Evaluating the Cytotoxicity of Functionalized MWCNT and Microbial Biofilm Formation on PHBV Composites
Abstract
1. Introduction
2. Materials and Methods
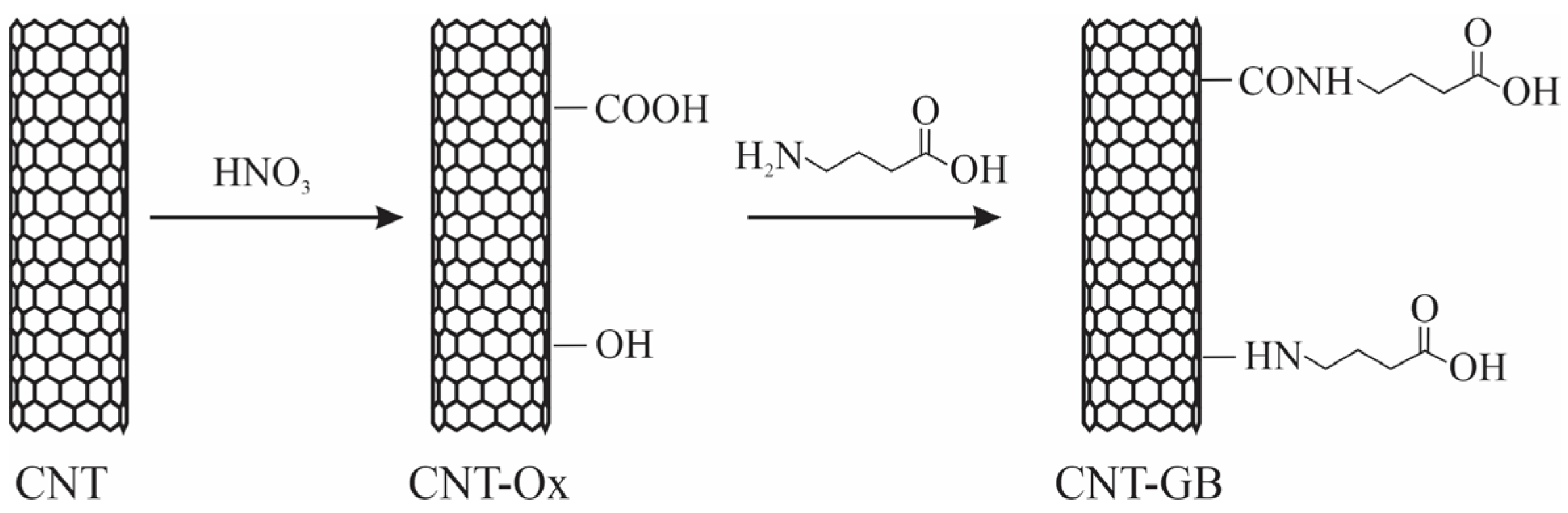
2.1. Functionalization of Carbon Nanotubes
2.1.1. Oxidation of Carbon Nanotubes (CNT-Ox)
2.1.2. Functionalization of Carbon Nanotubes with Gamma-Aminobutyric Acid (NTC-GB)
2.2. Production of PHBV/CNT Nanocomposites
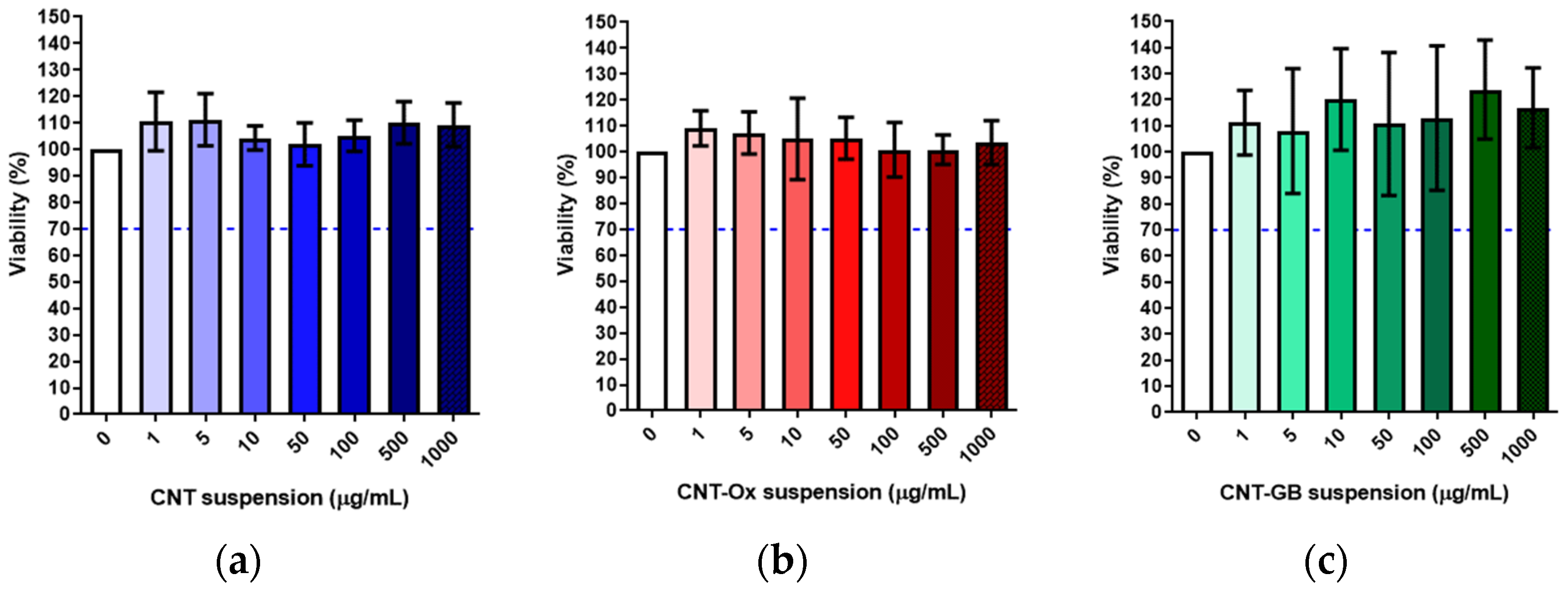
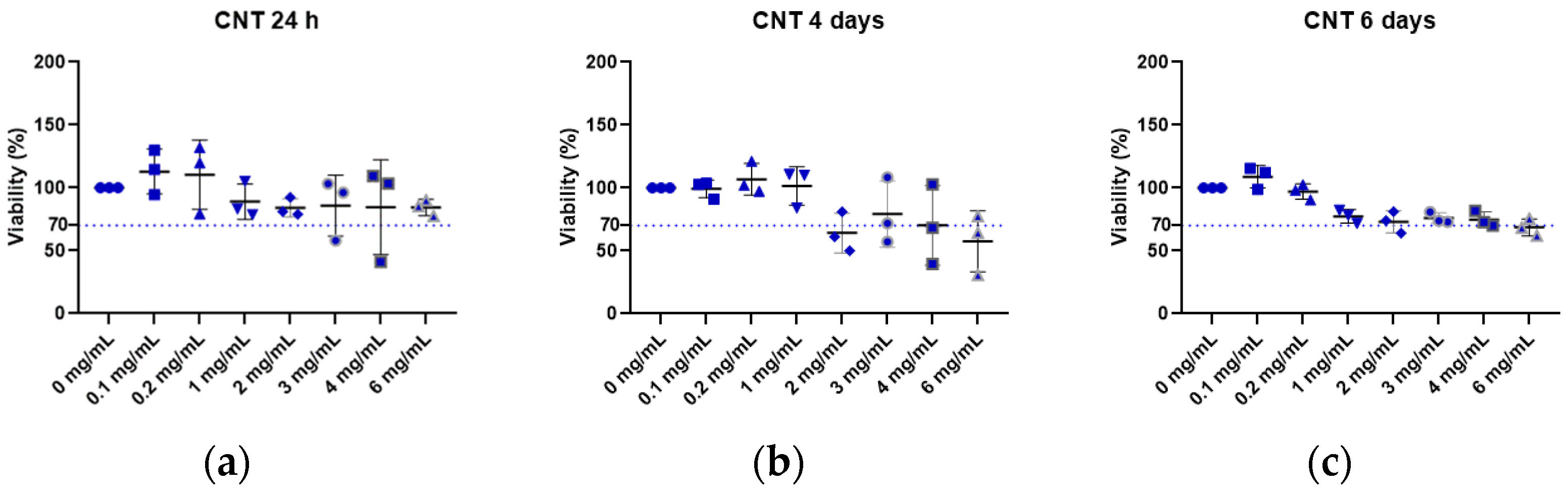
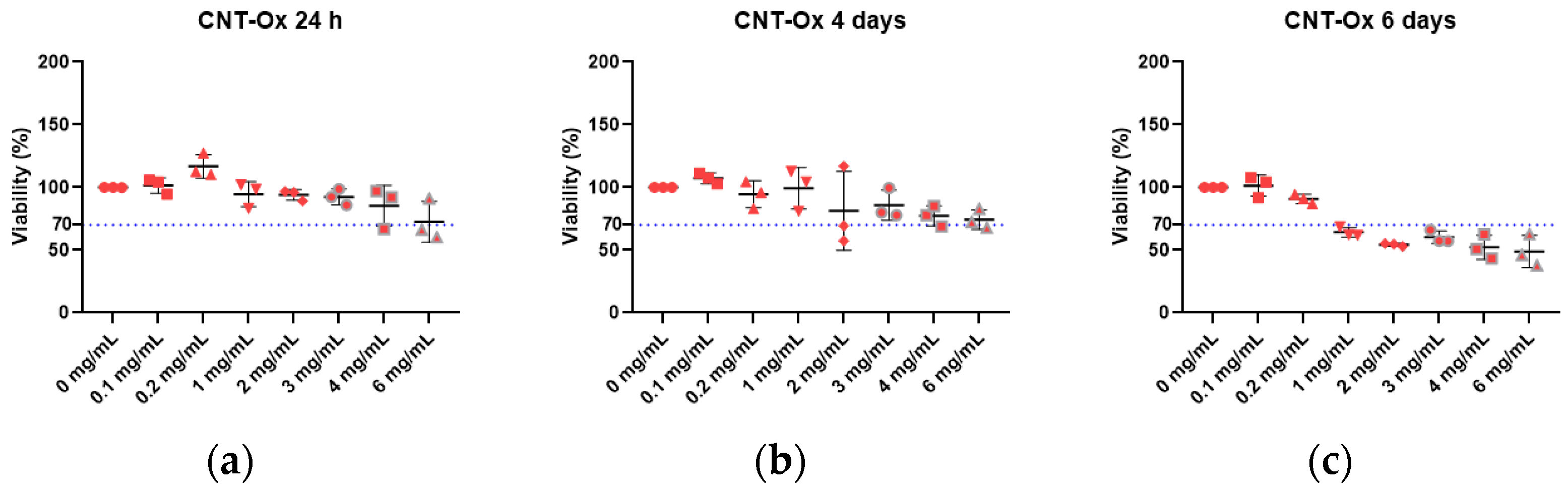
2.3. In Vitro Cytotoxicity Assay
2.3.1. Preparation of the Extracts
2.3.2. Cellular Incubation
2.3.3. MTT Assay
2.4. Antibiofilm Activity Test
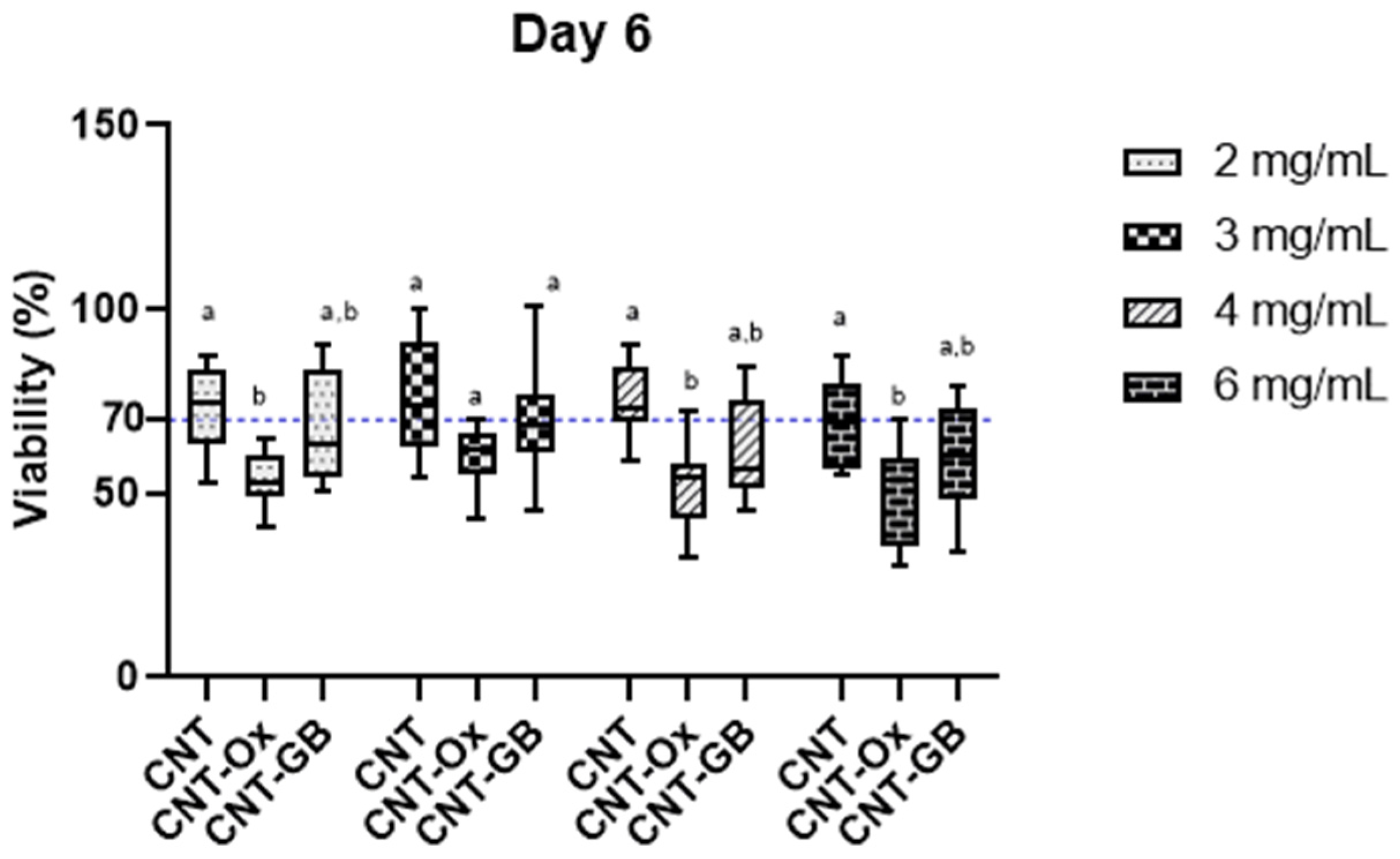
3. Results and Discussion
Antibiofilm Effect of PHBV and CNT Nanocomposites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- de Menezes, B.R.C.; Rodrigues, K.F.; Fonseca, B.C.S.; Ribas, R.G.; Montanheiro, T.L.A.; Thim, G.P. Recent advances in the use of carbon nanotubes as smart biomaterials. J. Mater. Chem. B 2019, 7, 1343–1360. [Google Scholar] [CrossRef] [PubMed]
- Montanheiro, T.L.A.; Cristóvan, F.H.; Machado, J.P.B.; Tada, D.B.; Durán, N.; Lemes, A.P. Effect of MWCNT functionalization on thermal and electrical properties of PHBV/MWCNT nanocomposites. J. Mater. Res. 2014, 30, 55–65. [Google Scholar] [CrossRef]
- Montanheiro, T.L.A.; de Menezes, B.R.C.; Ribas, R.G.; Montagna, L.S.; Campos, T.M.B.; Schatkoski, V.M.; Righetti, V.A.N.; Passador, F.R.; Thim, G.P. Covalently γ-aminobutyric acid-functionalized carbon nanotubes: Improved compatibility with PHBV matrix. SN Appl. Sci. 2019, 1, 1177. [Google Scholar] [CrossRef]
- Jin, Q.; Zheng, Z.; Feng, Y.; Tian, S.; He, Z. Multi-Walled Carbon Nanotubes Modified NiCo2S4 for the Efficient Photocatalytic Reduction of Hexavalent Chromium. C 2023, 9, 99. [Google Scholar] [CrossRef]
