Ferulic Acid and Clinoptilolite Affect In Vitro Rumen Fermentation Characteristics and Bacterial Abundance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Ruminal Inoculum and Experimental Diet Preparation
2.3. In Vitro Incubation, Sampling, and Chemical Analysis
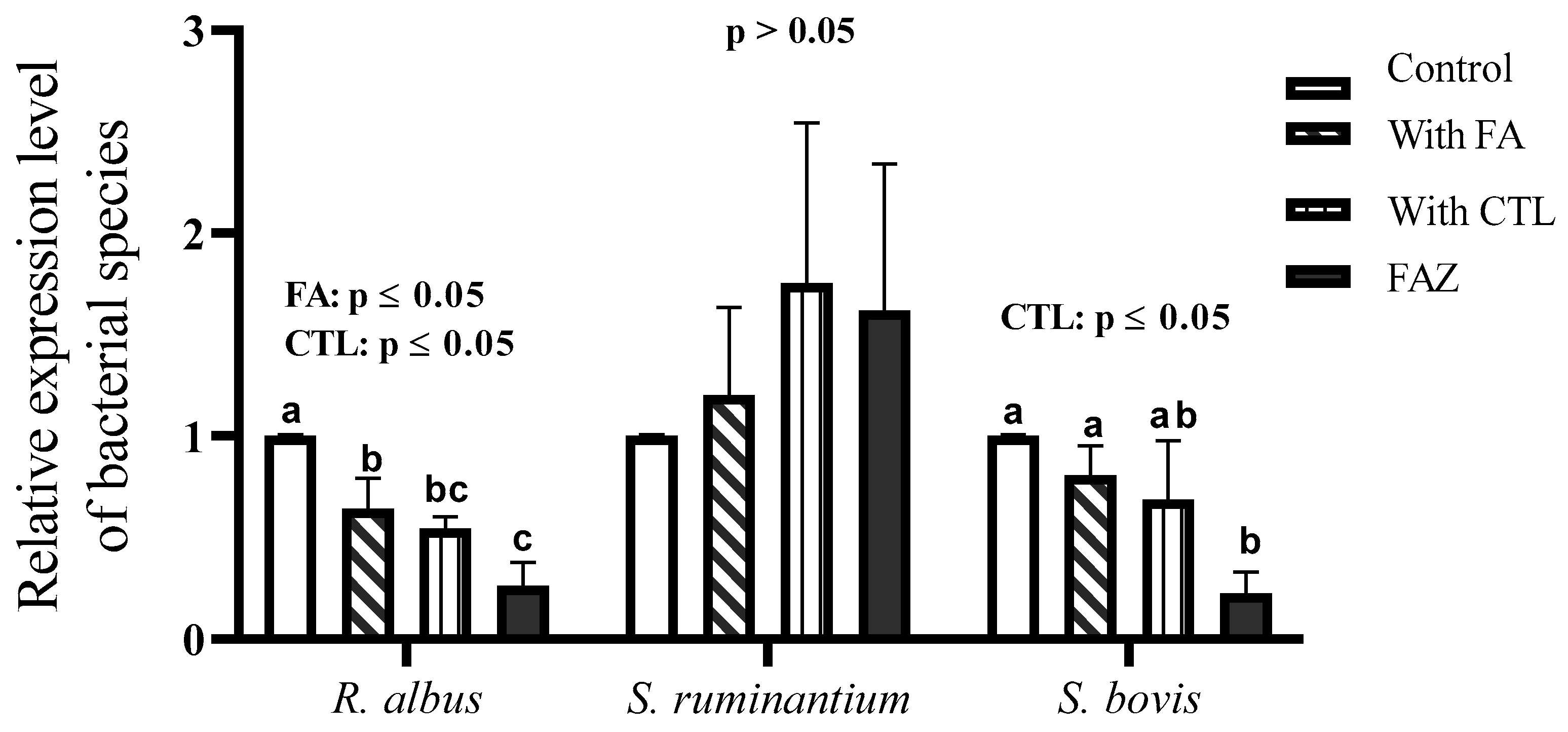
2.4. DNA Extraction and Relative Abundance of Bacterial Species by qPCR Analysis
| Target Name | Primer Sequences (5′–3′) a | Annealing Temperature (°C) | Amplicon Size (bp) | Source of Primer |
|---|---|---|---|---|
| Bacteria Universal (GOR) b | F: ACACTGGAACTGAGACACGG | 62 | 222 | This study |
| R: ATTACCGCGGCTGCTGG | ||||
| Streptococcus bovis | F: GAGTGCTAGGTGTTAGGCCC | 58 | 184 | This study |
| R: ATCGGGATGTCAAGACCTGG | ||||
| Ruminococcus albus | F: ACATTGGGACTGAGACACGG | 62 | 248 | This study |
| R: CCTACGCTCCCTTTACACCC | ||||
| Selenomonas ruminantium | F: GGCGGGAAGGCAAGTCAGTC | 63 | 83 | Khafipour et al. [26] |
| R: CCTCTCCTGCACTCAAGAAAGACAG |
2.5. Statistical Analysis
3. Results
In Vitro Rumen Fermentation Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baldi, A.; Gottardo, D. Livestock production to feed the planet: Animal protein: A forecast of global demand over the next years. Beyond Anthr. 2017, 5, 65. [Google Scholar] [CrossRef]
- Grazziotin, R.; Halfen, J.; Rosa, F.; Schmitt, E.; Anderson, J.; Ballard, V.; Osorio, J. Altered rumen fermentation patterns in lactating dairy cows supplemented with phytochemicals improve milk production and efficiency. J. Dairy Sci. 2020, 103, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Peña-Torres, E.F.; Dávila-Ramírez, J.L.; Peña-Ramos, E.A.; Valenzuela-Melendres, M.; Pinelli-Saavedra, A.; Avendaño-Reyes, L.; González-Ríos, H. Effects of dietary ferulic acid on growth performance, carcass traits and meat quality of heifers. J. Sci. Food Agric. 2021, 101, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Vaddella, V.; Zhou, D. Effects of chestnut tannins and coconut oil on growth performance, methane emission, ruminal fermentation, and microbial populations in sheep. J. Dairy Sci. 2011, 94, 6069–6077. [Google Scholar] [CrossRef]
- Singh, A.K.; Ojha, L.; Kumari, P.; Choubey, M.; Chaudhary, S.K. Phytochemicals as Natural Feed Additives for Ruminants. In Feed Additives and Supplements for Ruminants; Springer: Berlin/Heidelberg, Germany, 2024; pp. 167–196. [Google Scholar]
- Choi, Y.; Lee, S.J.; Kim, H.S.; Eom, J.S.; Jo, S.U.; Guan, L.L.; Seo, J.; Kim, H.; Lee, S.S.; Lee, S.S. Effects of seaweed extracts on in vitro rumen fermentation characteristics, methane production, and microbial abundance. Sci. Rep. 2021, 11, 24092. [Google Scholar] [CrossRef]
- Macías-Cruz, U.; Vicente-Pérez, R.; López-Baca, M.; González-Ríos, H.; Correa-Calderón, A.; Arechiga, C.; Avendano-Reyes, L. Effects of dietary ferulic acid on reproductive function and metabolism of pre-pubertal hairbreed ewes during the anestrous season. Theriogenology 2018, 119, 220–224. [Google Scholar] [CrossRef]
