Preferential Stripping Analysis of Post-Transition Metals (In and Ga) at Bi/Hg Films Electroplated on Graphene-Functionalized Graphite Rods
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus
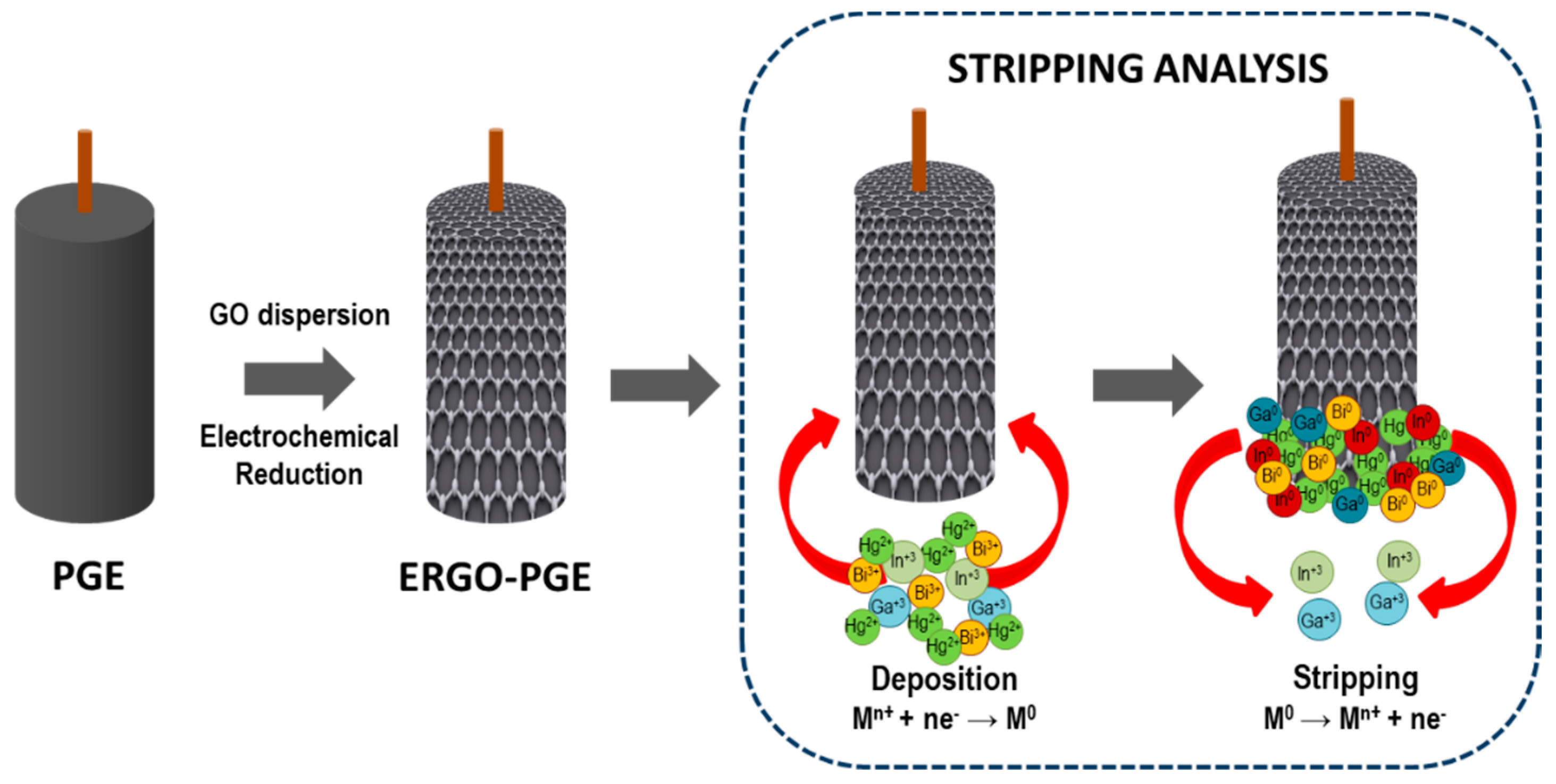
2.3. Electrochemical Reduction of Grapheme Oxide onto Pencil Graphite Electrodes (ERGO-PGE)
2.4. Voltammetric Analysis of Post-Transition Metals (Ga3+ and In3+)
3. Results and Discussion
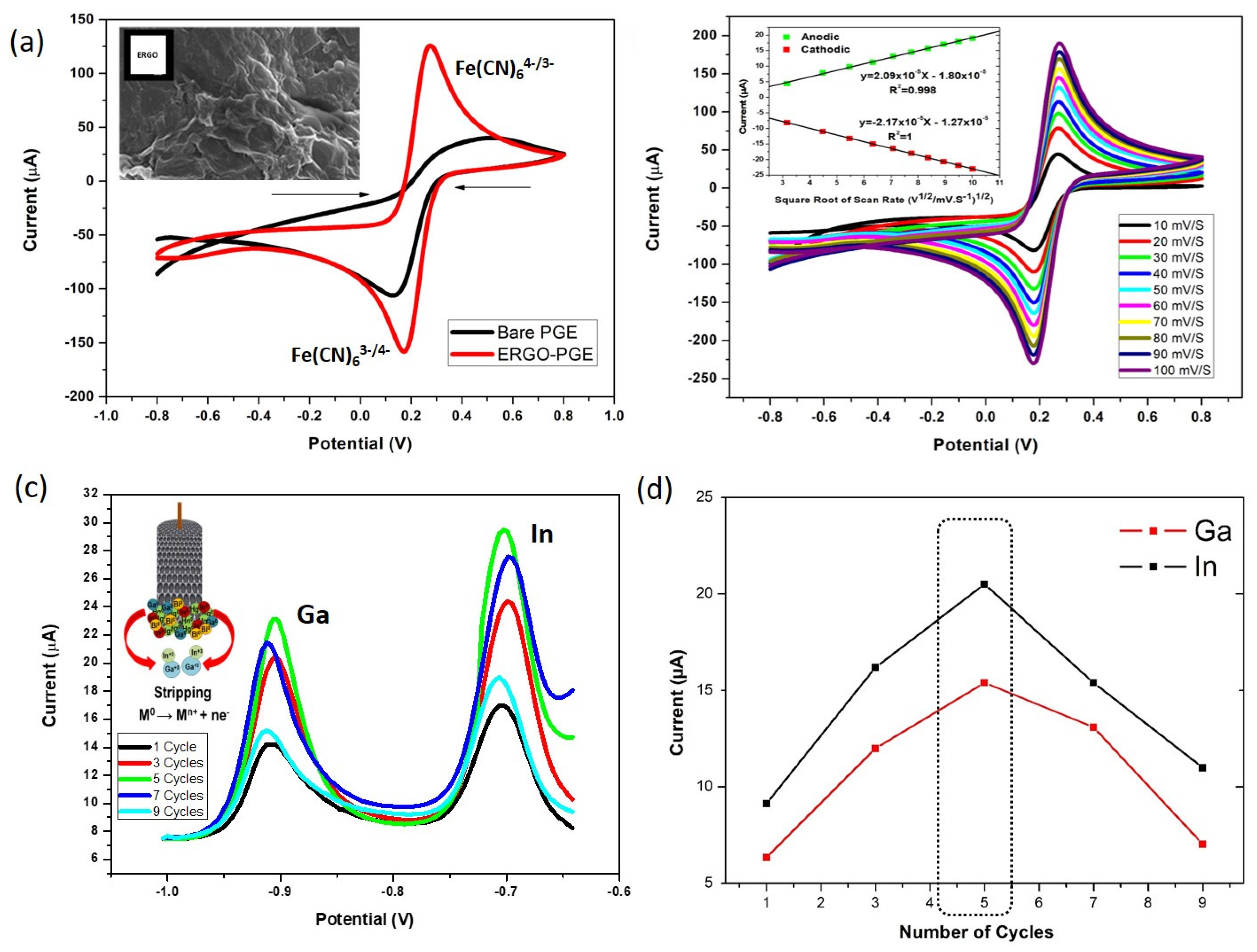
3.1. Electrochemical Characterization and Optimization of ERGO-Bi/Hg-Film PGEs for the Simultaneous Stripping Analysis of Post-Transition Metals
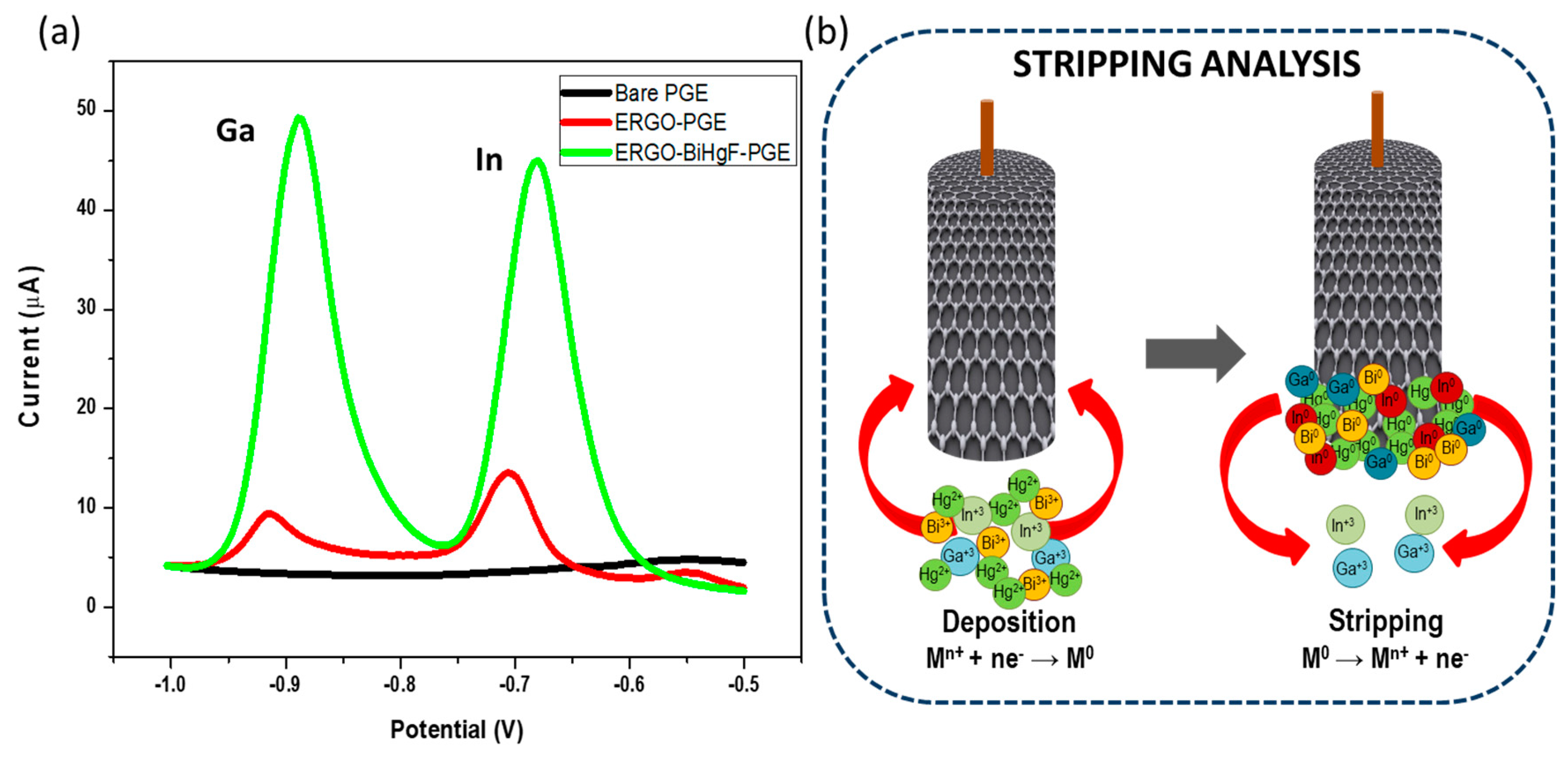
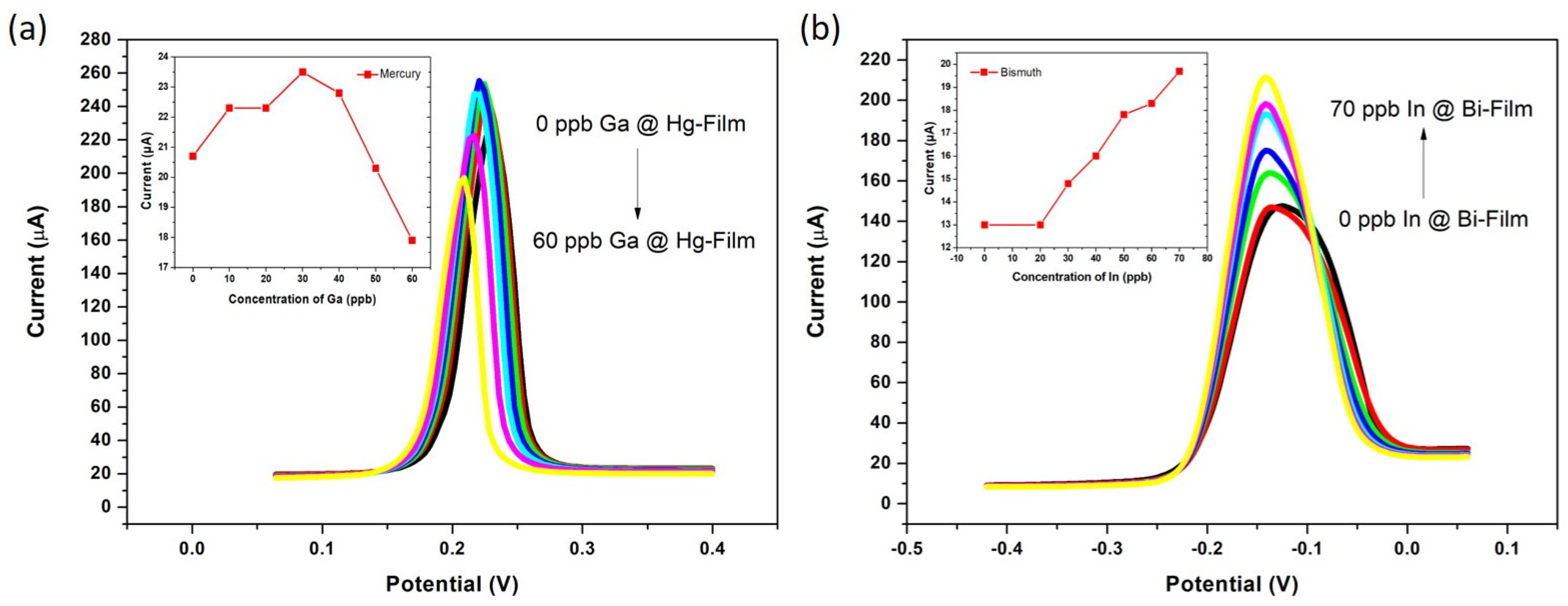
3.2. The Synergetic Effect of Graphene Sheets and Bismuth-Mercury Film on the Electrochemical Response of Ga3+ and In3+
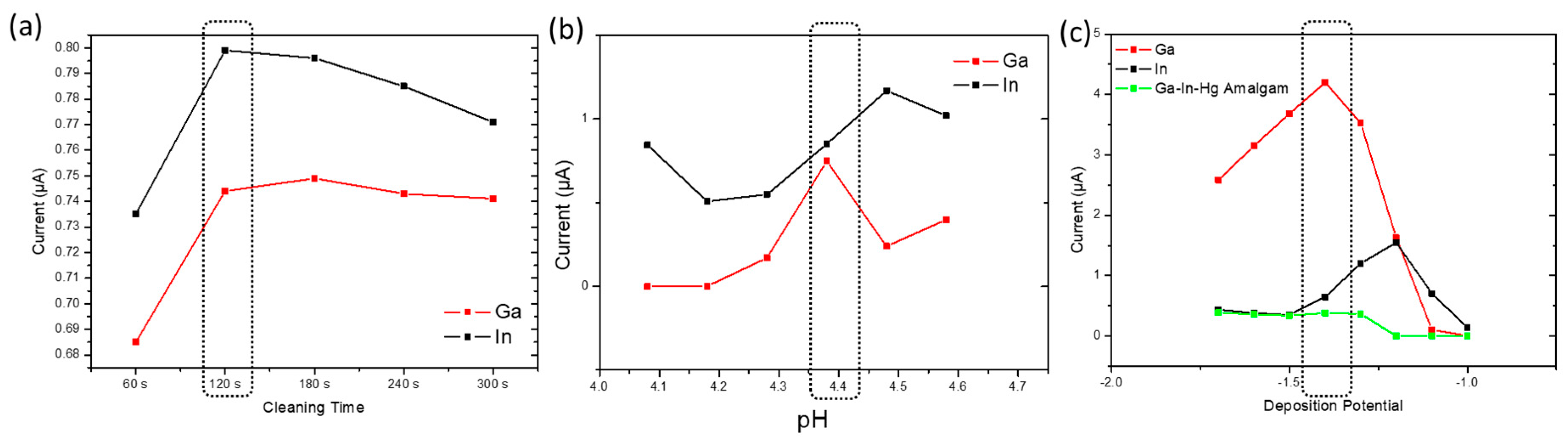
3.3. Experimental Parameter Optimization of ERGO-Bi/Hg-Film PGE for Ga3+ and In3+ Detection
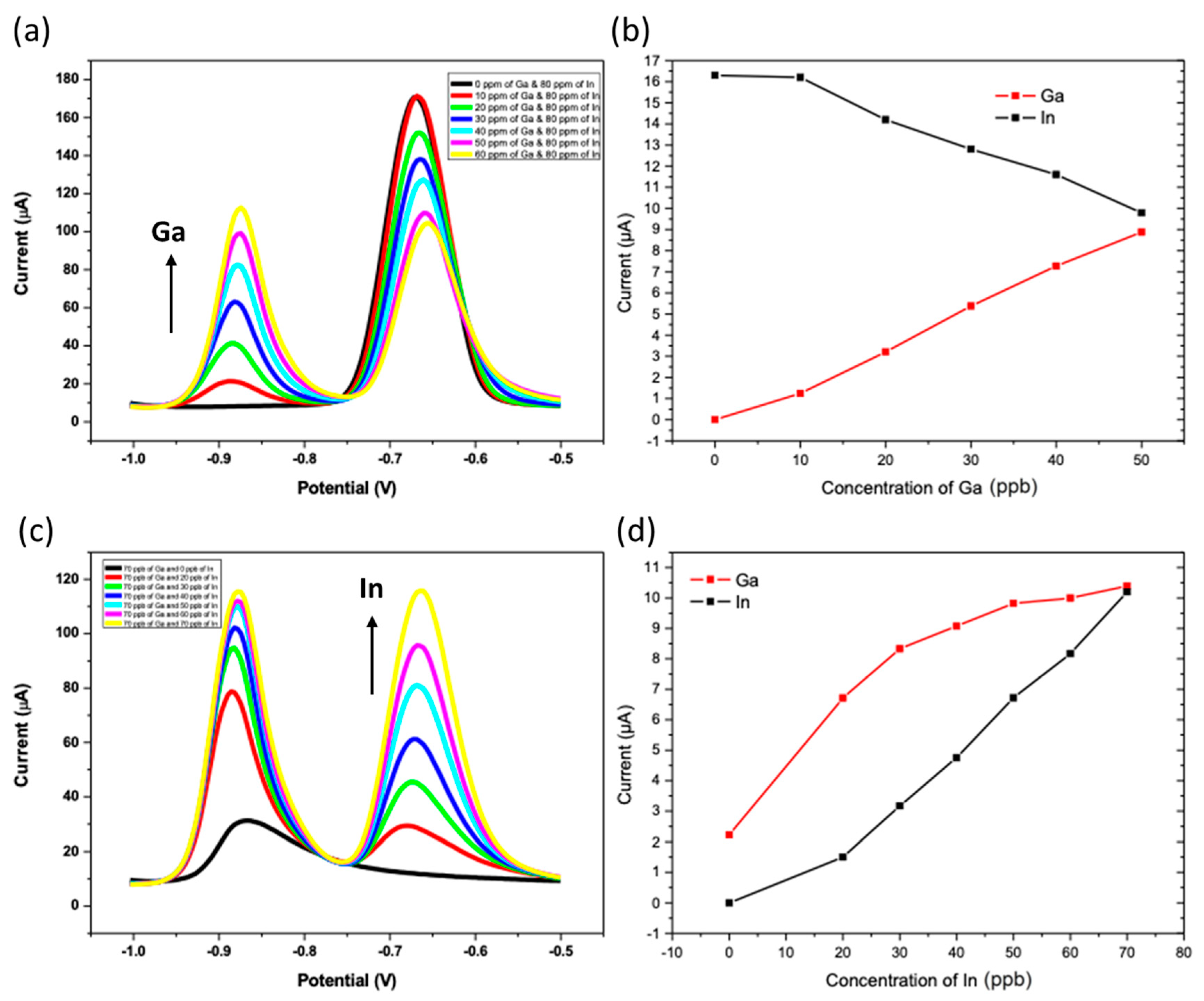
3.4. Intermetallic Interferences
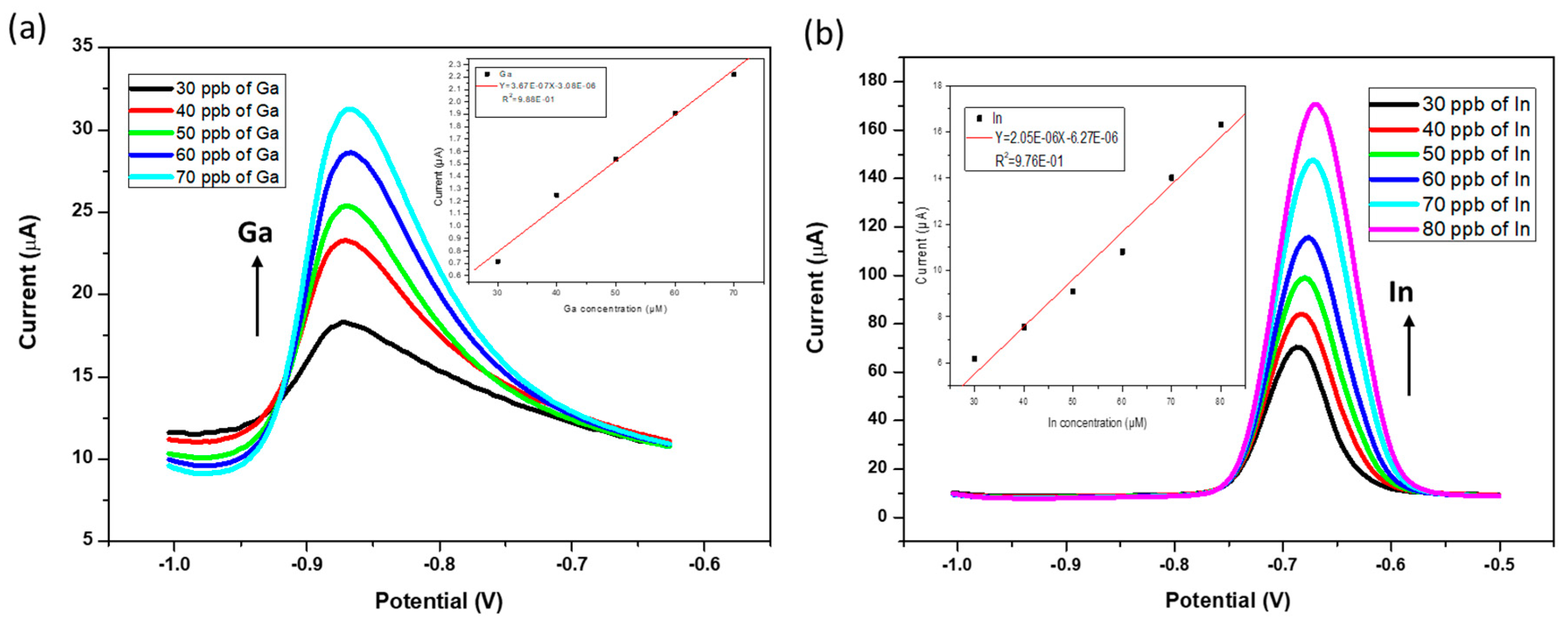
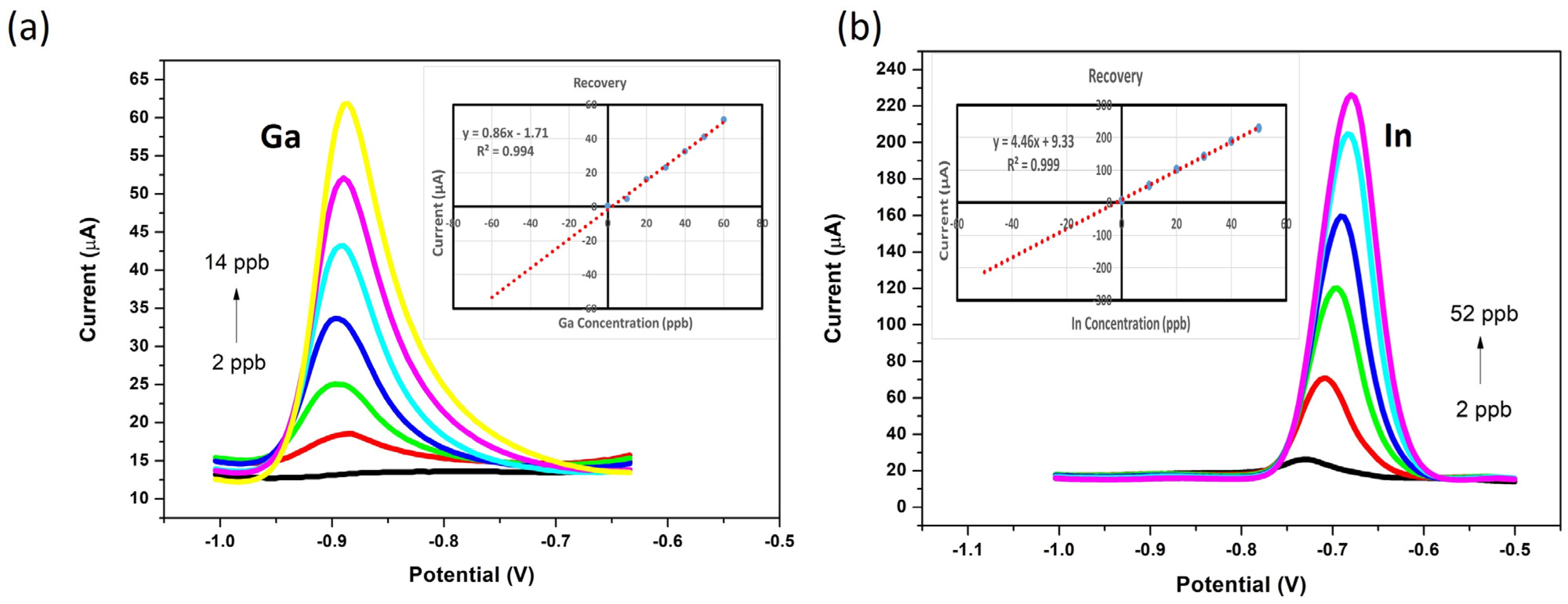
3.5. Analytical Performance of the ERGO-Bi/Hg-Film PGEs for the Individual Determination of Ga3+ and In3+
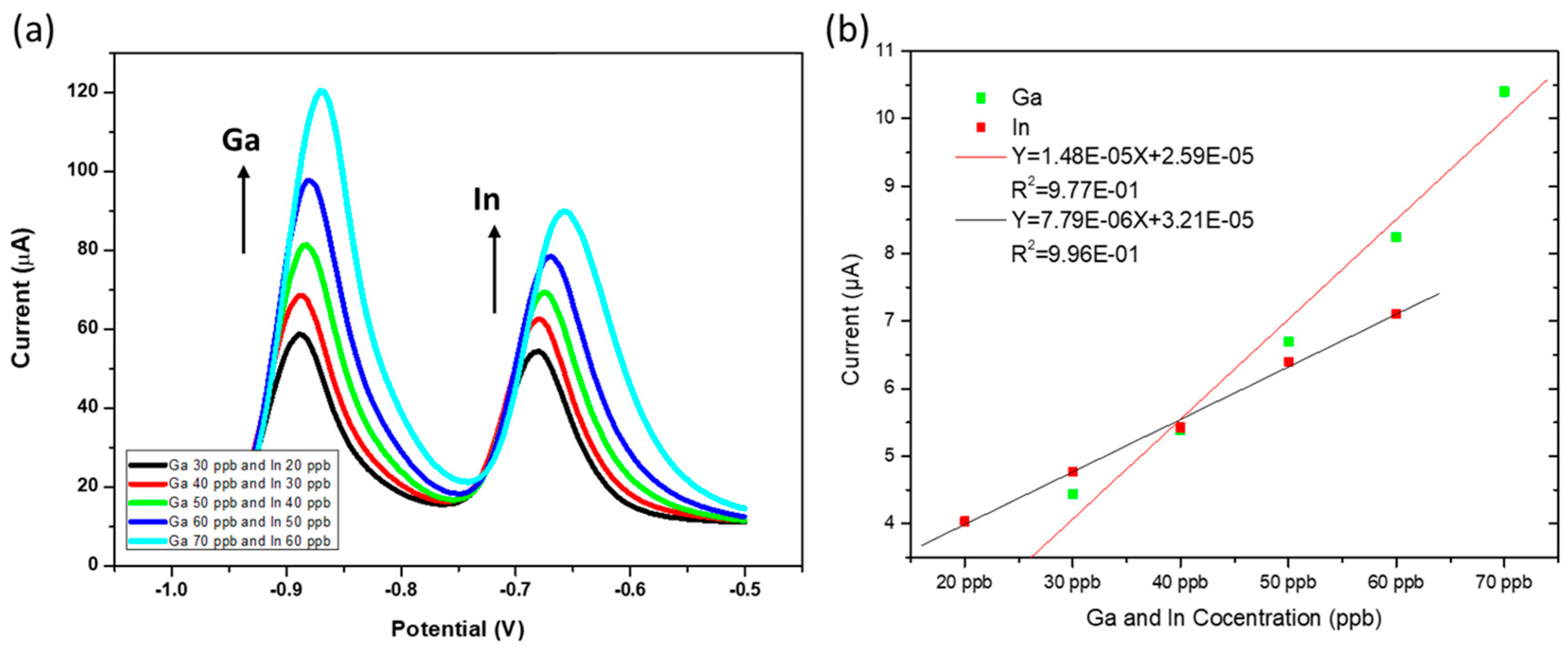
3.6. Simultaneous Analysis of In3+ and Ga3+ at ERGO-Bi/Hg-Film PGEs in Simulated Samples
3.7. Application to Tap Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hassanien, M.M.; Kenawy, I.M.; Mostafa, M.R.; El-Dellay, H. Extraction of gallium, indium and thallium from aquatic media using amino silica gel modified by gallic acid. Microchim. Acta 2011, 172, 137–145. [Google Scholar] [CrossRef]
- Mortada, W.I.; Kenawy, I.M.; Hassanien, M.M. A cloud point extraction procedure for gallium, indium and thallium determination in liquid crystal display and sediment samples. Anal. Methods 2015, 7, 2114–2120. [Google Scholar] [CrossRef]
- White, S.J.O.; Hemond, H.F. The anthrobiogeochemical cycle of indium: A review of the natural and anthropogenic cycling of indium in the environment. Crit. Rev. Environ. Sci. Technol. 2012, 42, 155–186. [Google Scholar] [CrossRef]
- Xiang, C.; Zou, Y.; Xie, J.; Fei, X.; Li, J. Nafion-modified glassy carbon electrode for trace determination of indium. Anal. Lett. 2005, 38, 2045–2055. [Google Scholar] [CrossRef]
- Piech, R. Novel Sensitive Voltammetric Detection of Trace Gallium(III) with Presence of Catechol Using Mercury Film Silver Based Electrode. Electroanalysis 2009, 21, 1842–1847. [Google Scholar] [CrossRef]
- Moskalyk, R.R. Gallium: The backbone of the electronics industry. Miner. Eng. 2003, 16, 921–929. [Google Scholar] [CrossRef]
- Kamat, J.V.; Guin, S.K.; Pillai, J.S.; Aggarwal, S.K. Scope of detection and determination of gallium(III) in industrial ground water by square wave anodic stripping voltammetry on bismuth film electrode. Talanta 2011, 86, 256–265. [Google Scholar] [CrossRef]
