1. Introduction
Lutein and its isomers zeaxanthin and meso-zeaxanthin play crucial roles as carotenoid pigments in the human eye’s macula and are recommended as essential nutraceuticals for addressing Age-Related Macular Degeneration (ARMD), a leading cause of vision loss in industrialized nations. Oxidative stress seems to be a primary factor responsible for the degeneration of the Retinal Pigmented Epithelium [
1]. The European Food Safety Authority (EFSA 2010) advocates a daily intake of 1 mg kg
−1 of lutein per body weight to replenish macular pigments. Consequently, lutein has gained popularity as a nutritional supplement and an antioxidant, also contributing to the prevention of cardiovascular disease and atherosclerosis [
2]. As a natural colorant (INS 161b), lutein is also used in the food industry [
3]. Originally extracted from marigold flowers (e.g.,
Tagetes erecta and
Tagetes patula), lutein has also become accessible from microalgae, akin to astaxanthin and PUFA-rich oils [
4,
5,
6].
The global expansion of microalgae production for diverse purposes, encompassing fuel, animal feed, food, and raw materials for nutraceuticals and pharmaceuticals, is evident [
7]. In microalgae, lutein (3R,3′R,6′R-β,ε-carotene-3,3′-diol) participates in photosystems as an accessory pigment, absorbing excess light energy in the blue region and quenching it, contributing to photoprotection, and even transferring a minor part to chlorophyll [
8,
9]. Unlike marigolds, lutein in microalgae is mainly in a free (unesterified) form [
10], and it can be produced 3 to 6 times more efficiently, reducing land and water usage, potentially transforming the lutein production industry [
11].
Figure 1 illustrates physicochemical characteristics of lutein essential for its extraction, spectrophotometric analysis, and quality control.
Microalgae are known for their ability to accumulate various bioproducts under specific cultivation conditions. Some examples, such as algae from the genera
Chlamydomonas,
Haematococcus,
Scenedesmus,
Arthrospira (
Spirulina), and
Chlorella, can produce high concentrations of lutein [
9,
12]. However, industrial-scale microalgal production currently lags behind the production of the carotenoids astaxanthin (a potent antioxidant) and β-carotene (an antioxidant and provitamin A) from
Haematococcus and
Dunaliella. Other strains can provide lipids for fuel production [
13] and fatty acids such as omega 3 [
5], as in the case of
Neochloris oleoabundans and
Schizochytrium sp., respectively.
Several studies address lutein production from microalgae, but most are limited to the laboratory scale. Factors such as culture medium and large-scale sterilization should be analyzed. Optimizing different conditions is crucial for deciding the stages and conditions for production [
14]. The main objective of this review is to comprehensively analyze data on the industrial production of lutein from microalgae. It covers material balances, production, and productivity, as well as the effective extraction and stabilization of lutein from microalgal biomass, providing an assessment of the technological evolution in recent years.
Figure 1.
Lutein proprieties. (
A) Solubility, (
B) Molecular Structure, (
C) physicochemical properties, and (
D) Absorption Spectra. 1 and 4—Craft and Soares [
15]. 2—Zang et al. [
16] and Sabitha [
17]. 3—National Center for Biotechnology Information [
18]. 5—Ingkasupart et al. [
19]. 6—Manupa et al. [
20].
Figure 1.
Lutein proprieties. (
A) Solubility, (
B) Molecular Structure, (
C) physicochemical properties, and (
D) Absorption Spectra. 1 and 4—Craft and Soares [
15]. 2—Zang et al. [
16] and Sabitha [
17]. 3—National Center for Biotechnology Information [
18]. 5—Ingkasupart et al. [
19]. 6—Manupa et al. [
20].
2. Lutein—A Key Molecule in Many Biological Systems: Roles and Bioactivity
Carotenoid biosynthesis in both microalgal and plant cells occurs predominantly in plastids. However, the structural aspects of the metabolic pathways of these pigments may vary depending on the tissue and plastid characteristics. Consequently, carotenoids synthesized through these pathways may specialize into apocarotenoids, serving in cell signaling or acting as structural components in photosynthesis or photoprotection of the thylakoid membrane [
21,
22].
The distribution of enzymes involved in carotenogenesis varies among plant and microalgal species. For instance, in the genus Arabidopsis, most carotenogenic enzymes are in the chloroplast membrane envelope, with fewer found in the thylakoid membrane. In addition, lutein gene expression can be influenced by abiotic factors such as light, temperature, stress conditions, and carbon sources. In microalgae, chemical inhibitors may also regulate carotenogenesis [
22,
23,
24]. The carbon molecules and energy required for lutein biosynthesis in microalgae come from the methylerythritol phosphate (MEP) and pentose phosphate pathways during heterotrophic nutrition. In contrast, in the autotrophic mode, these molecules are provided by the Calvin cycle (CBB). While the MEP pathway predominantly facilitates plant carbon flow, the mevalonate pathway is also utilized [
23,
25].
The precursor metabolite for carotene synthesis in microalgae and plants is isopentenyl diphosphate (IPP), derived primarily from the MEP pathway. IPP is isomerized to dimethylallyl pyrophosphate (DMAPP), catalyzed by IPP isomerase. Subsequently, IPP is condensed with DMAPP to form Geranylgeranyl pyrophosphate (GGPP). The condensation of two GGPP molecules gives rise to a linear, colorless carotene known as phytoene, converted to lycopene. The enzymes lycopene β-cyclase and lycopene ε-cyclase facilitate the addition of β- and ε-rings to the ends of lycopene molecules. The β-carotene is produced by the action of β-cyclase, while hydroxylation of the α- and β-carotenes gives rise to lutein and zeaxanthin, respectively. Zeaxanthin can be converted into violaxanthin via an epoxidase [
21,
25,
26,
27].
Figure 2 summarizes carotene synthesis in microalgae, highlighting the enzymes involved in the process.
Lutein is a crucial component of a light-harvesting protein complex, LHC-II, associated with photosystem II (PSII) in green plants and algae. Xanthophylls, including lutein, play a vital role in microalgal cells, acting as part of the antenna, capturing light for photosystem II reaction centers in the chloroplast. They also provide photoprotection for the thylakoid membrane, mainly against lipid peroxidation of the thylakoid, demonstrating high light tolerance [
8,
9].
Under high irradiance, xanthophylls can reduce light excitation to the center of the photosystem, thereby reducing oxidative stress. Two groups of xanthophylls present in the microalgal cell are α-carotene derivatives, such as lutein, and β-carotene derivatives, including zeaxanthin, antheraxanthin, and violaxanthin [
8,
28]. Lutein binds to proteins associated with the light-harvesting complex (LHC), part of photosystem II. The accumulation of the pigment is limited by the amount of LHC present in the chloroplast, linking increased lutein production in microalgae to expanded storage of this pigment [
29,
30].
Figure 3 illustrates the arrangement of xanthophylls in the microalgal cell.
3. Microalgae Cultivation and Lutein Production
3.1. Lutein Sources, Up-and-Coming Microalgae, and Cultivation Enhancement
The presence of lutein, and also zeaxanthin, in human blood and tissues is solely the result of ingesting foods containing these xanthophylls. Corn and eggs are significant sources of lutein and zeaxanthin in human diets, and many other foods, such as fruits, nuts, cereals, and vegetables, can contain fair amounts of these pigments. However, lutein can be found in higher concentrations in green leafy vegetables such as spinach and kale [
32], which have lutein contents of 1.1 mg g
−1 and 1.0 mg g
−1, respectively, on a dry basis. In comparison, the lutein content in some microalgae can reach up to 17 mg lutein g
−1 dry biomass [
33,
34].
There is research on industrial microalgae strains capable of high lutein productivity [
35,
36]. Although some microalgae are already produced for direct consumption (mainly
Spirulina sp. and
Chlorella sp.) or for extract valuable fractions (astaxanthin from
Haematococcus sp. and phycocyanin from
Arthrospira sp.), there is no consensus on the best strains that could be used to produce lutein-rich biomass.
Table 1 presents the microalgae with the highest lutein content in the biomass (mg g
−1) and those with the highest lutein productivity (mg L
−1 d
−1). Microalgae from the genus
Chlamydomonas are commonly used in genetic manipulation [
29,
37] to improve lutein content, while
Chlorella is the most studied so far for laboratory and industrial scales [
38].
In terms of cultivation, many advancements have been proposed for microalgae biomass and lutein production: light configuration strategies [
39], abiotic stress, and medium optimization [
23]. The color of light may affect the lutein content within cells. Li et al. [
40] noted that lutein content increased when
Chlorella sp. AE10 was cultivated under blue light, reaching 9.58 mg g
−1, a 63% rise compared to production under white light. The carotenoid protection role can explain this since blue light has higher energy than green and red light. In practical strategy, a two-step culture using white light for eight days, followed by ten days under white and blue lights, increased the lutein content from 1.04 mg g
−1 to 2.06 mg g
−1 in
Chlorococcum humicola [
41]. However, light color strategies may be impractical for large-scale lutein production due to the dependence on sunlight [
42].