- Tánori-Lozano, A.L.; Dávila-Ramírez, J.L.; Medrano-Vazquez, L.S.; Hernández-Mendoza, E.; González-Rios, H. Properties and uses of clinoptilolite. In Agricultural Research Updates; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2024; Volume 47, pp. 33–81. [Google Scholar]
- Goodarzi, M.; Nanekarani, S. The effects of calcic and potassic clinoptilolite on ruminal parameters in Lori breed sheep. APCBEE Procedia 2012, 4, 140–145. [Google Scholar] [CrossRef]
- Ghoneem, W.M.A.; El-Tanany, R.R.; Mahmoud, A.E.M. Effect of Natural Zeolite as a Rumen Buffer on Growth Performance and Nitrogen Utilization of Barki Lambs. Pak. J. Zool. 2022, 54, 1199–1207. [Google Scholar] [CrossRef]
- Mahdavirad, N.; Chaji, M.; Bojarpour, M.; Dehghanbanadaky, M. Comparison of the effect of sodium bicarbonate, sodium sesquicarbonate, and zeolite as rumen buffers on apparent digestibility, growth performance, and rumen fermentation parameters of Arabi lambs. Trop. Anim. Health Prod. 2021, 53, 465. [Google Scholar] [CrossRef]
- Russell, J.B.; Rychlik, J.L. Factors that alter rumen microbial ecology. Science 2001, 292, 1119–1122. [Google Scholar] [CrossRef]
- Ricke, S.; Martin, S.; Nisbet, D. Ecology, metabolism, and genetics of ruminal selenomonads. Crit. Rev. Microbiol. 1996, 22, 27–65. [Google Scholar] [CrossRef] [PubMed]
- Elolimy, A.A.; Arroyo, J.M.; Batistel, F.; Iakiviak, M.A.; Loor, J.J. Association of residual feed intake with abundance of ruminal bacteria and biopolymer hydrolyzing enzyme activities during the peripartal period and early lactation in Holstein dairy cows. J. Anim. Sci. Biotechnol. 2018, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Tánori-Lozano, A.; Quintana-Romandía, A.I.; Montalvo-Corral, M.; Pinelli-Saavedra, A.; Valenzuela-Melendres, M.; Dávila-Ramírez, J.L.; Islava-Lagarda, T.Y.; González-Ríos, H. Influence of ferulic acid and clinoptilolite supplementation on growth performance, carcass, meat quality, and fatty acid profile of finished lambs. J. Anim. Sci. Technol. 2022, 64, 274–290. [Google Scholar] [CrossRef] [PubMed]
- Tánori-Lozano, A.L. Modulación de la Fermentación Ruminal por el uso Combinado de Zeolita y Ácido Ferúlico en Dieta de Ovinos. Ph.D. Thesis, Centro de Investigación en Alimentación y Desarrollo, Repositorio CIAD, Hermosillo, Mexico, 2023. [Google Scholar]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- McDougall, E.I. Studies on ruminant saliva. 1. The composition and output of sheep’s saliva. Biochem. J. 1948, 43, 99. [Google Scholar] [CrossRef]
- Fleming, A.; Beck, M.R.; Bryant, R.H.; Dalley, D.; Edwards, G.; Gregorini, P. In vitro fermentation of fodder beet root increases cumulative gas production of methane and carbon dioxide. Livest. Sci. 2020, 241, 104225. [Google Scholar] [CrossRef]