- González, M.J.G.; Renedo, O.D.; Lomillo, M.A.A.; Martínez, M.J.A. Determination of gallium by adsorptive stripping voltammetry. Talanta 2004, 62, 457–462. [Google Scholar] [CrossRef]
- Chitambar, C.R. Medical applications and toxicities of gallium compounds. Int. J. Environ. Res. Public Health 2010, 7, 2337–2361. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Wlazlowska, E. An Activated Bismuth Layer Formed In Situ on a Solid Bismuth Microelectrode for Electrochemical Sensitive Determination of Ga(III). Membranes 2022, 12, 1267. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Wasąg, J. Determination of trace amounts of Ga(III) by adsorptive stripping voltammetry with in situ plated bismuth film electrode. Talanta 2015, 144, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Grabarczyk, M.; Wasąg, J. Adsorptive Cathodic Stripping Voltammetric Method for Determination of Gallium Using an In Situ Plated Lead Film Electrode. Electroanalysis 2015, 27, 2596–2600. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Adamczyk, M.; Wardak, C. Simultaneous AdSV determination of Ga and In on Hg(Ag)FE electrode by AdSV in presence of cupferron. Ionics 2020, 26, 1019–1027. [Google Scholar] [CrossRef]
- Chung, Y.; Lee, C.-W. Electrochemistry of Gallium. J. Electrochem. Sci. Technol. 2013, 4, 1–18. [Google Scholar] [CrossRef]
- Paolicchi, I.; Renedo, O.D.; Alonso Lomillo, M.A.; Arcos Martínez, M.J. Application of an optimization procedure in adsorptive stripping voltammetry for the determination of trace contaminant metals in aqueous medium. Anal. Chim. Acta 2004, 511, 223–229. [Google Scholar] [CrossRef]
- Gomdje, V.H.; Rosie, T.; Ngono, L.; Najih, R.; Chtaini, A. Electroanalytical Determination of Lead with Carbon Paste Modified Steel Electrode. Acta Tech. Corviniensis-Bull. Eng. 2013, 6, 139. [Google Scholar]
- Pokpas, K.; Zbeda, S.; Jahed, N.; Mohamed, N.; Baker, P.G.; Iwuoha, E.I. Electrochemically reduced graphene oxide pencil-graphite in situ plated bismuth-film electrode for the determination of trace metals by anodic stripping voltammetry. Int. J. Electrochem. Sci. 2014, 9, 736–759. [Google Scholar] [CrossRef]
- Wang, J.; Lu, J.; Kirgöz, Ü.A.; Hocevar, S.B.; Ogorevc, B. Insights into the anodic stripping voltammetric behavior of bismuth film electrodes. Anal. Chim. Acta 2001, 434, 29–34. [Google Scholar] [CrossRef]
- Franke, J.P.; de Zeeuw, R.A. Differential pulse anodic stripping voltammetry as a rapid screening technique for heavy metal intoxications. Arch. Toxicol. 1976, 37, 47–55. [Google Scholar] [CrossRef]
- Pokpas, K.; Jahed, N.; Tovide, O.; Baker, P.G.; Iwuoha, E.I. Nafion-graphene nanocomposite in situ plated bismuth-film electrodes on pencil graphite substrates for the determination of trace heavy metals by anodic stripping voltammetry. Int. J. Electrochem. Sci. 2014, 9, 5092–5115. [Google Scholar] [CrossRef]