- Estévez-Martínez, Y.; Quiroga-González, E.; Cuevas-Yañez, E.; Durón-Torres, S.; Alaníz-Lumbreras, D.; Chavira-Martínez, E.; Posada-Gómez, R.; Bravo-Tapia, J.; Castaño-Meneses, V. Membranes of Multiwall Carbon Nanotubes in Chitosan–Starch with Mechanical and Compositional Properties Useful in Li-Ion Batteries. C 2023, 9, 87. [Google Scholar] [CrossRef]
- Alshehri, R.; Ilyas, A.M.; Hasan, A.; Arnaout, A.; Ahmed, F.; Memic, A. Carbon Nanotubes in Biomedical Applications: Factors, Mechanisms, and Remedies of Toxicity. J. Med. Chem. 2016, 59, 8149–8167. [Google Scholar] [CrossRef]
- Vardharajula, S.; Ali, S.Z.; Tiwari, P.M.; Eroğlu, E.; Vig, K.; Dennis, V.; Singh, S.R. Functionalized carbon nanotubes: Biomedical applications. Int. J. Nanomed. 2012, 7, 5361–5374. [Google Scholar] [CrossRef]
- Wang, X.; Lim, E.G.; Hoettges, K.; Song, P. A Review of Carbon Nanotubes, Graphene and Nanodiamond Based Strain Sensor in Harsh Environments. C 2023, 9, 108. [Google Scholar] [CrossRef]
- Träger, D.; Słota, D.; Niziołek, K.; Florkiewicz, W.; Sobczak-Kupiec, A. Hybrid Polymer–Inorganic Coatings Enriched with Carbon Nanotubes on Ti-6Al-4V Alloy for Biomedical Applications. Coatings 2023, 13, 1813. [Google Scholar] [CrossRef]
- Aoki, K.; Ogihara, N.; Tanaka, M.; Haniu, H.; Saito, N. Carbon nanotube-based biomaterials for orthopaedic applications. J. Mater. Chem. B 2020, 8, 9227–9238. [Google Scholar] [CrossRef]
- Abdelrazek, E.M.; Hezma, A.M.; El-khodary, A.; Elzayat, A.M.; Rajeh, A. Modifying of Structural, Optical, Thermal, and Mechanical Properties of PCL/PMMA Biomaterial Blend Doped With MWCNTs as an Application in Materials Science. J. Inorg. Organomet. Polym. Mater. 2023, 33, 4117–4126. [Google Scholar] [CrossRef]