Moreover, using stress factors such as nutrient limitation, as is performed with
Haematococcus, usually does not work for the accumulation of lutein because the carotenoid is associated with the photosystem and thus can be seen as a primary metabolite, whose productivity is mainly correlated to biomass productivity, not to a secondary biosynthesis phase. In the same work cited before, nitrogen and phosphorus deprivation drastically reduced lutein content to 1.25 mg g
−1 against 9.58 mg g
−1 in the regular culture medium [
40]. Hence, abiotic stresses, such as high salinities or nutritional deprivation, are associated with decreased lutein content [
43,
44]. Lutein production can be intensified by optimizing biomass production, and this depends on tailored culture media, discussed in the next section.
Table 1.
Microalgae with high lutein content and productivity and important dietary sources of lutein for reference.
Table 1.
Microalgae with high lutein content and productivity and important dietary sources of lutein for reference.
| Microalgae | Culture Medium | Strategy | Lutein Content, Dry Basis (mg g−1) | Productivity (mg L−1 day−1) | Reference |
|---|
| Asterarcys quadricellulare PUMCC 511 | BBM | Optimization | 15.5 | 1.22 | [45] |
| Auxenochlorella protothecoides | Residual | Two stages | 4.99 | 34.13 | [46] |
| Chlamydomonas sp. JSC4 | BG11 | Two stages | 4.24 | 3.25 | [39] |
| Chlamydomonas reinhardtii | n.d. | Gene manipulation | 4.5 | n.d. | [38] |
| Chlorella vulgaris CS-41 | BG11 | 10× Nitrate | 10.5 | 1.3 | [44] |
| Chlorella salina | n.d. | Aeration and light | 10.15 | 5.74 | [47] |
| Chlorella protothecoides UTEX 29 | n.d. | Monascus residue | 9.11 | 4.13 | [48] |
| Chlorella sorokiniana FZU60 | BG11 | Fed-batch N and C | 9.57 | 17.35 | [49] |
| Chlorella zofingiensis CZ-bkt1 | n.d. | Mutant | 13.1 | 1.85 | [50] |
| Chlorella minutissima MCC-27 | BBM | Optimization | 5.58 | 0.65 | [51] |
| Muriellopsis sp. MCH35 | BBM | High-lutein-production strain | 4.2 | 3.81 | [52] |
| Egg yolk (raw) | | | 0.016 | | [32], recalculated * |
| Kale (cooked) | | | 1.011 | |
| Spinach (raw) | | | 1.1 | |
| Spinach (cooked) | | | 1.26 | |
3.2. Nutritional Requirements and Media Composition
Microalgae cultures are known to have similar requirements for growth as higher plants in terms of mineral nutrients. Consequently, soil water extracts initially formed the basis of culture media. From there, the traditional microalgal media used today were developed a century ago by phycology luminaries such as Arnon, Chu, Bold, and Pringsheim and further improved by many others in the following decades [
56]. These media have been successfully used for isolation, adaptation, and maintenance of strains for research but are unsuitable for mass production, being either unbalanced or too expensive.
Many algae are capable of autotrophy and heterotrophy [
57]. According to Arnon et al. [
58], almost 30 elements are required: macronutrients in a g to 100 mg L
−1 range and micronutrients in lower concentrations. If water is readily available, algal growth requires adding three main elements for autotrophic cultivation: C, N, and P. The Redfield ratio of 106C:16N:1P has been widely used as a first nutrient limitation [
59,
60]. Carbon is typically provided as CO
2 through aeration, while N and P are provided as added fertilizers.
Many studies are concerned with comparing traditional media for microalgae growth [
61,
62] and are not concerned with interactions or concentrations of specific compounds. Such comparisons do not represent the medium’s real potential for microalgae growth. Based on Yadav et al. [
63], a comparison between four media (BG11, modified BG11, BBM, and M-8) showed that, after 12 days of cultivation of
Chlorella vulgaris, phosphorus (P) and nitrogen (N) remained in excess. Laboratory culture media are frequently unbalanced regarding the N:P proportion [
64].
Culture conditions are linked to the type of carbon source, inorganic or organic. For example, while most microalgae readily assimilate organic carbon, this cannot be achieved in open photobioreactors because of bacterial and fungi competition for carbon sources. However, it is known that the carbon source does not directly affect lutein synthesis; it has an essential role in biomass production [
65]. Some works emphasize the importance of organic sources [
46,
66] for improving microalgae production, stating that, for mass production, just carbon dioxide is insufficient [
67]. For instance,
Auxenochlorella protothecoides was cultivated in two stages and showed a significant increase in biomass production from 0.66 g L
−1 to 12.65 g L
−1 after glucose supplementation, with a lutein content of 2.70 mg g
−1 and productivity of 12.36 mg L
−1 day
−1. Interestingly, this work indicated that a two-stage cultivation with a heterotrophic phase followed by a phototrophic condition resulted in 2.5× more lutein content in the cell than in the mixotrophic condition as the second stage [
46]. Two-stage cultivation seems to be a promising strategy for lutein production: using a heterotrophic or mixotrophic stage for high biomass production followed by a phototrophic stage for lutein production [
9].
Optimized media become more relevant in large-scale production, as large quantities of each compound are needed, impacting costs. In industry, the residues of nutrients must be treated before water disposal, and limiting compounds can adversely impact production or induce microalgae stress [
68,
69]. Laboratory (traditional) media are generally unsuitable for mass production. Moreover, the regional water quality must be characterized before nutrient supplementation.
3.2.1. Macro- and Micronutrient Optimization Strategies
The medium composition affects the biomass and lutein production yield and productivity [
62]. About 30 chemical elements are required for microalgae cultivation. In traditional media, those elements are combined as anions and cations, resulting in medium recipes mainly containing salts such as cation nitrates, sulfates, or phosphates. Nutrient excess affects the medium’s total salinity [
70], and even excess micronutrients can be toxic; on the other hand, its limitation can cause microalgae growth limitation [
71].
Media components are usually of analytical grade at the laboratory scale and are substituted by less expensive, food-grade reagents for large-scale production. Medium optimization involves evaluating and exploring the microalgae’s maximum potential for growth and lutein production. Laboratory culture media are often a starting point; Dineshkumar et al. [
51] optimized the BBM medium for newly isolated
Chlorella minutissima and improved biomass productivity and lutein content from 0.085 g L
−1 day
−1 and 2.67 mg g
−1 to 0.117 g L
−1 day
−1 and 5.58 mg g
−1, respectively. In another work, isolated
Chlorella vulgaris DSV77 had a lutein content increase of 1.6 mg g
−1 to 5.32 mg g
−1 after medium optimization [
72]. Related articles showed that micronutrients had significantly impacted biomass and lutein production for
C. minutissima, while macronutrients were more relevant for
C. vulgaris. Both media deviated considerably from the original recipes regarding minor components, again showing that medium optimization is critical in developing a new process.
Medium screening is laborious and time consuming but can avoid waste—using too much of a nutrient that will not be totally consumed. Advancements in automation increase throughput; Radzun et al. [
14] simultaneously optimized BM culture media for several newly isolated Chlorophytes, such as
Chlorella sp. and
Micractinium sp., using an automated system, improving the growth rate from less than 0.1 h
−1 to above 0.2 h
−1. As a comparison, the automated screening was performed in eighteen units of 96-well plates, which permitted 246 trials during 2 days for each strain analyzed. A conventional experiment (100 mL scale) with 12 trials took almost 10 days for a single strain screening [
51].
Finally, the water quality is crucial for scale-up and must be characterized before production. Soil extracts can be an excellent alternative for providing microelements.
3.2.2. Nitrogen Sources
Nitrogen is vital for microalgae growth as it is part of structural compounds: proteins and nucleic acids. Most cultivated microalgae can assimilate N as nitrate (NO
3−1), the central N- source in various synthetic media [
9]. Ammonium (NH
4+1) salts and urea ((NH
2)
2CO) have been reported as alternative nitrogen sources for cultivation.
Arthrospira platensis has presented an improved growth rate with both nitrogen sources, with a better biomass production of 1.18 g L
−1 (in 18 days) when the classical Zarrouk medium was supplemented with KNH
4SO
4 (ammonium potassium sulfate) [
73].
Chlorella minutissima cultivation showed that a medium supplemented with a cocktail prepared from effluent that contained different concentrations of NH
4+1, NO
3−1, and NO
2−1 resulted in 100%, 95.2%, and 100% substrate consumption, respectively. The lutein productivity reached 1.2 mg L
−1 day
−1, 1.4 times higher than the control [
74].
Nutritional stress conditions do not lead to lutein accumulation. For instance, the N deprivation showed an 18% loss in lutein content (original content of 4.2 mg g
−1) when
Dunaliella tertiolecta was cultivated with ammonium starvation [
75]. Similarly,
Chlorella protothecoides CS-41 [
76] and
Chlorella sorokiniana FZU60 [
77] did not improve lutein production with N deprivation, accumulating approximately 4.33 mg g
−1 and 9.51 mg g
−1, respectively. Moreover, no significant difference was found between these three N sources. Therefore, research supports the hypothesis that industrial processes should focus on maximizing biomass production. However, this is half of the process—after cultivation, microalgae biomass must be harvested and processed.
4. Biomass Downstream Processing Strategies
Typical lutein products are whole algal biomass or concentrated extracts used as nutraceuticals, feed, or color additives. The typical downstream process for these products starts with biomass harvesting, dewatering, cell disruption, and drying for biomass production; for lutein extracts, the process continues with solvent extraction and lutein recovery for purification and stabilization. The residual water content affects pigment extraction if non-polar solvents (e.g., hexane) are used [
52]. Moisture is less critical for more polar solvents such as ethanol or acetone. Furthermore, alkaline treatment can be performed before solvent extraction. This treatment is commonly applied to flower processing in the marigold industry [
11]. Each stage has different efficiency, energy consumption, and process duration depending on the microalgae type [
78].