- Ghaffari, N.; Pokpas, K.; Iwuoha, E.; Jahed, N. Sensitive Electrochemical Determination of Bisphenol a Using a Disposable, Electrodeposited Antimony-Graphene Nanocomposite Pencil Graphite Electrode (PGE) and Differential Pulse Voltammetry (DPV). Anal. Lett. 2023, 57, 1008–1025. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Feng, Y.; Yang, L.; Du, Z. Electrochemical behavior of graphene doped carbon paste electrode and its application for sensitive determination of ascorbic acid. Sens. Actuators B Chem. 2011, 157, 110–114. [Google Scholar] [CrossRef]
- Dervin, S.; Ganguly, P.; Dahiya, R.S. Disposable Electrochemical Sensor Using Graphene Oxide-Chitosan Modified Carbon-Based Electrodes for the Detection of Tyrosine. IEEE Sens. J. 2021, 21, 26226–26233. [Google Scholar] [CrossRef]
- Annu; Sharma, S.; Jain, R.; Raja, A.N. Review—Pencil Graphite Electrode: An Emerging Sensing Material. J. Electrochem. Soc. 2020, 167, 037501. [Google Scholar] [CrossRef]
- Özcan, A. Synergistic Effect of Lithium Perchlorate and Sodium Hydroxide in the Preparation of Electrochemically Treated Pencil Graphite Electrodes for Selective and Sensitive Bisphenol A Detection in Water Samples. Electroanalysis 2014, 26, 1631–1639. [Google Scholar] [CrossRef]
- Demetriades, D.; Economou, A.; Voulgaropoulos, A. A study of pencil-lead bismuth-film electrodes for the determination of trace metals by anodic stripping voltammetry. Anal. Chim. Acta 2004, 519, 167–172. [Google Scholar] [CrossRef]
- David, I.G.; Buleandra, M.; Popa, D.E.; Cheregi, M.C.; David, V.; Iorgulescu, E.E.; Tartareanu, G.O. Recent Developments in Voltammetric Analysis of Pharmaceuticals Using Disposable Pencil Graphite Electrodes. Processes 2022, 10, 472. [Google Scholar] [CrossRef]
- David, I.G.; Litescu, S.C.; Moraru, R.; Albu, C.; Buleandra, M.; Popa, D.E.; Riga, S.; Ciobanu, A.M.; Noor, H. Electroanalysis of Naringin at Electroactivated Pencil Graphite Electrode for the Assessment of Polyphenolics with Intermediate Antioxidant Power. Antioxidants 2022, 11, 2306. [Google Scholar] [CrossRef]
- Ishtiaq, S.; Sohail, M.; Rasul, S.; Zia, A.W.; Siller, L.; Chotana, G.A.; Sharif, M.; Nafady, A. Selenium Nanoneedles Deposited on a Pencil Graphite Electrode for Hydrazine Sensing. ACS Appl. Nano Mater. 2022, 5, 14336–14346. [Google Scholar] [CrossRef]
- Buleandră, M.; Popa, D.E.; Popa, A.; Codreanu, N.A.M.; David, I.G. Multi-Analyte Sensor Based on Pencil Graphite Electrode for Riboflavin and Pyridoxine Determination. J. Electrochem. Soc. 2022, 169, 017517. [Google Scholar] [CrossRef]
- Kumar Naik, T.S.S.; Kesavan, A.V.; Swamy, B.E.K.; Singh, S.; Anil, A.G.; Madhavi, V.; Ramamurthy, P.C. Low cost, trouble-free disposable pencil graphite electrode sensor for the simultaneous detection of hydroquinone and catechol. Mater. Chem. Phys. 2022, 278, 125663. [Google Scholar] [CrossRef]
