analysis and related metadata were deposited in the DNA Data Bank of Japan Sequence Read Archive (DRA) under the accession number DRA013173.
2.3. Real-Time Reverse-Transcription PCR for miRNA and mRNA
Quantitative RT-PCR (qRT–PCR) analysis using TaqMan® MicroRNA Assays (Thermo-Fisher Scientific, Waltham, MA, USA) was conducted to validate the results of miRNA-seq analysis. Because expression levels of target miRNAs miR-217-5p were low, PCR preamplification with 12 cycles was conducted using TaqMan® PreAmp Master Mix (Thermo-Fisher Scientific) before performing qRT–PCR. The expression of U6 small nuclear RNA (snRNA) was analyzed as an endogenous internal control to normalize expression levels of miRNAs. Monitoring the amplification of PCR products was conducted on a DICE Thermal Cycler Real-Time System (Takara-bio, Kusatsu, Japan).
Thunderbird SYBR qPCR Mix (Toyobo, Osaka, Japan) was used for the quantitative analysis of mRNA expression levels. GAPDH mRNA or 18S rRNA was used as an endogenous internal control to normalize mRNAs’ expression levels (the primers sequences are listed in Table S1). Monitoring of amplification of PCR products was conducted on a DICE Thermal Cycler Real-Time System (Takara-bio). Dissociation curve analyses were also performed to verify specific amplification.
2.4. miRNA Overexpression in E11 Podocytes
To overexpress miR-217-5p in E11 podocytes, the cells were transfected with mirVana miRNA Mimic-miR-217-5p (Thermo-Ficsher Scientific) at 50 nM using Lipofectamin RNAiMAX (Thermo-Ficsher Scientific). As a control, mirVana miRNA Mimic-Negative Control #1 (Thermo-Ficsher Scientific) was also used for transfection.
2.5. Cell Morphology Observation and Fluorescent Immunocytochemistry
Cell morphology in culture was routinely observed using an Olympus CKX41 inverted microscope (Olympus, Tokyo, Japan). For immunocytochemistry, cells were cultured on tissue culture-treated chamber slides, fixed with 10% formalin in PBS, permeabilized with 0.2% Triton-X100 in PBS, blocked with 1% bovine serum albumin (BSA) in PBS, and reacted with the rabbit anti-β-actin antibody (PM053-7) (MBL, Tokyo, Japan) followed by the Alexa488-labeled antirabbit IgG antibody (ab150077) (Abcam, Cambridge, UK). Fluorescent cells were observed under a BZ9000 fluorescence microscope (Keyence, Osaka, Japan).
2.6. Cell Viability Assay
E11 cells were seeded in 96-well plates at 2 × 103 cells/well, cultured overnight, and treated with PAN (Wako, Osaka, Japan) for 48 or 96 h. After changing to fresh medium, the viability of podocytes was evaluated using Cell Counting Reagent SF (Nacalai Tesque, Kyoto, Japan) according to the manufacturer’s instructions.
2.7. Western Blotting
Cells were lysed with a RIPA buffer (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1% NP-40; 0.5% sodium deoxycholate). Cell lysates were subjected to 8% SDS-polyacrylamide gel electrophoresis and transferred to Immobilon polyvinylidene difluoride membranes (Merck, Kenilworth, NJ, USA). The blots were then probed with the mouse anti-Myo1d monoclonal antibody (sc-515292) (Santa Cruz, Dallas, TX, USA) followed by the goat antimouse IgG antibody linked with horseradish peroxidase (HRP) (#330) (MBL). The same blots were also probed for β-actin using the HRP-linked rabbit anti-β-actin antibody (PM053-7) (MBL) as an internal control. Immobilon ECL Ultra Western HRP Substrate and Immobilon Western HRP Substrate (Merck-Millipore, Burlington, VT, USA) were used to detect Myo1d and β-actin, respectively. A LAS-4000 lumino-image analyzer (Thermo-Fisher Scientific) was used to detect chemiluminescent signals.
2.8. Reporter Plasmid Construction and Luciferase Assay
The 2.2-kbp 3′-UTR of mouse Myo1d mRNA was amplified by RT-PCR from the total RNA of E11 cells using PrimeScript II reverse transcriptase (Takara-bio) and KOD-Plus DNA polymerase (Toyobo). The primers used for RT-PCR amplification were: 5′-CTGCTGCACATCAGAGGCCT-3′ (forward) and 5′-TTTGTCGACAAGATTTAATGCTTTATTGC-3′ (reverse). The PCR product encompassing the 3′-UTRs of Myo1d was then subcloned into the pmirGLO vector (Promega, Madison, USA) at the DraI and SalI restriction sites to construct the pmirGLO-Myo1d 3′-UTR luciferase reporter plasmid. DNA ligation was conducted at 16 °C for 30 min using DNA Ligation Kit Ver.1 (Takara-bio). A reporter plasmid having a mutation at the predicted miR-217-5p binding site in the 3′-UTR of Myo1d mRNA was constructed using pmirGLO-Myo1d 3′-UTR as a template. First, the part between the predicted miR-217-5p binding site and the end of the 3′-UTR was PCR-amplified using KOD-Plus DNA polymerase (Toyobo) and the following primers: 5′-ACCTGGAATTCGGGGTGTGACTGACCACAGTAACAGCAGAGGAGAGGACACAGTGATTGTATGCATGGAGTAGGGGTCTCTTGAGTTAATGAAGATATCGTTATGGTTTG-3′ (forward) and 5′-TTTGTCGACAAGATTTAATGCTTTATTGC-3′ (reverse). The generated amplicon was digested using EcoRI and SalI at both termini. The amplicon then substituted the corresponding region of pmirGLO-Myo1d 3′-UTR. For reporter assays, E11 cells were transfected with these plasmids together with synthetic miRNA mimics (Thermo-Fisher Scientific) at 20 nM using Lipofectamin2000 (Thermo-Fisher Scientific). Twenty-four hours after transfection, the cells were processed using the Dual-Luciferase Reporter Assay System (Promega). Luminescence was detected using a TD-20/20 luminometer (Promega).
2.9. Urine Processing
PAN administration in Wistar rats and urine collection from rats were performed as described previously [25]. Urine samples were collected for 12 h at day 5 following the administration of PAN or saline. Collection on day 5 was chosen because we previously showed that urinary protein concentration, an index to evaluate the progression of glomerulopathy, was markedly increased from day 5 onwards [25]. The collected urine was filtered through a 0.22-μm filter to remove debris, then subjected to RNA isolation using an RNAiso Blood reagent (Takara, Tokyo, Japan). In parallel, to monitor the progression of glomerulopathy, the protein concentration of the filtered urine was measured as described previously [25].
2.10. Bioinformatics
mRNAs meeting the following criteria were selected as PAN-regulated mRNAs: (i) FPKM value of podocytes cultured with PAN >10, (ii) FPKM ratio (podocytes cultured with PAN/podocytes cultured without PAN) >1.2 or <0.8, and (iii) the false discovery rate (q-value) < 0.05. Putative miRNA targets were predicted using TargetScan v7.2 (http://www.targetscan.org/vert_72/, accessed on 6 June 2022) as described previously [28]. Enriched GO terms and interactive ontology corresponding to a specific gene were retrieved using GOnet [29], which predict biological processes associated with the predicted targets of miR-217-5p in podocytes.
2.11. Statistical Analysis
Student’s t-tests were used to assess the significance of differences among groups for comparisons between two groups, and one-way analysis of variance followed by Ryan’s test was used for comparisons among three or more groups. In all analyses, p < 0.05 was considered to be statistically significant.
3. Results
3.1. Identification of miRNAs Dysregulated by PAN-Induced Podocyte Injury
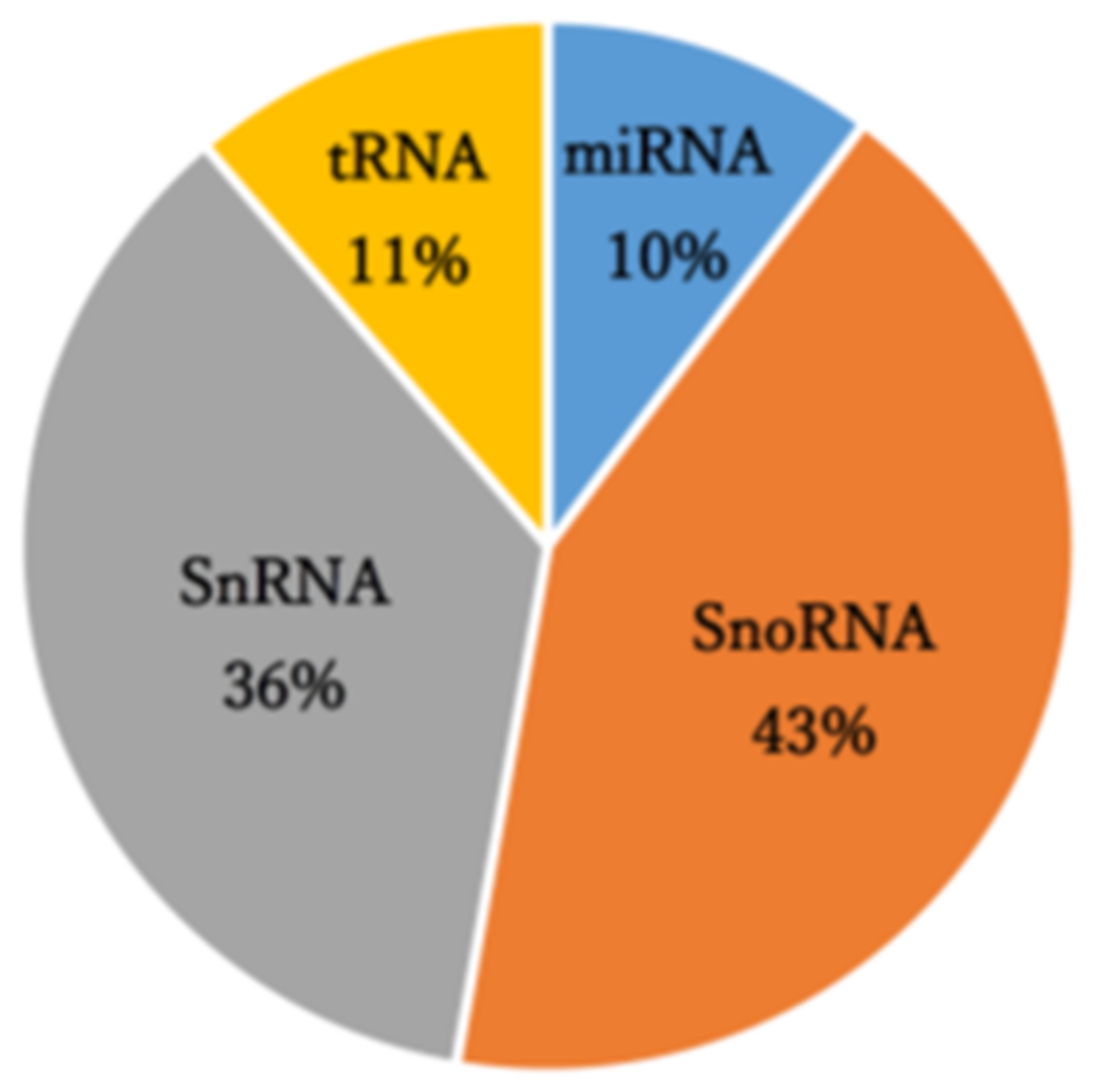
A small RNA sequencing analysis of control and PAN-treated primary rat podocytes that manufactured 8.64–13.5 million raw reads was performed. The mapping results of this analysis are summarized in Table S2. Reads obtained through small RNA-seq analysis were mapped to 3,948 annotated small RNAs comprising small nucleolar RNA (43%), small nuclear RNA (36%), transfer RNA-derived small RNAs (11%), and miRNAs (10%) (Figure 1). In the analysis of miRNA-derived reads, miR-217-5p, -216a-5p, -338-3p, and -3583-5p were identified as miRNAs with significantly enhanced expression (over 2-fold) following PAN-induced podocyte injury (Table 1). Conversely, miR-3572 expression was significantly reduced in response to PAN-induced podocyte injury (Table 1).
