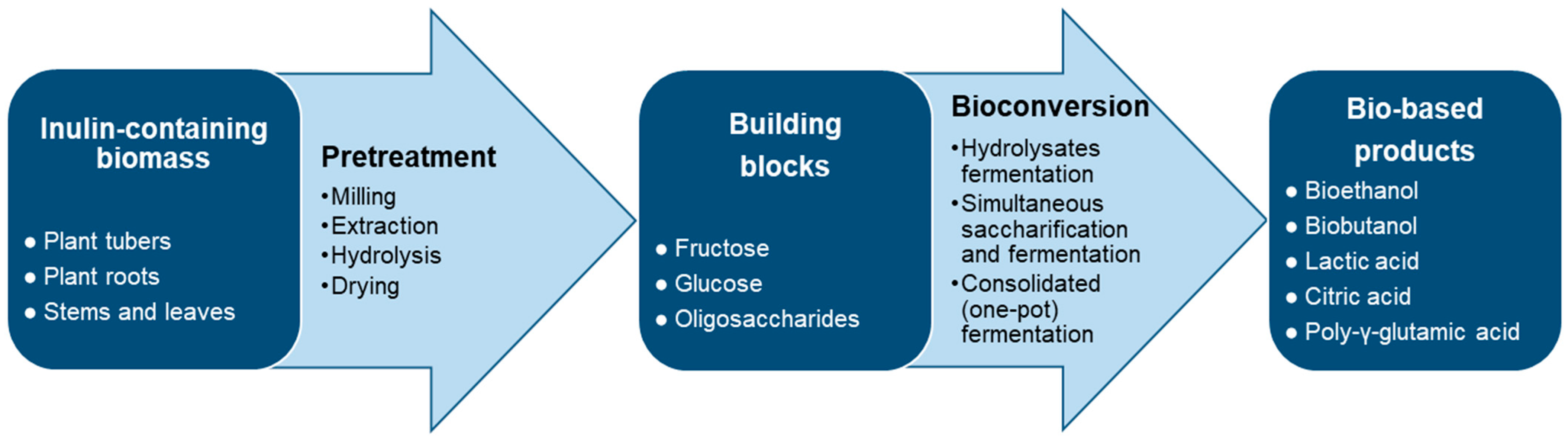
Microbial Conversion of Inulin to Valuable Products: The Biorefinery Concept
Abstract
1. Introduction
2. Pretreatment of Inulin-Containing Biomass
2.1. Extraction
2.2. Enzymatic Hydrolysis
2.3. Acidic Hydrolysis
2.4. Simultaneous Saccharification and Fermentation (SSF)
2.5. Consolidated Bioprocessing (CBP)
3. Biofuel Production from Inulin
3.1. Ethanol
3.2. Butanol
3.3. 2,3-Butanediol (2,3-BD)
4. Organic Acid Production from Inulin
4.1. Lactic Acid
| Inulin Source | Hydrolysis Type | Fermentation Mode | Strain | LA (g/L) | Conversion (%) | Yield (g/g) | Productivity (g/L/h) | Reference |
|---|---|---|---|---|---|---|---|---|
| JAT | Enzymatic | Batch | L. paracasei NJ | 144.08 | ND | 0.67 | 4.37 | [105] |
| JAT | Enzymatic | SSF, FB | L. paracasei KCTC 13169 | 92.5 | 98.0 | ND | ND | [106] |
| JAT | Enzymatic | SSF | A. niger SL-09, Lactobacillus sp. G-02 | 120.5 | 94.5 | ND | ND | [107] |
| JAT | Enzymatic | SSF | A. niger SL-09, Lactobacillus sp. G-02 | 141.5 | 93.6 | 0.524 | 4.7 | [108] |
| JAT | Enzymatic | Fed-batch | B. coagulans XZL4 | 134.0 | ND | 0.96 | 2.5 | [109] |
| JAT | Enzymatic | - | L. lactis | 142.0 | - | ND | ND | [110] |
| Chicory flour | Enzymatic | Batch | L. paracasei DSM 23505 | 123.7 | 91.0 | ND | ND | [113] |
| Chicory | Enzymatic | SSF | L. bulgaricus CGMCC 1.6970 | 123.6 | 97.9 | ND | ND | [114] |
4.2. Citric Acid
4.3. Poly-γ-glutamic Acid (γ-PGA)
5. Other Products from Inulin
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mignogna, D.; Szabó, M.; Ceci, P.; Avino, P. Biomass Energy and Biofuels: Perspective, Potentials, and Challenges in the Energy Transition. Sustainability 2024, 16, 7036. [Google Scholar] [CrossRef]
- González-Gloria, K.D.; Tomás-Pejó, E.; Amaya-Delgado, L.; Rodríguez-Jasso, R.M.; Loredo-Treviño, A.; Singh, A.; Hans, M.; Martín, C.; Kumar, S.; Ruiz, H.A. Biochemical and Biorefinery Platform for Second-Generation Bioethanol: Fermentative Strategies and Microorganisms. Fermentation 2024, 10, 361. [Google Scholar] [CrossRef]
- Tsigoriyna, L.; Arsov, A.; Gergov, E.; Petrova, P.; Petrov, K. Influence of pH on Inulin Conversion to 2,3-Butanediol by Bacillus licheniformis 24: A Gene Expression Assay. Int. J. Mol. Sci. 2023, 24, 14065. [Google Scholar] [CrossRef]
- Hughes, S.R.; Qureshi, N.; López-Núñez, J.C.; Jones, M.A.; Jarodsky, J.M.; Galindo-Leva, L.Á.; Lindquist, M.R. Utilization of Inulin-Containing Waste in Industrial Fermentations to Produce Biofuels and Bio-Based Chemicals. World J. Microbiol. Biotechnol. 2017, 33, 78. [Google Scholar] [CrossRef]
- Du, Y.; Kusama, K.; Hama, K.; Chen, X.; Tahara, Y.; Kajiwara, S.; Shibata, S.; Orihara, K. Protective Effects of Inulin on Stress-Recurrent Inflammatory Bowel Disease. Int. J. Mol. Sci. 2024, 25, 2494. [Google Scholar] [CrossRef]
- Wang, M.; Cheong, K.-L. Preparation, Structural Characterisation, and Bioactivities of Fructans: A Review. Molecules 2023, 28, 1613. [Google Scholar] [CrossRef]
- Baumgartner, S.; Dax, T.G.; Praznik, W.; Falk, H. Characterisation of the High-Molecular Weight Fructan Isolated from Garlic (Allium sativum L.). Carbohydr. Res. 2000, 328, 177–183. [Google Scholar] [CrossRef]
- Singh, R.S.; Bhermi, H.K. Production of Extracellular Exoinulinase from Kluyveromyces marxianus YS-1 Using Root Tubers of Asparagus officinalis. Bioresour. Technol. 2008, 99, 7418–7423. [Google Scholar] [CrossRef] [PubMed]
- Kango, N. Production of Inulinase Using Tap Roots of Dandelion (Taraxacum Officinale) by Aspergillus niger. J. Food Eng. 2008, 85, 473–478. [Google Scholar] [CrossRef]
- Leroy, G.; Grongnet, J.F.; Mabeau, S.; Corre, D.L.; Baty-Julien, C. Changes in Inulin and Soluble Sugar Concentration in Artichokes (Cynara scolymus L.) during Storage. J. Sci. Food Agric. 2010, 90, 1203–1209. [Google Scholar] [CrossRef]
- Singh, R.S.; Singh, T.; Hassan, M.; Larroche, C. Biofuels from Inulin-Rich Feedstocks: A Comprehensive Review. Bioresour. Technol. 2022, 346, 126606. [Google Scholar] [CrossRef]
- Rivera, A.; Pozo, M.; Sánchez-Moreno, V.E.; Vera, E.; Jaramillo, L.I. Pulsed Electric Field-Assisted Extraction of Inulin from Ecuadorian cabuya (Agave americana). Molecules 2024, 29, 3428. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, A.; Viera-Alcaide, I.; Guillén-Bejarano, R.; Rodríguez-Arcos, R.; Muñoz, M.J.; Monje Moreno, J.M.; Jiménez-Araujo, A. Asparagus Fructans as Emerging Prebiotics. Foods 2023, 12, 81. [Google Scholar] [CrossRef]
- Kurowska, A.; Ziemichód, W.; Herbet, M.; Piątkowska-Chmiel, I. The Role of Diet as a Modulator of the Inflammatory Process in the Neurological Diseases. Nutrients 2023, 15, 1436. [Google Scholar] [CrossRef] [PubMed]
- Petrov, K.; Popova, L.; Petrova, P. High lactic acid and fructose production via Mn2+-mediated conversion of inulin by Lactobacillus paracasei. Appl. Microbiol. Biotechnol. 2017, 101, 4433–4445. [Google Scholar] [CrossRef]
- Cornescu, G.M.; Panaite, T.D.; Soica, C.; Cismileanu, A.; Matache, C.C. Jerusalem Artichoke (Helianthus tuberosus L.) as a Promising Dietary Feed Ingredient for Monogastric Farm Animals. Appl. Sci. 2023, 13, 12748. [Google Scholar] [CrossRef]
- Lopes, S.M.S.; Krausová, G.; Rada, V.; Gonçalves, J.E.; Gonçalves, R.A.C.; De Oliveira, A.J.B. Isolation and Characterization of Inulin with a High Degree of Polymerization from Roots of Stevia rebaudiana (Bert.) Bertoni. Carbohydr. Res. 2015, 411, 15–21. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT 2024: Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QV (accessed on 2 November 2024).