- Zbeda, S.; Pokpas, K.; Titinchi, S.; Jahed, N.; Baker, P.G.; Iwuoha, E.I. Few-layer binder free graphene modified mercury film electrode for trace metal analysis by square wave anodic stripping voltammetry. Int. J. Electrochem. Sci. 2013, 8, 11125–11141. [Google Scholar] [CrossRef]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; Van Der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable atomic membranes from graphene sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Noveslov, K.S.; Geim, A.K.K.; Morozov, S.V.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.V.; Grigorieva, I.V.V.; Firsov, A.A.A.; Novoselov, K.S.; Geim, A.K.K.; et al. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Neto, A.C.; Guinea, F.; Peres, N.M. Drawing conclusions from graphene. Phys. World 2006, 19, 33–37. [Google Scholar] [CrossRef]
- Park, H.J.; Meyer, J.; Roth, S.; Skákalová, V. Growth and properties of few-layer graphene prepared by chemical vapor deposition. Carbon 2010, 48, 1088–1094. [Google Scholar] [CrossRef]
- Reina, A.; Thiele, S.; Jia, X.; Bhaviripudi, S.; Dresselhaus, M.S.; Schaefer, J.A.; Kong, J. Growth of large-area single- and Bi-layer graphene by controlled carbon precipitation on polycrystalline Ni surfaces. Nano Res. 2009, 2, 509–516. [Google Scholar] [CrossRef]
- Voutilainen, M.; Seppala, E.T.; Pasanen, P.; Oksanen, M. Graphene and carbon nanotube applications in mobile devices. IEEE Trans. Electron Devices 2012, 59, 2876–2887. [Google Scholar] [CrossRef]
- Patel, M.; Bisht, N.; Prabhakar, P.; Sen, R.K.; Kumar, P.; Dwivedi, N.; Ashiq, M.; Mondal, D.P.; Srivastava, A.K.; Dhand, C. Ternary nanocomposite-based smart sensor: Reduced graphene oxide/polydopamine/alanine nanocomposite for simultaneous electrochemical detection of Cd2+, Pb2+, Fe2+, and Cu2+ ions. Environ. Res. 2023, 221, 115317. [Google Scholar] [CrossRef]
- Rahman, H.A.; Rafi, M.; Putra, B.R.; Wahyuni, W.T. Electrochemical Sensors Based on a Composite of Electrochemically Reduced Graphene Oxide and PEDOT:PSS for Hydrazine Detection. ACS Omega 2023, 8, 3258–3269. [Google Scholar] [CrossRef] [PubMed]
- Pichún, B.; Núñez, C.; Arancibia, V.; Martí, A.A.; Aguirre, M.J.; Pizarro, J.; Segura, R.; Flores, E. Enhanced voltammetric sensing platform based on gold nanorods and electrochemically reduced graphene oxide for As(III) determination in seafood samples. J. Appl. Electrochem. 2024, 54, 1595–1606. [Google Scholar] [CrossRef]