4.1. Microalgae Harvesting
Harvesting is the first step after microalgae cultivation, aiming to separate the microalgae cells from the liquid cultivation media. Many solid–liquid separation techniques can be applied for microalgae harvesting, including centrifugation, filtration, coagulation and flocculation, flotation, or a combination of more than one technique [
79]. The choice of the microalgae harvesting process depends on factors such as scale, cost considerations, and the intended use of the harvested microalgae, requiring attention since this step can account for 20–30% of the total microalgae processing cost [
23].
Most processes to recover lutein-rich microalgal biomass use centrifugation. Due to its high separation efficiency, this method is widely applied on all scales, from laboratory to industry. Despite drawbacks such as high energy consumption, extended treatment times, and elevated maintenance and investment costs, it is feasible and efficient for high-value products, as in the case of lutein [
23,
79,
80]. Filtration is another method that presents high efficiency and usually costs less than centrifugation. However, it can take a long time, it requires pressure or vacuum, and membrane fouling or clogging issues can happen, increasing operation and maintenance costs on a large scale [
23,
79].
Flocculation is an adjuvant technology that aggregates microalgal cells, increasing the effective “particle” size and aiding processes like sedimentation, centrifugation, and filtration. It can be applied on a large scale and with various microalgae species [
79,
80]. Salt flocculation, an alternative to chemical flocculation, offers high harvesting efficiencies at a lower cost, demonstrating effectiveness. Furthermore, natural coagulants, like jackfruit seed starch, can be used to harvest
Chlorella sorokiniana for later lutein extraction [
81]. Electroflocculation technology provides broad species applicability and low power consumption but requires electrode maintenance, may introduce metal ion contamination, and can lead to temperature and pH changes [
23].
Certain microalgae can naturally undergo flocculation in response to environmental stresses like nitrogen levels, pH, and dissolved oxygen variations. This phenomenon is known as auto-flocculation, commonly induced at elevated pH, and is less disruptive to cells than centrifugation.
Desmodesmus sp.,
Chlorococcum sp.,
Scenedesmus sp., and
Chlorella sp. are examples of genera that present interesting lutein content and have already been harvested by auto-flocculation [
79,
80,
82]. The biomass concentration must be increased as much as possible, from the cultures’ original 1–10 g L
−1 to the 10–20% by weight that can be applied to the following process, usually a drying step [
83,
84].
4.2. Drying and Its Influence on Extraction
Drying is essential for biomass production but is optional for lutein extraction—in that case, it depends on the solvent used later in the process. Microalgae biomass is usually freeze-dried at the laboratory scale before the lutein extraction [
52,
81]. However, freeze-drying is usually too expensive for use in large-scale, commercial recovery of microalgal products [
80].
On a large scale, the drying process can streamline industry logistics. In an industry that does not perform lutein extraction immediately after harvesting, drying can extend the biomass shelf life until its processing [
85]. Spray drying is the preferred method for large-scale, high-value products, but it may lead to notable degradation of specific algal components, such as pigments. Carotenoids are heat sensitive and highly susceptible to drying temperature and time, e.g., in
Chlorella vulgaris, using spray drying, 30.8% of total carotenoids were lost [
85], presumably from oxidation. The same study proposed using diverse materials such as aquafaba, deactivated baker’s yeast, inulin, and maltodextrin in different ratios to encapsulate the microalgae. In most cases, minimal lutein losses were observed.
In certain instances, the solvent extraction of dry biomass has proven to be considerably more effective in recovering intracellular metabolites, such as lutein, than extracting from wet biomass [
80]. However, drying is hugely energy consuming, and, by eliminating this step, cost and energy savings can be expected [
86]. Studies on lutein extraction directly from wet biomass showed that the high water content is deleterious for non-polar solvent extraction. To avoid this problem, many solvent mixtures are proposed. Moreover, sequential extraction progressively removed the biomass water content, increasing the extraction yield [
86,
87,
88]. For example, extraction of dried and wet biomass of
Chlorella vulgaris showed that wet biomass yielded 2× more lutein (8.5 mg g
−1) than dry biomass (3.9 mg g
−1). The result was achieved with a single-step extraction with ether/ethanol binary solvent, which was higher than hexane single-solvent extraction [
86]. Therefore, a possible industrial option would be using moderately polar solvents, such as ethanol or acetone, in a multistage countercurrent process; that would not be efficient in extracting the non-polar beta-carotene but can be effective for lutein.
4.3. Alkaline Pretreatment
The following unitary process, alkaline treatment, also known as saponification, is applied for two reasons: for lutein transformation of esterified lutein into free lutein [
89] and chlorophyll removal from the biomass [
90]. In almost all references, KOH is the primary reagent for the saponification process [
86,
91,
92]. For saponification, the alkaline treatment uses a minimum of 10% of KOH for complete lutein conversion, although reported concentrations reached 60% of KOH [
93,
94,
95,
96]. For chlorophyll removal, this concentration is considerably lower; no more than 4% is related [
97,
98,
99].
In terms of lutein yield, in
Arthrospira platensis, a quantity of 115.08 µg g
−1 of free lutein could be obtained with 4% KOH treatment [
91], and
Chlorella sorokiniana NIOT-2 yielded 14.86 mg g
−1 of free lutein after 22% KOH treatment [
92]. The alkaline treatment for this process was optimized, and the importance of a temperature between 60 °C and 75 °C for maximum yield was highlighted. Despite the reports that include KOH treatment as a step for carotenoid extraction for microalgae [
91,
100], the practice seems inspired by the marigold industry, and tailored pretreatment should be developed for specific microalgal strains. The lutein esters predominate in marigold flowers, and saponification is necessary for feed or food applications; the ester isomers have an in vivo hydrolysis efficacy of less than 5% [
101]. Interestingly, pharmaceuticals and nutraceuticals benefit from having both free and esterified lutein as one has higher antioxidant properties and the second is more stable and remains for a long time in the blood [
102,
103]. Some microalgae contain mostly free lutein [
10], dispensing harsh alkaline treatment. Even so, alkaline treatment can be beneficial in dissolving the cytoplasmic membrane and improving the process speed in the extraction step. The cell membrane is the main barrier for the extraction of small molecules. Therefore, mechanical disruption of the cell wall is usually unnecessary, although tailored pretreatment for each microalgal species, depending on the cell wall composition, can be necessary for fractionation into multiple products [
7].
4.4. Extraction, Purification, and Stabilization of Lutein
Organic solvents are commonly used for carotenoid extraction. Ethanol, methanol, hexane, acetone, and diethyl-ether [
52,
81,
104] have been widely used for lutein extraction. As an innovative solvent, THF (tetrahydrofuran) is gaining attention due to its high affinity for lutein [
105]. In solvent extraction, the polarity of the solvent plays a significant role. Also, residual intracellular moisture drastically reduces the extractability of non-polar solvents [
86]. In terms of affinity, the relative solubility of lutein in ethanol, methanol, acetone, DMSO, and THF is 300 mg L
−1, 200 mg L
−1, 800 mg L
−1, 1000 mg L
−1, and 8000 mg L
−1, respectively [
15]. However, safe solvents must be used in nutraceutical lutein production, making ethanol a good choice [
9].
Many innovative processes have improved lutein extraction on the lab scale. Supercritical CO
2 extraction from
Haematococcus pluvialis yielded 4.03 mg g
−1 of lutein, and, despite an environmentally friendly solvent, only 52.32% of total lutein could be extracted [
106]. In a pressurized liquid extraction with ethanol, the optimized process for
Chlamydomonas sp. showed a low yield of lutein, only 26% [
107]. Ultrasound [
108] and microwave-assisted extraction [
109] can be highlighted to improve lutein recovery. However, supercritical and pressurized processes have high capital and operational costs. Ultrasound or microwave processing, popular in laboratory studies, are generally not applicable on a large scale [
110].
In conventional extraction (batch mode), multiple steps are necessary for the high-yield recovery of lutein [
111]. Each step is followed by centrifugation for solvent removal, and the biomass is subjected to the subsequent extraction. Yadav et al. [
111] reported that complete lutein extraction by ethanol from
Chlorella vulgaris wet biomass was attained in four consecutive steps. In another work, Gong et al. [
97] obtained 85% lutein recovery from wet and frozen biomass (−20 and −85, respectively) from
Chlorella vulgaris UTEX 265. Similarly, the wet biomass of
Scenedesmus obliquus yielded >95% lutein extracted by methanol after five consecutive extractions [
88]. After the last step of extraction, the residual solvent in the lutein must be purged with N
2 gas (99.9% purity) to avoid oxidation [
105]. In the case of non-GRAS solvent extraction, the lutein oleoresin is redissolved in acetone or ethanol and purged by nitrogen gas until the residual solvent level drops to GRAS-acceptable levels. These small-scale extractions can be readily scaled up since the mass transfer should occur similarly if the biomass-to-solvent ratio, temperature, and mixing conditions are replicated.
