Abstract
This study aimed to develop bioactive bi-layer edible films based on starch (primary layer) and LAB-fermented whey and/or mango pulp powder solutions (secondary layer). Bioactive bi-layer edible films were evaluated for their physical properties, mechanical properties, antioxidant capacity, and Lactobacillus rhamnosus availability for 28 days (4 and 20 °C). Selected bioactive bi-layer edible film was applied to sushi to evaluate its sensory acceptance. The results indicated that bi-layer edible films based on LAB-fermented whey/mango solutions presented a higher quantity of phenolic compounds (95.87–107.67 mg GAE/100 g) and higher antioxidant capacity (74.84–77.64%). In addition, the higher viability (106–107 CFU/g) of L. rhamnosus after edible film production was obtained in those formulated with whey. After the storage period, the antioxidant capacity of all edible films was significantly affected by the storage time, while edible films containing whey in their formulation and stored at 4 °C had a L. rhamnosus count higher than 6 log cycles, which is the minimum required threshold to exert its beneficial effects in humans. The sushi covered with the selected bi-layer edible film was well accepted by the consumers, showing acceptance values between “I like it” and “I like it much”. Therefore, the developed bi-layer edible films can serve as an alternative for adding health-promoting compounds to sushi with an adequate sensory acceptance of the consumers.
1. Introduction
Food packaging is one of the most important components of the food supply chain and is becoming an elemental factor in making decisions about the food manufacturing process [1]. Although the main characteristic of food packaging is to provide mechanical protection against physical, chemical, and microbiological damages, nowadays, it is considered the silent salesman because it presents valuable information like name, brand, labeling, claims, category, security, nutritional value, etc., being a communication vehicle between food processors and consumers [2,3]. Packaging materials such as glass, cartons, metal, plastic, and wood are commonly used in food products; however, due to their convenience and performance, low cost, versatility, barrier properties, and lightweight, plastic materials are the most used type of packaging [4]. The use of plastic material for food packaging presents significant environmental and health drawbacks because of their non-biodegradable and non-renewable nature and, from a health standpoint, the possibility of adding harmful chemical substances [5,6]. Therefore, the development of biopolymer-based functional films for packaging applications is gaining popularity due to their various advantages, for instance, biodegradability, biocompatibility, and non-toxic characteristics, over traditional packaging [7].
Recently, edible films and coatings have been developed and studied as alternative primary packaging because they can be consumed with food or easily degraded [8,9]. Edible films are a preformed thin layer (<0.3 mm) obtained from edible materials used for protecting food products from physical, chemical, and biological hazards [10]. Among edible materials, polysaccharides, proteins, and lipids, alone or in combination, have been used as the base of film formulation, and their uses depend on the food product to be applied [11]. In this sense, the protein-based edible films have good mechanical resistance and poor water vapor barrier properties, while polysaccharides improve the gas barrier properties of edible films [11]. Therefore, composite edible film formulation or the use of more than one edible layer to enhance the mechanical and physical characteristics of the edible film is an alternative method to increase the application of edible films to food products [12,13]. Moreover, edible films have also been used as bioactive or health-promoting compound carriers [8]. These bioactive edible films are gaining attention due to the additional benefits they provide to food consumers [6] and even as an intelligent indicator of food spoilage [14]. Among health-promoting compounds added to the edible films, antioxidants, antimicrobials, plant extracts, essential oils, probiotics, prebiotics, enzymes, and pigments are the most investigated compounds [11].
Mango is known as the king of fruits because it is the most popular fruit in tropical regions. It belongs to the Mangifera genus and is native to India and Southeast Asia [15]. However, nowadays, it is cultivated in different parts of the world, such as America, Africa, Australia, and Europe [16]. Although mango fruit is widely consumed due to its sensory (juicy and pleasant flavor) and nutritional properties, the health-beneficial compounds (phenolic compounds, carotenoids, fiber, and vitamins) that both its pulp and by-products contain are making it gain more consumers [17]. In this sense, mango fruit phytochemicals have proven benefits against some diseases, such as cardiovascular, type 2 diabetes, metabolic syndrome, cancer, etc. [17,18]. In addition, recent studies have indicated that mango has polyphenols and fiber, which can be used as a prebiotic for probiotic microorganisms, reducing inflammation processes and other symptoms associated with chronic intestinal diseases [19,20,21]. Therefore, looking for the incorporation of mango pulp in a probiotic matrix such as edible film is an attractive way to integrate its several benefits. This study aimed to develop and characterize a bi-layer edible film composed of a starch edible film and LAB-fermented whey/mango film and evaluate its sensory acceptance when the selected edible film was applied to sushi.
2. Materials and Methods
2.1. Vegetal Material
Mangoes (Mangifera indica) cv. Manila were obtained from a local supermarket in Puebla, Mexico. Ripe fruits with brilliant color, free of apparent physical and microbiological damage, were selected, washed, and manually peeled using a stainless steel knife, and cut in halves for drying (model 3926, Excalibur TB, Sacramento, CA, USA) at 55 °C for 24 h (moisture content = 18.82 ± 1.20%). Dried mangoes were ground, sieved (180 µm), and stored in glass bottles covered with aluminum at 20 ± 2 °C for further use.
2.2. Reagents
Reagents, broths, and agars were obtained from Sigma-Aldrich, Inc. (Toluca, Mexico), J.T. Baker (Mexico City, Mexico), and BD Bioxon (Mexico City, Mexico), respectively. Whey powder (10% protein, 75% carbohydrates, 1.5% fat, and 1.15% of sodium) and potato starch (0.05% protein and 80.70% carbohydrates) were obtained from Food Technologies Trading (Mexico City, Mexico) and Fabsa (Mexico City, Mexico), respectively.