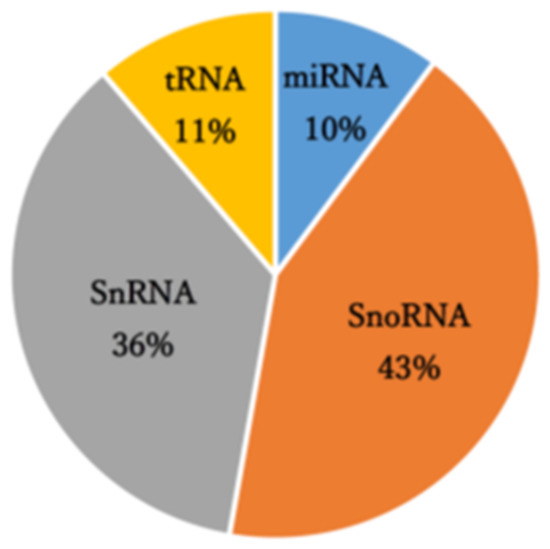
Figure 1.
Small RNA class composition of the small RNA sequencing-derived reads.

Table 1.
miRNAs dysregulated in PAN-treated podocytes.
3.2. Validation of PAN-Induced miR-217-5p Expression in Primary Rat Podocytes
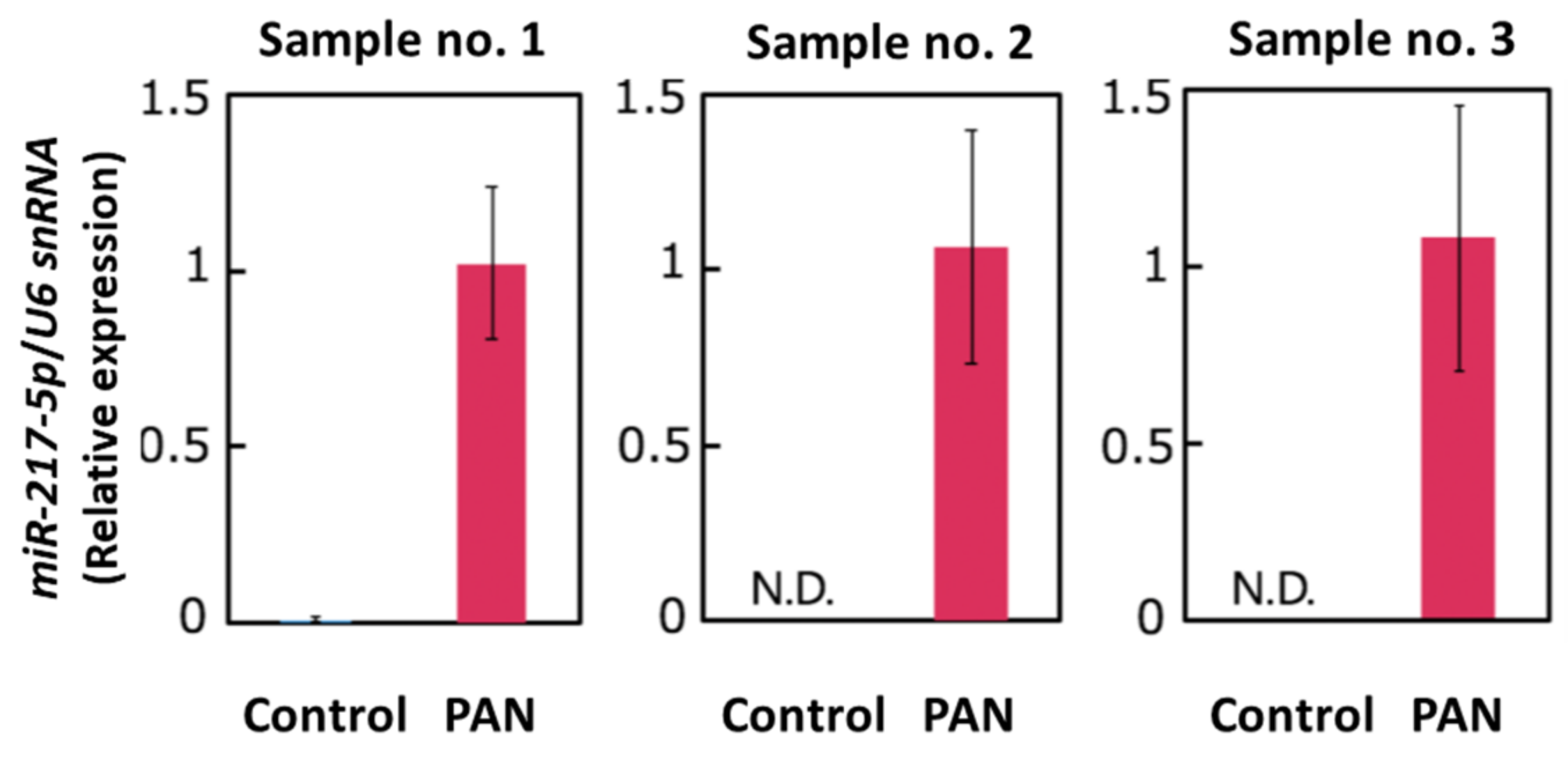
Our small RNA-seq analysis indicated that miR-217-5p is the most highly (5.7-fold) upregulated miRNA in PAN-treated podocytes; no other miRNAs were upregulated more than five times. We performed qRT–PCR analysis to verify PAN-induced miR-217-5p expression in primary rat podocytes. Analysis of qRT–PCR analysis revealed that miR-217-5p expression in rat podocytes was markedly increased following PAN treatment (Figure 2).
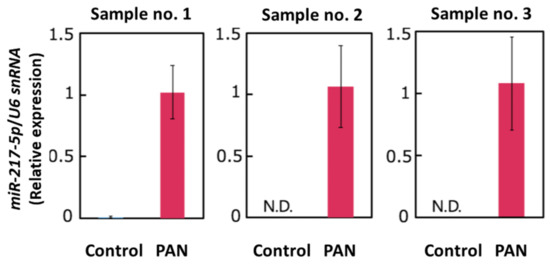
Figure 2.
Expression of miR-217-5p in PAN-treated rat podocytes. MiR-217-5p expression level was normalized relative to U6 snRNA expression. The normalized expression level of miR-217-5p at 3 μg/mL PAN was given an arbitrary value of 1. Data represent the means ± SD (n = 3).