- Zhang, X.; Zhu, X.; Shi, X.; Hou, Y.; Yi, Y. Extraction and Purification of Inulin from Jerusalem artichoke with Response Surface Method and Ion Exchange Resins. ACS Omega 2022, 7, 12048–12055. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, J.; Yu, C.; Li, Q.; Dong, F.; Wang, G.; Gu, G.; Guo, Z. Extraction, Degree of Polymerization Determination and Prebiotic Effect Evaluation of Inulin from Jerusalem Artichoke. Carbohydr. Polym. 2015, 121, 315–319. [Google Scholar] [CrossRef]
- Castellino, M.; Renna, M.; Leoni, B.; Calasso, M.; Difonzo, G.; Santamaria, P.; Gambacorta, G.; Caponio, F.; De Angelis, M.; Paradiso, V.M. Conventional and Unconventional Recovery of Inulin Rich Extracts for Food Use from the Roots of Globe Artichoke. Food Hydrocoll. 2020, 107, 105975. [Google Scholar] [CrossRef]
- Machado, M.T.C.; Eça, K.S.; Vieira, G.S.; Menegalli, F.C.; Martínez, J.; Hubinger, M.D. Prebiotic Oligosaccharides from Artichoke Industrial Waste: Evaluation of Different Extraction Methods. Ind. Crops Prod. 2015, 76, 141–148. [Google Scholar] [CrossRef]
- Toneli, J.T.C.L.; Park, K.J.; Ramalho, J.R.P.; Murr, F.E.X.; Fabbro, I.M.D. Rheological Characterization of Chicory Root (Cichorium intybus L.) Inulin Solution. Braz. J. Chem. Eng. 2008, 25, 461–471. [Google Scholar] [CrossRef]
- Laurenzo, K.S.; Navia, J.L.; Neiditch, D.S. Preparation of Inulin Products. U.S. Patent 5968365A, 19 October 1999. [Google Scholar]
- Redondo-Cuenca, A.; Herrera-Vázquez, S.E.; Condezo-Hoyos, L.; Gómez-Ordóñez, E.; Rupérez, P. Inulin Extraction from Common Inulin-Containing Plant Sources. Ind. Crops Prod. 2021, 170, 113726. [Google Scholar] [CrossRef]
- Rawat, H.K.; Ganaie, M.A.; Kango, N. Production of Inulinase, Fructosyltransferase and Sucrase from Fungi on Low-Value Inulin-Rich Substrates and Their Use in Generation of Fructose and Fructo-Oligosaccharides. Antonie Van Leeuwenhoek 2015, 107, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Soccol, C.R.; Selvakumar, P.; Soccol, V.T.; Krieger, N.; Fontana, J.D. Recent Developments in Microbial Inulinases: Its Production, Properties, and Industrial Applications. Appl. Biochem. Biotechnol. 1999, 81, 35–52. [Google Scholar] [CrossRef]
- Chi, Z.; Chi, Z.; Zhang, T.; Liu, G.; Yue, L. Inulinase-Expressing Microorganisms and Applications of Inulinases. Appl. Microbiol. Biotechnol. 2009, 82, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Rawat, H.K.; Soni, H.; Treichel, H.; Kango, N. Biotechnological Potential of Microbial Inulinases: Recent Perspective. Crit. Rev. Food Sci. Nutr. 2017, 57, 3818–3829. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Yamini, D.; Ambika, V.; Sravya Sowdamini, N. Trends in inulinase production—A review. Crit. Rev. Biotechnol. 2009, 29, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Abdella, A.; Al-Saman, M.; Abou-Elazm, F.I.; El-Far, S.W. Rhizopus oryzae Inulinase Production and Characterization with Application in Chicory Root Saccharification. Microbiol. Res. 2023, 14, 297–315. [Google Scholar] [CrossRef]
- Das, D.; Bhat, M.R.; Selvaraj, R. Review of Inulinase Production Using Solid-State Fermentation. Ann. Microbiol. 2019, 69, 201–209. [Google Scholar] [CrossRef]
- Eskandari Nasab, E.; Habibi-Rezaei, M.; Khaki, A.; Balvardi, M. Investigation on Acid Hydrolysis of Inulin: A Response Surface Methodology Approach. Int. J. Food Eng. 2009, 5, 3. [Google Scholar] [CrossRef]
- Świątek, K.; Gaag, S.; Klier, A.; Kruse, A.; Sauer, J.; Steinbach, D. Acid Hydrolysis of Lignocellulosic Biomass: Sugars and Furfurals Formation. Catalysts 2020, 10, 437. [Google Scholar] [CrossRef]
- Zong, X.; Lei, N.; Yin, J.; He, W.; Nie, S.; Xie, M. Exploration and Improvement of Acid Hydrolysis Conditions for Inulin-Type Fructans Monosaccharide Composition Analysis: Monosaccharide Recovery and By-Product Identification. Foods 2024, 13, 1241. [Google Scholar] [CrossRef]
- Gauss, W.F.; Suzuki, S.; Takagi, M. Manufacture of Alcohol from Cellulosic Materials Using Plural Ferments. U.S. Patent 3990944A, 9 November 1976. [Google Scholar]
- Olofsson, K.; Bertilsson, M.; Lidén, G. A Short Review on SSF—An Interesting Process Option for Ethanol Production from Lignocellulosic Feedstocks. Biotechnol. Biofuels 2008, 1, 7. [Google Scholar] [CrossRef]
- Patria, R.D.; Rehman, S.; Vuppaladadiyam, A.K.; Wang, H.; Lin, C.S.K.; Antunes, E.; Leu, S.-Y. Bioconversion of food and lignocellulosic wastes employing sugar platform: A review of enzymatic hydrolysis and kinetics. Biores. Technol. 2022, 352, 127083. [Google Scholar] [CrossRef]
- Banner, A.; Toogood, H.S.; Scrutton, N.S. Consolidated Bioprocessing: Synthetic Biology Routes to Fuels and Fine Chemicals. Microorganisms 2021, 9, 1079. [Google Scholar] [CrossRef] [PubMed]
- Padder, S.A.; Khan, R.; Rather, R.A. Biofuel Generations: New Insights into Challenges and Opportunities in Their Microbe-Derived Industrial Production. Biomass Bioenergy 2024, 185, 107220. [Google Scholar] [CrossRef]
- Bera, T.; Inglett, K.S.; Wilkie, A.C. Biofuel: Concepts and Considerations. EDIS 2020, 2020, 1–6. [Google Scholar] [CrossRef]
- A Global Food Crisis. Available online: https://www.wfp.org/global-hunger-crisis (accessed on 24 October 2024).