2.3. Fermentation Process of Whey and/or Mango Solutions
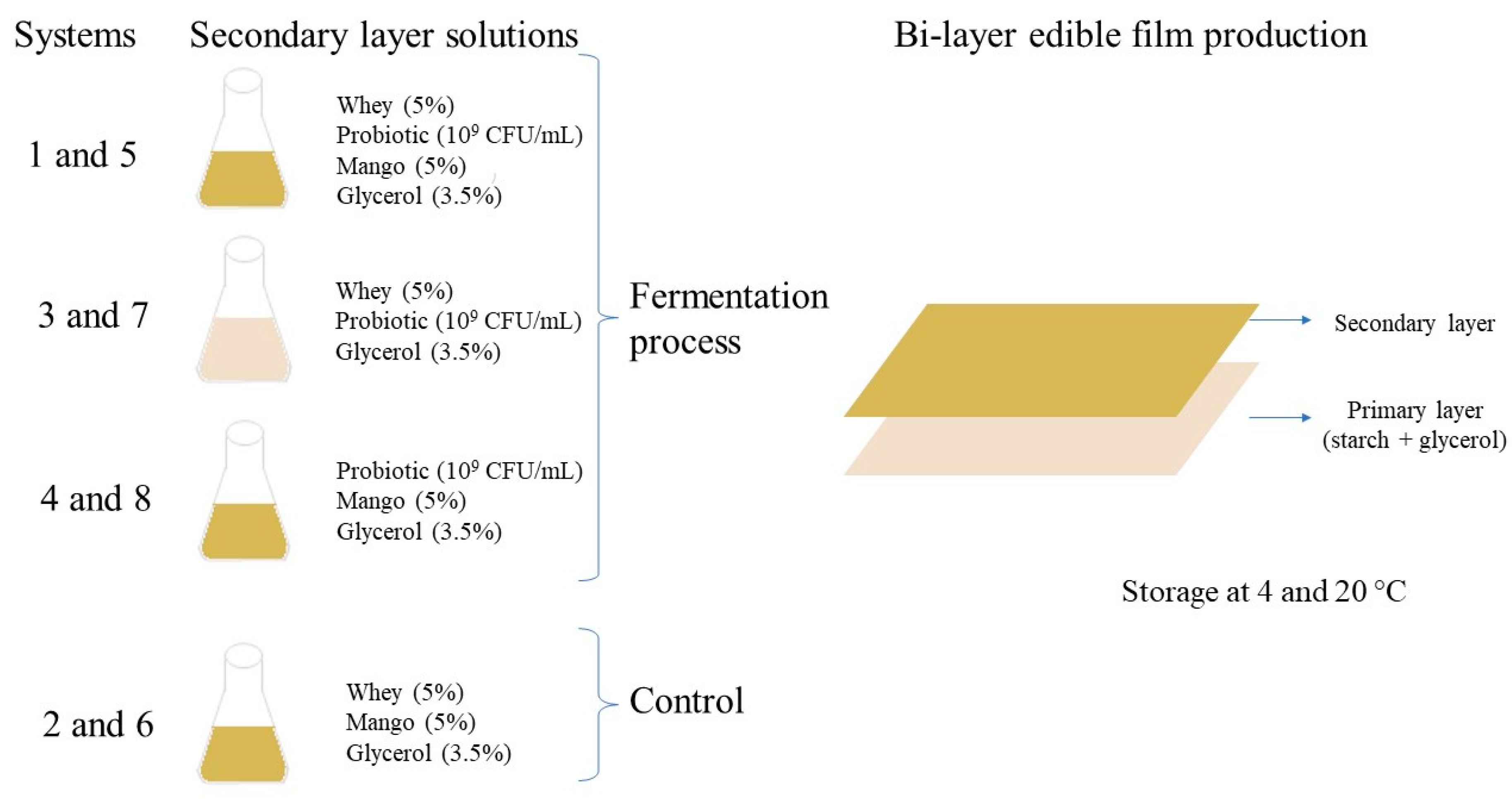
Figure 1 shows the formulation solutions to produce the secondary layer of edible films. All solutions were sterilized at 121 °C for 15 min, and systems 1, 3, 4, 5, 7, and 8 were inoculated with L. rhamnosus NRRL B442 (1 × 109 CFU/mL) previously cultured in MRS broth at 37 ± 2 °C for 24 h (the inoculated broth was plated on MRS agar and incubated at 37 ± 2 °C under an anaerobic environment to know its microbial count). After incubation (37 ± 2 °C for 24 h), the solutions with L. rhamnosus were immediately used for bi-layer edible film production. Systems 2 and 6 were non-inoculated solutions and were used as controls.
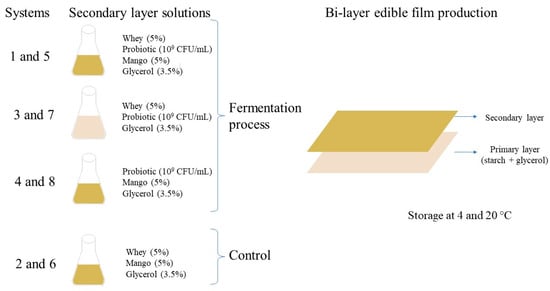
Figure 1.
Bi-layer edible films production.
2.4. Production of Bi-Layer Edible Films
The bi-layer edible films were produced in two steps: (1) The primary filmogenic solution (edible film 1) was made by mixing starch (5%) and glycerol (3.5%) with distilled water and then heated to 70 °C for 5 min. The solution was sterilized (at 121 °C for 15 min), poured (15 g) into wax paper (118 cm2), and dried at 40 °C for 2 h. (2) The secondary filmogenic solution (edible film 2) was then poured (60 g) on dried starch -based edible film (Figure 1) and dried again for 24 h at 40 °C (constant weight). Then, edible films were gently removed and stored (28 days at 45± 5 RH) in LLDPE (linear low-density polyethylene) bags at 4 ± 1 °C (systems 1, 2, 3, and 4) and 20 ± 2 °C (systems 5, 6, 7, and 8) to study their physical, mechanical, antioxidant, and probiotic survival stability. At the end of the storage, the edible film that maintained its quality characteristics was used to cover sushi for studying its sensory acceptance.
2.5. Moisture
The moisture content of bi-layer edible films was determined by oven-drying them at 105 °C until they reached a constant weight, according to the 925.25 methodology of the AOAC [22] Official Methods.
2.6. Color
The L*, a*, and b* color parameters of bi-layer edible films were evaluated using a Precise Colorimeter Reader (TCR 200, TIME High Technology, Beijing, China). The measurement was taken on the top of each edible film under room conditions of light and temperature. The L*, a*, and b* color parameters were used to calculate the total color change (∆E) using the following equation.
where , , and and , , and are the color parameters at the beginning and at the end of storage, respectively.
2.7. Thickness
The thickness (Th) of edible films was obtained by measuring five random areas using a millimeter micrometer (IP54, Qfun, Xi´an, China) with a sensitivity of 0.01 mm [23].
2.8. Water Vapor Permeability
The water vapor permeability (WVP) of edible films was measured following the ASTM E96 method [24]. Briefly, the bi-layer edible film was used to seal a glass vessel containing distilled water. The sealed vessel was placed at 4 ± 1 °C (45 ± 2% RH) and weighed every 1 h for 10 h. The WVP was calculated using the following equation.
where WVTR is the water vapor transmission rate (g/h m2), Th is the thickness (mm), and ∆P is the difference between partial water vapor pressures (kPa).
2.9. Mechanical Properties
Tensile strength (TS) and elongation at break (EAB) were evaluated using a texture analyzer (EZ-test, EZ-SX, Shimadzu Corp, Kyoto, Japan). The rectangles of bi-layer edible films (3.5 cm × 6.0 cm) were held tightly between mechanical grips at an initial distance of 3 cm. The force (MPa) and deformation (mm) were recorded during extension at 60 mm/min. Mechanical properties were determined using the interface software TRAPEZIUM X Material Testing Operation Software V 1.4.0. Both mechanical properties were calculated following the ASTM D882 method [25].