3.3. PAN-Induced Effects on Cell Viability, Cell Morphology, and miR-217-5p Expression in E11 Podocyte Cells
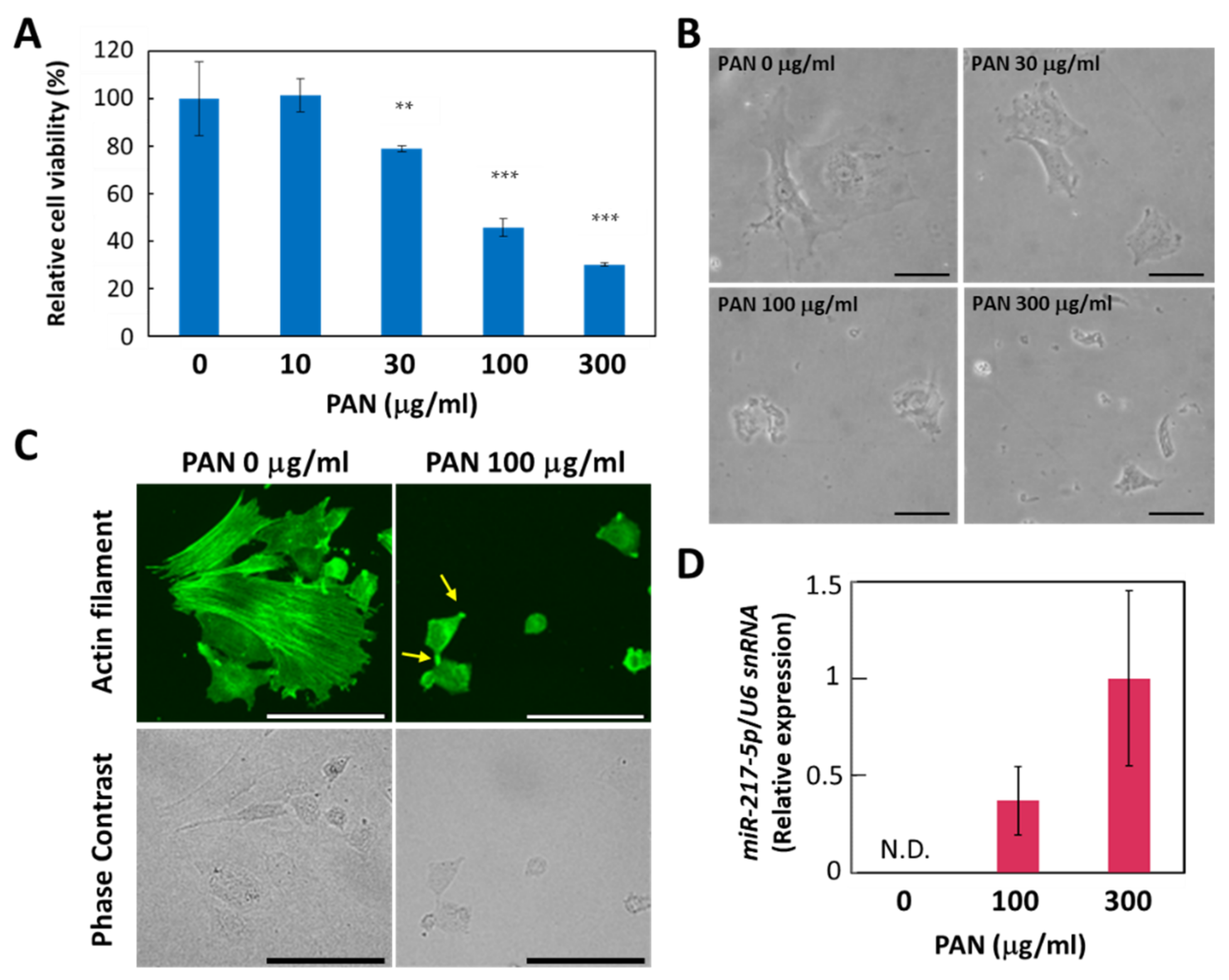
Considering the drastic induction of miR-217-5p expression in rat podocytes, we sought to determine whether the increased expression of miR-217-5p was involved in PAN-induced podocyte injury. However, primary rat podocytes are not suitable for this purpose because only a small number of podocytes can be isolated from a rat. Furthermore, it is generally difficult to efficiently transfect primary cells with DNA or RNA. Therefore, we evaluated whether E11, an immortalized cell line generated from mouse podocytes, could be used as an alternative in vitro model, since siRNA-mediated gene knockdown and vector-mediated gene overexpression have been previously conducted in this cell line [30]. First, we evaluated the effect of PAN on E11 cell viability. We found that PAN exerted a strong suppressive effect on E11 cell viability at >100 μg/mL (Figure 3A). This concentration is higher than effective PAN concentrations reported previously for primary rat podocytes (>10 μg/mL) [25]. The PAN-resistant properties of mouse podocytes compared to rat podocytes has been attributed to a deficiency in the adenosine deamination pathway [31] and low expression of plasma membrane amine transporter [32].
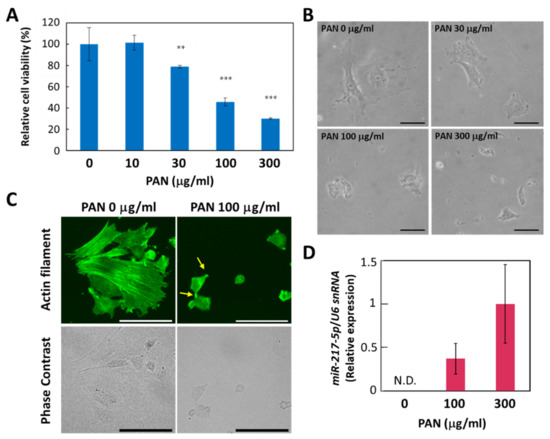
Figure 3.
PAN-induced effects on cell viability, cell morphology, and miR-217-5p expression in E11 podocytic cells. (A) PAN-induced reduction in the cell viability of E11 cells. Cell viability after PAN (0–300 μg/mL) exposure for 48 h was evaluated by WST-8 assay. Cell viability at 0 μg/mL PAN was given an arbitrary value of 100%. ** p < 0.01 versus 0 μg/mL PAN. *** p < 0.001 versus 0 μg/mL PAN. Data represent the means ± SD (n = 3). (B) Phase contrast images of E11 cells in culture after PAN (0–300 μg/mL) exposure for 48 h. Bars: 50 μm. (C) Actin cytoskeletons of E11 cells treated with or without PAN (100 μg/mL) for 48 h were visualized by immunostaining with anti-β-actin antibody (top). Phase contrast images of the corresponding cells are also shown (bottom). Representative processlike structures appearing in some PAN-treated cells are indicated by arrows. Bars: 100 μm. (D) Expression of miR-217-5p in PAN-treated E11 cells. MiR-217-5p expression in E11 cells treated with PAN (0–300 mg/mL) was normalized relative to U6 snRNA expression. The relative expression level of miR-217-5p at 300 μg/mL PAN was given an arbitrary value of 1. Data represent the means ± SD (n = 3). N.D. not detected.
We also found that PAN induced morphological changes in E11 cells. While E11 cells cultured in the absence of PAN largely exhibit a spread-out shape, cells appear shrunken when cultured with 100 μg/mL PAN (Figure 3B). In addition, immunostaining with an anti-β-actin antibody revealed that actin filaments lining appeared disorganized or shortened in E11 cells treated with 100 μg/mL PAN (Figure 3C).
Next, we evaluated whether miR-217-5p expression is upregulated in PAN-treated E11 cells. We found that miR-217-5p expression was upregulated in E11 cells treated with 100 and 300 μg/mL PAN (Figure 3D). These data suggest that PAN exerts similar effects on cell viability and miR-217-5p expression in both primary rat podocytes and E11 cells, indicating that E11 cells can be used as a model to investigate the role of miR-217-5p in PAN-induced podocyte injury.
3.4. MiR-217-5p-Induced Effects on Cell Viability and Cell Morphology in E11 Cells
We next evaluated whether miR-217-5p overexpression in E11 cells affects cell viability and morphology. MiR-217-5p overexpression did not significantly affect cell viability of E11 cells after 48 h (Figure 4A) and 96 h (Figure S1). However, E11 cells with miR-217-5p overexpression in culture exhibited shrunken shapes (Figure 4B). Furthermore, immunostaining with an anti-β-actin antibody revealed that actin filaments lining behind the cell membrane appeared disorganized or shortened in E11 cells with miR-217-5p overexpression (Figure 4C). These data imply that miR-217-5p is possibly involved in cell morphogenesis through the regulation of actin filament formation.
3.5. Integrative Analysis of miRNA and mRNA Expression in PAN-Treated Podocytes
Although miRNAs are known to silence their target genes by attenuating translation and degrading mRNAs, previous studies have shown that mammalian miRNAs predominantly reduce target mRNA levels [33,34]. Therefore, expression levels of PAN-regulated miRNAs and their target mRNAs in podocytes are thought to be reciprocally regulated. In the present study, we selected PAN-regulated mRNAs as described in the Materials and Methods section. Through screening based on these criteria, 226 and 480 mRNAs were identified as upregulated and downregulated mRNAs, respectively (Figure S2, Tables S3 and S4). Next, the PAN-regulated mRNAs were screened to identify miR-217-5p targets satisfying the following criteria: (i) expression inversely correlated with miR-217-5p and (ii) in silico-predicted targets of miR-217-5p. After proceeding with these procedures, 12 mRNAs were identified as the predicted targets of miR-217-5p (Figure S3 and Table 2).
3.6. Gene Ontologies Associated with the Predicted Targets of the PAN-Dysregulated miRNAs
GO enrichment analyses were performed using GOnet to predict biological processes associated with the predicted targets of miR-217-5p in podocytes (Table 2). Consistent with the mR-217-5p-induced abnormal morphology of E11 cells, interactive GO (biological process) terms including those related to cell morphogenesis were enriched with the predicted targets of miR-217-5p (Figure S4). This study focused on Myosin 1d (Myo1d) as a predicted target because it is annotated to GO terms related to actin filament organization (Table 2), and previous studies have shown the involvement of nonmuscle myosins in podocyte morphogenesis and injury [35].
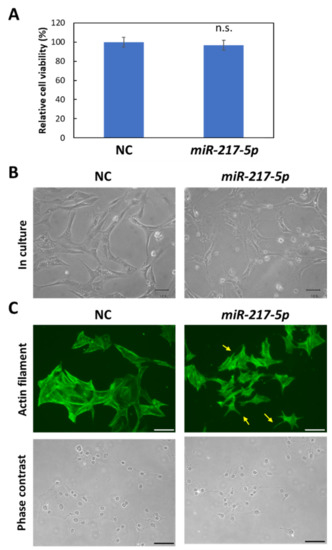
Figure 4.