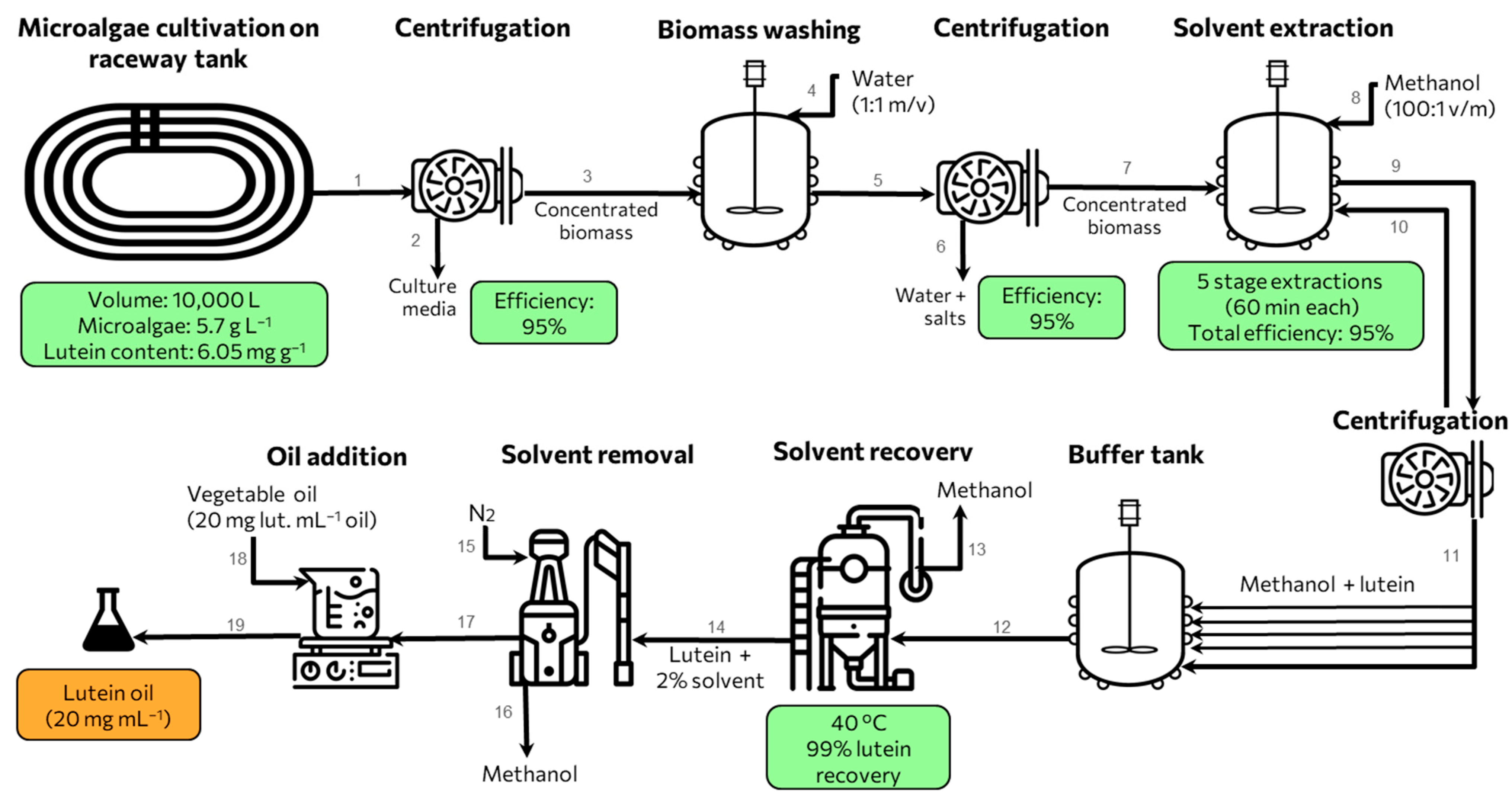
5. Industrial-Scale Microalgae-Based Lutein Production
A conceptual process for industrial lutein production from microalgae, based on average yields from the literature, is presented in
Figure 4. As previously reported, optimization for microalgae biomass production is relevant, being perhaps the most critical aspect. Strains to be used should have been explored in research regarding productivity and safe consumption. Consequently, the microalga
Chlorella minutissima MCC-27, previously optimized for lutein production [
51], was considered for this process. The expected biomass productivity for this strain is 0.57 g L
−1 day
−1, with a lutein content of 6.05 mg g
−1—within the average range among best-reported strains. A single photobioreactor module of 10 m
3 (i.e., a raceway with dimensions of 10 m length, 3 m width, and 0.3 m depth) could achieve, after 95% efficiency centrifugation [
112], a total of 54.15 kg of dry weight biomass after over ten days. Additionally, biomass washing is required to remove residual medium salt (
Figure 4—stream 4). This requires a new centrifugation step, resulting in clean, concentrated biomass (stream 7). However, it results in low salt biomass, enhancing purity and reducing equipment corrosion. To streamline production, several photobioreactors could be run in parallel, with daily sequenced harvesting or cold storage of wet biomass, as suggested by [
97].
Biomass drying is not used in this conceptual process; as discussed, direct extraction from wet biomass may reduce energy consumption. Sequential extraction can effectively overcome reduced extraction efficiency induced by the residual water. After five sequential methanol extractions with overall > 95% recovery (stream 10 represents the biomass return for re-extraction), the lutein solution in stream 12 proceeds to an evaporator where the solvent is recovered (stream 13), and 99% of the lutein remains in the extract [
88], yielding 616.2 g of lutein extract. This first lutein extract must be purged with N
2 to eliminate residual solvent. The lutein extract is then formulated by dissolving it into 28.3 L of vegetal oil for a final concentration of 20 mg mL
−1 lutein adequate for long-term storage or encapsulation into soft gel beads [
105].
To avoid unnecessary costs and steps due to methanol removal after extraction [
88], a suggested pressurized extraction by ethanol with 86.2% lutein yield [
105] could be more feasible due to the safety and GRAS classification. The final concentration of lutein oil can be adjusted for the desired final product. Typical lutein products contain 30 capsules with 25 mg of lutein + zeaxanthin. Based on that, the proposed industrial concept for lutein production could produce over 6000 capsules daily (24,600 capsules every four days).
6. Advancements in Microalgae-Based Lutein and Market Prospects
In the modern landscape of lutein production, marigold flowers (
Tagetes sp.) remain the predominant raw material. However, the high growth rate of microalgae, the ability to control culture conditions, and the ease of harvesting corroborate the advantages of producing lutein using photosynthetic microorganisms [
12,
23]. A notable distinction arises in lutein production rates between marigold flowers and microalgae. While marigold flowers yield a pigment production rate of 120 kg per hectare, microalgae can achieve 350–750 kg per hectare [
11].
GRAS microalgae products (generally recognized as safe by the American Food and Drug Administration) are developed and marketed with high added value. For instance,
Chlorella biomass, primarily produced by companies in Taiwan and Germany, is sold as a nutraceutical for food supplementation. Production levels range from 130 to 450 tons annually, with an average price of 44 USD kg
−1.
Haematococcus-derived astaxanthin is marketed at an average price of 2500 to 7000 USD kg
−1, predominantly produced in the United States and Japan, with around 300 tons per year produced worldwide. β-carotenes are extracted from the microalga
Dunaliella, with global production reaching 1200 tons per year, mainly in Australia and Israel, and sold for an average of 300 to 1500 USD kg
−1 [
113]. On the other hand, some GRAS microalgae are recognized as lutein producers, i.e.,
Haematococcus, Dunaliella, Scenedesmus, and
Chlorella; however, these studies have been conducted on a small scale [
11]. The European Union approved the lutein pigment obtained from Tagetes (E161b) as a colorant in food and for medical and pharmaceutical industries with a daily 1 mg kg
−1 body weight [
27].
The lutein market was approximately USD 135 million in 2015. The Compound Annual Growth Rate (CAGR) is expected to be 6.1% during 2020–2027, reaching USD 369 million in 2024 and USD 491.4 million in 2029 [
114,
115], and countries such as India, China, and Japan hold around 60% of the market; companies Biomed Ingredients, India Glycols Limited, Prakruti Products, and Bio-gen Extracts Private Limited are the major players, and the leading applications are beverages and health supplements [
9].
Knowledge and innovation are strictly related; therefore, patents and articles were analyzed to determine the status of innovations in producing lutein from microalgae. The search was performed for patents using the Derwent Innovations database, while the Scopus database was used for articles. One hundred and eight patents and one hundred and twelve articles (review and experimental) were retrieved.
Figure 5A illustrates the evolution of applied papers and articles, which follows an increasing trend with some natural fluctuations. It was observed that, since 2010, there has been a surge in technological growth mainly in genetic manipulation, production conditions, and extraction of lutein from microalgae due to the commercial interest in this pigment. The steady growth over the last few years has made lutein extraction an emerging technology developed jointly between companies, research centers, and universities (
Figure 5B). According to WIPO, China has the most patents applied, followed by France and India, which corroborates that the primary market is focused in Asia and Europe (
Figure 5C).