2.10. Total Phenolic Compounds and Antioxidant Capacity
The extracts of bi-layer edible films were conducted to evaluate the total phenolic compounds and antioxidant capacity. Five grams of edible film was blended with 100 mL of distilled water, cotton-filtered, and immediately used for antioxidant assays. Total phenolic compounds were evaluated following the methodology reported by Hernández-Carranza et al. [26]. In brief, 1 mL of extract was mixed with 1 mL of Folin–Ciocalteau reagent (0.1 N), and 3 min later, 1 mL of Na2CO3 (0.05%) was added. The mixture was allowed to stand for 30 min in a dark environment and read at 765 nm using a UV-Vis spectrophotometer (Jenway model 6405, Staffordshire, UK). Results were expressed as mg GAE (gallic acid equivalent) per 100 g of edible film using a standard curve of gallic acid. On the other hand, the antioxidant capacity was evaluated following the methodology proposed by Hernández-Carranza et al. [26]. One milliliter of sample was mixed with 1 mL of DPPH solution (0.004%) and allowed to stand for 30 min in a dark environment at room temperature. The antioxidant capacity was measured at 517 nm using a UV–Vis spectrophotometer. The results were expressed as mg of Trolox per 100 g of edible film using a standard curve of Trolox.
2.11. Probiotic Count
Probiotic microorganisms were quantified in bi-layer edible films formulated with fermented whey and/or mango solutions, following the methodology proposed by He et al. [27] with some modifications. Briefly, 1 g of edible film was placed in a sterilized bag with 9 mL of peptone water (0.1% w/v) and processed for 5 min using stomacher equipment (model 400, Seward, West Sussex, UK). Then, the sample was diluted until an appropriate count was reached (30–300 CFU/g), plated on MRS agar, and stored at 37 ± 2 °C under an anaerobic environment. A microbial count was performed after 24 h of incubation.
2.12. Bi-Layer Edible Film Application and Sensory Acceptance
The selected bi-layer edible film was applied to commercial sushi made of rice, avocado, cucumber, and cream cheese (Figure 4) to evaluate consumer acceptance. Sushi covered with the bi-layer edible film was evaluated for its sensory properties using a 7-point hedonic scale, where 1 means “dislike very much” and 7 means “like very much” [28]. A 1 cm slice of sushi was provided to fifty students from the Benemerita Universidad Autonoma de Puebla (20 years old on average, 30 women and 20 men) who consume sushi frequently (more than once a week) and do not have any problems with participating in the test. The odor, flavor, texture, and overall acceptance of sushi were evaluated after 28-day storage of the bi-layer edible film (4 ± 1 °C).
2.13. Statistical Analysis
Bi-layer edible films formulation was performed in triplicate, and each test was conducted in duplicate. Results were analyzed with analysis of variance (α = 0.05) using the Tukey’s test of Minitab 15 software (Minitab Inc., State College, PE, USA).
3. Results and Discussion
3.1. Physical and Mechanical Properties of Formulated Bi-Layer Edible Films
Table 1 shows the physical and mechanical properties of bi-layer edible films. Evidently, in the beginning of the storage, the moisture content (23.87 to 29.18%) of bi-layer edible films was higher than edible films (11.37–13.93%) based on starch and LAB-fermented whey solution [6], which may be due to the hydrophilic compounds (carbohydrates and fiber from the mango powder and proteins from whey) that interact with water molecules [29,30]. Moreover, both mango pulp powder and whey significantly (p < 0.05) increased the Th of edible films (systems 1, 2, 5, and 6), while the addition of probiotic microorganisms did not affect this mechanical property. In this sense, different studies have indicated that probiotics do not affect the Th of edible films; however, prebiotics and fiber affect it significantly [31,32]. On the other hand, none of the edible film components (whey, mango pulp powder, and probiotic) affected the WVP (p > 0.05); therefore, it is possible to infer that this property depends on the starch-based (primary) film, which is presented in all the edible films. Notably, the values of WVP, i.e., 0.36–6.46 × 10−10 gm (sm2Pa)−1, are lower than those reported for other edible films formulated with whey protein isolate- and carrageenan-based edible films added with different probiotics, i.e., 2.62–7.76 × 10−9 gm (sm2Pa)−1 [33]. The WVP is expected to be lower because increasing the number of layers in edible films reduces the water transmission through them [34]. Elongation at break (EAB) did not present any tendency about the effect of film components, showing values in the range of 19.20 to 42.36%, which are very similar to the results obtained by Azeredo et al. [35], who reported values of 31.54–42.07% in edible films made of mango puree reinforced with cellulose nanofiber and whey solution fermented by L. rhamnosus. Contrary to the EAB, the components of edible films affected the tensile strength (TS). Evidently, the edible films without mango pulp in their formulation (systems 3 and 7) presented a lower tensile strength (0.37–0.38 MPa). In this sense, the interaction between whey protein and carbohydrates from mango pulp and starch through hydrogen bonds and electrostatic interactions between amino and hydroxyl groups may increase the TS [36].

Table 1.
Physical and mechanical properties of formulated bi-layers edible films at the beginning and after 28 days of storage a.
During storage, although a reduction in moisture content was detected, no significant effect of time and temperature was observed, whereas Th decreased (p < 0.05) with storage time, regardless of temperature. Ramos et al. [37] pointed out that the reduction in Th may be associated with a weakness in the electrostatic repulsions of the edible film components, causing the aggregation phenomenon. Interestingly, the WVP of edible films was affected by the storage temperature, showing a reduction (p < 0.05) in edible films stored at 20 °C, which may be attributed to the increase in the aging process and the consequence aggregation of protein–carbohydrate interaction in the film matrix [38]. On the other hand, after 28 days of storage, both EAB and TS showed different behaviors, while EAB was reduced, and TS was increased, being significant in edible films formulated with whey and mango pulp powder without a fermentation process (systems 2 and 6), which indicates that fermentation maintained the mechanical properties of edible films during the storage. A similar behavior was reported by Hernández-Carranza et al. [6], who evaluated the stability of LAB-fermented starch-based edible films using two probiotic microorganisms (L. acidophilus and L. rhamnosus). They indicated that edible films fermented with L. rhamnosus did not present any changes in TS and EAB during 14 days of storage at 4 °C using LLDPE as a secondary package. The color characteristics of edible films are presented in Table 2. Edible films added with mango pulp powder (systems 1, 2, 4, 5, 6, and 8) showed L*, a*, and b* color parameters in the range of 67.51 to 76.17, −2.50 to 0.65, and 36.94 to 50.46, respectively, indicating a medium light shade of yellow (color code: #d1ba77) to a shade of yellow (color code: #c8af55) color of the hex color standard, which is expected due to the color characteristics of mango pulp. Viana et al. [39] indicated similar values for L* (63.55), a* (4.95), and b* (56.46) color parameters in edible films added with mango pulp. The variation in color parameters may be due to the amount of mango used; for instance, Rojas-Bravo et al. [40] indicated that as the amount of mango peel powder in the edible film formulation increased, a decrease in the luminosity (tends to darkness) and increments in a* (tends to red) and b* (tends to yellow) were observed. On the other hand, edible films without mango pulp (L*: 88.97–90.34, a*: 0.99–1.45, and b*: 11.35–12.06) displayed a very light shade of brown color (#ecdec9 code of the hex color standard) provided by whey and starch. A similar color code (a light shade of brown) was reported by Hernández-Carranza et al. [6] in edible films formulated with starch and LAB-fermented whey solution. Storage time significantly affected (p < 0.05) the color parameters of edible films, showing a browning process (the reduction in L* and b* and an increase in a* color parameters). This change was higher in the edible films that had mango pulp and was caused by the polyphenol oxidase enzyme, the main enzyme responsible for the browning process in fruits and vegetables [40]. The color change due to the storage time was calculated by the total color change (∆E), where a higher variation (>12.99) was observed in the bi-layer edible films formulated with mango pulp powder, while those films without mango presented less color change (6.41–7.72). Moreover, according to Piccirilli et al. [41], changes in color parameters of edible films formulated with proteins (ε-amino group in lysine) and carbohydrates (lactose) during storage could also be due to Maillard reactions.