- Economou, A.; Fielden, P.R. Mercury film electrodes: Developments, trends and potentialities for electroanalysis. Analyst 2003, 128, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Calvo, A.; Blanco-López, M.C.; Costa-García, A. Paper-based working electrodes coated with mercury or bismuth films for heavy metals determination. Biosensors 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Tefera, M.; Tessema, M.; Admassie, S.; Guadie, A. Electrochemical determination of endosulfan in vegetable samples using mercury film modified glassy carbon electrode. Sens. Bio-Sens. Res. 2021, 33, 100431. [Google Scholar] [CrossRef]
- Piech, R.; Bas, B. Sensitive voltammetric determination of gallium in aluminium materials using renewable mercury film silver based electrode. Int. J. Environ. Anal. Chem. 2011, 91, 410–420. [Google Scholar] [CrossRef]
- Kudr, J.; Nguyen, H.V.; Gumulec, J.; Nejdl, L.; Blazkova, I.; Ruttkay-Nedecky, B.; Hynek, D.; Kynicky, J.; Adam, V.; Kizek, R. Simultaneous automatic electrochemical detection of zinc, cadmium, copper and lead ions in environmental samples using a thin-film mercury electrode and an artificial neural network. Sensors 2015, 15, 592–610. [Google Scholar] [CrossRef]
- Yıldız, C.; Eskiköy Bayraktepe, D.; Yazan, Z. Highly sensitive direct simultaneous determination of zinc(II), cadmium(II), lead(II), and copper(II) based on in-situ-bismuth and mercury thin-film plated screen-printed carbon electrode. Monatshefte Fuer Chem./Chem. Mon. 2021, 152, 1527–1537. [Google Scholar] [CrossRef]
- Sanga, N.A.; Jahed, N.; Leve, Z.; Iwuoha, E.I.; Pokpas, K. Simultaneous Adsorptive Stripping Voltammetric Analysis of Heavy Metals at Graphenated Cupferron Pencil Rods. J. Electrochem. Soc. 2022, 169, 017502. [Google Scholar] [CrossRef]
- Pokpas, K.; Jahed, N.; Iwuoha, E. Tuneable, Pre-stored Paper-Based Electrochemical Cells (μPECs): An Adsorptive Stripping Voltammetric Approach to Metal Analysis. Electrocatalysis 2019, 10, 352–364. [Google Scholar] [CrossRef]
- Pokpas, K.; Jahed, N.; Bezuidenhout, P.; Smith, S.; Land, K.; Iwuoha, E. Nickel contamination analysis at cost-effective silver printed paper-based electrodes based on carbon black dimethyl-glyoxime ink as electrode modifier. J. Electrochem. Sci. Eng. 2022, 12, 153–164. [Google Scholar] [CrossRef]
- Finšgar, M.; Kovačec, L. Copper-bismuth-film in situ electrodes for heavy metal detection. Microchem. J. 2020, 154, 104635. [Google Scholar] [CrossRef]
- Albalawi, I.; Hogan, A.; Alatawi, H.; Moore, E. A sensitive electrochemical analysis for cadmium and lead based on Nafion-Bismuth film in a water sample. Sens. Bio-Sens. Res. 2021, 34, 100454. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Zhao, S.; Cui, G. Simultaneous determination of trace Pb(II), Cd(II), and Zn(II) using an integrated three-electrode modified with bismuth film. Microchem. J. 2021, 168, 106390. [Google Scholar] [CrossRef]
- Ai, Y.; Yan, L.; Zhang, S.; Ye, X.; Xuan, Y.; He, S.; Wang, X.; Sun, W. Ultra-sensitive simultaneous electrochemical detection of Zn(II), Cd(II) and Pb(II) based on the bismuth and graphdiyne film modified electrode. Microchem. J. 2023, 184, 108186. [Google Scholar] [CrossRef]