- Bahena-Molina, K.A.; Sunder, S.; Ganesan, A.; Saini, R.; Osorio-González, C.S.; Kaur Brar, S. Pretreatment Technologies for Second-Generation Bioethanol Production. In Liquid Biofuels: Bioethanol; Biofuel and Biorefinery Technologies; Soccol, C.R., Amarante Guimarães Pereira, G., Dussap, C.-G., Porto De Souza Vandenberghe, L., Eds.; Springer: Cham, Switzerland, 2022; Volume 12, pp. 209–241. ISBN 978-3-031-01240-2. [Google Scholar]
- Robak, K.; Balcerek, M. Review of Second-Generation Bioethanol Production from Residual Biomass. Food Technol. Biotechnol. 2018, 56, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, J.; Huang, Z.; Liu, T.; Li, H. Photothermal technique-enabled ambient production of microalgae biodiesel: Mechanism and life cycle assessment. Bioresour. Technol. 2023, 369, 128390. [Google Scholar] [CrossRef]
- Huang, J.; Liu, T.; Wang, K.; Huang, Z.; Wang, J.; Rokhum, S.L.; Li, H. Room-temperature and carbon-negative production of biodiesel via synergy of geminal-atom and photothermal catalysis. Environ. Chem. Lett. 2024, 22, 1607–1613. [Google Scholar] [CrossRef]
- Adeniyi, O.M.; Azimov, U.; Burluka, A. Algae Biofuel: Current Status and Future Applications. Renew. Sustain. Energy Rev. 2018, 90, 316–335. [Google Scholar] [CrossRef]
- Moravvej, Z.; Makarem, M.A.; Rahimpour, M.R. The Fourth Generation of Biofuel. In Second and Third Generation of Feedstocks; Elsevier: Amsterdam, The Netherlands, 2019; pp. 557–597. ISBN 978-0-12-815162-4. [Google Scholar]
- Malode, S.J.; Prabhu, K.K.; Mascarenhas, R.J.; Shetti, N.P.; Aminabhavi, T.M. Recent Advances and Viability in Biofuel Production. Energy Convers. Manag. X 2021, 10, 100070. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Pei, Z.; Fang, Z.; Yang, S.; Li, H. Bio-based deep eutectic solvent of enhanced lignin solubility for wheat straw fractionation and full-component utilization. Ind. Crops Prod. 2025, 223, 120054. [Google Scholar] [CrossRef]
- Tan, J.; Yu, D.; Yuan, J.; Wu, H.; Luo, H.; Zhang, H.; Li, X.; Li, H.; Yang, S. Efficient delignification of wheat straw for microbial lipid production enabled by a novel ternary deep eutectic solvent containing ethylene glycol. Fuel 2023, 347, 128485. [Google Scholar] [CrossRef]
- Tan, J.; Huang, J.; Yuan, J.; Chen, J.; Pei, Z.; Li, H.; Yang, S. Novel supramolecular deep eutectic solvent-enabled in-situ lignin protection for full valorization of all components of wheat straw. Bioresour. Technol. 2023, 388, 129722. [Google Scholar] [CrossRef]
- Song, Y.; Wi, S.G.; Kim, H.M.; Bae, H.-J. Cellulosic bioethanol production from Jerusalem artichoke (Helianthus tuberosus L.) using hydrogen peroxide-acetic acid (HPAC) pretreatment. Bioresour. Technol. 2016, 214, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Biofuel Basics. Available online: https://www.energy.gov/eere/bioenergy/biofuel-basics (accessed on 24 October 2024).
- Annual Ethanol Production. Available online: https://ethanolrfa.org/markets-and-statistics/annual-ethanol-production (accessed on 25 October 2024).
- Bedzo, O.K.K.; Mandegari, M.; Görgens, J.F. Techno-economic Analysis of Inulooligosaccharides, Protein, and Biofuel Co-production from Jerusalem Artichoke Tubers: A Biorefinery Approach. Biofuels Bioprod. Biorefining 2020, 14, 776–793. [Google Scholar] [CrossRef]
- Song, Y.; Oh, C.; Bae, H.-J. Simultaneous Production of Bioethanol and Value-Added d-Psicose from Jerusalem Artichoke (Helianthus Tuberosus L.) Tubers. Bioresour. Technol. 2017, 244, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chi, Z.; Zhao, C.H.; Chi, Z.M.; Gong, F. Bioethanol Production from Hydrolysates of Inulin and the Tuber Meal of Jerusalem Artichoke by Saccharomyces sp. W0. Bioresour. Technol. 2010, 101, 8166–8170. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Z.; Zou, S.-M.; He, M.-L.; Wang, C.-H. Bioethanol Production from the Dry Powder of Jerusalem Artichoke Tubers by Recombinant Saccharomyces cerevisiae in Simultaneous Saccharification and Fermentation. J. Ind. Microbiol. Biotechnol. 2015, 42, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, F.-L.; Wang, S.-A. Engineering a Natural Saccharomyces cerevisiae Strain for Ethanol Production from Inulin by Consolidated Bioprocessing. Biotechnol. Biofuels 2016, 9, 96. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, Z.-P.; Wang, X.-L.; Zhou, G.-Y.; Cao, Y.; Lu, P.; Liu, W.-F. Hydrolysis Kinetics of Inulin by Imidazole-Based Acidic Ionic Liquid in Aqueous Media and Bioethanol Fermentation. Chem. Eng. Sci. 2016, 151, 16–24. [Google Scholar] [CrossRef]
- Moshi, A.P.; Nyandele, J.P.; Ndossi, H.P.; Eva, S.M.; Hosea, K.M. Feasibility of Bioethanol Production from Tubers of Dioscorea sansibarensis and Pyrenacantha kaurabassana. Bioresour. Technol. 2015, 196, 613–620. [Google Scholar] [CrossRef]
- Negro, M.J.; Ballesteros, I.; Manzanares, P.; Oliva, J.M.; Sáez, F.; Ballesteros, M. Inulin-Containing Biomass for Ethanol Production Carbohydrate Extraction and Ethanol Fermentation. Appl. Biochem. Biotechnol. 2006, 132, 922–932. [Google Scholar] [CrossRef]
- Berthels, N.J.; Cordero Otero, R.R.; Bauer, F.F.; Thevelein, J.M.; Pretorius, I.S. Discrepancy in Glucose and Fructose Utilisation during Fermentation by Saccharomyces cerevisiae Wine Yeast Strains. FEMS Yeast Res. 2004, 4, 683–689. [Google Scholar] [CrossRef] [PubMed]
- N-Butanol Global Market Value 2015–2030. Available online: https://www.statista.com/statistics/1245211/n-butanol-market-volume-worldwide/ (accessed on 25 October 2024).