Table 2.
Color parameters of formulated bi-layers edible films at the beginning and after 28 days of storage a.
3.2. Effect of Storage Condition on Health-Promoting Compounds of Bi-Layer Edible Films
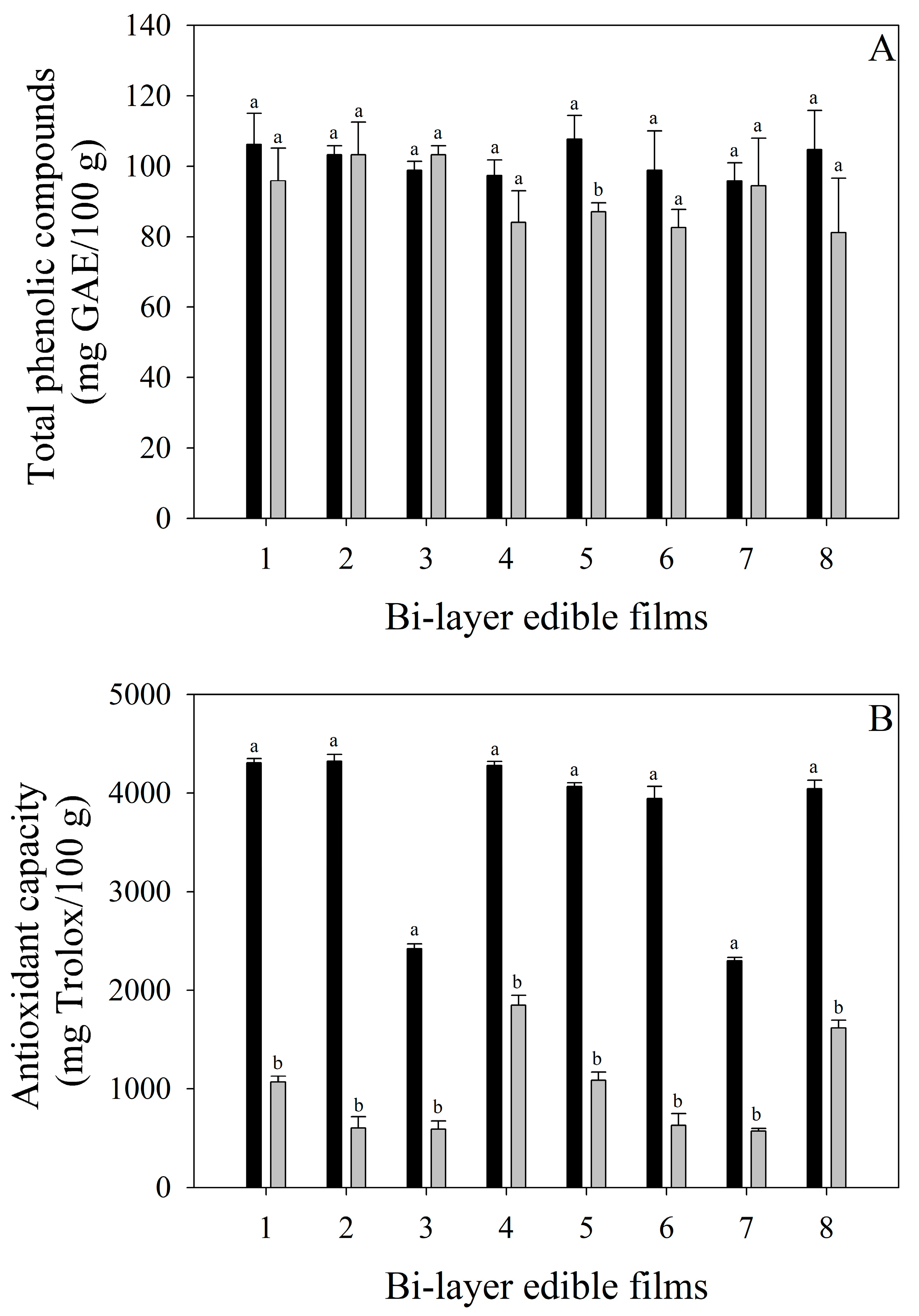
The total phenolic compounds and antioxidant capacity of bi-layer edible films are shown in Figure 2. The total phenolic compounds of all edible films were in the range of 95.87–107.67 mg GAE/100 g (regardless of the addition of mango pulp powder), indicating a possible affectation of other components of edible films (whey powder and starch) on the quantification of phenolic compounds. After the storage time, although the total phenolic compounds of edible films remained constant, the bi-layer edible films formulated with LAB-fermented whey and mango solution stored at 20 °C (system 5) presented a significant reduction (p < 0.05) in their total phenolic compounds. This reduction is quite interesting since system 5 also presented the highest total color change (∆E: 28.23), inferring that the polyphenol oxidase enzyme is responsible for the total phenolic compounds’ affectation. On the other hand, the antioxidant capacity of bi-layer edible films was affected by adding mango pulp powder, showing an increase in the antioxidant capacity of those edible films formulated with the fruit. After 28 days of storage, a significant reduction (p < 0.05) in all edible films without the effect of the storage temperature was observed, probably as antioxidant compounds are degraded by the oxidative reaction with oxygen, avoiding the oxidation of lipids present in whey. It is interesting to highlight that the reduction in antioxidant capacity without affecting the phenolic compounds is associated with other antioxidant compounds, such as ascorbic acid and carotenoids, also present in mango fruits. This phenomenon may be like those reported by Vithana et al. [42], who indicated that as the mango ripens, the total phenolic compounds decrease without affecting the antioxidant capacity due to the increase in ascorbic acid and carotenoids.
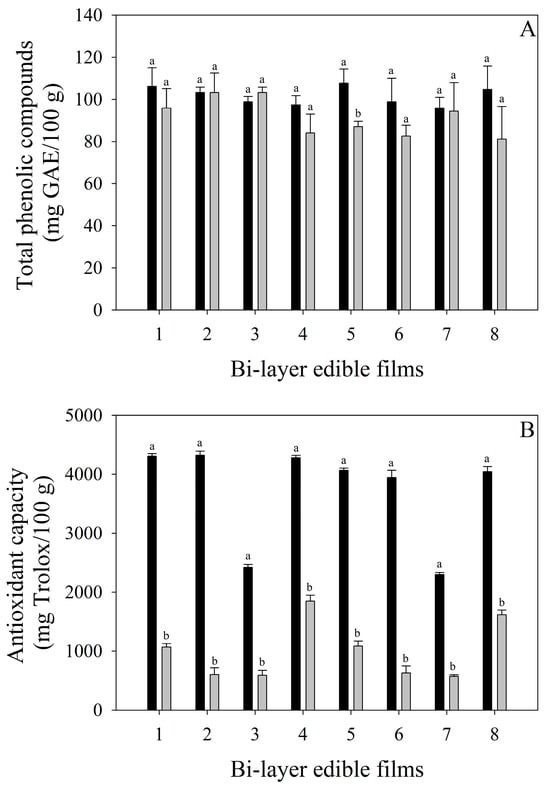
Figure 2.