7. Future Perspectives
Microalgal biomass is a rich source of pigments and other valuable bioproducts such as proteins, carbohydrates, essential vitamins, and fatty acids. Large-scale microalgae production is now a reality for selected strains that produce nutritional biomass (mainly Arthrospira and Chlorella) and carotenoid products (mainly Astaxanthin from Haematococcus sp. and β-carotene from Dunaliella). Lutein from microalgae has the potential to be the next big product in the microalgae market. Although the existing processes for microalgal products, especially carotenoids, can be adapted for lutein production, targeted process development can be more efficient. Based on the prior discussion, the following aspects of technological development seem particularly promising.
Strain selection—The lutein content varies considerably among different microalgal species. Algal strains with high lutein content, above 1% of the dry weight, and high productivities—above 5 mg L−1 d−1—have already been studied and can be used in large-scale processes. Novel strains can be obtained through random mutagenesis or genetic manipulation. However, there is much potential to be explored in nature, and the targeted isolation of strains—e.g., extremophiles that can grow with less microorganism contamination or strains that grow well in agroindustrial effluents—is a promising pathway for developing new strains, intensifying the process.
Culture media development—Research has shown that biomass titers are the most important factor for high lutein productivity after selecting suitable strains. Automation and artificial-intelligence-backed analysis can join classical statistical analysis of experiments for culture media optimization, leading to the rational use of fertilizers. Agroindustrial effluents are also a rich source of water and nutrients for microalgae production and must be evaluated whenever possible.
Culture media recovery—Not all nutrients are absorbed in microalgae cultivation, even if their concentrations are optimized. Some excess concentration may be necessary to facilitate transport and maintain high productivity. However, these nutrients can be partially recycled after biomass recovery, and their composition can be amended for a new production cycle. Recycling may bring back to the photobioreactors an excess of unwanted substances and microorganisms, and therefore must be optimized for each case, but can lead to resource optimization.
Minimal pretreatment and green solvents—As previously discussed, many processes at the laboratory scale borrowed from marigold processing the idea of alkaline treatment to facilitate lutein extraction. However, diversely from plant sources, microalgal biomass is already a suspension of small particles with a large specific surface area, facilitating solvent extraction. In addition, selected strains may have a predominance of unesterified lutein. Therefore, the minimal processing of lutein may prove effective and have a low cost, even if it means longer processing times for efficient extraction. As for the solvents, research shows that using moderate-polarity solvents such as ethanol can both dehydrate biomass and extract lutein but probably require more solvent than processes with previous drying. To compare wet and dry biomass processing and solvent recovery, process economics must be evaluated.
Biorefinery approaches—Downstream processing technologies developed for microalgae in general, such as harvesting, dewatering, and drying, can adapted for lutein production processes. Inverting this idea, existing processes can benefit from lutein extraction in a biorefinery approach. For example, biomass produced for protein production can be partially extracted with solvents for lutein production, and the dried meal can be further processed into proteinaceous meals or bioenergy. A complete economic evaluation must be performed based on preliminary data for this integrated process. It can increase the feasibility of otherwise uneconomical processes such as algal biodiesel production.
8. Conclusions
Lutein is gaining importance as a nutraceutical and pharmaceutical for ocular diseases and as an antioxidant. Lutein is poised to join other microalgal carotenoids on the market, such as β-carotene from Dunaliella sp. and astaxanthin from Haematococcus sp. However, although lutein production seems feasible and has higher productivity than land plant sources, there are challenges, and, thus, there is intensive ongoing research into its production.
Highly productive strains such as Asterarcys quadricellulare and many Chlorella strains were already isolated and can be used to develop efficient industrial processes. However, unlike secondary carotenoids, lutein production does not increase under stress conditions, although it can be modulated by the light quality used. Increasing biomass production is the primary pathway for process intensification and requires culture media optimization. On the lutein recovery side, existing processes (harvesting—drying—extraction—concentration) can be used, but research shows that drying could be skipped if moderately polar solvents are used.
Based on the patent and research landscape analysis, it is clear that lutein production from microalgae is still innovative and growing. Because of the many options, including the integration into biorefineries, lutein production requires a techno-economic evaluation that can be based on the already profuse research available.
Author Contributions
Conceptualization, J.C.d.C. and H.I.; writing—data compilation and original draft preparation, H.I., C.R.S., D.T.M.-A., J.C., G.V.d.M.P., L.P.d.S.V., M.C.M., R.R.A., G.A.R. and J.C.d.C.; writing—review and editing H.I., C.R.S., D.T.M.-A., G.V.d.M.P., L.P.d.S.V., R.R.A., G.A.R. and J.C.d.C.; visualization, H.I., D.T.M.-A., J.C., M.C.M. and J.C.d.C. All authors have read and agreed to the published version of the manuscript.
Funding
The researchers were funded by the Coordination for the Improvement of Higher Ed-ucation Personnel, CAPES foundation—PROEX Program, and the National Council for Scientific and Technological Development, CNPq, grant no.313276/2021-8.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nguyen, D.; Thrimawithana, T.; Piva, T.J.; Grando, D.; Huynh, T. Benefits of Plant Carotenoids against Age-Related Macular Degeneration. J. Funct. Foods 2023, 106, 105597. [Google Scholar] [CrossRef]
- Low, K.L.; Idris, A.; Mohd Yusof, N. Novel Protocol Optimized for Microalgae Lutein Used as Food Additives. Food Chem. 2020, 307, 125631. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, J.C.; Bicas, J.; Fernández, D.; Woiciechowski, A.; Medeiros, A.; Soccol, C. Natural Colorants from Microorganisms. In Biotechnological Production of Natural Ingredients for Food Industry; de Carvalho, J., Bicas, J., Fernández, D., Woiciechowski, A., Medeiros, A., Soccol, C., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2016; pp. 288–321. [Google Scholar]
- Merle, B. Nutrition et Dégénérescence Maculaire Liée à l’âge. J. Fr. Ophtalmol. 2023, 46, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Neylan, K.A.; Johnson, R.B.; Barrows, F.T.; Marancik, D.P.; Hamilton, S.L.; Gardner, L.D. Evaluating a Microalga (Schizochytrium sp.) as an Alternative to Fish Oil in Fish-Free Feeds for Sablefish (Anoplopoma fimbria). Aquaculture 2024, 578, 740000. [Google Scholar] [CrossRef]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, J.C.; Magalhães, A.I.; de Melo Pereira, G.V.; Medeiros, A.B.P.; Sydney, E.B.; Rodrigues, C.; Aulestia, D.T.M.; de Souza Vandenberghe, L.P.; Soccol, V.T.; Soccol, C.R. Microalgal Biomass Pretreatment for Integrated Processing into Biofuels, Food, and Feed. Bioresour. Technol. 2020, 300, 122719. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The Role of the Xanthophyll Cycle and of Lutein in Photoprotection of Photosystem II. Biochim. Biophys. Acta—Bioenerg. 2012, 1817, 182–193. [Google Scholar] [CrossRef]
- Kadri, M.S.; Singhania, R.R.; Anisha, G.S.; Gohil, N.; Singh, V.; Patel, A.K.; Patel, A.K. Microalgal Lutein: Advancements in Production, Extraction, Market Potential, and Applications. Bioresour. Technol. 2023, 389, 129808. [Google Scholar] [CrossRef]
- Aburai, N.; Ohkubo, S.; Miyashita, H.; Abe, K. Composition of Carotenoids and Identification of Aerial Microalgae Isolated from the Surface of Rocks in Mountainous Districts of Japan. Algal Res. 2013, 2, 237–243. [Google Scholar] [CrossRef]
- Lin, J.-H.; Lee, D.-J.; Chang, J.-S. Lutein Production from Biomass: Marigold Flowers versus Microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef]
- Saha, S.K.; Ermis, H.; Murray, P. Marine Microalgae for Potential Lutein Production. Appl. Sci. 2020, 10, 6457. [Google Scholar] [CrossRef]
- Khoo, K.S.; Ahmad, I.; Chew, K.W.; Iwamoto, K.; Bhatnagar, A.; Show, P.L. Enhanced Microalgal Lipid Production for Biofuel Using Different Strategies Including Genetic Modification of Microalgae: A Review. Prog. Energy Combust. Sci. 2023, 96, 101071. [Google Scholar] [CrossRef]
- Radzun, K.A.; Wolf, J.; Jakob, G.; Zhang, E.; Stephens, E.; Ross, I.; Hankamer, B. Automated Nutrient Screening System Enables High-Throughput Optimisation of Microalgae Production Conditions. Biotechnol. Biofuels 2015, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Craft, N.E.; Soares, J.H. Relative Solubility, Stability, and Absorptivity of Lutein and Beta-Carotene in Organic Solvents. J. Agric. Food Chem. 1992, 40, 431–434. [Google Scholar] [CrossRef]
- Zang, L.-Y.; Sommerburg, O.; van Kuijk, F.J.G. Absorbance Changes of Carotenoids in Different Solvents. Free Radic. Biol. Med. 1997, 23, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Thambavani, S.D.; Sabitha, M.A. The Spectral Determination of Chlorophylls a, b and Total Carotenoids Using Various Solvents for Tree Species Growing near Sugar Mill. Asian J. Exp. Chem. 2011, 7, 5–9. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5281243, Lutein. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lutein (accessed on 9 December 2023).