- Gwalwanshi, M.; Kumar, R.; Kumar Chauhan, M. A Review on Butanol Properties, Production and Its Application in Internal Combustion Engines. Mater. Today Proc. 2022, 62, 6573–6577. [Google Scholar] [CrossRef]
- Antoni, D.; Zverlov, V.V.; Schwarz, W.H. Biofuels from Microbes. Appl. Microbiol. Biotechnol. 2007, 77, 23–35. [Google Scholar] [CrossRef]
- Green, E.M. Fermentative Production of Butanol—The Industrial Perspective. Curr. Opin. Biotechnol. 2011, 22, 337–343. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Y.; Huang, J.; Liu, D. Metabolic engineering of Klebsiella pneumoniae for the de novo production of 2-butanol as a potential biofuel. Bioresour Technol. 2015, 197, 260–265. [Google Scholar] [CrossRef]
- Peralta-Yahya, P.P.; Zhang, F.; Del Cardayre, S.B.; Keasling, J.D. Microbial Engineering for the Production of Advanced Biofuels. Nature 2012, 488, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Trinh, C.T. Elucidating and Reprogramming Escherichia Coli Metabolisms for Obligate Anaerobic N-Butanol and Isobutanol Production. Appl. Microbiol. Biotechnol. 2012, 95, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Söhling, B.; Gottschalk, G. Molecular Analysis of the Anaerobic Succinate Degradation Pathway in Clostridium kluyveri. J. Bacteriol. 1996, 178, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Varman, A.M.; Xiao, Y.; Pakrasi, H.B.; Tang, Y.J. Metabolic Engineering of Synechocystis sp. Strain PCC 6803 for Isobutanol Production. Appl. Environ. Microbiol. 2013, 79, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, D.; Li, Y.; Wen, J.; Jia, X. Rational Improvement of the Engineered Isobutanol-Producing Bacillus subtilis by Elementary Mode Analysis. Microb. Cell Factories 2012, 11, 101. [Google Scholar] [CrossRef]
- Kondo, T.; Tezuka, H.; Ishii, J.; Matsuda, F.; Ogino, C.; Kondo, A. Genetic Engineering to Enhance the Ehrlich Pathway and Alter Carbon Flux for Increased Isobutanol Production from Glucose by Saccharomyces cerevisiae. J. Biotechnol. 2012, 159, 32–37. [Google Scholar] [CrossRef]
- Kolesinska, B.; Fraczyk, J.; Binczarski, M.; Modelska, M.; Berlowska, J.; Dziugan, P.; Antolak, H.; Kaminski, Z.J.; Witonska, I.A.; Kregiel, D. Butanol Synthesis Routes for Biofuel Production: Trends and Perspectives. Materials 2019, 12, 350. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, J.H.; Jang, S.H.; Nielsen, L.K.; Kim, J.; Jung, K.S. Fermentative Butanol Production by Clostridia. Biotechnol. Bioeng. 2008, 101, 209–228. [Google Scholar] [CrossRef]
- Jiang, Y.; Lv, Y.; Michenfelder, R.; Chen, T.; Wu, R.; Xin, F.; Jiang, M. Clostridium sp. Strain NJ4: A Promising Solventogenic Strain for Butanol Production from Jerusalem Artichoke through Consolidated Bioprocessing. Energy Fuels 2020, 34, 3406–3411. [Google Scholar] [CrossRef]
- Wu, Y.-D.; Xue, C.; Chen, L.-J.; Bai, F.-W. Impact of Zinc Supplementation on the Improved Fructose/Xylose Utilization and Butanol Production during Acetone–Butanol–Ethanol Fermentation. J. Biosci. Bioeng. 2016, 121, 66–72. [Google Scholar] [CrossRef]
- Ujor, V.; Bharathidasan, A.K.; Michel, F.C.; Ezeji, T.C.; Cornish, K. Butanol Production from Inulin-Rich Chicory and Taraxacum Kok-Saghyz Extracts: Determination of Sugar Utilization Profile of Clostridium saccharobutylicum P262. Ind. Crop. Prod. 2015, 76, 739–748. [Google Scholar] [CrossRef]
- Sarchami, T.; Rehmann, L. Optimizing Enzymatic Hydrolysis of Inulin from Jerusalem Artichoke Tubers for Fermentative Butanol Production. Biomass Bioenergy 2014, 69, 175–182. [Google Scholar] [CrossRef]
- Sarchami, T.; Rehmann, L. Optimizing Acid Hydrolysis of Jerusalem Artichoke-Derived Inulin for Fermentative Butanol Production. BioEnergy Res. 2015, 8, 1148–1157. [Google Scholar] [CrossRef]
- Bekers, M.; Bekers, M.; Grube, M.; Upite, D.; Kaminska, E.; Linde, R.; Scherbaka, R.; Danilevich, A. Carbohydrates in Jerusalem Artichoke Powder Suspension. Nutr. Food Sci. 2007, 37, 42–49. [Google Scholar] [CrossRef]
- Chen, L.; Xin, C.; Deng, P.; Ren, J.; Liang, H.; Bai, F. Butanol production from hydrolysate of Jerusalem artichoke juice by Clostridium acetobutylicum L7. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2010, 26, 991–996. [Google Scholar]
- 2,3-Butanediol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/262 (accessed on 25 October 2024).