- Neto, J.S.S.; Cavalcanti, D.K.K.; da Cunha Ferro, L.E.; de Queiroz, H.F.M.; Aguiar, R.A.A.; Banea, M.D. Effect of Multi-Walled Carbon Nanotubes on the Mechanical and Thermal Properties of Curauá Natural-Fiber-Reinforced Composites. C 2023, 9, 102. [Google Scholar] [CrossRef]
- Montanheiro, T.L.A.; Ribas, R.G.; Montagna, L.S.; de Menezes, B.R.C.; Schatkoski, V.M.; Rodrigues, K.F.; Thim, G.P. A brief review concerning the latest advances in the influence of nanoparticle reinforcement into polymeric-matrix biomaterials. J. Biomater. Sci. Polym. Ed. 2020, 31, 1869–1893. [Google Scholar] [CrossRef] [PubMed]
- Loura, N.; Gkartzou, E.; Trompeta, A.-F.; Konstantopoulos, G.; Klonos, P.A.; Kyritsis, A.; Charitidis, C.A. Development of CNT-Based Nanocomposites with Ohmic Heating Capability towards Self-Healing Applications in Extrusion-Based 3D Printing Technologies. C 2023, 9, 111. [Google Scholar] [CrossRef]
- Lemes, A.P.; Montanheiro, T.L.A.; da Silva, A.P.; Durán, N. PHBV/MWCNT Films: Hydrophobicity, Thermal and Mechanical Properties as a Function of MWCNT Concentration. J. Compos. Sci. 2019, 3, 12. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, J.; Chen, S.; Wu, S. Designing an Innovative Electrospinning Strategy to Generate PHBV Nanofiber Scaffolds with a Radially Oriented Fibrous Pattern. Nanomaterials 2023, 13, 1150. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cendal, A.I.; Gómez-Seoane, I.; de Toro-Santos, F.J.; Fuentes-Boquete, I.M.; Señarís-Rodríguez, J.; Díaz-Prado, S.M. Biomedical Applications of the Biopolymer Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV): Drug Encapsulation and Scaffold Fabrication. Int. J. Mol. Sci. 2023, 24, 11674. [Google Scholar] [CrossRef]