- Ingkasupart, P.; Manochai, B.; Song, W.T.; Hong, J.H. Antioxidant Activities and Lutein Content of 11 Marigold Cultivars (Tagetes spp.) Grown in Thailand. Food Sci. Technol. 2015, 35, 380–385. [Google Scholar] [CrossRef]
- Manupa, W.; Wongthanyakram, J.; Jeencham, R.; Sutheerawattananonda, M. Storage Stability and Antioxidant Activities of Lutein Extracted from Yellow Silk Cocoons (Bombyx mori) in Thailand. Heliyon 2023, 9, e16805. [Google Scholar] [CrossRef]
- Narang, P.K.; Dey, J.; Mahapatra, S.R.; Roy, R.; Kushwaha, G.S.; Misra, N.; Suar, M.; Raina, V. Genome-Based Identification and Comparative Analysis of Enzymes for Carotenoid Biosynthesis in Microalgae. World J. Microbiol. Biotechnol. 2022, 38, 8. [Google Scholar] [CrossRef]
- Shumskaya, M.; Wurtzel, E.T. The Carotenoid Biosynthetic Pathway: Thinking in All Dimensions. Plant Sci. 2013, 208, 58–63. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Yi, L.; Liu, J.; Yang, S.; Liu, B.; Chen, F.; Sun, H. Lutein Production from Microalgae: A Review. Bioresour. Technol. 2023, 376, 128875. [Google Scholar] [CrossRef]
- Park, Y.J.; Park, S.-Y.; Valan Arasu, M.; Al-Dhabi, N.A.; Ahn, H.-G.; Kim, J.K.; Park, S.U. Accumulation of Carotenoids and Metabolic Profiling in Different Cultivars of Tagetes Flowers. Molecules 2017, 22, 313. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, G. Plant Carotenoids: Genomics Meets Multi-Gene Engineering. Curr. Opin. Plant Biol. 2014, 19, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Clotault, J.; Peltier, D.; Berruyer, R.; Thomas, M.; Briard, M.; Geoffriau, E. Expression of Carotenoid Biosynthesis Genes during Carrot Root Development. J. Exp. Bot. 2008, 59, 3563–3573. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, Y.; Li, S.; Nagarajan, D.; Varjani, S.; Lee, D.-J.; Chang, J.-S. Recent Advances in Lutein Production from Microalgae. Renew. Sustain. Energy Rev. 2022, 153, 111795. [Google Scholar] [CrossRef]
- Pineau, B.; Gérard-Hirne, C.; Selve, C. Carotenoid Binding to Photosystems I and II of Chlamydomonas reinhardtii Cells Grown under Weak Light or Exposed to Intense Light. Plant Physiol. Biochem. 2001, 39, 73–85. [Google Scholar] [CrossRef]
- McQuillan, J.L.; Cutolo, E.A.; Evans, C.; Pandhal, J. Proteomic Characterization of a Lutein-Hyperaccumulating Chlamydomonas reinhardtii Mutant Reveals Photoprotection-Related Factors as Targets for Increasing Cellular Carotenoid Content. Biotechnol. Biofuels Bioprod. 2023, 16, 166. [Google Scholar] [CrossRef]
- Xie, Y.; Xiong, X.; Chen, S. Challenges and Potential in Increasing Lutein Content in Microalgae. Microorganisms 2021, 9, 1068. [Google Scholar] [CrossRef]
- Holtzegel, U. The Lhc Family of Arabidopsis thaliana. Endocytobiosis Cell Res. 2016, 27, 71–89. [Google Scholar]
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (Lutein, Zeaxanthin) Content in Fruits, Vegetables and Corn and Egg Products. J. Food Compos. Anal. 2009, 22, 9–15. [Google Scholar] [CrossRef]
- Mary Leema, J.T.; Kirubagaran, R.; Vinithkumar, N.V.; Dheenan, P.S.; Karthikayulu, S. High Value Pigment Production from Arthrospira (Spirulina) platensis Cultured in Seawater. Bioresour. Technol. 2010, 101, 9221–9227. [Google Scholar] [CrossRef]
- Kulkarni, S.; Nikolov, Z. Process for Selective Extraction of Pigments and Functional Proteins from Chlorella vulgaris. Algal Res. 2018, 35, 185–193. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A Review of High Value-Added Molecules Production by Microalgae in Light of the Classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Gonzalez, E.G.; de Carvalho, J.C.; Aulestia, D.T.M.; Gonzalez, O.I.M.; Soccol, C.R. Bioprospection of Green Microalgae Native to Paraná, Brazil Using a Multi-Criteria Analysis: Potential for the Production of Lipids, Proteins, and Carotenoids. Bioresour. Technol. Rep. 2020, 10, 100398. [Google Scholar] [CrossRef]
- Tokunaga, S.; Morimoto, D.; Koyama, T.; Kubo, Y.; Shiroi, M.; Ohara, K.; Higashine, T.; Mori, Y.; Nakagawa, S.; Sawayama, S. Enhanced Lutein Production in Chlamydomonas reinhardtii by Overexpression of the Lycopene Epsilon Cyclase Gene. Appl. Biochem. Biotechnol. 2021, 193, 1967–1978. [Google Scholar] [CrossRef]
- Cordero, B.F.; Couso, I.; León, R.; Rodríguez, H.; Vargas, M. Enhancement of Carotenoids Biosynthesis in Chlamydomonas reinhardtii by Nuclear Transformation Using a Phytoene Synthase Gene Isolated from Chlorella zofingiensis. Appl. Microbiol. Biotechnol. 2011, 91, 341–351. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, R.; Liu, X.; Ho, S.-H.; Xie, Y.; Chen, J. Strategies Related to Light Quality and Temperature to Improve Lutein Production of Marine Microalga Chlamydomonas sp. Bioprocess Biosyst. Eng. 2019, 42, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yuan, Y.; Cheng, D.; Zhao, Q. Effect of Light Quality on Growth Rate, Carbohydrate Accumulation, Fatty Acid Profile and Lutein Biosynthesis of Chlorella sp. AE10. Bioresour. Technol. 2019, 291, 121783. [Google Scholar] [CrossRef] [PubMed]
- Powtongsook, S.; Nootong, K. Photoautotrophic Cultivation of Chlorococcum humicola in Stirred Tank and Airlift Photobioreactors under Different Light Settings and Light Supplying Strategies for Biomass and Carotenoid Production. J. Chem. Technol. Biotechnol. 2019, 94, 3084–3092. [Google Scholar] [CrossRef]
- Gatamaneni Loganathan, B.; Orsat, V.; Lefsrud, M.; Wu, B.S. A Comprehensive Study on the Effect of Light Quality Imparted by Light-Emitting Diodes (LEDs) on the Physiological and Biochemical Properties of the Microalgal Consortia of Chlorella variabilis and Scenedesmus obliquus Cultivated in Dairy Wastewater. Bioprocess Biosyst. Eng. 2020, 43, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Paglia, G.; Magnúsdóttir, M.; Steinarsdóttir, E.A.; Gudmundsson, S.; Palsson, B.Ø.; Andrésson, Ó.S.; Brynjólfsson, S. Effects of Abiotic Stressors on Lutein Production in the Green Microalga Dunaliella salina. Microb. Cell Factories 2014, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Mcclure, D.D.; Nightingale, J.K.; Luiz, A.; Black, S.; Zhu, J.; Kavanagh, J.M. Pilot-Scale Production of Lutein Using Chlorella vulgaris. Algal Res. 2019, 44, 101707. [Google Scholar] [CrossRef]
- Singh, D.P.; Khattar, J.S.; Rajput, A.; Chaudhary, R.; Singh, R. High Production of Carotenoids by the Green Microalga Asterarcys quadricellulare PUMCC 5.1.1 under Optimized Culture Conditions. PLoS ONE 2019, 14, e0221930. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; He, X.; Ma, Q.; Lu, Y.; Bai, F.; Dai, J.; Wu, Q. Photosynthetic Accumulation of Lutein in Auxenochlorella protothecoides after Heterotrophic Growth. Mar. Drugs 2018, 16, 283. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, S.; Rajasree, S.R.R.; Suman, T.Y.; Aranganathan, L.; Thriuganasambandam, R.; Narendrakumar, G. Induction of β, ε-Carotene-3, 3′-Diol (Lutein) Production in Green Algae Chlorella salina with Airlift Photobioreactor: Interaction of Different Aeration and Light-Related Strategies. Biomass Convers. Biorefinery 2020, 11, 2003–2012. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, R.; Tang, Y.; Wang, Z.; Feng, B.; Li, Y. The Growth and Lutein Accumulation in Heterotrophic Chlorella protothecoides Provoked by Waste Monascus Fermentation Broth Feeding. Appl. Microbiol. Biotechnol. 2019, 103, 8863–8874. [Google Scholar] [CrossRef]
- Xie, Y.; Li, J.; Ma, R.; Ho, S.-H.; Shi, X.; Liu, L.; Chen, J. Bioprocess Operation Strategies with Mixotrophy/Photoinduction to Enhance Lutein Production of Microalga Chlorella sorokiniana FZU60. Bioresour. Technol. 2019, 290, 13. [Google Scholar] [CrossRef]