- Petrov, K.; Petrova, P. Current Advances in Microbial Production of Acetoin and 2,3-Butanediol by Bacillus spp. Fermentation 2021, 7, 307. [Google Scholar] [CrossRef]
- Hazeena, S.H.; Shurpali, N.J.; Siljanen, H.; Lappalainen, R.; Anoop, P.; Adarsh, V.P.; Binod, P. Bioprocess development of 2,3-butanediol production using agro-industrial residues. Bioprocess Biosyst. Eng. 2022, 45, 1527–1537. [Google Scholar] [CrossRef]
- 2,3-Butanediol Market Size, Share, Sale & Industry Demand. Available online: https://www.transparencymarketresearch.com/2-3-butanediol-market.html (accessed on 25 October 2024).
- Bai, Y.; Feng, H.; Liu, N.; Zhao, X. Biomass-Derived 2,3-Butanediol and Its Application in Biofuels Production. Energies 2023, 16, 5802. [Google Scholar] [CrossRef]
- Palaiogeorgou, A.M.; Delopoulos, E.I.M.; Koutinas, A.A.; Papanikolaou, S. Screening Bacterial Strains Capable of Producing 2,3-Butanediol: Process Optimization and High Diol Production by Klebsiella oxytoca FMCC-197. Fermentation 2023, 9, 1014. [Google Scholar] [CrossRef]
- Gergov, E.; Petrova, P.; Arsov, A.; Ignatova, I.; Tsigoriyna, L.; Armenova, N.; Petrov, K. Inactivation of sacB Gene Allows Higher 2,3-Butanediol Production by Bacillus licheniformis from Inulin. Int. J. Mol. Sci. 2024, 25, 11983. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, C.C.; Ujor, V.; Cornish, K.; Ezeji, T.C. Inactivation of the Levansucrase Gene in Paenibacillus polymyxa DSM 365 Diminishes Exopolysaccharide Biosynthesis during 2,3-Butanediol Fermentation. Appl. Environ. Microbiol. 2020, 86, e00196-e20. [Google Scholar] [CrossRef] [PubMed]
- Maina, S.; Prabhu, A.A.; Vivek, N.; Vlysidis, A.; Koutinas, A.; Kumar, V. Prospects on bio-based 2,3-butanediol and acetoin production: Recent progress and advances. Biotechnol. Adv. 2022, 54, 107783. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z. Recent Advances on Production of 2,3-Butanediol Using Engineered Microbes. Biotechnol. Adv. 2019, 37, 569–578. [Google Scholar] [CrossRef]
- Gao, J.; Xu, H.; Li, Q.; Feng, X.; Li, S. Optimization of Medium for One-Step Fermentation of Inulin Extract from Jerusalem Artichoke Tubers Using Paenibacillus polymyxa ZJ-9 to Produce R,R-2,3-Butanediol. Bioresour. Technol. 2010, 101, 7076–7082. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, C.; Li, K.; Wang, Y.; Gao, C.; Ma, C.; Xu, P. Efficient Simultaneous Saccharification and Fermentation of Inulin to 2,3-Butanediol by Thermophilic Bacillus licheniformis ATCC 14580. Appl. Environ. Microbiol. 2014, 80, 6458–6464. [Google Scholar] [CrossRef] [PubMed]
- Tsigoriyna, L.; Arsov, A.; Petrova, P.; Gergov, E.; Petrov, K. Heterologous Expression of Inulinase Gene in Bacillus licheniformis 24 for 2,3-Butanediol Production from Inulin. Catalysts 2023, 13, 841. [Google Scholar] [CrossRef]
- Park, J.M.; Oh, B.-R.; Kang, I.Y.; Heo, S.-Y.; Seo, J.-W.; Park, S.-M.; Hong, W.-K.; Kim, C.H. Enhancement of 2,3-Butanediol Production from Jerusalem Artichoke Tuber Extract by a Recombinant Bacillus sp. Strain BRC1 with Increased Inulinase Activity. J. Ind. Microbiol. Biotechnol. 2017, 44, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-H.; Wang, X.-D.; Dai, J.-Y.; Xiu, Z.-L. Microbial Production of 2,3-Butanediol from Jerusalem Artichoke Tubers by Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 2009, 82, 847–852. [Google Scholar] [CrossRef]
- Li, D.; Dai, J.-Y.; Xiu, Z.-L. A Novel Strategy for Integrated Utilization of Jerusalem Artichoke Stalk and Tuber for Production of 2,3-Butanediol by Klebsiella pneumoniae. Bioresour. Technol. 2010, 101, 8342–8347. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.-Y.; Guan, W.-T.; Xiu, Z.-L. Bioconversion of Inulin to 2,3-Butanediol by a Newly Isolated Klebsiella pneumoniae Producing Inulinase. Process Biochem. 2020, 98, 247–253. [Google Scholar] [CrossRef]
- Ojo, A.O.; De Smidt, O. Lactic Acid: A Comprehensive Review of Production to Purification. Processes 2023, 11, 688. [Google Scholar] [CrossRef]
- Rodrigues, C.; Vandenberghe, L.P.S.; Woiciechowski, A.L.; De Oliveira, J.; Letti, L.A.J.; Soccol, C.R. Production and Application of Lactic Acid. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 543–556. ISBN 978-0-444-63662-1. [Google Scholar]
- Andersen, A.A.; Greaves, J.E. D-Lactic Acid Fermentation of Jerusalem Artichokes. Ind. Eng. Chem. 1942, 34, 1522–1526. [Google Scholar] [CrossRef]
- Cai, Y.; Dun, Y.; Dong, W.; Chen, Q.; Lei, Y.; Hu, J.; Peng, N.; Zhao, S. Direct Lactic Acid Production from Raw Jerusalem Artichoke Powder by Lacticaseibacillus paracasei NJ and the Comparative Transcriptomic Profiling of Inulin and Monosaccharide Utilization. Food Biosci. 2024, 61, 104736. [Google Scholar] [CrossRef]
- Choi, H.-Y.; Ryu, H.-K.; Park, K.-M.; Lee, E.G.; Lee, H.; Kim, S.-W.; Choi, E.-S. Direct Lactic Acid Fermentation of Jerusalem Artichoke Tuber Extract Using Lactobacillus paracasei without Acidic or Enzymatic Inulin Hydrolysis. Bioresour. Technol. 2012, 114, 745–747. [Google Scholar] [CrossRef]
- Ge, X.-Y.; Qian, H.; Zhang, W.-G. Improvement of L-Lactic Acid Production from Jerusalem Artichoke Tubers by Mixed Culture of Aspergillus niger and Lactobacillus sp. Bioresour. Technol. 2009, 100, 1872–1874. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.-Y.; Qian, H.; Zhang, W.-G. Enhancement of L-Lactic Acid Production in Lactobacillus casei from Jerusalem Artichoke Tubers by Kinetic Optimization and Citrate Metabolism. J. Microbiol. Biotechnol. 2010, 20, 101–109. [Google Scholar] [CrossRef]