Total phenolic compounds (A) and antioxidant capacity (B) during the storage of bi-layers edible films. Black columns represent the values at the beginning of the storage. Gray columns represent the values after 28 days of storage. Bars indicate standard deviation. Different letters (a and b) for each edible film indicate significant differences (p > 0.05) between storage times.
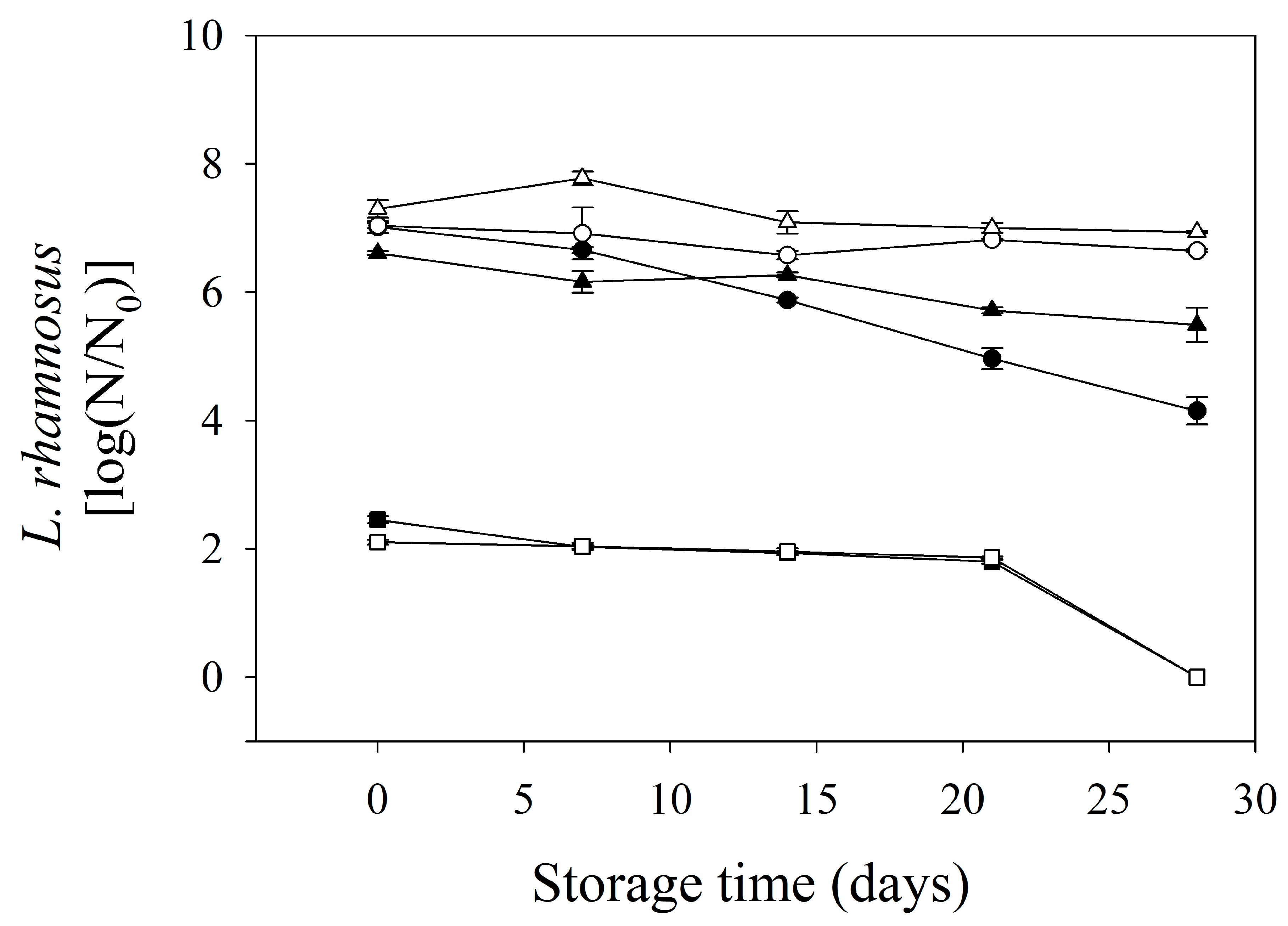
Figure 3 shows the viability of probiotics in formulated edible films during storage. Only LAB-fermented whey and mango solution edible films were evaluated (systems 1, 3, 4, 5, 7, and 8). Evidently, though all solutions to be fermented were inoculated with the same L. rhamnosus population (1 × 109 CFU/mL), bi-layer edible films presented different probiotic counts. In this sense, the edible films made of LAB-fermented mango solution without whey (systems 4 and 8) showed a reduction in their microbial load, which can be due to the absence of protein, which provides the essential nutrients for microbial survival and can scavenge free radicals produced during the drying process of edible film production [43]. After storage, the results indicated that storage time and temperature affected the probiotic viability. The edible films stored at 20 °C presented a reduction in the probiotic count between 1.11 and 2.86 log cycles, while these edible films, at 4 °C, showed reductions in the range of 0.36–0.39 log cycles; interestingly, edible films formulated with LAB-fermented whey solution showed the lowest microbial decrease during storage, regardless of the storage temperature. The mango acidity (pH = 3.5) probably affected the viability of L. rhamnosus during storage. Moreover, edible films without whey in their formulation did not contain probiotics at the end of the storage time, regardless of the temperature, while edible films stored at 4 °C formulated with LAB-fermented whey and mango (system 1) solution and LAB-fermented whey (system 3) solution showed a microbial count higher than six log cycles, which is the minimum required to exert its beneficial effects on the gastrointestinal system [44].
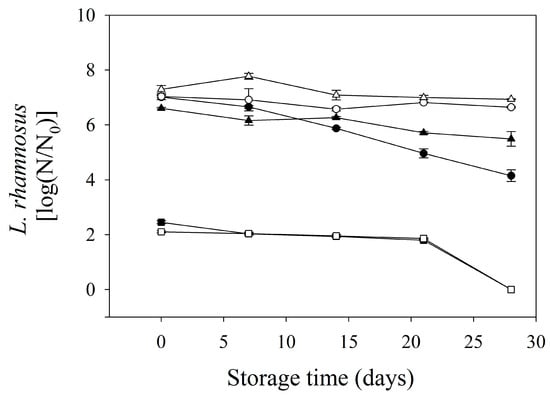
Figure 3.
Probiotic survival during storage in formulated bi-layers edible films. ○ and ● are the systems 1 and 5 stored at 4 and 20 °C, respectively. ∆ and▲ are the systems 3 and 7 stored at 4 and 20 °C, respectively. □ and ■ are the systems 4 and 8 stored at 4 and 20 °C, respectively.