- Rojas-Romo, C.; Aliaga, M.E.; Arancibia, V.; Gomez, M. Determination of Pb(II) and Cd(II) via anodic stripping voltammetry using an in-situ bismuth film electrode. Increasing the sensitivity of the method by the presence of Alizarin Red S. Microchem. J. 2020, 159, 105373. [Google Scholar] [CrossRef]
- Yaman, B.; Zaman, B.T.; Bakırdere, S.; Dilgin, Y. Sensitive, Accurate and Selective Determination of Cd(II) Using Anodic Stripping Voltammetry with in-situ Hg-Bi Film Modified Pencil Graphite Electrode After Magnetic Dispersive Solid Phase Microextraction. Electroanalysis 2021, 33, 2161–2168. [Google Scholar] [CrossRef]
- Ouyang, R.; Zhu, Z.; Tatum, C.E.; Chambers, J.Q.; Xue, Z.L. Simultaneous stripping detection of Zn(II), Cd(II) and Pb(II) using a bimetallic Hg–Bi/single-walled carbon nanotubes composite electrode. J. Electroanal. Chem. 2011, 656, 78–84. [Google Scholar] [CrossRef]
- Sharifian, P.; Aliakbar, A. Determination of Se(IV) by adsorptive cathodic stripping voltammetry at a Bi/Hg film electrode. Anal. Methods 2015, 7, 2121–2128. [Google Scholar] [CrossRef]
- William, S.; Hummers, J.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Silva, R.M.; Sperandio, G.H.; da Silva, A.D.; Okumura, L.L.; da Silva, R.C.; Moreira, R.P.L.; Silva, T.A. Electrochemically reduced graphene oxide films from Zn-C battery waste for the electrochemical determination of paracetamol and hydroquinone. Microchim. Acta 2023, 190, 273. [Google Scholar] [CrossRef] [PubMed]
- Leve, Z.D.; Jahed, N.; Sanga, N.A.; Iwuoha, E.I.; Pokpas, K. Determination of paracetamol at the electrochemically reduced graphene oxide-antimony nanocomposite modified pencil graphite electrode using adsorptive stripping differential pulse voltammetry. Sensors 2022, 22, 5784. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, B.; Gong, D.; Fan, J.; Li, Z.; Wang, C.; Wang, L.; Yu, C.; Wang, C.; Zeng, G. A cation–π interaction confined graphene oxide membrane for separation of light paraffins and olefins. Chem. Commun. 2023, 59, 5257–5260. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhu, H. Cation–π Interactions in Graphene-Containing Systems for Water Treatment and Beyond. Adv. Mater. 2020, 32, 1905756. [Google Scholar] [CrossRef]
- Medvecký, L.; Briančin, J. Possibilities of simultaneous determination of indium and gallium in binary InGa alloys by anodic stripping voltammetry in acetate buffer. Chem. Pap. 2004, 58, 93–100. [Google Scholar]
- Qu, L.; Jin, W. Adsorption voltammetry of the gallium morin system. Anal. Chim. Acta 1993, 274, 65–70. [Google Scholar] [CrossRef]
- Udisti, R.; Piccardi, G. Determination of gallium traces by differential pulse anodic stripping voltammetry. Anal. Bioanal. Chem. 1988, 331, 35–38. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhao, Q.L.; Huang, M.H. Cathodic Adsorptive Voltammetry of the Gallium-Alizarin Red S Complex at a Carbon Paste Electrode. Electroanalysis 2005, 17, 343–347. [Google Scholar] [CrossRef]