- Wang, L.; Xue, Z.; Zhao, B.; Yu, B.; Xu, P.; Ma, Y. Jerusalem Artichoke Powder: A Useful Material in Producing High-Optical-Purity l-Lactate Using an Efficient Sugar-Utilizing Thermophilic Bacillus coagulans Strain. Bioresour. Technol. 2013, 130, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wei, P.; Zhu, X.; Cai, J.; Huang, L.; Xu, Z. Efficient Production of L-Lactic Acid from Hydrolysate of Jerusalem Artichoke with Immobilized Cells of Lactococcus lactis in Fibrous Bed Bioreactors. Enzym. Microb. Technol. 2012, 51, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Shin, H.K. The Water-Soluble Extract of Chicory Reduces Glucose Uptake from the Perfused Jejunum in Rats. J. Nutr. 1996, 126, 2236–2242. [Google Scholar] [CrossRef] [PubMed]
- Rubel, I.A.; Pérez, E.E.; Genovese, D.B.; Manrique, G.D. In Vitro Prebiotic Activity of Inulin-Rich Carbohydrates Extracted from Jerusalem Artichoke (Helianthus tuberosus L.) Tubers at Different Storage Times by Lactobacillus paracasei. Food Res. Int. 2014, 62, 59–65. [Google Scholar] [CrossRef]
- Petrova, P.; Velikova, P.; Popova, L.; Petrov, K. Direct Conversion of Chicory Flour into l(+)-Lactic Acid by the Highly Effective Inulinase Producer Lactobacillus paracasei DSM 23505. Bioresour. Technol. 2015, 186, 329–333. [Google Scholar] [CrossRef]
- Xu, Q.; Zang, Y.; Zhou, J.; Liu, P.; Li, X.; Yong, Q.; Ouyang, J. Highly Efficient Production of D-Lactic Acid from Chicory-Derived Inulin by Lactobacillus bulgaricus. Bioprocess Biosyst. Eng. 2016, 39, 1749–1757. [Google Scholar] [CrossRef]
- Max, B.; Salgado, J.M.; Rodríguez, N.; Cortés, S.; Converti, A.; Domínguez, J.M. Biotechnological Production of Citric Acid. Braz. J. Microbiol. 2010, 41, 862–875. [Google Scholar] [CrossRef] [PubMed]
- Currie, J.N. The citric acid fermentation of Aspergillus niger. J. Biol. Chem. 1917, 31, 15–37. [Google Scholar] [CrossRef]
- Reena, R.; Sindhu, R.; Athiyaman Balakumaran, P.; Pandey, A.; Awasthi, M.K.; Binod, P. Insight into Citric Acid: A Versatile Organic Acid. Fuel 2022, 327, 125181. [Google Scholar] [CrossRef]
- Förster, A.; Aurich, A.; Mauersberger, S.; Barth, G. Citric Acid Production from Sucrose Using a Recombinant Strain of the Yeast Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2007, 75, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Drysdale, C.R.; McKay, A.M. Citric Acid Production by Aspergillus niger in Surface Culture on Inulin. Lett. Appl. Microbiol. 1995, 20, 252–254. [Google Scholar] [CrossRef]
- Lotfy, W.; Ghanem, K.; Elhelow, E. Citric Acid Production by a Novel Aspergillus niger Isolate: II. Optimization of Process Parameters through Statistical Experimental Designs. Bioresour. Technol. 2007, 98, 3470–3477. [Google Scholar] [CrossRef]
- Rakicka, M.; Wolniak, J.; Lazar, Z.; Rymowicz, W. Production of High Titer of Citric Acid from Inulin. BMC Biotechnol. 2019, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Rakicka, M.; Lazar, Z.; Rywińska, A.; Rymowicz, W. Efficient Utilization of Inulin and Glycerol as Fermentation Substrates in Erythritol and Citric Acid Production Using Yarrowia lipolytica Expressing Inulinase. Chem. Pap. 2016, 70, 1452–1459. [Google Scholar] [CrossRef]
- Förster, A.; Jacobs, K.; Juretzek, T.; Mauersberger, S.; Barth, G. Overexpression of the ICL1 Gene Changes the Product Ratio of Citric Acid Production by Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2007, 77, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Chi, Z.; Liu, G.-L.; Wang, F.; Madzak, C.; Chi, Z.-M. Inulin Hydrolysis and Citric Acid Production from Inulin Using the Surface-Engineered Yarrowia lipolytica Displaying Inulinase. Metab. Eng. 2010, 12, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qiu, Y.; Xu, H.; Wang, R.; Lei, P. Recent Advances in Poly-(γ-Glutamic Acid) Production by Microbial Fermentation. In Microbial Production of High-Value Products; Microbiology Monographs; Rehm, B.H.A., Wibowo, D., Eds.; Springer: Cham, Switzerland, 2022; Volume 37, pp. 237–269. ISBN 978-3-031-06599-6. [Google Scholar]
- Yoon, S.H.; Hwan Do, J.; Yup Lee, S.; Nam Chang, H. Production of poly-γ-glutamic acid by fed-batch culture of Bacillus licheniformis. Biotechnol. Lett. 2000, 22, 585–588. [Google Scholar] [CrossRef]
- Da Silva, S.B.; Cantarelli, V.V.; Ayub, M.A.Z. Production and Optimization of Poly-γ-Glutamic Acid by Bacillus subtilis BL53 Isolated from the Amazonian Environment. Bioprocess Biosyst. Eng. 2014, 37, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Candela, T.; Fouet, A. Poly-gamma-glutamate in Bacteria. Mol. Microbiol. 2006, 60, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Guo, Y.; Liu, J.; Qiu, H.; Zhao, M.; Zou, W.; Li, S. Microbial Synthesis of Poly-γ-Glutamic Acid: Current Progress, Challenges, and Future Perspectives. Biotechnol. Biofuels 2016, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Sirisansaneeyakul, S.; Cao, M.; Kongklom, N.; Chuensangjun, C.; Shi, Z.; Chisti, Y. Microbial Production of Poly-γ-Glutamic Acid. World J. Microbiol. Biotechnol. 2017, 33, 173. [Google Scholar] [CrossRef]
- Shih, I.L.; Van, Y.T.; Yeh, L.C.; Lin, H.G.; Chang, Y.N. Production of a Biopolymer Flocculant from Bacillus licheniformis and Its Flocculation Properties. Bioresour. Technol. 2001, 78, 267–272. [Google Scholar] [CrossRef]
- Elbanna, K.; Alsulami, F.S.; Neyaz, L.A.; Abulreesh, H.H. Poly (γ) Glutamic Acid: A Unique Microbial Biopolymer with Diverse Commercial Applicability. Front. Microbiol. 2024, 15, 1348411. [Google Scholar] [CrossRef] [PubMed]