Morphological abnormalities in miR-217-5p-transfected cells. (A) Effects of induced miR-217-5p expression in E11 cells on cell viability. Cell viability at day 2 following the transfection was evaluated. n.s.: not significant compared to the negative control miRNA mimic (NC)-transfected cells. (B) Phase contrast images of E11 cells transfected with the negative control miRNA (NC) or miR-217-5p mimics at 48 h posttransfection. Bars: 50 μm. (C) Actin cytoskeleton of E11 cells transfected with NC or miR-217-5p mimics at 48 h posttransfection. Actin filaments were visualized by immunostaining with anti-β-actin antibody (top). Processlike structures in the miR-217-5p-transfected cells are indicated by arrows. Phase contrast images of cells are also shown (bottom). Bars: 100 μm.
Figure 4.
Morphological abnormalities in miR-217-5p-transfected cells. (A) Effects of induced miR-217-5p expression in E11 cells on cell viability. Cell viability at day 2 following the transfection was evaluated. n.s.: not significant compared to the negative control miRNA mimic (NC)-transfected cells. (B) Phase contrast images of E11 cells transfected with the negative control miRNA (NC) or miR-217-5p mimics at 48 h posttransfection. Bars: 50 μm. (C) Actin cytoskeleton of E11 cells transfected with NC or miR-217-5p mimics at 48 h posttransfection. Actin filaments were visualized by immunostaining with anti-β-actin antibody (top). Processlike structures in the miR-217-5p-transfected cells are indicated by arrows. Phase contrast images of cells are also shown (bottom). Bars: 100 μm.
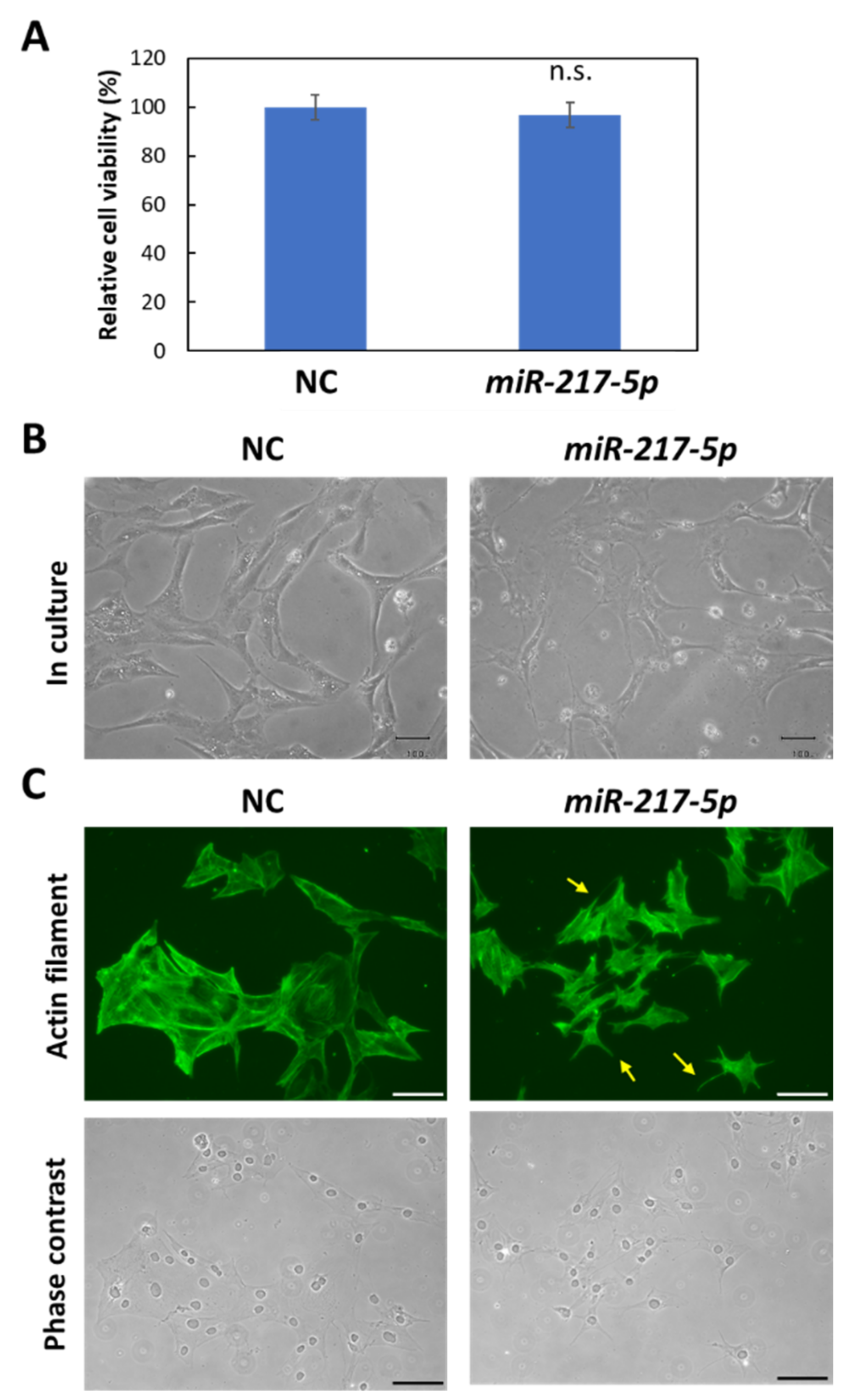

Table 2.
Predicted target mRNAs of miR-217-5p in PAN-treated podocytes and GO annotations.
Table 2.
Predicted target mRNAs of miR-217-5p in PAN-treated podocytes and GO annotations.
| mRNA | Fold Change | Representative GO (Biological Process) Annotations |
|---|---|---|
| Pard3b | 0.303 | cell adhesion, cell cycle, cell division, etc. |
| Fndc3b | 0.407 | integral component of membrane |
| Myo1d | 0.430 | actin filament organization, cellular localization, etc. |
| Akap2 | 0.493 | actin filament organization, protein localization, etc. |
| Peak1 | 0.532 | cell migration, focal adhesion assembly, etc. |
| Srgap1 | 0.551 | negative regulation of cell migration, etc. |
| Fn1 | 0.573 | acute-phase response, acute-phase response, etc. |
| Picalm | 0.604 | amyloid-beta clearance by transcytosis, axonogenesis, etc. |
| Meis1 | 0.610 | Angiogenesis, animal organ morphogenesis, etc. |
| Epha7 | 0.620 | Angiogenesis, animal organ morphogenesis, etc. |
| Crls1 | 0.716 | lens morphogenesis in camera-type eye, etc. |
| Rock1 | 0.733 | regulation of cell population proliferation, etc. |
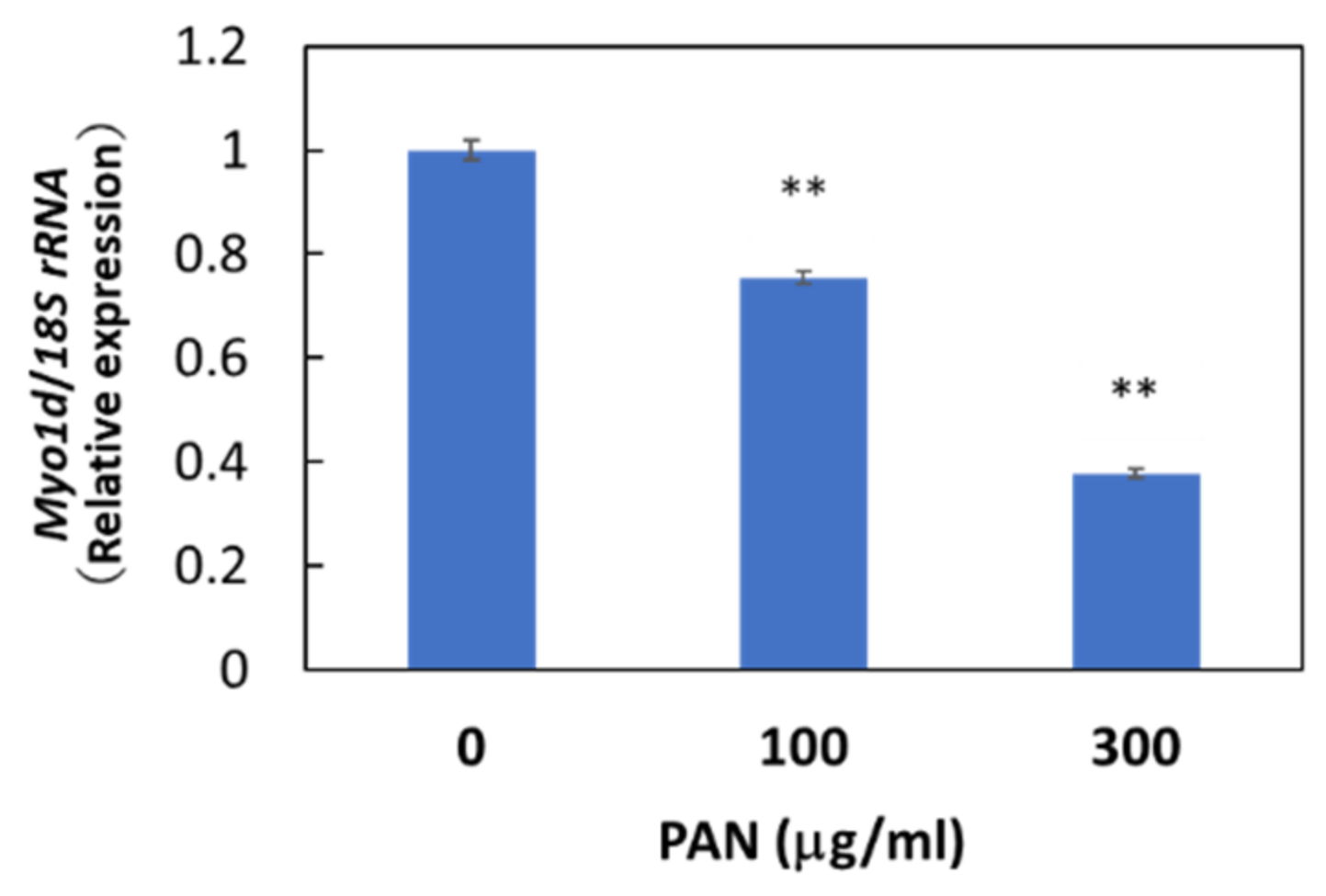
Immunohistochemical staining showed the cytosolic localization of Myo1d protein in E11 cells, consistent with the previously reported localization of Myo1d protein in the human bone osteosarcoma cell line U-2 OS (Figure S5). We then examined whether Myo1d mRNA expression in podocytes was downregulated by PAN as shown in our microarray analysis. As expected, PAN downregulated Myo1d mRNA expression levels in primary rat podocytes (Figure S6) and E11 cells (Figure 5), and PAN upregulated miR-217-5p expression in these cells (Figure 3D). The reciprocal regulation of miR-217-5p and Myo1d mRNA expression raises the possibility that the miR-217-5p-Myo1d axis may be involved in PAN-induced podocyte injury.