3.3. Sensory Acceptance of Sushi Covered with Selected Bi-Layer Edible Film
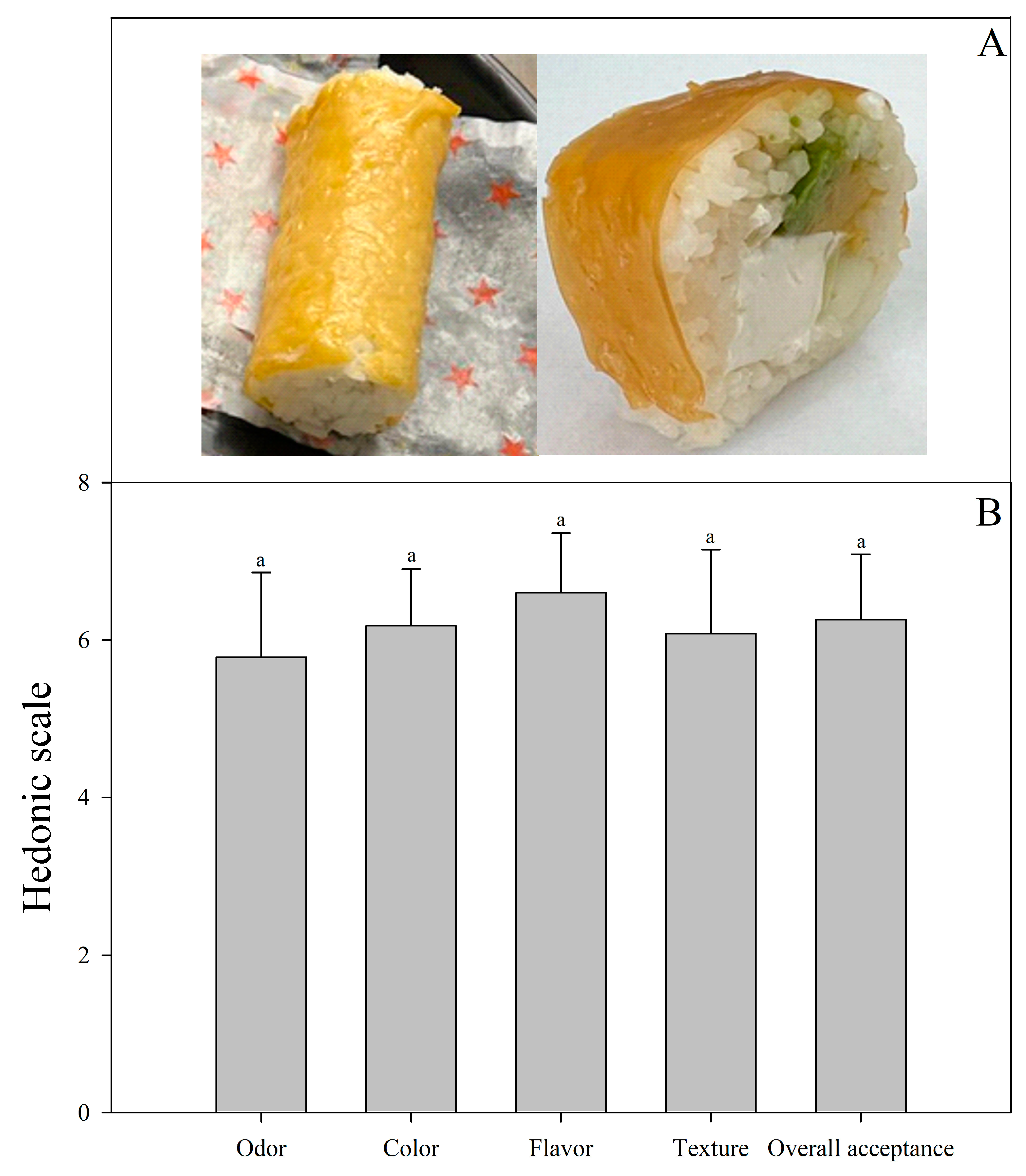
At the end of the storage, a bi-layer edible film formulated with LAB-fermented whey and mango solution stored at 4 °C (system 5) was used to cover sushi, as shown in Figure 4a. This edible film was selected because it had a higher antioxidant capacity and probiotic survival during storage. The results indicated that sushi covered with the bi-layer edible film was well accepted by consumers (Figure 4b), showing a response between I like it and I like it much, which is probably due to the pleasant and sour taste of the mango fruit and because consumers are used to eating sushi with a thin coating, generally made of nori algae, fish, or seafood.
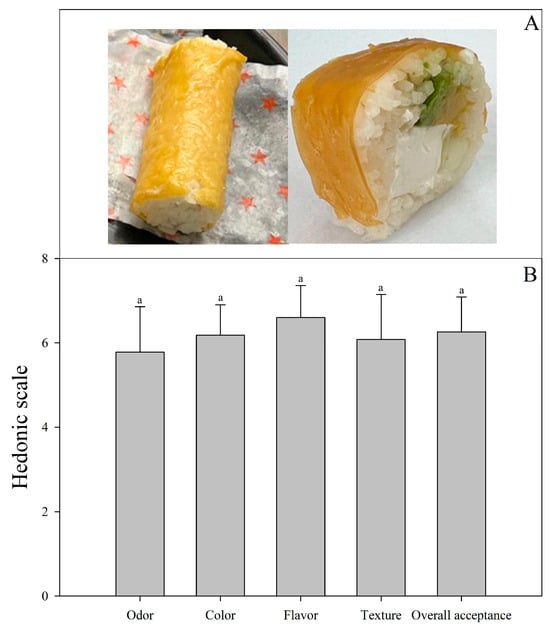
Figure 4.
Bi-layer edible film application (A) and sensory acceptance evaluation (B). Bars indicate standard deviation. Similar letters indicate insignificant differences (p > 0.05).
One of the main characteristics of the application of edible films is an adequate selection of the food to which it will be applied due to their direct contact with them; films must present adequate sensory properties [45,46]. In this sense, Fierro-Corona et al. [43] indicated that edible films based on LAB-fermented whey solution and starch were well-accepted (receiving responses such as I like it and I like it very much) by consumers of Manchego-type cheese; however, Kulawik et al. [47] pointed out that edible coatings based on furcellaran–gelatin applied to salmon sushi were detected by the consumers, indicating disagreements in terms of its color and general appearance.
4. Conclusions
Bi-layer edible films based on starch as the primary layer and LAB-fermented whey and/or mango solutions as the secondary layer were developed and applied (selected edible film) for covering sushi. The mango pulp powder addition in the formulation increased the yellow color (b* parameter) and the antioxidant capacity of edible films without affecting the moisture, WVP, EAB, and TS of edible films. After 28 days of storage, the browning phenomenon was observed in all edible films, being higher in those edible films containing mango powder; however, these bi-layer edible films presented a higher antioxidant capacity. On the other hand, at low temperatures of storage, probiotic microorganisms in edible films formulated with LAB-fermented whey and whey plus mango solutions presented adequate microbial load (106 CFU/g) to exert their beneficial effects. The application of selected bioactive bi-layer edible film to sushi was well accepted by consumers, which is paramount for adding health-promoting compounds to this product.