- Butron, A.; Llorente, O.; Fernandez, J.; Meaurio, E.; Sarasua, J.R. Morphology and mechanical properties of poly(ethylene brassylate)/cellulose nanocrystal composites. Carbohydr. Polym. 2019, 221, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Montanheiro, T.L.A.; Montagna, L.S.; Patrulea, V.; Jordan, O.; Borchard, G.; Ribas, R.G.; Campos, T.M.B.; Thim, G.P.; Lemes, A.P. Enhanced water uptake of PHBV scaffolds with functionalized cellulose nanocrystals. Polym. Test. 2019, 79, 106079. [Google Scholar] [CrossRef]
- Montanheiro, T.L.A.; Montagna, L.S.; Patrulea, V.; Jordan, O.; Borchard, G.; Lobato, G.M.M.; Catalani, L.H.; Lemes, A.P. Evaluation of cellulose nanocrystal addition on morphology, compression modulus and cytotoxicity of poly (3-hydroxybutyrate- co -3-hydroxyvalerate) scaffolds. J. Mater. Sci. Mater. life Sci. 2019, 54, 7198–7210. [Google Scholar] [CrossRef]
- Montanheiro, T.L.A.; Campos, T.M.B.; Montagna, L.S.S.; da Silva, A.P.; Ribas, R.G.; de Menezes, B.R.C.; Passador, F.R.; Thim, G. Influence of CNT pre-dispersion into PHBV/CNT nanocomposites and evaluation of morphological, mechanical and crystallographic features. Mater. Res. Express 2019, 6, 105375. [Google Scholar] [CrossRef]
- Montanheiro, T.L.A.; de Menezes, B.R.C.; Montagna, L.S.; Beatrice, C.A.G.; Marini, J.; Lemes, A.P.; Thim, G.P. Non-Isothermal Crystallization Kinetics of Injection Grade PHBV and PHBV/Carbon Nanotubes Nanocomposites Using Isoconversional Method. J. Compos. Sci. 2020, 4, 52. [Google Scholar] [CrossRef]
- Silva, A.P.; Amaral Montanheiro, T.L.; Stieven Montagna, L.; Andrade, P.F.; Durán, N.; Lemes, A.P. Effect of carbon nanotubes on the biodegradability of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanocomposites. J. Appl. Polym. Sci. 2019, 48020, 48020. [Google Scholar] [CrossRef]