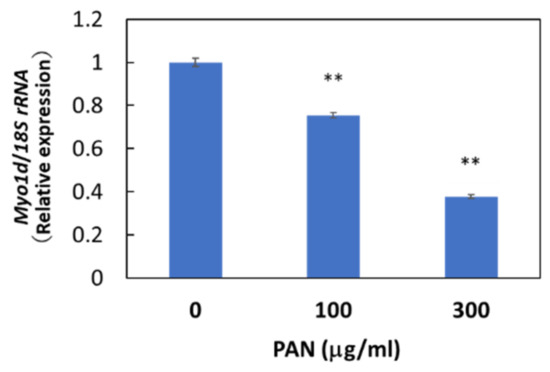
Figure 5.
Myo1d mRNA levels in PAN-treated E11 cells. E11 cells were treated with 0–300 μg/mL PAN. Values were normalized relative to 18S rRNA expression. The relative expression level of Myo1d mRNA at 0 μg/mL PAN was given arbitrary value of 1. ** p < 0.01 versus 0 μg/mL PAN. Data represent the means ± SD (n = 3).
This possibility was supported by analyses of publicly available datasets of podocyte injury-related transcriptomes. The analysis of the dataset GSE124622, created through microarray analysis of immortalized human podocytes treated with PAN and Adriamycin (ADR) to cause podocyte injury [36], showed that Myo1d mRNA levels in the PAN- and ADR-treated podocytes decreased 0.41-fold (p = 7.54E-06) and 0.64-fold (p = 0.0364) compared to control podocytes, respectively (Table S5). Furthermore, analysis of the dataset GSE108629 analysis, created through a microarray-based study using a mouse model of immunotoxin-inducible podocyte injury, showed that Myo1d mRNA levels were reduced 0.61-fold (p = 0.00158) after podocyte injury [37]. These results indicate that a reduction in Myo1d mRNA expression in injured podocytes was observed in our rat model and in in vitro or in vivo models from other organisms.
3.7. Target Validation of miR-217-5p
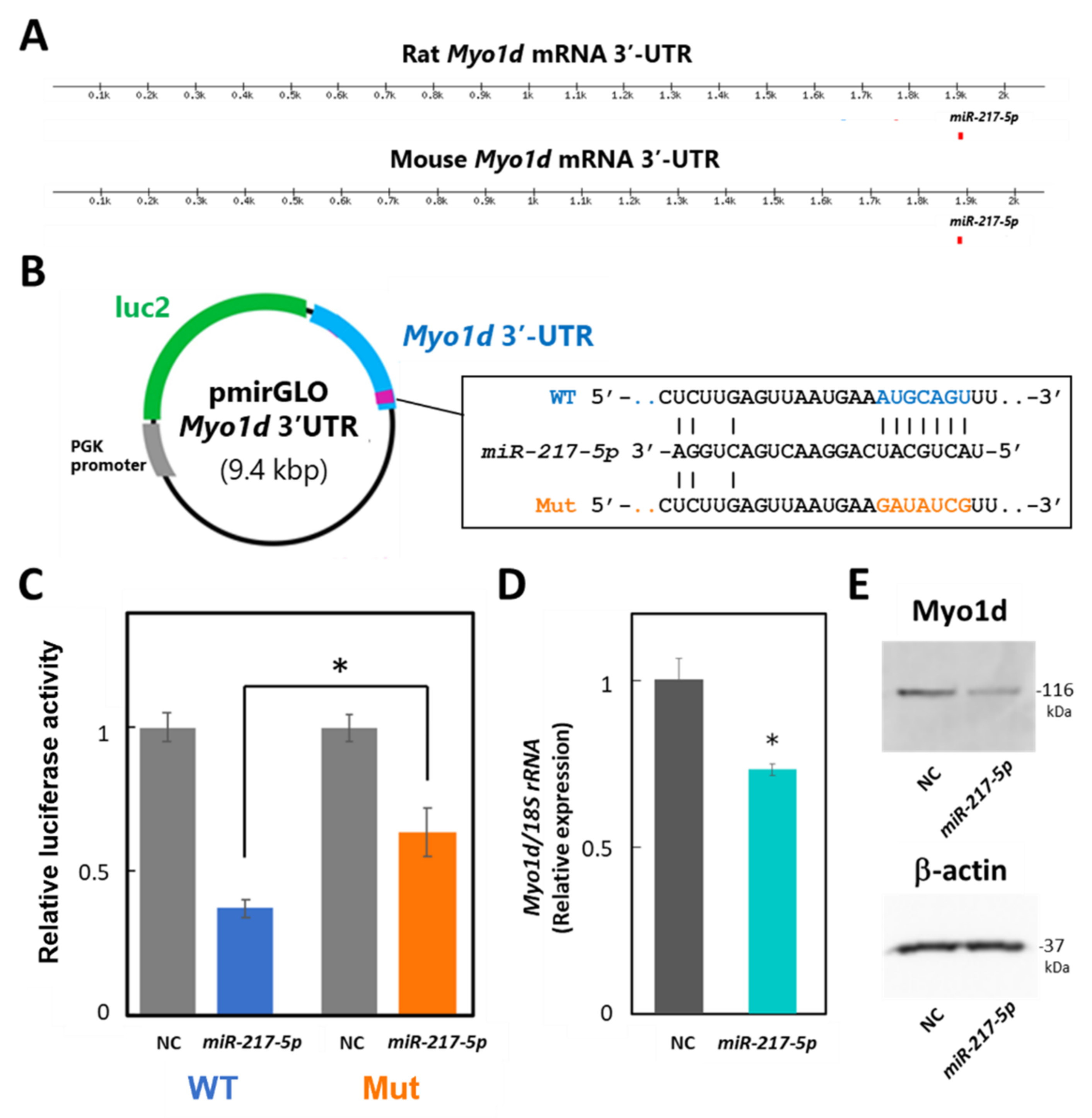
To evaluate whether miR-217-5p targets Myo1d mRNA in podocytes through its 3′-UTR, we performed luciferase assays in E11 cells. Because the 3′-UTRs of mouse and rat Myo1d mRNAs are highly similar (84%) and have a predicted common miR-217-5p binding site at identical positions of their 3′-UTRs (Figure 6A), we constructed reporter plasmid containing the mouse Myo1d 3′-UTR, designated as pmirGLO-Myo1d-3′-UTR (Figure 6B). Cotransfection of miR-217-5p showed significantly downregulated luciferase activity in the reporter plasmid-transfected cells compared to the control (Figure 6C). Alternatively, when E11 cells were transfected with the reporter plasmid with a mutation at the predicted miR-217-5p-binding site in the Myo1d 3′-UTR (Figure 6B), the inhibitory effect of miR-217-5p on luciferase activity was weakened (Figure 6C). However, the mutation only partly restored the luciferase activity attenuated by miR-217-5p. Therefore, there may be other miR-217-5p recognition site(s) in the 3′-UTR of Myo1d mRNA, which were not identified by the target prediction tool.
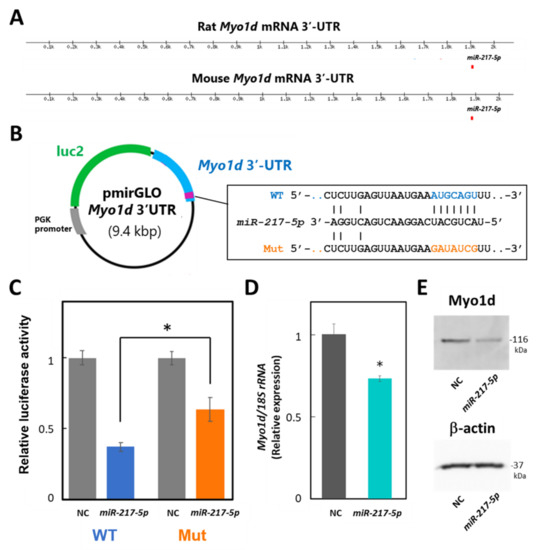
Figure 6.
Target validation of miR-217-5p in PAN-treated rat podocytes. (A) Schematic illustration showing the putative position of miR-217-5p binding sites in the 3′-UTRs of mouse and rat Myo1d mRNAs predicted by TargetScan Human 8.0. (B) Schematic illustration showing the structure of the reporter plasmid with the wild-type (WT) and mutated (mut) 3′-UTR of mouse Myo1d. (C) A luciferase reporter assay examining targeting of the Myo1d 3′-UTR by rno-miR-217-5p. The relative light units (RLUs) of firefly luciferase activity under the control of the Myo1d 3′-UTR were normalized to those of constitutively controlled Renilla luciferase. The average relative RLUs of luciferase activity in the negative control miRNA mimic (NC)-transfected cells was set at 1. Data represent the means ± SD of triplicate measurements. * p < 0.05 versus control. (D) Expression levels of the Myo1d mRNA in E11 cells transfected with the miR-217-5p mimic or NC. Myo1d mRNA levels were analyzed using qRT-PCR, and the data were normalized to the expression level of 18S rRNA. The average normalized Myo1d mRNA expression level in NC-transfected cells was set at 1. (E) Expression levels of the Myo1d protein in E11 cells transfected with the miR-217-5p mimic or NC. Myo1d protein expression was analyzed using Western blotting. The expression of β-actin protein as an internal control was analyzed on the same blot. The original images of blots from two independent experiments are shown in Figure S7.