- Weatherburn, M. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, D.; Wang, L.; Hao, J.; Wang, J.; Zhou, X.; Wang, W.; Qiu, Q.; Huang, X.; Zhou, J. Convergent evolution of rumen microbiomes in high-altitude mammals. Curr. Biol. 2016, 26, 1873–1879. [Google Scholar] [CrossRef]
- Wolin, M.J. A theoretical rumen fermentation balance. J. Dairy Sci. 1960, 43, 1452–1459. [Google Scholar] [CrossRef]
- Larue, R.; Yu, Z.; Parisi, V.A.; Egan, A.R.; Morrison, M. Novel microbial diversity adherent to plant biomass in the herbivore gastrointestinal tract, as revealed by ribosomal intergenic spacer analysis and rrs gene sequencing. Environ. Microbiol. 2005, 7, 530–543. [Google Scholar] [CrossRef]
- Yu, Z.; Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 2004, 36, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Khafipour, E.; Li, S.; Plaizier, J.C.; Krause, D.O. Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis. Appl. Environ. Microbiol. 2009, 75, 7115–7124. [Google Scholar] [CrossRef] [PubMed]
- Menci, R.; Coppa, M.; Torrent, A.; Natalello, A.; Valenti, B.; Luciano, G.; Priolo, A.; Niderkorn, V. Effects of two tannin extracts at different doses in interaction with a green or dry forage substrate on in vitro rumen fermentation and biohydrogenation. Anim. Feed Sci. Technol. 2021, 278, 114977. [Google Scholar] [CrossRef]
- Nowak, B.; Moniuszko-Szajwaj, B.; Skorupka, M.; Puchalska, J.; Kozłowska, M.; Bocianowski, J.; Kołodziejski, P.A.; Szumacher-Strabel, M.; Patra, A.K.; Stochmal, A. Effect of Paulownia leaves extract levels on in vitro Ruminal fermentation, microbial population, methane production, and fatty acid biohydrogenation. Molecules 2022, 27, 4288. [Google Scholar] [CrossRef]
- McCollum, F.T.; Galyean, M.L. Effects of clinoptilolite on rumen fermentation, digestion and feedlot performance in beef steers fed high concentrate diets. J. Anim. Sci. 1983, 56, 517–524. [Google Scholar] [CrossRef]
- Getachew, G.; Robinson, P.; DePeters, E.; Taylor, S. Relationships between chemical composition, dry matter degradation and in vitro gas production of several ruminant feeds. Anim. Feed Sci. Technol. 2004, 111, 57–71. [Google Scholar] [CrossRef]
- Jin, L.; Dunière, L.; Lynch, J.; Zaheer, R.; Turkington, K.; Blackshaw, R.; Lupwayi, N.; O’Donovan, J.; Harker, K.; McAllister, T. Impact of ferulic acid esterase-producing lactobacilli and fibrolytic enzymes on ensiling and digestion kinetics of mixed small-grain silage. Grass Forage Sci. 2017, 72, 80–92. [Google Scholar] [CrossRef]
- Min, B.; Wright, C.; Ho, P.; Eun, J.; Gurung, N.; Shange, R. The effect of phytochemical tannins-containing diet on rumen fermentation characteristics and microbial diversity dynamics in goats using 16S rDNA amplicon pyrosequencing. Agric. Food Anal. Bacteriol. 2014, 4, 141909. [Google Scholar]
- Barakat, A.Z.; Hamed, A.R.; Bassuiny, R.I.; Abdel-Aty, A.M.; Mohamed, S.A. Date palm and saw palmetto seeds functional properties: Antioxidant, anti-inflammatory and antimicrobial activities. J. Food Meas. Charact. 2020, 14, 1064–1072. [Google Scholar] [CrossRef]