- Huang, W.; Lin, Y.; He, M.; Gong, Y.; Huang, J. Induced High-Yield Production of Zeaxanthin, Lutein, and β-Carotene by a Mutant of Chlorella zofingiensis. J. Agric. Food Chem. 2018, 66, 891–897. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Dhanarajan, G.; Dash, S.K.; Sen, R. An Advanced Hybrid Medium Optimization Strategy for the Enhanced Productivity of Lutein in Chlorella minutissima. Algal Res. 2015, 7, 24–32. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Marticorena, P.; Sepúlveda, C.; Salinas, F.; Cerezal, P.; Riquelme, C. Effect of Drying Methods on Lutein Content and Recovery by Supercritical Extraction from the Microalga Muriellopsis sp. (MCH35) Cultivated in the Arid North of Chile. Mar. Drugs 2020, 18, 528. [Google Scholar] [CrossRef]
- Zhang, R.; Yao, F.; Ning, Z. Characterization of Four Thermogelled Egg Yolk Varieties Based on Moisture and Protein Content. Poult. Sci. 2023, 102, 102499. [Google Scholar] [CrossRef]
- Haytowitz, D.B.; Ahuja, J.K.C.; Wu, X.; Somanchi, M.; Nickle, M.; Nguyen, Q.A.; Roseland, J.M.; Williams, J.R.; Patterson, K.Y.; Li, Y.; et al. USDA National Nutrient Database for Standard Reference, Legacy Release; United States Department of Agriculture: Washington, DC, USA, 2019. [Google Scholar]
- TACO. Nepa—Núcleo de Estudos e Pesquisas em Alimentação; NEPA-UNICAMP: Campinas, Brazil, 2011; p. 161. [Google Scholar]
- Andersen, R.A. Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier Academic Press: Burlington, MA, USA, 2005; Volume 1, ISBN 0-12-088426-7. [Google Scholar]
- Richmond, A. Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Science: Hoboken, NJ, USA, 2004; ISBN 0-632-05953-2. [Google Scholar]
- Arnon, D.I.; McSwain, B.D.; Tsujimoto, H.Y.; Wada, K. Photochemical Activity and Components of Membrane Preparations from Blue-Green Algae. I. Coexistence of Two Photosystems in Relation to Chlorophyll a and Removal of Phycocyanin. Biochim. Biophys. Acta—Bioenerg. 1974, 357, 231–245. [Google Scholar] [CrossRef]
- Geider, R.; La Roche, J. Redfield Revisited: Variability of C:N:P in Marine Microalgae and Its Biochemical Basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef]
- Shurtz, B.K.; Wood, B.; Quinn, J.C. Nutrient Resource Requirements for Large-Scale Microalgae Biofuel Production: Multi-Pathway Evaluation. Sustain. Energy Technol. Assess. 2017, 19, 51–58. [Google Scholar] [CrossRef]
- Ram, S.; Paliwal, C.; Mishra, S. Growth Medium and Nitrogen Stress Sparked Biochemical and Carotenogenic Alterations in Scenedesmus sp. CCNM 1028. Bioresour. Technol. Rep. 2019, 7, 100194. [Google Scholar] [CrossRef]
- Xie, Y.; Lu, K.; Zhao, X.; Ma, R.; Chen, J.; Ho, S.-H. Manipulating Nutritional Conditions and Salinity-Gradient Stress for Enhanced Lutein Production in Marine Microalga Chlamydomonas sp. Biotechnol. J. 2018, 14, 1800380. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.K.; Yadav, M.; Rani, P.; Yadav, A.; Bhardwaj, N.; Bishnoi, N.R.; Singh, A. Screening of Best Growth Media for Chlorella vulgaris Cultivation and Biodiesel Production. Biofuels 2023, 1–7. [Google Scholar] [CrossRef]
- de Carvalho, J.C.; Sydney, E.B.; Assú Tessari, L.F.; Soccol, C.R. Culture Media for Mass Production of Microalgae. In Biofuels from Algae; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. [Google Scholar]
- Chen, J.-H.; Chen, C.-Y.; Chang, J.-S. Lutein Production with Wild-Type and Mutant Strains of Chlorella sorokiniana MB-1 under Mixotrophic Growth. J. Taiwan Inst. Chem. Eng. 2017, 79, 66–73. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lu, I.-C.; Nagarajan, D.; Chang, C.-H.; Ng, I.-S.; Lee, D.-J.; Chang, J.-S. A Highly Efficient Two-Stage Cultivation Strategy for Lutein Production Using Heterotrophic Culture of Chlorella sorokiniana MB-1-M12. Bioresour. Technol. 2018, 253, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.; Mehariya, S.; Karatza, D.; Chianese, S.; Iovine, A.; Casella, P.; Marino, T.; Musmarra, D. Bench-Scale Cultivation of Microalgae Scenedesmus almeriensis for CO2 Capture and Lutein Production. Energies 2019, 12, 2806. [Google Scholar] [CrossRef]
- Rauytanapanit, M.; Janchot, K.; Kusolkumbot, P.; Sirisattha, S.; Waditee-Sirisattha, R.; Praneenararat, T. Nutrient Deprivation-Associated Changes in Green Microalga Coelastrum sp. TISTR 9501RE Enhanced Potent Antioxidant Carotenoids. Mar. Drugs 2019, 17, 328. [Google Scholar] [CrossRef]
- Lv, H.; Cui, X.; Wahid, F.; Xia, F.; Zhong, C.; Jia, S. Analysis of the Physiological and Molecular Responses of Dunaliella salina to Macronutrient Deprivation. PLoS ONE 2016, 11, e0152226. [Google Scholar] [CrossRef]
- De Paepe, J.; Garcia Gragera, D.; Arnau Jimenez, C.; Rabaey, K.; Vlaeminck, S.E.; Gòdia, F. Continuous Cultivation of Microalgae Yields High Nutrient Recovery from Nitrified Urine with Limited Supplementation. J. Environ. Manag. 2023, 345, 118500. [Google Scholar] [CrossRef]
- Randall, D.G.; Naidoo, V. Urine: The Liquid Gold of Wastewater. J. Environ. Chem. Eng. 2018, 6, 2627–2635. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Kwon, J.S.; Kang, S.T.; Kim, B.R.; Jung, Y.; Han, J.G.; Park, J.H.; Hwang, J.K. Optimization of Culture Media for Large-Scale Lutein Production by Heterotrophic Chlorella vulgaris. Biotechnol. Prog. 2014, 30, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, M.; Mehrzad, J.; Najafi, M.F.; Zhiani, R.; Shamsian, S.A.A. Nitrate and Ammonia: Two Key Nitrogen Sources for Biomass and Phycocyanin Production by Arthrospira (Spirulina) platensis. J. Appl. Phycol. 2022, 34, 2271–2281. [Google Scholar] [CrossRef]
- De Bhowmick, G.; Sen, R.; Sarmah, A.K. Consolidated Bioprocessing of Wastewater Cocktail in an Algal Biorefinery for Enhanced Biomass, Lipid and Lutein Production Coupled with Efficient CO2 Capture: An Advanced Optimization Approach. J. Environ. Manag. 2019, 252, 109696. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, Z.E. Ammonium Nutrition Induces Triacylglycerol, β-Carotene, and Lutein Production in Dunaliella tertiolecta Butcher. Turk. J. Fish. Aquat. Sci. 2019, 19, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.M.; Zhang, X.W.; Chen, F. Heterotrophic Production of Biomass and Lutein by Chlorella protothecoides on Various Nitrogen Sources. Enzym. Microb. Technol. 2000, 27, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, J.; Ho, S.-H.; Ma, R.; Shi, X.; Liu, L.; Chen, J. Pilot-Scale Cultivation of Chlorella sorokiniana FZU60 with a Mixotrophy/Photoautotrophy Two-Stage Strategy for Efficient Lutein Production. Bioresour. Technol. 2020, 314, 123767. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.A.; Esteves, A.F.; Pires, J.C.M. Optimization of Microalgal Harvesting with Inorganic and Organic Flocculants Using Factorial Design of Experiments. Processes 2022, 10, 1124. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S.K. Microalgae Harvesting Techniques: A Review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef]
- Molina Grima, E.; Belarbi, E.-H.; Acién Fernández, F.; Robles Medina, A.; Chisti, Y. Recovery of Microalgal Biomass and Metabolites: Process Options and Economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Khoo, K.S.; Chong, Y.M.; Chang, W.S.; Yap, J.M.; Foo, S.C.; Khoiroh, I.; Lau, P.L.; Chew, K.W.; Ooi, C.W.; Show, P.L. Permeabilization of Chlorella sorokiniana and Extraction of Lutein by Distillable CO2-Based Alkyl Carbamate Ionic Liquids. Sep. Purif. Technol. 2021, 256, 117471. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, X.; Chen, J.; Yang, X.; Ho, S.-H.; Wang, B.; Chang, J.-S.; Shen, Y. Enhancing Cell Growth and Lutein Productivity of Desmodesmus sp. F51 by Optimal Utilization of Inorganic Carbon Sources and Ammonium Salt. Bioresour. Technol. 2017, 244, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Kirnev, P.C.S.; de Carvalho, J.C.; Miyaoka, J.T.; Cartas, L.C.; Vandenberghe, L.P.S.; Soccol, C.R. Harvesting Neochloris Oleoabundans Using Commercial Organic Flocculants. J. Appl. Phycol. 2018, 30, 2317–2324. [Google Scholar] [CrossRef]