- Buescher, J.M.; Margaritis, A. Microbial Biosynthesis of Polyglutamic Acid Biopolymer and Applications in the Biopharmaceutical, Biomedical and Food Industries. Crit. Rev. Biotechnol. 2007, 27, 1–19. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhu, Y.; Zhan, Y.; Zhang, Y.; Sha, Y.; Zhan, Y.; Xu, Z.; Li, S.; Feng, X.; Xu, H. Systematic Unravelling of the Inulin Hydrolase from Bacillus amyloliquefaciens for Efficient Conversion of Inulin to Poly-(γ-Glutamic Acid). Biotechnol. Biofuels 2019, 12, 145. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhu, Y.; Sha, Y.; Lei, P.; Luo, Z.; Feng, X.; Li, S.; Xu, H. Development of a Robust Bacillus amyloliquefaciens Cell Factory for Efficient Poly(γ-Glutamic Acid) Production from Jerusalem Artichoke. ACS Sustain. Chem. Eng. 2020, 8, 9763–9774. [Google Scholar] [CrossRef]
- Qiu, Y.; Sha, Y.; Zhang, Y.; Xu, Z.; Li, S.; Lei, P.; Xu, Z.; Feng, X.; Xu, H. Development of Jerusalem Artichoke Resource for Efficient One-Step Fermentation of Poly-(γ-Glutamic Acid) Using a Novel Strain Bacillus amyloliquefaciens NX-2S. Bioresour. Technol. 2017, 239, 197–203. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, Y.; Zhu, Y.; Sha, Y.; Xu, Z.; Feng, X.; Li, S.; Xu, H. Improving Poly-(γ-Glutamic Acid) Production from a Glutamic Acid-Independent Strain from Inulin Substrate by Consolidated Bioprocessing. Bioprocess Biosyst. Eng. 2019, 42, 1711–1720. [Google Scholar] [CrossRef]
- Sha, Y.; Zhang, Y.; Qiu, Y.; Xu, Z.; Li, S.; Feng, X.; Wang, M.; Xu, H. Efficient Biosynthesis of Low-Molecular-Weight Poly-γ-Glutamic Acid by Stable Overexpression of PgdS Hydrolase in Bacillus amyloliquefaciens NB. J. Agric. Food Chem. 2019, 67, 282–290. [Google Scholar] [CrossRef]
- Singh, R.S.; Singh, T.; Hassan, M.; Kennedy, J.F. Updates on inulinases: Structural aspects and biotechnological applications. Int. J. Biol. Macromol. 2020, 164, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Chauhan, K.; Kennedy, J.F. A panorama of bacterial inulinases: Production, purification, characterization and industrial applications. Int. J. Biol. Macromol. 2017, 96, 312–322. [Google Scholar] [CrossRef]
- Lara-Fiallos, M.; Ayala Chamorro, Y.T.; Espín-Valladares, R.; DelaVega-Quintero, J.C.; Olmedo-Galarza, V.; Nuñez-Pérez, J.; Pais-Chanfrau, J.-M.; Martínez, A.P. Immobilised Inulinase from Aspergillus niger for Fructose Syrup Production: An Optimisation Model. Foods 2024, 13, 1984. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.L.; da Silva, S.P.; Converti, A.; Porto, T.S. Production, Biochemical Characterization, and Kinetic/Thermodynamic Study of Inulinase from Aspergillus terreus URM4658. Molecules 2022, 27, 6418. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Gerliani, N.; Aïder, M. Kluyveromyces marxianus: An emerging yeast cell factory for applications in food and biotechnology. Int. J. Food Microbiol. 2020, 333, 108818. [Google Scholar] [CrossRef]
- Belmonte-Izquierdo, Y.; Salomé-Abarca, L.F.; González-Hernández, J.C.; López, M.G. Fructooligosaccharides (FOS) Production by Microorganisms with Fructosyltransferase Activity. Fermentation 2023, 9, 968. [Google Scholar] [CrossRef]
- Singh, R.S.; Singh, R.P.; Kennedy, J.F. Recent insights in enzymatic synthesis of fructooligosaccharides from inulin. Int. J. Biol. Macromol. 2016, 85, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Chavan, A.R.; Singh, A.K.; Gupta, R.K.; Nakhate, S.P.; Poddar, B.J.; Gujar, V.V.; Purohit, H.J.; Khardenavis, A.A. Recent trends in the biotechnology of functional non-digestible oligosaccharides with prebiotic potential. Biotechnol. Gen. Eng. Rev. 2023, 39, 465–510. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Kim, H.S. Continuous production of gluconic acid and sorbitol from Jerusalem artichoke and glucose using an oxidoreductase of Zymomonas mobilis and inulinase. Biotechnol. Bioeng. 1992, 39, 336–342. [Google Scholar] [CrossRef]
- Liang, Z.X.; Li, L.; Li, S.; Cai, Y.H.; Yang, S.T.; Wang, J.F. Enhanced propionic acid production from Jerusalem artichoke hydrolysate by immobilized Propionibacterium acidipropionici in a fibrous-bed bioreactor. Bioprocess Biosyst. Eng. 2012, 35, 915–921. [Google Scholar] [CrossRef]
- Gunnarsson, I.B.; Karakashev, D.; Angelidaki, I. Succinic acid production by fermentation of Jerusalem artichoke tuber hydrolysate with Actinobacillus succinogenes 130Z. Ind. Crop. Prod. 2014, 62, 125–129. [Google Scholar] [CrossRef]
- Xia, J.; Xu, J.; Liu, X.; Xu, J.; Wang, X.; Li, X. Economic co-production of poly (malic acid) and pullulan from Jerusalem artichoke tuber by Aureobasidium pullulans HA-4D. BMC Biotechnol. 2017, 17, 20–30. [Google Scholar] [CrossRef] [PubMed]


| Inulin-Containing Plant | Latin Name | Inulin-Containing Plant Part | % Inulin 1 | Reference |
|---|---|---|---|---|
| Agave | Agave americana, Agave tequilana | Lobes | 7.0–12.0 | [12] |
| Asparagus | Asparagus officinalis | Stems | 2.0–3.0 | [8,13] |
| Barley | Hordeum vulgare | Seed | 0.5–1.5 | [14] |
| Burdock | Arctium sp. | Roots | 3.5–4.0 | [14] |
| Chicory | Cichorium intybus L. | Root | 14.9–68.0 | [15,16] |
| Dahlia | Dahlia pinnata | Tuber | 10–20.0 | [13] |
| Dandelion | Taraxacum officinale L. | Root | 12.0–15.0 | [11,14] |
| Garlic | Allium sativum | Cloves | 16.6–24.9 | [7,14] |
| Jerusalem artichoke | Helianthus tuberosus L. | Tuber | 14.0–23.0 | [16] |
| Leek | Allium ampeloprasum | Bulb | 3.0–10.0 | [14] |
| Onion | Allium cepa L. | Bulb | 3.1–6.0 | [14] |
| Sweet leaf | Stevia rebaudiana | Root | 18.0–23.0 2 | [17] |