- East, G.; Cofre, P. Determination of gallium by square-wave voltammetry anodic stripping, based on the electrocatalytic action of 2,2′-bipyridine in dimethylsulphoxide: Comparison with an aqueous NaSCN/NaClO4 electrolyte. Talanta 1993, 40, 1273–1281. [Google Scholar] [CrossRef]
- Farias, P.A.M.; Martin, C.M.L.; Ohara, A.K.; Gold, J.S. Cathodic adsorptive stripping voltammetry of indium complexed with morin at a static mercury drop electrode. Anal. Chim. Acta 1994, 293, 29–34. [Google Scholar] [CrossRef]
- Benvidi, A.; Ardakani, M.M. Subnanomolar Determination of Indium by Adsorptive Stripping Differential Pulse Voltammetry Using Factorial Design for Optimization. Anal. Lett. 2009, 42, 2430–2443. [Google Scholar] [CrossRef]
- Nosal-Wiercińska, A.; Dalmata, G. Application of the catalytic properties of N-methylthiourea to the determination of In(III) at low levels by square wave voltammetry. Monatshefte Fuer Chem./Chem. Mon. 2009, 140, 1421–1424. [Google Scholar] [CrossRef]
- Bobrowski, A.; Putek, M.; Zarebski, J. Antimony Film Electrode Prepared In Situ in Hydrogen Potassium Tartrate in Anodic Stripping Voltammetric Trace Detection of Cd(II), Pb(II), Zn(II), Tl(I), In(III) and Cu(II). Electroanalysis 2012, 24, 1071–1078. [Google Scholar] [CrossRef]
- Florence, T.M.; Batley, G.E.; Farrar, Y.J. The determination of indium by anodic stripping voltammetry: Application to natural waters. J. Electroanal. Chem. Interfacial Electrochem. 1974, 56, 301–309. [Google Scholar] [CrossRef]
- Taher, M.A. Differential pulse polarography determination of indium after column preconcentration with [1-(2-pyridylazo)-2-naphthol]-naphthalene adsorbent or its complex on microcrystalline naphthalene. Talanta 2000, 52, 301–309. [Google Scholar] [CrossRef]
- Zhang, J.; Shan, Y.; Ma, J.; Xie, L.; Du, X. Simultaneous determination of indium and thallium ions by anodic stripping voltammetry using antimony film electrode. Sens. Lett. 2009, 7, 605–608. [Google Scholar] [CrossRef]

| Target Analyte | Electrode | Linear Range (mol. L−1) | Detection Limit (mol. L−1) | Reference |
|---|---|---|---|---|
| Ga | Hg(Ag)FE | 2 × 10−9–1 × 10−7 | 1.0 × 10−10 | [5] |
| Ga | BiFe | 3 × 10−10–3 × 10−7 | 1 × 10−10 | [7] |
| Ga | HDME | 1.4 × 10−8–2.7 × 10−7 | 5 × 10−8 | [8] |
| Ga | BiSME | 2 × 10−8 – 2 × 10−6 | 7 × 10−9 | [10] |
| Ga | PbFE | 1 × 10−8–2 × 10−7 | 3.8 × 10−9 | [11] |
| Ga | Hg(Ag)FE | 1.25 × 10−9–9 × 10−8 | 1.6 × 10−9 | [13] |
| Ga | Ag-HgFE | 5 × 10−9–8×10−8 | 1.4 × 10−9 | [46] |
| Ga | HMDE | 1×10−9–1 × 10−7 | 4 × 10−10 | [66] |
| Ga | HDME | 1 × 10−8–1.7 × 10−6 | 5.7 × 10−11 | [67] |
| Ga | CPE | 2.9 × 10−10–8.6 × 10−8 | 1.4 × 10−10 | [68] |