- Montagna, L.S.; Oyama, I.C.; Lamparelli, R.C.B.C.; Da Silva, A.P.; Montanheiro, T.L.A.; Lemes, A.P. Evaluation of Biodegradation in Aqueous Medium of Poly(Hydroxybutyrate-Co-Hydroxyvalerate)/Carbon Nanotubes Films in Respirometric System. J. Renew. Mater. 2019, 7, 117–128. [Google Scholar] [CrossRef]
- Song, Y.; Lin, K.; He, S.; Wang, C.; Zhang, S.; Li, D.; Wang, J.; Cao, T.; Bi, L.; Pei, G. Nano-biphasic calcium phosphate / polyvinyl alcohol composites with enhanced bioactivity for bone repair via low-temperature three-dimensional printing and loading with platelet-rich fibrin. J. Nanomed. 2018, 13, 505–523. [Google Scholar] [CrossRef] [PubMed]
- Molaei, A.; Yousefpour, M. Electrophoretic deposition of chitosan–bioglass®–hydroxyapatite–halloysite nanotube composite coating. Rare Met. 2022, 41, 3850–3857. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Sami, N.; Yasin, D.; Ahmad, N.; Fatma, T. Biomedical applications of environmental friendly poly-hydroxyalkanoates. Int. J. Biol. Macromol. 2021, 183, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Râpă, M.; Stefan, L.M.; Seciu-Grama, A.-M.; Gaspar-Pintiliescu, A.; Matei, E.; Zaharia, C.; Stănescu, P.O.; Predescu, C. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (P(3HB-co-3HV))/Bacterial Cellulose (BC) Biocomposites for Potential Use in Biomedical Applications. Polymers 2022, 14, 5544. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Sivan, A. New perspectives in plastic biodegradation. Curr. Opin. Biotechnol. 2011, 22, 422–426. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Arcentales-Vera, B.; Estrella-Nuñez, J.; Yánez-Vega, H.; Bucio, E. Antimicrobial Activity of Composites-Based on Biopolymers. Macromol 2022, 2, 258–283. [Google Scholar] [CrossRef]