- Soltan, Y.; Hashem, N.; Morsy, A.; El-Azrak, K.; El-Din, A.N.; Sallam, S. Comparative effects of Moringa oleifera root bark and monensin supplementations on ruminal fermentation, nutrient digestibility and growth performance of growing lambs. Anim. Feed Sci. Technol. 2018, 235, 189–201. [Google Scholar] [CrossRef]
- Chesson, A.; Stewart, C.S.; Wallace, R.J. Influence of plant phenolic acids on growth and cellulolytic activity of rumen bacteria. Appl. Environ. Microbiol. 1982, 44, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Wang, W.-K.; Wu, Q.-C.; Yang, H.-J. The release and catabolism of ferulic acid in plant cell wall by rumen microbes: A review. Anim. Nutr. 2022, 9, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Borneman, W.S.; Akin, D.; VanEseltine, W. Effect of phenolic monomers on ruminal bacteria. J. Appl. Environ. Microbiol. 1986, 52, 1331–1339. [Google Scholar] [CrossRef]
- Weimer, P.; Digman, M. Fermentation of alfalfa wet-fractionation liquids to volatile fatty acids by Streptococcus bovis and Megasphaera elsdenii. Bioresour. Technol. 2013, 142, 88–94. [Google Scholar] [CrossRef]
- Amanzougarene, Z.; Fondevila, M. Rumen Fermentation of Feed Mixtures Supplemented with Clay Minerals in a Semicontinuous In Vitro System. Animals 2022, 12, 345. [Google Scholar] [CrossRef]
- White, J.L.; Ohlrogge, A.J. Ion Exchange Materials to Increase Consumption of Non-Protein Nitrogen by Ruminants. U.S. Patent 4,393,082, 12 July 1983. [Google Scholar]
- Zhang, X.; Ke, W.; Ding, Z.; Xu, D.; Wang, M.; Chen, M.; Guo, X. Microbial mechanisms of using feruloyl esterase-producing Lactobacillus plantarum A1 and grape pomace to improve fermentation quality and mitigate ruminal methane emission of ensiled alfalfa for cleaner animal production. J. Environ. Manag. 2022, 308, 114637. [Google Scholar] [CrossRef]
- Roque-Jiménez, J.; Pinos-Rodríguez, J.; Rojo-Rub, R.; Mendoza, G.; Vazquez, A.; De Jesus, J.C.; Lee-Rangel, H. Effect of natural zeolite on live weight changes, ruminal fermentation and nitrogen metabolism of ewe lambs. S. Afr. J. Anim. Sci. 2018, 48, 1148–1155. [Google Scholar] [CrossRef]
- Ebeid, H.M.; Mengwei, L.; Kholif, A.E.; Hassan, F.-u.; Lijuan, P.; Xin, L.; Chengjian, Y. Moringa oleifera oil modulates rumen microflora to mediate in vitro fermentation kinetics and methanogenesis in total mix rations. Curr. Microbiol. 2020, 77, 1271–1282. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, J.; Lang, X.; Liu, L.; Casper, D.P.; Wang, C.; Zhang, L.; Wei, S. Effects of oregano essential oil on in vitro ruminal fermentation, methane production, and ruminal microbial community. J. Dairy Sci. 2020, 103, 2303–2314. [Google Scholar] [CrossRef]