Next, we evaluated whether miR-217-5p overexpression in E11 cells affects Myo1d mRNA and protein expression levels through qRT–PCR and Western blotting, respectively. As expected, expression levels of both Myo1d mRNA and Myo1d protein were reduced by overexpression of miR-217-5p compared to the control (Figure 6D,E).
3.8. Detection of miR-217-5p in Urine from PAN-Administrated Rats
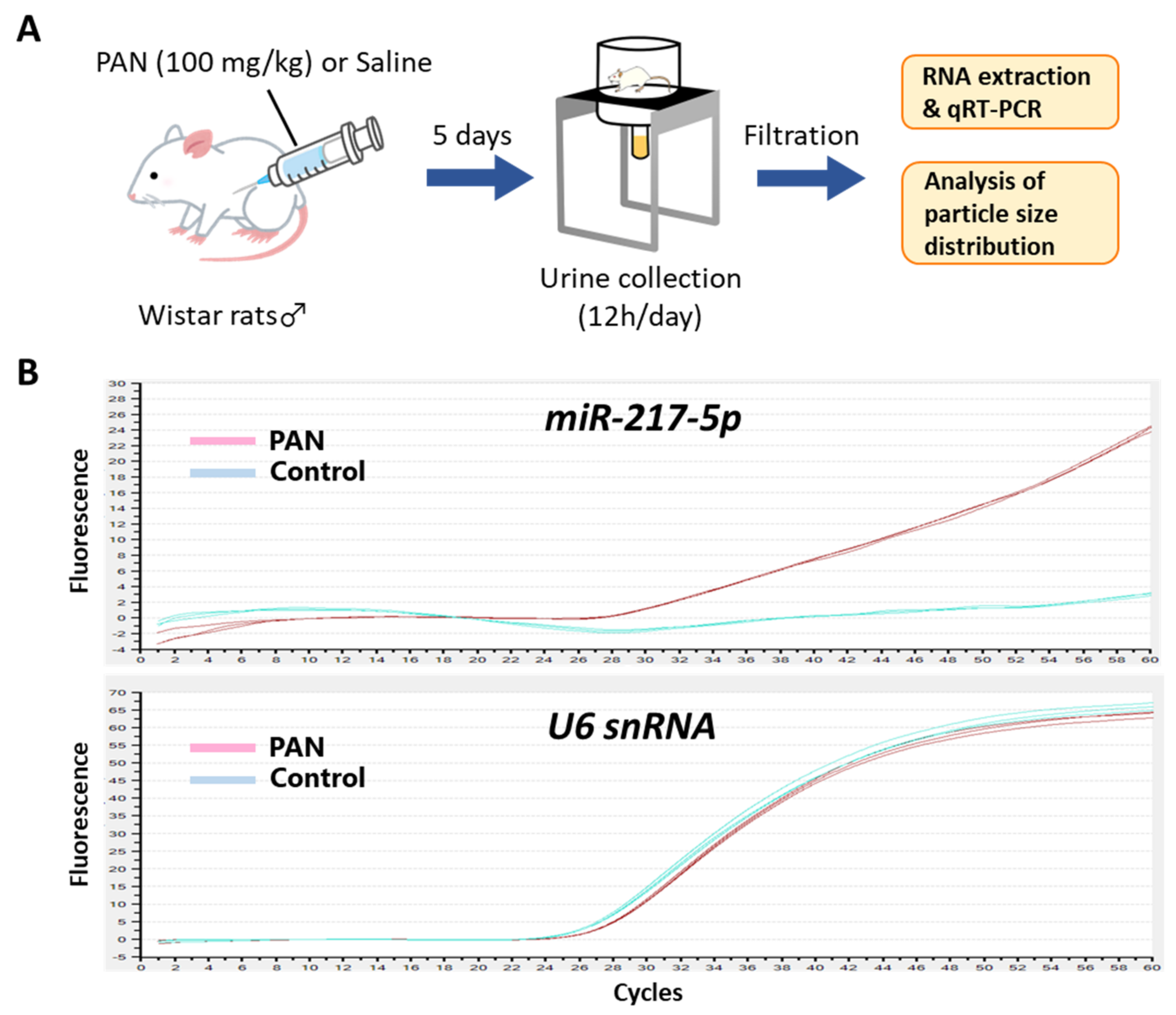
Because podocytes face the urinary space of the Bowman’s capsule, it is conceivable that miRNAs in podocytes are secreted into glomerular filtrate. We evaluated the amount of miR-217-5p in urine from PAN- and saline-administrated rats (Figure 7A). Consistent with the marked increase of miR-217-5p expression in PAN-treated rat podocytes (Figure 2), miR-217-5p was detected in cell-free urine from PAN-administrated rats but not in that from saline-administrated rats (Figure 7B). By contrast, U6 snRNA, a small nuclear RNA ubiquitously expressed in many cell types including podocytes, was detected in urine at similar levels from both PAN- and saline-administrated rats (Figure 7B).
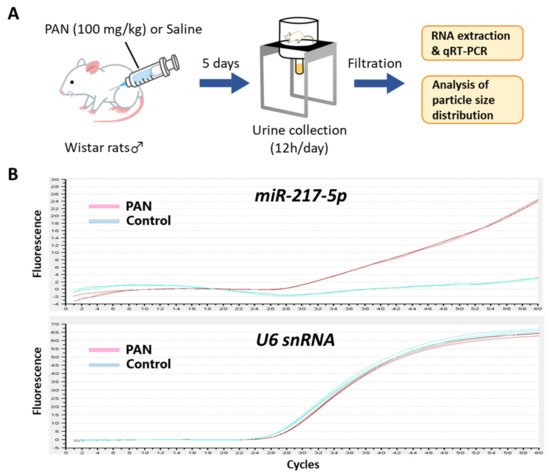
Figure 7.
Detection of miR-217-5p in urine from PAN-administrated rats. (A) Schematic illustration of the experimental procedure. Seven-week-old male Wistar rats were administrated with 100 mg/kg PAN or saline. (B) Amplification plots of qRT–PCR for the detection of miR-217-5p and U6 snRNA in urine from the PAN- or saline-administrated rats. Urine samples collected at day 5 following PAN/saline administration were used for the analysis.
4. Discussion
This study performed miRNA-seq analysis of primary culture rat podocytes treated with or without PAN. The miRNA-seq dataset was combined with RNA-seq datasets of PAN-treated rat podocytes, which were acquired previously from the same RNA samples [25], and subjected to integrative bioinformatical analysis of miRNA and mRNA expression. MiR-217-5p was identified as the miRNA most strongly upregulated by PAN, with Myo1d as its possible target. We propose that the miR-217-5p-Myo1d axis may be associated with PAN-induced podocyte injury.
Many miRNAs have been previously associated with podocyte injury [23,24]. This study identified miR-217-5p as having the most highly upregulated expression (5.73-fold) following PAN-induced podocyte injury. The second most highly upregulated miRNA was miR-216a-5p (4.64-fold). These results are consistent with the fact that miR-217, -216a, and -216b are generated from a common host gene designated as MIR217HG, which spans an approximately 40-kbp region in the rat, mouse, and human genomes, allowing simultaneous regulation at a transcriptional level. Furthermore, the expression of MIR217HG-derived miRNAs is simultaneously dysregulated in different tissues under pathological conditions, such as in acute pancreatic injury [38,39,40,41,42,43], pancreatic cancer [44,45], and gastric cancers [46]; this may also be the case with podocyte injury.
Of the five dysregulated miRNAs, only miR-217-5p has been associated with podocyte injury in previous studies. Sun et al. reported that the expression of miR-217-5p is upregulated in podocytes stimulated with high glucose, and miR-217-5p is involved in high glucose-induced podocyte injury by attenuating the PTEN expression as its direct target [47]. These data are consistent with our findings that miR-217-5p expression is upregulated in response to podocyte injury. However, Jin et al. showed that reduced expression of the long noncoding RNA XIST prevents podocyte apoptosis in membranous nephropathy through the miR-217-TLR4 axis [48]. This report contrasts with our finding because claiming that miR-217-5p exerts a protective effect on podocyte injury. The inconsistency may be attributed to different pathological conditions between studies. The PAN nephropathy model exhibits pathological conditions similar to a minimal change disease rather than membranous nephropathy. However, whether miR-217-5p expression is differently regulated in various nephropathy models requires further investigation.
Although dysregulation of miR-217-5p expression to podocyte injury has been reported previously, the mechanism underlying the relationship is not understood. The GO enrichment analysis of our RNA-seq dataset showed that the predicted targets of miR-217-5p were closely associated with cell morphogenesis. Consistent with this analysis, E11 cells with miR-217-5p overexpression exhibited shrunken cell shapes with shortened or disorganized actin cytoskeletons. Previously, Solanki et al. demonstrated that the apoptosis-related p53 pathway is involved in actin cytoskeleton damage in PAN- or ADR-treated podocytes [36]. However, in our study, the p53 pathway was not associated with predicted targets of miR-217-5p in PAN-treated podocytes. Furthermore, unlike PAN, miR-217-5p overexpression did not affect E11 cell viability. Therefore, it is possible that PAN induces podocyte cell death through a mechanism independent of miR-217-5p. Of note, pathway analysis using DAVID (the Database for Annotation, Visualization and Integrated Discovery) revealed that mRNAs identified to be upregulated by PAN in our mRNA-seq analysis are most significantly associated with the p53 signaling pathway (Table S6). Therefore, we suggest that PAN may also induce morphological changes of podocytes as a result of p53-mediated apoptosis activated by the upregulated mRNAs. On the other hand, the mRNAs identified to be downregulated by PAN were not significantly associated with the p53 signaling pathway. Instead, these mRNAs were strongly associated with cell morphology-related pathways such as the “regulation of actin cytoskeleton” and “focal adhesion” pathways (Table S7), which were not associated with the upregulated mRNAs. It is possible that these pathways are partly associated with mRNAs negatively regulated by the PAN-induced miRNAs including miR-217-5p.