- Vagos, M.R.; Gomes, M.; Moreira, J.M.R.; Soares, O.S.G.P.; Pereira, M.F.R.; Mergulhão, F.J. Carbon nanotube/poly(Dimethylsiloxane) composite materials to reduce bacterial adhesion. Antibiotics 2020, 9, 434. [Google Scholar] [CrossRef]
- Goodwin, D.G.; Marsh, K.M.; Sosa, I.B.; Payne, J.B.; Gorham, J.M.; Bouwer, E.J.; Fairbrother, D.H. Interactions of Microorganisms with Polymer Nanocomposite Surfaces Containing Oxidized Carbon Nanotubes. Environ. Sci. Technol. 2015, 49, 5484–5492. [Google Scholar] [CrossRef]
- Mohammed, M.K.A.; Mohammad, M.R.; Jabir, M.S.; Ahmed, D.S. Functionalization, characterization, and antibacterial activity of single wall and multi wall carbon nanotubes. IOP Conf. Ser. Mater. Sci. Eng. 2020, 757, 012028. [Google Scholar] [CrossRef]
- Talodthaisong, C.; Plaeyao, K.; Mongseetong, C.; Boonta, W.; Srichaiyapol, O.; Patramanon, R.; Kayunkid, N.; Kulchat, S. The Decoration of ZnO Nanoparticles by Gamma Aminobutyric Acid, Curcumin Derivative and Silver Nanoparticles: Synthesis, Characterization and Antibacterial Evaluation. Nanomaterials 2021, 11, 442. [Google Scholar] [CrossRef]
- Yurtdaş Kırımlıoğlu, G.; Menceloğlu, Y.; Erol, K.; Yazan, Y. In vitro/in vivo evaluation of gamma-aminobutyric acid-loaded N, N -dimethylacrylamide-based pegylated polymeric nanoparticles for brain delivery to treat epilepsy. J. Microencapsul. 2016, 33, 625–635. [Google Scholar] [CrossRef]
- Immich, A.P.S.; Pennacchi, P.C.; Naves, A.F.; Felisbino, S.L.; Boemo, R.L.; Maria-Engler, S.S.; Catalani, L.H. Improved tympanic membrane regeneration after myringoplastic surgery using an artificial biograft. Mater. Sci. Eng. C 2017, 73, 48–58. [Google Scholar] [CrossRef] [PubMed]
- ISO/EN10993-5; International Standard ISO 10993-5 Biological Evaluation of Medical Devices: Tests for In Vitro Cytotoxicity. Part 5 Tests Cytotox. Vitr. Methods; ISO: Genève, Switzerland, 2009.