- Hartinger, T.; Gresner, N.; Südekum, K.-H. Does intra-ruminal nitrogen recycling waste valuable resources? A review of major players and their manipulation. J. Anim. Sci. Biotechnol. 2018, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Kardaya, D.; Sudrajat, D.; Dihansih, E. Efficacy of dietary urea-impregnated zeolite in improving rumen fermentation characteristics of local lamb. Media Paternakan 2012, 35, 207. [Google Scholar] [CrossRef]
- Dschaak, C.; Eun, J.-S.; Young, A.; Stott, R.; Peterson, S. Effects of supplementation of natural zeolite on intake, digestion, ruminal fermentation, and lactational performance of dairy cows. Prof. Anim. Sci. 2010, 26, 647–654. [Google Scholar] [CrossRef]
- Wang, Y.; Alexander, T.W.; McAllister, T.A. In vitro effects of phlorotannins from Ascophyllum nodosum (brown seaweed) on rumen bacterial populations and fermentation. J. Sci. Food Agric. 2009, 89, 2252–2260. [Google Scholar] [CrossRef]
- Russell, J.B.; Wilson, D.B. Why are ruminal cellulolytic bacteria unable to digest cellulose at low pH? J. Dairy Sci. 1996, 79, 1503–1509. [Google Scholar] [CrossRef]
- Russell, J.B.; Wallace, R.J. Energy-yielding and energy-consuming reactions. Rumen Microb. Ecosyst. 1997, 246–282. [Google Scholar] [CrossRef]

| Item | Incubation Time (h) | No CTL | CTL 2 | SEM | p-Value 3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No FA | FA 1 | No FA | FA | FA | CTL | FA × CTL | FA × CTL × T | |||
| pH | 12 | 6.4 a | 6.4 a | 6.45 b | 6.4 a | 0.01 | 1.000 | 0.030 | 0.030 | <0.0001 |
| 24 | 6.3 a | 6.4 b | 6.4 b | 6.3 a | ||||||
| 48 | 6.4 a | 6.4 a | 6.4 a | 6.45 b | ||||||
| 72 | 6.3 a | 6.3 a | 6.35 b | 6.35 b | ||||||
| NH3-N 4 (mg/L) | 12 | 34.4 a | 39.17 ac | 48.96 b | 42.65 c | 1.84 | 0.169 | 0.707 | 0.008 | <0.0001 |
| 24 | 50.84 a | 50.12 a | 49.80 a | 51.54 a | ||||||
| 48 | 40.34 a | 35.19 ab | 34.66 b | 39.07 ab | ||||||
| 72 | 57.19 a | 38.92 b | 37.81 b | 44.97 c | ||||||
| CH4 5 (moles/mol VFA) | 12 | 22.50 | 22.56 | 22.37 | 22.57 | 1.40 | 0.432 | 0.823 | 0.093 | 0.1173 |
| 24 | 26.14 | 25.88 | 25.32 | 25.28 | ||||||
| 48 | 24.35 | 29.80 | 29.00 | 28.01 | ||||||
| 72 | 28.80 | 32.43 | 30.48 | 27.91 | ||||||
| VFA (mmol/L) | Incubation Time (h) | No CTL | CTL 2 | SEM | p-Value 3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No FA | FA 1 | No FA | FA | FA | CTL | FA × CTL | FA × CTL × T | |||
| Acetate | 12 | 43.05 a | 43.61 a | 42.87 a | 43.33 a | 2.127 | 0.185 | 0.888 | 0.003 | 0.002 |
| 24 | 49.64 a | 49.43 a | 48.02 a | 48.15 a | ||||||
| 48 | 45.12 a | 56.87 b | 55.50 b | 53.72 b | ||||||
| 72 | 50.57 a | 61.96 b | 58.39 b | 48.96 a | ||||||
| Propionate | 12 | 25.89 a | 26.55 a | 26.08 a | 26.25 a | 2.101 | 0.517 | 0.879 | 0.100 | 0.006 |
| 24 | 28.60 a | 28.95 a | 27.84 a | 28.18 a | ||||||
| 48 | 24.27 a | 30.83 b | 30.63 b | 30.48 b | ||||||
| 72 | 25.06 a | 32.95 b | 31.51 b | 24.11a | ||||||
| Butyrate | 12 | 14.89 | 14.78 | 14.91 | 14.94 | 1.276 | 0.758 | 0.917 | 0.821 | 0.647 |