| Ga | HMDE | 1 × 10−4–1 × 10−5 | 2 × 10−8 | [69] |
| Ga | ERGO-Bi/HgF–PGE | 0.43 × 10−6–1.14 × 10−6 | 2.53 × 10−9 | This study |
| Target Analyte | Electrode | Linear Range (mol. L−1) | Detection Limit (mol. L−1) | Reference |
|---|---|---|---|---|
| In | Nafion-GCE | 1.0 × 10−10–1.0 × 10−9 | 7.5 × 10−10 | [4] |
| In | Hg(Ag)FE | 1.25 × 10−9–9 × 10−8 | 1.4 × 10−9 | [13] |
| In | Static Hg drop | 34–340 × 10−9 | 4.3 × 10−11 | [70] |
| In | HMDE | 3.4–87 × 10−10 | 1.1 × 10−12 | [71] |
| In | HDME | 3.9 × 10−7–5 × 10−4 | 1 × 10−7 | [72] |
| In | SbFe | 8.4–84 × 10−10 | 1.2 × 10−11 | [73] |
| In | TMFE | - | 1 × 10−14 | [74] |
| In | HMDE | 0 –3.2 × 10−7 | 1.7 × 10−9 | [75] |
| In | Metal thin-film | 4.3–21.7 ×10−6 | 0.012 | [65] |
| In | SbFe | 1.7–17 × 10−7 | 6.9 × 10−11 | [76] |
| In | ERGO-Bi/HgF–PGE | 0.17 × 10−6–0.60 × 10−6 | 7.27 × 10−9 | This study |
| Target Analyte | Repetitive Cycles | Original (ppb) | Added (ppb) | Found (ppb) | RSD (%) | Recovery (%) |
|---|---|---|---|---|---|---|
| Gallium | First | ND * | 2 | 2.07 | 2.43 | 103.5 |
| Second | ND * | 2 | 1.95 | 1.79 | 97.5 | |
| Third | ND * | 2 | 2.1 | 3.45 | 105 | |
| Indium | First | ND * | 2 | 1.9 | 3.62 | 95 |
| Second | ND * | 2 | 2.05 | 1.74 | 102.5 | |
| Third | ND * | 2 | 1.95 | 1.79 | 97.5 |
| Target Analyte | Repetitive Cycles | Original (ppb) | Added (ppb) | Found (ppb) | RSD (%) | Recovery (%) |
|---|---|---|---|---|---|---|
| Indium | First | ND * | 2 | 2.08 | 2.77 | 104 |
| Second | ND * | 2 | 2.11 | 3.78 | 105.5 | |
| Third | ND * | 2 | 1.9 | 3.62 | 95 | |
| Gallium | First | ND * | 10 | 10.5 | 3.45 | 105 |
| Second | ND * | 10 | 9.6 | 2.88 | 96 | |
| Third | ND * | 10 | 2.1 | 1.40 | 102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaffari, N.; Jahed, N.; Abader, Z.; Baker, P.G.L.; Pokpas, K. Preferential Stripping Analysis of Post-Transition Metals (In and Ga) at Bi/Hg Films Electroplated on Graphene-Functionalized Graphite Rods. C 2024, 10, 95. https://doi.org/10.3390/c10040095
Ghaffari N, Jahed N, Abader Z, Baker PGL, Pokpas K. Preferential Stripping Analysis of Post-Transition Metals (In and Ga) at Bi/Hg Films Electroplated on Graphene-Functionalized Graphite Rods. C. 2024; 10(4):95. https://doi.org/10.3390/c10040095
Chicago/Turabian StyleGhaffari, Nastaran, Nazeem Jahed, Zareenah Abader, Priscilla G. L. Baker, and Keagan Pokpas. 2024. "Preferential Stripping Analysis of Post-Transition Metals (In and Ga) at Bi/Hg Films Electroplated on Graphene-Functionalized Graphite Rods" C 10, no. 4: 95. https://doi.org/10.3390/c10040095
APA StyleGhaffari, N., Jahed, N., Abader, Z., Baker, P. G. L., & Pokpas, K. (2024). Preferential Stripping Analysis of Post-Transition Metals (In and Ga) at Bi/Hg Films Electroplated on Graphene-Functionalized Graphite Rods. C, 10(4), 95. https://doi.org/10.3390/c10040095