- Gutiérrez-Praena, D.; Pichardo, S.; Sánchez, E.; Grilo, A.; Cameán, A.M.; Jos, A. Influence of carboxylic acid functionalization on the cytotoxic effects induced by single wall carbon nanotubes on human endothelial cells (HUVEC). Toxicol. Vitr. 2011, 25, 1883–1888. [Google Scholar] [CrossRef]
- Fraczek-Szczypta, A.; Menaszek, E.; Syeda, T.B.; Misra, A.; Alavijeh, M.; Adu, J.; Blazewicz, S. Effect of MWCNT surface and chemical modification on in vitro cellular response. J. Nanoparticle Res. 2012, 14, 1181. [Google Scholar] [CrossRef]
- Mohammadi, E.; Zeinali, M.; Mohammadi-Sardoo, M.; Iranpour, M.; Behnam, B.; Mandegary, A. The effects of functionalization of carbon nanotubes on toxicological parameters in mice. Hum. Exp. Toxicol. 2020, 39, 1147–1167. [Google Scholar] [CrossRef]
- Malmir, S.; Barral, L.; Bouza, R.; Esperanza, M.; Seoane, M.; Feijoo-Bandín, S.; Lago, F. Poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/cellulose nanocrystal films: Artificial weathering, humidity absorption, water vapor transmission rate, antimicrobial activity and biocompatibility. Cellulose 2019, 26, 2333–2348. [Google Scholar] [CrossRef]
- Braga, N.F.; Vital, D.A.; Guerrini, L.M.; Lemes, A.P.; Formaggio, D.M.D.; Tada, D.B.; Arantes, T.M.; Cristovan, F.H. PHBV-TiO2 mats prepared by electrospinning technique: Physico-chemical properties and cytocompatibility. Biopolymers 2018, 109, e23120. [Google Scholar] [CrossRef]
- Piedade, A.P.; Pinho, A.C.; Branco, R.; Morais, P.V. Evaluation of antimicrobial activity of ZnO based nanocomposites for the coating of non-critical equipment in medical-care facilities. Appl. Surf. Sci. 2020, 513, 145818. [Google Scholar] [CrossRef]
- Singh, V.K.; Kumari, P.; Som, A.; Rai, S.; Mishra, R.; Singh, R.K. Design, synthesis and antimicrobial activity of novel quinoline derivatives: An in silico and in vitro study. J. Biomol. Struct. Dyn. 2023, 1–21, online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.A.A.; Muddathir, A.M.; Osman, M.A. Antimicrobial activity, phytochemical screening of crude extracts, and essential oils constituents of two Pulicaria spp. growing in Sudan. Sci. Rep. 2020, 10, 17148. [Google Scholar] [CrossRef]
- Anandhi, S.; Leo Edward, M.; Jaisankar, V. Synthesis, characterization and antimicrobial activity of polyindole/ZrO2 nanocomposites. Mater. Today Proc. 2021, 40, S93–S101. [Google Scholar] [CrossRef]
- Rohde, M. The Gram-Positive Bacterial Cell Wall. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Pasquina-Lemonche, L.; Burns, J.; Turner, R.D.; Kumar, S.; Tank, R.; Mullin, N.; Wilson, J.S.; Chakrabarti, B.; Bullough, P.A.; Foster, S.J.; et al. The architecture of the Gram-positive bacterial cell wall. Nature 2020, 582, 294–297. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnology 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.I.; Kim, D.A.; Patel, K.D.; Shin, U.S.; Kim, H.W.; Lee, J.H.; Lee, H.H. Carbon nanotube incorporation in PMMA to prevent microbial adhesion. Sci. Rep. 2019, 9, 4921. [Google Scholar] [CrossRef]