Interestingly, pathway analysis using DAVID showed that the predicted targets of miR-217-5p in podocytes are significantly associated with the ‘axon guidance’ pathway. Previous studies have suggested there is common molecular machinery for the formation of podocyte foot processes and the axon guidance of neurons [49,50,51]. Taken together with the fact that the myosin I protein family was previously associated with both podocyte morphology [52] and axon guidance [53], we decided to focus on Myo1d, an actin-binding protein, as a possible target of miR-217-5p. As expected, Myo1d is a direct target of miR-217-5p; therefore, the miR-217-5p-Myo1d axis could be involved in podocyte injury and warrants further investigation as a research target for clinical applications.
Finally, we showed that miR-217-5p was present in urine from PAN-administrated rats but not in control rats. When podocytes are severely damaged, they occasionally become detached from the glomeruli and moved into urine [54]. Furthermore, urine is rich in exosomes, unilamellar small vesicles (50–100 nm in diameter) secreted from many cell types including podocytes [54]. Consistently, we detected small vesicles with varying diameter peaking at 100 nm in the PAN- and saline-administrated rats by dynamic light scattering (DLS) (Figure S8). Previous work demonstrated that intracellular miRNAs are loaded into exosomes and secreted into the extracellular environment [55]; therefore, it is possible that miR-217-5p is present in not only urinary podocytes but also urinary exosomes from PAN-administrated rats. Taken together, miR-217-5p may be a promising biomarker of some kidney diseases involving podocyte injury.
In the present study, we could identify a novel miRNA-mRNA axis associated with podocyte injury. Nonetheless, this study has some limitations. First, it should be demonstrated that decreased Myo1d expression in E11 and rat primary podocytes leads to their morphological changes. Furthermore, the loss-of-function studies of miR-217-5p, e.g., the phenotypic analysis of miR-217-5p-knockout podocytes generated using a genome editing technique, is necessarily performed to support our findings. We hope that the results of such additional experiments will increase the clinical significance of our findings.
Supplementary Materials
The following information can be downloaded at https://www.mdpi.com/article/10.3390/ncrna8030043/s1. Figure S1. Effect of induced miR-217-5p expression on cell viability of E11 cells. Cell viability was evaluated at day 4 after transfection. n.s.: not significant compared to the NC-transfected cells. Figure S2. Selection of mRNAs dysregulated in PAN-treated podocytes. The numbers of upregulated (A) and downregulated (B) mRNAs selected based on the criteria are shown. Figure S3. Selection of mRNAs possibly targeted by miR-217-5p. Figure S4. Interactive GO terms enriched with the predicted targets of miR-217-5p in PAN-treated podocytes. Enriched GO terms (biological process) were filtered with a threshold of p-values greater than 8.35 × 10−5 and analyzed for interconnection using the GOnet software. Of the 12 predicted targets, only Cris1 is not connected to any of the enriched GO terms. The more intense color of the GO term node indicates the more significant enrichment of the term (the smaller p-value). Figure S5. Myo1d mRNA levels in PAN-treated primary rat podocytes. Values were normalized relative to GAPDH mRNA expression. Primary rat podocytes were treated with 0–30 μg/mL PAN. The relative expression level of Myo1d mRNA at 0 μg/mL PAN was given an arbitrary value of 1. * p < 0.05 versus 0 mg/mL PAN. Data represent the means ± SD. Figure S6. Immunocytochemical detection of Myo1d protein in E11 cells. Myo1d proteins were detected in the cytosol of E11 cells. The cytosolic localization of Myo1d proteins have been also shown in the human bone osteosarcoma cell line U-2 OS (The Human Protein Atlas database; https://www.proteinatlas.org/ENSG00000176658-MYO1D/subcellular#, accessed on 6 June 2022). Bar: 100 μm. Figure S7. Western blot images showing the detection of Myo1d protein in E11 cells with induced miR-217-5p expression. Arrowheads indicate the immunodetected bands of Myo1d protein. Figure S8. Size distribution of particles in urine from Wistar rats administrated with PAN (100 mg/kg) or saline. Whole particles in the urine were isolated by ultracentrifugation and analyzed by dynamic light scattering. Table S1. Primers designed for SYBR qRT-PCR. Table S2. Summary of small RNA sequencing results. Table S3. mRNAs upregulated in response to PAN-induced podocyte injury. Table S4. mRNAs downregulated in response to PAN-induced podocyte injury. Table S5. Decreased Myo1d mRNA expression in injured human podocytes based on the RNA sequencing datasets registered under the accession GSE124622 in Gene Expression Omnibus. Table S6. Pathways significantly associated with the mRNAs upregulated by PAN in podocytes. Table S7. Pathways significantly associated with the mRNAs downregulated by PAN in podocytes.
Author Contributions
Experiments, O.I., M.H., A.H., H.O. and R.M.; bioinformatic analyses, O.I., M.H. and A.H.; manuscript preparation, O.I. and M.H.; review and editing, O.I., N.M. and T.I.; resources, O.I. and T.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The small RNA sequencing data obtained for this study are deposited in DRA of DDBJ under the accession identifier DRA013173.
Acknowledgments
The authors thank Daisuke Okuzaki (the Genome Information Research Center, Osaka University) for supporting the small RNA sequencing analysis in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Kidney Day: Chronic Kidney Disease. 2015. Available online: http://www.worldkidneyday.org/faqs/chronic-kidney-disease/ (accessed on 6 June 2022).
- Whaley-Connell, A.; Nistala, R.; Chaudhary, K. The importance of early identification of chronic kidney disease. Mo. Med. 2011, 108, 25–28. [Google Scholar] [PubMed]
- Darshi, M.; Van Espen, B.; Sharma, K. Metabolomics in Diabetic Kidney Disease: Unraveling the Biochemistry of a Silent Killer. Am. J. Nephrol. 2016, 44, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, S.R.; Aeddula, N.R. Chronic Renal Failure. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Zhong, J.; Yang, H.C.; Fogo, A.B. A perspective on chronic kidney disease progression. Am. J. Physiol. Renal. Physiol. 2017, 312, F375–F384. [Google Scholar] [CrossRef] [PubMed]
- Turin, T.C.; Tonelli, M.; Manns, B.J.; Ravani, P.; Ahmed, S.B.; Hemmelgarn, B.R. Chronic kidney disease and life expectancy. Nephrol. Dial. Transplant. 2012, 27, 3182–3186. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.K.; Li, P.K. Chronic kidney disease epidemic: How do we deal with it? Nephrology (Carlton) 2018, 23 (Suppl. S4), 116–120. [Google Scholar] [CrossRef] [PubMed]
- Whalen, H.; Clancy, M.; Jardine, A. Future challenges in renal transplantation. Minerva Chir. 2012, 67, 15–30. [Google Scholar] [PubMed]
- Neipp, M.; Jackobs, S.; Klempnauer, J. Renal transplantation today. Langenbecks Arch. Surg. 2009, 394, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Coates, P.T.; Wong, G. Current controversies in nephrology—how to crossmatch for transplantation? Kidney Int. 2020, 97, 662–663. [Google Scholar] [CrossRef]
- Sandip, K. Challenges & Controversies in Kidney Transplantation, 1st ed.; JAYPEE Digital: New Delhi, India, 2015; pp. 1–172. [Google Scholar]
- Shrestha, B.M. Strategies for reducing the renal transplant waiting list: A review. Exp. Clin. Transplant. 2009, 7, 173–179. [Google Scholar]
- Voora, S.; Adey, D.B. Management of Kidney Transplant Recipients by General Nephrologists: Core Curriculum 2019. Am. J. Kidney. Dis. 2019, 73, 866–879. [Google Scholar] [CrossRef]
- Murray, I.; Paolini, M.A. Histology, Kidney and Glomerulus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Liapis, H.; Romagnani, P.; Anders, H.J. New insights into the pathology of podocyte loss: Mitotic catastrophe. Am. J. Pathol. 2013, 183, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Garg, P. A Review of Podocyte Biology. Am. J. Nephrol. 2018, 47 (Suppl. S1), 3–13. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M. Podocyte injury and its consequences. Kidney Int. 2016, 89, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Wakiyama, M.; Takimoto, K.; Ohara, O.; Yokoyama, S. Let-7 microRNAmediated mRNA deadenylation and translational repression in a mammalian cell-free system. Genes Dev. 2007, 21, 1857–1862. [Google Scholar] [CrossRef]