Author Contributions
Conceptualization, data acquisition, and methodology: P.H.-C. Data acquisition, investigation, and methodology: B.A.M.-G. Validation and supervision: K.H.E.-S. Data acquisition and supervision: C.R.-L. Validation and supervision: S.d.C.B.-B. Validation and supervision: S.V.A.-R. Supervision, validation, visualization, and writing—original draft: I.I.R.-L. Conceptualization, formal analysis, funding acquisition, and writing—original draft: C.E.O.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Vicerrectoría de Investigación y Estudios de Posgrado of the Benemérita Universidad Autónoma de Puebla (project number: 000234-VIEP2023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Petkoska, A.T.; Daniloski, D.; D’Cunha, N.M.; Naumovski, N.; Broach, A.T. Edible packaging: Sustainable solutions and novel trends in food packaging. Food Res. Int. 2021, 140, 109981. [Google Scholar] [CrossRef] [PubMed]
- Bou-Mitri, C.; Adbessater, M. Food packaging design and consumer perception of the product quality, safety, healthiness and preference. Nutr. Food Sci. 2021, 51, 71–86. [Google Scholar] [CrossRef]
- Mahmud, J.; Sarmast, E.; Shankar, S.; Lacroix, M. Advantages of nanotechnology developments in active food packaging. Food Res. Int. 2022, 154, 111023. [Google Scholar] [CrossRef] [PubMed]
- Sid, S.; Mor, R.S.; Kishore, A.; Singh, V. Bio-sourced polymers as alternatives to conventional food packaging materials: A review. Trends Food Sci. Technol. 2021, 115, 87–104. [Google Scholar] [CrossRef]
- Das, D.; Panesar, P.; Saini, C.S.; Kennedy, J.F. Improvement in properties of edible film through non-thermal treatments and nanocomposite materials: A review. Food Packag. Shelf Life 2022, 32, 100843. [Google Scholar] [CrossRef]
- Hernández-Carranza, P.; Fierro-Corona, G.; Tapia-Maruri, D.; Ruíz-Martínez, I.; Ávila-Reyes, S.V.; Ruiz-López, I.I.; Ochoa-Velasco, C.E. Bioactive edible films based on LAB-fermented whey solution and potato starch: Characterization and storage behavior. Food Bioproc. Technol. 2023, 16, 3045–3056. [Google Scholar] [CrossRef]
- Mujtaba, M.; Lipponen, J.; Ojanen, M.; Puttonen, S.; Vaittinen, H. Trends and challenges in the development of bio-based barrier coating materials for paper/cardboard food packaging; a review. Sci. Total Environ. 2022, 851, 158328. [Google Scholar] [CrossRef]
- Ceylan, H.G.; Atasoy, A.F. New bioactive edible packing systems/symbiotic edible films/coatings as carries of probiotics and prebiotics. Food Bioproc. Technol. 2023, 16, 1413–1428. [Google Scholar] [CrossRef]
- Chavan, P.; Lata, K.; Kaur, T.; Jambrak, A.R.; Sharma, S.; Riy, S.; Sinhmar, A.; Theory, R.; Singh, G.P.; Aayush, K.; et al. Recent advances in the preservation of postharvest fruits using edible films and coatings: A comprehensive review. Food Chem. 2023, 148, 135916. [Google Scholar] [CrossRef]
- Yildirim-Yalcin, M.; Tornuk, F.; Toker, O.S. Recent advances in the improvement of carboxymethyl cellulose-based edible films. Trends Food Sci. Technol. 2022, 129, 179–193. [Google Scholar] [CrossRef]
- Chen, W.; Ma, S.; Wang, Q.; McClements, D.J.; Liu, X.; Ngai, T.; Liu, F. Fortification of edible films with bioactive agents: A review of their formation, properties, and application in food preservation. Crit. Rev. Food Sci. Nutr. 2022, 62, 5029–5055. [Google Scholar] [CrossRef]
- Yan, J.; Luo, Z.; Ban, Z.; Lu, H.; Li, D.; Yang, D.; Aghdam, M.S.; Li, L. The effect of the layer-by-layer (LBL) edible coating on strawberry quality and metabolites during storage. Postharvest Biol. Technol. 2019, 147, 29–38. [Google Scholar] [CrossRef]
- Ajesh Kumar, V.; Pravitha, M.; Yadav, A.; Pandiselvam, R.; Srivastav, P.P. Influence of ultrasonic application on soybean aqueous extract based composite edible film: Characterization and their food application. Food Hydrocoll. 2023, 135, 108210. [Google Scholar] [CrossRef]
- Han, B.; Che, P.; Guo, J.; Yu, H.; Zhong, S.; Li, D.; Liu, C.; Feng, Z.; Jiang, B. A novel intelligent indicator film: Preparation, characterization, and application. Molecules 2023, 28, 3384. [Google Scholar] [CrossRef]
- Lauricella, M.; Emanuele, S.; Calvaruso, G.; Giuliano, M.; D’Anneo, A. Multifaceted health benefits of Mangifera indica L. (Mango): The inestimable value of orchads recently planted in Sicilian rural areas. Nutrients 2017, 9, 525. [Google Scholar] [CrossRef] [PubMed]
- Tirado-Kulieva, V.A.; Gutiérrez-Valverde, K.S.; Villegas-Yarlequé, M.; Camacho-Orbegoso, E.W.; Villegas-Aguilar, G.F. Research trends on mango by-products: A literature review with bibliometric analysis. J. Food Meas. Character 2022, 16, 2760–2771. [Google Scholar] [CrossRef]
- Lebaka, V.R.; Wee, Y.-J.; Ye, W.; Korivi, M. Nutritional composition and bioactive compounds in three different parts of mango fruit. Int. J. Environ. Res. Public Health 2021, 18, 741. [Google Scholar] [CrossRef] [PubMed]
- Wall-Medrano, A.; Olivas-Aguirre, F.J.; Ayala-Zavala, J.F.; Domínguez-Avila, J.A.; González-Aguilar, G.A.; Herrera-Cazares, L.A.; Gaytan-Martínez, M. Health benefits of mango by-products. In Food Wastes and By-Products: Nutraceutical and Health Potential; Campos-Vega, R., Oomah, D., Vergara-Castañeda, H.A., Eds.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Ordoñez-Díaz, J.L.; Moreno-Ortega, A.; Roldán-Guerra, F.J.; Ortíz-Somovilla, V.; Moreno-Rojas, J.M.; Pereira-Caro, G. In vitro gastrointestinal digestion and colonic catabolism of mango (Mangifera indica L.) pulp polyphenols. Foods 2020, 9, 1836. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Castellon-Chicas, M.J.; Arbizu, S.; Talcott, S.T.; Drury, N.L.; Smith, S.; Mertens-Talcott, S.U. Mango (Mangifera indica L.) polyphenols: Anti-inflammatory intestinal microbial health benefits, and associated mechanisms of actions. Molecules 2021, 26, 2732. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Charoensiddhi, S.; Xue, X.; Sun, B.; Liu, Y.; El-Seedi, H.R.; Wang, K. A review on the gastrointestinal protective effects of tropical fruit polyphenols. Crit. Rev. Food Sci. Nutr. 2022, 17, 7197–7223. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 20th ed.; AOAC International: Washington, DC, USA, 2000. [Google Scholar]
- Aparicio-Fernández, X.; Vega-Ahuatzin, A.; Ochoa-Velasco, C.E.; Cid-Pérez, S.; Hernández-Carranza, P.; Ávila-Sosa, R. Physical and antioxidant characterization of edible films added with red prickly pear (Opuntia ficus-indica L.) cv. San Martín peel and/or its aqueous extracts. Food Bioproc. Technol. 2018, 11, 368–379. [Google Scholar] [CrossRef]
- Method E96; Standard Test Method for Water Vapor Transmission of Materials. American Society for Testing and Materials, ASTM: West Conshohocken, PA, USA, 1980.