- Dong, L.; Henderson, A.; Field, C. Antimicrobial Activity of Single-Walled Carbon Nanotubes Suspended in Different Surfactants. J. Nanotechnol. 2012, 2012, 928924. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Kourinou, M.; Velidakis, E.; Mountakis, N.; Fischer-Griffiths, P.E.; Grammatikos, S.; Tzounis, L. Additive manufacturing of multifunctional polylactic acid (PLA)—Multiwalled carbon nanotubes (MWCNTs) nanocomposites. Nanocomposites 2021, 7, 184–199. [Google Scholar] [CrossRef]
- Benincasa, M.; Pacor, S.; Wu, W.; Prato, M.; Bianco, A.; Gennaro, R. Antifungal Activity of Amphotericin B Conjugated to Carbon Nanotubes. ACS Nano 2011, 5, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Zare-Zardini, H.; Amiri, A.; Shanbedi, M.; Memarpoor-Yazdi, M.; Asoodeh, A. Studying of antifungal activity of functionalized multiwalled carbon nanotubes by microwave-assisted technique. Surf. Interface Anal. 2013, 45, 751–755. [Google Scholar] [CrossRef]
- David, M.E.; Ion, R.-M.; Grigorescu, R.M.; Iancu, L.; Holban, A.M.; Nicoara, A.I.; Alexandrescu, E.; Somoghi, R.; Ganciarov, M.; Vasilievici, G.; et al. Hybrid Materials Based on Multi-Walled Carbon Nanotubes and Nanoparticles with Antimicrobial Properties. Nanomaterials 2021, 11, 1415. [Google Scholar] [CrossRef] [PubMed]
- McCall, A.D.; Pathirana, R.U.; Prabhakar, A.; Cullen, P.J.; Edgerton, M. Candida albicans biofilm development is governed by cooperative attachment and adhesion maintenance proteins. npj Biofilms Microbiomes 2019, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Reyes-García, M.G.; Hernández-Hernández, F.; García-Tamayo, F. Gamma-aminobutyric acid (GABA) increases in vitro germ-tube formation and phospholipase B1 mRNA expression in Candida albicans. Mycoscience 2012, 53, 36–39. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, X.; Ren, B.; Cheng, L. The regulation of hyphae growth in Candida albicans. Virulence 2020, 11, 337–348. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montanheiro, T.L.d.A.; Schatkoski, V.M.; Camarena, D.E.M.; de Oliveira, T.C.; da Silva, D.M.; Vegian, M.R.d.C.; Catalani, L.H.; Koga-Ito, C.Y.; Thim, G.P. Evaluating the Cytotoxicity of Functionalized MWCNT and Microbial Biofilm Formation on PHBV Composites. C 2024, 10, 33. https://doi.org/10.3390/c10020033
Montanheiro TLdA, Schatkoski VM, Camarena DEM, de Oliveira TC, da Silva DM, Vegian MRdC, Catalani LH, Koga-Ito CY, Thim GP. Evaluating the Cytotoxicity of Functionalized MWCNT and Microbial Biofilm Formation on PHBV Composites. C. 2024; 10(2):33. https://doi.org/10.3390/c10020033
Chicago/Turabian StyleMontanheiro, Thaís Larissa do Amaral, Vanessa Modelski Schatkoski, Denisse Esther Mallaupoma Camarena, Thais Cardoso de Oliveira, Diego Morais da Silva, Mariana Raquel da Cruz Vegian, Luiz Henrique Catalani, Cristiane Yumi Koga-Ito, and Gilmar Patrocínio Thim. 2024. "Evaluating the Cytotoxicity of Functionalized MWCNT and Microbial Biofilm Formation on PHBV Composites" C 10, no. 2: 33. https://doi.org/10.3390/c10020033
APA StyleMontanheiro, T. L. d. A., Schatkoski, V. M., Camarena, D. E. M., de Oliveira, T. C., da Silva, D. M., Vegian, M. R. d. C., Catalani, L. H., Koga-Ito, C. Y., & Thim, G. P. (2024). Evaluating the Cytotoxicity of Functionalized MWCNT and Microbial Biofilm Formation on PHBV Composites. C, 10(2), 33. https://doi.org/10.3390/c10020033