- de Carvalho, J.C.; Sydney, E.B.; de Souza Kirnev, P.C.; Medeiros, A.B.P.; Soccol, C.R. Technologies for Separation and Drying of Algal Biomass for Varied Applications. In Handbook of Algal Technologies and Phytochemicals; CRC Press: Boca Raton, FL, USA, 2019; Volume II, pp. 241–249. [Google Scholar]
- Stramarkou, M.; Papadaki, S.; Kyriakopoulou, K.; Krokida, M. Effect of Drying and Extraction Conditions on the Recovery of Bioactive Compounds from Chlorella vulgaris. J. Appl. Phycol. 2017, 29, 2947–2960. [Google Scholar] [CrossRef]
- Gong, M.; Wang, Y.; Bassi, A. Process Analysis and Modeling of a Single-Step Lutein Extraction Method for Wet Microalgae. Appl. Microbiol. Biotechnol. 2017, 101, 8089–8099. [Google Scholar] [CrossRef] [PubMed]
- Luengo, E.; Condón-Abanto, S.; Álvarez, I.; Raso, J. Effect of Pulsed Electric Field Treatments on Permeabilization and Extraction of Pigments from Chlorella vulgaris. J. Membr. Biol. 2014, 247, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Di Caprio, F.; Altimari, P.; Pagnanelli, F. Sequential Extraction of Lutein and Β-carotene from Wet Microalgal Biomass. J. Chem. Technol. Biotechnol. 2020, 95, 3024–3033. [Google Scholar] [CrossRef]
- Mc Gee, D.; Archer, L.; Paskuliakova, A.; Mc Coy, G.R.; Fleming, G.T.A.; Gillespie, E.; Touzet, N. Rapid Chemotaxonomic Profiling for the Identification of High-Value Carotenoids in Microalgae. J. Appl. Phycol. 2018, 30, 385–399. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Iovine, A.; Casella, P.; Marino, T.; Karatza, D.; Chianese, S.; Musmarra, D. Enhancing Biomass and Lutein Production from Scenedesmus almeriensis: Effect of Carbon Dioxide Concentration and Culture Medium Reuse. Front. Plant Sci. 2020, 11, 415. [Google Scholar] [CrossRef]
- Sam, K.J.; Nair, M.S.; Velmurugan, S.; Rajarathinam, R.; Arumugam, L. Extraction of Lutein/Zeaxanthin from Arthrospira platensis and Optimisation of the Saponification Process Using the Response Surface Methodology. Indian Chem. Eng. 2023, 65, 249–259. [Google Scholar] [CrossRef]
- Mary Leema, J.T.; Persia Jothy, T.; Dharani, G. Rapid Green Microwave Assisted Extraction of Lutein from Chlorella sorokiniana (NIOT-2)—Process Optimization. Food Chem. 2022, 372, 131151. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.R.; Manirafasha, E.; Pan, X.; Chen, B.-Y.; Lu, Y.; Jing, K. Exploring Biostimulation of Plant Hormones and Nitrate Supplement to Effectively Enhance Biomass Growth and Lutein Production with Thermo-Tolerant Desmodesmus sp. F51. Bioresour. Technol. 2019, 291, 121883. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, E.B.; Soares, A.T.; Da Costa, D.C.; Silva Neto, H.D.A.; Fernandes, V.D.O.; Antoniosi Filho, N.R. A Thermal Water Microalga: Eutetramorus planctonicus as a Promising Source of Fatty Acids and Lutein. J. Environ. Chem. Eng. 2018, 6, 6707–6713. [Google Scholar] [CrossRef]
- Ma, R.; Zhao, X.; Xie, Y.; Ho, S.-H.; Chen, J. Enhancing Lutein Productivity of Chlamydomonas sp. via High-Intensity Light Exposure with Corresponding Carotenogenic Genes Expression Profiles. Bioresour. Technol. 2019, 275, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Othman, R.; Noh, N.; Nurrulhidayah, A.F.; Anis Hamizah, H.; Jamaludin, M.A. Determination of Natural Carotenoid Pigments from Freshwater Green Algae as Potential Halal Food Colorants. Int. Food Res. J. 2017, 24, 468–471. [Google Scholar]
- Gong, M.; Li, X.; Bassi, A. Investigation of Simultaneous Lutein and Lipid Extraction from Wet Microalgae Using Nile Red as Solvatochromic Shift Probe. J. Appl. Phycol. 2018, 30, 1617–1627. [Google Scholar] [CrossRef]
- De Bhowmick, G.; Sarmah, A.K.; Sen, R. Performance Evaluation of an Outdoor Algal Biorefinery for Sustainable Production of Biomass, Lipid and Lutein Valorizing Flue-Gas Carbon Dioxide and Wastewater Cocktail. Bioresour. Technol. 2019, 283, 198–206. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Sen, R. A Sustainable Perspective of Microalgal Biorefinery for Co-production and Recovery of High-value Carotenoid and Biofuel with CO2 Valorization. Biofuels Bioprod. Biorefining 2020, 14, 879–897. [Google Scholar] [CrossRef]
- Gong, Y.; Huang, J. Characterization of Four Untapped Microalgae for the Production of Lipids and Carotenoids. Algal Res. 2020, 49, 101897. [Google Scholar] [CrossRef]
- Spinola, M.V.; Díaz-Santos, E. Microalgae Nutraceuticals: The Role of Lutein in Human Health. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M.A., Xu, J.-L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 243–263. ISBN 978-981-15-0169-2. [Google Scholar]
- Ochoa Becerra, M.; Mojica Contreras, L.; Hsieh Lo, M.; Mateos Díaz, J.; Castillo Herrera, G. Lutein as a Functional Food Ingredient: Stability and Bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- do Nascimento, T.C.; Pinheiro, P.N.; Fernandes, A.S.; Murador, D.C.; Neves, B.V.; de Menezes, C.R.; de Rosso, V.V.; Jacob-Lopes, E.; Zepka, L.Q. Bioaccessibility and Intestinal Uptake of Carotenoids from Microalgae Scenedesmus obliquus. LWT 2021, 140, 110780. [Google Scholar] [CrossRef]
- Rearte, T.A.; Figueroa, F.L.; Gómez-Serrano, C.; Vélez, C.G.; Marsili, S.; Iorio, A.d.F.; González-López, C.V.; Cerón-García, M.C.; Abdala-Díaz, R.T.; Acién-Fernández, F.G. Optimization of the Production of Lipids and Carotenoids in the Microalga Golenkinia Aff. brevispicula. Algal Res. 2020, 51, 102004. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Jesisca; Hsieh, C.; Lee, D.-J.; Chang, C.-H.; Chang, J.-S. Production, Extraction and Stabilization of Lutein from Microalga Chlorella sorokiniana MB-1. Bioresour. Technol. 2016, 200, 500–505. [Google Scholar] [CrossRef]
- Di Sanzo, G.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical Carbon Dioxide Extraction of Astaxanthin, Lutein, and Fatty Acids from Haematococcus pluvialis Microalgae. Mar. Drugs 2018, 16, 334. [Google Scholar] [CrossRef] [PubMed]
- Montero, L.; Sedghi, M.; García, Y.; Almeida, C.; Safi, C.; Engelen-Smit, N.; Cifuentes, A.; Mendiola, J.A.; Ibáñez, E. Pressurized Liquid Extraction of Pigments from Chlamydomonas sp. and Chemical Characterization by HPLC–MS/MS. J. Anal. Test. 2018, 2, 149–157. [Google Scholar] [CrossRef]
- Yaiche Achour, H.; Blanco Llamero, C.; Saadi, S.A.; Zitouni, A.; Señoráns, F.J. Ultrasound Assisted Extraction of Carotenoids from Green and Orange Biomasses of Dunaliella salina DunaDZ1. Ann. Univ. Dunarea Jos Galati. Fascicle VI—Food Technol. 2022, 46, 22–34. [Google Scholar] [CrossRef]
- Gayathri, S.; Rajasree Radhika, S.R.; Suman, T.Y.; Aranganathan, L. Ultrasound-Assisted Microextraction of β, ε-Carotene-3, 3′-Diol (Lutein) from Marine Microalgae Chlorella salina: Effect of Different Extraction Parameters. Biomass Convers. Biorefinery 2018, 8, 791–797. [Google Scholar] [CrossRef]
- Sarkarat, R.; Mohamadnia, S.; Tavakoli, O. Recent Advances in Non-Conventional Techniques for Extraction of Phycobiliproteins and Carotenoids from Microalgae. Braz. J. Chem. Eng. 2023, 40, 321–342. [Google Scholar] [CrossRef]
- Yadav, S.; Bansal, S.; Chaithra, M.L.; Sibi, G. Assessment of Optimal Growth Conditions for Specific Carotenoids Production by Chlorella vulgaris. J. Appl. Nat. Sci. 2020, 12, 550–555. [Google Scholar] [CrossRef]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C.M. Harvesting Techniques Applied to Microalgae: A Review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Markets and Markets. Lutein Market by Form (Powder & Crystalline, Oil Suspension, Beadlet, Emulsion), Source (Synthetic, Natural), Application (Dietary Supplements, Animal Feed, Food, Beverages,), Production Process and Region—Global Forecast to 2028. Available online: https://www.marketsandmarkets.com/Market-Reports/lutein-market-69753879.html (accessed on 10 October 2023).
- Kumar, S.; Deshmukh, R. Microalgae Market: Global Opportunity Analysis and Industry Forecast 2021–2028; Allied Market Research: Portland, OR, USA, 2021. [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).