- Method D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. American Society for Testing and Materials, ASTM: West Conshohocken, PA, USA, 1995.
- Hernández-Carranza, P.; Ávila-Sosa, R.; Guerrero-Beltrán, J.A.; Navarro-Cruz, A.R.; Corona-Jiménez, E.; Ochoa-Velasco, C.E. Optimization of antioxidant compounds extraction from fruit by-products: Apple pomace, orange and banana peel. J. Food Process. Preserv. 2016, 40, 103–115. [Google Scholar] [CrossRef]
- He, C.; Sampers, I.; Raes, K. Dietary fiber concentrates recovered from agro-industrial by-products: Functional properties and application as physical carriers for probiotics. Food Hydrocoll. 2021, 111, 106175. [Google Scholar] [CrossRef]
- Greis, M.; Sainio, T.; Katina, K.; Nolden, A.; Partanen, R.; Seppä, L. Dynamic texture perception in plant-based yogurt alternatives: Identifying temporal drivers of liking by TDS. Food Qual. Prefer. 2020, 86, 104019. [Google Scholar] [CrossRef]
- Shahrampour, D.; Khomeiri, M.; Razavi, S.M.; Kashiri, M. Development and characterization of alginate/pectin edible films containing Lactobacillus plantarum KMC. LWT—Food Sci. Technol. 2020, 118, 108758. [Google Scholar] [CrossRef]
- Susmitha, A.; Sasikumar, K.; Rajan, D.; Padmakumar, A.; Nampoothiri, K.M. Development and characterization of corn starch-gelatin based edible films incorporated with mango and pineapple for active packaging. Food Biosci. 2021, 41, 100977. [Google Scholar] [CrossRef]
- Sáez-Orviz, S.; Marcet, I.; Rendueles, M.; Díaz, M. Preparation of edible films with Lactobacillus plantarum and lactobionic acid produced by sweet whey fermentation. Membranes 2022, 12, 115. [Google Scholar] [CrossRef]
- Ceylan, H.G.; Atasoy, A.F. Optimization and characterization of prebiotic concentration of edible films containing Bifidobacterium animalis subs. lactis BB-12® and its application to block type processed cheese. Int. Dairy J. 2022, 134, 105443. [Google Scholar] [CrossRef]
- Sogut, E.; Filiz, E.; Seydim, A.C. Whey protein isolate- and carrageenan-based edible films as carriers of different probiotic bacteria. J. Dairy Sci. 2022, 105, 4829–4842. [Google Scholar] [CrossRef]
- Chen, X.; Cui, F.; Zi, H.; Zhou, Y.; Liu, H.; Xiao, J. Development and characterization of hydroxypropyl starch/zein bilayer edible film. Int. J. Biol. Macromol. 2019, 141, 1175–1182. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Mattoso, L.H.; Wood, D.; Williams, T.G.; Avena-Bustillos, R.J.; McHugh, T.H. Nanocomposite edible films from mango puree reinforced with cellulose nanofibers. J. Food Sci. 2009, 74, N31–N35. [Google Scholar] [CrossRef]
- Farajpour, R.; Emam, D.Z.; Moeini, S.; Tavañolipour, H.; Safayan, S. Structural and physico-mechanical properties of potato starch-olive oil edible films reinforced with zein nanoparticles. Int. J. Biol. Macromol. 2020, 149, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Ramos, Ó.L.; Pereira, J.O.; Silva, S.I.; Fernandes, J.C.; Franco, M.I.; Lopes da Silva, J.A.; Pintado, M.E.; Malcata, F.X. Evaluation of antimicrobial edible coatings from a whey protein isolate base to improve the shelf life of cheese. J. Dairy Sci. 2012, 95, 6282–6292. [Google Scholar] [CrossRef] [PubMed]
- Theerawitayaart, W.; Prodpran, T.; Benjakul, S.; Nilsuwan, K.; de la Caba, K. Storage stability of fish gelatin films by molecular modification or direct incorporation of oxidized linoleic acid: Comparative studies. Food Hydrocoll. 2021, 113, 106481. [Google Scholar] [CrossRef]
- Viana, R.M.; Sá, N.M.S.M.; Barros, M.O.; Borges, M.F.; Azeredo, H.M.C. Nanofibrillated bacterial cellulose and pectin edible films added with fruit purees. Carbohydr. Polym. 2018, 196, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Bravo, M.; Rojas-Zenteno, E.G.; Hernández-Carranza, P.; Ávila-Sosa, R.; Aguilar-Sánchez, R.; Ruiz-López, I.I.; Ochoa-Velasco, C.E. A potential application of mango (Mangifera indica L. cv Manila) peel powder to increase the total phenolic compounds and antioxidant capacity of edible films and coatings. Food and Bioproc. Technol. 2019, 12, 1584–1592. [Google Scholar] [CrossRef]
- Piccirilli, G.N.; Soazo, M.; Pérez, L.M.; Delorenzi, N.J.; Verdini, R.A. Effect of storage conditions on the physicochemical characteristics of edible films based on whey protein concentrate and liquid smoke. Food Hydrocoll. 2019, 87, 221–228. [Google Scholar] [CrossRef]
- Vithana, M.D.K.; Singh, Z.; Johnson, S.K. Harvest maturity stage affects the concentrations of health-promoting compounds: Lupeol, mangiferin and phenolic acids in the pulp and peel of ripe ‘Kensington Pride’ mango fruit. Sci. Hortic. 2019, 243, 125–130. [Google Scholar] [CrossRef]
- Fierro-Corona, G.; Ruiz-López, I.I.; Ochoa-Velasco, C.E.; Hernández-Carranza, P. Effect of edible films’ application on the quality characteristics of Manchego-type cheese during storage. Food Bioproc. Technol. 2023, 16, 2910–2920. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Dalbhagat, C.G.; Mishra, H.N. Emerging technologies and coating materials for improved probiotication in food products: A review. Food Bioproc. Technol. 2022, 15, 998–1039. [Google Scholar] [CrossRef]
- Bizymis, A.P.; Tzia, C. Edible films and coatings: Properties for the selection of the components, evolution through composites and nanomaterials, and safety issues. Crit. Rev. Food Sci. Nutr. 2022, 62, 8777–8792. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.M.; Estevinho, B.; Rocha, F. Preparation and incorporation of functional ingredients in edible films and coatings. Food Bioproc. Technol. 2021, 14, 209–231. [Google Scholar] [CrossRef]
- Kulawik, P.; Jamróz, E.; Zając, M.; Guzik, P.; Tkaczewska, J. The effect of furcellaran-gelatin edible coatings with green and pu-erh tea extracts on the microbiological, physicochemical and sensory changes of salmon sushi stored at 4 °C. Food Control 2019, 100, 83–91. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).