- Meister, G.; Tuschl, T. Mechanisms of gene silencing by double-stranded RNA. Nature 2004, 431, 343–349. [Google Scholar] [CrossRef]
- Shi, S.; Yu, L.; Chiu, C.; Sun, Y.; Chen, J.; Khitrov, G.; Merkenschlager, M.; Holzman, L.B.; Zhang, W.; Mundel, P.; et al. Podocyte-selective deletion of dicer induces proteinuria and glomerulosclerosis. J. Am. Soc. Nephrol. 2008, 19, 2159–2169. [Google Scholar] [CrossRef]
- Harvey, S.J.; Jarad, G.; Cunningham, J.; Goldberg, S.; Schermer, B.; Harfe, B.D.; McManus, M.T.; Benzing, T.; Miner, J.H. Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. J. Am. Soc. Nephrol. 2008, 19, 2150–2158. [Google Scholar] [CrossRef]
- Ho, J.; Ng, K.H.; Rosen, S.; Dostal, A.; Gregory, R.I.; Kreidberg, J.A. Podocyte-specific loss of functional microRNAs leads to rapid glomerular and tubular injury. J. Am. Soc. Nephrol. 2008, 19, 2069–2075. [Google Scholar] [CrossRef]
- Ishii, H.; Kaneko, S.; Yanai, K.; Aomatsu, A.; Hirai, K.; Ookawara, S.; Ishibashi, K.; Morishita, Y. MicroRNAs in Podocyte Injury in Diabetic Nephropathy. Front. Genet. 2020, 11, 993. [Google Scholar] [CrossRef]
- Li, J.Y.; Yong, T.Y.; Michael, M.Z.; Gleadle, J.M. Review: The role of microRNAs in kidney disease. Nephrology (Carlton) 2010, 15, 599–608. [Google Scholar] [CrossRef]
- Horikawa, A.; Yoneda, T.; Yaoita, E.; Yamaguchi, K.; Shigenobu, S.; Kuramochi, M.; Yamate, J.; Inui, T.; Ishibashi, O. A novel splicing variant of small nucleolar RNA host gene 4 is a podocyte-selective non-coding RNA upregulated in response to puromycin aminonucleoside-induced podocyte injury. J. Biochem. 2019, 165, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Katsuya, K.; Yaoita, E.; Yoshida, Y.; Yamamoto, Y.; Yamamoto, T. An improved method for primary culture of rat podocytes. Kidney Int. 2006, 69, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Yamada, E.; Okada, S.; Shimoda, Y.; Tagaya, Y.; Hashimoto, K.; Satoh, T.; Mori, M.; Okada, J.; Pessin, J.E.; et al. Nucleobindin-2 is a positive regulator for insulin-stimulated glucose transporter 4 translocation in fenofibrate treated E11 podocytes. Endocr. J. 2014, 61, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, O.; Akagi, I.; Ogawa, Y.; Inui, T. MiR-141-3p is upregulated in esophageal squamous cell carcinoma and targets pleckstrin homology domain leucine-rich repeat protein phosphatase-2, a negative regulator of the PI3K/AKT pathway. Biochem. Biophys. Res. Commun. 2018, 501, 507–513. [Google Scholar] [CrossRef]
- Pomaznoy, M.; Ha, B.; Peters, B. GOnet: A tool for interactive Gene Ontology analysis. BMC Bioinform. 2018, 19, 470. [Google Scholar] [CrossRef]
- Thilo, F.; Liu, Y.; Loddenkemper, C.; Schuelein, R.; Schmidt, A.; Yan, Z.; Zhu, Z.; Zakrzewicz, A.; Gollasch, M.; Tepel, M. VEGF regulates TRPC6 channels in podocytes. Nephrol. Dial. Transplant. 2012, 27, 921–929. [Google Scholar] [CrossRef][Green Version]
- Nosaka, K.; Takahashi, T.; Nishi, T.; Imaki, H.; Suzuki, T.; Suzuki, K.; Kurokawa, K.; Endou, H. An adenosine deaminase inhibitor prevents puromycin aminonucleoside nephrotoxicity. Free Radic. Biol. Med. 1997, 22, 597–605. [Google Scholar] [CrossRef]
- Xia, L.; Zhou, M.; Kalhorn, T.F.; Ho, H.T.; Wang, J. Podocyte-specific expression of organic cation transporter PMAT: Implication in puromycin aminonucleoside nephrotoxicity. Am. J. Physiol. Renal. Physiol. 2009, 296, F1307–F1313. [Google Scholar] [CrossRef]
- Guo, H.; Ingolia, N.T.; Weissman, J.S.; Bartel, D.P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010, 466, 835–840. [Google Scholar] [CrossRef]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef]
- Noris, M.; Remuzzi, G. Non-muscle myosins and the podocyte. Clin. Kidney J. 2012, 5, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Solanki, A.K.; Srivastava, P.; Rahman, B.; Lipschutz, J.H.; Nihalani, D.; Arif, E. The Use of High-throughput transcriptomics to identify pathways with therapeutic significance in podocytes. Int. J. Mol. Sci. 2019, 21, 274. [Google Scholar] [CrossRef] [PubMed]
- Okabe, M.; Motojima, M.; Miyazaki, Y.; Pastan, I.; Yokoo, T.; Matsusaka, T. Global polysome analysis of normal and injured podocytes. Am. J. Physiol. Renal. Physiol. 2019, 316, F241–F252. [Google Scholar] [CrossRef]
- Erdos, Z.; Barnum, J.E.; Wang, E.; DeMaula, C.; Dey, P.M.; Forest, T.; Bailey, W.J.; Glaab, W.E. Evaluation of the Relative Performance of Pancreas-Specific MicroRNAs in Rat Plasma as Biomarkers of Pancreas Injury. Toxicol. Sci. 2020, 173, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rouse, R. Co-sequencing and novel delayed anti-correlation identify function for pancreatic enriched microRNA biomarkers in a rat model of acute pancreatic injury. BMC Genom. 2018, 19, 297. [Google Scholar] [CrossRef]
- Wang, J.; Huang, W.; Thibault, S.; Brown, T.P.; Bobrowski, W.; Gukasyan, H.J.; Evering, W.; Hu, W.; John-Baptiste, A.; Vitsky, A. Evaluation of miR-216a and miR-217 as Potential Biomarkers of Acute Exocrine Pancreatic Toxicity in Rats. Toxicol. Pathol. 2017, 45, 321–334. [Google Scholar] [CrossRef]
- Rouse, R.; Rosenzweig, B.; Shea, K.; Knapton, A.; Stewart, S.; Xu, L.; Chockalingam, A.; Zadrozny, L.; Thompson, K. MicroRNA biomarkers of pancreatic injury in a canine model. Exp. Toxicol. Pathol. 2017, 69, 33–43. [Google Scholar] [CrossRef]
- Calvano, J.; Edwards, G.; Hixson, C.; Burr, H.; Mangipudy, R.; Tirmenstein, M. Serum microRNAs-217 and -375 as biomarkers of acute pancreatic injury in rats. Toxicology 2016, 368–369, 1–9. [Google Scholar] [CrossRef]
- Goodwin, D.; Rosenzweig, B.; Zhang, J.; Xu, L.; Stewart, S.; Thompson, K.; Rouse, R. Evaluation of miR-216a and miR-217 as potential biomarkers of acute pancreatic injury in rats and mice. Biomarkers 2014, 19, 517–529. [Google Scholar] [CrossRef]
- Rachagani, S.; Macha, M.A.; Menning, M.S.; Dey, P.; Pai, P.; Smith, L.M.; Mo, Y.Y.; Batra, S.K. Changes in microRNA (miRNA) expression during pancreatic cancer development and progression in a genetically engineered KrasG12D; Pdx1-Cre mouse (KC) model. Oncotarget 2015, 6, 40295–40309. [Google Scholar] [CrossRef]
- Egeli, U.; Tezcan, G.; Cecener, G.; Tunca, B.; Demirdogen Sevinc, E.; Kaya, E.; Ak, S.; Dundar, H.Z.; Sarkut, P.; Ugras, N.; et al. Pancreas. miR-216b Targets FGFR1 and Confers Sensitivity to Radiotherapy in Pancreatic Ductal Adenocarcinoma Patients Without EGFR or KRAS Mutation. Pancreas 2016, 45, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Safaralizadeh, R.; Ajami, N.; Nemati, M.; Hosseinpourfeizi, M.; Azimzadeh Isfanjani, A.; Moaddab, S.Y. Disregulation of miR-216a and miR-217 in Gastric Cancer and Their Clinical Significance. J. Gastrointest. Cancer 2019, 50, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Z.P.; Zhang, R.Q.; Zhang, H.M. Repression of miR-217 protects against high glucose-induced podocyte injury and insulin resistance by restoring PTEN-mediated autophagy pathway. Biochem. Biophys. Res. Commun. 2017, 483, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.W.; Pan, M.; Ye, H.Y.; Zheng, Y.; Chen, Y.; Huang, W.W.; Xu, X.Y.; Zheng, S.B. Down-regulation of the long non-coding RNA XIST ameliorates podocyte apoptosis in membranous nephropathy via the miR-217-TLR4 pathway. Exp. Physiol. 2019, 104, 220–230. [Google Scholar] [CrossRef]
- Kobayashi, N. Mechanism of the process formation; podocytes vs. neurons. Microsc. Res. Tech. 2002, 57, 217–223. [Google Scholar] [CrossRef]
- Tapia, R.; Guan, F.; Gershin, I.; Teichman, J.; Villegas, G.; Tufro, A. Semaphorin3a disrupts podocyte foot processes causing acute proteinuria. Kidney Int. 2008, 73, 733–740. [Google Scholar] [CrossRef]
- Fan, X.; Li, Q.; Pisarek-Horowitz, A.; Rasouly, H.M.; Wang, X.; Bonegio, R.G.; Wang, H.; McLaughlin, M.; Mangos, S.; Kalluri, R.; et al. Inhibitory effects of Robo2 on nephrin: A crosstalk between positive and negative signals regulating podocyte structure. Cell Rep. 2012, 2, 52–61. [Google Scholar] [CrossRef]
- Arif, E.; Wagner, M.C.; Johnstone, D.B.; Wong, H.N.; George, B.; Pruthi, P.A.; Lazzara, M.J.; Nihalani, D. Motor protein Myo1c is a podocyte protein that facilitates the transport of slit diaphragm protein Neph1 to the podocyte membrane. Mol. Cell Biol. 2011, 31, 2134–2150. [Google Scholar] [CrossRef]
- Benesh, A.E.; Fleming, J.T.; Chiang, C.; Carter, B.D.; Tyska, M.J. Expression and localization of myosin-1d in the developing nervous system. Brain Res. 2012, 1440, 9–22. [Google Scholar] [CrossRef]
- Zeng, L.; Szeto, C.C. Urinary podocyte markers in kidney diseases. Clin. Chim. Acta 2021, 523, 315–324. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).