| Salsify | Tragopogon porrifolius L. | Root | 4.0–11.0 | [13] |
| Yacón | Polymnia sonchifolia | Root | 3.1–19.0 | [14] |
| Inulin Source | Pretreatment | Fermentation Mode | Species/Strain | Ethanol (g/L) | Yield (g/g) | Conversion (%) | Productivity (g/L/h) | Reference |
|---|---|---|---|---|---|---|---|---|
| JAT | Enzymatic | Batch | S. cerevisiae | 18.0 | ND 1 | 71.8 | ND | [57] |
| JAT | Enzymatic | Batch | Saccharomyces sp. W0 | 14.6 | 0.384 | 98.8 | ND | [58] |
| JAT extract (50%) | Enzymatic | SSF | Saccharomyces sp. W0 | 12.1 | 0.319 | 96.3 | ND | [58] |
| JAT powder | No | SSF, FB 3 | S. cerevisiae 6525 | 84.3 | 0.453 | 88.6 | ND | [59] |
| Chicory 2 | No | CBP 4 | S. cerevisiae JZD-InuMKCP | 95.19 | 0.486 | 95.0 | 3.20 | [60] |
| JAT | No | CBP 4 | S. cerevisiae JZD-InuMKCP | 81.76 | 0.469 | 91.7 | 3.13 | [60] |
| JAT | Acidic/Imidazole | Batch | S. cerevisiae | 137.4 | 0.421 | 92.47 | 2.38 | [61] |
| D. sansibarensis | Acidic/0.2 N H2SO4 | Batch | I. terricola | 56.0 | 0.49 | 95.0 | 1.3 | [62] |
| P. kaurabassana | Acidic/0.2 N H2SO4 | Batch | I. terricola | 35.0 | 0.49 | 96.0 | 0.8 | [62] |
| JAT stalks | Acidic/0.05 N HCl | Batch | S. cerevisiae | 32.5 | 0.49 | 88–92 | ND | [63] |
| JAT stalks | Acidic/0.05 N HCl | Batch | K. marxianus | 32.0 | 0.49 | 96–100 | ND | [64] |
| Inulin Source | Pretreatment | Fermentation Mode | Species | Butanol (g/L) | Conversion (%) | Yield (g/g) | Productivity (g/L/h) | Reference |
|---|---|---|---|---|---|---|---|---|
| JAT | Enzymatic | CBP 1 | Clostridium sp. NJ4 | 13.25 | ND 2 | ND | 0.09 | [78] |
| JAT 3 | Enzymatic | CBP 1 | Clostridium sp. NJ4 | 14.4 (24.6) 4 | ND | ND | ND | [78] |
| JAT | Acid | Batch | C. acetobutylicum L7 | 4.5 | ND | 0.048 | 0.028 | [79] |
| JAT 5 | Acid | Batch | C. acetobutylicum L7 | 12.8 | ND | 0.122 | 0.089 | [79] |
| JAT | Steam and pressure | Batch | C. saccharobutylicum P262 | 8.6 (12.5) 4 | ND | 0.32 | 0.21 | [80] |
| Chicory roots 6 | Steam and pressure | Batch | C. saccharobutylicum P262 | 4.88 (8.5) 4 | ND | 0.33 4 | 0.12 | [80] |
| JAT | Enzymatic | Batch | C. saccharobutylicum DSM 13864 | 9.6 | ND | 0.33 4 | ND | [81] |
| JAT | Acid (H2SO4) | Batch | C. saccharobutylicum DSM 13864 | 9.8 (15.1) 3 | 80.0 | 0.31 | 0.25 | [81] |
| JA juice 7 | Acid | Batch | C. acetobutylicum L7 | 8.67 | 93.6 | 0.192 | ND | [84] |
| JA juice 7 | Acid | Batch | C. acetobutylicum L7 | 11.21 | 94.8 | ND | [84] |
| Inulin Source | Pretreatment | Fermentation Mode | Species/Strain | 2,3-BD (g/L) | Yield (g/g) | Productivity (g/L/h) | Reference |
|---|---|---|---|---|---|---|---|
| Frutafit® CLR | No | SSF | B. licheniformis ΔsacB | 128.7 | 0.429 | 1.65 | [91] |
| JAT | No | Batch | P. polymyxa ZJ-9 | 36.92 | ND | 0.88 | [95] |
| JAT | Enzymatic | Fed-batch SSF | B. licheniformis ATCC 14580 | 103.0 | ND | 3.4 | [96] |
| Frutafit® HD | No | SSF | B. licheniformis T26 | 18.5 | 0.1 | ND | [97] |
| JAT | Enzymatic | Fed-batch | Bacillus sp. BRC1 | 28.6 | ND | ND | [98] |
| JAT | Enzymatic | SSF, FB | K. pneumoniae | 81.58 | ND | ND | [99] |
| JAT | Enzymatic | Fed-batch SSF | K. pneumoniae | 91.63 | ND | ND | [99] |
| JAT | Acid | Batch | K. pneumoniae | 80.5 * | ND | ND | [100] |
| JAT stalks | No | Batch | K. pneumoniae H3 | 80.4 * | 0.426 * | 2.23 * | [101] |
| Inulin Source | Fermentation Type | Species/Strain | Yield (g/L) | Reference |
|---|---|---|---|---|
| Chicory flour | Surface fermentation | A. niger ATCC 9142 | 14 | [113] |
| Chicory flour | Air-passed surface fermentation | A. niger ATCC 9142 | 29 | [113] |
| JAT | Repeated-batch | Y. lipolytica AWG7 INU 8 | 200 | [115] |
| Inulin and glycerol | Fed-batch | Y. lipolytica Wratislavia K1 INU 6 | 105.2 | [116] |
| JAT | Flask-batch | Y. lipolytica SWJ-1b | 77.9 (5.3) * | [118] |
| JAT | 2 L fermentation system | Y. lipolytica SWJ-1b | 68.9 (4.1) * | [118] |
| Inulin Source | Fermentation Mode | Species/Strain | Yield (g/L) | Productivity (g/L/h) | Reference |
|---|---|---|---|---|---|
| JAT | Batch | B. amyloliquefaciens NB | 18.95 | 0.29 | [134] |
| JAT | Fed-batch | B. amyloliquefaciens NB | 32.14 | ND | [135] |
| JAT | Batch | B. amyloliquefaciens NX-2S | 6.85 | ND | [136] |
| JAT | Batch | B. amyloliquefaciens NX-2S | 39.4 | 0.43 | [136] |
| JAT | Fed-batch | B. amyloliquefaciens NX-2S154 | 14.83 | ND | [137] |
| JAT | Batch | B. amyloliquefaciens NB | 17.62 1 | ND | [138] |
| JAT | SSF + inulinase (40 IU/g inulin) | B. amyloliquefaciens NX-2S154 | 18.54 | ND | [139] |
| JAT | Repeated batch | B. amyloliquefaciens NX-2S154 | 19.93 | 0.28 | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsigoriyna, L.; Stefanov, S.; Armenova, N.; Petrova, P.; Petrov, K. Microbial Conversion of Inulin to Valuable Products: The Biorefinery Concept. Fermentation 2024, 10, 640. https://doi.org/10.3390/fermentation10120640
Tsigoriyna L, Stefanov S, Armenova N, Petrova P, Petrov K. Microbial Conversion of Inulin to Valuable Products: The Biorefinery Concept. Fermentation. 2024; 10(12):640. https://doi.org/10.3390/fermentation10120640
Chicago/Turabian StyleTsigoriyna, Lidia, Stefan Stefanov, Nadya Armenova, Penka Petrova, and Kaloyan Petrov. 2024. "Microbial Conversion of Inulin to Valuable Products: The Biorefinery Concept" Fermentation 10, no. 12: 640. https://doi.org/10.3390/fermentation10120640
APA StyleTsigoriyna, L., Stefanov, S., Armenova, N., Petrova, P., & Petrov, K. (2024). Microbial Conversion of Inulin to Valuable Products: The Biorefinery Concept. Fermentation, 10(12), 640. https://doi.org/10.3390/fermentation10120640