| 24 | 16.94 | 16.80 | 16.53 | 16.50 | ||||||
| 48 | 15.72 | 18.14 | 17.81 | 17.53 | ||||||
| 72 | 19.56 | 19.37 | 18.32 | 18.91 | ||||||
| Isobutyrate | 12 | 2.86 | 2.81 | 2.70 | 2.75 | 0.171 | 0.886 | 0.962 | 0.402 | 0.862 |
| 24 | 3.59 | 3.55 | 3.60 | 3.57 | ||||||
| 48 | 3.53 | 3.53 | 3.58 | 3.59 | ||||||
| 72 | 3.61 | 3.40 | 3.44 | 3.62 | ||||||
| Valerate | 12 | 3.55 a | 3.56 a | 3.61 a | 3.59 a | 0.701 | 0.784 | 0.969 | 0.178 | 0.012 |
| 24 | 4.31 a | 4.29 a | 4.15 a | 4.17 a | ||||||
| 48 | 3.84 a | 5.06 a | 4.93 a | 4.77 a | ||||||
| 72 | 3.56 a | 5.96 b | 5.67 b | 3.41 a | ||||||
| Isovalerate | 12 | 2.55 | 2.55 | 2.56 | 2.55 | 0.127 | 0.547 | 0.778 | 0.123 | 0.124 |
| 24 | 3.77 | 3.74 | 3.66 | 3.44 | ||||||
| 48 | 3.43 | 3.84 | 4.00 | 3.66 | ||||||
| 72 | 4.02 | 3.99 | 4.00 | 3.83 | ||||||
| Total VFA | 12 | 91.66 a | 92.76 a | 91.71 a | 92.34 a | 4.116 | 0.229 | 0.996 | 0.005 | 0.0009 |
| 24 | 105.64 a | 105.56 a | 102.51 a | 102.75 a | ||||||
| 48 | 94.47 a | 117.33 b | 115.44 b | 112.69 b | ||||||
| 72 | 105.17 a | 126.92 b | 120.47 b | 101.52 a | ||||||
| A:P 4 | 12 | 0.92 | 0.90 | 0.91 | 0.91 | 0.11 | 0.927 | 0.862 | 0.766 | 0.594 |
| 24 | 0.94 | 0.92 | 0.94 | 0.93 | ||||||
| 48 | 1.04 | 1.01 | 1.00 | 0.97 | ||||||
| 72 | 1.13 | 1.03 | 1.02 | 1.15 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tánori-Lozano, A.; López-Baca, M.Á.; Muhlia-Almazán, A.; Montalvo-Corral, M.; Pinelli-Saavedra, A.; Islava-Lagarda, T.Y.; Dávila-Ramírez, J.L.; Valenzuela-Melendres, M.; González-Rios, H. Ferulic Acid and Clinoptilolite Affect In Vitro Rumen Fermentation Characteristics and Bacterial Abundance. Fermentation 2024, 10, 549. https://doi.org/10.3390/fermentation10110549
Tánori-Lozano A, López-Baca MÁ, Muhlia-Almazán A, Montalvo-Corral M, Pinelli-Saavedra A, Islava-Lagarda TY, Dávila-Ramírez JL, Valenzuela-Melendres M, González-Rios H. Ferulic Acid and Clinoptilolite Affect In Vitro Rumen Fermentation Characteristics and Bacterial Abundance. Fermentation. 2024; 10(11):549. https://doi.org/10.3390/fermentation10110549
Chicago/Turabian StyleTánori-Lozano, Ana, M. Ángeles López-Baca, Adriana Muhlia-Almazán, Maricela Montalvo-Corral, Araceli Pinelli-Saavedra, Thalia Y. Islava-Lagarda, José Luis Dávila-Ramírez, Martín Valenzuela-Melendres, and Humberto González-Rios. 2024. "Ferulic Acid and Clinoptilolite Affect In Vitro Rumen Fermentation Characteristics and Bacterial Abundance" Fermentation 10, no. 11: 549. https://doi.org/10.3390/fermentation10110549
APA StyleTánori-Lozano, A., López-Baca, M. Á., Muhlia-Almazán, A., Montalvo-Corral, M., Pinelli-Saavedra, A., Islava-Lagarda, T. Y., Dávila-Ramírez, J. L., Valenzuela-Melendres, M., & González-Rios, H. (2024). Ferulic Acid and Clinoptilolite Affect In Vitro Rumen Fermentation Characteristics and Bacterial Abundance. Fermentation, 10(11), 549. https://doi.org/10.3390/fermentation10110549






